Abstract
Objective
Persons with congenital heart disease (CHD) are at increased risk of neurodevelopmental disabilities, including impairments to executive function. Sulcal pattern features correlate with executive function in adolescents with single‐ventricle heart disease and tetralogy of Fallot. However, the interaction of sulcal pattern features with genetic and participant factors in predicting executive dysfunction is unknown.
Methods
We studied sulcal pattern features, participant factors, and genetic risk for executive function impairment in a cohort with multiple CHD types using stepwise linear regression and machine learning.
Results
Genetic factors, including predicted damaging de novo or rare inherited variants in neurodevelopmental disabilities risk genes, apolipoprotein E genotype, and principal components of sulcal pattern features were associated with executive function measures after adjusting for age at testing, sex, mother's education, and biventricular versus single‐ventricle CHD in a linear regression model. Using regression trees and bootstrap validation, younger participant age and larger alterations in sulcal pattern features were consistently identified as important predictors of decreased cognitive flexibility with left hemisphere graph topology often selected as the most important predictor. Inclusion of both sulcal pattern and genetic factors improved model fit compared to either alone.
Interpretation
We conclude that sulcal measures remain important predictors of cognitive flexibility, and the model predicting executive outcomes is improved by inclusion of potential genetic sources of neurodevelopmental risk. If confirmed, measures of sulcal patterning may serve as early imaging biomarkers to identify those at heightened risk for future neurodevelopmental disabilities.
Introduction
Congenital heart disease (CHD), the most common major congenital anomaly, is associated with disrupted fetal brain growth, structural brain abnormalities, and neurodevelopmental disabilities (NDD). 1 , 2 In particular, compared with the normative population, individuals with CHD have a greater risk of impaired executive function, which may lead to poor school performance and reduced quality of life. 3 , 4 , 5 , 6 Treatment of executive dysfunction with early educational and neurodevelopmental support services may help to mitigate its impact on employability later in life. 2
Brain magnetic resonance imaging (MRI) can quantify many traits such as area, thickness, and volumes, which are themselves influenced by the cellular and molecular maturation of the brain throughout development and in response to brain injuries. Previous studies have characterized brain MRI differences in participants with CHD to improve our understanding of the association between CHD and NDD. 7 , 8 , 9 , 10 As impairments are likely to be specific to both brain function and neuroanatomic areas or tracts, identification of any contributing neuroanatomical basis for such associations would provide potential biomarkers for studies of therapeutic interventions. Advanced analytic approaches can characterize more complex structures such as the folding pattern of sulci on the surface of the brain and provide insight into the mechanisms by which CHD impacts brain development. 11 Primary cortical sulci develop prior to the third trimester 12 , 13 , 14 and are stable across the first two decades of life, indicating they could serve as early biomarkers of later outcomes. 13 , 15 , 16 , 17 , 18 Previous work has shown an association between sulcal patterns and executive function in two independent cohorts with single‐ventricle CHD and tetralogy of Fallot. 19 , 20 , 21 Whether these findings are generalizable to other CHD types has not been determined.
Genetic variation also contributes to CHD risk. 22 Recent studies show that participants with CHD have more de novo variants predicted to damage gene function (dDNVs) than participants without CHD. 23 , 24 , 25 , 26 In a study of 2645 parent–offspring trios, investigators identified an excess of predicted protein‐damaging de novo variants in individuals with CHD, NDD, and congenital abnormalities (28%) compared with individuals with isolated CHD (3%). 24 dDNVs in participants with CHD and NDD were enriched for known genetic variants associated with NDD and for genes with functions related to chromatin modification. In a recent study, participants with dDNVs in chromatin‐modifying genes had impairments with lower verbal comprehension and working memory as well as higher likelihood of autism spectrum disorder. 27 Common variants may also play a role, as the Apolipoprotein E (ApoE) ε2 allele has been associated with worse Psychomotor Development Index scores in infants undergoing cardiac surgery. 28 Genetic factors may impact brain structure and microstructure, including sulcal measures.
The interaction of sulcal pattern features with genetic and participant factors in predicting executive dysfunction is unknown. Using machine learning models, we studied sulcal pattern features, together with genetic and participant factors, to predict risk of executive dysfunction in a cohort with multiple CHD types. Specifically, we first assessed whether sulcal patterns are associated with executive function. We then quantified the relative contribution of sulcal measures and genetic variants as predictors of executive function measures.
Methods
Cohort
We performed a secondary analysis of the Genomic Basis of Neurodevelopmental and Brain Outcomes in CHD Study (ClinicalTrials.gov NCT03070197), a multi‐center observational cohort study of individuals with CHD. This study was approved by the central Institutional Review Board and reliance agreements were approved at each study center. Written informed consent was obtained from participants or their parents; written informed assent was obtained from competent older children. All participants included in this secondary analysis are also enrolled in CHD GENES (ClinicalTrials.gov NCT01196182). Inclusion criteria included diagnosis of CHD, age ≥8 years, available DNA sequencing data, heterozygous for a dDNV not previously associated with neurodevelopmental risk or absence of such variants, and informed consent/assent. Exclusion criteria included history of cardiac transplant, a cardiac surgical procedure within 6 months of enrollment, known genetic syndrome due to a pathogenic variant identified in a gene associated with abnormalities of the brain structure or function, structural heart disease, or potentially other associated features, presence of copy number variant (CNV) known to be clinically pathogenic, overwhelming acquired brain injury, such as a major stroke or severe ischemic injury, or lack of ability to communicate in either English or Spanish. Participants were further excluded from receiving a brain MRI if they had a contraindication to having brain MRI scan, were claustrophobic or unable to lie still while in the MRI scanner for the required time, or pregnant. Of 199 participants in the parent study, 97 completed a brain MRI scan.
Executive function outcomes
Three Delis‐Kaplan Executive Function System (D‐KEFS) subtests were used as primary endpoints in our analysis. 29 Tests were performed by a licensed psychologist or supervised psychometrist at each site. As a measure of executive function measure, the D‐KEFS provides a comprehensive assessment of higher‐level thinking and cognitive flexibility. Specific scores used in the current study were related to cognitive flexibility (verbal fluency category switching accuracy, trail making number–letter switching) and overall executive function (tower total achievement). The category switching accuracy subtest measures verbal behavioral productivity, working memory, set shifting, and cognitive flexibility. The number–letter switching subtest measures cognitive flexibility, working memory, and processing speed by means of a visuomotor sequencing task. The tower total achievement subtest measures spatial planning, rule‐learning, and inhibition of impulsive response. The expected mean score for all subtests is 10 with a standard deviation (SD) of 3, with a higher score indicating better performance.
