Abstract
The cytotoxic necrotizing factor 1 (CNF1) activates Rho GTPases by deamidation of glutamine-63 and thereby induces redistribution of the actin cytoskeleton and formation of stress fibers. Here, we have studied the effects of CNF1 on the transepithelial resistance of Caco-2 cells, a human intestinal epithelial cell line, in comparison with the Rho-inactivating toxin B of Clostridium difficile. Whereas toxin B decreased the transepithelial resistance of Caco-2 cells by about 80% after 4 h, CNF1 reduced it by about 40%. Significant changes of the transepithelial resistance induced by CNF1 were detected after 3 h of incubation. Half-maximal effects were observed with 10 and 41 ng of CNF1 and toxin B per ml, respectively. Flux measurement revealed no CNF1-induced increase of fluorescein isothiocyanate-dextran permeation within the first 4 h of incubation and a 2.9-fold increase after 24 h of incubation. In contrast, toxin B induced a 28-fold increase of permeation after 24 h. As detected by rhodamine-phalloidin staining, CNF1 increased polymerization of F actin at focal contacts of adjacent cells and induced formation of stress fibers. The data indicate that not only depolymerization but also polymerization of actin and subsequent reorganization of the actin cytoskeleton alter the barrier function of intestinal tight junctions.
The organization of actin filaments is crucial for the assembly of intestinal tight junctions (31, 32, 35). It is now well-established that Rho GTPases regulate the organization of the cytoskeleton (14, 37, 40). Several communications report on the effects of Rho-modifying bacterial toxins on intestinal epithelial cells (7, 8). The Rho-inactivating toxin Clostridium difficile toxins A and B, which glucosylate Rho GTPases (15, 21, 22), or Clostridium botulinum C3 exoenzyme, which ADP-ribosylates Rho (1, 4, 5), was used to show that breakdown of the actin cytoskeleton causes an increase in intestinal permeability (28, 34). Recently, it was reported that cytotoxic necrotizing factor 1 (CNF1), an ∼115-kDa protein produced by pathogenic Escherichia coli strains (10), activates Rho GTPases by deamidation of glutamine at position 63 (12, 41). This activation of Rho GTPases results in polymerization of F actin, increased formation of stress fibers, and multinucleation of cells.
Here, we show that treatment of Caco-2 cells with CNF1 induces a decrease in transepithelial electrical resistance which is accompanied by an increased paracellular permeability. Thus, activation of Rho GTPases by CNF1 does not support intestinal barrier function, but, similar to inactivation of Rho proteins, leads to disintegration of monolayers.
MATERIALS AND METHODS
Cell culture.
Caco-2 cells of passage 52 were obtained from the German Cancer Research Center (Heidelberg, Germany). Cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. The cells were subcultured every week and seeded on filter inserts (12-mm diameter) at a density of approximately 4 × 104 cells cm−2 for flux studies and determination of transepithelial resistance. For phase-contrast and fluorescence microscopy, cells were seeded on poly-l-lysine-coated coverslips.
Expression and purification of the CNF1 and CNF1-C866S mutant.
The GST-CNF1 vector (pGEX-2T) was transformed into E. coli (BL21-DE3 strain) by heat shock at 42°C (42). Expression of the glutathione S-transferase (GST) fusion protein in BL21 cells growing at 37°C was induced by adding isopropyl-β-d-thiogalactopyranoside (final concentration, 0.2 mM) at an optical density at 600 nm (OD600) of 0.5. Six hours after induction, cells were collected and lysed by sonication in lysis buffer (20 mM Tris-HCl [pH 7.4], 10 mM NaCl, 5 mM MgCl2, 1% Triton, 2.5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) and purified by affinity chromatography with glutathione-Sepharose (Pharmacia). Loaded beads were washed five times in washing buffer A (20 mM Tris-HCl [pH 7.4], 10 mM NaCl, 5 mM MgCl2) and washing buffer B (150 mM NaCl, 50 mM Tris-HCl [pH 7.5]) at 4°C. GST-CNF1 was eluted from the beads by glutathione elution (10 mM glutathione, 50 mM Tris-HCl [pH 8.0]) for 15 min at room temperature. CNF1 was eluted from the beads by thrombin digestion (200 μg of thrombin per ml, 150 mM NaCl, 50 mM triethanolamine-HCl [pH 7.5], 2.5 mM CaCl2) for 45 min at room temperature. Thrombin was then removed by incubation with benzamidine-Sepharose beads.
Purification of C. difficile toxin B and Clostridium limosum exoenzyme C3.
C. difficile toxin B (9, 24) and C. limosum exoenzyme C3 (20) were purified as described elsewhere.
Measurement of transepithelial resistance.
