Abstract
IMPORTANCE
The rising threat of antibiotic resistance and other adverse consequences resulting from the misuse of antibiotics requires a better understanding of antibiotic use in hospitals in the United States.
OBJECTIVE
To use proprietary administrative data to estimate patterns of US inpatient antibiotic use in recent years.
DESIGN, SETTING, AND PARTICIPANTS
For this retrospective analysis, adult and pediatric in-patient antibiotic use data was obtained from the Truven Health MarketScan Hospital Drug Database (HDD) from January 1, 2006, to December 31, 2012. Data from adult and pediatric patients admitted to 1 of approximately 300 participating acute care hospitals provided antibiotic use data for over 34 million discharges representing 166 million patient-days.
MAIN OUTCOMES AND MEASURES
We retrospectively estimated the days of therapy (DOT) per 1000 patient-days and the proportion of hospital discharges in which a patient received at least 1 dose of an antibiotic during the hospital stay. We calculated measures of antibiotic usage stratified by antibiotic class, year, and other patient and facility characteristics. We used data submitted to the Centers for Medicare and Medicaid Services Healthcare Cost Report Information System to generate estimated weights to apply to the HDD data to create national estimates of antibiotic usage. A multivariate general estimating equation model to account for interhospital covariance was used to assess potential trends in antibiotic DOT over time.
RESULTS
During the years 2006 to 2012, 300 to 383 hospitals per year contributed antibiotic data to the HDD. Across all years, 55.1% of patients received at least 1 dose of antibiotics during their hospital visit. The overall national DOT was 755 per 1000 patient-days. Overall antibiotic use did not change significantly over time. The multivariable trend analysis of data from participating hospitals did not show a statistically significant change in overall use (total DOT increase, 5.6; 95% CI, −18.9 to 30.1; P = .65). However, the mean change (95% CI) for the following antibiotic classes increased significantly: third- and fourth-generation cephalosporins, 10.3 (3.1–17.5); macrolides, 4.8 (2.0–7.6); glycopeptides, 22.4 (17.5–27.3); β-lactam/β-lactamase inhibitor combinations, 18.0 (13.3–22.6); carbapenems, 7.4 (4.6–10.2); and tetracyclines, 3.3 (2.0–4.7).
CONCLUSIONS AND RELEVANCE
Overall DOT of all antibiotics among hospitalized patients in US hospitals has not changed significantly in recent years. Use of some antibiotics, especially broad spectrum agents, however, has increased significantly. This trend is worrisome in light of the rising challenge of antibiotic resistance. Our findings can help inform national efforts to improve antibiotic use by suggesting key targets for improvement interventions.
Owing to the rising threat of antibiotic resistance and other consequences resulting from unnecessary antibiotic use, ensuring appropriate antibiotic usage in the United States has become a national priority.1–4 In response, the US government has developed The National Strategy for Combating Antibiotic-Resistant Bacteria.2,5 Antibiotic use surveillance is a key objective within that strategy and a core element of hospital antibiotic stewardship programs identified by the Centers for Disease Control and Prevention (CDC).6 Appropriate antibiotic prescribing improves patient safety, slows development of antibiotic resistance, and reduces wasted resources.1
A better understanding of antibiotic use in US hospitals can inform stewardship efforts by identifying targets for reducing inappropriate or unnecessary prescribing. Previous national surveys of antibiotic usage in acute care hospitals were conducted in 20023,7,8 and 20119 by the CDC. Furthermore, several studies have capitalized on electronic or administrative data from US hospitals to characterize antibiotic use.10–16 However, past efforts have been limited by diversity in patient populations and facilities that precluded national estimates and provided limited longitudinal estimates. To address these gaps, we used a proprietary administrative data set from a large and diverse population of US hospitals to estimate patterns of in-patient antibiotic use over several years and extrapolate these findings to all US hospitals.
Methods
Adult and pediatric drug use data were obtained from the Truven Health MarketScan Hospital Drug Database (HDD). The HDD contains hospital discharge records for all patients discharged from participating acute care, general, nonfederal hospitals in the United States. These records include discharge-specific diagnostic and procedure codes, demographic information, inpatient drug usage data based on billing records, admission and discharge dates, and facility descriptors. We included visits from contributing hospitals for patients discharged from January 1, 2006, to December 31, 2012, including in-hospital deaths. For each discharge, we identified antibiotic doses charged to the patient during the inpatient stay. We searched the HDD for doses of any antibiotic listed in the US Food and Drug Administration’s National Drug Code Directory17 or the World Health Organization’s Anatomical Therapeutic Chemical (ATC)/Defined Daily Dose (DDD) classification system.18 We eliminated antibiotics not administered by oral, parental, or inhalation routes. Each antibiotic was categorized into 1 of 15 classes: aminoglycoside, first- and second-generation cephalosporin, third- and fourth-generation cephalosporin, lincosamide, fluoroquinolone, macrolide, glycopeptide, sulfonamide, β-lactam/β-lactamase inhibitor combination, carbapenem, penicillin, tetracycline, metronidazole, and other antibacterial agents. We further eliminated hospitals not submitting any antibiotic usage data within a given year (2% of participating hospitals). This study did not require review by an investigational review board as the HDD contains no personally identifiable data, and the data use agreement precluded any access by the investigators to such identifiers; therefore, the analysis did not involve human subjects.
We retrospectively estimated measures of antibiotic use as both days of therapy per 1000 patient-days (DOT) and the proportion of hospital discharges in which a patient received at least 1 dose of an antibiotic during their stay. One DOT represents the administration of a single agent on a given day regardless of the dosage strength or number of doses. For example, administration of a single 1000-mg dose of cefazolin or 3 1000-mg doses given 8 hours apart both constitute 1 DOT, while a patient receiving doses of vancomycin and ceftazidime would count as 2 DOTs.10 In addition to calculating overall usage measures, we stratified measures by antibiotic class, year, or other patient and facility characteristics. All hospital locations were included.
