Abstract
Objective
A long‐term decline in health‐related quality of life (HRQoL) has been reported after coronavirus disease 2019 (COVID‐19). Studies with people with persistent symptoms showed inconsistent outcomes. Cognition and emotion are important determinants in HRQoL, but few studies have examined their prognostic significance for HRQoL and functionality in post‐COVID patients with persisting symptoms. We aimed to describe QoL, HRQoL, and functioning in individuals post‐COVID with varying COVID‐19 severities and to investigate the predictive value of cognitive and emotional variables for QoL, HRQoL, and functioning.
Methods
In total, 492 participants (398 post‐COVID and 124 healthy controls) underwent a neurobehavioral examination that included assessments of cognition, mood, QoL/HRQoL (WHOQOL‐BREF, EQ‐5D), and functioning (WHODAS‐II). Analysis of covariance and linear regression models were used to study intergroup differences and the relationship between cognitive and emotional variables and QoL and functioning.
Results
The Physical and Psychological dimensions of WHOQoL, EQ‐5D, and WHODAS Cognition, Mobility, Life Activities, and Participation dimensions were significantly lower in post‐COVID groups compared with a control group. Regression models explaining 23.9%–53.9% of variance were obtained for the WHOQoL‐BREF dimensions and EQ‐5D, with depressive symptoms, post‐COVID symptoms, employment status, income, and mental speed processing as main predictors. For the WHODAS, models explaining 17%–60.2% of the variance were obtained. Fatigue, depressive symptoms, mental speed processing, and post‐COVID symptoms were the main predictors.
Interpretation
QoL/HRQoL and functioning after COVID‐19 in individuals with persistent symptoms were lower than in non‐affected persons. Depressive symptoms, fatigue, and slower mental processing speed were predictors of lower QoL/HRQoL and functioning.
Introduction
Post‐coronavirus disease 2019 (COVID‐19) condition (PCC) is defined as the continuance or onset of new symptoms 3 months after the initial SARS‐CoV‐2 infection, which last at least 2 months and cannot be attributed to another cause. 1 The prevalence of PCC is unknown, as it varies according to study cohorts, methodology, SARS‐CoV‐2 variants, and vaccination status, but it is estimated that one in eight people with COVID‐19 will develop PCC. 2 , 3 Fatigue, dyspnea, and altered smell and taste senses are frequent symptoms. 4 Poor memory, executive dysfunction, and slow processing speed 5 , 6 , 7 have been reported in people with PCC, and depression and anxiety symptoms are also prevalent, sometimes going above clinical cutoff values. 8 , 9 , 10
Quality of life (QoL) is a general concept influenced by a person's physical health, psychological condition, level of independence, social relationships, and relationship to the environment. 11 Health‐related QoL (HRQoL) can indicate how a patient sees their place in life as it changes because of sickness and its treatment. 12 , 13 HRQoL focuses on the effects of disease on QoL, whereas QoL encompasses all aspects of living. 14 Functioning refers to a person's bodily functions, activities, and engagement concerning environmental circumstances. 15
Studies have documented a decrease in HRQoL and functioning in individuals more than 6 months after discharge or recovery. This has been observed in both patients post‐hospitalization 16 , 17 , 18 and individuals experiencing persistent symptoms. 19 , 20 , 21 , 22 , 23 , 24 Nevertheless, research involving persons experiencing persistent symptoms following moderate or mild COVID‐19 revealed that the average HRQoL fell within the range of the general population mean. Only 4.2% of participants reported their HRQoL as very poor or poor. 25
The relationship between COVID‐19 severity and HRQoL has been investigated over the short, medium, and long terms. One month after recovery, there were no differences between hospitalized and non‐hospitalized patients in a sample of patients under 50 years of age. 26 However, participants with severe COVID‐19 had significantly worse HRQoL than those with moderate COVID‐19 27 one and a half months after recovery, and 1–2 months after hospital discharge, the HRQoL for patients in the ICU was lower than for those non‐ICU. 28 On the other hand, further studies reported that, 3 months post‐recovery, no differences were found between ICU, moderate, and mild groups 29 or between ICU and non‐ICU groups. 30 In the long term, 12 months post‐infection, people with mild COVID‐19 had QoL scores within population norms, whereas those with moderate or severe/critical disease had scores below. 31
Synthesizing previous research can be challenging due to the diversity of cohorts or the variation in time between disease onset and HRQoL assessment. Furthermore, most studies have compared HRQoL scales with population norms. As a direct consequence of the COVID‐19 epidemic, the QoL of the entire population has decreased, 32 , 33 therefore, when compared to these norms, the QoL experienced by healthy individuals may be overestimated. On the other hand, only a small number of studies have been conducted on QoL in people with PCC, and many of these studies were conducted through online surveys. 21 , 23 , 25 In terms of whether having more severe COVID‐19 affects QoL, comparisons between severity groups reveal inconsistent findings that do not appear to be associated with the time elapsed between diagnosis and evaluation.
Cognitive impairment is related to poor HRQoL in neurological, 34 psychiatric, 35 systemic, 36 metabolic, 37 cardiovascular, 38 and infectious 39 diseases. Few studies have examined the relationship between cognition, QoL/HRQoL, and functioning in individuals after COVID‐19, with none including PCC groups. Improvements in verbal fluency and executive function after cognitive rehabilitation were accompanied by improvements in QoL. 40 Impairments in verbal memory, fluency, mental processing speed, and executive function affected the self‐care dimension, and executive function affected the mobility dimension of the EuroQoL‐5D HRQoL scale. 41 Lastly, cognitive dysfunction, anxiety, fatigue, and hyposmia/hypogeusia were responsible for 28.8% of the functioning variance. 42
Several studies have demonstrated an increase in anxious–depressive symptoms in people between 3 and 12 months after recovery from COVID‐19. 43 , 44 , 45 , 46 Post‐COVID‐19 anxiety and depression symptoms are associated with poorer HRQol, both in the medium 47 and long terms. 20 , 45 , 48 , 49 Both an increase in depressive symptoms and reduction of QoL occur simultaneously; however, little research has been conducted focusing on the predictive value of emotional factors on QoL, HRQoL, and functioning among individuals with PCC. 48
The aims of the present study were (1) to describe QoL, HRQoL, and functioning in a large sample of people with PCC with various COVID‐19 severities, including comparison with healthy individuals, and (2) to investigate the predictive value of cognitive and emotional variables for QoL, HRQoL, and functioning in people with PCC.
Materials and Methods
Study design and settings
We conducted a cross‐sectional, exploratory multicenter study of patients recruited from neuropsychology and COVID‐19 units across 19 hospitals in Catalonia, Madrid, Canarias, (Spain), and Andorra, coordinated by the Consorci Sanitari de Terrassa (Barcelona, Spain). The study was conducted with the approval of the Drug Research Ethics Committee (CEIm) of Consorci Sanitari de Terrassa (CEIm code: 02‐20‐107‐070) and the Ethics Committee of the University of Barcelona (IRB00003099).
Sample size
We used preliminary data from two projects (ClinicalTrials.gov IDs: NCT05307549 and NCT05307575). The appropriate sample size was calculated using the G*Power V3.1.9.6.1. 50 To meet the sample size requirements of the power analysis with a β‐error of 95%, and a significance level of 5%, 492 participants were required. Based on previous research, 6 we estimate the effect size of the difference in cognitive tests between post‐COVID people and controls as small to moderate. Due to the unequal size of the groups, which entails a loss of statistical power, 51 we chose a β‐error of 95%.
Participants recruitment
Three hundred ninety‐four people with PCC who had either been hospitalized or who attended different hospitals and health centers with persistent symptoms and met the inclusion criteria were recruited consecutively from June 2021 to December 2022. The patients were referred mainly by general practitioners, internists, neurologists, or allergists to neuropsychology units. Twenty‐six recruited individuals were excluded from the analysis due to incomplete study information. As a control sample, 134 persons who had not been infected were recruited. Ten of these lacked the complete data and were excluded from the analysis. Figure 1 shows the flowchart for the sample selection.
Figure 1.
Flowchart for the sample selection. (A) Post‐COVID condition individuals; (B) Health control. Participants were selected from the following hospitals: Consorci Sanitari de Terrassa (Terrassa, Barcelona, Spain); Hospital Sant Joan Despí Moisès Broggi‐Consorci Sanitari Integral (Sant Joan Despí, Barcelona, Spain); Hospital Universitari Arnau de Vilanova (Lleida, Spain); Hospital Universitari de Santa Maria (Lleida, Spain); Consorci Sanitari Alt Penedès‐Garraf (Vilafranca de Penedés, Barcelona, Spain); Hospital Verge de la Cinta, (Tortosa, Tarragona, Spain); Fundació Sant Hospital (La Seu d'Urgell, Lleida, Spain); Consorci Hospitalari de Vic (Vic, Barcelona, Spain); Hospital Universitari Germans Trias i Pujol (Badalona, Barcelona, Spain); Hospital Universitari de Bellvitge (Barcelona, Spain); Hospital Universitari Mútua de Terrassa (Terrassa, Barcelona, Spain); Hospital Clinic de Barcelona (Barcelona, Spain); Hospital Municipal Badalona (Badalona, Barcelona, Spain); Institut d'Assistència Sanitària (Girona, Spain); Hospital de Figueres (Figueres, Girona, Spain); Hospital de Puigcerdà (Puigcerdà, Girona, Spain); Hospital General de la Cruz Roja San José y Santa Adela (Madrid, Spain); Hospital Nostra Senyora de Meritxell (Andorra); Hospitales San Roque (Gran Canaria, Islas Canarias, Spain).
The inclusion criteria for the PCC group were (a) confirmed diagnosis of COVID‐19 according to WHO criteria with signs and symptoms of the disease during the acute phase; (b) a post‐infection period of at least 12 weeks; and (c) age between 18 and 65 years. The exclusion criteria were (a) established diagnosis before COVID‐19 of psychiatric, neurological, or neurodevelopmental disorders or systemic pathologies known to cause cognitive deficits; and (b) motor or sensory alterations that could impede neuropsychological examinations.
Individuals in the control group were volunteers recruited among relatives and friends of people with PCC and disseminating information through hospitals and health centers. The inclusion criteria were (a) age between 18 and 65 years; (b) not having had COVID‐19 (no compatible symptoms or no positive test). The exclusion criteria were (a) established diagnosis of psychiatric, neurological, or neurodevelopmental disorders or systemic pathologies known to cause cognitive deficits before participation in the study and (b) motor or sensory alterations that could impede neuropsychological examinations. All participants were native Spanish speakers.
