Anorexia nervosa (AN) is characterized by underweight, and the primary goal of treatment is weight restoration. Treatment approaches (ie, hospitalization for weight recovery vs for medical stabilization) and settings (ie, medical/pediatric or psychiatric units) for patients with AN vary between and also within countries. Several specialized eating disorder units worldwide have established high-caloric refeeding (HCR) protocols for patients with AN. In observational studies, HCR shortens hospital stays and increases initial weight gain, the latter being associated with a favorable long-term prognosis. However, clinicians may still remain reluctant to accept this approach for fear of medical complications of HCR, including the risk of refeeding syndrome (RS).1 Research is building toward the development of evidence-based recommendations for safe and effective re-nutrition of underweight patients with AN. This focused review was based on clinical experience and describes 3 different protocols for nutritional management devised by experts from 3 different parts of the world (Australia, Germany, and the United States), in medical refeeding of patients with AN who have established HCR in their clinical units. In addition, and in order to understand energy requirements, empirical data on energy turnover of patients with AN from former metabolic studies are presented. To the best of our knowledge, there is no study reporting on HCR in a cohort of severely malnourished adolescents with AN (ie, with a mean body mass index [BMI] of <15 kg/m2). Therefore, to provide information about the treatment of extremely malnourished patients with AN, we included a recently published HCR protocol for adults with a BMI of <13 kg/m2.2
CONCERN FOR REFEEDING SYNDROME HAS HISTORICALLY SHAPED NUTRITIONAL TREATMENT STRATEGIES FOR PATIENTS WITH AN
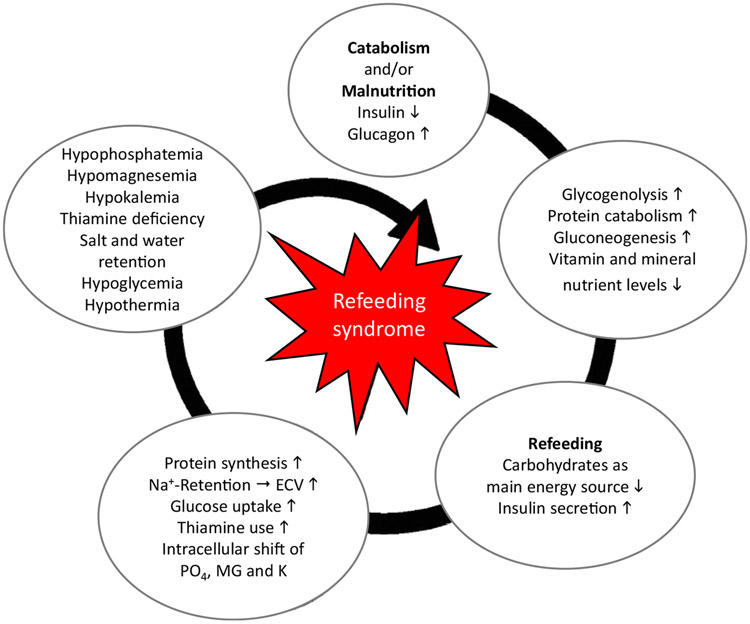
The Refeeding Syndrome (RS), originally described in famine and war victims, is characterized by severe clinical complications during initial refeeding of malnourished patients, including those of organ failure and coma, and, in the worst albeit extremely rare case, death.1 The pathophysiological mechanisms underlying the RS are shown in Figure 1.3 For a long time, there has been no commonly accepted definition of RS, and subsequently prevalence rates and clinical outcomes are still unclear. In June 2020, the American Society for Parenteral and Enteral Nutrition (ASPEN) proposed a new clinical definition: namely, a decrease in serum phosphorus, and/or potassium, and/or magnesium levels, and/or organ dysfunction resulting from a decrease in any of these and/or due to thiamine deficiency, occurring within 5 days of reintroduction of calories.4 This consensus definition is intended as a basis for further research into the incidence, consequences, pathophysiology, avoidance, and treatment of RS. An important indicator of risk is refeeding hypophosphatemia, as electrolytes move from the extracellular to the intracellular space with glucose uptake.1 Some authors link RS to cardiac changes and delirium, others to electrolyte disturbances. Therefore, one of the goals of refeeding is to maintain serum phosphate levels within the normal range, which can be achieved by oral phosphate supplementation, to reduce the risk of RS and its associated complications. Over time, a number of case reports have documented the RS in patients with AN during initial refeeding. These reports have shaped low-caloric refeeding (LCR) protocols starting with low initial energy intake, that is, <1,400 kcal/24 h,5 based on the belief that low energy intake would minimize/abolish the risk of RS.1 More recently, LCR has been linked to poor weight gain and protracted hospital stays 6 and has been described to lead to the “underfeeding syndrome.” Unlike patients without eating disorders but with protein calorie malnutrition (PCM), patients with AN do not generally have increased gastrointestinal losses or increased energy demands related to critical illness or infection. Therefore, caution needs to be exercised in generalizing findings from refeeding of malnourished