Abstract
Objective
Although acute brain infarcts are common after surgical aortic valve replacement (SAVR), they are often unassociated with clinical stroke symptoms. The relationship between clinically “silent” infarcts and in‐hospital delirium remains uncertain; obscured, in part, by how infarcts have been traditionally summarized as global metrics, independent of location or structural consequence. We sought to determine if infarct location and related structural connectivity changes were associated with postoperative delirium after SAVR.
Methods
A secondary analysis of a randomized multicenter SAVR trial of embolic protection devices (NCT02389894) was conducted, excluding participants with clinical stroke or incomplete neuroimaging (N = 298; 39% female, 7% non‐White, 74 ± 7 years). Delirium during in‐hospital recovery was serially screened using the Confusion Assessment Method. Parcellation and tractography atlas‐based neuroimaging methods were used to determine infarct locations and cortical connectivity effects. Mixed‐effect, zero‐inflated gaussian modeling analyses, accounting for brain region‐specific infarct characteristics, were conducted to examine for differences within and between groups by delirium status and perioperative neuroprotection device strategy.
Results
23.5% participants experienced postoperative delirium. Delirium was associated with significantly increased lesion volumes in the right cerebellum and temporal lobe white matter, while diffusion weighted imaging infarct‐related structural disconnection (DWI‐ISD) was observed in frontal and temporal lobe regions (p‐FDR < 0.05). Fewer brain regions demonstrated DWI‐ISD loss in the suction‐based neuroprotection device group, relative to filtration‐based device or standard aortic cannula.
Interpretation
Structural disconnection from acute infarcts was greater in patients who experienced postoperative delirium, suggesting that the impact from covert perioperative infarcts may not be as clinically “silent” as commonly assumed.
Introduction
Surgical aortic valve replacement (SAVR) for aortic valve stenosis is common with ~100,000 patients/year undergoing the procedure or its less invasive alternative, transcatheter aortic valve replacement (TAVR). Despite improved surgical outcomes, survival and quality of life (QoL), a high proportion of patients undergoing both procedures demonstrate perioperative acute brain infarcts on diffusion weighted imaging (DWI; range 32%–90%). 1 , 2 , 3 Depending upon the rigor of ascertainment and evaluation, infarction leading to clinical stroke is as high as 17% in patients undergoing SAVR, 1 but the vast majority of DWI infarcts are often deemed clinically “silent.” These covert infarcts are most often discrete and small (<1 cm3), and generally deemed of little clinical consequence despite their co‐occurrence with perioperative neurocognitive disorders (PND), 4 which carry serious short‐ and long‐term consequences.
PND, which include postoperative delirium (POD) and postoperative cognitive dysfunction (POCD), occur with a high prevalence after cardiac surgery (~11%–46% POD; ~15%–60% POCD). 5 PND risk is multifactorial, reflecting a combination of surgical complexity, presurgical health and cognitive status, perioperative embolic damage, and postoperative complications. The consequences of POD can be profound, including a high likelihood of requiring discharge to a skilled nursing facility and increased 1‐year mortality and rehospitalization risk. 6 Given the seriousness of stroke and POD, it is critical to identify cardiac surgery patients at high risk for delirium and to develop interventions to prevent or mitigate perioperative cerebral infarction.
Cardiac surgical trials involving neuroradiographic endpoints have commonly used total lesion volume or count as summary variables of perioperative neurological damage. These summary metrics fail to account for lesion location, which, independent of aggregate lesion volume, may have a disproportionate impact on functioning (e.g., thalamocapsular infarcts). Aggregate whole‐brain volume metrics obscure valuable information about affected cerebral vascular territories, which may have particular relevance for neuroprotection strategy development. In addition, perioperative DWI infarcts result in white matter structural connectivity changes, which can be measured as the percentage of lost white matter tract connections to specific brain regions due to spatially overlapping and intervening lesions. A DWI lesion‐related structural disconnection (DWI‐ISD) metric in SAVR patients, thus, reflects perioperative infarct impact on brain structural connectivity, often in regions linked, yet remote to the spatial location of an infarct (i.e., diaschisis).
In this secondary analysis of randomized controlled clinical trial data, we used advanced neuroimaging analysis methods and zero‐inflated linear mixed‐effects (ZILME) 7 modeling to examine for differences in DWI infarct location, regional infarct volume extent and regional DWI‐ISD among older patients who underwent SAVR, with and without embolic protection. These regional neuroradiographic outcome variables were then compared in patients who did and did not experience POD to elucidate brain regions where SAVR‐related lesion damage or disconnection could be related to delirium. In addition, we examined whether suction or filtration embolic protection devices relative to standard aortic cannula control were associated with a reduction in regional lesion prevalence, volume, or DWI‐ISD.
Methods
Study cohort
The CTSN Neuroprotection Clinical Trial (NCT02389894) enrolled 383 patients, age 60 years and older, at 18 North American centers, who were scheduled to undergo SAVR. Participants were excluded if they had endocarditis, clinical stroke within 3 months prior to randomization, or underwent either cardiac catheterization, or cerebral/aortic arch angiography within 3 days of the planned SAVR. 8 This secondary analysis only includes trial participants who completed both neuroimaging and delirium screening and who did not experience clinically evident stroke or possible stroke symptoms [NIH Stroke Scale (NIHSS) ≤ 2]. These exclusions were established to narrow focus to only those patients who experienced clinically covert DWI infarcts (n = 298; see CONSORT diagram, Fig. 1A). The demographics of this sub‐cohort and the parent trial are depicted in Table 1.
Figure 1.
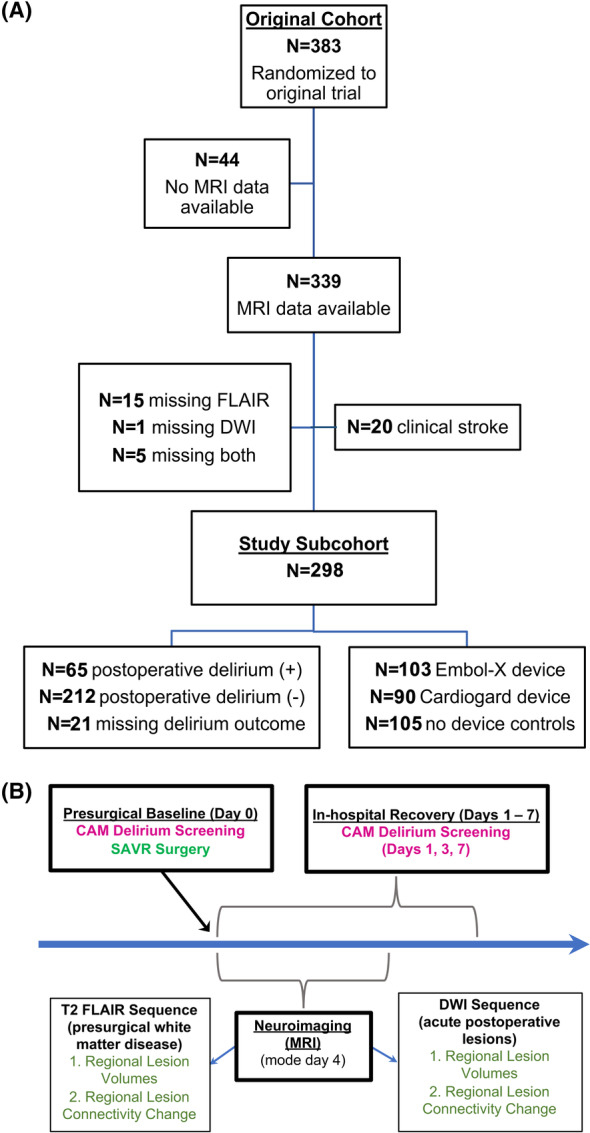
Study CONSORT diagram and schematic of trial operations and outcome timing. (A) CONSORT diagram demonstrating the flow of patients from the original parent trial through to the current image‐based study sub‐cohort. (B) Illustrates the trial design, including timing of interventions and outcome measures.
Table 1.
