Abstract
Aim
To describe and correlate electroretinographic responses with clinical and angiographic findings in retinal vasculitis (RV).
Methods
Medical records of patients with diagnosis of RV at a tertiary eye centre from December 2017 to May 2021 were reviewed. Cases in which fluorescein angiography (FFA) and full field electroretinography (ffERG) were done within 1 month were included. FFAs were graded according to the Angiography Scoring for Uveitis Working Group from 0 to 40, where 0 is normal. A novel ffERG grading system was implemented where individual waves were graded for timing and amplitude and general ffERG score was determined with 6 being a perfect score.
Results
20 patients (34 eyes) were included. Mean age was 43.9±19.8 years; 70% were female. Median best-corrected visual acuity was 0.8 (0.08–1). Mean FFA score was 12.6±6.5. Median general ffERG score was 5 (0–6). 68% and 91% of eyes had responses with general ffERG scores ≥5 and 4, respectively. Flicker timing was most commonly affected.
FFA scores weakly correlated with delayed photopic cone b-wave and flicker timing (p=0.03 and 0.016, respectively). Vitreous haze moderately correlated with delayed cone b-wave timing (p<0.001), delayed flicker timing (p=0.002) and weakly correlated with lower flicker amplitude (p=0.03). Underlying systemic disease was associated with poor ffERG responses.
Conclusion
In this study, RV was not frequently associated with severe global retinal dysfunction Higher FFA scores, and vitreous haze grading were weakly, but significantly, correlated with cone-generated ffERG responses.
INTRODUCTION
Retinal vasculitis is defined as inflammation of retinal vessels, including arterioles, venules and capillaries. It can be isolated or associated with other categories of ocular inflammation such as intermediate uveitis or panuveitis. Underlying systemic diseases have been associated with retinal vasculitis in 25%–55% of cases.1 2 Fundus fluorescein angiography (FFA) is routinely used for diagnosis and evaluation of retinal vasculitis.3 Complications of retinal vasculitis include macular oedema, optic nerve inflammation, retinal ischaemia and neovascularisation.4
Full field electroretinography (ffERG) is an objective method which evaluates global retinal function by measuring the retinal electrical responses to light stimulation. It is useful for diagnosis and monitoring of a wide range of inherited and acquired retinal diseases.5 The initial negative wave (a-wave) is generated by the photoreceptors in the dark-adapted state and by both photoreceptors and Off-bipolar cells in the light-adapted state.6 7 The subsequent positive wave (b-wave) is generated by the inner retina, mainly bipolar and Muller cells. The status of retinal dark or light adaptation can differentiate between the rod and cone pathways, respectively. Flicker, which is a fast light stimulation at a rate of 30 Hz, is also used to isolate cone pathway responses as rods cannot respond to fast rates of light stimulation.5 8 ffERG has been used to evaluate retinal function in ocular inflammation. Studies have shown that ocular inflammation is associated with variable ffERG responses that were normal, subnormal and less commonly, extinguished.9-12 In the index study, we aim to evaluate retinal function in cases with retinal vasculitis as well as correlate between the severity and location of inflammation, and ffERG findings.
MATERIALS AND METHODS
Setting of the study and subject selection
We conducted a retrospective study of patients who were diagnosed with retinal vasculitis between December 2017 and February 2021 at Byers Eye Institute, Stanford (California, USA). The initial cohort of patients was identified using The STAnford Research Repository (STARR) tool. We included patients where ffERG and wide-angle FFA were done within 1 month. We excluded patients (and eyes) with FFA and ffERG done more than 1 month apart, significant media opacity, previous vitrectomy, previous retinal detachment, previous retinal laser, diffuse chorioretinal lesions, multifocal chorioretinal lesions and poor FFA image quality.