Transepithelial resistance was determined with an epithelial volt-ohm meter (World Precision Instruments, Sarasota, Fla.) equipped with a chamber for filter inserts. Filters with confluent cell monolayers were used at days 6 to 9 after seeding. For long-term experiments, we used standard medium as described above for apical and basolateral bath solutions. Only filters with an initial resistance of ≥100 Ω cm2 were used. The mean of transepithelial resistance of all experiments was 524 ± 803 Ω cm2 (n = 79). Due to high standard deviation of initial resistances, the results were expressed as percentages of the means of initial resistances of each data set. Statistical analyses were performed by the two-tailed unpaired Student t test, for which values of P < 0.05 were considered significant. Values are expressed as means ± standard deviations.
Flux measurement.
For paracellular flux studies, filters were incubated in bicarbonate-buffered Ringer solution (115 mM NaCl, 50 mM NaHCO3, 2.8 mM KH2PO4-K2HPO4 [pH 7.4], 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM glucose) added to the apical and basolateral reservoir. Fluorescein isothiocyanate (FITC)-labeled dextran (molecular mass of 11,000 Da) given to the apical reservoir at a final concentration of 100 μM was used as the marker substance. After 4 and 24 h of incubation, samples were taken from the apical and the basolateral reservoir and the marker substance was measured in a fluorescence spectrophotometer at 475- and 520-nm excitation and emission wavelengths, respectively.
[32P]ADP ribosylation of Rho proteins.
Modification of Rho proteins of Caco-2 cells after treatment with CNF1 and toxin B was checked by subsequent in vitro [32P]ADP ribosylation. Therefore, Caco-2 cells were incubated for 4 h with 100 ng of CNF1 or 100 ng of toxin B per ml. Incubation was stopped by rinsing cells with ice-cold phosphate-buffered saline (PBS). Cells were harvested and homogenized by sonification. The cell lysate was resuspended in ADP ribosylation buffer to a final concentration of 50 mM HEPES (pH 7.4), 5 mM MgCl2, 2.5 mM dithiothreitol, 2.5 mM NAD, 10 mM thymidine, and 0.5 μCi of [32P]NAD in a final volume of 50 μl. To start ribosylation, 0.25 μg of C. limosum C3 toxin was added. Labeled proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent phosphorimaging.
Rhodamine-phalloidin staining.
Cells on coverslips were incubated with CNF1 or toxin B in cell culture medium for the indicated times. After washing with PBS, the cells were fixed in 3% formaldehyde and 0.05% Triton X-100 in PBS for 10 min. The cells were washed three times and incubated in 30 μg of rhodamine-phalloidin per ml in PBS for 30 min. Thereafter, the cells were washed and subjected to fluorescence microscopy.
RESULTS
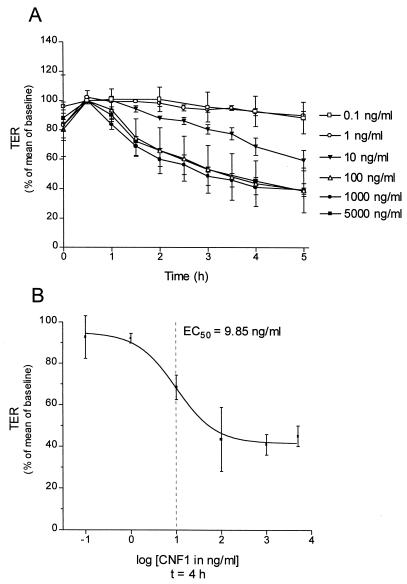
As shown in Fig. 1A, GST-CNF1 decreased transepithelial resistance in a time- and concentration-dependent manner. The effects of GST-CNF1 occurred with a lag phase of 30 to 60 min. Differences in transepithelial resistance during this time were caused by changing the medium. Therefore, the transepithelial resistance value at the time point 1 h was taken as 100%. No effects were observed at concentrations of ≤1 ng of GST-CNF1 per ml within 5 h of incubation. Maximum effects on transepithelial resistance were observed at a concentration of 100 ng of GST-CNF1 per ml. At this concentration, transepithelial resistance decreased by 39% of baseline resistance after 5 h of incubation. The rate of the decrease in transepithelial resistance was slightly accelerated at 50 times-higher concentrations (5 μg of GST-CNF1 per ml). The half-maximal effect (EC50) of GST-CNF1 to decrease transepithelial resistance after 4 h was calculated to be 9.85 ng/ml (Fig. 1B).
FIG. 1.
Effect of GST-CNF1 on the transepithelial resistance of Caco-2 cells. (A) Caco-2 cells incubated for the indicated times with increasing concentrations of GST-CNF1. Thereafter, transepithelial resistance (TER) was determined as described in Materials and Methods. (B) EC50 of GST-CNF1 (9.85 ng/ml) calculated from changes in transepithelial resistance after 4 h of incubation with the toxin. The effect of GST-CNF showed a lag period of 0.5 to 1 h, in which no significant changes in transepithelial resistance were detectable. Data are means ± standard deviations (n = 4).