Parent clinical trial and sub‐study cohort demographic variables and demographic variables by postoperative delirium status.
| Demographic and clinical variables | Postoperative delirium (POD) status | |||||
|---|---|---|---|---|---|---|
| Parent trial cohort | Sub‐study cohort | p‐value | POD | No POD | p‐value | |
| N (%) | 383 (100%) | 298 (77.8%) | 65/277 a (23.5%) | 212/277 a (76.5%) | ||
| Sex = Female (%) | 147 (38.4%) | 117 (39.3%) | p = 0.88 | 23 (21.7%) | 83 (78.3%) | p = 0.69 |
| Age (mean; SD) | 73.89 (6.7) | 74.02 (6.7) | p = 0.69 | 75 (6.7) | 74 (6.8) | p = 0.28 |
| Race (%) | p = 0.99 | p = 0.37 | ||||
| Asian | 5 (1.3%) | 4 (1.3%) | 1 (25.0%) | 3 (75.0%) | ||
| Black | 19 (5.0%) | 14 (4.7%) | 1 (8.3%) | 11 (91.6%) | ||
| Not reported | 5 (1.3%) | 4 (1.3%) | 2 (50.0%) | 2 (50.0%) | ||
| White | 352 (91.9%) | 276 (92.6%) | 61 (23.7%) | 196 (76.3%) | ||
| Surgical device (%) | p = 0.98 | p = 0.43 | ||||
| Embol‐X (filtration device) | 133 (34.7%) | 103 (34.6%) | 26 (26.5%) | 72 (73.5%) | ||
| CardioGard (suction device) | 118 (30.8%) | 90 (30.2%) | 21 (25.0%) | 63 (75.0%) | ||
| Cannula control | 132 (34.5%) | 105 (35.2%) | 18 (18.9%) | 77 (81.1%) | ||
| History of stroke (%) | 21 (5.5%) | 15 (5.0%) | p = 0.93 | 4 (30.8%) | 9 (69.2%) | p = 0.76 |
| History of hypertension (%) | 320 (83.6%) | 245 (82.2%) | p = 0.72 | 59 (26.1%) | 167 (73.9%) | p = 0.04 |
t‐test were used for continuous variables and Fisher test for discrete variables.
Percentages reflect number of study patients in each group, minus patients with incomplete POD outcome data (n = 16) or screened positive for delirium prior to surgery (n = 5).
Surgical procedure and neuroprotection intervention
Participants undergoing SAVR were randomized to neuroprotection intervention with participants receiving either a filtration‐based device, Embol‐X, an aortic cannula that used intra‐aortic filtration to remove particulate emboli >120 μm via a heparin‐coated polyester mesh filter (i.e., an intra‐aortic filtration device), or a suction‐based device, CardioGard, which extracted particulate and air emboli through a suction side port located posterior to the aortic perfusion cannula main port (i.e., suction‐based extraction device). The control group received a standard aortic perfusion cannula. 8 Randomization occurred intraoperatively and stratified patients by whether their planned surgery included concomitant procedures (SAVR alone or SAVR with coronary artery bypass and/or mitral valve repair).
Delirium screening and stroke ascertainment
Postoperative delirium screening was conducted at preoperative baseline and postoperatively on Days 1, 3, and 7 (±3) using the 3‐minute Confusion Assessment Method (3D‐CAM) 9 or, for nonverbal/intubated patients, the Confusion Assessment Method for intensive care unit (CAM‐ICU). 10 Screening was conducted by study coordinators or neurology trainees, who had undergone quality assurance training and certification procedures. POD was assigned if any of the post‐procedure CAM screens were positive. If no post‐procedure CAM screens were positive, the POD outcome was designated as missing if participants did not undergo at least two out of the three postoperative POD screening visits. Serial NIHSS screening was conducted on all participants prior to surgery and postoperatively on Days 1, 3, and 7. Clinical stroke outcome was assigned to patients who experienced stroke as an adverse event and were adjudicated by neurologists blinded to trial assignment.
Neuroimaging procedures
All participants underwent 1.5 or 3.0 Tesla MR‐based neuroimaging prior to hospital discharge (mode, MRI 4 days post‐surgery). Participant T1‐weighted anatomical, fluid‐attenuated recovery (FLAIR), and diffusion weighted imaging (DWI/ADC; b1000) sequences were acquired. Baseline, preoperative white matter disease volumes were derived from FLAIR sequence data, minus any spatially overlapping acute DWI lesion volumes. A well‐validated supervised learning‐based method was used for segmentation of FLAIR white matter hyperintensities. 11 The method used a large and diverse archival external image training set of adult multimodal (T1‐weighted + FLAIR) scans collected at 1.5 and 3.0 Tesla strength, from which semiautomatic delineated reference labels for hyperintensities and other tissue types were generated. Perioperative infarct volume and location were derived from the DWI sequence and apparent diffusion coefficient (ADC) maps, independent of the FLAIR sequence data. A semiautomated segmentation method was used to generate participants' DWI lesion maps. This involved manual detection of participants' DWI infarcts by a neuroradiologist using structural imaging and DWI/ADC lesion maps that were linearly aligned and upon which lesion “seed points” were placed. 12 These seed points were then used as input for an automated region growing algorithm that delineated DWI infarct boundaries from 1.5 and 3 Tesla strength training data to generate regional DWI volumes for subsequent analyses. 13 Raw lesion volumes and their regional extent (i.e., lesion volume/ROI volume) were calculated within each ROI for subsequent comparison by postoperative delirium status and neuroprotection device assignment.
Neuroimaging metrics that measure structural connectivity between brain regions have been proposed as more refined measures for assessing neurocognitive and functional damage following stroke. 14 , 15 To assess the structural impact of preoperative white matter disease and perioperative infarctions on disruption of white matter tracts connecting proximal and distal brain regions, we used the Network Modification (NeMo) Toolbox. 16 NeMo utilizes lesion volume masks to estimate the mean percentage of lesion‐related loss of cortical and subcortical connections in diffusion tensor imaging (DTI)‐derived white matter tract streamlines of normative healthy adults (n = 73; 33 women, 30.2 + 6.7 years). NeMo has been used to demonstrate patterns of structural connectivity change associated with white matter disease and lesions in Alzheimer's disease dementia, traumatic brain injury and long‐term outcomes following ischemic stroke. 17 Change in connectivity (ChaCo) scores were measured for each brain atlas ROI, which reflect the percentage of lost DTI streamline connections to a ROI due to intervening white matter lesions.
Statistical analyses
Patient demographics were compared across the three treatment arms (Supplementary Data) (i.e., filtration‐based neuroprotection device, suction‐based device or standard cannula control), between individuals who did and did not experience POD and between cohorts (i.e., between the full trial cohort and secondary analysis cohort), using t‐tests for continuous variables and chi‐squared tests for categorical variables (Table 1).
Modeling approach
The lesion volumetric data was “zero‐inflated” for many of the brain ROIs (i.e., those that did not demonstrate acute preoperative FLAIR or perioperative DWI infarction). As such, and to account for the correlation between FLAIR and FLAIR+DWI from the same patients, we adopted a zero‐inflated linear mixed‐effects (ZILME) modeling approach for ROI's showing zero‐inflated outcomes. 7 The ZILME approach simultaneously fits a logistic‐regression model for a binary outcome (e.g., lesion presence vs. absence), and a linear mixed‐effects (LME) model for a continuous outcome (e.g., magnitude of infarct‐related cortical connectivity change or increase in volume). This approach provides unbiased estimates and inferences of the SAVR induced changes and differences between patient groups.
Prior to ZILME analysis, neuroimaging data were log10 transformed to normalize nonzero values. Perioperative infarct volumes and infarct‐mediated cortical disconnection (i.e., ChaCo values) were evaluated overall, by neuroprotection strategy and by delirium status. When modeling the association of infarct damage with POD or neuroprotection device, we did not include SAVR patients that experienced acutely debilitating clinical stroke during their in‐hospital recovery (n = 23; mean total DWI infarct volume 1687 mm3), as we were primarily interested in the spatial distribution and associated effects of more common clinically “silent” perioperative DWI infarcts in participants from the original trial population (n = 310; median total DWI volume 41 mm3). Fixed effects for the LME and logistic models within the ZILME included MRI sequence type (FLAIR, FLAIR+DWI), POD status and neuroprotection device group, including interaction terms between MRI‐type and POD and MRI‐type and neuroprotection device, as well as age, sex and hypertension. A random intercept for patient was considered for both continuous and logistic models. ROIs that did not show zero‐inflation were analyzed using LME models with the same covariates. Odds ratios (OR), fold‐change (FCH) and p‐values for comparisons of interest were estimated using contrasts, p‐values were adjusted for multiple comparisons using the Benjamini–Hochberg correction, which controls the false discovery rate (p‐FDR). A significance threshold of p‐FDR < 0.05 was established for all group‐wise comparisons and p‐FDR < 0.01 for within‐group change comparisons, unless otherwise stated. All analyses were performed using R (version 4.2.1).
