Abstract
Background
Paget Disease (PD) is usually asymptomatic and discovered incidentally, it is known that it is exhibited low to high grade increased F-18 FDG uptake.
Aim
In this study, we investigated the distinguishability of FDG PET/CT in incidental PD cases from other bone diseases and at different stages of the disease.
Patients and Methods
In this cross-sectional, descriptive study, “Paget” identification associated with PET/CT reports was found in 69 of 18,119 studies (~3.8%). Of the 45 patients (33 males and 12 females) eligible for inclusion in the study, 35.6% had monostotic and 64.4% had polyostotic disease (p>0.5). There was no statistically significant difference in biochemical parameters between groups.
Results
According to the radiological appearance of the patients, 36 were in the mixed stage and 9 were in the blastic stage. Only the difference in ALP and creatinine values between the groups was statistically significant. SUVmax, SUVmean and HU values were found to be statistically significantly higher in pagetoid bones compared to control bone lesions. For SUVmax for PD bone lesion we found the 2.55 cutoff point with a sensitivity of 91% and a specificity of 84%.
Conclusion
The specific radiological appearance of bone lesions and the evaluation of metabolic activity compared to normal bone seem to help differentiate PD from other lesions. Prospective studies are needed in the differentiation of FDG's disease stage and treatment response evaluation. The ability to differentiate between benign and malignant FDG avid bone lesions in oncological patients’ enables appropriate patient management, including avoiding unnecessary additional invasive procedures such as bone biopsy.
Keywords: Paget disease, F-18 FDG PET/CT, incidental bone lesion
INTRODUCTION
Paget disease (osteitis deformans) (PD) is a chronic non-malignant skeletal disorder characterized by abnormal and excessive remodelling of the bone. Although the highest prevalence is seen in European countries, particularly in the UK (4.6%) (1), significant regional variations have been reported (2, 3). The prevalence and incidence of PD in the Turkish population have not been determined yet, only a few case studies are available. The extraordinary geographical distribution of the disease cannot be explained by known industrial, geological or climatic features.
The onset of PD in the population younger than 50 years is uncommon (5, 6). PD is a localized disorder that may be monostotic (affecting only one bone), or polyostotic (affecting 2 or more bones). The cause of PD remains largely unknown; however the main aethiopathogenesis on the development of disease has been defined as slow virus infection that triggers the osteoclast of the pagetic bone (3, 6). The other purported aetiological conditions of PD are vascular diseases, genetic diseases, an immunologic or metabolic disorder, or true neoplastic process (6).
Three major phases are recognized: the lytic phase, in which osteoclastic resorption predominates the mixed phase (active), in which there is both osteoclastic and osteoblastic hyperplasia with predominant osteoblastic activity; and finally, the blastic phase (late inactive), in which osteoblastic activity gradually declines (3, 7). PD is generally considered as a progressive disorder that evolves through various phases of activity followed by an inactive or quiescent stage. Most of the untreated patients have elevated serum ALP, reflecting the extent and the activity of the disease. It should be kept in mind that biochemical data may be within normal limits in cases with limited skeletal involvement.
Imaging is considered to be essential in establishing diagnose of PD, evaluating the type and extent of complications, and monitoring the effects of therapy. The main techniques available for imaging patients with PD are radiography, bone scintigraphy, CT, and MRI. The imaging appearance of PD is dependent on the disease’s stage. The four primary radiographic features of PD that are particularly important in the overall evaluation and aid that helps distinguishing Paget's from other diseases such as fibrous dysplasia, infection, and blastic metastases are 1. Advancing wedge of resorption, 2. Accentuation and coarsening of trabecular pattern along stress lines, 3. Cortical thickening, and 4. Enlargement of bone contours. Scintigraphy provides an excellent detection of disease distribution and local activity, therefore complements plain radiographs. Another major advantage of scintigraphy in comparison to conventional radiography is its ability to dynamically measure response to therapeutic agents. Bone scintigraphy is more sensitive than conventional radiography, revealing up to 30% more bone lesions, especially in sites with multiple overlapping radiographic shadows such as the ribs, sternum, and scapula (8). CT or MRI can also be helpful if the diagnosis is uncertain, however neither is usually required.
It is mentioned that PD is usually asymptomatic and discovered incidentally, therefore it does not usually require treatment. However, in a comprehensive retrospective study involving 899 patients, it was stated that the majority of patients (74.4%) were symptomatic and 2.4% of them presented with complications (5).
Although rare, it is known that PD exhibites low to high grade increased F-18 FDG uptake (9-23). Therefore, PD could be described as a possible cause of false-positive F-18 FDG PET/CT, especially in elderly patients who are being assessed for oncologic imaging (24).
We had two goals in this study. The first was to investigate the role of PET/CT in differentiating bone PD from other benign bone diseases and malignant/metastatic bone diseases. The second was to evaluate the visual, biochemical and metabolic parameters obtained by F-18 FDG PET/CT in terms of the possibility of distinguishing the stage of the disease and the number of affected bones (poly or monostotic) in PD.