FFA grading
We used the Angiography Scoring for Uveitis Working Group (ASUWOG) grading system to quantify the FFA severity from 0 to 40, where 0 is no inflammation.13 The scoring system accounted for optic disc leakage, macular leakage, central non-macular leakage, peripheral capillary leakage, central and peripheral vascular staining, retinal ischaemia and pooling. FFA scores were assigned by two independent graders (HHG and WM). Table 1 details the ASUWOG grading system.
Table 1.
Fundus fluorescein angiography individual signs and total scores per Angiography Scoring for Uveitis Working Group grading system
| Angiographic sign | Maximum score |
|---|---|
| Optic disc hyperfluorescence | 3 |
| Macular leakage | 4 |
| Central retinal vascular staining/leakage | 3 |
| Peripheral retinal vascular staining/leakage | 4 |
| Central capillary leakage (excluding fovea) | 2 |
| Peripheral capillary leakage | 8 |
| Macular ischaemia | 2 |
| Peripheral ischaemia | 4 |
| Neovascularisation of disc | 2 |
| Neovascularisation elsewhere | 2 |
| Pinpoint leaks | 2 |
| Retinal staining/pooling | 4 |
| Total | 40 |
ffERG grading
All patients had ffERG recordings in compliance with the International Society for Clinical Electrophysiology of Vision standard.8 Amplitude (A) and timing (T) of individual ffERG responses were analysed including scotopic 0.01 b-wave (rod) response (A&T), scotopic 3.0 (mixed) a-wave (A) and b-wave (A&T) responses, photopic 3.0 b-wave (cone) response (A&T) and 30 Hz 3.0 photopic cone flicker (A&T) response.
A novel grading method (online supplemental table 1) was used as normative ffERG values vary with patient age, and the same value could not be interpreted similarly across patients with different ages. Amplitude and timing of individual waves were graded based on their corresponding z scores from 0 to 5, where 5 is normal (within 95% of distribution) and 0 is an absent response. The general ffERG score was determined by the mean of all individual scores, with an additional 1 point if oscillatory potentials were present under both scotopic and photopic conditions (0.5 point if present under only one condition). b/a ratios were measured by dividing the absolute amplitude values of scotopic 3.0 mixed b-waves over their corresponding a-waves.
Data collection and outcomes
Clinical and demographical data were collected including presence of concomitant systemic diseases, types of ocular inflammation, best-corrected visual acuity (BCVA), vitreous cell grading according to the Multicenter Uveitis Steroid Treatment Trial criteria,14 vitreous haze grading according to the standardisation of uveitis nomenclature (SUN) criteria.15 Optical coherence tomography (OCT) findings in addition to ffERG readings and FFA data were also collected. All relevant clinical data were collected within 1 month from the ffERG.
Statistical analysis
Descriptive statistics were calculated for the variables of interest. Continuous variables were expressed in mean and SD or median and range. Tests of normality, including Shapiro’s test and histograms, were performed. Pearson’s correlation and Spearman’s correlation were used for data with normal and non-normal distribution, respectively.
Data analysis was performed using RStudio software (V.1.3.1093).
RESULTS
Demographic and baseline clinical criteria
A total of 174 patients were initially identified using the STARR tool. Only 20 patients (34 eyes) met the inclusion and exclusion criteria and were included in the study (figure 1). Mean age (SD) was 43.9±19.8 years and 70% were female. Underlying ocular diagnoses were isolated vasculitis (70%), intermediate uveitis (20%) and panuveitis (10%). Thirty-two per cent of eyes exhibited signs of retinal ischaemia. Fifty per cent of patients did not have associated systemic diseases or infections and were considered idiopathic. Summary of baseline criteria and associated systemic diseases can be found in table 2.
Figure 1.