To create national estimates of inpatient antibiotic use, we performed a weighted extrapolation of data from the subset of US hospitals reporting to the HDD. We generated estimated weights to apply to HDD data using data submitted to the Centers for Medicare and Medicaid Services Healthcare Cost Report Information System (HCRIS). The HCRIS includes reports submitted from all Medicare-certified US hospitals.19 We used the HCRIS to determine the number of patient-days and discharges among all US nonfederal acute care hospitals for each year of the study. Furthermore, we stratified by bed size (<300, ≥300), US census division, urban or rural status, and teaching status. In a similar fashion, we determined the number of patient-days and discharges for each hospital participating in the HDD by year, stratified by the same characteristics. To determine hospital-specific weights for the extrapolation, we divided year-specific total patient-days or discharges in each of the HCRIS strata by the number of patient-days or discharges for each participating HDD hospital to generate a hospital-specific weight. There were 72 possible strata based on these variables, but certain strata were combined due to the small number of participating hospitals in the HDD. As a result, 35 strata were created to generate weights. For the DOT measure, weights based on patient-days were used and further stratified by critical care days vs all other inpatient-days; for the proportion of discharges during which an antibiotic was used, weights based on discharges were used. Results are reported as the weighted national estimates except where otherwise noted.
To validate the extrapolated national estimates, we compared our national estimates based on the HDD with independent national estimates derived from the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (Agency for Healthcare Research and Quality, Rockville, Maryland; http://hcupnet.ahrq.gov/). Specifically, we compared results from our extrapolated national estimates with those of the HCUP with regard to the following: number of annual in-patient discharges and inpatient-days, the mean and median length of stay (LOS), age and sex distribution of discharged patients, payer distribution, distribution of certain common diagnostic and procedure codes, and proportion of inpatient deaths. Comparisons were limited to nonfederal hospitals in the HCUP because our weights were based on nonfederal hospitals and to the year 2012 for this report.
To assess potential trends in antibiotic use over time, we developed a multivariate model using linear regression based on the approximately normal distribution of the outcome. Our model used generalized estimating equations (GEE) to account for interhospital covariance. Our outcome was DOT per 1000 patient-days for each facility and used facility weights as described previously. Our main variable of interest was calendar year, and we included facility characteristics such as case mix index, average age, bed size category, teaching status, facility urban or rural location, proportion of surgical discharges, average comorbidity score for the facility,20 and facility geographic location as determined by US census division. Furthermore, we used revenue codes to identify days in which patients received care from intensive care units; for those days, antibiotic usage was attributed to a critical care location. For all other patient-days, antibiotic usage was attributed to a noncritical care location. We also included in the model the proportion of inpatient-days in which the primary International Classification of Diseases, Ninth Revision, Clinical Modification21 diagnosis code for that admission was related to an infection, which we previously found to be an important predictor of hospital antibiotic usage.22 To examine possible region-specific and age-specific trends, we included interaction terms between calendar year and census division or age category instead of average age.
All data were analyzed using SAS version 9.3 (SAS Institute Inc).
Results
From January 1, 2006, to December 31, 2012, data on over 34 million discharges representing 166 million patient-days from 552 hospitals were included in the study. Over the 7 years, the number of hospitals contributing data for a given year ranged from 300 to 383 (Table 1). The proportion of hospitals varied by geographic location, with the largest number of hospitals participating in the South Atlantic region (29%) and the lowest in the New England region (3%); a distribution that was slightly but significantly different than that of all US hospitals. Hospitals were generally larger and more frequent in urban areas.
Table 1.
Characteristic of the Hospitals Reporting Antibiotic Use Data to the Truven MarketScan Hospital, 2006–2012
| Characteristic | Hospitals, No. | HDD Hospitals, % | US Hospitals, %a |
| Year | |||
| 2006 | 383 | NA | 7.9 |
| 2007 | 336 | NA | 6.9 |
| 2008 | 311 | NA | 6.5 |
| 2009 | 364 | NA | 7.6 |
| 2010 | 323 | NA | 6.7 |
| 2011 | 308 | NA | 6.4 |
| 2012 | 300 | NA | 6.5 |
| All years | 552 | NA | NA |
| Hospital characteristics | |||
| Urban | 464 | 84.1 | 57.3 |
| Rural | 88 | 15.9 | 42.7 |
| Teaching | 121 | 21.9 | 23.2 |
| Nonteaching | 431 | 78.1 | 76.8 |
| No. of beds, <300 | 365 | 66.1 | 86.7 |
| No. of beds, ≥300 | 187 | 33.9 | 13.3 |
| No. of beds, mean (SD) | 284 (NA) | 196 (NA) | 139 (171) |
| US Census division | |||
| New England (CT, MA, ME, NH, RI, VT) | 14 | 2.5 | 3.7 |
| Mid-Atlantic (NJ, NY, PA) | 96 | 17.4 | 9.0 |
| South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV) | 159 | 28.8 | 14.3 |
| Northeast Central (IL, IN, MI, OH, WI) | 83 | 15.0 | 15.0 |
| Southeast Central (AL, KY, MS, TN) | 51 | 9.2 | 8.6 |
| Northwest Central (IA, KS, MN, MO, ND, NE, SD) | 26 | 4.7 | 13.9 |
| Southwest Central (AR, LA, OK, TX) | 58 | 10.5 | 16.0 |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, WY) | 26 | 4.7 | 8.3 |
| Pacific (AK, CA, HI, OR, WA) | 39 | 7.1 | 11.3 |
Abbreviations: HDD, Truven Health MarketScan Hospital Drug Database.
Estimated US proportions based on data from the Centers for Medicare and Medicaid Services Healthcare Cost Report Information System for acute care hospitals.
National estimates from our analysis were compared with those from the HCUP (Table 2). While patient-days and dis charges among reporting hospitals decreased over time, the weighted national estimates of total patient-days and discharges were similar to those from the HCUP across study years, differing by −0.7% to 5.6% for any given year. In addition, our extrapolated estimates were similar to the HCUP estimates for median LOS, sex distribution, age categories, distribution of common diagnostic and procedure codes, and proportion of inpatient stays resulting in death. Estimates of the proportion of discharges with a private source primary payer was higher than the HCUP (38.8% vs 31.5%).
Table 2.