METHODS
Patients
This cross-sectional, descriptive study was approved by the local ethics committee (No: TUTF-BAEK-2016/290).
We performed a database search for PET/CT records generated from 10/2009 to 12/2016 which included the word “Paget” in reports for patients who underwent PET/CT examination for the workup of recurrent or metastatic disease with a known malignancy elsewhere, or for cancer screening. For this purpose, the reports of a total of 18,119 subjects were reviewed. The medical records of identified cases were reviewed to determine whether the following inclusion/exclusion criteria were met. PET/CT images of patients with “Paget” identification in the reports were first evaluated by a nuclear medicine specialist (FU) and then by a radiologist experienced in the skeletal system (FEU) to get a second opinion. Patients who provided these features were included in this study. Exclusion criteria were the presence of a lesion with increased or physiological F-18 FDG uptake that was not radiologically suggestive of Paget's. Patients were excluded from this study if clinical data were not complete, or viewed with Ga-68 PSMA, Ga-68 DOTATE or NAF PET/CT.
PET/CT Images
PET/CT imaging was performed using a combined whole-body PET/CT system (Discovery STE; GE Medical Systems, Milwaukee, WI). All patients fasted with oral contrast hydration for at least 6 h (blood glucose level <200 mg/dL) prior to imaging, which was started approximately 60 minutes after intravenous injection of 370–555 MBq (10–15 mCi) of FDG. After a low-dose CT study for attenuation correction and precise anatomical localization without contrast enhancement, patients were examined from the top of the skull to the upper thigh, using a three-dimensional mode with a 3-min acquisition and six or seven bed positions. In the presence of bone lesions, all leg images were also added.
Image Analysis
The incidentally found lesions were re-evaluated by an experienced radiologist. The distribution, localization, mono/polyostatic nature and stage (lytic / blastic / mixed) of the lesions were evaluated.
The PET/CT images were reviewed by one of the authors (FU, a board-certified nuclear medicine physician with over 10 years of experience in PET/CT imaging). The distribution of bones affected by PD in the patient group varied considerably. Control of a healthy bone area and metabolic evaluation was preferred according to the localization of the primary Paget's bone lesion (humerus in the presence of pelvic and/or vertebral lesion, femur in the presence of primary lesion in the humerus, and vertebrae in the presence of extensive PD). Paget bone lesions radiologically suggestive and approved by the radiologist were defined either when the FDG uptake had increased more than that of background activity / the surrounding normal tissue or when a lesion revealed an SUVmax value of more than 2.5 g/mL. F-18 FDG accumulation was analysed semi-quantitatively by calculating the maximum standardized uptake value (SUVmax) in the regions of interest placed over the suspected lesions.
Standardized uptake values (SUVs) were calculated as follows: SUV = radioactivity in ROI (Bq/ml) × lean body mass (kg) / injected radioactivity (Bq).
The values of SUVmax and SUVmean in the liver and spleen, which are hematopoietic organs, and bone lesions containing Paget were also compared.
Statistical analysis
Data analysis was conducted with IBM SPSS Statistics 22. Descriptive analysis was carried out to assess the patients’ demographic and clinical characteristics. Continuous variables were expressed as the mean ± standard deviation (SD), and analysed by Student t test and Mann-Whitney U test. ROC analysis was performed for differential diagnosis of Paget lesion and normal bone. p<0.05 were considered as statistically significant.
RESULTS
Of the 18,119 studies, 69 (~0.0038%) had the word "Paget" associated with PET/CT reports. Re-examining 69 reports containing the word "Paget"; images of 10 missed, 5 reports belonged to Ga-68 PSMA and 2 reports of NaF imaging, while 7 cases examined by radiology were not considered Paget. As a result, 45 patients, 33 men and 12 women, whose data were available and accepted as PD, were included in this study. The mean age was 68.9 ± 8.6 in male patients and 59.7 ± 9.3 in females, the difference was statistically significant (p= 0.005). While 18.6% of the patients included in the study were using alcohol, 51.6% were smoking. Nineteen of the patients were farmers, 13 were housewives, and 23 were from other professions (cook, mine worker, furniture maker, truck driver, etc.).
Bone scintigraphy was available for nine patients. When images were re-evaluated, images of 8 cases were consistent between bone scintigraphy and F-18 FDG PET/CT. However, in only one case, more lesions (in costa and occipital bone) were identified at bone scintigraphy.
Sixteen (35.6%) of the patients had monostotic disease and 29 (64.4%) had polyostotic disease (Fig. 1). Having single or multiple bone involvement was not statistically significant (p>0.5). When the patients were divided into two groups as monostotic and polyostotic, there was no statistically significant difference between their biochemical parameters (Table 1).