patients in developing nations or different patient populations, such as those with surgical complications or cancer, to those with AN. Given these differences, specific, evidenced-based refeeding strategies are needed for patients with AN to ensure safety while avoiding the negative consequences of underfeeding.7 Such evidence-based recommendations are pressing, given that official guidelines for the treatment of AN, such as from the American Psychiatric Association, the American Dietetic Association, the National Institute for Health and Care Excellence, and the German S3-Guidelines, still recommend conservative, LCR for patients with AN. More recent clinical evidence indicates that HCR is feasible and is not associated with RS in specialized settings that implement HCR jointly with close medical monitoring and phosphate and thiamine supplementation. An informative, systematic review found that electrolyte abnormalities were not increased with HCR in mildly and moderately malnourished patients (75%–85% median body mass index), but that the safety of HCR had not been established, highlighting the lack of prospective studies in severely malnourished patients.5
FIGURE 1. Pathophysiology of refeeding syndrome.
Note: Adapted and republished from Reber et al.3 (https://doi.org/10.3390/jcm8122202; published under Creative Commons license CC BY: https://creativecommons.org/licenses/by/4.0). ECV = extracellular volume.
ENERGY REQUIREMENTS OF PATIENTS WITH AN
During inpatient treatment of patients with AN, the prescription of sufficient calories is required to support medically necessary weight gain for nutritional improvement. Some eating disorder programs aim for providing sufficient calories to achieve weekly weight gains between 500 and 1,500 g, whereas others prescribe a set amount of initial daily calories (eg, 1,400 kcal5) without setting a fixed target of weekly weight gains. Eating disorder–specific treatment guidelines from different countries still recommend low-to-moderate energy intakes between 600 and 1,600 kcal/d (or 5–40 kcal/kg/d) with slow caloric advances, leaving significant room for differences in interpretation. However, even for experienced clinicians, it is difficult to estimate the individual caloric requirements that will continuously support adequate renutrition. Formulas to guide clinicians working in these settings are derived mostly from the healthy and normal-weight population. However, basal metabolic rate and therefore energy expenditure in acutely malnourished patients before refeeding is down-regulated due to starvation. Subsequently, during refeeding, patients with AN may experience increases in energy needs that are markedly beyond baseline and above what would be estimated. This hypermetabolic change has been attributed to a switch of substrate metabolism from the catabolic to the anabolic state, increased resting and diet-induced thermogenesis, increased energy losses as heat and cellular repair processes, as well as physical (hyper-)activity. These processes, along with fluid shifts and possibly, at times, with covert food restriction, all challenge the calculation of individual energy needs as well as the interpretation of weight gain in patients with AN. Very few, small studies using the gold standard technique of doubly labeled water have estimated average total daily energy expenditure at 1,972 kcal/d or 46 kcal/kg/d in underweight patients with AN who met hospitalization criteria,8 and 2,602 kcal/d (41 kcal/kg/d) in long-term weight-recovered patients.9 These studies estimated total daily energy expenditure; however, in order to gain weight, energy intake has to be markedly higher than energy expenditure. Using 24-hour surveillance and weighing of consumed food to calculate caloric intake, energy needs during short-term refeeding have been estimated to be as high as 80 kcal/kg/d, which would correspond to 3,600 kcal/d in a patient weighing 45 kg.10 In summary, the biological range of energy expenditure in patients with AN during refeeding is both dynamic and high, making individual energy requirements difficult to estimate during refeeding.