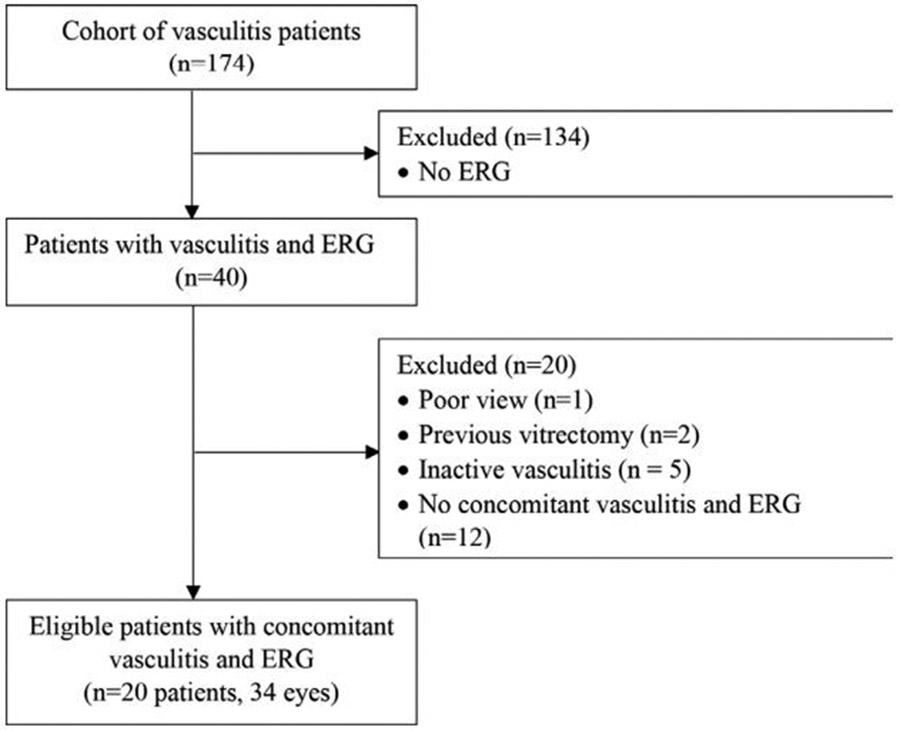
Consolidated Standards of Reporting Trials Cohort Selection Diagram. ERG, electroretinography.
Table 2.
Baseline patient criteria and associated systemic diseases and markers
| Patient | Age range | Sex | Race/ethnicity | Underlying ocular condition | Laterality | Associated systemic disease/marker |
|---|---|---|---|---|---|---|
| 1 | 50 s | F | Hispanic | Panuveitis | OU | HLA B27, tuberculosis |
| 2 | 20 s | F | South Asian | Isolated vasculitis | OU | Takayasu arteritis |
| 3 | 60 s | F | Hispanic | Intermediate uveitis | OU | – |
| 4 | 60 s | F | Non-Hispanic white | Isolated vasculitis | OU | – |
| 5 | 10 s | F | South Asian | Isolated vasculitis | OU | – |
| 6 | 40 s | F | East Asian | Isolated vasculitis | OU | – |
| 7 | 10 s | M | Hispanic | Isolated vasculitis | OD | – |
| 8 | 30 s | F | Hispanic | Intermediate uveitis | OU | – |
| 9 | 70 s | F | Hispanic | Isolated vasculitis | OU | Cold agglutinin disease, CLL |
| 10 | 40 s | F | Non-Hispanic white | Isolated vasculitis | OS | – |
| 11 | 20 s | F | Non-Hispanic white | Isolated vasculitis | OS | Multiple sclerosis |
| 12 | 30 s | M | Non-Hispanic white | Isolated vasculitis | OU | HLA A29 |
| 13 | 50 s | F | East Asian | Intermediate uveitis | OU | – |
| 14 | 30 s | M | East Asian | Isolated vasculitis | OU | HLA B27, antiretinal antibodies |
| 15 | <10 | M | Hispanic | Panuveitis | OD | – |
| 16 | 20 s | F | Non-Hispanic white | Intermediate uveitis | OU | Sjogren Syndrome |
| 17 | 40 s | F | Non-Hispanic white | Isolated vasculitis | OU | Relapsing polychondoritis |
| 18 | 70 s | M | Non-Hispanic white | Isolated vasculitis | OU | – |
| 19 | 30 s | F | East Asian | Isolated vasculitis | OS | Tuberculosis |
| 20 | 80 s | M | Non-Hispanic white | Isolated vasculitis | OD | Herpetic |
CLL, chronic lymphocytic leukaemia; OD, right eye; OS, left eye; OU, both eyes.
