Keywords: exercise, high-intensity interval training, lipid droplet, lipophagy, skeletal muscle
Abstract
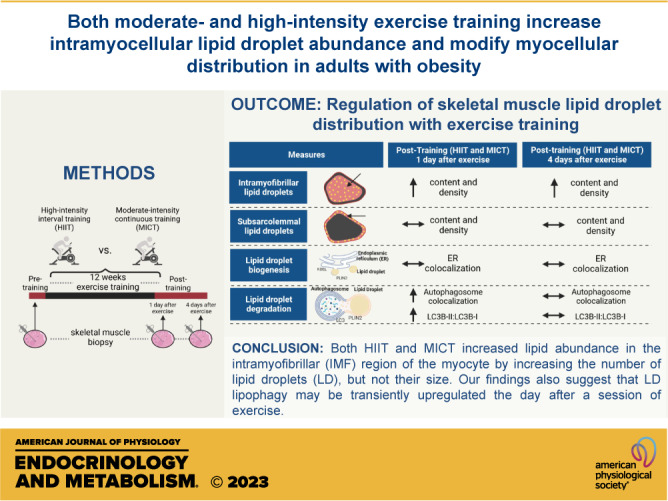
Exercise training modifies lipid metabolism in skeletal muscle, but the effect of exercise training on intramyocellular lipid droplet (LD) abundance, size, and intracellular distribution in adults with obesity remains elusive. This study compared high-intensity interval training (HIIT) with more conventional moderate-intensity continuous training (MICT) on intramyocellular lipid content, as well as LD characteristics (size and number) and abundance within the intramyofibrillar (IMF) and subsarcolemmal (SS) regions of type I and type II skeletal muscle fibers in adults with obesity. Thirty-six adults with obesity [body mass index (BMI) = 33 ± 3 kg/m2] completed 12 wk (4 days/wk) of either HIIT (10 × 1 min, 90% HRmax + 1-min active recovery; n = 19) or MICT (45-min steady-state exercise, 70% HRmax; n = 17), while on a weight-maintaining diet throughout training. Skeletal muscle biopsies were collected from the vastus lateralis before and after training, and intramyocellular lipid content and intracellular LD distribution were measured by immunofluorescence microscopy. Both MICT and HIIT increased total intramyocellular lipid content by more than 50% (P < 0.01), which was attributed to a greater LD number per µm2 in the IMF region of both type I and type II muscle fibers (P < 0.01). Our findings also suggest that LD lipophagy (autophagy-mediated LD degradation) may be transiently upregulated the day after the last exercise training session (P < 0.02 for both MICT and HIIT). In summary, exercise programs for adults with obesity involving either MICT or HIIT increased skeletal muscle LD abundance via a greater number of LDs in the IMF region of the myocyte, thereby providing more lipid in close proximity to the site of energy production during exercise.
NEW & NOTEWORTHY In this study, 12 wk of either moderate-intensity continuous training (MICT) or high-intensity interval training (HIIT) enhanced skeletal muscle lipid abundance by increasing lipid droplet number within the intramyofibrillar (IMF) region of muscle. Because the IMF associates with high energy production during muscle contraction, this adaptation may enhance lipid oxidation during exercise. Despite differences in training intensity and energy expenditure between MICT and HIIT, their effects on muscle lipid abundance and metabolism were remarkably similar.
INTRODUCTION
Intramyocellular lipids provide an energy dense fuel to support muscle contraction during exercise (1, 2). Intramyocellular lipid content is often found to be elevated in well-trained endurance athletes, which enhances the local availability of fat within skeletal muscle to fuel prolonged exercise. This favorable adaptive response may be largely attributed to an exercise-induced increase in skeletal muscle triacylglycerol synthesis within the hours after each exercise session (3). Somewhat paradoxically, intramyocellular lipid content in sedentary adults with obesity is also often found to be relatively high (4, 5), likely stemming from chronically elevated lipolytic rates from adipose tissue (6), providing an overabundance of fatty acids to skeletal muscle for lipid synthesis. The excessive intramyocellular lipid accumulation in sedentary adults with obesity is causally linked with the development of insulin resistance (7–9). Findings from previous studies examining the effects of exercise training on muscle lipid content in adults with obesity are equivocal; most report either an increase (10–15) or no change in total muscle lipid content (16–19). However, the response to exercise training on muscle lipid content for individuals with obesity have been confounded by several factors, including weight loss during training, differences in the mode and intensity of exercise, and limited dietary control, all of which can impact interpretations of the effects of exercise training on the regulation of muscle lipid storage.