Procedure
The entire process was comprised of two sessions. During the initial session, we collected data on demographic characteristics, prior comorbidities, COVID‐19, and post‐COVID‐19 symptoms. Each participant underwent a cognitive assessment in the second session with a comprehensive neuropsychological battery as described in Ariza et al. 5 In this investigation, the frequency of cognitive impairment tests was determined. Each participant's score was considered affected if it fell below 1.5 SD when standardized by age and educational level. In addition, participants completed questionnaires that measured fatigue, anxiety, and depressive symptoms, QoL, health‐related QoL (HRQoL), and functioning. The Chalder Fatigue Scale (CFQ) 52 was used to assess fatigue, the Generalized Anxiety Disorder 7‐item scale (GAD‐7) 53 to assess anxiety, and the Patient Health Questionnaire‐9 (PHQ‐9) to assess depressive symptoms. 54 The 26‐item modified version of the validated World Health Organization Quality of Life Scale‐Short Form (WHOQOL‐BREF) evaluated QoL. The WHOQOL‐BREF questionnaire includes questions about physical, psychological, social, and environmental dimensions of QoL. 55 HRQoL was assessed with the validated Spanish version of the EQ‐5D‐3L provided by the EuroQoL group. 56 The EQ‐5D‐3L has two separate elements: a descriptive system and a visual analog scale (EQ‐VAS). The descriptive system includes five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. The scores on the five dimensions of the EQ‐5D‐3L were translated into a single index value (Spanish value set) for all health states, with higher values indicating greater HRQoL. EQ‐VAS assesses the self‐evaluated health status of the participants ranging from 0 to 100. Finally, the level of functioning was evaluated utilizing the full version of the World Health Organization Disability Assessment Schedule‐II (WHODAS‐II). Scores ranging from 0 to 100 were obtained for a total and six domains (cognition, mobility, self‐care, getting along, life activities, and participation) scores, with higher values indicating lower levels of functioning. 57
Statistical analyses
Descriptive statistics were obtained for all variables of the study. Graphical representations and descriptive statistics were used to study the assumptions. Group differences in demographics were examined using analysis of variance. The chi‐squared test was performed to compare categorical measures between groups. One‐way analysis of covariance with Bonferroni‐adjusted post hoc comparisons was performed to determine differences in QoL, HRQoL, and level of functioning, cognitive and emotional variables among groups, including age, sex, educational level, income (gross salary per year), and change of employment status as nuisance variables. The effect size was calculated using the value partial eta squared (ήp 2).
Correlations between QoL, HRQoL, level of functioning, demographic (age, sex, formal education, income level, and change in employment status), clinical variables (severity of COVID‐19 and the time since onset to assessment), comorbidities (heart, respiratory and liver disease, high blood pressure, dyslipidemia, diabetes, and obesity), post‐COVID symptoms, anxiety, depression, fatigue, and cognitive variables were assessed using Pearson correlation. To reduce the number of symptoms, an index was calculated by adding the number of symptoms (1 for presence and 0 for absence) and dividing by the total number of symptoms collected (excluding cognitive, depressive, and anxiety symptoms to avoid overlapping symptoms).
Stepwise multiple regression analyses were conducted, including the variables that correlated significantly with QoL, HRQoL, and level of functioning in the previous correlation tests as covariates that were used to adjust the regression analyses in Step 1 (enter method). In Step 2 (forward method), cognitive and emotional variables were included in the models. Analyses were performed using IBM SPSS Statistics 27.0 (IBM Corp., Armonk, NY, USA). The critical level for statistical significance was set at α = 0.05.
Results
Sample demographics
Three hundred sixty‐eight participants with PCC were classified into three severity groups: severe–intensive care unit (ICU‐PCC) (n = 81), hospitalized (H‐PCC) (n = 80), and mild (M‐PCC) (n = 207). 58 The participants' sociodemographic characteristics and comorbidities are shown in Table 1. The M‐PCC and HC groups were equivalent in age and sex and had a higher proportion of women and were younger than the ICU‐PCC and the H‐PCC groups. There were no differences in the estimated IQ across the three PCC groups. However, there were differences in the levels of formal education, with the M‐PCC having the highest level of education. The estimated IQ and the education level in the HC group were higher than those in all three PCC groups. The gross annual salary distribution varied among the four groups. ICU‐PCC had a more substantial number of individuals with salaries in the lowest range than the HC group, which had the highest proportion of individuals in the highest range. The three PCC groups showed a larger proportion of changes in job situation than the HC, although the PCC‐H and M‐PCC groups were significantly higher.
Table 1.
Sociodemographic characteristics and comorbidities of the PCC and HC groups.
| ICU‐PCC | H‐PCC | M‐PCC | HC | F | p | η 2 | Post hoc Bonferroni | |
|---|---|---|---|---|---|---|---|---|
| n = 81 | n = 80 | n = 207 | n = 124 | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
| Age (years) | 52.82 (8.47) | 53.24 (8.68) | 47.55 (9.54) | 46.78 (10.03) | 13.907 | 0.0001 | 0.079 | ICU > HC |
| ICU > M | ||||||||
| H > HC | ||||||||
| H > M | ||||||||
| Education (years) | 13.14 (3.14) | 13.28 (3.46) | 14.33 (3.24) | 15.73 (3.31) | 14.681 | 0.0001 | 0.083 | HC > ICU |
| HC > H | ||||||||
| HC > M | ||||||||
| M > ICU | ||||||||
| Estimated IQ a | 99.68 (8.44) | 100.98 (7.64) | 101.43 (10.12) | 104.96 (6.66) | 6.921 | 0.0001 | 0.077 | HC > ICU |
| HC > H | ||||||||
| HC > M | ||||||||
| Time since onset to assessment (months) | 8.8 (3.8) | 10.10 (5) | 12.09 (6.7) | 10.225 | 0.0001 | 0.053 | M > ICU | |
| M > H |
| N (%) | N (%) | N (%) | N (%) | χ 2 | p | |
|---|---|---|---|---|---|---|
| Sex (female) | 38 (46.9) | 40 (50) | 165 (79.7) | 93 (75) | 44.498 | 0.0001 |
| Gross salary (year) | 27.400 | 0.007 | ||||
| <14,922€ | 27 (33.3) | 20 (25) | 38 (18.4) | 15 (12.2) | ||
| 14,922–24,869€ | 19 (23.5) | 25 (31.3) | 64 (30.9) | 34 (27.6) | ||
| 24.869–34.817€ | 21 (25.9) | 18 (22.5) | 61 (29.5) | 31 (25.2) | ||
| 34,817–39,790€ | 2 (2.5) | 7 (8.8) | 22 (10.6) | 19 (15.4) | ||
| >39,790€ | 12 (14.8) | 10 (12.5) | 22 (10.6) | 24 (19.5) | ||
| Change in employment status | 35 (14.8) | 29 (36.3) | 79 (38.2) | 11 (8.9) | 39.800 | 0.0001 |
| Comorbidities | ||||||
| Heart disease | 3 (3.7) | 3 (3.8) | 5 (2.4) | 2 (1.6) | ||
| Respiratory disease | 13 (16) | 11 (13.8) | 26 (12.6) | 6 (4.8) | 7.743 | 0.052 |
| High blood pressure | 24 (29.6) | 13 (16.3) | 16 (7.7) | 5 (4) | 36.778 | 0.0001 |
| Dyslipidemia | 18 (22.2) | 14 (17.5) | 19 (9.2) | 12 (2.4) | 11.537 | 0.009 |
| Diabetes mellitus | 5 (6.2) | 7 (8.8) | 1 (0.5) | 3 (2.4) | ||
| Obesity | 44 (55.7) | 28 (34.9) | 34 (17.6) | 14 (12.8) | 62.892 | 0.0001 |
| Chronic liver disease | 3 (3.7) | 3 (3.8) | 1 (0.5) | 0 | ||
| Tobacco smoking | 4 (4.9) | 4 (5) | 19 (9.2) | 29 (23.4) | 25.279 | 0.0001 |
HC, healthy control; ICU, intensive care unit; PCC, post‐COVID condition.
Intelligence quotient estimated using Word Accentuation Test.
On average, all PCC participants had shown a positive test 328 days before their neuropsychological evaluation (SD = 178.81 days), and the ICU‐PCC group had shorter intervals since testing positive than the other two groups. Comorbidities were more prevalent in the PCC groups than in the HC group, and their prevalence rose as COVID‐19 severity increased. Specifically, ICU individuals had a higher prevalence of premorbid hypertension, dyslipidemia, and obesity than other PCC and HC groups. Figure 2 and Table S1 show symptoms reported by people with PCC at the assessment time. CU‐PCC showed significantly more limb weakness than H‐PCC and more posttraumatic stress than the other two groups. However, M‐PCC reported more cognitive symptoms, headache, dizziness, and altered smell/taste than the other groups and more pain than H‐PCC.
Figure 2.
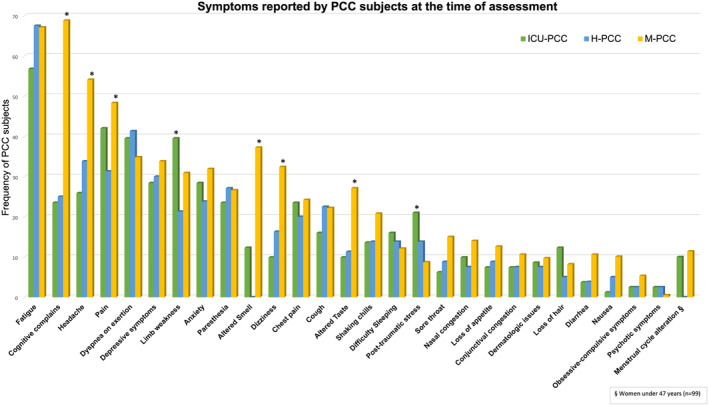
Symptoms reported by people with PCC at time of assessment. The graph illustrates the frequency of symptoms exhibited by the participants. Asterisks denote significant differences (p < 0.05).