Median (range) BCVA was 20/25 (20/20–20/250). Median (range) vitreous cells and haze were 0 (0–3) and 0 (0–2), respectively. OCT was normal in 59% of eyes. Foveal thinning (15%), epiretinal membranes (12%), macular oedema (9%) and subfoveal deposits (3%) were seen on OCT. Ninety-one per cent of eyes were phakic, 6% were pseudophakic and 3% (one eye) was aphakic. Only four eyes had 1+ nuclear cataract, which was considered non-visually significant. All patients were under treatment except for two patients (three eyes). Online supplemental table 2 shows baseline clinical findings.
FFA gradings
Mean (SD) overall FFA score was 12.6 (6.5). Online supplemental table 3 shows FFA’s overall and individual signs’ scores. Figure 2 shows the frequencies of overall FFA scores in our cohort. Overall FFA scores were not statistically different across different underlying ocular diagnoses (p=0.42).
Figure 2.
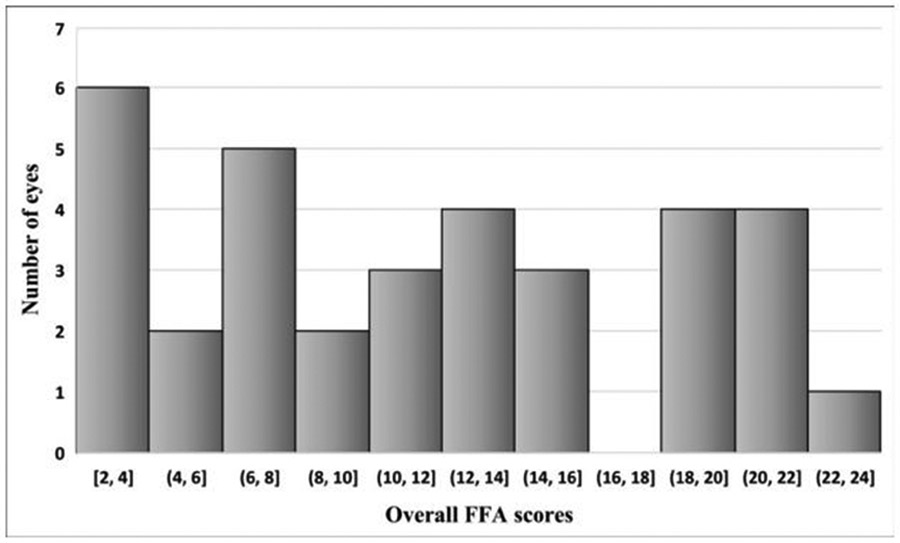
Frequencies of overall FFA scores. FFA, fundus fluorescein angiography.
ffERG gradings
Median (range) overall ffERG score was 5 (0–6). Sixty-eight per cent of eyes showed responses with general scores ≥5 and 91% showed responses with general scores ≥4. Eighteen per cent of eyes showed normal ffERG with perfect score of 6. Overall, our cohort of retinal vasculitis did not show severely abnormal retinal function except in 9% (three eyes of two patients) whose overall ffERG scores were ≤1 (figure 3). Of those, one patient had cryoglobulinaemia and chronic lymphocytic leukaemia and the other had tuberculous uveitis.
Figure 3.