MEDICAL MONITORING OF HCR IN AN: CLINICAL PRACTICE APPROACHES OF 3 EATING DISORDER UNITS IN AUSTRALIA, GERMANY, AND THE UNITED STATES
First, in Sydney, Australia, HCR has routinely been implemented with close medical monitoring for more than 10 years. The 2 Sydney units described here are medically based programs, based in a medical hospital. One unit is without psychiatric support and is managed by Adolescent and Young Adult consultants with nursing, dietetic, and psychosocial support in the treating team. The other unit has co-leads in Psychiatry and Adolescent Medicine, supported by a full multi-disciplinary team. Both units admit patients predominantly to a dedicated adolescent ward. Kohn and Madden11 reported refeeding more than 400 adolescents with AN, leading to a mean weight gain in the first week of treatment of >2 kg without any serious medical complications. Today in clinical practice, all patients with medical instability treated in this unit initially receive a standard 1-kcal/mL feed through a nasogastric tube, typically commencing with 2,400 kcal/d. Once vital signs have been stabilized for 24 hours, oral feeds (1,800 kcal meal plan) are commenced and nasogastric feeding continues over night (1 L of 1 kcal/mL for 10 hours at 100 mL/h). Routinely patients are given a multivitamin daily. Oral phosphate is supplemented if the serum phosphate level is <1.0 mmol/L. Other electrolytes (anions) are supplemented to maintain normal values, although this is rarely required. Continuous nasogastric tube feeding is used to avoid periods of “fasting” during overnight sleep until vital signs (pulse, temperature, and blood pressure) are stabilized. Details regarding the patient population and refeeding strategy for the units in Sydney are shown in Table 1.11,12
TABLE 1.
High-Caloric Refeeding Algorithms in 3 Specialized Units
| Sydney, Australia (adolescents)11,13 | Prien, Germany (adults)2 | University of California, San Francisco (adolescents)6 |
|
|---|---|---|---|
| Population | Age, mean (SD) [range]: 14.8 (1.5) [12–18] y BMI, mean (SD) [range]: 15.2 (5.1) [9.5–21] kg/m2 %mBMI, mean: 78.4, SD and range not reported |
Age: mean (SD) [range]: 23.8 (5.3) [18–47] y BMI: mean (SD) [range]: 11.5 (0.9) [9.5–13] kg/m2 Exclusion: age >40 years, chronic renal failure, heart insufficiency |
Age: mean (SEM) [range]: 16.1(0.4) [9–20] y BMI: mean (SEM) [range] 16.2 (0.5) [range not reported] %mBMI: mean (SEM): 79.8 (2.1) [range not reported] Patients <60% median BMI (by US Centers for Disease Control and Prevention data) are excluded from high-caloric refeeding due to concerns of medical fragility and are refed via low-caloric refeeding |
| Food intake | Started with 2,400 kcal and nasogastric tube feeding, which reduces according to a standardized protocol Initial intake was lower in patients with a BMI of <12 kg/m2 Weight gain goal: >1 kg/wk |
Starting with 2,000 kcal/day orally, spread over 3 meals and adjusting energy intake to achieve weight gain of 0.7–1 kg/wk This caloric intake can subsequently be increased meal by meal or with additional snacks in between the three main meals. Usual, stepwise increases (by 200 kcal per step) are from 2,000 kcal to 3,000 kcal and from 3,000 kcal to 4,000 kcal. Managing exercise and nonexercise physical activity along with caloric intake is important in order to normalize both. The general aim is to steadily maintain weight gain of 700 to 1,000 g/wk. If weight cannot be increased with 4,000 kcal, excessive exercising seems likely. In this case, additional increase of meals risks reinforcing purging behavior, thus potentially harming patients. Management of physical activity should go hand in hand with the management of caloric intake and weight restoration. This approach requires obligatory resting time and support to perform alternative activities and regulation of anxiety and emotions |
Started with 1,764 ± 60 kcal (1,400–2,400) with 6 meals per day; high-calorie liquid formula [oral)] to replace refused calories in meals kcal increased 122 ± 8 per day; no weight gain goal Currently, there is a fixed kcal/d starting point for all patients |
| Fluids | The use of continuous nasogastric tube feeds assisted in providing the fluid and calories to reestablish circulation | Care is taken not to use fluid replacement per se, as cardiac failure can be induced, by volume overload, in patients with protein calorie malnutrition Circulatory changes are corrected slowly; the adaptive physiological changes producing a low-volume, low-pressure circulation are corrected over several days to 1 week Diuretics are used with caution |
Fluid balance calculated daily as the difference between total intake and output recorded over 24 h All beverages were weighed and measured before serving; free water was restricted to 1 L/d Intake represents beverages only (no intravenous fluids); output represents urine only, using bedside commode |