Quantification of sulcal pattern features
MR images were used to quantify sulcal patterns as a graph structure with sulcal pits and their surrounding catchment basins as nodes. MRI data acquisition closely followed the approach of Adolescent Brain Cognitive Development Study; preprocessing was done (e.g., gradwarp, bias field correction) as previously described. 27 , 30 , 31 Sulcal catchment basins are concavities on the cortical surface that are separated by convex ridges and sulcal pits are the deepest points in sulcal catchment basins. 32 , 33 Maps of sulcal depth were generated using FreeSurfer v5.3.0, and images were processed to generate quantitative measures of sulcal pattern similarity as described previously. 19 , 20 , 34 , 35 , 36 Harmonization was not performed as sulcal pit extraction and graph construction are highly reproducible across scan sessions and scanners 37 .
Each individual's hemispheric and lobar regional sulcal pattern similarities were quantitatively calculated, with a score ranging from 0 to 1, relative to a group of 174 healthy controls without CHD described previously. 19 , 20 In brief, the control sample consisted of 80 participants from the Human Connectome Project (age range 22–25 years for all included participants, 48% female sex) and 49 participants (median age [IQR] 15 [14–16] years, 44% female sex) and 45 participants (age 15.5 [14–17] years, 58% female sex) from the control groups of two separate CHD studies. 3 , 7 , 38 For each participant, a mean similarity score relative to each of the 174 healthy controls was measured, with a total of 50 measures computed for each individual. After measuring the similarity to controls with all features combined (sulcal position, depth, area, and graph topology), we further measured the similarity to controls of each feature separately to evaluate their relative importance on the composite sulcal pattern similarity. These methodological procedures are explained in more detail previously. 39
Identification of genetic variants
Exome sequencing was used to determine whether each participant did or did not have rare (allele frequency < 1 × 10−5 in gnomAD) predicted loss‐of‐function (LoF) variants in genes in four previously defined independent categories: high brain expression (HBE; upper quartile of mouse brain expression at E14.5 23 4420 genes), chromatin modifiers (620 genes), associated with neurodevelopmental disabilities (based on literature review through April 2017; 720 genes 27 ), or genetically constrained with a high probability of intolerance to LoF (high pLI; pLI > 0.5 in gnomAD 2.0 cohort, 4543 genes). Genome sequencing was available for 67 participants and was used to determine Apolipoprotein E (ApoE) genotype based on single nucleotide polymorphisms at chr19:44908684 and chr19:44908822 (hg38).
Statistical analysis
We considered demographic and medical characteristics as covariates in our analysis. These included age at testing, sex, mother's education, and biventricular/single‐ventricle CHD type. We did not adjust for additional MRI features, such as volumetrics and surface area, as these may be influenced by sulcal patterns. Although CHD diagnosis was originally categorized into four groups (single ventricle with arch obstruction, single ventricle without arch obstruction, biventricular with arch obstruction, and biventricular without arch obstruction), we used a simplified biventricular/single‐ventricle categorization due to the low prevalence of arch obstruction in our sample.
Associations between patient demographic characteristics, genetic variants, and sulcal pattern similarity with neurodevelopmental outcomes were assessed using separate linear regression models to predict category switching accuracy, number–letter switching, and tower total achievement. Sulcal pattern similarity measures had high dimensionality and collinearity, so the sulcal pattern similarity measures for the entire sample were decomposed into principal components (PC) using the R package prcomp. 40 To further focus on the most impactful sulcal pattern features, we calculated the PC loading values for the subset of measures with absolute loadings >0.25 to determine the relationship between features and PCs. For genetic characteristics, we considered presence or absence of ε2 or ε4 ApoE genotype as opposed to the reference ε3 ApoE genotype, chromatin LoF variant, HBE LoF variant, and NDD LoF variant. Stepwise Akaike information criterion (AIC) was used to select independent genetic and sulcal predictors of executive function. 41 Stepwise AIC selection will not necessarily select candidate predictors with statistically significant p‐values, but rather considers the performance of candidate models as a whole. After candidate predictors were selected, we additionally adjusted for age at testing, sex, mother's education, and biventricular versus single‐ventricle heart disease. As a sensitivity analysis, we refit models additionally adjusting for MRI scanner model. For neurodevelopmental outcome models for which the sulcal pattern similarity or genetic characteristic factors were statistically significant (p < 0.05), we considered additional models to explore their relative contributions. First, we used stepwise AIC selection with demographic variables, genetic variants, and sulcal similarity to control measures as candidate predictors (Model 3). We then fit two additional models: one with the sulcal measures excluded (Model 1) and another with the genetic characteristics excluded (Model 2) for comparison.
For neurodevelopmental outcome models for which the sulcal pattern similarity or genetic characteristic factors were statistically significant (p < 0.05), we used regression trees as a machine learning technique to develop alternative classification models that may be more clinically useful. 42 We considered one tree with demographic variables, genetic variants, and sulcal similarity to control measures as candidate predictors and another with sulcal measures excluded. This technique is particularly well‐suited to exploring potentially nonlinear relationships and interactions between variables and identifying population subgroups who are homogeneous with respect to outcome. Recursive partitioning, wherein data are partitioned along the predictor axes into subsets with similar values of the dependent variable until a stopping criterion is reached, was used to create pruned regression trees in the entire sample (minimum terminal node size = 7, complexity parameter = 0.02). For robustness to outliers and interpretability, rather than use the sulcal PCs we used as input dichotomized versions of each original sulcal measure with their respective median values as cut points. To compare model performance, we reported the percent variability in outcome explained by the predictors, or R 2, and the root mean squared error (RMSE). To assess for possible overfitting, we computed a fivefold cross‐validation RMSE for each model. 43 , 44 , 45 This procedure involves randomly dividing the data into five equal‐sized sets and repeatedly performing the fitting procedure, excluding 20% of the data, or one “set”, each time starting from step 0. The fitting procedure is performed on the remaining 80% of the data, and the RMSE is computed using the excluded 20% as a testing set. We then average over the five computed RMSE to compute a cross‐validation RMSE. For the regression models, we performed stepwise AIC selection once for the entire sample and then calculated the cross‐validation RMSE for the final model using the same folds as those used for the regression trees. To assess the stability of the predictors selected for our regression trees, we used a bootstrapping approach. We generated 1000 bootstrapped samples (sampling with replacement from the original sample stratified by diagnosis group) and fit regression trees to each bootstrapped sample as described previously. 43 , 44 , 45 We then report which predictors occurred more than 5% of the time as a first level or a first‐or‐second level split. The regression tree analyses were performed using the rpart and rpart.plot libraries in R version 4.2.1.