Intramyocellular lipids are largely stored within lipid droplets (LDs), which are dynamic organelles composed of a triacylglycerol-rich core surrounded by a phospholipid monolayer. The abundance of triacylglycerol stored within LDs is primarily regulated by the balance between of triacylglycerol hydrolysis (lipolysis) and esterification. However, the abundance of LD organelles themselves are largely regulated by nascent LD biogenesis from the endoplasmic reticulum (ER; 20) and their degradation (i.e., lipophagy), which entails recruitment of the autophagosome specifically to the LD and transport to the lysosome for degradation (21, 22). The location of the LD also has an important impact on its cellular function. For example, LDs localized to the intramyofibrillar (IMF) region of the myocyte are recognized to support energy requirements for skeletal muscle contraction (23, 24). Interestingly, endurance training has been found to increase LD contact with the mitochondria in the IMF region, which can help support fatty acid oxidation for the contracting muscle (14, 16). Less is known about LDs localized to the subsarcolemmal (SS) region, but since LDs in the SS region are often found in close proximity to SS mitochondria (24), they may play a role to help support the metabolic demand for processes occurring at or near the cell membrane. Perhaps, not surprisingly, LDs are more abundant in type I versus type II muscle fibers, which aligns with the high oxidative capacity of type I fibers to support prolonged and relatively low-intensity muscle activation (25). Although exercise training may favorably modify intramyocellular lipid storage and distribution (10–19), how exercise modifies LD storage is still not completely understood.
High-intensity interval training (HIIT) has gained considerable attention as a time-efficient exercise prescription to increase aerobic capacity (26, 27) and improve metabolic health in obesity (28). However, the effects of HIIT versus more conventional, moderate-intensity continuous training (MICT) on intramyocellular lipid content and LD distribution in adults with obesity remain unclear. The primary aim of this study was to compare the effects of 12-wk MICT versus HIIT on the size, number, and cellular distribution of LDs within skeletal muscle in adults with obesity. We hypothesized skeletal muscle lipid content will increase similarly after 12 wk of HIIT and MICT, and this increase in lipid content will be largely due to an increase in LDs in the IMF region of the myocyte (i.e., near the site of highest energy production during exercise). Although we anticipate total muscle lipid content will increase similarly after HIIT and MICT, because the intensity of exercise dictates muscle fiber recruitment patterns (i.e., type II muscle fibers recruited during high-intensity exercise), we hypothesize the increase in lipid content in type II fibers will be greater after HIIT versus MICT, whereas the increase in lipid content in type I fibers will be greater in MICT versus HIIT. In addition, an exploratory aim of this project was to examine the effects of training on the regulation of LD turnover by assessing LD colocalization with proteins specific to the ER (index of LD biogenesis) and proteins specific to the autophagosome (index of lipophagy).
METHODS
Participants
Thirty-six adults with obesity [body mass index (BMI) = 33 ± 3 kg/m2] participated in this study. Participants enrolled into this clinical trial were sedentary (not participating in aerobic or resistance-training programs for the previous 6 mo) and weight stable (±2 kg for the previous 6 mo). Participants were not taking medications known to affect glucose or lipid metabolism; they did not smoke and did not have a history of heart disease. All females were premenopausal, eumenorrheic, and not pregnant or lactating. Volunteers in this study also participated in previously published studies from our laboratory, focusing on the effect of 12-wk MICT and HIIT on whole body insulin sensitivity (hyperinsulinemic-euglycemic clamp) and adipose tissue metabolism (29, 30). Findings from the present study complement but do not overlap with main findings from previous publications. Participants provided written informed consent before participation. The study protocol was approved by the University of Michigan Institutional Review Board and registered at clinicaltrials.gov (NCT02706093).