QoL, HRQoL, and functioning
Table 2 presents the differences in QoL, HRQoL, and level of functioning between groups after adjusting for age, sex, level of education, income, and change in employment status. The physical health dimension score of the WHOQoL‐BREF was significantly lower in all PCC groups compared with the HC group. The psychological dimension score was statistically significantly lower in the H‐PCC and M‐PCC compared with the HC group and lower in the M‐PCC group compared with the ICU‐PCC group. The environment dimension score of the WHOQoL‐BREF was significantly lower in the M‐PCC group than the HC group (Fig. 3). The EQ‐5D index and EQ‐5D VAS scores were significantly lower in all PCC groups than in the HC group. In addition, the EQ‐5D index and VAS scores were lower in the M‐PCC group than in the ICU and H‐PCC groups. The PCC groups scored higher than the HC group on the WHODAS total, cognition, mobility, life activities, and participation dimensions. The H‐PCC and M‐PCC groups scored higher on the WHODAS self‐care scale than the HC group, and the M‐PCC group scored higher on the Getting Along scale than the HC group. The life activities score of M‐PCC was higher than the ICU and H‐PCC groups (see Fig. 4).
Table 2.
Intergroup differences in QoL, HRQoL, and level of functioning adjusted for age, sex, educational level, comorbidities, income, and change of employment status.
| ICU‐PCC | H‐PCC | M‐PCC | HC | F | p | η 2 | Post hoc Bonferroni | p | |
|---|---|---|---|---|---|---|---|---|---|
| Madj (SE) | Madj (SE) | Madj (SE) | Madj (SE) | ||||||
| PH‐WHOQoL‐BREF | 51.64 (1.22) | 51.43 (1.16) | 48.63 (0.72) | 57.98 (0.97) | 20.280 | 0.0001 | 0.113 | ICU < HC | 0.0001 |
| H < HC | 0.0001 | ||||||||
| M < HC | 0.0001 | ||||||||
| PS‐WHOQoL‐BREF | 60.53 (1.41) | 57.68 (1.34) | 54.69 (0.83) | 62.59 (1.12) | 12.354 | 0.0001 | 0.072 | H < HC | 0.042 |
| M < HC | 0.0001 | ||||||||
| M < ICU | 0.003 | ||||||||
| SR‐WHOQoL‐BREF | 63.86 (2.38) | 62.66 (2.27) | 59.47 (1.41) | 65.67 (1.90) | 2.614 | 0.051 | 0.016 | ||
| E‐WHOQoL‐BREF | 66.38 (1.80) | 65.98 (1.71) | 64.47 (1.06) | 69.63 (1.43) | 2.857 | 0.037 | 0.018 | M < HC | 0.022 |
| EQ‐5D‐3L index | 0.752 (0.022) | 0.787 (0.021) | 0.677 (0.013) | 0.864 (0.018) | 25.474 | 0.0001 | 0.138 | ICU < HC | 0.001 |
| H < HC | 0.042 | ||||||||
| M < HC | 0.0001 | ||||||||
| M < ICU | 0.032 | ||||||||
| M < H | 0.0001 | ||||||||
| EQ‐5D‐3L VAS | 67.83 (2.30) | 67.32 (2.20) | 59.81 (1.35) | 76.82 (1.81) | 19.551 | 0.0001 | 0.110 | ICU < HC | 0.023 |
| H < HC | 0.008 | ||||||||
| M < HC | 0.0001 | ||||||||
| M < ICU | 0.023 | ||||||||
| M < H | 0.027 | ||||||||
| WHODAS total | 27.58 (2.00) | 26.54 (1.90) | 31.98 (1.18) | 12.35 (1.59) | 33.348 | 0.0001 | 0.174 | ICU < HC | 0.0001 |
| H > HC | 0.0001 | ||||||||
| M > HC | 0.0001 | ||||||||
| WHODAS cognition | 26.70 (2.30) | 26.12 (2.19) | 32.56 (1.36) | 11.44 (1.83) | 29.160 | 0.0001 | 0.155 | ICU > HC | 0.0001 |
| H > HC | 0.0001 | ||||||||
| M > HC | 0.0001 | ||||||||
| WHODAS mobility | 23.02 (2.43) | 20.93 (2.30) | 24.57 (1.43) | 9.12 (1.92) | 14.494 | 0.0001 | 0.084 | ICU > HC | 0.0001 |
| H > HC | 0.0001 | ||||||||
| M > HC | 0.0001 | ||||||||
| WHODAS self‐care | 8.64 (1.68) | 10.32 (1.60) | 8.54 (0.99) | 2.72 (1.33) | 5.521 | 0.0001 | 0.034 | H > HC | 0.003 |
| M > HC | 0.003 | ||||||||
| WHODAS getting along | 16.26 (2.39) | 16.98 (2.27) | 20.88 (1.41) | 12.04 (1.89) | 4.920 | 0.002 | 0.030 | M > HC | 0.001 |
| WHODAS life activities | 27.69 (2.84) | 28.88 (2.69) | 38.08 (1.67) | 12.11 (2.25) | 29.232 | 0.0001 | 0.155 | ICU > HC | 0.0001 |
| H > HC | 0.0001 | ||||||||
| M > HC | 0.0001 | ||||||||
| M > ICU | 0.014 | ||||||||
| M > H | 0.028 | ||||||||
| WHODAS participation | 29.93 (2.05) | 28.94 (2.03) | 34.28 (1.26) | 14.85 (1.68) | 28.969 | 0.0001 | 0.154 | ICU > HC | 0.0001 |
| H > HC | 0.0001 | ||||||||
| M > HC | 0.0001 |
η 2 effect size is as follows: η 2 = 0.009, small; η 2 = 0.059, medium; η 2 = 0.139, large.
E, environment; H, hospitalized; HC, healthy control; ICU, intensive care unit; M, mild; PCC, post‐COVID condition; PH, physical health; PS, psychological; SR, social relationships; WHODAS, World Health Organization Disability Assessment Schedule; WHOQOL‐BREF, World Health Organization Quality of Life Scale.
Figure 3.
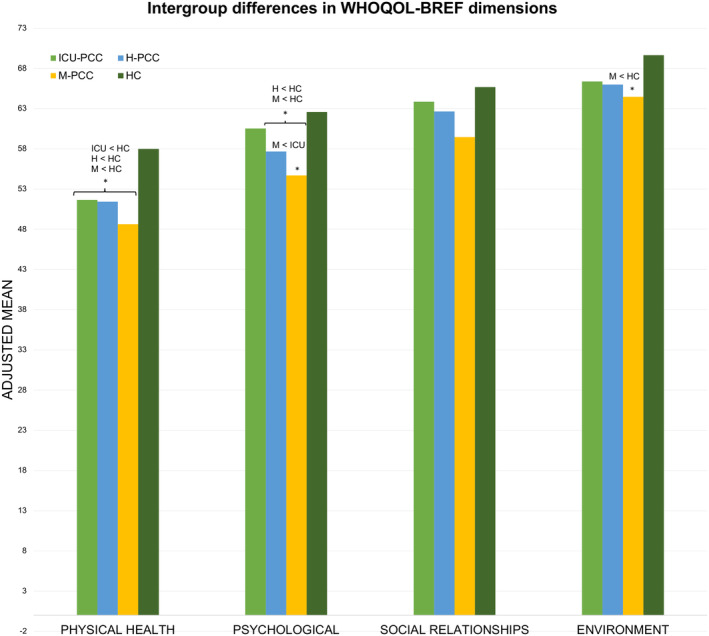
Intergroup differences in WHOQoL‐BREF adjusted for age, sex, educational level, comorbidities, income, and employment status change. Bars represent the adjusted mean of the WHOQoL‐BREF dimension scores for each group. Asterisks denote significant differences. The Bonferroni comparisons are shown.
Figure 4.
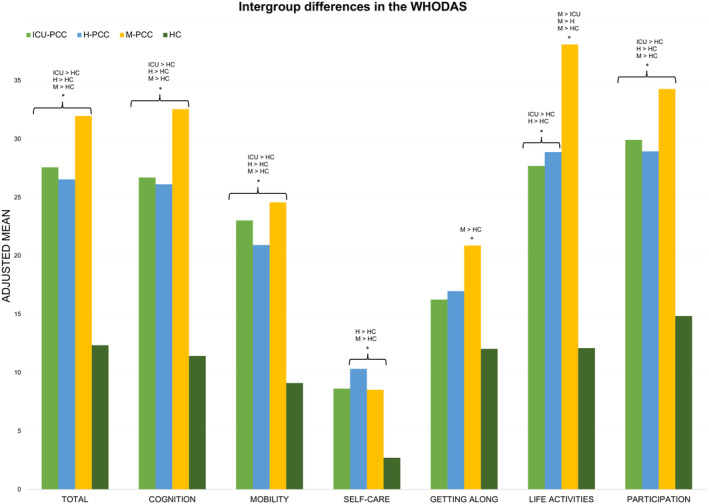
Intergroup differences in WHODAS‐II adjusted for age, sex, educational level, comorbidities, income, and employment status change. Bars represent each group's adjusted mean of the WHODAS Total and dimension scores. Asterisks denote significant differences. The Bonferroni comparisons are shown.
Mental health scales and fatigue of PCC and healthy control participants
After adjustment for age, sex, education level, income, employment status change, and comorbidities, there were statistically significant differences between groups on the GAD‐7 (F = 8.891, p = .0001, partial η 2 = 0.053), PHQ‐9 (F = 24.961, p = 0.0001, partial η 2 = 0.135), and CFQ (F = 61.649, p = 0.0001, partial η 2 = 0.279). Posthoc Bonferroni analysis showed that all PCC groups scored significantly higher than the HC group on the GAD‐7, PHQ‐9, and CFQ questionnaires. In addition, the M‐PCC group had higher PHQ‐9 scores than the H‐PCC group and higher CFQ scores than the ICU and H‐PCC groups (Table S2).
Table 3 displays the percentage of subjects with impairment for each neuropsychological test, while Table 4 displays the same percentage for people with PCC categorized by COVID‐19 severity. As can be seen, several tests were impaired in people with PCC compared to HCs: general cognition (MoCA), verbal memory (RAVLT sum, RAVLT immediate recall, RAVLT delay recall, RAVLT recognition), mental processing speed (TMT A, SCWT word, SCWT color), executive function (SCWT color word, phonetic and semantic fluency), and social cognition (RMET).
Table 3.