| Vital signs | Monitored either continuously over 24–48 hours, then every 4–6 hours until normal vital sign and electrolytes have been consistently recorded for 72 hours | Measurement of blood pressure, heart rate, and detection of edema Monitoring of vital signs during the first 3–4 weeks for patients with BMI up to 13 kg/m2 |
Heart rate and blood pressure were assessed with continuous cardiac monitoring, and temperature was measured orally. Postural changes in heart rate and blood pressure were assessed starting with supine measurements (after 5 min in position), followed by standing measurements (after 2 min in position) |
| Supplements | To prevent refeeding syndrome, oral phosphate (SANDOZ phosphate, 500 mg) is supplemented to correct serum phosphate levels >1.0 mmol/L The dose is adjusted empirically from serial measurements of serum phosphate Serial measurements of electrolytes, blood sugar, and urine pH continue daily until levels are maintained within normal ranges. Urinalysis included measure of specific gravity indicating hydration and pH reflecting metabolism |
Phosphate (PH; REDUCTO spezial 602 mg/360 mg equivalent of 612 mg PH twice per day, 0-1-1) and thiamine (200 mg, 1-0-0) when initial BMI is >13 kg/m2 is given prophylactically over 2 wk after admission when beginning re-nutrition in order to prevent RS. After 2 weeks, PH supplementation is adjusted based on measurements of serum PH. Serial measurements of electrolytes, blood sugar on a daily basis for the first week, then weekly for 4 wk and continued according to normalized ranges |
Electrolytes were measured daily to monitor refeeding risk Low serum phosphate (>3.0 mg/dL) or declining trend was supplemented in packets of 250 mg phosphate Daily multivitamin with minerals, 500 mg calcium carbonate twice per day, and zinc sulfate or zinc acetate once per day |
| Body temperature | Maintained above 35.5°C, for example by providing continuous caloric intake and/or increasing environmental temperature or using overhead heating or heated waterbeds in hypothermic patients | Maintained by clothes and heated rooms | |
| Mental state | Should be assessed daily for mood symptoms as well as the development of delirium, associated with the RS | Psychiatric evaluation upon admission | |
| Further monitoring | Containment of exercising or purging behaviors | Containment of exercising or purging behaviors | Bedrest |
| Usual treatment after initial refeeding phase and discharge criteria | After an average admission of 28 days, patients are supported to continue a meal plan of 2,700–3,500 kcal/d Following medical stabilization (using criteria established by the Society for Adolescent Medicine,11) patients are transitioned to oral intake only. Discharge occurs after ongoing weight gain has been established through oral feeding both in the hospital and during leave for family meals outside the hospital Weight gain after discharge is typically supported through family-based therapy |
The average duration of stay in the special ward to treat extreme AN is 2 months. After the initial refeeding phase, the weight target remains at 700–1,000 g/wk, and nutrition is adjusted to achieve this goal, with a maximum meal plan of up to 4000 kcal/d. After discharge from the unit, patients are transferred to a less intensive, inpatient setting, are moved to a residential home, allowed to return home and receive outpatient psychotherapy or receive daypatient treatment The German medical system allows for a length of inpatient treatment as required to achieve weight recovery and improvement of eating behavior. Apart from these weight recovery aspects, the most common discharge criteria are discontinuation of therapy decided by adult patients (28% among extremely underweight patients, 11% among all patients with an eating disorder as main diagnosis), substance abuse, excessive therapy-offending behaviors, contiunous lack of motivation, or therapy decision | Length of stay was 11.9 ± 1.0 days Patients are discharged home with medical/nutritional care and psychoptherapy in our outpatient program or in the community Discharge criteria are based on the reversal of published vital sign instabilities (Society for Adolescent Medicine, Position Statement, 201511: 1) heart rate ≥ 45 beats/min for 24 hours; 2) normotension (>90/50 mm Hg) 3) temperature ≥35.6°C 4) orthostatic heart rate increase in HR ≤35 beats/min 5) orthostatic BP decrease in systolic BP of ≤20 mm Hg or in diastolic BP of >10 mm Hg 6) ≥75% of mBMI |
Note: BMI = body mass index; %mBMI = percent median BMI; BP = blood pressure; HR = heart rate; PH = phosphate; RS = refeeding syndrome; SEM = standard error of the mean.