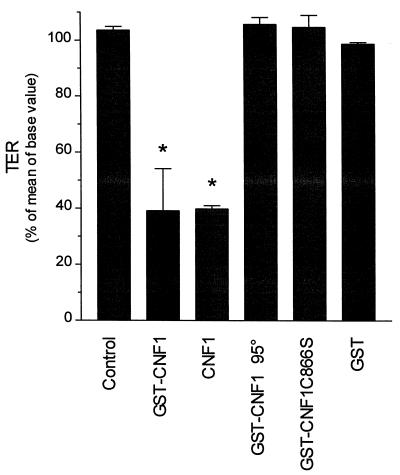
To prove that the effect on transepithelial resistance resulted from activation of Rho GTPases and not from receptor-mediated signals, we applied an inactive GST-CNF1 mutant (CNF1-C866S) (42) to Caco-2 monolayers (Fig. 2). Like heat-inactivated GST-CNF1 (10 min at 95°C), GST-CNF1-C866S caused no decrease in transepithelial resistance (105.6% ± 2.5% and 104.6% ± 4.2%, respectively) after an incubation period of 4 h. We also tested whether GST-CNF1 shows the same activity as CNF1 liberated from GST by thrombin. Like GST-CNF1, CNF1 decreased transepithelial resistance to 39.8% ± 1.1% after 4 h. Purified GST had no effect on transepithelial resistance (98.6% ± 0.6%).
FIG. 2.
Effects of GST-CNF1 and GST-CNF1-C866S on transepithelial resistance. Caco-2 cells were incubated with GST-CNF1 (100 ng/ml), CNF1 liberated from GST (100 ng/ml), heat-inactivated (10 min, 95°C) GST-CNF1 (200 ng/ml) and the enzymatically inactive mutant GST-CNF1-C866S (200 ng/ml), and purified GST (200 ng/ml) for 4 h. Thereafter, transepithelial resistance (TER) was determined. Data are means ± standard deviations (n = 4, *P < 0.05).
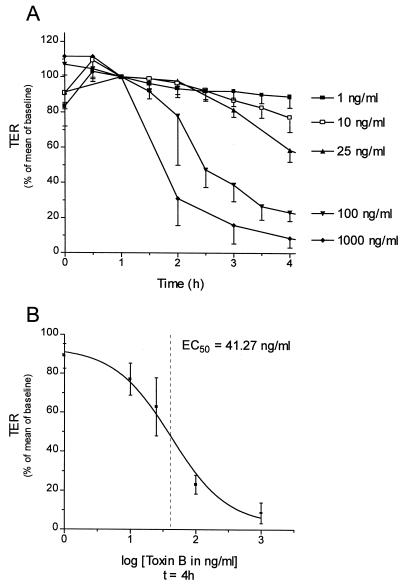
C. difficile toxin B, which inactivates Rho by glucosylation (22), decreased the transepithelial resistance of Caco-2 cells (Fig. 3A). The effects of toxin B on Caco-2 cells were observed at concentrations of higher than 1 ng/ml. A maximal reduction of transepithelial resistance of about 90% was observed at a concentration of 1,000 ng/ml after 4 h of incubation. The EC50 of toxin B was calculated to be 41 ng/ml (Fig. 3B). The effects of GST-CNF1 and of toxin B could not be reversed when the cells were washed 15 min after the addition of toxin (data not shown).
FIG. 3.
Effect of C. difficile toxin B on transepithelial resistance (TER). (A) Caco-2 cells treated with increasing concentrations of toxin B for the indicated times. Thereafter, transepithelial resistance was determined. (B) EC50 (41 ng/ml) of toxin B calculated for changes in the transepithelial resistance occurring after 4 h of incubation with the toxin. Data are means ± standard deviations. (n = 5 to 10).