Results
Cohort characteristics
The sample included 97 individuals with a median age [interquartile range (IQR)] of 16.2 [9.8–22.4] years (Table 1). The cohort had a majority of males (59%) and included 29 (40%) individuals with ε2 or ε4 ApoE genotype, 9 (9%) individuals with a chromatin LoF variant, 61 (63%) individuals with a HBE LoF variant, and 8 (8%) individuals with an NDD LoF variant. There were 24 (25%) individuals with a single‐ventricle CHD (4 with arch obstruction) and 73 (75%) individuals with biventricular CHD (7 with arch obstruction). The highest maternal education level was high school for 14%, some college or college for 57%, and a postgraduate degree for 29%.
Table 1.
Participant characteristics.
| Category | Variable | Value | Overall, n = 97 |
|---|---|---|---|
| Demographic and medical characteristics | Female sex | 40 (41%) | |
| Mother's education | High school or less/other | 14 (14%) | |
| Some college/college graduate | 55 (57%) | ||
| Postgraduate degree | 28 (29%) | ||
| Congenital heart disease diagnosis | Single ventricle with arch obstruction | 4 (4%) | |
| Single ventricle without arch obstruction | 20 (21%) | ||
| Biventricular with arch obstruction | 7 (7%) | ||
| Biventricular without arch obstruction | 66 (68%) | ||
| MRI model | General electric MR750 | 5 (5%) | |
| Siemens Prisma | 65 (68%) | ||
| Siemens Prisma fit | 25 (26%) | ||
| Cortical thickness | 2.66 ± 0.15 | ||
| MRI infarct number | 0 | 86 (90%) | |
| 1–5 | 10 (10%) | ||
| Genetic characteristics | ApoE ε2/ε4 genotype | 29 (40%) | |
| Chromatin LoF variant | 9 (9%) | ||
| HBE LoF variant | 61 (63%) | ||
| NDD LoF variant | 8 (8%) | ||
| Total sulcal similarity measures | Left hemisphere | 0.7503 ± 0.0069 | |
| Left frontal | 0.7626 ± 0.0076 | ||
| Left temporal | 0.7481 ± 0.0124 | ||
| Left parietal | 0.7331 ± 0.0148 | ||
| Left occipital | 0.7432 ± 0.0145 | ||
| Right hemisphere | 0.7379 ± 0.0228 | ||
| Right frontal | 0.7530 ± 0.0198 | ||
| Right temporal | 0.7379 ± 0.0271 | ||
| Right parietal | 0.7190 ± 0.0224 | ||
| Right occipital | 0.7389 ± 0.0222 | ||
| Executive function testing | Age at testing (years) | 16.2 [9.8–22.4] | |
| Category switching accuracy | 10.6 ± 3.3 | ||
| Number–letter switching | 9.3 ± 3.6 | ||
| Tower total achievement | 9.8 ± 2.5 |
Values are mean ± SD, number (%), or median [interquartile range]. Data complete except for MRI model (n = 95), cortical thickness (n = 95), MRI infarct number (n = 96), ApoE ε2/ε4 genotype (n = 73), and total sulcal similarity measures (n = 92).
Abbreviations: ApoE, Apolipoprotein E; CHD, congenital heart disease; HBE, high brain expression; LoF, loss‐of‐function; MRI, magnetic resonance imaging; NDD, neurodevelopmental disability.
There was a high degree of correlation between the 50 left and right hemisphere sulcal pattern similarity to average control measures, with intrahemispheric correlations greater within the right hemisphere than the left (Fig. 1). Due to the high dimensionality and autocorrelation of the sulcal measures, we used a PC decomposition in our subsequent regression analyses. The first five PCs accounted for 57% of the total variance in sulcal measures (Table 2). The first PC accounted for 28% of the total variance with the right hemisphere total measure its most relevant feature. The most relevant variables for the second PC were several left hemisphere measures. The third, fourth, and fifth PCs were also primarily composed of left hemisphere measures.
Figure 1.
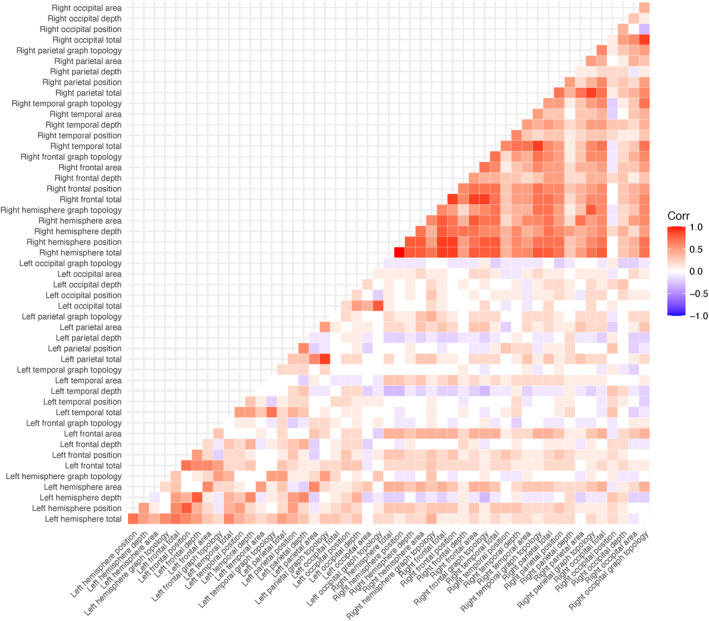
Pearson correlation coefficient matrix between all sulcal similarity measures.
Table 2.