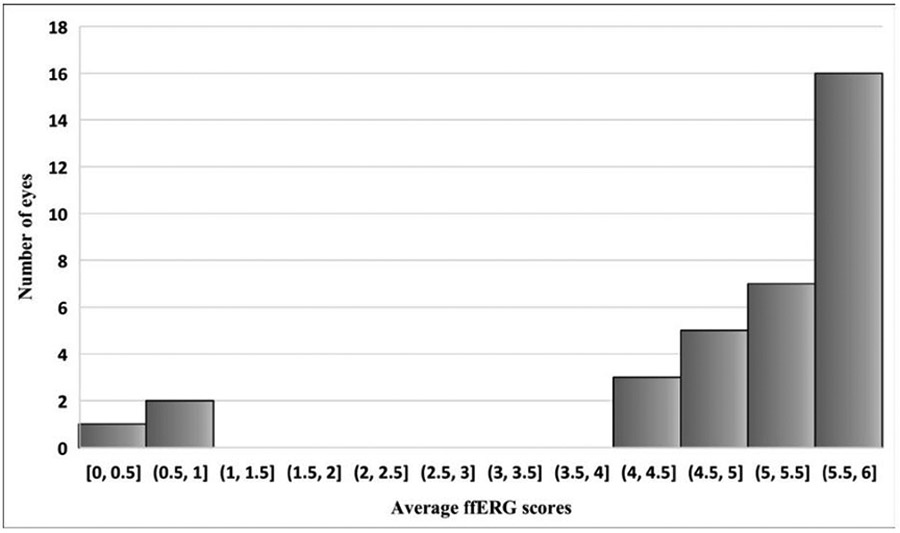
Frequencies of average ffERG scores ffERG, full field electroretinography.
A 3.0 photopic 30 Hz flicker timing was the most commonly affected parameter of the individual ffERG responses with 65% of eyes showing delayed responses and 50% showing severe delay with scores ≤2. A 3.0 scotopic mixed response was the least affected with 79% showing normal amplitude and 94% showing normal timing. Figure 4 shows means of individual ffERG responses in our cohort. b/a ratio was above 1 in all except for one eye. This eye was associated with chronic lymphocytic leukaemia and cryoglobulinaemia, and the electronegative response might be attributed to a form of cancer associated retinopathy. Online supplemental table 4 shows overall and individual ffERG wave scores for each eye. Overall ffERG scores were not statistically different across different ocular diagnoses (P=0.52).
Figure 4.
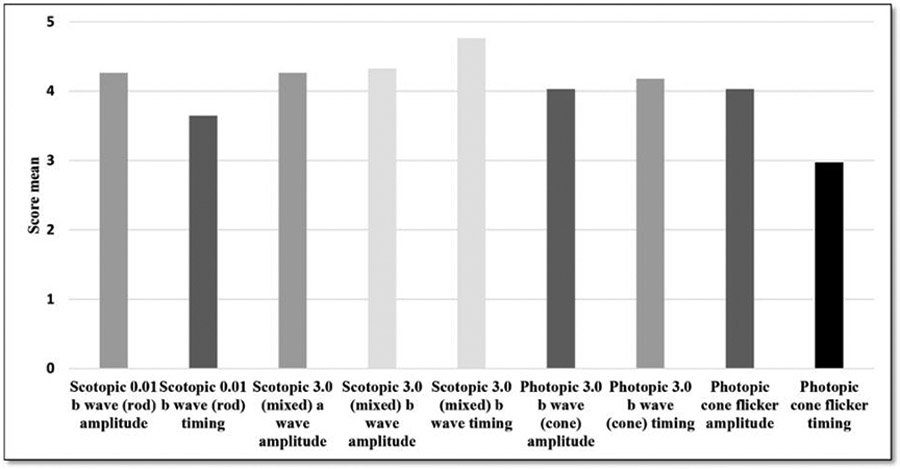
Mean scores of individual ffERG responses. ffERG, full field electroretinography.