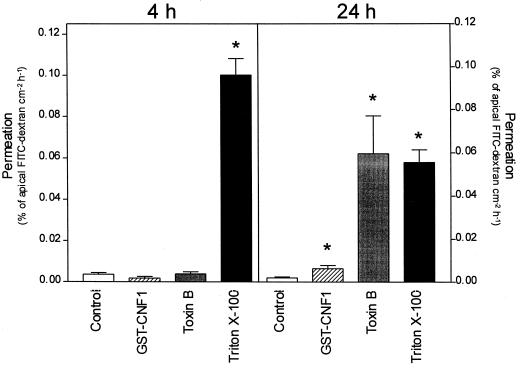
Corresponding to the observed decrease in transepithelial resistance, the paracellular permeability for macromolecules increased after GST-CNF1 or toxin B treatment as measured by fluxes of FITC-dextran. In the controls, FITC-dextran (11,000 Da) exhibited a permeation of 0.0036% ± 0.0008% cm−2 h−1 within the first 4 h and 0.0022% ± 0.00043% cm−2 h−1 after 24 h of incubation under control conditions (Fig. 4). Although GST-CNF1 and toxin B decreased transepithelial resistance after 4 h of incubation, the permeability for FITC-dextran was not increased during this period (0.0018% ± 0.0008% cm−2 h−1 and 0.0038% ± 0.011% cm−2 h−1). As a positive control, cells were disrupted by the addition of 0.05% Triton X-100. Under this condition, increase of flux was 0.1% ± 0.08% cm−2 h− after 4 h. After 24 h of incubation, the fluxes of dextran were significantly increased to 0.0064% ± 0.0016% cm−2 h−1 for GST-CNF1, 0.062% ± 0.018% cm−2 h−1 for toxin B, and 0.058% ± 0.06% cm−2 h−1 for Triton X-100-disrupted cells, compared to control values for the same data set. Differences in permeation values between the 4- and 24-h data sets are due to decreasing gradients of marker during incubation. These flux data were confirmed by measurement of transepithelial resistance after 24 h of incubation. Whereas GST-CNF1-treated cells exhibited a residual transepithelial resistance of 46.6 ± 2.4 Ω cm2, no transepithelial resistance was detected after incubation for 24 h with toxin B (data not shown).
FIG. 4.
Flux measurement with FITC-dextran. Caco-2 cell monolayers were treated with GST-CNF1 (100 ng ml−1) or toxin B (100 ng ml−1) for 4 and 24 h, respectively. At the indicated times, the permeability of the monolayer was determined by measurement of FITC-dextran fluxes as described in Materials and Methods. As a positive control, the cells were treated with 0.05% Triton X-100. Data are means ± standard deviations (n = 4 to 5; ∗, P < 0.05).
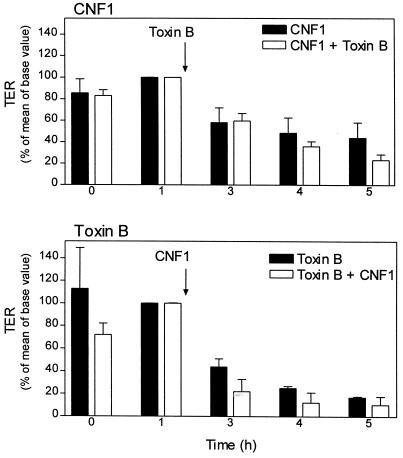
Because Rho proteins are activated by CNF1 and inactivated by toxin B, we investigated the effects of both toxins applied in combination. Preincubation of cells with GST-CNF1 for 60 min showed no alteration in the time course and extent of toxin B-induced decrease in transepithelial resistance compared to nonpreincubated cells (Fig. 5). Addition of GST-CNF1 1 h after application of toxin B did not reduce the effect of toxin B on transepithelial resistance.
FIG. 5.
Effects of CNF1 on toxin B-induced decrease in transepithelial resistance (TER). Caco-2 cell monolayers were incubated for 1 h with CNF1 (100 ng/ml [upper panel]) and toxin B (ToxB, [100 ng/ml [lower panel]), respectively. Thereafter, toxin B (100 ng/ml [upper panel]) or CNF1 (100 ng/ml [lower panel]) was added, and the incubation was continued for the indicated times. At the indicated time points, transepithelial resistance was determined. Data are means ± standard deviations (n = 5).
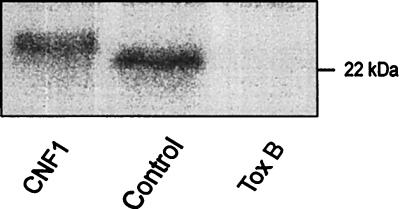
Modifications of Rho proteins of Caco-2 cells treated with CNF1 and toxin B for 4 h were detected by subsequent [32P]ADP ribosylation of Rho. In this assay, C. limosum C3 transferase and [32P]NAD were added to the lysate of toxin-treated cells, and the labeled proteins were analyzed by SDS-PAGE. As shown in Fig. 6, [32P]ADP-ribosylated Rho proteins of GST-CNF1-treated cells were shifted to a higher apparent molecular mass compared to [32P]ADP-ribosylated Rho proteins of control cells, indicating covalent modification of Rho proteins by deamidation (41). In contrast to CNF1-modified GTPases, Rho proteins, which are glucosylated by toxin B, are no longer substrates for C3-catalyzed ADP ribosylation. Accordingly, in Caco-2 cells which were preincubated with toxin B, Rho was not labeled by C3-catalyzed [32P]ADP ribosylation (Fig. 6). Thus, the data indicate that after 4 h of incubation of Caco-2 cells, Rho proteins are completely modified by CNF1 or toxin B.
FIG. 6.