Results
Patients characteristics
The sub‐cohort of SAVR patients with complete neuroimaging data and no clinical stroke (n = 298) was representative of the original cohort of 383 patients (see CONSORT diagram Fig. 1A; Table 1). Mean age was 74 years (6.7), 39% were female and 7% non‐white. The incidence of POD was 23.5%, excluding 21 patients who were missing complete presurgical or postoperative delirium screening data or screened positive for delirium prior to surgery (i.e., 65/277). Nine patients (13.8%) were screened using the CAM‐ICU, of which three were positive for POD during in‐hospital recovery. Among common risk factors for perioperative stroke, a history of hypertension was significantly higher in those who experienced POD (p = 0.04), while the prevalence of hyperlipidemia and diabetes mellitus type II were not significantly different between groups (p = 0.89 and p = 0.83, respectively).
Presurgical FLAIR hyperintensities
Consistent with advanced age and an increased prevalence of hypertension, all SAVR patients displayed presurgical FLAIR white matter hyperintensities in at least one white matter ROI. Likewise, the prevalence of presurgical FLAIR hyperintensity‐mediated structural connectivity alteration was high in all patients. Although the incidence of presurgical white matter hyperintensities and structural connectivity alteration was high, the extent and magnitude of damage and/or connectivity loss within each ROI was considerably more variable. Average presurgical FLAIR hyperintensity volume (expressed as a percentage of the ROI volume in mL) ranged from 0.0001% to 7% with the greatest hyperintensity burden observed in bilateral caudate nuclei, fornix, occipital and frontal lobe white matter ROIs. This finding is consistent with the periventricular hyperintensity expression pattern commonly observed in patients with chronic ischemic small vessel white matter disease. 18 The magnitude of FLAIR hyperintensity‐mediated structural connectivity loss (expressed as a percentage of total streamline connections to a ROI) ranged from 0.04% to 8%. Bilateral occipital/calcarine cortices, bilateral caudate, right anterior cingulum cortex, bilateral insular cortices, bilateral putamen, and right thalamus ROIs all showed >1% presurgical connectivity loss relative to normative NeMo tractography atlas data. 16
There were no statistically significant presurgical differences in regional FLAIR hyperintensity volumes, regional prevalence of FLAIR hyperintensities or in FLAIR hyperintensity‐mediated structural disconnection between SAVR patients with and without POD or between standard cannula control and neuroprotection device groups.
Perioperative DWI infarctions
Following SAVR, there were 18 ROIs with significantly increased acute DWI infarct incidence (p‐FDR < 0.01). Bilateral cerebellum ROIs showed the greatest change in lesion number, prior to surgery 7% of patients had FLAIR hyperintensities impacting the left cerebellum, but DWI lesion incidence in this region increased significantly to 20% of patients following surgery (OR = 11.0; 95% CI: 9.8–12.4) after adjusting for age, sex and hypertension. Similarly, 18% of patients had FLAIR hyperintensities in the right cerebellum, significantly increasing to 29% with DWI lesion in this region following surgery (adjusted OR = 7.6; 95% CI: 6.6–8.8). The bilateral precentral gyri, right middle frontal gyrus and right superior parietal lobe ROIs all showed a significant OR > 2, indicating that the number of SAVR patients with lesions in these ROIs more than doubled after surgery. Six ROIs demonstrated a significant increase in lesion volume, most notably, the left and right cerebellum which both showed >33% increase (FCH > 1.3) in lesion volume after surgery.
Infarct‐mediated structural disconnection (DWI‐ISD) analyses revealed broad, incremental connectivity loss following SAVR, including 86 ROIs showing significant (p‐FDR < 0.01) increases in structural disconnectivity from perioperative DWI infarcts (Fig. 2). Aggregated into broad brain territories, of the 86 ROIs showing significant increases, most were grouped in strategic subcortical regions (e.g., thalamus) and the limbic, frontal and parietal cortices. Overrepresentation analysis (ORA) showed that these 86 ROI were significantly enriched (p‐FDR < 0.1, hypergeometric test) with left hemispheric regions, parietal lobe regions, and subcortical strategic regions.
Figure 2.
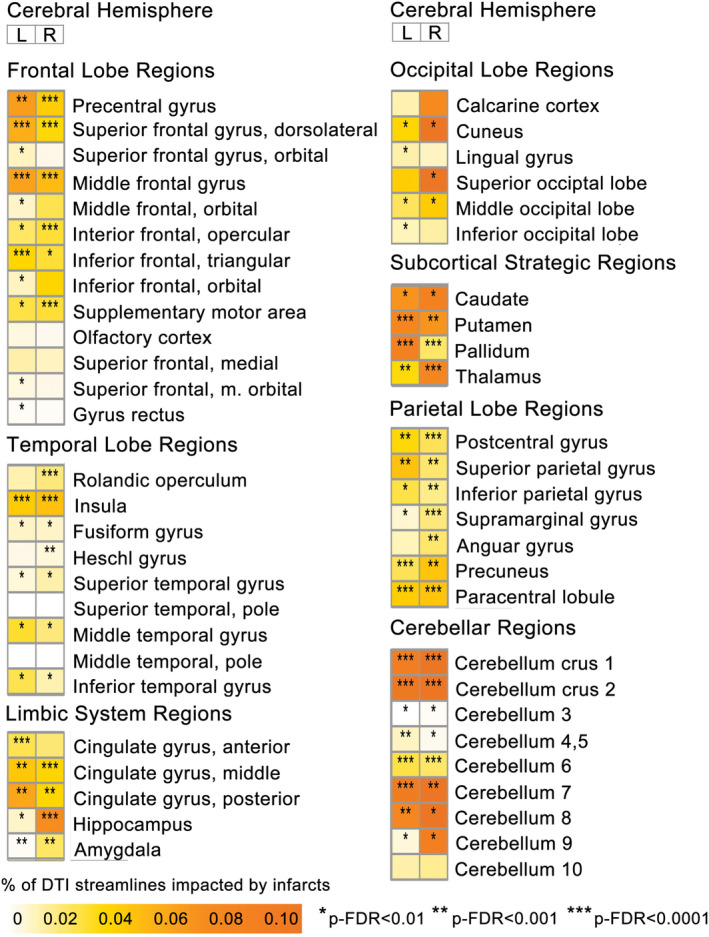
Regional perioperative acute ischemic DWI infarct‐mediated cortical disconnectivity in all groups. Heatmap of cortical disconnectivity associated with acute ischemic diffusion‐weighted imaging (DWI) lesions for each ROI, separated by cerebral hemisphere. Measured using change in connectivity (ChaCo) values, which reflect the % of afferent DTI streamlines impacted within each region‐of‐interest (ROI). Asterisks indicate significant change in connectivity from presurgical baseline; *p‐FDR < 0.01, **p‐FDR < 0.001, ***p‐FDR < 0.0001.
Perioperative DWI infarctions and postoperative delirium status
The SAVR patients who experienced POD tended to exhibit larger fold increases in perioperative cerebrovascular neurological damage with two ROIs (right cerebellum and right temporal lobe white matter) reaching statistical significance (p‐FDR < 0.05; see Fig. 3A.1). Lesion volume in the right cerebellum increased 2.1‐fold in patients who experienced POD relative to 1.3‐fold in non‐POD patients, while lesion volume in the right temporal lobe white matter increased 1.3‐fold in POD cases but did not increase in non‐POD patients. Statistically significant (p‐FDR < 0.01) within‐group lesion volume extent increases were observed in the right cerebellum, right frontal and temporal lobe white matter and left parietal lobe white matter in patients who experienced POD. A single ROI in the left frontal lobe white matter showed a significant within‐group lesion volume extent increase in non‐POD patients (Fig. 4).
Figure 3.