Second, a specialized unit in Prien, Germany, manages significantly underweight adults with extreme (ie, BMI <15 kg/m2) and often enduring AN, that is, an illness duration of >10 years and repeated prior hospitalizations for AN. This adult psychiatric unit has the ability to admit patients with AN for longer periods of time and also to work on behavioral issues. Close medical surveillance by an Internal Medicine specialist is combined with psychotherapy from the beginning of inpatient treatment. The transition from medical stabilization, which is initially the main focus in patients with extreme underweight, to more intensive psychotherapy takes place gradually within the hospital. In this unit, in 103 adult patients with AN, and an average admission BMI of 11.5 kg/m2, HCR started with oral intake of 2,000 kcal/d combined with routine thiamine and phosphate supplementation for 2 weeks, followed by individual phosphate supplementation according to blood phosphate levels.2 This protocol led to an average weekly weight gain of 1.05 kg/wk in the first 4 weeks, with this accelerated weight gain improving vital signs (blood pressure, heart rate), blood parameters (creatine kinase, sodium, liver enzymes, leukocytes, and thrombocytes) as well as the physical performance (assessed by a Sit-up Squat Stand [SUSS] test) of the patients and without occurrence of RS in any of these patients.2 This protocol includes replacement of fluid, energy, electrolytes, protein, and micronutrients, spread over 3 daily meals. Details regarding the patient population and refeeding strategy for the unit in Prien are shown in Table 1. In general, in this German hospital, treatment protocols for adults and adolescents are similar with regard to the nutritional and medical management as well as psychotherapeutic treatments, which are based on a multimodal approach with cognitive behavioral treatment delivered in individual and group sessions. Also, drop-out rates from treatment are similar in adolescents and adults. Differences essentially concern only a few aspects. First, for adolescents, the involvement of their families or legal guardians is an essential part of the management, and all adolescents receive at least 3 sessions of family therapy during their hospitalization. Second, treatment is voluntary for both adults and adolescents, but for minors up to the age of 18 years, the consent of a parent or legal guardian is required. Furthermore, the overall aim of weight gain in adults is a BMI of 20 kg/m2, whereas in adolescents it is the 25th BMI percentile.
Third, and at another specialized unit (University of California, San Francisco [UCSF], USA), different refeeding regimens are studied. The UCSF is a medical unit in a pediatric hospital with some psychiatric support. Once a patient meets Society for Adolescent Health and Medicine [SAHM] criteria for medical stability,12 the patient is typically discharged. A prospective study from this unit5 of moderately malnourished adolescents with AN receiving meal-based HCR beginning with 1,764 ± 60 (range, 1,400–2,400) kcal/d and oral electrolyte supplementation to correct low or declining serum levels did not find increased electrolyte abnormalities compared to those receiving LCR, that is, starting with 1,093 ± 28 kcal, an approach that would be consistent with the above-mentioned current recommendations in several national guidelines. Compared to the LCR group that gained 0.14 kg/d over a 15-day period, the HCR group gained approximately 0.23 kg/d during the same time, with a significantly shorter hospital stay of nearly 6 days. A randomized clinical trial is underway to compare the efficacy, safety, and cost-effectiveness of meal-based LCR versus HCR in adolescents with AN (ClinicalTrials.gov Identifier: NCT02488109). Details regarding the patient population and refeeding strategy for the unit in San Francisco are shown in Table 1.13
These protocols from single sites in Australia, the United States, and Germany all support the feasibility of HCR in specialty care units, both in adolescents and in adults with AN who are moderately to extremely underweight and malnourished, and when accompanied by close medical supervision. The fact that these HCR approaches varied regarding (1) daily caloric intake (starting from 1,400 to 2,400 kcal/d), (2) routes of administration (oral vs. nasogastric tube), and (3) rates of advancement in caloric increases and electrolyte replacement (from prophylaxis to repletion of low serum levels) is a clear limitation of the present focused review. However, all 3 regimens demonstrated satisfactory weight gain with no reported cases of RS in patients with AN. Taking the experiences and data from these 3 diversely located, specialized AN units into account that are embedded in different care systems, there is preliminary evidence suggesting that an HCR protocol carried out under close medical supervision is feasible. However, randomized controlled trials with predefined caloric intake and protocolized electrolyte correction are needed to further establish the safety and efficacy of HCR across different patient populations with AN and to craft evidence-based guidelines.
Acknowledgments
This work was supported by funding from the Christina Barz Foundation, Essen, Germany (Project Number T0155 -31.954). The sponsors had no role in design, conduct or data interpretation of the study.