Comparison of Unweighted and Extrapolated Estimates From the Truven MarketScan Hospital Drug Database to National Estimates From HCUPnet,a 2012
| Characteristic | Unweighted Estimates | Extrapolated Estimates | HCUPb Estimates |
|---|---|---|---|
| Male, % | 43.3 | 43.2 | 42.1 |
| Age category, y, % | |||
| 0–17 | 13.5 | 13.5 | 15.7 |
| 18–44 | 24.3 | 24.4 | 24.3 |
| 45–64 | 26.2 | 26.1 | 24.4 |
| 65–84 | 27.5 | 27.5 | 27.1 |
| ≥65 | 35.9 | 36.0 | 35.5 |
| ≥85 | 8.4 | 8.5 | 8.4 |
| Primary ICD9 DX, % | |||
| V30.00 (Single liveborn without cesarean delivery birth) | 6.0 | 6.3 | 6.8 |
| V30.01 (Single liveborn by cesarean section birth) | 3.1 | 3.1 | 3.3 |
| 486 (Pneumonia, organism unspecified) | 2.1 | 2.2 | 2.3 |
| 038.9 (Unspecified septicemia) | 2.0 | 2.2 | 2.2 |
| 414.01 (Coronary atherosclerosis of native coronary artery) | 1.4 | 1.3 | 1.3 |
| 91.21 (Obstructive chronic bronchitis with acute exacerbation) | 1.3 | 1.4 | 1.3 |
| Primary ICD9 PX, % | |||
| 74.1 (Low cervical cesarean delivery birth) | 3.3 | 3.2 | 3.2 |
| 73.59 (Other manually assisted delivery) | 3.2 | 3.1 | 3.3 |
| 99.55 (Prophylactic administration of vaccine) | 3.0 | 3.1 | 2.7 |
| 64.0 (Circumcision) | 2.2 | 2.5 | 2.6 |
| Missing | 36.2 | 36.7 | 37.0 |
| Payer, % | |||
| Medicare | 36.1 | 36.8 | 39.8 |
| Medicaid | 16.7 | 17.3 | 20.1 |
| Private | 40.4 | 38.8 | 31.5 |
| Died,% | 1.7 | 1.6 | 1.8 |
| Length of stay, d | |||
| Mean days, 2012 | 5 | 4.8 | 4.5 |
| Median days, 2012 | 3 | 3 | 3 |
| Total No. of discharges | |||
| 2006 | 5 798 833 | 33 966 046 | 33 894 820 |
| 2007 | 5 185 506 | 34 046 058 | 33 874 702 |
| 2008 | 4 923 823 | 33 776 051 | 34 218 028 |
| 2009 | 4 966 148 | 33 516 719 | 33 591 290 |
| 2010 | 4 807 413 | 33 228 563 | 32 998 046 |
| 2011 | 4 612 634 | 32 986 834 | 33 987 722 |
| 2012 | 4 389 496 | 31 200 547 | 32 102 480 |
| Total No. of inpatient-days | |||
| 2006 | 27 896 463 | 150 786 507 | 155 916 172 |
| 2007 | 25 055 255 | 150 360 541 | 152 963 613 |
| 2008 | 23 845 456 | 148 557 139 | 157 402 929 |
| 2009 | 23 656 222 | 147 766 396 | 152 837 457 |
| 2010 | 23 000 641 | 148 091 321 | 152 292 154 |
| 2011 | 22 027 345 | 149 092 086 | 154 584 146 |
| 2012 | 20 881 215 | 141 291 753 | 143 409 982 |
| Days of therapy/1000 patient-days, 2012 | 745.5 | 767.5 | NA |
| Proportion of discharges with at least 1 antibiotic in 2012, % | 55.0 | 55.3 | NA |
| Days of therapy/1000 patient-days by class in 2012, % | |||
| Aminoglycosides | 19.2 | 19.8 | NA |
| First- and second-generation cephalosporins | 80.8 | 83.1 | NA |
| Third- and fourth-generation cephalosporins | 102.0 | 105.6 | NA |
| Lincosamide | 19.3 | 19.8 | NA |
| Fluoroquinolones | 109.9 | 117.0 | NA |
| Macrolides | 40.1 | 42.1 | NA |
| Glycopeptide | 102.4 | 103.4 | NA |
| Sulfa | 13.7 | 13.8 | NA |
| β-Lactam/β-lactamase inhibitor combinations | 99.0 | 102.6 | NA |
| Carbapenems | 31.6 | 32.3 | NA |
| Penicillins | 28.1 | 29.0 | NA |
| Tetracyclines | 12.8 | 13.2 | NA |
| Metronidazole | 48.6 | 49.3 | NA |
| Other | 38.1 | 36.7 | NA |
Abbreviations: ICD9 DX, International Classification of Diseases, Ninth Revision, Clinical Modification21 diagnosis code; ICD9 PX, International Classification of Diseases, Ninth Revision, Clinical Modification21 procedure code; NA, not applicable.
Healthcare Cost and Utilization Project (Agency for Healthcare Research and Quality, Rockville, Maryland, http://hcupnet.ahrq.gov/).
All estimates are for the year 2012 except for the comparisons of the number of discharges and inpatient-days, which include years 2006 to 2012.
Overall, 55.1% of patients discharged received at least 1 dose of an antibiotic during their hospital visit. The overall rate of antibiotic use for all study years was 755 per 1000 patient-days (Table 3). Both the proportion of discharges during which an antibiotic was received and the DOT per 1000 patient-days varied by antibiotic class. Days of therapy per 1000 patient-days were highest for fluoroquinolones, third- and fourth-generation cephalosporins, and β-lactam/β-lactamase inhibitor combinations. The proportion of discharges in which an antibiotic was given during the hospital visit was highest for first- and second-generation cephalosporins, fluoroquinolones, and third- and fourth-generation cephalosporins.
Table 3.