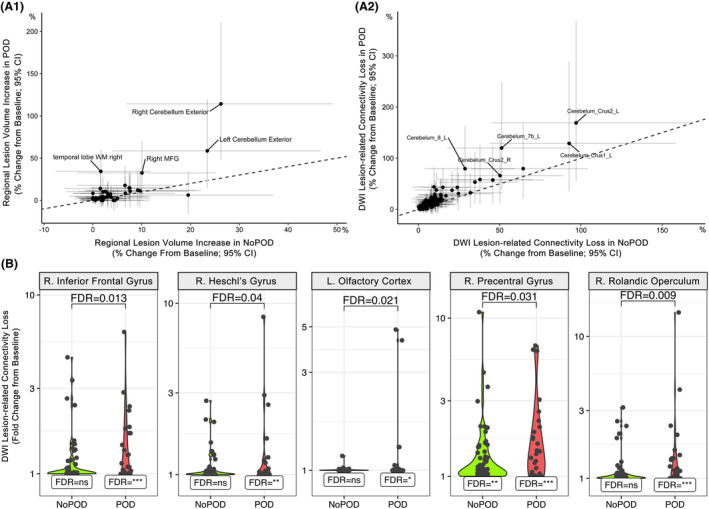
Comparing SAVR‐induced infarct volume and connectivity loss between patients with and without POD. Scatterplots show average percent change (95% CI) from presurgical baseline in lesion volume (A.1) or DWI lesion‐related connectivity loss (A.2) for each ROI in patients with POD (y‐axis) versus without POD (x‐axis). Labels indicate ROIs with POD‐change minus NoPOD‐change >5% (A.1; right cerebellum and temporal lobe ROIs significantly different between POD groups, p‐FDR < 0.05). The dashed line represents the origin line. Violin plots (B) show DWI lesion‐related connectivity loss change from presurgical baseline within delirium groups for ROIs with significant group‐wise differences (p‐FDR < 0.05). Within group change significance denoted as p‐FDR: *<0.01, **<0.001, ***<0.0001.
Figure 4.
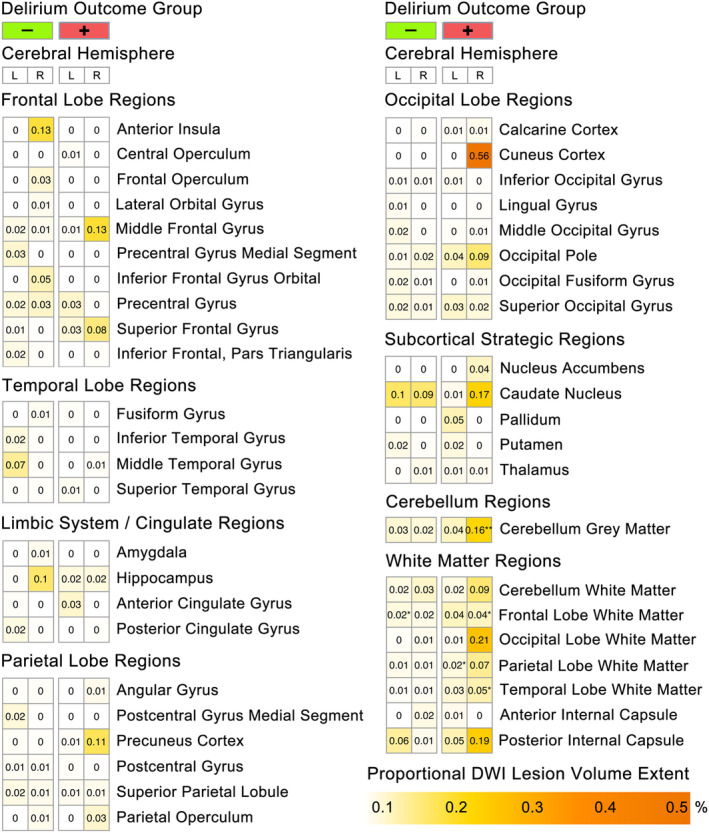
Regional perioperative diffusion‐weighted imaging (DWI) lesion extent by cerebral hemisphere and delirium outcome group. Heatmap of regional perioperative diffusion‐weighted imaging (DWI) lesion volumes corrected for individual ROI volumes (i.e., DWI lesion volume/ROI volume = regional lesion extent), separated by cerebral hemisphere and delirium outcome group. ROIs with lesion extent >0.01% are shown. Asterisks indicate significant change in regional lesion extent from presurgical baseline; *p‐FDR < 0.01, **p‐FDR < 0.001, ***p‐FDR < 0.0001.
Although only two ROI showed higher DWI lesion volume in patients who experienced POD, wider differences in lesion incidence were observed. A comparison of group‐wise differences in DWI lesion incidence showed nine ROIs with significantly (p‐FDR < 0.05) greater increase in lesion incidence in POD relative to non‐POD (i.e., right superior frontal gyrus, left superior frontal gyrus, right middle frontal gyrus, right precuneus, right calcarine cortex, left calcarine cortex, posterior limb of the internal capsule, left hippocampus, and right accumbens area). For example, the right superior frontal gyrus showed a significant increase in lesion incidence in POD patents (OR = 2.2; 95% CI: 1.8–2.6), but in non‐POD patients the increase in lesion incidence was notably smaller (OR = 1.1; 95% CI: 1.8–2.6). Conversely, only two ROIs (i.e., right precentral gyrus and brain stem) showed significantly greater incidence increase in non‐POD relative to POD patients (p‐FDR < 0.05). Thus, although SAVR‐induced increases in lesion incidence are widespread in patients who did and did not experience POD, patients with POD showed greater increase in lesion incidence in frontal lobe and occipital cortices, as well as in limbic cortex of the left mesial temporal lobe.
Patients with and without delirium exhibited statistically significant (p‐FDR < 0.01) within‐group fold‐increases in DWI‐ISD (e.g., POD 23 ROIs; NoPOD 25 ROIs; Fig. 5), but there was a general trend in a greater magnitude of DWI‐ISD in the cerebellar regions of patients who experienced POD (Fig. 3A.2). In a direct comparison of DWI‐ISD loss between delirium groups (p‐FDR < 0.05), patients who experienced POD demonstrated significantly greater connectivity loss in right temporal lobe (i.e., rolandic operculum, Heschl's gyrus) and frontal lobe (i.e., right inferior frontal gyrus, left olfactory cortex, right precentral gyrus) regions (see Figs. 3B and 5).
Figure 5.
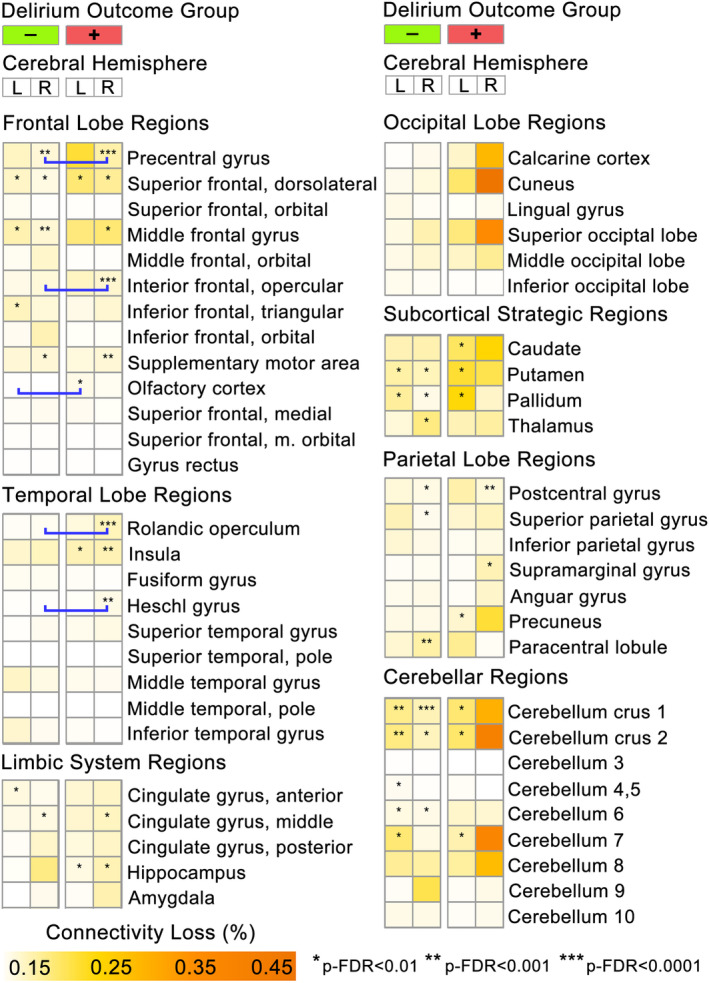
Significant SAVR‐associated DWI infarct changes in structural connectivity are more frequently observed in patients who experienced postoperative delirium. Heatmap shows the mean perioperative DWI lesion‐mediated connectivity loss (DWI‐ISD) for each ROI, separated by hemisphere and delirium outcome. Stars indicate ROIs with significant within‐group fold‐change in SAVR‐induced connectivity loss from presurgical baseline (p‐FDR corrected). Blue bracket bars indicate ROIs with significant differences (p‐FDR < 0.05) in DWI‐ISD between POD and no POD groups (see Fig. 3).