[32P]ADP ribosylation of Rho in lysates of CNF1- and toxin B-treated Caco-2 cells. Caco-2 cells were treated for 4 h without (control) and with GST-CNF1 (100 ng/ml) and toxin B (100 ng/ml), respectively. Thereafter, cells were lysed and Rho proteins were [32P]ADP ribosylated in the presence of [32P]NAD and C3 exoenzyme. The labeled proteins were analyzed by SDS-PAGE and phosphorimaging (shown). C3 labeled Rho with an apparent molecular mass of 23 kDa. Treatment of cells with GST-CNF1, which deamidates Rho, causes a gel shift to higher apparent molecular mass. Toxin B-induced glucosylation of Rho prevents subsequent ADP ribosylation by C3.
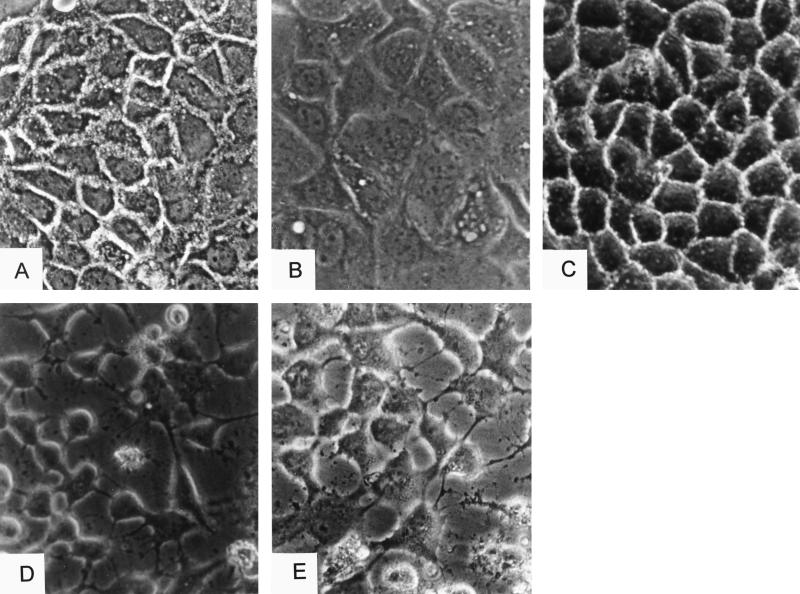
Then, we studied the effects of CNF1 and toxin B on the morphology of confluent Caco-2 cells by phase-contrast microscopy. CNF1 treatment of Caco-2 cells for 5 h caused cell swelling and multinucleation; however, attachment to adjacent cells was still observed. Cell borders of control cells appeared as light dotted lines (Fig. 7A) but were hardly visible after CNF1 treatment by phase-contrast microscopy (Fig. 7B). Cells incubated with the inactive mutant GST-CNF1C866S showed the same morphology as control cells (Fig. 7C). Toxin B induced shrinking and rounding of cells, with complete disintegration of the cell monolayer (Fig. 7D). Cells preincubated for 1 h with CNF1 and subsequently incubated with toxin B for an additional 4 h exhibited the typical toxin B phenotype (Fig. 7E).
FIG. 7.
Phase-contrast microscopy of CNF1- and toxin B-treated Caco2 cells. Caco-2 cells were untreated (A) or treated with GST-CNF1 (100 ng ml−1) (B), with GST-CNF1C866S (100 ng ml−1) (C), or with toxin B (100 ng ml−1) (D) for 6 h. (E) Cells were also treated with CNF1 (100 ng ml−1) for 1 h, and then toxin B was added for a further 5 h. After treatment, the cells were fixed and applied for phase-contrast microscopy.
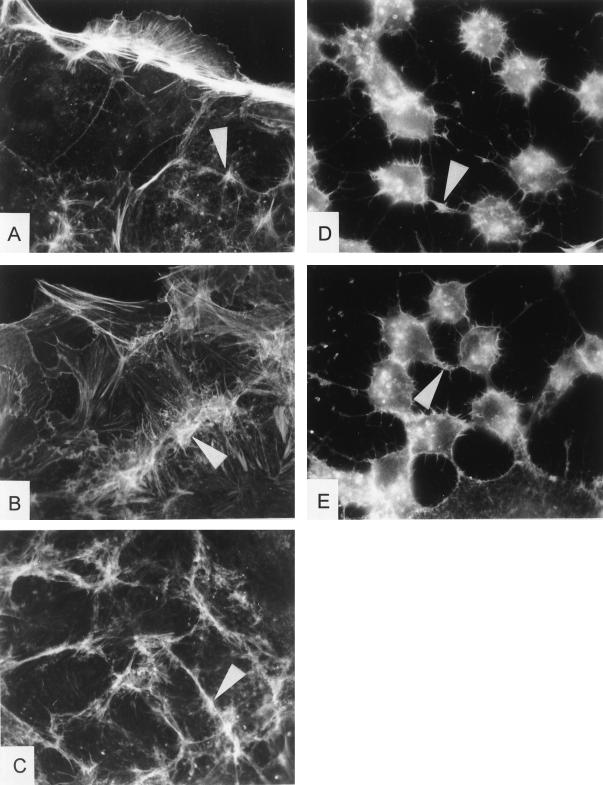
The rhodamine-phalloidin staining of control cells exhibited a strong actin staining at the cortex or growth zone of cells, whereas almost no cytoplasmic filaments or stress fibers were observable (Fig. 8). CNF1 induced elongation of cortical actin cables and formation of stress fibers. Additionally, overlapping growth zones of cells are visible. The CNF1 effect was accompanied by strong formation of actin filaments in the focal contacts of adjacent cells, whereas the actin filaments of GST-CNF1C866S-treated cells remained unaffected. Treatment with toxin B induced a retraction of actin filaments to the perinuclear region with only few actin cables left. When cells were first treated with CNF1 and afterward incubated in the presence of toxin B, cells revealed the toxin B phenotype of the cytoskeleton.