Extrapolated Estimates of Antibiotic Usage in the Truven MarketScan Hospital Drug Database by Year and Various Characteristics, 2006–2012
| DOT/1000 PDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | All Years | |
| Antibiotic class | ||||||||
| All | 732.5 | 736.9 | 755.6 | 766.8 | 755.4 | 770.0 | 767.5 | 754.8 |
| Aminoglycosides | 30.7 | 29.2 | 27.6 | 25.4 | 23.3 | 20.9 | 19.8 | 25.3 |
| First- and second-generation cephalosporins | 96.8 | 93.0 | 90.5 | 90.0 | 87.9 | 85.8 | 83.1 | 89.6 |
| Third- and fourth-generation Cephalosporins | 90.2 | 88.1 | 89.2 | 93.1 | 96.7 | 103.7 | 105.6 | 95.2 |
| Lincosamide | 23.1 | 22.9 | 22.3 | 21.6 | 20.4 | 20.2 | 19.8 | 21.5 |
| Fluoroquinolones | 143.7 | 141.0 | 139.4 | 134.3 | 126.6 | 123.0 | 117.0 | 132.3 |
| Macrolides | 35.2 | 34.2 | 36.9 | 38.7 | 37.6 | 42.0 | 42.1 | 38.1 |
| Glycopeptide | 72.0 | 77.1 | 85.0 | 91.7 | 93.6 | 100.1 | 103.4 | 88.8 |
| Sulfa | 15.4 | 16.0 | 16.5 | 16.0 | 15.4 | 14.5 | 13.8 | 15.4 |
| β-Lactam/β-lactamase inhibitor combinations | 75.5 | 80.5 | 88.0 | 93.4 | 94.5 | 99.1 | 102.6 | 90.4 |
| Carbapenems | 22.2 | 23.8 | 27.0 | 29.8 | 29.6 | 31.6 | 32.3 | 28.0 |
| Penicillins | 35.8 | 34.6 | 33.0 | 32.0 | 30.8 | 29.1 | 29.0 | 32.1 |
| Tetracyclines | 8.5 | 10.1 | 12.3 | 14.8 | 13.7 | 13.5 | 13.2 | 12.3 |
| Metronidazole | 53.7 | 53.1 | 52.4 | 51.0 | 50.0 | 49.7 | 49.3 | 51.3 |
| Other | 29.8 | 33.0 | 35.4 | 35.1 | 35.3 | 36.8 | 36.7 | 34.6 |
| US Census division | ||||||||
| New England (CT, MA, ME, NH, RI, VT) | 703.5 | 733.0 | 754.8 | 749.7 | 751.4 | 787.3 | 792.6 | 753.0 |
| Mid-Atlantic (NJ, NY, PA) | 644.4 | 648.5 | 650.5 | 652.6 | 642.4 | 659.1 | 647.3 | 649.3 |
| South Atlantic (DC, DE, FL, GA, MD, NC, SC, VA, WV) | 799.3 | 789.5 | 804.3 | 798.9 | 785.1 | 806.2 | 819.4 | 800.2 |
| Northeast Central (IL, IN, MI, OH, WI) | 784.7 | 769.9 | 800.4 | 831.6 | 810.6 | 823.4 | 863.0 | 811.6 |
| Southeast Central (AL, KY, MS, TN) | 733.7 | 726.7 | 738.8 | 741.5 | 739.0 | 758.7 | 768.0 | 743.4 |
| Northwest Central (IA, KS, MN, MO, ND, NE, SD) | 684.7 | 722.8 | 741.2 | 749.0 | 741.2 | 748.9 | 673.2 | 723.4 |
| Southwest Central (AR, LA, OK, TX) | 798.2 | 830.1 | 851.7 | 850.8 | 818.5 | 802.7 | 807.7 | 823.1 |
| Mountain (AZ, CO, ID, MT, NM, NV, UT, WY) | 804.7 | 775.1 | 822.5 | 802.5 | 788.6 | 799.9 | 757.2 | 793.9 |
| Pacific (AK, CA, HI, OR, WA) | 568.6 | 592.9 | 601.2 | 690.8 | 711.9 | 747.1 | 712.1 | 665.0 |
| Age category, y | ||||||||
| 0–17 | 424.5 | 417.5 | 412.9 | 416.5 | 397.3 | 373.7 | 347.9 | 400.1 |
| 18–44 | 671.0 | 670.1 | 680.5 | 689.9 | 674.0 | 676.8 | 673.4 | 676.5 |
| 45–64 | 807.9 | 814.5 | 834.5 | 856.4 | 839.8 | 850.6 | 850.1 | 836.4 |
| 65–84 | 818.1 | 824.6 | 847.4 | 856.9 | 846.2 | 869.5 | 868.4 | 846.9 |
| ≥85 | 769.1 | 769.2 | 802.0 | 813.2 | 817.0 | 850.9 | 852.5 | 811.4 |
| Sex | ||||||||
| Male | 761.7 | 766.5 | 785.6 | 795.5 | 779.8 | 792.1 | 786.8 | 781.1 |
| Female | 708.5 | 712.3 | 730.5 | 743.0 | 734.9 | 751.2 | 750.8 | 732.7 |
| Critical care location | ||||||||
| Yes | 1072.1 | 1073.2 | 1086.4 | 1073.8 | 1103.2 | 1124.0 | 1117.2 | 1092.3 |
| No | 698.7 | 702.7 | 720.9 | 733.7 | 719.5 | 735.3 | 732.7 | 720.3 |
| Urban | 730.9 | 739.6 | 754.9 | 764.9 | 753.4 | 767.2 | 761.6 | 753.2 |
| Rural | 745.4 | 712.4 | 762.0 | 784.4 | 774.4 | 798.2 | 827.9 | 769.9 |
| Teaching | 713.8 | 725.3 | 734.0 | 741.3 | 724.3 | 735.3 | 740.7 | 730.5 |
| Nonteaching | 752.9 | 749.1 | 778.8 | 792.9 | 787.2 | 805.7 | 796.9 | 780.4 |
| No. of beds, <300 | 739.0 | 739.1 | 768.1 | 790.2 | 776.0 | 804.1 | 818.3 | 775.5 |
| No. of beds, ≥300 | 726.7 | 734.9 | 744.9 | 747.4 | 738.6 | 742.2 | 725.4 | 737.2 |
| Large, urban teaching hospital | 713.6 | 724.7 | 729.1 | 733.1 | 717.3 | 722.7 | 711.8 | 721.8 |
| Hospitals other than large, urban, teaching | 744.2 | 744.2 | 771.9 | 786.9 | 777.9 | 797.0 | 801.6 | 774.6 |
| Proportion of discharges by antibiotic class, % | ||||||||
| All | 53.8 | 54.0 | 54.9 | 55.7 | 55.7 | 56.0 | 55.3 | 55.6 |
| Aminoglycosides | 5.7 | 5.6 | 5.3 | 4.6 | 4.3 | 4.0 | 3.9 | 4.8 |
| First- and second-generation cephalosporins | 20.4 | 20.3 | 20.1 | 20.2 | 20.1 | 19.5 | 18.9 | 20.0 |
| Third- and fourth-generation cephalosporins | 10.9 | 10.9 | 11.1 | 11.6 | 12.1 | 13.3 | 13.4 | 11.9 |
| Lincosamide | 3.4 | 3.4 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Fluoroquinolones | 16.8 | 16.7 | 16.9 | 16.4 | 15.8 | 15.7 | 15.0 | 16.2 |
| Macrolides | 4.9 | 4.9 | 5.3 | 5.7 | 5.5 | 6.1 | 6.0 | 5.5 |
| Glycopeptide | 8.2 | 8.9 | 9.9 | 10.7 | 11.3 | 12.3 | 12.9 | 10.6 |
| Sulfa | 1.8 | 1.9 | 2.0 | 2.0 | 1.9 | 1.8 | 1.8 | 1.9 |
| β-Lactam/β-lactamase inhibitor combinations | 7.5 | 8.0 | 8.6 | 9.1 | 9.5 | 10.2 | 10.4 | 9.0 |
| Carbapenems | 1.7 | 2.0 | 2.3 | 2.6 | 2.7 | 2.9 | 3.0 | 2.4 |
| Penicillins | 6.0 | 5.7 | 5.5 | 5.1 | 4.9 | 4.6 | 4.6 | 5.2 |
| Tetracyclines | 0.9 | 1.1 | 1.3 | 1.6 | 1.5 | 1.5 | 1.5 | 1.4 |
| Metronidazole | 5.1 | 5.2 | 5.1 | 5.1 | 5.2 | 5.3 | 5.3 | 5.2 |
| Other | 2.9 | 3.1 | 3.2 | 3.4 | 3.5 | 3.6 | 3.6 | 3.3 |
Abbreviations: DOT, days of therapy; PDs, patient-days.