Perioperative DWI infarctions and neuroprotection device uses
As reported previously in the parent clinical trial, neither suction‐ nor filter‐based neuroprotection devices had a statistically significant effect on aggregate perioperative DWI lesion volume following SAVR. 8 Similarly, in this secondary analysis, there were no statistically significant (p‐FDR < 0.05) regionally specific changes in DWI infarct volumes between standard aortic cannula control and neuroprotection device groups.
However, the suction‐based neuroprotection device did appear to be protective in terms of regional lesion incidence in five ROIs relative to controls (p‐FDR < 0.05; i.e., right cerebellum exterior, left cerebellum exterior, right precentral gyrus, right hippocampus, and right superior parietal lobule). Conversely, three ROIs showed higher lesion incidence in the suction‐based neuroprotection group relative to control (i.e., right middle frontal gyrus, right precuneus, and left calcarine cortex). The filtration‐based neuroprotection group showed four ROIs with lower surgery‐induced lesion incidence relative to control (i.e., left cerebellum white matter, right cerebellum white matter, right hippocampus, and right cerebellum exterior). Seven ROIs demonstrate worse lesion incidence in the filtration‐based neuroprotection group relative to control (p‐FDR < 0.05), predominantly in posterior middle cerebral artery territory watershed regions (i.e., right superior parietal lobule, left superior parietal lobule, right precuneus), as well as in the left superior frontal gyrus, brain stem, right calcarine cortex, and right posterior limb of the internal capsule.
There were no statistically significant differences between neuroprotection groups in DWI‐ISD. However, an examination of change in DWI‐ISD within treatment groups found 10 ROIs with significant perioperative decline in the standard aortic cannula control group (FCH > 1.1, p‐FDR < 0.01), 14 ROIs with significant loss in the filter‐based neuroprotection group (FCH > 1.1, p‐FDR < 0.01), but only 1 ROI (right cerebellum crus 1; FCH = 2.47, p‐FDR = 0.003) with significant perioperative reduction in the suction‐based neuroprotection group (Fig. 6).
Figure 6.
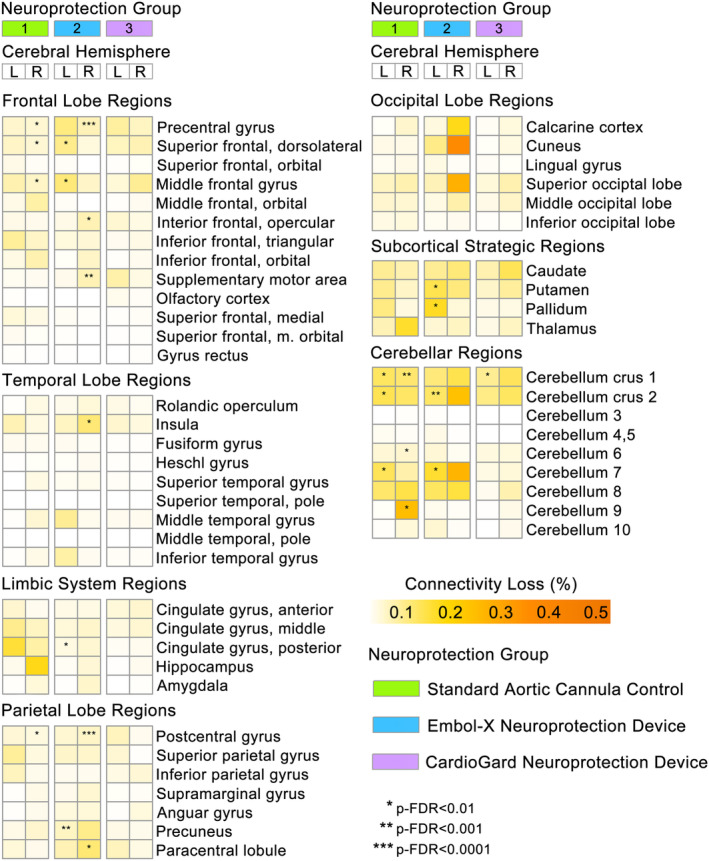
Reduced DWI infarct‐mediated structural connectivity loss with embolic capture neuroprotection device use during SAVR. Brain region heatmap shows the mean perioperative DWI lesion‐mediated connectivity loss for each ROI, separated by hemisphere and surgical device group. Stars indicate ROIs with significant within‐group fold‐change in connectivity loss from presurgical baseline (p‐FDR < 0.01).
Discussion
In this study, we describe the spatial distribution, regional lesion incidence, and structural disconnection effects of presurgical FLAIR and postoperative DWI abnormalities in a cohort of older adults who underwent SAVR stratified by delirium status and perioperative embolic neuroprotection type.
We found DWI lesion incidence and volume increased significantly in select cortical regions subserved by posterior and middle cerebral artery territories following SAVR. There were significantly greater perioperative infarct volume increases in the right cerebellum and temporal lobe white matter of patients who experienced POD after SAVR (Fig. 3A.1). DWI lesion‐mediated structural disconnection (DWI‐ISD) effects were also more prevalent in patients who experienced POD. In these patients, greater loss of structural connectivity due to DWI infarcts was observed in frontal and temporal lobe regions (i.e., right precentral gyrus, inferior frontal lobe, Rolandic operculum, Heschl gyrus, and left olfactory cortex; see Fig. 3B). Finally, we observed that use of a suction‐based neuroprotection device, which captures both particulate and gaseous materials, may be associated with fewer brain regions impacted by DWI‐ISD (i.e., 1 ROI) compared to use of filtration‐based neuroprotection or standard aortic cannulation (i.e., 14 and 10 ROIs, respectively; Fig. 6).
Preoperative white matter hyperintensity burden may pose a potential POD risk in both cardiac and non‐cardiac surgical populations, 19 , 20 , 21 but the literature is inconclusive on this point. 20 , 22 , 23 Preoperative cerebral infarcts have been found to be significant predictor of POD following isolated CABG (n = 153; 10.5% POD incidence; odd ratio 2.26, 95% CI 1.10–4.78), along with preoperative cognitive decline and the presence of ascending aortic atherosclerosis. 24 However, this finding may reflect more acute cerebrovascular damage than incrementally accumulating chronic ischemic white matter hyperintensities. We found that presurgical white matter hyperintensity volumes and associated structural disconnectivity, while present and considerable, did not significantly differ between patients who did or did not experience POD following SAVR. Presurgical WMH lesion prevalence revealed some variation between neuroprotection groups and aortic cannula controls in a small number of regions, but there were no statistically significant differences between these groups for total WMH volume or associated structural disconnection.
To date, an association between acute brain DWI infarct location and delirium has been posited by case studies suggesting the importance of strategic lesion location in the basal ganglia and cerebellum. 25 The observed spatial distribution of DWI infarcts in this study were consistent with neuroimaging results from CABG, 26 , 27 TAVR 2 , 28 and the recent ACE Cardiolink‐3 aortic arch repair trials. 29 DWI infarcts were found to be more highly concentrated in posterior cerebral and middle cerebral circulation territories and watershed border zones. Perioperative infarction damage is often expressed in the cerebellum, which is consistent with our study results. The enhanced risk for perioperative cerebellar infarctions could be related to the increased vascular density of the cerebellum, combined with the possible presence of vertebral stenosis, on the infarct distribution in patients undergoing aortic cannulation. 30 Additionally, right hemispheric frontal lobe motor, inferior frontal and temporal lobe sensory regions appear to be preferentially impacted in SAVR patients who developed POD. It is known that pre‐existing motor and sensory impairments are risk factors for POD. Reduced motor and supplemental motor area functional connectivity is associated with increased risk for frailty, 31 , 32 which, in turn, is a salient risk factor for delirium after cardiac 33 and noncardiac surgery. It is possible that the observed reduced primary motor region disconnectivity in POD patients reflects diaschisis from their greater incidence of DWI infarction in the cerebellum (Figs. 3D and 4).