FIG. 8.
Rhodamine-phalloidin staining of CNF1- and toxin B-treated Caco-2 cells. Caco-2 cells were untreated (control) (A) or were treated with GST-CNF1 (100 ng ml−1) (B), GST-CNF1C866S (100 ng ml−1) (C), toxin B (100 ng ml−1) (D), and toxin B after 1 h of preincubation with GST-CNF1 (each 100 ng ml−1) (E) for 6 h. Thereafter, the cells were washed, and F actin was stained with rhodamine-phalloidin. Arrows indicate prominent formation of stress fibers and actin filaments located at the adherens junctions of adjacent cells.
DISCUSSION
Here, we report that treatment of Caco-2 cells with CNF1, which activates Rho by deamidation of glutamine-63 (12), induced an increased permeability of the cell monolayer. The effects of CNF1 on the transepithelial resistance were time and dose dependent and occurred with a lag phase of about 60 min. This delay is most likely due to internalization processes. The effects of CNF1 appear to be specific and depend on the catalytic activity of CNF1, because the inactive mutant GST-CNF1C866S (42) has no effect on transepithelial resistance. This mutant, which has no catalytic activity but still harbors the receptor-binding domain at its N terminus (29), did not induce any morphological changes or alteration of the transepithelial resistance. Therefore, we suggest that all CNF1 effects observed are exclusively due to modification of Rho GTPases and conclude that the activation of Rho GTPases by CNF1 induces a decrease in transepithelial resistance and an increase in paracellular permeability. The mechanism by which CNF1 causes the disintegration of the monolayer is not yet known. It has been reported that CNF1 induces a delayed mortality of HeLa cells after 5 days, due to inhibition of mitosis (6). Because the maximal CNF1-induced reduction (∼40%) of the transepithelial resistance was determined 5 h after the start of toxin treatment of cells, we do not believe that secondary effects (e.g., inhibition of proliferation) significantly contribute to this change in transepithelial resistance.
Staining by F actin of CNF1-treated Caco-2 cells revealed enhanced formation of actin filaments along junctions and cell-cell contact sites of adjacent cells and showed a large increase in stress fibers. Similar effects of CNF1 for other cell lines and tissues were previously reported (36). These findings are in agreement with a role of Rho in formation of cell adhesions (37), adherens junctions (3), and stress fibers (38, 39). The main barriers for paracellular fluxes appear to be tight junctions (33). In addition to the transmembrane protein occludin, which connects the adjacent cells in the region of tight junctions, several proteins located at the cytosolic sites of tight junctions have been identified, including ZO-1 and ZO-2 (13, 17, 19). Rho proteins are involved in the regulation of tight junctions. Specific inactivation of Rho by C3 decreased transepithelial resistance and caused redistribution of ZO-1 proteins (35). In turn, one expects that the activation of Rho by CNF1 increases rather than decreases transepithelial resistance. This discrepancy can possibly be explained as follows. Recent studies in our laboratory suggest that CNF1 modifies not only Rho but also Rac and Cdc42 in intact cells (29a). Moreover, it was reported that depending on the cell type, Rho and Rac/Cdc42 may regulate the actin cytoskeleton in an opposite manner (18, 30). Therefore, one can speculate that the persistent activation of Rac and Cdc42 induced by CNF1 may be important for the increase in transepithelial resistance observed after CNF1 treatment of Caco-2 cells. However, not in line with this assumption are recent studies by Takaishi and coworkers (45) showing that in MDCK cells, Rho but not Rac or Cdc42 is involved in regulation of tight junctions, whereas all three GTPases play important roles in the regulation of cell-cell adhesion sites. CNF1 was shown to stimulate tyrosine phosphorylation of focal adhesion proteins and assembly of focal adhesion plaques (27). Again, both processes would rather support organization of epithelial cell monolayers. Interestingly, lysophosphatidic acid, which is also known to activate RhoA (18, 26), causes recruitment of tyrosine-phosphorylated proteins and actin formation at focal adhesion plaques but decreases transepithelial resistance when given to brain endothelial cells (43). Furthermore, Turner et al. described the importance of the phosphorylation of the myosin light chain in the regulation of epithelial tight junctions and hypothesized that increased tension of the perijunctional actomyosin ring may decrease transepithelial resistance (46). Thus, the effects of CNF1 on transepithelial resistance may be due to activation of MLC phosphorylation via RhoA and Rho-kinase, which was shown to inhibit myosin phosphatase (25).