The multivariable trend analysis of data from participating hospitals did not show a statistically significant change in overall use (total DOT increase, 5.6; 95% CI, −18.9 to 30.1; P = .65). Temporal trends varied by antibiotic class (Figure 1). Estimates of usage decreased for aminoglycosides, first and second generation cephalosporins, fluoroquinolones, sulfa, metronidazole (P < .001 for preceding medications), and penicillins (P = .01) with the greatest decrease among fluoroquinolones. Macrolides, third- and fourth-generation cephalosporins, glycopeptides, β-lactam/β-lactamase inhibitor combinations, carbapenems, tetracyclines, and other types of antibacterials all increased (P < .001 for all except third- and fourth-generation cephalosporins [P = .001]).
Figure 1. Mean DOT per 1000 Patient-days for All Antibiotics.
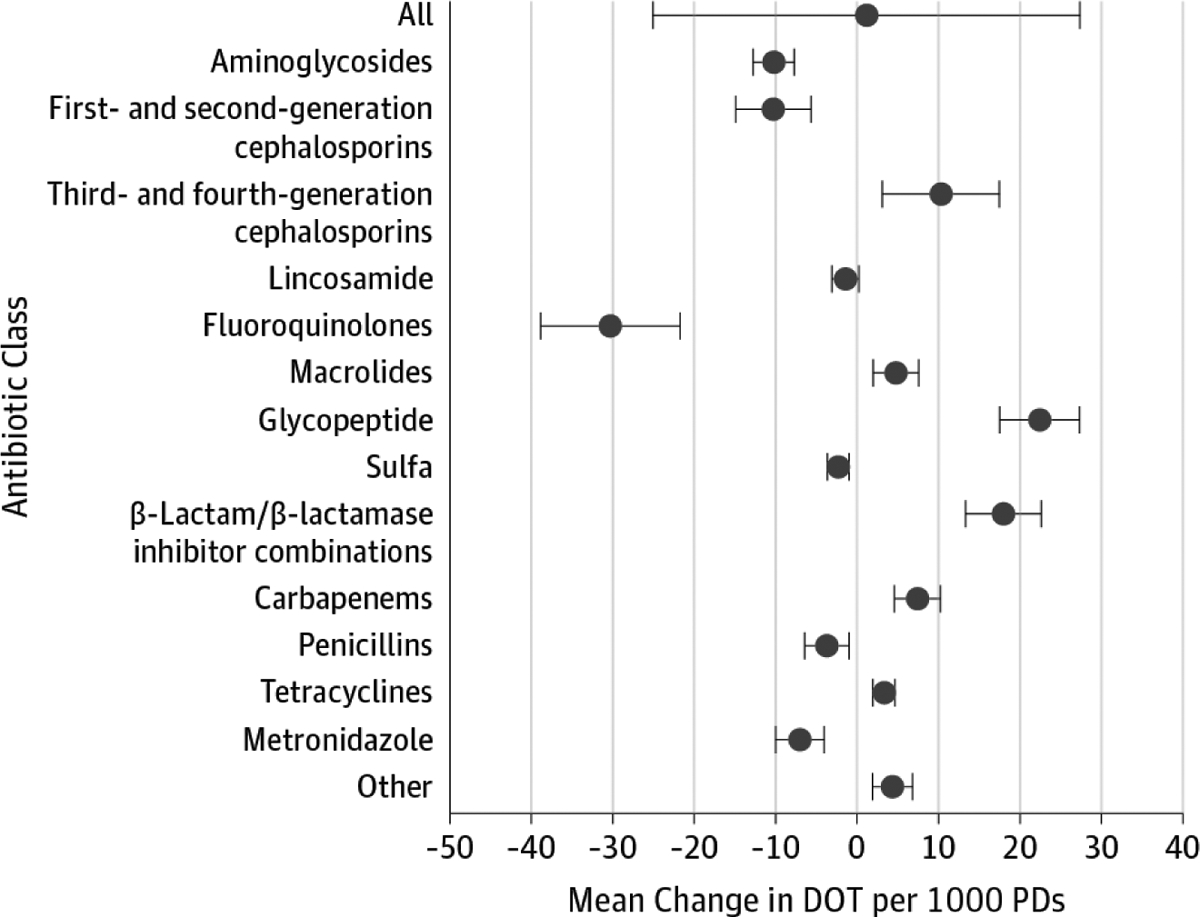
Across all hospitals, the change in mean DOT per 1000 patient-days were estimated by generalized estimating equation models controlling for case mix index, average patient age, bed size category, teaching status, urban or rural facility location, proportion of surgical discharges, average comorbidity score, facility geographic location, critical care setting, and proportion of inpatient-days in which the International Classification of Diseases, Ninth Revision, Clinical Modification21 diagnosis code was related to an infection. Data points represent mean DOT per 1000 patient-days for each antibiotic class, and the whiskers represent 95% CIs. DOT indicates days of therapy.