Factor loadings from the first five principal components of sulcal similarity measures (n = 92) with only factors with an absolute value loading >0.25 in at least one principal component shown.
| Sulcal similarity measure | Principal component (% variability explained) | ||||
|---|---|---|---|---|---|
| 1 (28%) | 2 (12%) | 3 (8%) | 4 (5%) | 5 (4%) | |
| Left hemisphere total | 0.08 | −0.33 | 0.17 | 0.04 | −0.09 |
| Left hemisphere position | 0.09 | −0.27 | −0.09 | −0.02 | −0.10 |
| Left hemisphere depth | −0.02 | −0.33 | −0.11 | −0.03 | 0.16 |
| Left hemisphere area | 0.13 | −0.02 | 0.25 | −0.01 | 0.03 |
| Left hemisphere graph topology | 0.02 | −0.15 | 0.33 | 0.12 | −0.13 |
| Left frontal total | 0.09 | −0.27 | 0.11 | −0.14 | 0.16 |
| Left frontal depth | 0.01 | −0.29 | −0.08 | −0.07 | 0.15 |
| Left frontal area | 0.14 | −0.06 | 0.15 | −0.10 | 0.27 |
| Left frontal graph topology | 0 | −0.04 | 0.28 | −0.11 | 0.14 |
| Left temporal total | 0.01 | −0.26 | 0.02 | 0.22 | −0.17 |
| Left temporal graph topology | 0 | −0.12 | 0.09 | 0.33 | −0.19 |
| Left parietal total | 0.08 | −0.15 | 0.29 | 0.23 | 0.11 |
| Left parietal area | 0.08 | 0.05 | 0.33 | 0.04 | 0.09 |
| Left parietal graph topology | 0.08 | −0.05 | 0.34 | 0.14 | 0.13 |
| Left occipital total | 0.02 | −0.10 | 0.16 | −0.40 | −0.37 |
| Left occipital depth | 0.04 | −0.14 | 0.07 | −0.31 | 0.03 |
| Left occipital graph topology | −0.02 | −0.04 | 0.13 | −0.19 | −0.49 |
| Right hemisphere total | 0.26 | 0.04 | −0.04 | 0.01 | −0.05 |
| Right temporal area | 0.15 | 0.03 | 0.02 | 0.28 | −0.02 |
Bold text indicates the principal component with absolute value loading >0.25.
Associations of sulcal measures and genetic factors with executive function
Predictors of category switching accuracy included presence of NDD LoF variant and the first and fifth PCs of the sulcal measures (Table 3). None of the four demographic characteristics were statistically significantly associated with category switching accuracy, though the fifth sulcal PC was positively associated with category switching accuracy (p = 0.007). Independent predictors of number–letter switching included ε2 or ε4 ApoE genotype and the fourth sulcal PC, which both had a negative association with number–letter switching. Mother's education was positively associated with number–letter switching, with the expected score [β ± SE] 2.8 ± 1.2 (p = 0.02) points higher for maternal education level of college versus high school and 4.3 ± 1.3 (p = 0.002) points higher for maternal education level of postgraduate degree versus high school. Since ApoE sequencing was not available for all participants, we refit this model with the full sample and results were similar; the effect of the fourth sulcal PC was −0.3 ± 0.2 (p = 0.28). Independent predictors of total tower achievement included presence of NDD LoF variant and the fifth sulcal PC. All three models had a cross‐validation RMSE within 11% of the respective RMSE. Participants with single‐ventricle CHD had a higher expected total tower achievement than those with biventricular CHD, [β ± SE] 2.1 ± 0.6 (p < 0.001) points. We additionally assessed the sensitivity of our results to adjustment by MRI model. Here, the effect of the fifth sulcal PC on category switching accuracy was similar: 0.7 ± 0.2 (p = 0.009).
Table 3.
Regression results for three executive function outcomes, all additionally adjusted for age at test, sex, mother's education, and biventricular versus single‐ventricle CHD. Genetic and sulcal variables were selected using stepwise AIC selection.
| Category switching accuracy (n = 92) | Number–letter switching (n = 71 a ) | Tower total achievement (n = 92) | |
|---|---|---|---|
| Female sex | −0.34 ± 0.68 (0.61) | −0.76 ± 0.81 (0.35) | −0.95 ± 0.50 (0.06) |
| Bi‐ventricular CHD | 0.58 ± 0.80 (0.47) | −0.43 ± 1.00 (0.67) | −2.12 ± 0.59 (<0.001*) |
| Mother's education (some college/college graduate vs. high school or less/other) | 0.93 ± 1.02 (0.36) | 2.82 ± 1.15 (0.02*) | −0.33 ± 0.76 (0.66) |
| Mother's education (postgraduate degree vs. high school or less/other) | 0.73 ± 1.09 (0.51) | 4.31 ± 1.32 (0.002*) | 0.20 ± 0.81 (0.81) |
| Age at testing | 0.07 ± 0.04 (0.13) | 0.01 ± 0.05 (0.90) | 0.03 ± 0.03 (0.38) |
| ApoE ε2/ε4 genotype | – | −1.35 ± 0.83 (0.11) | – |
| NDD LoF variant | −1.98 ± 1.18 (0.10) | – | −1.54 ± 0.88 (0.08) |
| First principal component | 0.14 ± 0.09 (0.13) | – | – |
| Fourth principal component | – | −0.34 ± 0.24 (0.17) | – |
| Fifth principal component | 0.65 ± 0.23 (0.007*) | – | 0.23 ± 0.17 (0.19) |
| Adjusted R 2 | 0.11 | 0.15 | 0.13 |
| R 2 | 0.19 | 0.23 | 0.20 |
| RMSE | 2.96 | 3.07 | 2.22 |
| Cross‐validation RMSE | 3.31 | 3.37 | 2.47 |
Data are presented as β ± SE (p‐value).
Abbreviations: AIC, Akaike information criterion; ApoE, Apolipoprotein E; CHD, congenital heart disease; LoF, loss‐of‐function; NDD, neurodevelopmental disability; RMSE, root mean squared error.
Excluding 21 individuals for which ApoE genotyping was not performed.
p < 0.05.
Associations of sulcal measures, genetic features, and participant characteristics with category switching accuracy
As category switching accuracy was the only outcome with a significant association with sulcal measures, we explored this relationship further. We performed three separate multiple linear regressions predicting category switching accuracy that varied based on inclusion of sulcal pattern measures and genetic variants (Table 4). Age at testing was consistently positively associated with category switching accuracy, with the effect decreasing after adjustment for sulcal similarity measures. Presence of NDD LoF variance was negatively associated with category switching accuracy, and the magnitude of the effect increased after adjustment for the sulcal PCs. Furthermore, including the sulcal PCs caused the adjusted R 2 to change from 0.03 (in the genetics only model) to 0.11. The adjusted R 2 for the model with only sulcal PCs was 0.09.