Recently, Hofman et al. reported that CNF1 decreases transmigration of leukocytes in the T84 monolayer without affecting tight junction permeability (16). The reason for these discrepancies is not known at present. However, in these studies a different cell line revealing higher transepithelial resistances was used, and CNF1 was applied at a concentration (10 ng/ml) lower than that described here.
Fiorentini et al. reported that preincubation with CNF1 for 18 h impairs toxin B-induced effects (11). For studies of the combined actions of toxins on transepithelial resistance, we preincubated Caco-2 cells with CNF1 for only 1 h. A longer preincubation period reduced transepithelial resistance to values which no longer allowed differentiation between the effects of CNF1 and toxin B. Although the EC50 of CNF1 to decrease transepithelial resistance was sixfold lower than that of toxin B, preincubation with CNF1 did not alter the effect of toxin B when it was applied at the same concentrations. These data indicate that toxin B exhibits a dominant effect on Rho. This finding is in line with recent observations that glucosylation of Rho (44) and Ras (23) by C. difficile toxin B and Clostridium sordellii lethal toxin, respectively, uncouples the interaction of the GTPases with their effectors.
In summary, our findings indicate that not only inhibition of Rho proteins by bacterial toxins but also activation of these GTPases by CNF1 causes significant changes in transepithelial resistance and in the permeability of epithelial monolayers. Further studies are necessary to clarify whether these effects are of relevance for a role of CNF1 as a virulence factor of E. coli.
ACKNOWLEDGEMENT
We thank R. Mueller (Medical College Wisconsin) for critical reading of the manuscript.
Footnotes
Corresponding author. Mailing address: Institut für Pharmakologie und Toxikologie, Albert-Ludwigs-Universität Freiburg, Hermann-Herder-Str. 5, D-79104 Freiburg, Germany. Phone: 49 761 203 5313. Fax: 49 761 203 5311. E-mail: aktories@ruf.uni-freiburg.de.
REFERENCES
- 1.Aktories K, Braun U, Rösener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989;158:209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- 2.Aktories K. Bacterial toxins that target Rho proteins. J Clin Invest. 1997;99:827–829. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga V M, Machesky L M, Hall A, Hotchin N A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun U, Habermann B, Just I, Aktories K, Vandekerckhove J. Purification of the 22 kDa protein substrate of botulinum ADP-ribosyltransferase C3 from porcine brain cytosol and its characterization as a GTP-binding protein highly homologous to the rho gene product. FEBS Lett. 1989;243:70–76. doi: 10.1016/0014-5793(89)81220-7. [DOI] [PubMed] [Google Scholar]
- 5.Chardin P, Boquet P, Madaule P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein rho C is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilament in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Rycke J, Mazars P, Nougayrede J-P, Tasca C, Boury M, Herault F, Valette A, Oswald E. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect Immun. 1996;64:1694–1705. doi: 10.1128/iai.64.5.1694-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon S T, Rubin E J, Yakubovich M, Pothoulakis C, LaMont J T, Feig L A, Gilbert R J. Involvement of Ras-related Rho proteins in the mechanisms of action of Clostridium difficile toxin A and toxin B. Infect Immun. 1995;63:1421–1426. doi: 10.1128/iai.63.4.1421-1426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donelli G, Fabbri A, Fiorentini C. Bacteroides fragilis enterotoxin induces cytoskeletal changes and surface blebbing in HT-29 cells. Infect Immun. 1996;64:113–119. doi: 10.1128/iai.64.1.113-119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichel-Streiber C, Harperath U, Bosse D. Purification of two high molecular weight toxins of Clostridium difficile which are antigenetically related. Microb Pathog. 1989;2:307–318. doi: 10.1016/0882-4010(87)90073-8. [DOI] [PubMed] [Google Scholar]
- 10.Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentini C, Donelli G, Matarrese P, Fabbri A, Paradisi S, Boquet P. Escherichia coli cytotoxic necrotizing factor 1: evidence for induction of actin assembly by constitutive activation of the p21 Rho GTPase. Infect Immun. 1995;63:3936–3944. doi: 10.1128/iai.63.10.3936-3944.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 13.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 15.Hecht G, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman P, Flatau G, Selva E, Gauthier M, Le Negrate G, Fiorentini C, Rossi B, Boquet P. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect Immun. 1998;66:2494–2500. doi: 10.1128/iai.66.6.2494-2500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalink K, Van Corven E J, Hengeveld T, Morii N, Narumiya S, Moolenaar W H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jesaitis L A, Goodenough D A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Just I, Mohr C, Schallehn G, Menard L, Didsbury J R, Vandekerckhove J, van Damme J, Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J Biol Chem. 1992;267:10274–10280. [PubMed] [Google Scholar]