Usage of antibiotics was about 52% greater in critical care locations (1092 vs 720 DOT per 1000 patient-days) (Table 3) compared with noncritical care locations (P < .001) but not consistently for every class. Usage was significantly greater in critical care locations for aminoglycoside, third- and fourth-generation cephalosporin, glycopeptide, β-lactam/β-lactamase inhibitor combination, carbapenem, penicillin, metronidazole, and other types of antibacterials (eFigure 1 in the Supplement).
Antibiotic usage varied by geographic location of the hospital even after controlling for various facility characteristics in the multivariable model (P < .001) (Figure 2). Divisions in the Southeast Central, Northeast Central, Southwest Central, and Mountain areas of the United States had the highest DOT per 1000 patient-days overall, while divisions in the New England, Mid Atlantic, and Pacific had the lowest. Trends in usage over time did not vary significantly by geographic region (P = .25).
Figure 2. Mean DOT per 1000 Patient-days by US Census Division Between January 1, 2006, and December 31, 2012.
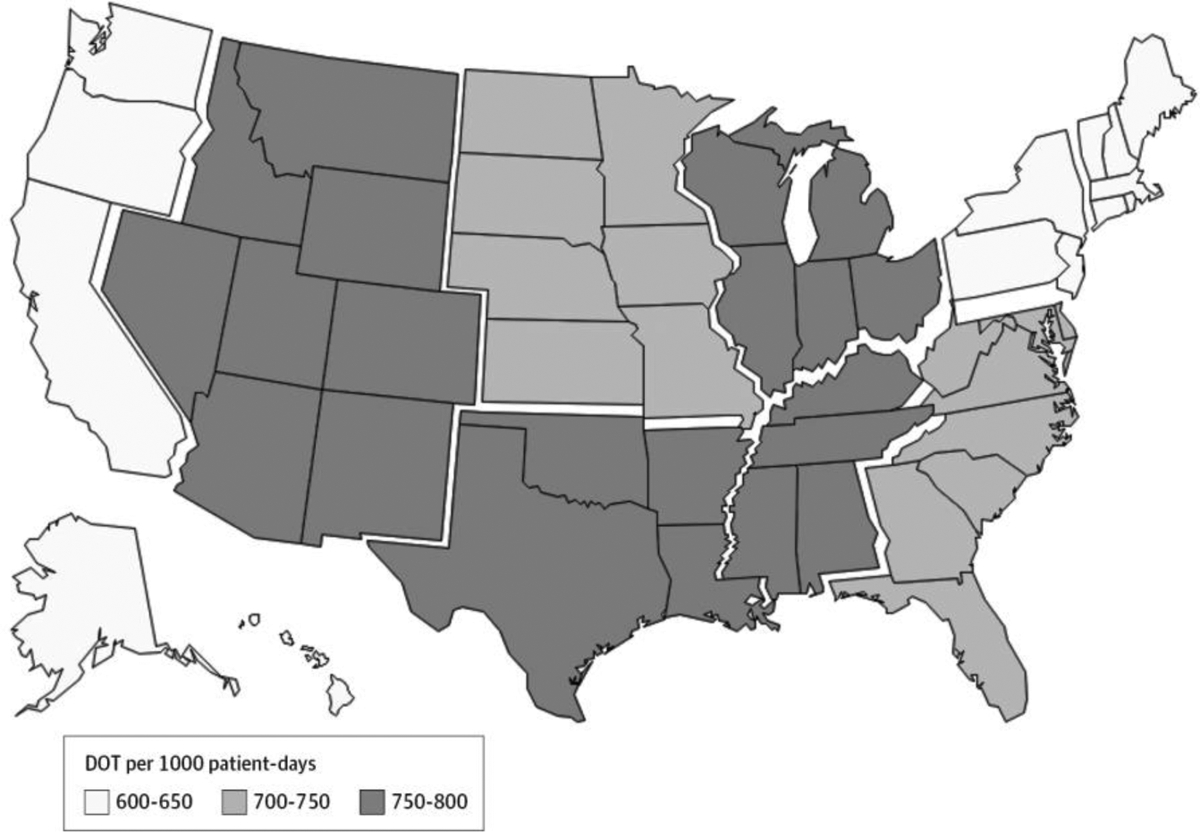
Across all hospitals, the mean DOT per 1000 patient-days by census division were estimated by generalized estimating equation models controlling for year, case mix index, average patient age, bed size category, teaching status, urban or rural facility location, proportion of surgical discharges, average comorbidity score, facility geographic location, critical care setting, and proportion of inpatient-days in which the International Classification of Diseases, Ninth Revision, Clinical Modification21 diagnosis code was related to an infection. DOT indicates days of therapy.
Antibiotic usage did not vary significantly by facility bed size (P = .17) or urban or rural location (P = .34). Based on our GEE model, hospitals identified as nonteaching had slightly higher DOT per 1000 patient-days compared with teaching hospitals (P = .004). Similarly hospitals denoted as large urban teaching hospitals had a lower but not significantly different DOT per 1000 patient-days compared with other hospitals (P = .16).
Antibiotic usage varied by age group. Usage was lowest for those younger than 18 years and highest for those 45 years and older. Among the youngest age group, aminoglycosides, third- and fourth-generation cephalosporin, and penicillin were the most commonly used antibiotics, while the most commonly used classes for older age groups were third- and fourth-generation cephalosporin, fluoroquinolones, glycopeptide, and β-lactam/β-lactamase inhibitor combinations. The distribution of usage by age group was consistent among census divisions, and usage over time did not vary significantly by age group in GEE models (P = .12).
Discussion
Our study is the first, to our knowledge, to provide national estimates of temporal trends in antibiotic use among US hospitals. These observations build upon previous studies, including those from a recent national point-prevalence survey of inpatient antibiotic use,9 by demonstrating important changes in use over time and quantifying days of antibiotic therapy. While overall rates of antibiotic use in US hospitals did not change significantly from 2006 to 2012, we identified important trends within individual antibiotic classes. There were significant decreases in fluoroquinolones (20%) and first- and second-generation cephalosporins (7%) usage, but these decreases were offset by significant increases in vancomycin (32%) and agents with broad-spectrum activity against gram-negative bacteria, including carbapenem (37%), third- and fourth-generation cephalosporin (12%), and β-lactam/β-lactamase inhibitor combination antibiotics (26%). Despite substantial reduction in fluoroquinolone use, this class remained the most commonly used antibiotic class in US hospitals in 2012 (eFigure 2 in the Supplement).