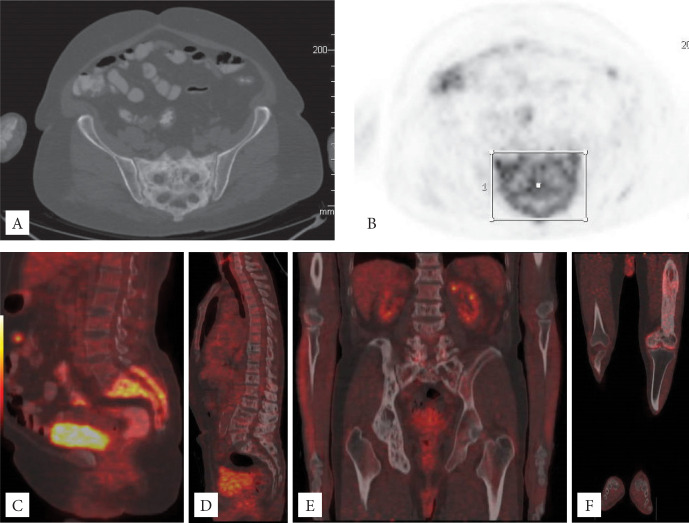
Figure 1.
A-B-C: 61-year-old female patient, monostatic PD (SUVmax 11; HU 128) with significant monostatic FGD accumulation in the sacrum (axial CT and PET images and sagittal fusion images). D-E: A 68-year-old male patient was diagnosed with thyroid cancer. It has polyostatic involvement in the thoracolumbar vertebrae, sacrum, right ilium, ischium and pubis, and exhibits low FDG affinity (vertebra SUVmax 3.4 and HU 282, sacrum SUVmax 2 and HU 176, right hemipelvis SUVmax 1.9 and HU 217) (Sagittal and coronal fusion image’). F: A 61-year-old male patient with a head and neck tumor. Polyostatic PD with involvement of both ilium, left ischium and pubis, entire left femur, and right femoral head and neck. There is increased FDG accumulation in the left femur (SUVmax 3.6 and HU 319).
Table 1.
Comparison of biochemical data of patients divided into two groups as mixed and blastic phases, and monostatic and polyostatic
| Parameters (mean ± Std. Deviation) |
According to affected bone | According to phases | ||
|---|---|---|---|---|
| Monostatic | Polyostatic | Mixed Phase | BlasticPhase | |
| n | 16 | 29 | 36 | 9 |
| ALP* | 186 ± 154 | 150 ± 72 | 133 ± 57 | 370 ± 229 |
| Ca mg/dl | 9 ± 0.6 | 9 ± 1.5 | 9.04 ± 0.99 | 9.28 ±0.94 |
| Phosphate (mg/dl) | 3.3 ± 0.7 | 3.7 ± 0.7 | 3.35 ± 0.69 | 3.77 ±0.72 |
| PTH pg/ml | 113.7 ± 94 | 34£ | 95.5 ±107 | 128.4 ±0.85 |
| 25 OHD | 21.6 ± 1.3 | 6.8£ | 17.55 ± 7.24 | 22.27 ±0.93 |
| Uric acid mg/dl | 5.9 ± 1.4 | 5.6 ± 2.4 | 5.66 ± 2.07 | 6.23 ± 0.79 |
| Osteocalcin ng/ml | 19.8 ±8 | 9.1£ | 11.62 ± 3.5 | 25.4 |
| Creatinine* mg/dl | 1.46 ± 1.5 | 0.8 ± 0.17 | 1.03 ± 0.62 | 2.47 ± 2.86 |
| Urea mg/dl | 42 ± 19 | 35 ± 12 | 38 ± 17 | 51 ± 19 |
| Albumin g/dl | 3.8 ± 0.57 | 3.9 ± 0.8 | 3.83 ± 0.65 | 3.98 ± 0.59 |
| Fasting blood glucose mg/dl | 115 ± 31 | 122 ± 52 | 118±39 | 113±36 |
| CRP mg/dl | 3.3 ± 4.3 | 0.59 ± 0.45 | 2.75±4.06 | 1.07±1.21 |
| Sedimentation mm/h | 58 ± 37 | 18 ± 17 | 43.4±41.6 | 62±10.6 |
| WBC cells/mm3 | 7544.5 ± 2936.4 | 11373 ± 13961.9 | 9345.2±8627.1 | 7238±3952.2 |
| Hb g/dl | 12.1 ± 2.3 | 12.9 ± 2.5 | 12.6±2.2 | 11.1±3.1 |
| Htc % | 36.9 ± 6.3 | 37.9 ± 6.9 | 37.7±5.8 | 34.6±9.4 |
| Platelets x103/mm3 | 296 ± 115 | 258 ± 60 | 278±93 | 311±149 |
: p<0.05 (Indicates of statistically significant difference). Statistically significant results are in bold. £: This is only single case data.
When these 45 patients were examined radiologically, they were divided into 2 stages as mixed and blastic. No patients were detected in the pure lytic stage. According to the radiological appearance of the patients, 36 were in the mixed stage, and 9 were in the blastic stage. When the effect of the disease stage being mixed or blastic on biochemical parameters was examined, it was observed that the parameters were high in the blastic stage, but only the difference in ALP and creatinine values was found to be statistically significant (Mixed / blastic ALP: 133 vs. 370; creatinine 1.03 vs. 2.47) (Table 1).