Frequency of impaired cognitive tests.
| PCC | HC | χ 2 | p | |
|---|---|---|---|---|
| N | N | |||
| MoCA | 68 (18.5%) | 0 | ||
| RAVLT total | 75 (20.5%) | 7 (5.7%) | 14.450 | 0.0001 |
| RAVLT immediate recall | 68 (18.6%) | 9 (7.3%) | 8.860 | 0.003 |
| RAVLT delay recall | 50 (13.7%) | 2 (1.6%) | 14.032 | 0.0001 |
| RAVLT recognition | 151 (43.3%) | 25 (20.3%) | 20.469 | 0.0001 |
| Digits forward | 50 (13.7%) | 9 (7.3%) | 3.492 | 0.062 |
| Digit span backwards | 37 (10.1%) | 6 (4.9%) | 3.141 | 0.076 |
| Digit symbol | 13 (3.6%) | 1 (0.8%) | 2.494 | 0.114 |
| TMT A | 53 (14.5%) | 8 (6.5%) | 5.365 | 0.021 |
| TMT B | 51 (14.1%) | 10 (8.1%) | 2.964 | 0.085 |
| SCWT words | 92 (25.3%) | 20 (16.4%) | 4.064 | 0.044 |
| SCWT colors | 80 (22%) | 10 (8.2%) | 11.501 | 0.0001 |
| SCWT color word | 71 (19.5%) | 8 (6.6%) | 11.254 | 0.0001 |
| Phonetic fluency (“P”) | 41 (11.2%) | 3 (2.4%) | 8.634 | 0.003 |
| Semantic fluency (“animals”) | 56 (15.3%) | 8 (6.5%) | 6.262 | 0.012 |
| BNT | 20 (5.5%) | 4 (3.3%) | 0.966 | 0.326 |
| RMET | 71 (19.6%) | 10 (8.1%) | 8.640 | 0.003 |
Numbers represent values below 1.5 SD of the standardized age and educational level score.
BNT, Boston naming test; HC, healthy control; MoCA, Montreal Cognitive Assessment; PCC, post‐COVID condition; RAVLT, Rey's auditory verbal learning test; RMET, Reading the Mind in the Eyes Test; SCWT, Stroop color word test; TMT, trail making test.
Table 4.
Frequency of impaired neuropsychological test for severity of COVID‐19 groups.
| ICU | Hospital | Mild | χ 2 | p | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| MoCA | 21 (25.9%) | 18 (22.5%) | 29 (14%) | 6.586 | 0.037 |
| RAVLT sum | 15 (18.5%) | 17 (21.3%) | 43 (21%) | 0.251 | 0.882 |
| RAVLT immediate recall | 15 (18.5%) | 16 (20%) | 37 (18.1%) | 0.132 | 0.936 |
| RAVLT delay recall | 11 (13.6%) | 11 (13.8%) | 28 (13.7%) | 0.001 | 1.00 |
| RAVLT recognition | 35 (43.2%) | 33 (44.6%) | 83 (42.8%) | 0.072 | 0.965 |
| Digit span forward | 13 (16%) | 9 (11.3%) | 28 (13.7%) | 0.786 | 0.675 |
| Digit span backward | 12 (14.8%) | 7 (8.8%) | 18 (8.8%) | 2.535 | 0.282 |
| Digit symbol | 4 (4.9%) | 0 | 9 (4.4%) | 3.831 | 0.147 |
| TMT A | 15 (18.5%) | 9 (11.3%) | 29 (14.1%) | 1.759 | 0.415 |
| TMT B | 15 (18.8%) | 9 (11.5%) | 27 (13.2%) | 1.978 | 0.372 |
| SCWT words | 17 (21.5%) | 16 (20%) | 59 (28.8%) | 3.103 | 0.212 |
| SCWT color | 13 (16.5%) | 13 (16.3%) | 45 (21.9%) | 1.594 | 0.451 |
| SCWT word color | 13 (16.5%) | 13 16.3%) | 45 (22%) | 1.789 | 0.409 |
| Phonetic fluency (“P”) | 9 (11.1%) | 8 (10%) | 24 (11.7%) | 1.169 | 0.919 |
| Semantic fluency (“animals”) | 13 (16%) | 8 (10%) | 35 (17.1%) | 2.266 | 0.322 |
| BNT | 8 (9.9%) | 2 (2.5%) | 10 (4.9%) | 4.550 | 0.103 |
| RMET | 22 (27.8%) | 16 (20.3%) | 33 (16.1%) | 5.035 | 0.081 |
Numbers represent values below 1.5 SD of the standardized age and educational level score.
BNT, Boston naming test; H, hospitalized; ICU, intensive care unit; M, mild; MoCA, Montreal Cognitive Assessment; PCC, post‐COVID condition; RAVLT, Rey's auditory verbal learning test; RMET, Reading the Mind in the Eyes Test; SCWT, Stroop color word test; TMT, trail making test.
Similarly, significant differences existed across groups on the MoCA (F = 7.759, p = 0.0001, partial η 2 = 0.047), RAVLT learning (F = 7.111, p = 0.0001, partial η 2 = 0.044), RAVLT delay recall (F = 4.860, p = 0.0002, partial η 2 = 0.030), RAVLT recognition (F = 3.577, p = 0.014, partial η 2 = 0.023), digit span forward (F = 4.331, p = 0.005, partial η 2 = 0.027), digit symbol (F = 6.478, p = 0.0001, partial η 2 = 0.040), trail making test (TMT) B (F = 4.123, p = 0.007, partial η 2 = 0.026), SCWT color (F = 4.490, p = 0.004, partial η 2 = 0.028), SCWT word‐color (F = 5.622, p = 0.0001, partial η 2 = 0.035), phonetic fluency (F = 6.515, p = 0.0001, partial η 2 = 0.040), semantic fluency (F = 5.005, p = 0.002, partial η 2 = 0.031), BNT (F = 4.714, p = 0.003, partial η 2 = 0.029), and RMET (F = 4.746, p = 0.003, partial η 2 = 0.030) despite adjusting for age, sex, level of education, income, change in employment status, and comorbidities. Post hoc Bonferroni analysis showed that all PCC groups performed worse than the HC group on the MoCA, RAVLT delay recall, digit symbol, SCWT word‐color, and semantic fluency tests. The H‐PCC and M‐PCC groups showed poorer performance in RAVLT learning and recognition than the HC group, whereas ICU‐PCC performed worse than HCs on digit span forward, BNT, and RMET tests. The TMT B and phonetic fluency scores were poorer for the ICU and M‐PCC groups than the HC group. Finally, the performance on SCWT color in the M‐PCC group was lower than in HCs (Table S3).
Predictors of QoL, HRQoL, and functioning in people with PCC
Table 5 displays the predictors for the models of QoL and HRQoL variables. PHQ‐9, post‐COVID symptoms, and change in employment status were significant predictors for a model that explained 35.1% of the variance for the PH‐WHOQoL‐BREF. As the Psychological‐WHOQoL‐BREF dimension incorporates aspects related to negative and positive emotions and self‐esteem, we excluded the GAD‐7 and PHQ‐9 variables to prevent redundancy in the emotional measures. CFQ, income, post‐COVID symptoms, and TMT A added statistical significance to a model that accounted for 32.8% of the variance. PHQ‐9, income, and RAVLT recognition were significant predictors in a model explaining 23.9% of the variance for the Social Relationships‐WHOQoL‐BREF. A model explaining 34.4% of the variance in the Environment‐WHOQoL‐BREF was significantly predicted by PHQ‐9, SCWT color–word, income, post‐COVID symptoms, and hypertension. For the EQ‐5D index, significant predictors of a model explaining 53.9% of the variance were PHQ‐9, post‐COVID symptoms, CFQ, and digit symbol score.
Table 5.
Multiple linear regression models testing the association between cognitive variables QoL and HRQoL.
| WHOQoL‐BREF physical health (controlled for sex, income, change in employment status, severity of COVID‐19, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 64.958 | 0.0001 | 35.1% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | 66.609 | 0.0001 | ||||||
| PHQ‐9 score | −0.361 | −6.890 | 0.0001 | 0.669 | 1.495 | |||
| Post‐COVID symptoms | −0.227 | −4.352 | 0.0001 | 0.652 | 1.487 | |||
| Change in employment status | −0.154 | −3.309 | 0.001 | 0.850 | 1.176 | |||
| WHOQoL‐BREF psychological (without depression and anxiety) (controlled for sex, education, income, change in employment status, time since onset to assessment, severity of COVID‐19, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 44.272 | 0.0001 | 32.8% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | 35.951 | 0.0001 | ||||||
| CFQ score | −0.390 | −7.246 | 0.0001 | 0.654 | 1.530 | |||
| Income | 0.152 | 3.379 | 0.0001 | 0.938 | 1.066 | |||
| Post‐COVID symptoms | −0.158 | −2.874 | 0.004 | 0.625 | 1.600 | |||
| TMT A (time) | −0.111 | −2.445 | 0.015 | 0.918 | 1.089 | |||
| WHOQoL‐BREF social relationships (controlled for income and change in employment status) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 38.099 | 0.0001 | 23.9% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | 23.267 | 0.0001 | ||||||
| PHQ‐9 score | −0.430 | −8.854 | 0.0001 | 0.910 | 1.099 | |||
| Income | 0.110 | 2.294 | 0.022 | 0.943 | 1.061 | |||
| RAVLT recognition score | 0.102 | 2.150 | 0.032 | 0.963 | 1.038 | |||
| WHOQoL‐BREF environment (controlled for sex, education, income, change in employment status, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 38.030 | 0.0001 | 34.4% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | 30.411 | 0.0001 | ||||||
| PHQ‐9 score | −0.340 | −6.426 | 0.0001 | 0.663 | 1.509 | |||
| SCWT CW score | 0.199 | 4.354 | 0.0001 | 0.889 | 1.125 | |||
| Income | 0.163 | 3.630 | 0.0001 | 0.920 | 1.086 | |||
| Post‐COVID symptoms | −0.135 | −0.135 | 0.010 | 0.684 | 1.463 | |||
| HBP | −0.109 | −0.109 | 0.012 | 0.984 | 1.016 | |||
| EQ‐5D index (controlled for sex, income, change in employment status, time since onset to assessment, severity of COVID‐19, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 104.623 | 0.0001 | 53.9% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | 60.269 | 0.0001 | ||||||
| PHQ‐9 score | −0.343 | −6.494 | 0.0001 | 0.466 | 2.146 | |||
| Post‐COVID symptoms | −0.251 | −5.398 | 0.0001 | 0.601 | 1.665 | |||
| CFQ score | −0.218 | −4.000 | 0.0001 | 0.437 | 2.287 | |||
| Digit symbol score | 0.104 | 2.772 | 0.006 | 0.917 | 1.091 | |||
CFQ, Chalder Fatigue Questionnaire; CW, color word; HBP, high blood pressure; PHQ‐9, Patient Health Questionnaire‐9; RAVLT, Rey's auditory verbal learning test; SCWT, Stroop color word test; TMT, trail making test; WHOQoL‐BREF, Health Organization Quality of Life Scale‐Short Form.