Relationship of overall ffERG score with other baseline and clinical criteria
Overall ffERG score did not show significant correlation with overall FFA score (p=0.35). No cut-off value was found in overall FFA score above which ffERG scores were significantly low. Overall FFA score for eyes with normal and abnormal ffERG was 8.2±3.9 and 12.4±6.8, respectively, and the difference was not statistically significant (p=0.18). Vitreous haze and the presence of associated systemic disease showed statistically significant but weak correlation with lower overall ffERG score (Cor=−0.47 to −0.44; p=0.008, 0.014, respectively). Older age, infectious aetiology, vitreous cells, lower BCVA and OCT abnormalities were not associated with lower overall ffERG score. Online supplemental table 5 summarises correlation analysis between overall ffERG scores and different baseline and clinical criteria. b/a ratios were not correlated with the overall ffERG scores or any of the clinical findings.
Correlation analysis between clinical findings with individual ffERG wave scores
Lower BCVA was only weakly correlated with delayed photopic 3.0 b-wave (cone) implicit timing (Cor=−0.37; p=0.046). Higher vitreous haze readings were moderately correlated with delayed photopic 3.0 b-wave (cone) implicit timing (Cor=−0.62; p<0.001), delayed 3.0 photopic 30 Hz flicker implicit timing (Cor=−0.53; p=0.002) and weakly correlated with lower amplitudes of 3.0 photopic 30 Hz flicker (Cor=−0.39; p=0.03). Online supplemental table 6 summarises significant correlation analyses between individual ffERG wave scores and clinical findings and FFA scores.
Correlation analysis between FFA and individual ffERG scores
Higher overall FFA scores showed a significant but weak correlation with delayed 3.0 photopic 30 Hz flicker implicit timings (Cor=−0.43; p=0.016) and delayed photopic 3.0 b-wave (cone) implicit timings (Cor=−0.37; p=0.03). Overall FFA scores were not correlated with any other individual ffERG responses.
Peripheral capillary leakage and macular leakage also showed a significant but weak correlation with delayed 3.0 photopic 30 Hz flicker implicit timing (Cor=−0.42 and −0.39; p=0.018 & 0.028, respectively). Macular leakage in FFA was not necessarily associated with intra or subretinal fluid in OCT. No other individual ffERG response was correlated with any other individual FFA findings.
DISCUSSION
The effect of uveitis on retinal function as evaluated by ffERG has been addressed in different studies.16 However, ffERG findings in retinal vasculitis have rarely been described. Our study shows that in cases of retinal vasculitis, retinal function is, in general, mildly affected or even normal regardless of the severity of angiographic findings.
Ikeda et al17 have studied 21 eyes with ocular inflammation and classified the presence of retinal vasculitis into 4 categories: vitreous cells with no fluorescein leakage from retinal vessels, fluorescein leakage from peripheral retinal vessels, fluorescein leakage from the disc or macular vessels, and fluorescein leakage from retinal vessels associated with pigment epithelial and choroidal changes. They found that b-wave amplitudes were depressed in the latter two categories.17 However, their study predates the establishment of international standards for ffERG. In our study, macular and peripheral leakage were weakly correlated only with delayed 3.0 photopic 30 Hz flicker timing. Our study is different as we used the age-adjusted ffERG gradings, which was not accounted for in the former study.
Brouwer et al18 have studied ffERG responses in various types of uveitis. Similar to our study, the authors used the ASUWOG grading system They reported that prolonged cone b implicit timing was the most commonly affected ffERG response and was associated with higher FFA scores, presence of vitritis and anterior chamber cells. These findings are complementary to our study but with some differences. Our study showed that the most commonly affected parameter was also a cone generated response, namely the flicker implicit timing, in contrast to the cone b-wave implicit time in the aforementioned study. In addition, we have found that cone generated responses, in the form of either flicker or cone b-wave implicit timing (or both), were correlated with BCVA, overall FFA scores and the activity of inflammation. Their study, however, did not find a correlation between BCVA and cone b-wave implicit timing. In addition, they did not explore the relationship between individual FFA signs and ffERG waves. The differences with our study can be attributed to fact that the authors used the aggregate absolute ffERG values which did not account for age differences and that our study has looked specifically into cases with retinal vasculitis.18 Nevertheless, both sets of results support the notion that cones are more affected in uveitis and its associated responses are more correlated with clinical findings.