Table 4.
Results for category switching accuracy based on three multiple linear regression models.
| Model 1 (n = 92) | Model 2 (n = 92) | Model 3 (n = 92) | |
|---|---|---|---|
| Female sex | −0.29 ± 0.70 (0.68) | −0.24 ± 0.68 (0.73) | −0.34 ± 0.68 (0.61) |
| Bi‐ventricular CHD | 0.91 ± 0.82 (0.27) | 0.73 ± 0.80 (0.36) | 0.58 ± 0.80 (0.47) |
| Mother's education (some college/college graduate vs. high school or less/other) | 0.79 ± 1.06 (0.46) | 0.92 ± 1.03 (0.37) | 0.93 ± 1.02 (0.36) |
| Mother's education (postgraduate degree vs. high school or less/other) | 0.90 ± 1.14 (0.43) | 0.76 ± 1.10 (0.50) | 0.73 ± 1.09 (0.51) |
| Age at testing | 0.10 ± 0.04 (0.03*) | 0.07 ± 0.04 (0.12) | 0.07 ± 0.04 (0.13) |
| NDD LoF variant | −1.55 ± 1.22 (0.21) | – | −1.98 ± 1.18 (0.10) |
| First principal component | – | 0.14 ± 0.09 (0.13) | 0.14 ± 0.09 (0.13) |
| Fifth principal component | – | 0.59 ± 0.23 (0.01*) | 0.65 ± 0.23 (0.007*) |
| Adjusted R 2 | 0.03 | 0.09 | 0.11 |
| R 2 | 0.09 | 0.16 | 0.19 |
| RMSE | 3.13 | 3.01 | 2.96 |
| Cross‐validation RMSE | 3.31 | 3.23 | 3.31 |
Data are presented as β ± SE (p‐value).
Abbreviations: CHD, congenital heart disease; LoF, loss‐of‐function; NDD, neurodevelopmental disability; RMSE, root mean squared error.
p < 0.05.
Classification model of category switching accuracy by regression tree
As category switching accuracy was the only outcome with a significant association with sulcal measures, we explored this relationship with regression trees. In the first regression tree (Fig. 2A), age emerged as the strongest predictor of category switching accuracy, appearing as the root node of the tree. Presence of NDD LoF variant was also selected as an important predictor. The lowest (most impairment) mean category switching accuracy (6.8) was found in seven individuals who were <25 years of age and had an NDD LoF variant. The highest (least impairment) mean category switching accuracy (13.2) was found in 13 individuals who were >25 years of age. This model explained 12% of the total variability in category switching accuracy and had a cross‐validation RMSE 1% higher than the RMSE. In the second regression tree (Fig. 2B), the patient demographic characteristics, genetic variants, and sulcal pattern symmetry measures were included as candidate predictors. Only age and sulcal measures, but not genetic characteristics, were selected as important predictors in the regression tree and all were positively associated with category switching accuracy. Age and left hemisphere sulcal pattern similarity were the strongest predictors of category switching accuracy; right temporal depth and graph topology and occipital depth were also selected to be in the tree. The lowest mean category switching accuracy (7.5) was found in 15 individuals who were aged <25 years and had low similarity to controls in left hemisphere graph topology, right temporal depth, and right temporal graph topology, with lower sulcal pattern similarity values indicating more abnormalities in sulcal pattern. The highest mean category switching accuracy (13.2) was found in 13 individuals who were over 25 years old. The inclusion of sulcal measures causes the percent variability explained to increase from 12% to 30%. There is no evidence of overfitting as the cross‐validation RMSE was 14% higher than the RMSE, which is a similar inflation factor that of the regression analysis. In the bootstrap resamples, first‐level splits occurred most often with age (25%), left hemisphere graph topology (23%), right occipital depth (11%), and right temporal area (6%).
Figure 2.
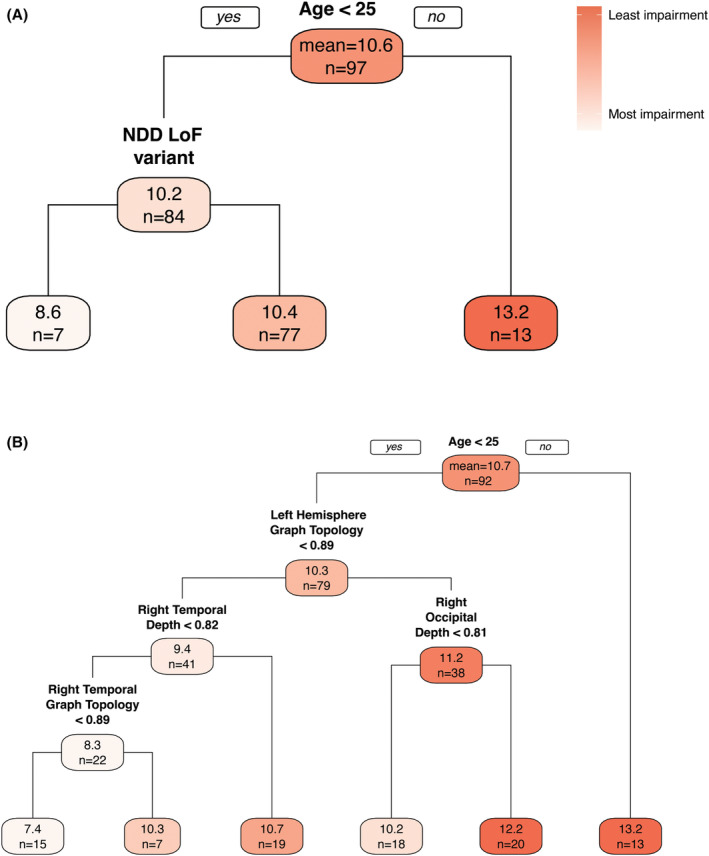
Regression trees predicting category switching accuracy with various candidate predictors. Tree (A) (n = 97, R 2 = 0.12, RMSE = 3.08, cross‐validation RMSE = 3.12) considers only patient demographic characteristics and genetic variants while Tree (B) (n = 92, R 2 = 0.30, RMSE = 2.76, cross‐validation RMSE = 3.12) additionally considers all sulcal measures. Each node indicates the mean category switching accuracy (top number) and sample size (bottom number) for participants with the characteristics along the paths above the node. For example, in tree A, 7 individuals aged <25 with an NDD LoF variant had the lowest mean score of 8.6 and 13 individuals aged >25 had the highest mean score of 13.2. LoF, loss‐of‐function; NDD, neurodevelopmental disability; RMSE, root mean squared error.