PHQ‐9, post‐COVID symptoms, CFQ, digit symbol, and change in employment status added statistical significance to the model of WHODAS Mobility dimension, accounting for 38.2% of the variance. For the WHODAS Self‐Care dimension, a model emerged that explained 17% of the variance, with CFQ, digit symbol score, and post‐COVID symptoms being significant predictors. PHQ‐9 score and SCWT word score were the predictors that added significance to a model that explained 23.7% of the variance of the WHODAS Getting Along dimension. The WHODAS Life Activities dimension model was predicted by PHQ‐9, CFQ, post‐COVID symptoms, female sex, and digit span forward, which explained 48.3% of the variance. Statistically significant predictors for the WHODAS Participation dimension model (60.2% of variance) included PHQ‐9, CFQ, change in employment status, post‐COVID symptoms, education level, and digit symbol score (see Table 6).
Table 6.
Multiple linear regression models testing the association between cognitive variables and level of functioning.
| WHODAS Mobility (controlled for sex, income, change in employment status, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 44.726 | 0.0001 | 38.2% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | 0.414 | 0.679 | ||||||
| PHQ‐9 score | 0.232 | 3.774 | 0.0001 | 0.463 | 2.161 | |||
| Post‐COVID symptoms | 0.181 | 3.331 | 0.0001 | 0.595 | 1.681 | |||
| CFQ score | 0.199 | 3.087 | 0.002 | 0.421 | 2.378 | |||
| Digit symbol score | −0.113 | −2.553 | 0.011 | 0.894 | 1.118 | |||
| Change in employment status | 0.109 | 2.323 | 0.021 | 0.795 | 1.258 | |||
| WHODAS self‐care (controlled for sex, income, change in employment status, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 25.219 | 0.0001 | 17% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | −0.169 | 0.866 | ||||||
| CFQ score | 0.232 | −3.867 | 0.0001 | 0.649 | 1.542 | |||
| Digit symbol score | −0.180 | −3.578 | 0.0001 | 0.928 | 1.078 | |||
| Post‐COVID symptoms | 0.145 | 2.375 | 0.018 | 0.626 | 1.598 | |||
| WHODAS getting along (controlled for sex, income, change in employment status, and time since onset to assessment) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 55.702 | 0.0001 | 23.7 | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | ||||||||
| PHQ‐9 score | 0.451 | 9.255 | 0.0001 | 0.912 | 1.096 | |||
| SCWT W score | −0.103 | −2.109 | 0.036 | 0.912 | 1.096 | |||
| WHODAS life activities (controlled for sex, income, change in employment status, time since onset to assessment, severity of COVID‐19, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 67.236 | 0.0001 | 48.3% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | −0.127 | 0.899 | ||||||
| PHQ‐9 score | 0.284 | 4.992 | 0.0001 | 0.453 | 2.210 | |||
| CFQ score | 0.258 | 4.381 | 0.0001 | 0.420 | 2.381 | |||
| Post‐COVID symptoms | 0.195 | 3.961 | 0.0001 | 0.601 | 1.664 | |||
| Sex (female) | 0.099 | 2.461 | 0.014 | 0.900 | 1.111 | |||
| Digit span forward | −0.091 | −2.259 | 0.024 | 0.909 | 1.100 | |||
| WHODAS participation (controlled for age, sex, income, change in employment status, time since onset to assessment, severity of COVID‐19, and post‐COVID symptoms) | ||||||||
|---|---|---|---|---|---|---|---|---|
| F | p | R 2 adj (%) | ||||||
| 77.200 | 0.0001 | 60.2% | Predictors | Beta | t | p | Tolerance | VIF |
| Constant | −1.100 | 0.272 | ||||||
| PHQ‐9 score | 0.403 | 8.052 | 0.0001 | 0.451 | 2.218 | |||
| CFQ score | 0.223 | 4.299 | 0.0001 | 0.419 | 2.387 | |||
| Change employment status | 0.149 | 3.939 | 0.0001 | 0.788 | 1.269 | |||
| Post‐COVID symptoms | 0.112 | 2.571 | 0.011 | 0.596 | 1.679 | |||
| Education | 0.117 | 3.233 | 0.001 | 0.861 | 1.161 | |||
| Digit symbol score | −0.088 | −2.097 | 0.037 | 0.635 | 1.576 | |||
CFQ, Chalder Fatigue Questionnaire; CW, color word; PHQ‐9, Patient Health Questionnaire‐9; SCWT, Stroop color word test; W, word; WHODAS, World Health Organization Disability Assessment Schedule‐II.
Discussion
Our first aim was to describe QoL, HRQoL, and functioning in a large sample of people with PCC with different COVID‐19 severities and compare these data with those from healthy control participants. Very few studies have examined QoL in individuals post‐COVID‐19, 59 with the majority of them primarily focusing on health care workers. 60 , 61 When testing QoL using the WHOQoL‐BREF, we found that participants with PCC scored lower than HCs on a subset of QoL measures that address physical and mental health. Moreover, we found that participants who had mild COVID‐19 reported worse QoL than those who were admitted to an ICU.
No additional effects on QoL beyond those related to health were found (except for a lower environmental QoL in the M‐PCC group compared with controls). Consistently, results obtained with the specific HRQoL indicator, the EQ‐5D index, were significantly lower in all PCC groups compared with HC group. Lower HRQoL after COVID‐19 has been reported in previous studies. 16 , 17 , 18 , 19 , 22 , 23 , 24 The average EQ‐5D index value for the three groups under investigation exceeded the values previously documented by researchers from the other countries. 17 , 23 , 24 In contrast, a prior investigation with participants from Spain reported a higher mean value than ours. 22 The observed disparities likely stem from variations in cultural norms and the differential effects of the COVID‐19 pandemic experienced by different countries and regions.
Differences were also observed in the EQ‐5D index across PCC groups. Like the observed pattern in the Psychological‐WHOQoL‐BREF dimension, the differences identified in this study were unexpected, as those with a less severe manifestation of COVID‐19 exhibited lower EQ‐5D scores. These differences went in the opposite direction of what had been seen in previous studies. 27 , 31 Because participants in the M‐PCC group had been experiencing symptoms longer than the participants in the other groups, they may have perceived their HRQoL to be poorer. Hospitalized people reported fewer depression symptoms than those not hospitalized. The M‐PCC group may have worried more about illness progression and consequences. 48 This may have increased their perception of symptoms and caused more psychological symptoms. Current hypotheses on the pathogenesis of persistent symptoms in mild and moderate/severe acute cases could explain why people with M‐PCC reported lower QoL. In severe instances, symptoms may be secondary to the acute process, whereas symptoms in M‐PCC cases may be due to later‐activated processes, such as inflammatory or autoimmune phenomena. Although these causative mechanisms may coexist regardless of disease severity, 62 we may be comparing two distinct conditions.
The results of the functioning variables were consistent with those obtained from the two QoL/HRQoL scales. All participants with PCC had a decline in their general functioning. In addition, they obtained lower scores in the areas of cognition, mobility, life activities, and participation. In the Self‐Care dimension, participants with H‐PCC and M‐PCC reported the most difficulties, while the M‐PCC group showed the lowest scores in the Getting Along component. The only dimension of functioning in which the PCC groups differed was activities of daily living. The M‐PCC group's functioning was worse than those of the other two groups. The ideas discussed previously regarding HRQoL may explain why these individuals performed worse on activities of daily living.
The second aim of the present study was to investigate the influence of cognitive and emotional variables on QoL/HRQoL and functioning in a sample of participants with PCC. People with cognitive deficits may experience difficulties performing basic activities of daily living or solving complex problems. Being unable to accomplish these tasks may reduce autonomy and negatively impact QoL. Patients with other medical conditions have had their QoL and functional status predicted by their neurocognitive performance. 36 , 37 , 38 , 63 , 64 , 65 The participants with PCC in the present study, regardless of the severity of acute COVID‐19, showed impairments in global cognition (MoCA), verbal memory (RAVLT), and mental speed processing–executive function (Digit symbol test, TMT B, SCWT) compared with the control group.
Depressive symptoms, anxiety, and fatigue are also common in people with PCC. 43 , 44 , 45 , 46 , 66 In the present study, all PCC groups showed higher levels of anxiety, depressive symptoms, and fatigue than HCs. Additionally, participants who had less severe COVID‐19 experienced higher levels of depressive symptoms than participants who had more severe forms of COVID‐19 (hospitalized). Some previous studies have linked the severity of COVID‐19 to a worse emotional outcome, 8 , 10 while others have not. 67 , 68 As far as we know, no study has shown that people with PCC who had mild COVID‐19 had more depressive symptoms than other people with PCC who had more serious COVID‐19. This finding fit with the fact that people with less serious COVID‐19 reported lower HRQoL than those with more severe disease. In fact, this may explain why HRQoL was more affected. On the other hand, fatigue is a major manifestation of PCC regardless of the severity of the acute stage of COVID‐19. 66 However, in the present sample, COVID‐19 severity appeared to be inversely related to fatigue measured at 12 months after positive testing.
Memory, executive function, and mental speed processing emerged as predictors of WHOQoL‐BREF Psychological, Social Relationships, and Environment domains and EQ‐5D. The cognitive predictors for the functioning models (WHODAS) were mental speed processing (mobility, self‐care, getting along, and participation) and attention (life activities). These cognitive variables are precisely the functions that have been found to be impaired relative to healthy subjects in the current study and previous research. 6 , 7
The WHOQoL‐BREF Social Relationships domain assesses respondents' personal and social fulfillment. Recognition memory explained part of the variance of this model. Memory deficits may influence an individual's functional independence, health perceptions, and life happiness by making it difficult to remember crucial work assignments, meaningful social activities, medical appointments, or errands. Poor memory performance is linked to low QoL in patients with HIV, 63 severe mental illness, 34 , 35 and Parkinson's disease. 34 In individuals infected with SARS‐CoV2, impaired verbal memory affected self‐care dimension scores on the EQ‐5D HRQoL scale. 41
The Environment domain of the WHOQoL‐BREF encompasses safety and security, property and physical environment satisfaction, economics, access to care, information, and transportation. The variance of this variable was partially explained by executive function (SCWT CW). Executive function is a crucial cognitive ability for most daily activities and has been linked to QoL in a variety of conditions. 36 , 37 Palladini et al. 40 found an effect of improvements in executive function on QoL, and Poletti et al. 41 found that impaired executive function in SARS‐CoV2‐infected people affected the Self‐Care and Mobility dimensions of the EQ‐5D.