Footnotes
Disclosure: Dr. Haas has received grant support from the Swiss Anorexia Nervosa Foundation. Prof. Le Grange has received grant funding from the National Institutes of Health and royalties from Routledge and Guilford Press. He is the co-director of the Training Institute for Child and Adolescent Eating Disorders, LLC. Dr. Voderholzer has received grant support from the Swiss Anorexia Nervosa Foundation. Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, the Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/JandJ, Karuna, LB Pharma, Lundbeck, MedAvanteProPhase, MedInCell, Medscape, Merck, Misubishi Tanabe Oharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He provided expert testimony for Janssen, and Otsuka. He served on a Data Safety Monitoring Board for , Lundbeck, Rovi, Supernus, and Teva. He has received rgrant support from Bendheim Foundation, Berlin Institute of Health, Janssen, National Institute of Mental Health, USA, Patient Centered Outcomes Research Institute, and Takeda. He is also a stock option holder of LB Pharma. Prof. Kohn and Drs. Körner, Cuntz, Garber have reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Verena Haas, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Michael Kohn, Centre for Research into Adolescent’S Health, Westmead Hospital, University of Sydney, Australia..
Thorsten Körner, Schön Klinik Roseneck, Prien am Chiemsee, Germany..
Ulrich Cuntz, Schön Klinik Roseneck, Prien am Chiemsee, Germany.; PMU Medizinische Privatuniversität Salzburg, Austria..
Andrea K. Garber, University of Califorinia at San Francisco, California..
Daniel Le Grange, University of Califorinia at San Francisco, California.; The University of Chicago, Illinois..
Ulrich Voderholzer, Schön Klinik Roseneck, Prien am Chiemsee, Germany.; Department of Psychiatry and Psychotherapy, Ludwigs-Maximilian-University, Munich, Germany..
Christoph U. Correll, Charité — Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.; Zucker School of Medicine at Hofstra/Northwell, Hempstead and The Zucker Hillside Hospital, New York..
REFERENCES
- 1.Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition. 2001;17:632–637. [DOI] [PubMed] [Google Scholar]
- 2.Koerner T, Haas V, Heese J, et al. Outcomes of an accelerated inpatient refeeding protocol in 103 extremely underweight adults with anorexia nervosa at a specialized clinic in Prien, Germany. J Clin Med. 2020;9:1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reber E, Friedli N, Vasiloglou MF, et al. Management of refeeding syndrome in medical inpatients. J Clin Med. 2019;8:2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva JSV, Seres DS, Sabino K, et al. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract. 2020;35:178–195. [DOI] [PubMed] [Google Scholar]
- 5.Garber AK, Sawyer SM, Golden NH, et al. A systematic review of approaches to refeeding in patients with anorexia nervosa. Int J Eat Disord. 2016;49:293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garber AK, Mauldin K, Michihata N, Buckelew SM, Shafer M-A, Moscicki A-B. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J Adolesc Health. 2013;53:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohn MR, Madden S, Clarke SE. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr. 2011;23:390–394. [DOI] [PubMed] [Google Scholar]
- 8.Casper RC, Schoeller DA, Kushner R, Hnilicka J, Gold ST. Total daily energy expenditure and activity level in anorexia nervosa. Am J Clin Nutr. 1991;53:1143–1150. [DOI] [PubMed] [Google Scholar]
- 9.Platte P, Pirke KM, Trimborn P, Pietsch K, Krieg JC, Fichter MM. Resting metabolic rate and total energy expenditure in acute and weight recovered patients with anorexia nervosa and in healthy young women. Int J Eat Disord. 1994;16:45–52. [DOI] [PubMed] [Google Scholar]
- 10.Kaye WH, Gwirtsman HE, Obarzanek E, Geroge DT. Relative importance of calorie intake needed to gain weight and level of physical activity in anorexia nervosa. Am J Clin Nutr. 1988;47:989–994. [DOI] [PubMed] [Google Scholar]
- 11.Kohn M, Madden S. Re: critical appraisal of the management of severe malnutrition. J Paediatr Child Health. 2007;43. 329–329. [DOI] [PubMed] [Google Scholar]
- 12.Golden NH, Katzman DK, Sawyer SM, et al. Eating disorders in adolescents: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:496–503. [DOI] [PubMed] [Google Scholar]
- 13.Madden S, Miscovic-Wheatley J, Clarke S, Touyz S, Hay P, Kohn MR. Outcomes of a rapid refeeding protocol in adolescent anorexia nervosa. J Eat Disord. 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]