Most of our cohort was associated with either normal or mildly depressed ffERG scores, except for three eyes belonging to wo subjects. The first patient (#14) had bilateral retinal vasculitis that was associated with antiretinal antibodies. Interestingly, retinal vasculitis has been reported to be associated with autoimmune retinopathy, and in that case, ffERG was significantly affected.19 The second patient (#19) had retinal vasculitis in one eye associated with QuantiFERON positivity. Patient #9 had bilateral retinal vasculitis associated with cold agglutinin disease and CLL, and the ffERG scores were the lowest in our cohort excluding the two aforementioned cases. These findings support the notion that significantly reduced ffERG responses in the setting of retinal vasculitis may suggest the presence of an underlying systemic condition. Such suggestion is further supported by the statistically significant correlation between lower overall ffERG scores and the presence of systemic disease or marker. The correlation may also raise suspicion to certain masquerade syndromes such as retinal dystrophies with vascular leakage, which can be challenging diagnostically.
Our study did not find strong clinical predictors of suboptimal retinal function as evaluated by ffERG. Although we have found several statistically significant correlations with depressed ffERG responses, all of them, except for vitreous haze, were weakly correlated. Therefore, we suggest using ffERG to evaluate retinal function in retinal vasculitis, and not just rely on clinical criteria to assess its severity.
The main limitation of our study is the imperfect ffERG grading system; ffERG response values do not follow normal distribution; hence, the grading system, which is based on z scores, is not optimal. The proposed grading system is an interval, not ratio, semiquantitative scale, which makes it less accurate than a true quantitative one. We could have used the absolute ffERG response values for the correlation analysis, but the differences in normative ffERG values per age, which are significant, would not be accounted for, and that would have raised great concerns on the accuracy of the analysis. We opted to choose the proposed ffERG grading system, despite its limitations, since it accounted for the age differences.
FFA and ffERG were not performed on the same day, and we have set a maximum interval of 1 month between the two modalities for inclusion. Although changes in vasculitis severity may occur in less than 1 month if treatment was changed, this is unlikely the case in our cohort, as we made treatment decisions based on both modalities (FFA and ffERG) rather than a single one. There is also a concern for selection bias since most cases in the initial cohort did not have ffERG. However, we only recommend ERG for cases with significant vasculitis and not for any degree of vasculitis. Therefore, we believe the potential bias is not very concerning since, in our cohort, only cases with minimal vasculitis did not have ffERG.
Other limitations in our study include the retrospective design, non-linear grading systems for FFA severity, relatively small number of patients and the few cases with occlusive retinal vasculitis. Further studies are required to clearly elucidate our findings.
CONCLUSION
In this study, retinal vasculitis was not frequently associated with severe retinal dysfunction as measured with the ffERG. The presence of systemic diseases or markers might be associated with lower overall ffERG score. Lower BCVA, higher FFA scores and vitreous haze gradings were weakly correlated with cone related ffERG abnormalities. ffERG may provide a functional assessment of the retina not otherwise predicted by clinical or angiographic finding in retinal vasculitis.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
Little is known about full field electroretinography (ffERG) findings in retinal vasculitis and its correlation with clinical and angiographic findings.
WHAT THIS STUDY ADDS
Retinal function is relatively preserved in retinal vasculitis. The severity of vasculitis is weakly correlated with select cone generated ffERG responses. Vasculitis in the setting of systemic disease is associated with poor ffERG responses.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Poor retinal function in the setting of vasculitis may be suggestive of systemic disease. Further research can be implemented to investigate the predilection of cone dysfunction in retinal vasculitis.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Additional supplemental material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/bjo-2022-321716).