- 21.Just I, Fritz G, Aktories K, Giry M, Popoff M R, Boquet P, Hegenbarth S, Von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- 22.Just I, Selzer J, Wilm M, Von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 23.Just I, Selzer J, Hofmann F, Green G A, Aktories K. Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- 24.Just I, Selzer J, Hofmann F, Aktories K. Clostridium difficile toxin B as a probe for Rho GTPases. In: Aktories K, editor. Bacterial toxins. Chapman & Hall; 1997. pp. 160–161. [Google Scholar]
- 25.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 26.Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenaar W H. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J Cell Sci. 1997;110:2417–2427. doi: 10.1242/jcs.110.19.2417. [DOI] [PubMed] [Google Scholar]
- 27.Lacerda H M, Pullinger G D, Lax A J, Rozengurt E. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21(rho)-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J Biol Chem. 1998;272:9587–9596. doi: 10.1074/jbc.272.14.9587. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence J P, Brevetti L, Obiso R J, Wilkins T D, Imura K, Oper R. Effects of epidermal growth factor and Clostridium difficile toxin B in a model of mucosal injury. J Pediatr Surg. 1998;32:430–433. doi: 10.1016/s0022-3468(97)90598-4. [DOI] [PubMed] [Google Scholar]
- 29.Lemichez E, Flatau G, Bruzzone M, Boquet P, Gauthier M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol Microbiol. 1997;24:1061–1070. doi: 10.1046/j.1365-2958.1997.4151781.x. [DOI] [PubMed] [Google Scholar]
- 29a.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. Deamidation of Cdc42 and Rac1 by Escherichia coli cytotoxic necrotizing factor 1-activation of c-Jun-N-terminal kinase in HeLa cells. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 30.Lim L, Hall C, Monfries C. Regulation of actin cytoskeleton by Rho-family GTPases and their associated proteins. Cell Dev Biol. 1996;7:699–706. [Google Scholar]
- 31.Madara J L, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madara J L, Moore R, Carlson S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am J Physiol. 1987;253:C854–C861. doi: 10.1152/ajpcell.1987.253.6.C854. [DOI] [PubMed] [Google Scholar]
- 33.Madara J L. Tight junction dynamics: is paracellular transport regulated? Cell. 1988;53:497–498. doi: 10.1016/0092-8674(88)90562-4. . (Review.) [DOI] [PubMed] [Google Scholar]
- 34.Mahida Y R, Makh S, Hyde S, Gray T, Borriello S P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nusrat A, Giry M, Turner J R, Colgan S P, Parkos C A, Carnes D, Lemichez E, Boquet P, Madara J L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oswald E, Sugai M, Labigne A, Wu H C, Fiorentini C, Boquet P, O’Brien A D. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci USA. 1994;91:3814–3818. doi: 10.1073/pnas.91.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 38.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 39.Ridley A J, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley A J. Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev. 1995;5:24–30. doi: 10.1016/s0959-437x(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Deamidation of Gln63 of Rho induced by Escherichia coli cytotoxic necrotizing factor 1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt G, Selzer J, Lerm M, Aktories K. The Rho-deamidating cytotoxic-necrotizing factor CNF1 from Escherichia coli possesses transglutaminase activity: cysteine-866 and histidine-881 are essential for enzyme activity. J Biol Chem. 1998;273:13669–13674. doi: 10.1074/jbc.273.22.13669. [DOI] [PubMed] [Google Scholar]
- 43.Schulze C, Rubin L L, Staddon J M. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem. 1997;68:991–1000. doi: 10.1046/j.1471-4159.1997.68030991.x. [DOI] [PubMed] [Google Scholar]
- 44.Sehr P, Gili J, Genth H, Just I, Pick E, Aktories K. Glucosylation and ADP-ribosylation of Rho proteins—effects on nucleotide binding, GTPase activity, and effector-coupling. Biochemistry. 1998;57:5296–5304. doi: 10.1021/bi972592c. [DOI] [PubMed] [Google Scholar]
- 45.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner J R, Rill B K, Carlson S L, Carnes D, Kerner R, Mrsny R J, Madara J. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]