SUVmax, SUVmean and HU data according to the stage of the patients are presented in the Table 2. No statistically significant difference was found in the parameters of the patients at these two different stages (Mann–Whitney U test; P >0.05). However, when the lesions of the patients were compared with their control bone values, SUVmax, SUVmean and HU values were statistically significantly higher in the pagetoid bones (Student t test; p<0.001) (Table 2).
Table 2.
Comparison of metabolic parameters according to disease stage, and patient and healthy (control) bone structures in PET/CT
| According to phases | According to control bone | |||||
|---|---|---|---|---|---|---|
| Mixed Phase (n=36) |
Blastic Phase (n=9) |
p | Paget Bone (n = 45) |
Control Bone (n = 45) |
p | |
| SUVmax | 4.96±2.63 | 5.67±2.16 | ns | 5.1 ± 2.54 | 2.09 ± 0.61 | 0.000 |
| SUVmean | 1.28±0.58 | 1.6±0.6 | ns | 1.34 ± 0.59 | 0.79 ± 0.31 | 0.000 |
| HU | 133.17 ± 130.45 | 58.67±210.23 | ns | 118.27 ± 149.94 | 67.07 ± 132.84 | 0.000 |
ns = nonsignificant.
Correlations of SUVmax and SUVmean of Pagetoid bone, organ and biochemical parameters are demonstrated at Table 3. When Paget bone lesions were compared with liver and spleen parameters, it was observed that the lesion’s SUVmax and SUVmean values were positively correlated with both liver and spleen values. When the relationship between biochemical parameters and pagetoid bone’s SUVmax and SUVmean values, a significant positive correlation was found between CRP, sedimentation and platelet values with Pagetoid bone’s values (Table 3). As SUVmax and SUVmean increase, Serum, CRP and Platelet values increase. No significant correlation was found between other parameters and pagetoid bone lesions.
Table 3.
Correlations of SUVmax and SUVmean of Pagetoid bone, organ and biochemical parameters
| SUVmax | SUVmean | ||||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Organ parameters | |||||
| Liver | 0.290 | 0.053 | 0.342 | 0.021* | |
| Spleen | 0.296 | 0.049* | 0.228 | 0.131 | |
| Biochemical parameters | |||||
| CRP | 0.594 | 0.019* | 0.750 | 0.001* | |
| Sedimantation | 0.534 | 0.074 | 0.532 | 0.075 | |
| Plt | 0.495 | 0.004* | 0.580 | 0.001* | |
Indicates of statistically significant difference.
When HU, biochemical parameters were compared with FDG PET/CT values, the only significant negative correlation was between ALP and HU (r =0.737, p=0.001).
When we performed the ROC analysis to use the SUVmax, SUVmean and HU for differential diagnosis of Paget lesion and normal bone, we found the cut-off point of 2.55 (Sensitivity 91% and specifity 84%) (Figure 2). The statistical data obtained for SUVmean and HU are also presented in Figure 2.
Figure 2.
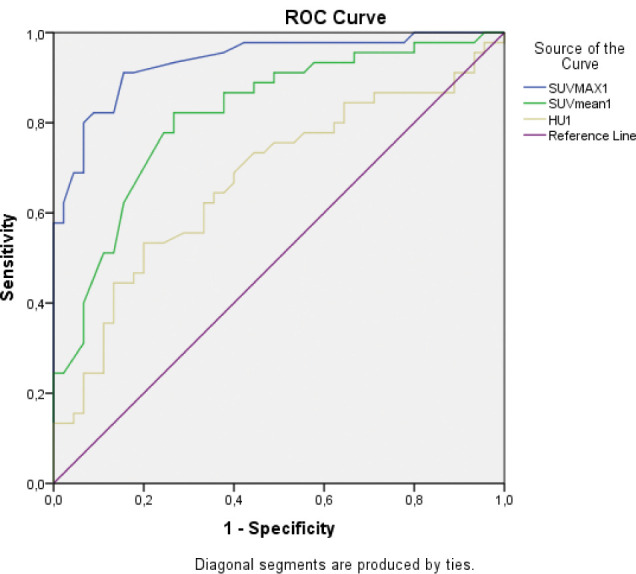
Receiver-operating curve analysis. Cut-off of 2.55 for SUVmax, 0.920 for SUVmean and 108.5 for HU yielded sensitivity of 97%, 82, 69 and specificity of 90% (area under curve, 0.936, 0.819, and 0.674, respectively).
DISCUSSION
PET/CT with F-18 FDG is not a cancer specific imaging agent. It also plays an important role in the evaluation of bone pathology. However, differential diagnosis of benign and malignant FDG-avid lesions such as bone Paget lesions is an assertive approach. When this differentiation is possible, it ensures proper patient management, including avoiding unnecessary additional invasive procedures such as bone biopsy.