Ethics approval The study was conducted in compliance with the Declaration of Helsinki, the United States Code of Federal Regulations Title 21, and the Harmonized Tripartite Guidelines for Good Clinical Practice (1996). Stanford University institutional review board approved the study under the protocol number 48178, and an informed consent waiver was obtained as the charts of enrolled patients were retrospectively reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1.Graham EM, Stanford MR, Sanders MD, et al. A point prevalence study of 150 patients with idiopathic retinal vasculitis: 1. diagnostic value of Ophthalmological features. Br J Ophthalmol 1989;73:714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum JT, Ku J, Ali A, et al. Patients with retinal vasculitis rarely suffer from systemic vasculitis. Semin Arthritis Rheum 2012;41:859–65 published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum JT, Sibley CH, Lin P. Retinal vasculitis. Curr Opin Rheumatol 2016;28:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanford MR, Verity DH. Diagnostic and therapeutic approach to patients with retinal vasculitis. Int Ophthalmol Clin 2000;40:69–83. [DOI] [PubMed] [Google Scholar]
- 5.Creel DJ. Electroretinograms. Handb Clin Neurol 2019;160:481–93. [DOI] [PubMed] [Google Scholar]
- 6.Sieving PA, Murayama K, Naarendorp F. Push-Pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 1994;11:519–32. [DOI] [PubMed] [Google Scholar]
- 7.Robson JG, Saszik SM, Ahmed J, et al. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 2003;547:509–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 2015;130:1–12. [DOI] [PubMed] [Google Scholar]
- 9.Algvere P. Electroretinographic studies on posterior uveitis. Acta Ophthalmol 1967;45:299–313. [DOI] [PubMed] [Google Scholar]
- 10.Martenet AC, Niemeyer G. [The value of electroretinography in uveitis]. Ophtalmologie 1990;4:169–72. [PubMed] [Google Scholar]
- 11.Sobrin L, Lam BL, Liu M, et al. Electroretinographic monitoring in birdshot chorioretinopathy. Am J Ophthalmol 2005;140:52.e1–52.e18. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W, Zhou C, Cao Q, et al. Longitudinal study of visual function in Vogt-Koyanagi-Harada disease using Full-Field electroretinography. Am J Ophthalmol 2018;191:92–9. [DOI] [PubMed] [Google Scholar]
- 13.Tugal-Tutkun I, Herbort CP, Khairallah M, et al. Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). Int Ophthalmol 2010;30:539–52. [DOI] [PubMed] [Google Scholar]
- 14.Multicenter Uveitis Steroid Treatment Trial Research Group, Kempen JH, Altaweel MM, et al. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol 2010;149:550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature working G. standardization of uveitis Nomenclature for reporting clinical data. Results of the first International workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moschos MM, Gouliopoulos NS, Kalogeropoulos C. Electrophysiological examination in uveitis: a review of the literature. Clin Ophthalmol 2014;8:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda H, Franchi A, Turner G, et al. Electroretinography and electro-oculography to localize abnormalities in early-stage inflammatory eye disease. Doc Ophthalmol 1989;73:387–94. [DOI] [PubMed] [Google Scholar]
- 18.Brouwer AH, de Wit GC, Ten Dam NH, et al. Prolonged cone b-wave on electroretinography is associated with severity of inflammation in noninfectious uveitis. Am J Ophthalmol 2019;207:121–9. [DOI] [PubMed] [Google Scholar]
- 19.Anastasakis A Dick AD, Damato EM. et al. Cancer-Associated retinopathy presenting as retinal vasculitis with a negative ERG suggestive of on-bipolar cell pathway dysfunction. Doc Ophthalmol 2011;123:59–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