Mental speed processing (TMT A) explained a portion of variance of the WHOQoL‐BREF Psychological dimension, which examines participants' beliefs, emotions, self‐esteem, body image, thinking, and learning. For the EQ‐5D index, a model was generated in which mental processing speed (digit symbol score) was a predictor variable. This model explained 53.9% of the variance in this measure. Participants post‐COVID‐19 with impaired mental speed processing showed lower Self‐Care dimension scores on the EQ‐5D according to Poletti et al. 69 Slow mental processing speed has also been linked to poor HRQoL in people with HIV. 39
Except for activities of daily living, which can be explained by attention, all aspects of functioning measured by the WHODAS could be predicted by mental processing speed (digit symbol, SCWT word). Processing speed is an essential component of cognitive ability and is essential for responding quickly and correctly in complicated situations. It is reduced in a variety of types of neurological disorders, such as multiple sclerosis 70 and mild cognitive impairment. 71 An effect on information processing speed is seen in sleep 72 and mood disorders, 73 as well as in systemic diseases such as chronic fatigue syndrome 74 and fibromyalgia. 75
Anxiety and depression are common comorbidities of chronic conditions. 76 , 77 Comorbidity between a chronic disease and depression is worse for health than depression alone, chronic disease alone, or chronic disease without depression. 78 The persistence of symptoms has been linked to depression following COVID‐19. 79 We found that depressive symptomatology worsened Physical Health, Social Relationships, and Environment QoL, EQ‐5D, and Mobility, Getting Along, Life Activities, and Participation and functioning in the present participants with PCC. Conversely, anxiety did not explain any QoL/HRQoL dimensions or functioning. On the other hand, contrary to Calabria et al., 80 fatigue had no explanatory value in the QoL dimensions, except for the Psychological dimension (which precluded anxiety and depression). In contrast, fatigue played an essential role in HRQoL and virtually all functioning domains. These findings were in agreeance with previous post‐COVID‐19 studies. Fatigue 24 , 80 , 81 and depression 48 were reported to be predictors of low HRQoL and poorer functioning. 24
Post‐COVID fatigue is correlated with cognitive impairment in most studies. 80 , 82 , 83 However, the direction of this relationship has shown conflicting results in people with PCC. While fatigue accounted for up to 10% of the variability observed in visual memory, phonetic fluency, and visuoconstructive ability in individuals with PCC, 82 other studies have shown that a model containing executive dysfunction explained about 40% of the variability of post‐COVID‐19 fatigue. 80 In this regard, it has been reported that fatigue acts as a mediator between cognitive impairment and its manifestation, namely cognitive complaints. 83
This association between fatigue and cognitive impairment could mask the impact of cognition on QoL and functioning. Nonetheless, the indices, tolerance, and variance inflation factor results suggested that collinearity was not a problem.
The limitations and strengths of the present study require consideration while interpreting the findings. The cross‐sectional nature of our study design prevented us from determining causal relationships with precision. Moreover, our severity groups varied with respect to demographic variables (age, sex, level of education, income, change in employment status), comorbidities, post‐COVID‐19 symptoms, and time from infection to evaluation. Some of these variations cannot be avoided and are risk factors for a more or less severe form of COVID‐19 or the development of PCC. However, we had to control for these variables in our analyses. This study was conducted across multiple centers, while maintaining a consistent cultural environment. Due to the presence of cultural variations in QoL, it is plausible that the findings may lack generalizability to other countries.
One of the strengths of this study was its large sample size, which included participants from several health centers. The sample underwent a comprehensive assessment on multiple levels, including clinical, the neuropsychological, and the emotional dimension. QoL was examined in great detail, taking into account aspects of QoL that are unrelated to health, and functionality. We compared QoL, HRQoL, and functionality in patients with PCC with a group of disease‐free HCs, with all data collected concurrently. In addition to never having COVID‐19, the present control sample lacked pathologies that could affect HRQoL other than the PCC group's comorbidities.
In conclusion, the results of the present study showed that between 8 and 12 months after infection with SARS‐CoV2, QoL/HRQoL, and functioning in individuals with persistent symptoms was lower relative to non‐affected persons. It was apparent that the health issues associated with PCC were the primary source of concern for persons who have the condition, whereas other areas of their QoL were unaffected by the condition. Although individuals with PCC who had a milder form of the disease tended to report lower HRQoL and functioning than those who were hospitalized, the severity of COVID‐19 was not a factor that could explain this difference. On the other hand, all aspects of QoL, HRQoL, and functioning could be represented by a model, with a set of shared predictors. These included post‐COVID symptoms, depressive symptoms, income or employment status change, fatigue, and speed of mental processing. Future therapeutic studies should emphasize the assessment and treatment of these symptoms to improve the quality of life and functioning of people with PCC.
Author Contributions
Mar Ariza, Maite Garolera, Carme Junqué, and Bàrbara Segura designed the study. Neus Cano collected the data. Mar Ariza performed the statistical analyses and wrote the first version of the manuscript. Carme Junqué revised the manuscript critically for important intellectual content. All authors revised the manuscript drafts and approved the final manuscript.
NAUTILUS‐Project Collaborative Group List of Affiliation
Jose A. Bernia, Servei d'Anestesia Reanimació i Clinica del Dolor, Consorci Sanitari de Terrassa (CST) (Terrassa, Barcelona, Spain).
Vanesa Arauzo, Servei de Medicina Intensiva, Consorci Sanitari de Terrassa (CST) (Terrassa, Barcelona, Spain).
Marta Balague‐Marmaña, Hospital Sant Joan Despí Moisès Broggi, Consorci Sanitari Integral (Sant Joan Despí, Spain).
Cristian Pérez‐Pellejero, Hospital Sant Joan Despí Moisès Broggi, Consorci Sanitari Integral (Sant Joan Despí, Barcelona, Spain).
Silvia Cañizares, Hospital Clinic de Barcelona (Barcelona, Spain).
Jose Antonio Lopez Muñoz, Occupational Health Care Service, Hospital Clínic (Barcelona, Spain).
Jesús Caballero, Hospital Universitari Arnau de Vilanova (Lleida, Spain).
Anna Carnes‐Vendrell, Hospital Universitari de Santa Maria (Lleida, Spain).
Gerard Piñol‐Ripoll, Hospital Universitari de Santa Maria (Lleida, Spain).
Ester Gonzalez‐Aguado, Consorci Sanitari Alt Penedès‐Garraf (Vilafranca de Penedés, Barcelona, Spain).
Carme Tayó‐Juli, Consorci Sanitari Alt Penedès‐Garraf (Vilafranca de Penedés, Barcelona, Spain).
Eva Forcadell‐Ferreres, Hospital Verge de la Cinta, (Tortosa, Tarragona, Spain).
Silvia Reverte‐Vilarroya, Hospital Verge de la Cinta, (Tortosa, Tarragona, Spain).
Susanna Forné, Fundació Sant Hospital de la Seu d'Urgell (La Seu d'Urgell, Lleida, Spain).
Jordina Muñoz‐Padros, Consorci Hospitalari de Vic (Vic, Barcelona, Spain).
Anna Bartes‐Plan, Consorci Hospitalari de Vic (Vic, Barcelona, Spain).
Jose A. Muñoz‐Moreno, Servei de Malalties Infeccioses, Fundació Lluita contra les Infeccions ‐ Hospital Universitari Germans Trias i Pujol (Badalona, Barcelona, Spain).
Anna Prats‐Paris, Servei de Malalties Infeccioses, Fundació Lluita contra les Infeccions ‐ Hospital Universitari Germans Trias i Pujol (Badalona, Barcelona, Spain).
Anna Gasa‐Roqué, Hospital Universitari de Bellvitge (L'Hospitalet de Llobregat, Barcelona, Spain).
Laura Casas Valls, Hospital Universitari de Bellvitge (L'Hospitalet de Llobregat, Barcelona, Spain).
Marta Almeria, Hospital Universitari Mútua Terrassa (Terrassa, Barcelona, Spain).
Judith Castejon, Hospital Universitari Mútua Terrassa (Terrassa, Barcelona, Spain).
Maria José Ciudad, Badalona Serveis Assistencials (Badalona, Barcelona, Spain).
Anna Ferré, Badalona Serveis Assistencials (Badalona, Barcelona, Spain).
Manuela Lozano, Institut d'Assistència Sanitària (Girona, Spain).
Tamar Garzon, Institut d'Assistència Sanitària (Girona, Spain).
Marta Cullell, Fundació Salut Empordà (Figueres, Girona, Spain).
Sonia Vega, Fundació Salut Empordà (Figueres, Girona, Spain).
Sílvia Alsina, Fundació Hospital de Puigcerdà (Puigcerdà, Girona, Spain).
Maria J. Maldonado‐Belmonte, Hospital Universitario Central de la Cruz Roja San José y Santa Adela (Madrid, Spain).
Susana Vazquez‐Rivera, Hospital Universitario Central de la Cruz Roja San José y Santa Adela (Madrid, Spain).
Eloy García‐Cabello, Clínica Universitaria de Psicología, Facultad de Ciencias de la Salud, Universidad Fernando Pessoa (La Palmas de Gran Canaria, Islas Canarias, Spain).
Yaiza Molina, Clínica Universitaria de Psicología, Facultad de Ciencias de la Salud, Universidad Fernando Pessoa (La Palmas de Gran Canaria, Islas Canarias, Spain).
Sandra Navarro, Servei Andorrà d'Atenció Sanitària (SAAS) (Andorra).
Eva Baillès, Servei Andorrà d'Atenció Sanitària (SAAS) (Andorra).