Discussion
We describe the associations between sulcal patterns, genetic variants, and demographic characteristics with cognitive flexibility in participants with a variety of CHD types. Across analyses, sulcal patterns were associated with executive function measures. Sulcal pattern similarity to controls was positively associated with category switching accuracy, a measure of cognitive flexibility, and this association remained after adjustment for presence of NDD LoF variant and age at testing. In our regression analysis, left occipital graph topology, total, and left frontal area measures were the predominant contributors to the sulcal pattern feature PC that was positively associated with category switching accuracy. In our regression trees and bootstrap validation, which allow for complex nonlinear interactions between predictors, sulcal pattern similarity to control measures and age at testing were consistently identified as important predictors of category switching accuracy, with left hemisphere graph topology and right‐sided sulcal biomarkers often selected as important predictors. We conclude that sulcal measures and genetic sources of NDD risk are independently important predictors of cognitive flexibility in individuals with CHD.
We additionally assessed the impact of participant demographic characteristics on executive function. Age emerged as an important predictor of category switching accuracy in both the regression models and trees. This could be due to improvement in category switching accuracy that occurs during early adulthood as people take on additional responsibilities, or adults with lower functioning may be less likely to come for evaluation than children who are generally brought in by parents. Although a strong association between socioeconomic status and executive function has been observed previously, 45 , 46 maternal education only emerged as an important predictor for one of the executive function scores considered, number–letter switching. This may be because a combination of maternal and paternal education would have been more relevant for our cohort, or the higher‐than‐average and narrow range of socioeconomic status represented in our study may have limited the ability to observe an association. Finally, participants with biventricular CHD had lower tower total achievement than participants with single‐ventricle CHD. Though the sample size here is small, previous studies of executive function in persons with CHD saw no differences in tower total achievement between participants with single‐ventricle CHD and biventricular transposition of the great arteries but did observe poorer performance in visual spatial executive function tasks among participants with single‐ventricle CHD. 6
Correlation of frontal lobe sulcal features with category switching accuracy fits with the previous observation that executive function is disrupted with frontal lobe injury. 47 Supporting a role for the frontal and occipital lobes in executive function, a study of 300 mostly college‐aged participants revealed a correlation between the white matter volume of the fronto‐occipital fasciculus and executive function. 48 Further, a previous study also found more left‐sided abnormalities of sulcal folding depth in infants with complex CHD prior to cardiac surgery. 49 In addition, decreased left‐sided white matter microstructure was correlated with poorer mathematic skill and increased inattention/hyperactivity. 50
Previous studies have demonstrated an effect of specific risk genes, such as chromatin‐modifying genes or the Alzheimer's‐related gene ApoE, 51 , 52 on neurodevelopmental outcomes in people with CHD. Damaging variants in chromatin‐modifying genes that reduce the functional protein are associated with an increased risk of neurodevelopmental delay in persons with CHD. Here, we found that the presence of an NDD LoF variant was included as a predictor in the regression tree for category switching accuracy. Some genes may have pleiotropic effects and the rare variants included in the model may be associated with NDD due to their influence on both heart and brain development, thereby accounting for differential outcomes. Alternatively, ApoE ε2 genotype has previously been associated with worse neurodevelopmental outcomes at 12–14 months after CHD surgery and increased risk of behavioral problems at 4 years of age. 28 , 51 , 53 However, the ApoE ε2 genotype in the same cohort was also associated with a reduced risk of ADHD in adolescence. 52 Presence of ε2 or ε4 ApoE genotype was positively associated with number–letter switching, supporting a positive association with executive function measures in the school‐age or older cohort studied here. Finally, inclusion of genetic risk factors in our models strengthened the association between sulcal pattern symmetry and category switching accuracy, indicating the association between sulcal patterns and executive function is not completely explained by known genetic factors. If genetic influence on sulcal pattern features were the only mechanism of neurodevelopmental risk, the inclusion of genetic risk would not be expected to strengthen the association of sulcal pattern features with executive function measures.
There are several limitations to this current analysis. This study excluded participants with known genetic syndrome due to a either a pathogenic variant identified in a gene associated with abnormalities of the brain structure or function, structural heart disease, or presence of a pathogenic CNV. Therefore, the relationship of known genetic syndromes with sulcal pattern features and executive function was not assessed this study. Further, it could be that individuals with some genetic disorders may be less likely to tolerate brain MRI acquisition, limiting detection of some genetic associations with executive function. Regression analysis on a cohort of this size can result in overfitting. We attempted to remedy this by using model selection, PC analysis, and regression trees, but the exploratory findings of this study need independent replication. Additionally, we did not have information on patient handedness. Given the higher association observed with sulcal patterns in the left hemisphere, it would be interesting to assess if patient handedness impacts executive function. Other limitations to this analysis include its primary focus on only three measures of executive function. Replication of these findings with other measures of executive function is needed. Further, the cohort includes few participants with arch obstruction in either the single‐ventricle or two‐ventricle groups, precluding generalization to those with coarctation. We did not have sufficient sample size and power to analyze the impact of specific forms of CHD associated with diminished cerebral oxygen delivery in utero. The cohorts represent the period from school age to young adulthood and could therefore be less sensitive to detect associations that are limited to a more narrow developmental window. Finally, the cohort analyzed had higher‐than‐average socioeconomic status, limiting generalizability. It would be useful to validate results on a cohort with a wider range of social class.