Primary malignant bone tumours are rare and heterogeneous groups. They originate from bone tissue consisting of cartilage, osteoid and fibrous tissue and bone marrow elements, whereas cancer-related bone metastases are much more common and frequently encountered in the clinic. Survival rates are low in these patients. Radiological datas are of limited value as metastases cannot be detected without more than 50% structural changes in bone. Commonly used in oncological patients, F-18 FDG shows varying levels of uptake in both primary and secondary bone lesions, often presenting a diagnostic challenge for clinicians, radiologists, and nuclear medicine specialist. Therefore, FDG uptake cannot be used reliably to determine or exclude malignancy in primary osseous lesions. It is clinically valuable to distinguish such incidental lesions, especially in patients screened for other purposes. Evaluation of morphological CT findings together with the knowledge of varying levels of FDG PET uptake in malignant bone tumours and incidental benign bone lesions supports the differential diagnosis in patients. Thus, it guides the clinical management of patients by avoiding unnecessary examinations and invasive procedures.
In our retrospective study of 45 patients, we found that the metabolic parameters (SUVmax and SUVmean) and HU values obtained by F-18 FDG PET/CT were insufficient to distinguish the disease’s stage. However, when the lesions of the patients were compared with their control bone values; SUVmax, SUVmean and HU values were statistically significantly higher in the pagetoid bones. With the analysis, we found the optimal SUVmax cut-off to be 2.55. We agree that these cut-off values are not universally applicable to all PET centres because SUV is affected by many factors. The different intensity of metabolic appearance in our patient group is due to the variable nature of FDG uptake in PD. The potential for fusion imaging to differentiate PD between other pathological bone findings should not be forgotten. Thus, we can distinguish benign lesion by comparing it with CT and the aforementioned cut-off value.
The three stages of the disease process results in exhibition of variability and often characteristic radiographic manifestations of the disease. As a result of this anarchic bone behaviour, which produces irregular new bone (mosaic), an increased or decreased external bone contour and a narrowed or enlarged bone marrow cavity are seen, in contrast to bone enlargement, which has long been considered a universal feature of the disease. The mean extension rate of the lesions with in the cortical bone was 8 ± 0.5 mm/year (25). There is notable variability in the rate of disease progression from one patient to another or in a single individual.
Underlying osteoclastic and osteoblastic changes in bone(s) are reflected in the patient's serum and urine laboratory values. Biochemical parameters that help diagnose PD are (i) elevated serum alkaline phosphatase (ALP) level due to intense osteoblastic activity, (ii) increased urinary total hydroxyproline excretion secondary to bone resorption, (iii) usually normal serum calcium level unless a fracture or secondary hyperparathyroidism develops due to extensive bone turnover. ALP activity is a general indicator of the bone formation rate in skeletal tissue. Bone-specific ALP is synthesized by osteoblasts and is assumed to be involved in the calcification of the bone matrix; however its precise role in the formation process is unknown. ALP levels can be normal when there is only a small focus, monostatic PD or after successful medical treatment. Therefore, it is inappropriate to conclude that normal biochemistry indicates the absence of active PD when skeletal involvement is limited. In our study population, when the biochemical parameters were compared according to the number of affected bones, no data were found to differ, whereas ALP and creatinine levels differed according to the stage of the disease. In PD, osteoblasts action following bone destruction by the uncontrolled activity of osteoclasts caused increased amount of serum ALP activity. These results were consistent with the nature of the disease and the previous study (26). However, there is a need for comprehensive prospective studies comparing all biochemical data with disease stage and the number of involved bones. The biochemical data of the patients were compared with the pagetoid bone SUVmax, SUVmean and HU values. While there is a significant positive correlation with CRP, sedimentation and platelet values, there is significant inverse correlation between ALP and HU. The blastic group has higher ALP, which is also related to the disease. Increased serum and urine levels of hydroxyproline are seen in the lytic phase (related to increased rate of bone resorption) and are an accurate marker of resorptive activity, even in monostotic disease. We could not reach this parameter due to the retrospective study in sufficient cases.
In the study by Cook et al. (26), the largest series on PD, it compares bone scintigraphy and F-18 FDG PET images of 18 patients. Thirteen of these patients had been treated with disodium pamidronate (range 1 to 48 months) prior to screening. In this study, the metabolic parameters of PET were not available and anatomical correlations were not included. However, most patients with PD had no abnormal F-18 FDG deposition in bones that had proved to be affected by MDP bone scintigraphy. FDG uptake was low in most cases and this appearance was inconsistent with bone scintigraphy. ALP levels were found to be significantly higher in FDG positive patients. However, this study is fundamentally different from our study because these patients were receiving bisphosphonate therapy. The reason for this may be that the metabolic response appears earlier in F-18 FDG PET compared to bone scintigraphy, or it may be a reflection of the already low metabolic uptake of F-18 FDG in PD. The response to drug therapy in PD is a long process, and the response to bisphosphonate therapy may differ both among affected individuals and in different regions in the same individual. Treatment response assessment is a very different study and PET/CT can provide comprehensive whole-body imaging with anatomical correlation in this context. For these reasons, serial studies are needed.