Distinct from standard radiographic FLAIR and DWI lesions, white matter fractional anisotropy (FA) reduction in the cerebellum has been detected in the postoperative MRIs of those who have experienced ICU‐related delirium relative to matched healthy controls, 34 as well as in the presurgical MRI data of patients who subsequently developed delirium after noncardiac surgery. 35 Thus, independent of surgery type, vulnerability of the cerebellum to perioperative infarction and the associated risk for subsequent delirium appears to be high. The pathophysiological mechanisms of this association are still relatively obscured, but the role of the cerebellum in brain network oscillatory activity via the cortico‐thalamo‐cerebellar pathway is one hypothesis, as dysfunction in this circuit can have a negative impact on interhemispheric communication and has been suggested as the basis for “dysmetria of thought.” 36 , 37 , 38 Non‐motor deficits arising from lesions damaging the cortico‐cerebellar connection, such as the cognitive abnormalities seen in patients with cerebellar cognitive affective syndrome (aka. Schmahmann's syndrome) 39 are routinely observed during delirium states (e.g., executive dysfunction, affective dysregulation, attention, and working memory impairment). 40 , 41
Our finding of increased perioperative DWI‐ISD loss to the right inferior frontal gyrus of patients who developed POD is also intriguing, as this region has been identified as a central “hub” of the brain's salience functional network. 42 The salience network assigns importance to incoming external stimuli, acting as an intermediary network mediating switching from internal, self‐referential processing (i.e., default mode network), and task‐related functional network processing demands. Pertinent to our findings, salience network functional connectivity has been found to be negatively impacted by chronic cerebrovascular white matter disease, 43 and inter‐network discoordination between salience and other task‐related functional brain networks and the default mode network has been proposed as a pathophysiological expression of delirium state. 44 , 45
As with the parent clinical trial, 8 there were no statistically significant differences in aggregate, whole brain DWI infarction volumes, and we did not detect any significant regional distribution differences in infarcts between neuroprotection device groups and controls. Neuroprotection use, however, appeared to reduce the overall level of DWI‐ISD loss relative to controls (see Fig. 6) with the suction‐based device showing minimal significant perioperative increases in structural disconnectivity. In the original CTSN Neuroprotection Trial, it was observed that those patients who received the suction‐based device demonstrated less delirium late in the in‐hospital recovery period compared to controls. 8 Together, these results suggest that suction‐based devices may better protect against perioperative embolic damage during SAVR, but in a regionally specific manner that may have some association with the type of particulate matter being collected or the extent of protective coverage of the ascending aortic vasculature.
It should be noted that our study results are associative and not causal, but they do provide potential insights into greater lesion‐mediated structural disconnection changes in POD patients involving regions largely subserved by posterior and middle cerebral arteries. It is important to note that these findings do not necessarily indicate a protective role of surgical device against POD pathophysiology, since additional factors such as inflammation and reperfusion injury likely contribute to POD and connectivity loss, independently of surgical device. Selection bias in studies with mixed participation in neuroimaging has been raised with respect to possible differences between those who do develop delirium compared to those who do not and the implications of the bias on possible POD‐related neuroimaging associations. 46 While selection bias cannot be completely ruled out as a possible factor in our results, there were no significant demographic differences between our study sub‐cohort (i.e., those with complete neuroimaging data and without clinical stroke) and the parent clinical trial cohort (Table 1). Our use of a stringent NIHSS exclusionary threshold (NIHSS ≥2) to ensure that no clinical stroke patients were included in the study analyses also introduced selection bias for patients with possible milder stroke symptoms, but this was done to restrict analyses to better understand the possible clinical consequences of more common “covert” DWI infarcts and their possible association with delirium. It is also important to note the narrow racial diversity in the clinical trial cohort. Participants were 93% white, thus limiting the wider generalizability of our findings to diverse surgical groups. Lastly, it is important to note that postoperative delirium was not screened daily in the parent clinical trial. Screening with CAM‐based instruments occurred on in‐hospital recovery Days 1, 3, and 7, which allows for the possibility that some in the non‐POD group may have experienced that condition and went undetected by study personnel. This, in turn, may have diluted our ability to detect additional significant differences between POD and non‐POD groups in some of our analyses.
Region specific connectivity metrics, coupled with zero‐inflated modeling have revealed key insights into the relationship between POD and SAVR‐induced covert infarcts.
Findings of cerebral embolic load and acute postoperative neuropsychological task decline associations have been mixed, 47 , 48 but intriguingly, the resolution of DWI infarcts upon subsequent neuroimaging tends to track positively with the resolution of any acute postoperative cognitive decline. 49 The association of DWI infarcts and acute neurocognition may be stronger in procedures where there is greater risk of gaseous emboli (e.g., SAVR), 50 and it is notable that of the neuroprotection device groups in this study, the loss of DWI‐ISD appeared to be the least in those randomized to a device that captured both particulate and gaseous emboli. Furthermore, our results suggest that postoperative delirium may be associated with widely distributed and sudden loss in structural connectivity in multiple regions, but notably prevalent in the cerebellum and salience network‐related cortical regions.
Future cardiac surgery clinical trials involving neuroimaging data should consider lesion‐related structural disconnection metrics, like DWI‐ISD, as potential primary or secondary trial outcomes. Our study suggests that regional structural disconnectivity from acute perioperative infarction may play a role in the expression of postoperative delirium, which, in turn, present avenues for additional targeted neuroprotection of POD‐vulnerable brain regions.
Author Contributions
J.N.B: conception and design of study, results interpretation, drafting of manuscript. L.E.T.: data analysis, manuscript preparation, results interpretation. G.E.: data acquisition and analysis. J.R.O.: data analysis, data management. A.K.: data analysis and statistical consultation. A.J.M., A.I., and J.M.: drafting of manuscript. E.B.: acquisition and analysis of data, statistical consultation. M.A., M.M., P.O., and A.C.G.: conception and design of study, drafting of manuscript. M.S‐F.: conception of data analysis, manuscript preparation, results interpretation. S.R.M.: conception and design of study, manuscript preparation, results interpretation.
Conflict of Interest
None of the authors have commercial relationships with the companies whose products were used in the study or may be affected by its outcomes.
Trial Registration
Acknowledgments
The authors would like to acknowledge the dedication and efforts of the many CTSN research coordinators without whom this research project would not have been possible and our patients who so generously participated in this study. This research was supported, in part, by funding provided by NHLBI U01‐HL088942, NIA R01‐AG074185, and NHLBI R01‐HL130443.
Cardiothoracic Surgical Trials Network (CTSN)
The members of the Cardiothoracic Surgical Trials Network (CTSN) who were involved in the parent clinical trial (NCT02389894) from which this manuscript secondary analyses were conducted are as follows:
National Heart, Lung and Blood Institute: Kathleen Fenton, Marissa A. Miller (ret), Wendy C. Taddei‐Peters, Neal O. Jeffries, Dennis Buxton, Nancy L. Geller, David Gordon, Catherine Burke, Albert Lee, Tyrone Smith;
National Institute of Neurological Disorders and Stroke: Claudia S. Moy (ret);
Canadian Institutes of Health Research: Ilana Kogan Gombos;
Network Chairs: Cleveland Clinic, Marc Gillinov (Chair); American Heart Association, Mariell Jessup (Vice Chair); Toronto General Hospital, Richard Weisel, (Chair‐Emeritus); Christiana Care Health System, Timothy J. Gardner, (Chair‐Emeritus); Mount Sinai Health System, Eric A. Rose, (Vice Chair‐Emeritus);
Data Coordinating Center: International Center for Health Outcomes and Innovation Research at Icahn School of Medicine at Mount Sinai, Annetine C. Gelijns, Ellen Moquete, Helena L. Chang, Melissa Chase, Seth D. Goldfarb, Lopa Gupta, Edlira Dobrev, Ron Levitan, Milerva Santos, Xia Ye Michael K. Parides, Deborah D. Ascheim, Kinjal Shah, Stephanie Pan, Katherine Kirkwood, Karen O'Sullivan;
Clinical Site Investigators: Baylor Research Institute: Michael Mack (PI), Rachelle Winkle; Cleveland Clinic Foundation: Marc Gillinov (PI), Carrie Geither; Columbia University: Michael Argenziano (PI), Sowmya Sreekanth; Dartmouth‐Hitchcock Medical Center: Jock N. McCullough (PI), Henry Stokes; Duke University: Peter K. Smith (PI), Stacey Welsh; Emory University: Vinod H. Thourani (PI), Kim Baio; Hôpital Laval: Pierre Voisine (PI), Gladys Dussault; Mission Hospital: Mark A. Groh (PI), Ralph Mangusan; Montefiore‐Einstein Heart Center: Robert E. Michler (PI), Rebecca Meli; Montreal Heart Institute: Louis P. Perrault (PI), Sophie Robichaud; NIH Heart Center at Suburban Hospital: Keith A. Horvath (PI), Margaret Iraola; Ohio State University Medical Center: Bryan A. Whitson (PI), Denise Fadorsen; Toronto General Hospital: Maral Ouzounian (PI), Shakira Christie; University of Alberta Hospital: John C. Mullen (PI), Alexandra Hripko; University of Maryland: James S. Gammie (PI), Manal Al‐Suqi; University of Pennsylvania: Michael A. Acker (PI), Steven Messé, Mary Lou Mayer; University of Southern California: Michael Bowdish (PI), Edward Lozano; University of Virginia: Gorav Ailawadi (PI), Sandra Burks.