Funding information
This research was supported by the Agency for Management of University and Research Grants (AGAUR) from the Generalitat de Catalunya (Pandemies, 202PANDE00053) and La Marató de TV3 Foundation (202111‐30‐31‐32).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Supplementary Table 1.
Acknowledgments
This research was supported by the Agency for Management of University and Research Grants (AGAUR) from the Generalitat de Catalunya (Pandemies, 202PANDE00053) and La Marató de TV3 Foundation (202111‐30‐31‐32).
Study Registration: www.ClinicalTrials.gov, identifier NCT05307549 and NCT05307575.
Funding Statement
This work was funded by Agencia de Gestió d'Ajuts Universitaris i de Recerca grant Pandemies 2020PANDE00053; Fundació la Marató de TV3 grant 20211303132.
Contributor Information
Maite Garolera, Email: mgarolera@cst.cat.
NAUTILUS Project Collaborative Group:
Jose A. Bernia, Vanesa Arauzo, Marta Balague‐Marmaña, Silvia Cañizares, Jose Antonio Lopez Muñoz, Jesús Caballero, Anna Carnes‐Vendrell, Gerard Piñol‐Ripoll, Ester Gonzalez‐Aguado, Carme Tayó‐Juli, Eva Forcadell‐Ferreres, Silvia Reverte‐Vilarroya, Susanna Forné, Jordina Muñoz‐Padros, Anna Bartes‐Plan, Jose A. Muñoz‐Moreno, Anna Prats‐Paris, Anna Gasa‐Roqué, Laura Casas Valls, Marta Almeria, Judith Castejon, Maria José Ciudad, Anna Ferré, Manuela Lozano, Tamar Garzon, Marta Cullell, Sonia Vega, Sílvia Alsina, Maria J. Maldonado‐Belmonte, Susana Vazquez‐Rivera, Eloy García‐Cabello, Yaiza Molina, Sandra Navarro, and Eva Baillès
References
- 1. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post‐COVID‐19 Condition . A clinical case definition of post‐COVID‐19 condition by a Delphi consensus [Internet]. Lancet Infect Dis. 2022;22(4):e102‐e107. http://www.thelancet.com/article/S1473309921007039/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ballering AV, van Zon SKR, olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID‐19 in The Netherlands: an observational cohort study [Internet]. Lancet. 2022;400(10350):452‐461. doi: 10.1016/S0140-6736(22)01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ECDC . Prevalence of post COVID‐19 condition symptoms: a systematic review and meta‐analysis of cohort study data, stratified by recruitment setting [Internet]. Europe Centre for Disease Prevention and Control; 2022. https://www.ecdc.europa.eu/sites/default/files/documents/Prevalence‐post‐COVID‐19‐condition‐symptoms.pdf [Google Scholar]
- 4. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact [Internet]. EClinicalMedicine. 2021;38:101019. http://www.thelancet.com/article/S2589537021002996/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariza M, Cano N, Segura B, et al. Neuropsychological impairment in post‐COVID condition individuals with and without cognitive complaints. Front Aging Neurosci. 2022;14:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delgado‐Alonso C, Valles‐Salgado M, Delgado‐Álvarez A, et al. Cognitive dysfunction associated with COVID‐19: a comprehensive neuropsychological study. J Psychiatr Res. 2022;150(February):40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. García‐Sánchez C, Calabria M, Grunden N, et al. Neuropsychological deficits in patients with cognitive complaints after COVID‐19 [Internet]. Brain Behav. 2022;12:e2508. doi: 10.1002/brb3.2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magnúsdóttir I, Lovik A, Unnarsdóttir AB, et al. Acute COVID‐19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health. 2022;7(5):e406‐e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sampogna G, Di Vincenzo M, Giallonardo V, et al. The psychiatric consequences of long‐COVID: a scoping review. J Pers Med. 2022;12(11):1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badinlou F, Lundgren T, Jansson‐Fröjmark M. Mental health outcomes following COVID‐19 infection: impacts of post‐COVID impairments and fatigue on depression, anxiety, and insomnia—a web survey in Sweden [Internet]. BMC Psychiatry. 2022;22(1):1‐11. doi: 10.1186/s12888-022-04405-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Power M, Kuyken W. World Health Organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46(12):1569‐1585. [DOI] [PubMed] [Google Scholar]
- 12. Schipper H. Quality of life: principles of the clinical paradigm. J Psychosoc Oncol. 1990;8(2–3):71‐185. [Google Scholar]
- 13. Sosnowski R, Kulpa M, Ziȩtalewicz U, et al. Basic issues concerning health‐related quality of life [Internet]. Cent European J Urol. 2017;70(2):206. /pmc/articles/PMC5510334/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karimi M, Brazier J. Health, health‐related quality of life, and quality of life: what is the difference? [Internet]. Pharmacoeconomics. 2016;34(7):645‐649. doi: 10.1007/s40273-016-0389-9 [DOI] [PubMed] [Google Scholar]
- 15. Kostanjsek N. Use of the international classification of functioning, disability and health (ICF) as a conceptual framework and common language for disability statistics and health information systems [Internet]. BMC Public Health. 2011;11(SUPPL. 4):1‐6. doi: 10.1186/1471-2458-11-S4-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gil D, Tiscar C, Gómez M, et al. Health‐related quality of life and stress‐related disorders in COVID‐19 ICU survivors: are they worse than with other causes of ARDS? [Internet]. J Intens Med. 2022;2(2):103. /pmc/articles/PMC8907013/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moens M, Duarte RV, De Smedt A, et al. Health‐related quality of life in persons post‐COVID‐19 infection in comparison to normative controls and chronic pain patients. Front Public Health. 2022;10:3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Brien K, Townsend L, Dowds J, et al. 1‐year quality of life and health‐outcomes in patients hospitalised with COVID‐19: a longitudinal cohort study. Respir Res. 2022;23:115. doi: 10.1186/s12931-022-02032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D'ávila KG, Monaiar LR, Dantas LDP, et al. Decrease in health‐related quality of life and post‐COVID‐19 syndrome in health care workers after SARS‐CoV‐2 infection: a cohort study. J Occup Environ Med. 2023;65(1):E1‐E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim Y, Bae S, Chang HH, et al. Long COVID prevalence and impact on quality of life 2 years after acute COVID‐19. Sci Rep. 2023;13:11207. doi: 10.1038/s41598-023-36995-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsson SB, von Feilitzen GS, Andersson ME, et al. Self‐reported symptom severity, general health, and impairment in post‐acute phases of COVID‐19: retrospective cohort study of Swedish public employees [Internet]. Sci Rep. 2022;12(1):1‐12. doi: 10.1038/s41598-022-24307-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sacristán‐Galisteo C, López‐de‐Uralde‐Villanueva I, Del Corral T, Martín‐Casas P. Functional status and quality of life in nonhospitalized COVID‐19 survivors. Acad J Health Sci. 2022;37:58‐64. [Google Scholar]
- 23. Tak CR. The health impact of long COVID: a cross‐sectional examination of health‐related quality of life, disability, and health status among individuals with self‐reported post‐acute sequelae of SARS CoV‐2 infection at various points of recovery [Internet]. J Patient Rep Outcomes. 2023;7(1):31 /pmc/articles/PMC10029785/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker S, Goodfellow H, Pookarnjanamorakot P, et al. Impact of fatigue as the primary determinant of functional limitations among patients with post‐COVID‐19 syndrome: a cross‐sectional observational study. BMJ Open. 2023;13(6):e069217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lemhöfer C, Sturm C, Loudovici‐Krug D, Best N, Gutenbrunner C. The impact of post‐COVID‐Syndrome on functioning—results from a community survey in patients after mild and moderate SARS‐CoV‐2‐infections in Germany. J Occup Med Toxicol. 2021;16(1):45. doi: 10.1186/s12995-021-00337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Temperoni C, Grieco S, Pasquini Z, et al. Clinical characteristics, management and health related quality of life in young to middle age adults with COVID‐19. MBC Infectious Diseases. 2021;21:1‐10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7848882/pdf/12879_2021_Article_5841.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iqbal A, Iqbal K, Arshad Ali S, et al. The COVID‐19 Sequelae: a cross‐sectional evaluation of post‐recovery symptoms and the need for rehabilitation of COVID‐19 survivors. Cureus. 2021;13(2):e13080. doi: 10.7759/cureus.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pappa S, Barmparessou Z, Athanasiou N, et al. Depression, insomnia and post‐traumatic stress disorder in COVID‐19 survivors: role of gender and impact on quality of life. J Pers Med. 2022;12(3):486. doi: 10.3390/jpm12030486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rass V, Beer R, Schiefecker AJ, et al. Neurological outcome and quality of life 3 months after COVID‐19: a prospective observational cohort study [Internet]. Eur J Neurol. 2021;28(10):3348. /pmc/articles/PMC8250725/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albu S, Zozaya NR, Murillo N, García‐Molina A, Chacón CAF, Kumru H. What's going on following acute COVID‐19? Clinical characteristics of patients in an out‐patient rehabilitation program. NeuroRehabilitation. 2021;48(4):469‐480. [DOI] [PubMed] [Google Scholar]
- 31. Verveen A, Wynberg E, van Willigen HDG, et al. Health‐related quality of life among persons with initial mild, moderate, and severe or critical COVID‐19 at 1 and 12 months after infection: a prospective cohort study. BMC Med. 2022;20(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lehmann J, Holzner B, Giesinger JM, et al. Functional health and symptoms in Spain before and during the COVID‐19 pandemic. BMC Public Health. 2021;21(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malesevic S, Sievi NA, Baumgartner P, et al. Impaired health‐related quality of life in long‐COVID syndrome after mild to moderate COVID‐19 [Internet]. Sci Rep. 2023;13(1):1‐11. doi: 10.1038/s41598-023-34678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pirogovsky E, Woods SP, Vincent Filoteo J, Gilbert PE. Prospective memory deficits are associated with poorer everyday functioning in Parkinson's disease. J Int Neuropsychol Soc. 2012;18(6):986‐995. doi: 10.1017/S1355617712000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsui M, Sumiyoshi T, Arai H, Higuchi Y, Kurachi M. Cognitive functioning related to quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):280‐287. [DOI] [PubMed] [Google Scholar]
- 36. Ratiu I, Virden TB, Baylow H, Flint M, Esfandiarei M. Executive function and quality of life in individuals with Marfan syndrome [Internet]. Qual Life Res. 2018;27(8):2057‐2065. doi: 10.1007/s11136-018-1859-7 [DOI] [PubMed] [Google Scholar]