When the literature on PD was searched for F-18 FDG PET, studies on incidental cases were found (9-23). Details of these cases are presented in Table 4.Very different results were observed in these studies. Although some cases did not have a SUVmax value, most of them exhibited an increased metabolic appearance. Of these 15 cases, only 4 were confirmed by biopsy, and only two cases were polyostatic. Different results were presented between whether the case was monoostatic or polyostatic and ALP and SUVmax values. It is a fact that these studies do not reflect the results of a large group of cases, since all of them contain results presented on a single case. A large series of cases like our study has not been found in the literature. Therefore, it is not possible to compare the outcome of such extensive cases with the one-to-one case series. However, individual case results are also valuable, and guiding. The variable FDG activity has been demonstrated in PD (Table 4). While the majority of patients with PD showed no abnormal F-18 FDG uptake, a few patients had marked F-18 FDG uptake, mimicking malignant lesions. However, F-18 FDG PET/CT played an important role in excluding bony metastases or sarcomatous changes of Paget’s disease (27-29), F-18 FDG PET/CT can be used for separation, taking into account the defined visual analysis and metabolic values.
Table 4.
| SUVmax value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative (≤2.5) | Positive (>2.5) | No data available | FDG uptake | Bone scan | PET vs. PET/ CT | Confirmation with biopsy | Poli vs. monostatic | ALP | Malignancy | |
| Schmid et al. (2002) (9) |
+ | Increased | + | PET | No | Mono | No data available | Tongue | ||
| Spieth et al. (2003) (10) |
+ | Increased | No | PET | No | Mono | Normal | Colorectal and Lung | ||
| Bhargava et al. (2005) (11) |
+ | Normal | + | PET/CT | No | Mono | Normal | Colorectal | ||
| Mahmood et al. (2008) (12) |
+ | Faint | No | PET/CT | No | Mono | No data available | Malignant mesothelioma |
||
| Aslan et al. (2009) (13) |
+ | Normal | + | PET | + | Mono | No data available | Thyroid | ||
| Park et al. (2010) (14) |
+ | Faint | + | PET/CT | No | Mono | Increased | No | ||
| Woo et al. (2010) (15) |
+ | Faint | + | PET/CT | No | Mono | Increased | No | ||
| Chakraborty et al. (2011) (16) | + | Normal | + | PET/CT | No | Mono | Increased | Bladder | ||
| Mena et al. (2013) (17) |
+ | Faint | + | PET/CT | No | Mono | No data available | Rectum | ||
| Adams et al. (2015) (18) |
+ | Increased | + | PET/CT | + | Poli | Increased | Lymphoma | ||
| Kamaleshwaran et al. (2015)(19) | + | Increased | No | PET/CT | No | Mono | Increased | Lung | ||
| Sharma et al. (2015) (20) |
+ | Faint | No | PET/CT | + | Mono | Increased | No | ||
| SáPinto et al. (2017) (21) |
+ | Normal | + | PET/CT | No | Mono | Normal | Lung | ||
| Sasikumar et al. (2017) (22) | + | Mild | No | PET/CT | + | Poli | No data available | Rectum and prostate | ||
| Zhong et al. (2017) (23) | + | Increased | + | PET/CT | No | Mono | No data available | Multiple myeloma | ||
There was no case suggestive of osteosarcoma transformation due to PD in our study group. Osteosarcoma secondary to PD is a rare but aggressive malignancy with a worse prognosis than traditional osteosarcoma (7). The presence of pathological fractures and the increase in ALP level compared to previous values are factors that cast doubt on the osteosarcoma transformation process (28). Many different histological subtypes of sporadic cases have been reported in the literature. Depending on the histopathological subtype of the tumour, the lesion may not exhibit metabolic involvement in F-18 FDG PET/CT (27, 29, 30). Patients typically presented with a devastating lytic lesion and associated with soft tissue mass, sarcomatous degeneration tend to have a medullary origin, and the tumour mass envelops the bone in a circular fashion and particularly prefers the pelvis, humerus, and femur. Metastases due to various malignancies can also be seen in Pagetoid bone and their appearance can be quite variable. MRI is helpful in demonstrating pathological bone marrow infiltration, which helps distinguishing Paget's osteosarcoma from non-neoplastic mimics of malignant degeneration (30).
F-18 FDG is not specific for cancer and can accumulate in inflammatory processes. However, F-18 FDG accumulation in benign lesions and inflammatory processes may be different from malignancy, because of the different level and extent of GLUT and hexokinase expressions in normal tissue, inflammatory lesions, and cancer. For these reasons, when the Pagetoid bone SUVmax and SUV mean values are compared with the biochemical parameters; the significant positive correlation with CRP, sedimentation and platelet values may have an effect on the retention mechanism of FDG. Similar correlations were found in haematological organ parameters, lesion SUVmax and SUVmean values were significantly increased in both liver and spleen values. In this regard, cell culture studies will be appropriate.
Polyostotic disease (65–90%) is more frequent than monostotic disease (7). The distribution ratio of patients in our study group (poliostotic/monoostotic (64.4 vs 35.6%)) was similar to previous studies. The fact that the patients in our study group were male predominant, were seen at an advanced age, and smoking was common. These features are consistent with previous studies. However, since this study is not an epidemiological study, it is not correct to make a comparison.