Protocol Review Committee: David A. Bull (Chair); Patrice Desvigne‐Nickens, Executive Secretary; Dennis O. Dixon, Rebecca Gottesman, Mark Haigney, Richard Holubkov, Constantino Iadecola, Alice Jacobs, Eric M. Meslin, John M. Murkin, John A. Spertus;
Data and Safety Monitoring Board: Frank Sellke (Chair); Cheryl L. McDonald, Executive Secretary; John Canty, Neal Dickert, Dennis O. Dixon, John S. Ikonomidis, KyungMann Kim, David O. Williams, Clyde W. Yancy, Seemant Chaturvedi, Marc Chimowitz;
Medical Monitors: James C. Fang, Wayne Richenbacher;
Overall Event Adjudication Committee: Vivek Rao (Chair); Karen L. Furie, Rachel Miller, Jennifer Cook, David D'Alessandro, Frederick Han, Sean Pinney, Mary N. Walsh;
EAC sub‐committee: David Greer (Chair); Koto Ishida, Christian Stapf.
Echo Core Lab, Massachusetts General Hospital: Judy Hung, Xin Zeng, David Hung, Sudarat Satitthummanid;
MRI Core Lab, University of Pennsylvania: Michel Billelo, Christos Davatzikos, Guray Erus, Lauren Karpf, Lisa Desiderio;
Neurocognitive Core Lab, Duke University Medical Center: Joseph P. Mathew, Jeffrey N. Browndyke, Michael L. James, Yanne Toulgoat‐Dubois, Rachele Brassard;
Histopathology Core Lab, CVPath Institute, Inc., Gaithersburg, MD: Renu Virmanu, Maria E. Romero, Ryan Braumann.
Funding Statement
This work was funded by National Heart, Lung, and Blood Institute grants R01‐HL130443 and U01‐HL088942; National Institute on Aging grant R01‐AG074185.
Contributor Information
Jeffrey N. Browndyke, Email: j.browndyke@duke.edu.
for the Cardiothoracic Surgical Trials Network (CTSN) Investigators:
Kathleen Fenton, Marissa A. Miller, Wendy C. Taddei‐Peters, Neal O. Jeffries, Dennis Buxton, Nancy L. Geller, David Gordon, Catherine Burke, Albert Lee, Tyrone Smith, Claudia S. Moy, Ilana Kogan Gombos, Marc Gillinov, Mariell Jessup, Richard Weisel, Timothy J. Gardner, Eric A. Rose, Annetine C. Gelijns, Ellen Moquete, Helena L. Chang, Melissa Chase, Seth D. Goldfarb, Lopa Gupta, Edlira Dobrev, Ron Levitan, Milerva Santos, Xia Ye Michael K. Parides, Deborah D. Ascheim, Kinjal Shah, Stephanie Pan, Katherine Kirkwood, Karen O'Sullivan, Michael Mack, Rachelle Winkle, Marc Gillinov, Carrie Geither, Michael Argenziano, Sowmya Sreekanth, Jock N. McCullough, Henry Stokes, Peter K. Smith, Stacey Welsh, Vinod H. Thourani, Kim Baio, Pierre Voisine, Gladys Dussault, Mark A. Groh, Ralph Mangusan, Robert E. Michler, Rebecca Meli, Louis P. Perrault, Sophie Robichaud, Keith A. Horvath, Margaret Iraola, Bryan A. Whitson, Denise Fadorsen, Maral Ouzounian, Shakira Christie, John C. Mullen, Alexandra Hripko, James S. Gammie, Manal Al‐Suqi, Michael A. Acker, Steven Messé, Mary Lou Mayer, Michael Bowdish, Edward Lozano, Gorav Ailawadi, Sandra Burks, David A. Bull, Patrice Desvigne‐Nickens, Dennis O. Dixon, Rebecca Gottesman, Mark Haigney, Richard Holubkov, Constantino Iadecola, Alice Jacobs, Eric M. Meslin, John M. Murkin, John A. Spertus, Frank Sellke, Cheryl L. McDonald, John Canty, Neal Dickert, Dennis O. Dixon, John S. Ikonomidis, KyungMann Kim, David O. Williams, Clyde W. Yancy, Seemant Chaturvedi, Marc Chimowitz, James C. Fang, Wayne Richenbacher, Vivek Rao, Karen L. Furie, Rachel Miller, Jennifer Cook, David D'Alessandro, Frederick Han, Sean Pinney, Mary N. Walsh, David Greer, Koto Ishida, Christian Stapf, Judy Hung, Xin Zeng, David Hung, Sudarat Satitthummanid, Michel Billelo, Christos Davatzikos, Guray Erus, Lauren Karpf, Lisa Desiderio, Joseph P. Mathew, Jeffrey N. Browndyke, Michael L. James, Yanne Toulgoat‐Dubois, Rachele Brassard, Renu Virmanu, Maria E. Romero, and Ryan Braumann
References
- 1. Messe SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation. 2014;129(22):2253‐2261. doi: 10.1161/CIRCULATIONAHA.113.005084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samim M, Hendrikse J, van der Worp HB, et al. Silent ischemic brain lesions after transcatheter aortic valve replacement: lesion distribution and predictors. Clin Res Cardiol. 2015;104(5):430‐438. doi: 10.1007/s00392-014-0798-8 [DOI] [PubMed] [Google Scholar]
- 3. Stolz E, Gerriets T, Kluge A, Klovekorn WP, Kaps M, Bachmann G. Diffusion‐weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: implications for future neuroprotective trials? Stroke. 2004;35(4):888‐892. doi: 10.1161/01.STR.0000120306.82787.5A [DOI] [PubMed] [Google Scholar]
- 4. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery‐2018. J Alzheimers Dis. 2018;66(1):1‐10. doi: 10.3233/JAD-189004 [DOI] [PubMed] [Google Scholar]
- 5. Shadvar K, Baastani F, Mahmoodpoor A, Bilehjani E. Evaluation of the prevalence and risk factors of delirium in cardiac surgery ICU. J Cardiovasc Thorac Res. 2013;5(4):157‐161. doi: 10.5681/jcvtr.2013.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443‐451. doi: 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Guo B, Yi N. Zero‐inflated gaussian mixed models for analyzing longitudinal microbiome data. PLoS ONE. 2020;15(11):e0242073. doi: 10.1371/journal.pone.0242073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mack MJ, Acker MA, Gelijns AC, et al. Effect of cerebral embolic protection devices on CNS infarction in surgical aortic valve replacement: a randomized clinical trial. JAMA. 2017;318(6):536‐547. doi: 10.1001/jama.2017.9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcantonio ER, Ngo LH, O'Connor M, et al. 3D‐CAM: derivation and validation of a 3‐minute diagnostic interview for CAM‐defined delirium: a cross‐sectional diagnostic test study. Ann Intern Med. 2014;161(8):554‐561. doi: 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703‐2710. doi: 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 11. Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11(Pt 1):620‐627. doi: 10.1007/978-3-540-85988-8_74 [DOI] [PubMed] [Google Scholar]
- 12. Doshi J, Erus G, Ou Y, et al. MUSE: MUlti‐atlas region segmentation utilizing ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186‐195. doi: 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams RL, Bischof L. Seeded region growing. IEEE Trans Pattern Anal Mach Intell. 1994;16(6):641‐647. doi: 10.1109/34.295913 [DOI] [Google Scholar]
- 14. Johansen‐Berg H, Scholz J, Stagg CJ. Relevance of structural brain connectivity to learning and recovery from stroke. Front Syst Neurosci. 2010;4:146. doi: 10.3389/fnsys.2010.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sotelo MR, Kalinosky BT, Goodfriend K, Hyngstrom AS, Schmit BD. Indirect structural connectivity identifies changes in brain networks after stroke. Brain Connect. 2020;10(8):399‐410. doi: 10.1089/brain.2019.0725 [DOI] [PubMed] [Google Scholar]
- 16. Kuceyeski A, Maruta J, Relkin N, Raj A. The network modification (NeMo) tool: elucidating the effect of white matter integrity changes on cortical and subcortical structural connectivity. Brain Connect. 2013;3(5):451‐463. doi: 10.1089/brain.2013.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuceyeski A, Navi BB, Kamel H, et al. Exploring the brain's structural connectome: a quantitative stroke lesion‐dysfunction mapping study. Hum Brain Mapp. 2015;36(6):2147‐2160. doi: 10.1002/hbm.22761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age‐related white matter changes of the brain. Dement Geriatr Cogn Disord. 1998;9(suppl. 1):2‐5. doi: 10.1159/000051182 [DOI] [PubMed] [Google Scholar]
- 19. Hatano Y, Narumoto J, Shibata K, et al. White‐matter hyperintensities predict delirium after cardiac surgery. Am J Geriatr Psychiatry. 2013;21(10):938‐945. doi: 10.1016/j.jagp.2013.01.061 [DOI] [PubMed] [Google Scholar]