In conclusion, we have shown an association between sulcal pattern similarity to controls and executive function in a cohort of different types of CHD. This association remained, and strengthened, after adjustment for genetic variants in the multiple linear regression and explained more of the variation in scores than genetic variants alone. However, the regression tree analysis identified that sulcal measures and genetic sources of NDD risk are important independent predictors of executive function outcomes. Sulcal pattern features explained more variability in category switching accuracy than genetic factors. Sulcal measures may reflect underlying changes in neuroanatomy and/or brain microstructure that are functionally associated with executive function but not uniformly affected by the classes of genes studied here. Additional sources of variation may include degree of fetal hypoxia‐ischemia, which vary by CHD type and may also be impacted by placental health, independent of the CHD type and postnatal acquired brain injuries. If confirmed, such measures of sulcal patterning can be studied at earlier ages for the potential to serve as early markers of those at risk for neurodevelopmental complications. Identifying MRI biomarkers of NDD also allows clarification of the timing of postnatal neurodevelopmental critical periods, which is a crucial step in elucidating intervention periods for children living with CHD.
Author Contributions
LM, JWN, DW, NHT, WKC, PEG, KI, and SUM conceived and designed the study. LM, JWN, DW, NHT, EA, MK, WKC, JC, SC, BDG, EG, DJH, HH, EK, PM, TAM, AN‐B, GAP, AER, PEG, KI, and SUM contributed to the acquisition and analysis of the data. LM, JWN, DW, PEG, KI, and SUM drafted a substantial portion of the manuscript or figures. All authors participated in editing of the manuscript.
Conflict of Interest
The authors have no relevant commercial activities to report.
Acknowledgements
The authors would like to thank all participants and their families. We would also like to thank Heather R. Adams, David C. Bellinger, Somer Bishop, Henry Buswell, Christopher Cannistraci, Johanna Calderon, Shiran Chen, Victor Chen, Lauren Christopher, James F. Cnota, Todd Constable, Nancy Cross, Anders M. Dale, Cecelia DeSoto, Lazar Fleysher, John Foxe, Ed Freedman, Michele Frommelt, Borjan Gagoski, Anne Snow Gallagher, Judith Geva, Caren S. Goldberg, Emily Griffin, Dorota Gruber, Abha Gupta, Brandi Henson, Rick Kim, Alex Kolevzon, Joshua Kuperman, Linda Lambert, Kristen Lanzilotta, Brande Latney, Christina Layton, Jennifer S. Li, Chunyan Liu, Derek Lundahl, Shannon Lundy, Stacy Lurie, Meghan MacNeal, Laura Ment, Julith S. Miller, Leona Oakes, Sharon O'Neill, Minhui Ouyang, Ashok Panigrahy, Emily Richardson, Angela Romano‐Adesman, Mark W. Russell, Kelly Sadamitsu, Hedy Sarofin, Anjali Sadhwani, Dustin Scheinost, Christine E. Seidman, Zoey Shaw, Paige Siper, Deepak Srivastava, Sherin Stahl, Eileen Taillie, Allison Thomas, Alexandra Thompson, Madalina E. Tivarus, Nhu Tran, Marti Tristani, Henry Wang, Ting Wang, Sarah Winter, Julie Wolf, Han Yin, Duan Xu, Amy Young, Yensy Zetino, and Brandon Zielinski for their contributions to the study. This project was supported by the National Center for Research Resources (U01 HL098153), the National Center for Advancing Translational Sciences (UL1 TR000003, UL1 TR002541), the National Institutes of Health (U01 HL098123, U01 HL098147, U01 HL098153, U01 HL098162, U01 HL098163, U01 HL098188, and U01 HL131003), and the National Institute of Health Centers for Mendelian Genomics (U54 HG006504), in addition to K08 HL157653 (S.U.M), R01 HL128818 (A.P.), R01 NS114087 (K.I.), Canadian Institutes of Health Research Canada Resaearch Chair in Translational Therapeutics in Autism Spectrum Disorders and Dr. Stuard D Sims Chair in Autism Spectrum Disorders (E.A.), Boston Children's Hospital Office of Faculty Development Career Development Award and American Heart Association Career Development Award (S.U.M.), the Farb Family Fund (J.W.N.), and the Kostin Family Innovation Fund (J.W.N.).
Funding Statement
This work was funded by American Heart Association ; Boston Children's Hospital; Farb Family Fund; Kostin Family Innovation Fund; National Center for Advancing Translational Sciences grants UL1 TR000003 and UL1 TR002541; National Center for Research Resources grant U01 HL098153; National Heart, Lung, and Blood Institute grants K08 HL157653, R01 HL128818, U01 HL098123, U01 HL098147, U01 HL098153, U01 HL098162, U01 HL098163, U01 HL098188, and U01 HL131003; National Institute of Neurological Disorders and Stroke grant R01 NS114087; National Institutes of Health grant U54 HG006504.
Contributor Information
Kiho Im, Email: kiho.im@childrens.harvard.edu.
Sarah U. Morton, Email: sarah.morton@childrens.harvard.edu.