We can only hypothesize why we observed certain antibiotic class-specific trends. For instance, the decrease in fluoroquinolone use may be related to Clostridium difficile infection prevention efforts23 or changes to empirical therapy due to the emergence of widespread resistance to fluoroquinolones among several clinically important bacteria.24 Increases in third- and fourth-generation cephalosporin, β-lactam/β-lactamase inhibitor combination, and carbapenem antibiotics may reflect increasing concern about infections caused by antibiotic-resistant gram-negative bacteria. Likewise, increases in glycopeptide use, mainly vancomycin, may reflect a response to the emergence of methicillin-resistant Staphylococcus aureus as a common cause of community-acquired skin and soft tissue infections.25,26
The current distribution of inpatient antibiotic use by antibiotic class in the United States appears to differ considerably in comparison to the United Kingdom and France. For example, in 2012, 47% of total inpatient use in the United States was accounted for by 4 classes: third- and fourth-generation cephalosporin, fluoroquinolone, carbapenem, and glycopeptide. In contrast, these 4 classes accounted for only 11% of in-patient use in the United Kingdom from 2012 to 201327 and for 27%, an estimate including all cephalosporins, in France in 2007.28 Similarly, penicillin antibiotics account for 30% of in-patient use in the United Kingdom27 and 21% in France28 but only 4% in US hospitals. It is possible that differences in prevalence of antibiotic-resistant pathogens observed in these countries, which tend to be lower than the United States, could be, in part, attributable to different patterns of antibiotic use, although other factors, such as different infection control practices or patient movement patterns may also play a role.25,29 Between 2005 and 2009, the United Kingdom observed dramatic reductions in C difficile infection, which is hypothesized to be attributable to replacing a large proportion of fluoroquinolone and cephalosporin use with antibiotics such as penicillin that may be less likely to result in C difficile over-growth. Overall rates of antibiotic use in the United Kingdom did not change during this time period.30,31 While the optimal distribution of antibiotic use by class is unknown, the United Kingdom’s experience suggests a similar shift in distribution might be beneficial for US patients. Such a change is likely feasible given that approximately 50% of inpatient antibiotic use in US hospitals is for treatment of lower respiratory and urinary tract infections9 and that inpatient prescrib ing for these and other infections is often inappropriate.2,32 While current treatment guidelines for lower respiratory tract infections include fluoroquinolones or cephalosporins, non-fluoroquinolone and noncephalosporin regimens are also recommended as first-line therapy.33 Enhanced efforts to improve diagnosis of urinary tract infections could lead to deep reductions in unnecessary antibiotic use directed at urinary tract infections that are commonly treated with third-generation cephalosporin or fluoroquinolone antibiotics. A shift away from inpatient fluoroquinolone and cephalosporin use may have major implications for prevention of C difficile infections in the United States, especially because use of these 2 categories of antibiotics was particularly common among older patients at the highest risk of severe outcomes from C difficile infections.
Our study had a number of strengths. Of studies estimating inpatient antibiotic use in the United States, ours is the largest to date that we know of, containing information from at least 300 hospitals each year, representing a diversity of sizes, teaching status, and urban or rural locations in each geographic division. In addition, our study contained data across several years allowing for trend analysis. Even though our sample of hospitals contained a wider variety of hospital types, our point estimates were consistent with results from past studies with regard to overall and class-specific use.9–11,15 Detailed data contained information on a range of hospital and patient-specific demographic and clinical characteristics, allowing for this information to be included in multivariate models. Furthermore, we used data from the HCRIS to develop weighting information to extrapolate our estimates to the national level. Since nonweighted estimates were similar to those produced by our extrapolation, we believe the sample of hospitals participating in the HDD may represent a national sample. When we validated our national estimates from the HDD with independent national estimates derived from the HCUP, those measures matched well.
Limitations
This study has certain limitations. Administrative data are collected primarily for billing purposes and adapted for research. There is likely misclassification in pharmacy, clinical, and facility information; however, this bias is most likely nondifferential. Furthermore, administrative data has been shown to be useful in other areas of health care research.34–38 Use of pharmacy charge data to estimate medication usage in hospitals was previously validated in a small sample with excellent agreement11 and more recently in a small group of pediatric hospitals.39 When limited to hospitals submitting data for at least 5 years of the study period, our analysis found similar trends in the multivariate analyses. Likewise, some geographic regions are over or under represented by the distribution of hospitals in this sample compared with the US distribution of hospitals. We sought to overcome this through regional adjustment of estimates using nationally representative data from the HCRIS; however, the small number of hospitals in some regions may still limit the reliability of the estimates. Furthermore, our extrapolated estimates were limited to acute, nonfederal hospitals in the United States and do not adequately represent children’s hospitals, which represent less than 1% of included hospitals. Nonetheless, we were encouraged to find that in aggregate, our national estimates appear quite consistent with other studies. Finally, due to changes in the data source, we were unable to generate national estimates after 2012; however, we do not believe major changes in antibiotic use in acute care hospitals have occurred. In a sensitivity analysis including a sample of 137 hospitals with data through 2014, we observed similar trends. Unfortunately, the hospitals available for this analysis were not representative of the entire sample because not all geographic regions were represented in this limited sample of hospitals.
Conclusions
Overall rates of antibiotic use in US hospitals did not change significantly from 2006 to 2012. However, we identified significant changes in specific antibiotic classes and regional variation that may have important implications for reducing antibiotic-resistant infections. Improved monitoring of antibiotic use is critical to direct the work of antibiotic stewardship programs in the United States. In 2012, the CDC launched the Antibiotic Use Option of the National Healthcare Safety Network that provides real-time monitoring of antibiotics administered in UShospitals. As enrollment in the antibiotic use option grows, it will provide ongoing systematic assessment at the hospital, state, regional, and national levels. Because inappropriate antibiotic use increases the risk of antibiotic resistance and other adverse patient outcomes,2 continued monitoring of antibiotic use is critical to future improvements in patient safety.