- 20. Kant IMJ, de Bresser J, van Montfort SJT, et al. Preoperative brain MRI features and occurrence of postoperative delirium. J Psychosom Res. 2021;140:110301. doi: 10.1016/j.jpsychores.2020.110301 [DOI] [PubMed] [Google Scholar]
- 21. Shibagaki K, Shirasaka T, Sawada J, et al. Silent cerebral ischemia detected by magnetic resonance imaging can predict postoperative delirium after total arch replacement for aneurysm. JTCVS Open. 2022;10:87‐96. doi: 10.1016/j.xjon.2022.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown CH, Faigle R, Klinker L, et al. The association of brain MRI characteristics and postoperative delirium in cardiac surgery patients. Clin Ther. 2015;37(12):2686‐2699.e9. doi: 10.1016/j.clinthera.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cavallari M, Hshieh TT, Guttmann CR, et al. Brain atrophy and white‐matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging. 2015;36(6):2122‐2129. doi: 10.1016/j.neurobiolaging.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otomo S, Maekawa K, Goto T, Baba T, Yoshitake A. Pre‐existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;17(5):799‐804. doi: 10.1093/icvts/ivt304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Irimia P, Martinez‐Vila E, Martinez‐Cuesta A, Zulueta J. Delirium due to brain microembolism: diagnostic value of diffusion‐weighted MRI. J Neuroimaging. 2007;17(2):175‐177. doi: 10.1111/j.1552-6569.2006.00067.x [DOI] [PubMed] [Google Scholar]
- 26. Bendszus M, Stoll G. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol. 2006;5(4):364‐372. doi: 10.1016/S1474-4422(06)70412-4 [DOI] [PubMed] [Google Scholar]
- 27. Sun X, Lindsay J, Monsein LH, Hill PC, Corso PJ. Silent brain injury after cardiac surgery: a review: cognitive dysfunction and magnetic resonance imaging diffusion‐weighted imaging findings. J Am Coll Cardiol. 2012;60(9):791‐797. doi: 10.1016/j.jacc.2012.02.079 [DOI] [PubMed] [Google Scholar]
- 28. Meller SM, Baumbach A, Brickman AM, Lansky AJ. Clinical implications for diffusion‐weighted MRI brain lesions associated with transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2014;83(3):502‐508. doi: 10.1002/ccd.24904 [DOI] [PubMed] [Google Scholar]
- 29. Chen CH, Peterson MD, Mazer CD, et al. Acute infarcts on brain MRI following aortic arch repair with circulatory arrest: insights from the ACE CardioLink‐3 randomized trial. Stroke. 2022;54:67‐77. doi: 10.1161/STROKEAHA.122.041612 [DOI] [PubMed] [Google Scholar]
- 30. Ito H, Uchida M, Kaji T, et al. Risk factors of cerebellar microembolic infarctions after carotid artery stenting. World Neurosurg. 2020;142:e290‐e296. doi: 10.1016/j.wneu.2020.06.207 [DOI] [PubMed] [Google Scholar]
- 31. Lammers F, Zacharias N, Borchers F, Morgeli R, Spies CD, Winterer G. Functional connectivity of the supplementary motor network is associated with Fried's modified frailty score in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(12):2239‐2248. doi: 10.1093/gerona/glz297 [DOI] [PubMed] [Google Scholar]
- 32. Lammers‐Lietz F, Zacharias N, Morgeli R, Spies CD, Winterer G. Functional connectivity of the supplementary and presupplementary motor areas in postoperative transition between stages of frailty. J Gerontol A Biol Sci Med Sci. 2022;77:2464‐2473. doi: 10.1093/gerona/glac012 [DOI] [PubMed] [Google Scholar]
- 33. Nomura Y, Nakano M, Bush B, et al. Observational study examining the association of baseline frailty and postcardiac surgery delirium and cognitive change. Anesth Analg. 2019;129(2):507‐514. doi: 10.1213/ANE.0000000000003967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song R, Song G, Xie P, et al. Diffusion tensor imaging and resting‐state functional magnetic resonance imaging in patients with delirium in intensive care unit. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(1):88‐93. doi: 10.3760/cma.j.cn121430-20190905-00016 [DOI] [PubMed] [Google Scholar]
- 35. Cavallari M, Dai W, Guttmann CR, et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain. 2016;139(Pt 4):1282‐1294. doi: 10.1093/brain/aww010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Georgescu Margarint EL, Georgescu IA, Zahiu CD, et al. Reduced interhemispheric coherence after cerebellar vermis output perturbation. Brain Sci. 2020;10(9):621. doi: 10.3390/brainsci10090621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037‐1066. doi: 10.1016/j.cortex.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019;42:337‐364. doi: 10.1146/annurev-neuro-070918-050258 [DOI] [PubMed] [Google Scholar]
- 39. Abderrakib A, Ligot N, Naeije G. Cerebellar cognitive affective syndrome after acute cerebellar stroke. Front Neurol. 2022;13:906293. doi: 10.3389/fneur.2022.906293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bodranghien F, Bastian A, Casali C, et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. 2016;15(3):369‐391. doi: 10.1007/s12311-015-0687-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taskiran‐Sag A, Uzuncakmak Uyanik H, Uyanik SA, Oztekin N. Prospective investigation of cerebellar cognitive affective syndrome in a previously non‐demented population of acute cerebellar stroke. J Stroke Cerebrovasc Dis. 2020;29(8):104923. doi: 10.1016/j.jstrokecerebrovasdis.2020.104923 [DOI] [PubMed] [Google Scholar]
- 42. Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180‐190. doi: 10.1016/j.neuroimage.2014.05.052 [DOI] [PubMed] [Google Scholar]
- 43. Chen H, Li Y, Liu Q, et al. Abnormal interactions of the salience network, central executive network, and default‐mode network in patients with different cognitive impairment loads caused by leukoaraiosis. Front Neural Circuits. 2019;13:42. doi: 10.3389/fncir.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choi SH, Lee H, Chung TS, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012;169(5):498‐507. doi: 10.1176/appi.ajp.2012.11060976 [DOI] [PubMed] [Google Scholar]
- 45. Nitchingham A, Kumar V, Shenkin S, Ferguson KJ, Caplan GA. A systematic review of neuroimaging in delirium: predictors, correlates and consequences. Int J Geriatr Psychiatry. 2018;33(11):1458‐1478. doi: 10.1002/gps.4724 [DOI] [PubMed] [Google Scholar]
- 46. Cavallari M, Fong TG, Touroutoglou A, et al. Assessment of potential selection bias in neuroimaging studies of postoperative delirium and cognitive decline: lessons from the SAGES study. Brain Imaging Behav. 2022;16(4):1732‐1740. doi: 10.1007/s11682-022-00644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Browndyke JN, Moser DJ, Cohen RA, et al. Acute neuropsychological functioning following cardiosurgical interventions associated with the production of intraoperative cerebral microemboli. Clin Neuropsychol. 2002;16(4):463‐471. doi: 10.1076/clin.16.4.463.13910 [DOI] [PubMed] [Google Scholar]
- 48. Patel N, Minhas JS, Chung EM. Intraoperative embolization and cognitive decline after cardiac surgery: a systematic review. Semin Cardiothorac Vasc Anesth. 2016;20(3):225‐231. doi: 10.1177/1089253215626728 [DOI] [PubMed] [Google Scholar]
- 49. Knipp SC, Matatko N, Schlamann M, et al. Small ischemic brain lesions after cardiac valve replacement detected by diffusion‐weighted magnetic resonance imaging: relation to neurocognitive function. Eur J Cardiothorac Surg. 2005;28(1):88‐96. doi: 10.1016/j.ejcts.2005.02.043 [DOI] [PubMed] [Google Scholar]
- 50. Abu‐Omar Y, Balacumaraswami L, Pigott DW, Matthews PM, Taggart DP. Solid and gaseous cerebral microembolization during off‐pump, on‐pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127(6):1759‐1765. doi: 10.1016/j.jtcvs.2003.09.048 [DOI] [PubMed] [Google Scholar]