Limitations of the study
Our study has limitations due to the outdated data, such as the lack of biochemical data, the inability to follow up the patient because our hospital serves a wide area as a regional hospital. Another limitation was that all patients who were considered to be diagnosed with PD did not undergo further investigation for the diagnosis and treatment of the disease.
Recent studies have described the utility of F-18 FDG PET/CT in monitoring treatment with bisphosphonates in patients with PD, particularly in the monostotic form. It would be appropriate to conduct a prospective study in a large patient group on this subject.
Because of the specificity of the radiological appearance and the diagnosis of concomitant malignancy, histopathological evaluation was not performed in these patients.
In conclusion, PET/CT is widely used especially in oncological diseases, however FDG is not a cancer-specific agent, and its uptake may increase in some physiological and benign conditions. Being aware of the appearance and presence of PD, especially in patients with a diagnosis of malignancy, should prevent false positive metastasis. In this patient group, it is of special importance to make a correct diagnosis in order to make correct patient management and to save patients from unnecessary treatments, such as the avoidance of unnecessary additional invasive tests as bone biopsy.
Acknowledgements
We thank Mr. M.Arda Üstün for linguistic analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Cooper C, Dennison E, Schafheutle K, Kellingray S, Guyer P, Barker D. Epidemiologyof Paget’s disease of bone. Bone. 1999;24([Suppl 5]):3S–5S. doi: 10.1016/s8756-3282(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 2.Ralston SH, Corral-Gudino L, Cooper C, Francis RM, Fraser WD, Gennari L, Guañabens N, Javaid MK, Layfield R, O'Neill TW, Russell RGG, Stone MD, Simpson K, Wilkinson D, Wills R, Zillikens MC, Tuck SP. Diagnosis and Management of Paget's Disease of Bone in Adults: A Clinical Guideline. J Bone Miner Res. 2019;34(4):579–604. doi: 10.1002/jbmr.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whyte MP. Clinical practice. Paget's disease of bone. N Engl J Med. 2006;355(6):593–600. doi: 10.1056/NEJMcp060278. [DOI] [PubMed] [Google Scholar]
- 4.Singer FR. The evaluation and treatment of Paget's disease of bone. Best Pract Res Clin Rheumatol. 2020;34(3):101506. doi: 10.1016/j.berh.2020.101506. [DOI] [PubMed] [Google Scholar]
- 5.Davie M, Davies M, Francis R, Fraser W, Hosking D, Tansley R. Paget's disease of bone: a review of 889 patients. Bone. 1999;24(5 Suppl):11S–12S. doi: 10.1016/s8756-3282(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 6.Siris ES. Paget's disease of bone. J Bone Miner Res. 1998;13(7):1061–1065. doi: 10.1359/jbmr.1998.13.7.1061. [DOI] [PubMed] [Google Scholar]
- 7.Smith SE, Murphey MD, Motamedi K, Mulligan ME, Resnik CS, Gannon FH. From the archives of the AFIP. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation. Radiographics. 2002;22(5):1191–1216. doi: 10.1148/radiographics.22.5.g02se281191. [DOI] [PubMed] [Google Scholar]
- 8.Mirra JM, Brien EW, Tehranzadeh J. Paget's disease of bone: review with emphasis on radiologic features, Part I. Skeletal Radiol. 1995;24(3):163–171. doi: 10.1007/BF00228918. [DOI] [PubMed] [Google Scholar]
- 9.Schmid RA, Schwenzer K, Weiss M, Rock C, Rink FJ, Hahn K, Dresel S. Monostotic Paget's Disease of a cervical vertebra: differential diagnosis with F-18 FDG positron emission tomography using a coincidence technique and with Tc-99m dicarboxy propane diphosphonate. Clin Nucl Med. 2002;27(7):537–538. doi: 10.1097/00003072-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Spieth ME, Kasner DL, Manor WF. Positron emission tomography and Paget disease: hot is not necessarily malignant. Clin Nucl Med. 2003;28(9):773–774. doi: 10.1097/01.rlu.0000082671.73091.db. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava P, Naydich M, Ghesani M. Normal F-18 FDG vertebral uptake in Paget's disease on PET scanning. Clin Nucl Med. 2005;30(3):191–192. doi: 10.1097/00003072-200503000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood S, Martinez de Llano SR. Paget disease of the humerus mimicking metastatic disease in a patient with metastatic malignant mesothelioma on whole body F-18 FDG PET/CT. Clin Nucl Med. 2008;33(7):510–512. doi: 10.1097/RLU.0b013e318177928a. [DOI] [PubMed] [Google Scholar]
- 13.Arslan N, Guvenc I, Alagoz E, Yıldırım D, Deveci S, Ozguven MA. Paget's Disease mimicking vertebral metastases. Balkan Military Medical Review. 2009;12(2):95–99. [Google Scholar]