References
- 1. Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143‐1172. [DOI] [PubMed] [Google Scholar]
- 2. Calderon J, Willaime M, Lelong N, et al. Population‐based study of cognitive outcomes in congenital heart defects. Arch Dis Child. 2018;103(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 3. Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d‐transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creighton DE, Robertson CM, Sauve RS, et al. Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120(3):e478‐e486. [DOI] [PubMed] [Google Scholar]
- 5. Neufeld RE, Clark BG, Robertson CM, et al. Five‐year neurocognitive and health outcomes after the neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2008;136(6):1413‐1421.e2. [DOI] [PubMed] [Google Scholar]
- 6. Cassidy AR, White MT, DeMaso DR, et al. Executive function in children and adolescents with critical cyanotic congenital heart disease. J Int Neuropsychol Soc. 2015;21(1):34‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellinger DC, Watson CG, Rivkin MJ, et al. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc. 2015;4(12):e002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Asis‐Cruz J, Donofrio MT, Vezina G, Limperopoulos C. Aberrant brain functional connectivity in newborns with congenital heart disease before cardiac surgery. Neuroimage Clin. 2018;17:31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rollins CK, Ortinau CM, Stopp C, et al. Regional brain growth trajectories in fetuses with congenital heart disease. Ann Neurol. 2021;89(1):143‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watson CG, Stopp C, Wypij D, Bellinger DC, Newburger JW, Rivkin MJ. Altered white matter microstructure correlates with IQ and processing speed in children and adolescents post‐fontan. J Pediatr. 2018;200:140‐149.e4. [DOI] [PubMed] [Google Scholar]
- 11. Im K, Grant PE. Sulcal pits and patterns in developing human brains. Neuroimage. 2019;185:881‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1(1):86‐93. [DOI] [PubMed] [Google Scholar]
- 13. Garel C, Chantrel E, Brisse H, et al. Fetal cerebral cortex: Normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol. 2001;22(1):184‐189. [PMC free article] [PubMed] [Google Scholar]
- 14. White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72(1):36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rakic P. Genetic control of cortical convolutions science. Neuroscience. 2004;303(5666):1983‐1984. [DOI] [PubMed] [Google Scholar]
- 16. Hill J, Inder T, Neil J, Dierker D, Harwell J, van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107(29):13135‐13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rash BG, Rakic P. Genetic resolutions of brain convolutions science. Neuroscience. 2014;343(6172):744‐745. [DOI] [PubMed] [Google Scholar]
- 18. Cachia A, Borst G, Tissier C, et al. Longitudinal stability of the folding pattern of the anterior cingulate cortex during development. Dev Cogn Neurosci. 2016;19:122‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morton SU, Maleyeff L, Wypij D, et al. Abnormal left‐hemispheric sulcal patterns correlate with neurodevelopmental outcomes in subjects with single ventricular congenital heart disease. Cereb Cortex. 2020;30(2):476‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morton SU, Maleyeff L, Wypij D, et al. Abnormal right‐hemispheric sulcal patterns correlate with executive function in adolescents with tetralogy of fallot. Cereb Cortex. 2021;31(10):4670‐4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asschenfeldt B, Evald L, Yun HJ, et al. Abnormal left‐hemispheric sulcal patterns in adults with simple congenital heart defects repaired in childhood. J Am Heart Assoc. 2021;10(7):e018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pierpont ME, Brueckner M, Chung WK, et al. Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation. 2018;138(21):e653‐e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Homsy J, Zaidi S, Shen Y, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350(6265):1262‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin SC, Homsy J, Zaidi S, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017;49(11):1593‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sifrim A, Hitz MP, Wilsdon A, et al. Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat Genet. 2016;48(9):1060‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Page DJ, Miossec MJ, Williams SG, et al. Whole exome sequencing reveals the major genetic contributors to nonsyndromic tetralogy of fallot. Circ Res. 2019;124(4):553‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morton SU, Norris‐Brilliant A, Cunningham S, et al. Association of potentially damaging de novo gene variants with neurologic outcomes in congenital heart disease. JAMA Netw Open. 2023;6(1):e2253191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein e genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126(6):1736‐1745. [DOI] [PubMed] [Google Scholar]
- 29. Delis DC, Kaplan E, Kramer JH, Psychological Corporation . Delis‐Kaplan executive function system. Psychological Corp.; 2001. [Google Scholar]
- 30. Casey BJ, Cannonier T, Conley MI, et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagler DJ Jr, Hatton S, Cornejo MD, et al. Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage. 2019;202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lohmann G, von Cramon DY. Automatic labelling of the human cortical surface using sulcal basins. Med Image Anal. 2000;4(3):179‐188. [DOI] [PubMed] [Google Scholar]
- 33. Im K, Jo HJ, Mangin JF, Evans AC, Kim SI, Lee JM. Spatial distribution of deep sulcal landmarks and hemispherical asymmetry on the cortical surface. Cereb Cortex. 2010;20(3):602‐611. [DOI] [PubMed] [Google Scholar]
- 34. Nattel SN, Adrianzen L, Kessler EC, et al. Congenital heart disease and neurodevelopment: clinical manifestations, genetics, mechanisms, and implications. Can J Cardiol. 2017;33(12):1543‐1555. [DOI] [PubMed] [Google Scholar]
- 35. Im K, Raschle NM, Smith SA, Ellen Grant P, Gaab N. Atypical sulcal pattern in children with developmental dyslexia and at‐risk kindergarteners. Cereb Cortex. 2016;26(3):1138‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Essen DC. A tension‐based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313‐318. [DOI] [PubMed] [Google Scholar]
- 37. Im K, Lee JM, Jeon S, et al. Reliable identification of deep sulcal pits: the effects of scan session, scanner, and surface extraction tool. PLoS ONE. 2013;8(1):e53678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Essen DC, Ugurbil K, Auerbach E, et al. The human connectome project: a data acquisition perspective. Neuroimage. 2012;62(4):2222‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Im K, Pienaar R, Lee JM, et al. Quantitative comparison and analysis of sulcal patterns using sulcal graph matching: a twin study. Neuroimage. 2011;57(3):1077‐1086. [DOI] [PubMed] [Google Scholar]
- 40. Naik GR. Advances in Principal Component Analysis: Research and Development. Springer; 2017. [Google Scholar]
- 41. Yamashita T, Yamashita K, Kamimura R. A stepwise AIC method for variable selection in linear regression. Commun Stat Theory Methods. 2007;36(13):2395‐2403. [Google Scholar]
- 42. Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Routledge; 2017. [Google Scholar]
- 43. Efron B, Hastie T. Computer Age Statistical Inference, Student Edition: Algorithms, Evidence, and Data Science. Cambridge University Press; 2021. [Google Scholar]
- 44. Hastie T, Tibshirani R, Friedman JH, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; 2009. [Google Scholar]
- 45. James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning. Springer; 2013. [Google Scholar]
- 46. Last BS, Lawson GM, Breiner K, Steinberg L, Farah MJ. Childhood socioeconomic status and executive function in childhood and beyond. PLOS ONE. 2018;13(8):e0202964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav. 2014;123:45‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takeuchi H, Taki Y, Sassa Y, et al. Brain structures associated with executive functions during everyday events in a non‐clinical sample. Brain Struct Funct. 2013;218:1017‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ortinau C, Alexopoulos D, Dierker D, van Essen D, Beca J, Inder T. Cortical folding is altered before surgery in infants with congenital heart disease. J Pediatr. 2013;163(5):1507‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rollins CK, Watson CG, Asaro LA, et al. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165(5):936‐944.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaynor JW, Kim DS, Arrington CB, et al. Validation of association of the apolipoprotein e ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg. 2014;148(6):2560‐2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmithorst VJ, Panigrahy A, Gaynor JW, et al. Organizational topology of brain and its relationship to adhd in adolescents with d‐transposition of the great arteries. Brain Behav. 2016;6(8):e00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gaynor JW, Nord AS, Wernovsky G, et al. Apolipoprotein e genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124(1):241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]