Supplementary Material
Key Points.
Question
How much antibiotic use occurs in US hospitals, and has the pattern of use changed over time?
Findings
Overall rates of antibiotic use in US hospitals did not change significantly from 2006 to 2012; however, use of certain antibiotic classes, including glycopeptides, β-lactam/β-lactamase inhibitor combinations, and carbapenems, increased significantly.
Meaning
Patterns of antibiotic use in US hospitals may have important implications for reducing antibiotic-resistant infections; these findings can help inform national efforts to improve antibiotic use by suggesting key targets for improvement.
Funding/Support:
This study was funded by the Centers for Disease Control and Prevention (CDC).
Role of the Funder/Sponsor:
This manuscript underwent the CDC clearance process, but otherwise the CDC had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis. 2014; 58(3):401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridkin S, Baggs J, Fagan R, et al. ; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63 (9):194–200. [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob JT, Gaynes RP. Emerging trends in antibiotic use in US hospitals: quality, quantification and stewardship. Expert Rev Anti Infect Ther. 2010; 8(8):893–902. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, US Department of Health and Human Services. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed August 11, 2016.
- 5.The White House. National Strategy for Combating Antibiotic-Resistant Bacteria. https://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf. Accessed August 11, 2016.
- 6.Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis. 2014;59(suppl 3):S97–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 to June 2002, issued August 2002. Am J Infect Control. 2002;30(8): 458–475. [DOI] [PubMed] [Google Scholar]
- 8.Fridkin SK, Steward CD, Edwards JR, et al. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis. 1999;29(2):245–252. [DOI] [PubMed] [Google Scholar]
- 9.Magill SS, Edwards JR, Beldavs ZG, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014; 312(14): 1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44(5):664–670. [DOI] [PubMed] [Google Scholar]
- 11.Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168 (20):2254–2260. [DOI] [PubMed] [Google Scholar]
- 12.MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol. 2008; 29(3):203–211. [DOI] [PubMed] [Google Scholar]
- 13.Pakyz AL, Gurgle HE, Ibrahim OM, Oinonen MJ, Polk RE. Trends in antibacterial use in hospitalized pediatric patients in United States academic health centers. Infect Control Hosp Epidemiol. 2009;30 (6):600–603. [DOI] [PubMed] [Google Scholar]
- 14.Lasky T, Ernst FR, Greenspan J, Wang S, Gonzalez L. Estimating pediatric inpatient medication use in the United States. Pharmacoepidemiol Drug Saf. 2011;20(1):76–82. [DOI] [PubMed] [Google Scholar]
- 15.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis. 2011;53(11):1100–1110. [DOI] [PubMed] [Google Scholar]
- 16.Pakyz AL, Carroll NV, Harpe SE, Oinonen M, Polk RE. Increase in use of vancomycin for Clostridium difficile infection in US hospitals. Infect Control Hosp Epidemiol. 2010;31(8):867–868. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. National Drug Code Directory. http://www.fda.gov/Drugs/informationondrugs/ucm142438.htm. Accessed June 9, 2014.
- 18.WHO Collaborating Centre for Drug Statistics Methodology. International Language for Drug Utilization Research ATC/DDD. http://www.whocc.no/. Accessed June 9, 2014.
- 19.Centers for Medicare & Medicaid Services. Cost Reports. http://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/costreports/index.html. Accessed June 9, 2014. [PubMed]
- 20.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7): 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. International Classification of Diseases, Ninth Revision (ICD-9). Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 22.Baggs J, Fridkin S, Pollack LA, Srinivasan A, Jernigan J. Factors Associated with Inter-Hospital Variability of Inpatient Antibiotic Use in a Cohort of US Hospitals. Previous presentation: idweek; October 7–11, 2015; San Diego, CA. [Google Scholar]
- 23.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sievert DM, Ricks P, Edwards JR, et al. ; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial -resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34 (1):1–14. [DOI] [PubMed] [Google Scholar]
- 25.CDC’s Antibiotic Resistance Patient Safety Atlas http://www.cdc.gov/hai/surveillance/ar-patient-safety-atlas.html. Accessed June 20, 2016, 2016.
- 26.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147–159. [DOI] [PubMed] [Google Scholar]
- 27.Cooke J, Stephens P, Ashiru-Oredope D, et al. Longitudinal trends and cross-sectional analysis of English national hospital antibacterial use over 5 years (2008–13): working towards hospital prescribing quality measures. J Antimicrob Chemother. 2015;70(1):279–285. [DOI] [PubMed] [Google Scholar]
- 28.Dumartin C, L’Hériteau F, Péfau M, et al. Antibiotic use in 530 French hospitals: results from a surveillance network at hospital and ward levels in 2007. J Antimicrob Chemother. 2010;65(9): 2028–2036. [DOI] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance interactive database (EARS-Net) http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/database.aspx. Accessed June 20, 2016, 2016.
- 30.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart--Then Focus. J Antimicrob Chemother. 2012; 67(suppl 1):i51–i63. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55(8):1056–1063. [DOI] [PubMed] [Google Scholar]
- 32.Dellit TH, Owens RC, McGowan JE Jr, et al. ; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44 (2):159–177. [DOI] [PubMed] [Google Scholar]
- 33.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(suppl 1): S45–S53. [DOI] [PubMed] [Google Scholar]
- 35.Lieu TA, Hinrichsen VL, Moreira A, Platt R. Collaborations in population-based health research: the 17th annual HMO Research Network Conference, March 23–25, 2011, Boston, Massachusetts, USA. Clin Med Res. 2011;9(3–4): 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platt R, Davis R, Finkelstein J, et al. Multicenter epidemiologic and health services research on therapeutics in the HMO Research Network Center for Education and Research on Therapeutics. Pharmacoepidemiol Drug Saf. 2001;10(5): 373–377. [DOI] [PubMed] [Google Scholar]
- 37.Platt R, Carnahan RM, Brown JS, et al. The U.S. Food and Drug Administration’s Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):1–8. [DOI] [PubMed] [Google Scholar]
- 38.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21(4):578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beus JM, Ross R, Metjian TA, Gerber JS. The Performance of Administrative Data for Measurement of Antibiotic Use Varies Across Antibiotics. Previous presentation: idweek; October 7–11, 2015; San Diego, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