- 14.Park ET, Kim SE. Radiography, Bone Scan, and F-18 FDG PET/CT Imaging Findings in a Patient with Paget's Disease. Nucl Med Mol Imaging. 2010;44(1):87–89. doi: 10.1007/s13139-009-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo JH, Kim S, Choi SJ, Lee YH, Ji JD, Song GG. Diagnosis of Paget's disease of the pelvis using F-18 FDG PET/CT. Int J Rheum Dis. 2010;13(4):e51–e54. doi: 10.1111/j.1756-185X.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty D, Mittal BR, Kamaleshwaran KK, Kashyap R, Bhattacharya A, Kumar S. Urinary bladder carcinoma associated with Paget's disease of skull: Imaging findings on Tc99m-MDP bone scintigraphy, F18-Fluoride PET/CT and F18-FDG PET/CT. Indian J Nucl Med. 2011;26(1):42–43. doi: 10.4103/0972-3919.84614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mena LM, Hernández AC, Gallego M, Martínez T, Contreras JF. Hallazgo casual de enfermedad de Paget en un estudio PET/TC con 18F-FDG en pacientecon cáncer de recto [Incidental detection of Paget disease on 18F-FDG PET/CT scan in a patient with rectal cancer] Rev Esp Med Nucl Imagen Mol. 2013;32(2):117–118. doi: 10.1016/j.remn.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Adams HJ, de Klerk JM, Fijnheer R, Dubois SV, Nievelstein RA, Kwee TC. Paget's disease of the bone mimicking lymphomatous bone marrow involvement at FDG-PET. Am J Hematol. 2015;90(3):269–270. doi: 10.1002/ajh.23921. [DOI] [PubMed] [Google Scholar]
- 19.Kamaleshwaran KK, Natarajan S, Shibu D, Malaikkal A, Shinto AS. Paget's disease of pelvis mimicking metastasis in a patient with lung cancer evaluated using staging and follow-up imaging with fluorine-18 fluorodeoxyglucose-positron emission tomography/computed tomography. Indian J Nucl Med. 2015;30(2):151–153. doi: 10.4103/0972-3919.152980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Chatterjee P. Response monitoring to bisphosphonate therapy in monostotic paget disease using (18)F-FDG PET/CT. Clin Nucl Med. 2015;40(6):499–500. doi: 10.1097/RLU.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 21.SáPinto A, Alves VM, Oliveira A, Castro RH, Pereira JG. Incidental finding of a monostotic form of Paget Disease of the scapula in a lung cancer patient. Radiography (Lond) 2017;23(3):e72–e74. doi: 10.1016/j.radi.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Sasikumar A, Joy A, Pillai MR, Raman V, Vasudevan A, Madhavan J. Erratumto: A rare case of rectal carcinoma and prostate carcinoma with coexistent Paget's disease mimicking bone metastases in both 18F-FDG and 68Ga PSMA PET/CT. Eur J Nucl Med Mol Imaging. 2017;44(4):738–739. doi: 10.1007/s00259-017-3633-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhong X, Ye Y, Ou X. Impressive Paget Disease of the Lumbar Spine Masks the Coexisting Multiple Myeloma. Clin Nucl Med. 2017;42(9):e417–e421. doi: 10.1097/RLU.0000000000001733. [DOI] [PubMed] [Google Scholar]
- 24.Kwee TC, de Klerk JMH, Nix M, Heggelman BGF, Dubois SV, Adams HJA. Benign Bone Conditions That May Be FDG-avid and Mimic Malignancy. SeminNucl Med. 2017;47(4):322–351. doi: 10.1053/j.semnuclmed.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Reid IR. Management of Paget's disease of bone. Osteoporos Int. 2020;31(5):827–837. doi: 10.1007/s00198-019-05259-1. [DOI] [PubMed] [Google Scholar]
- 26.Cook GJ, Maisey MN, Fogelman I. Fluorine-18-FDG PET in Paget's disease of bone. J Nucl Med. 1997;38(9):1495–1497. [PubMed] [Google Scholar]
- 27.Davis MA, Scalcione LR, Gimber LH, Thompson RB, Avery RJ, Taljanovic MS. Paget sarcoma of the pelvic bone with widespread metastatic disease on radiography, CT,MRI, and 18F-FDG PET/CT with pathologic correlation. Clin Nucl Med. 2014;39(4):371–373. doi: 10.1097/RLU.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 28.Wick MR, Siegal GP, Unni KK, McLeod RA, Greditzer HG., 3rd Sarcomas of bone complicating osteitis deformans (Paget's disease): fifty years' experience. Am J Surg Pathol. 1981;5(1):47–59. doi: 10.1097/00000478-198101000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Bush LA, Toresdahl B, Hoch B, Chew FS. Falsely Negative F-18 FDG PET of Osteosarcoma Arising In Paget Disease. Radiol Case Rep. 2015;4(3):295. doi: 10.2484/rcr.v4i3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilden W, Saifuddin A. An update on imaging of Paget's sarcoma. Skeletal Radiol. 2021;50(7):1275–1290. doi: 10.1007/s00256-020-03682-8. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Chatterjee P. Response monitoring to bisphosphonate therapy in monostotic paget disease using (18)F-FDG PET/CT. Clin Nucl Med. 2015;40(6):499–500. doi: 10.1097/RLU.0000000000000721. [DOI] [PubMed] [Google Scholar]