- 37. Ho HT, Lin SI, Guo NW, Yang YC, Lin MH, Wang CS. Executive function predict the quality of life and negative emotion in older adults with diabetes: a longitudinal study [Internet]. Prim Care Diabetes. 2022;16(4):537‐542. doi: 10.1016/j.pcd.2022.05.002 [DOI] [PubMed] [Google Scholar]
- 38. Kiessling A, Henriksson P. Perceived cognitive function is a major determinant of health related quality of life in a non‐selected population of patients with coronary artery disease—a principal components analysis. Qual Life Res. 2004;13(10):1621‐1631. [DOI] [PubMed] [Google Scholar]
- 39. Tozzi V, Costa M, Sampaolesi A, et al. Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res Hum Retroviruses. 2003;19(8):643‐652. [DOI] [PubMed] [Google Scholar]
- 40. Palladini M, Bravi B, Colombo F, et al. Cognitive remediation therapy for post‐acute persistent cognitive deficits in COVID‐19 survivors: a proof‐of‐concept study. Neuropsychol Rehabil. 2023;33(7):1207‐1224. doi: 10.1080/09602011.2022.2075016 [DOI] [PubMed] [Google Scholar]
- 41. Poletti S, Palladini M, Mazza MG, et al. Long‐term consequences of COVID‐19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur Arch Psychiatry Clin Neurosci. 2022;272(5):773‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cacciatore M, Raggi A, Pilotto A, et al. Neurological and mental health symptoms associated with post‐COVID‐19 disability in a sample of patients discharged from a COVID‐19 ward: a secondary analysis. Int J Environ Res Public Health. 2022;19:4242. doi: 10.3390/ijerph19074242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fernández‐de‐las‐Peñas C, Gómez‐Mayordomo V, De‐la‐Llave‐Rincón AI, et al. Anxiety, depression and poor sleep quality as long‐term post‐COVID sequelae in previously hospitalized patients: a multicenter study [Internet]. J Inf. 2021;83(4):496‐522. http://www.journalofinfection.com/article/S0163445321003194/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID‐19 outbreak in China: a web‐based cross‐sectional survey. Psychiatry Res. 2020;288:112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huarcaya‐Victoria J, Alarcon‐Ruiz CA, Barzola‐Farfán W, et al. One‐year follow‐up of depression, anxiety, and quality of life of Peruvian patients who survived COVID‐19 [Internet]. Qual Life Res. 2023;32(1):139‐149. doi: 10.1007/s11136-022-03208-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mazza MG, Palladini M, De Lorenzo R, et al. One‐year mental health outcomes in a cohort of COVID‐19 survivors. J Psychiatr Res. 2022;145(August 2021):118‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rass V, Ianosi BA, Zamarian L, et al. Factors associated with impaired quality of life three months after being diagnosed with COVID‐19 [Internet]. Qual Life Res. 2022;31(5):1401‐1414. doi: 10.1007/s11136-021-02998-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mastrorosa I, Del Duca G, Pinnetti C, et al. What is the impact of post‐COVID‐19 syndrome on health‐related quality of life and associated factors: a cross‐sectional analysis [Internet]. Health Qual Life Outcomes. 2023;21(1):28. /pmc/articles/PMC10031164/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Si TL, Chen P, Zhang L, et al. Depression and quality of life among Macau residents in the 2022 COVID‐19 pandemic wave from the perspective of network analysis. Front Psychol. 2023;14(April):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Faul F, Erdfelder E, Lang A‐G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175‐191. [DOI] [PubMed] [Google Scholar]
- 51. Rusticus SA, Lovato CY. Impact of sample size and variability on the power and type I error rates of equivalence tests: a simulation study. Pract Assess Res Eval. 2014;19(11):1‐10. [Google Scholar]
- 52. Jackson C. The chalder fatigue scale (CFQ 11) [Internet]. Occup Med. 2015;65(1):86. https://academic.oup.com/occmed/article/65/1/86/1433061 [DOI] [PubMed] [Google Scholar]
- 53. García‐Campayo J, Zamorano E, Ruiz MA, et al. Cultural adaptation into Spanish of the generalized anxiety disorder‐7 (GAD‐7) scale as a screening tool [Internet]. Health Qual Life Outcomes. 2010;8:8. /pmc/articles/PMC2831043/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Diez‐Quevedo C, Rangil T, Sanchez‐Planell L, Kroenke K, Spitzer RL. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679‐686. [DOI] [PubMed] [Google Scholar]
- 55. WHO . WHOQOL‐BREF: Spanish version. World Health Organization. 2020;1‐3.
- 56. Foundation ER . EQ‐5D‐3L User Guide [Internet]. 2018. https://euroqol.org/publications/user‐guides
- 57. Organización Mundial de la Salud. Medición de la Salud y la Discapacidad [Internet] . 2015. https://apps.who.int/iris/bitstream/handle/10665/170500/9874573309_spa.pdf?sequence=1&isAllowed=y%0Ahttps://apps.who.int/iris/handle/10665/170500
- 58. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20(8):e192‐e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sarda R, Kumar A, Chandra A, et al. Prevalence of long COVID‐19 and its impact on quality of life among outpatients with mild COVID‐19 disease at Tertiary Care Center in North India. J Patient Exp. 2022;9:23743735221117358. doi: 10.1177/23743735221117358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pires BMFB, Bosco PS, Nunes AS, et al. Post‐COVID‐19 health professionals' quality of LIFE: a cross‐sectional study. Cogitare Enfermagem. 2021;26:e78275. [Google Scholar]
- 61. Rashid MU, Khan MAS, Dalal K, et al. Quality of life (QoL) among COVID‐19 recovered healthcare workers in Bangladesh [Internet]. BMC Health Serv Res. 2022;22(1):1‐13. doi: 10.1186/s12913-022-07961-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Castanares‐Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review [Internet]. Ann Med. 2022;54(1):1473. /pmc/articles/PMC9132392/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Doyle K, Weber E, Atkinson JH, et al. Aging, prospective memory, and health‐related quality of life in HIV infection. AIDS Behav. 2012;16(8):2309‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sanchez‐Luengos I, Lucas‐Jiménez O, Ojeda N, et al. Predictors of health‐related quality of life in Parkinson's disease: the impact of overlap between health‐related quality of life and clinical measures [Internet]. Qual Life Res. 2022;31(11):3241‐3252. doi: 10.1007/s11136-022-03187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ueoka Y, Tomotake M, Tanaka T, et al. Quality of life and cognitive dysfunction in people with schizophrenia [Internet]. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):53‐59. doi: 10.1016/j.pnpbp.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 66. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS‐CoV‐2 infection is common and independent of severity of initial infection [Internet]. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ollila H, Pihlaja R, Koskinen S, et al. Long‐term cognitive functioning is impaired in ICU‐treated COVID‐19 patients: a comprehensive controlled neuropsychological study [Internet]. Crit Care. 2022;26(1):1‐11. doi: 10.1186/s13054-022-04092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Renaud‐Charest O, Lui LMW, Eskander S, et al. Onset and frequency of depression in post‐COVID‐19 syndrome: a systematic review [Internet]. J Psychiatr Res. 2021;144(July):129‐137. doi: 10.1016/j.jpsychires.2021.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID‐19 survivors: role of inflammatory and clinical predictors [Internet]. Brain Behav Immun. 2020;89:594‐600. doi: 10.1016/j.bbi.2020.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barker‐Collo SL. Quality of life in multiple sclerosis: does information‐processing speed have an independent effect? Arch Clin Neuropsychol. 2006;21(2):167‐174. [DOI] [PubMed] [Google Scholar]
- 71. Wadley VG, Bull TP, Zhang Y, et al. Cognitive processing speed is strongly related to driving skills, financial abilities, and other instrumental activities of daily living in persons with mild cognitive impairment and mild dementia. J Gerontol Ser A Biol Sci Med Sci. 2021;76(10):1829‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kilpinen R, Saunamäki T, Jehkonen M. Information processing speed in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2014;129(4):209‐218. [DOI] [PubMed] [Google Scholar]
- 73. Khanahmadi M, Malmir M, Eskandari H, Orang T. Evaluation of visual information processing speed in depressed people. Iran J Public Health. 2013;42(11):1266‐1273. [PMC free article] [PubMed] [Google Scholar]
- 74. Ocon AJ. Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front Physiol. 2013;4:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Veldhuijzen DS, Sondaal SFV, Oosterman JM. Intact cognitive inhibition in patients with fibromyalgia but evidence of declined processing speed. J Pain. 2012;13(5):507‐515. [DOI] [PubMed] [Google Scholar]
- 76. Cassileth BR, Lusk EJ, Strouse TB, et al. Psychosocial status in chronic illness. N Engl J Med. 1984;311(8):506‐511. [DOI] [PubMed] [Google Scholar]
- 77. Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders. Prev Chronic Dis. 2005;2(1):1‐10. [PMC free article] [PubMed] [Google Scholar]
- 78. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2017;370(8):851‐858. [DOI] [PubMed] [Google Scholar]
- 79. Bottemanne H, Gouraud C, Hulot JS, et al. Do anxiety and depression predict persistent physical symptoms after a severe COVID‐19 episode? A prospective study. Front Psychiatry. 2021;12(November):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Calabria M, García C, Nicholas S, et al. Post‐COVID‐19 fatigue: the contribution of cognitive and neuropsychiatric symptoms [Internet]. J Neurol. 2022;269:3990‐3999. doi: 10.1007/s00415-022-11141-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Skinner JP, Moran LV. Persistent effects of COVID‐19 in patients hospitalized during the first wave of the pandemic: the impact of persistent fatigue on quality of life in a cross‐sectional study. J Med Virol. 2023;95(2):e28491. [DOI] [PubMed] [Google Scholar]
- 82. Azcue N, Gómez‐Esteban JC, Acera M, et al. Brain fog of post‐COVID‐19 condition and chronic fatigue syndrome, same medical disorder? [Internet]. J Transl Med. 2022;20(1):1‐16. doi: 10.1186/s12967-022-03764-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Delgado‐Alonso C, Díez‐Cirarda M, Pagán J, et al. Unraveling brain fog in post‐COVID syndrome: relationship between subjective cognitive complaints and cognitive function, fatigue, and neuropsychiatric symptoms. Eur J Neurol. 2023. doi: 10.1111/ene.16084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.