Preliminary Assessment
Participants arrived to the Clinical Research Unit for a body composition assessment using dual-energy X-ray absorptiometry (Lunar DPX DEXA scanner; GE Healthcare, Madison, WI). A graded exercise test was then performed to determine maximal aerobic capacity (V̇o2peak) on a cycle ergometer (Lode Corvival; Groningen, Netherlands), as described previously by our laboratory (29).
Study Design
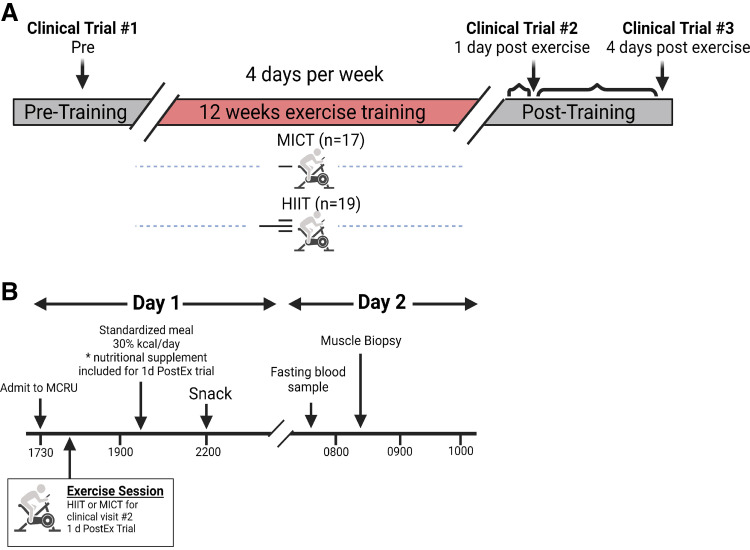
Enrolled participants were randomly assigned to either MICT (n = 17) or HIIT (n = 19; Table 1). Before training, participants completed their pretraining clinical trial, which included the collection of a skeletal muscle biopsy sample. Participants then completed 12 wk (4 sessions/wk) of their assigned exercise training program (MICT or HIIT; details of these training programs provided in the Exercise Training Interventions section). After completing the 12-wk training programs, participants completed two more clinical trials; the first of these posttraining clinical trials was completed the day after the last exercise session (1d PostEx). The second posttraining clinical trial was conducted 4 days after the last exercise session (4d PostEx), to examine the chronic adaptive responses to exercise training without the potent confounding effects of a recent session of exercise (31, 32; Fig. 1A). Importantly, participants were required to maintain their body weight throughout the entire 12-wk intervention.
Table 1.
Participant characteristics and plasma glucose, insulin, and lipid concentration before and after exercise training
| MICT (n = 17) |
HIIT (n = 19) |
|||||
|---|---|---|---|---|---|---|
| Sex, f/m | 12/5 |
12/7 |
||||
| Age, yr | 30 ± 6 |
31 ± 7 |
||||
| Height, m | 1.7 ± 0.08 |
1.71 ± 0.09 |
||||
| Pretraining | Trained 1d PostEx | Trained 4d PostEx | Pretraining | Trained 1d PostEx | Trained 4d PostEx | |
| Body mass, kg | 98.0 ± 11.7 | 97.3 ± 11.5 | 97.6 ± 11.7 | 96.4 ± 13.7 | 96.6 ± 13.7 | 96.6 ± 13.6 |
| Fat mass, kg | 42.5 ± 7.7 | 41.8 ± 7.7* | 42.0 ± 7.7* | 40.5 ± 7.1 | 40.0 ± 7.1* | 40.1 ± 7.2* |
| Fat free mass, kg | 55.5 ± 9.3 | 55.5 ± 9.5 | 55.7 ± 9.6 | 55.9 ± 10.5 | 56.5 ± 10.4 | 56.5 ± 10.3 |
| BMI, kg/m2 | 34.0 ± 3.2 | 33.7 ± 3.0 | 33.8 ± 2.9 | 32.9 ± 2.9 | 32.9 ± 3.0 | 33.0 ± 3.0 |
| Body fat % | 43.4 ± 6.0 | 43.1 ± 6.4* | 42.1 ± 5.4 | 41.6 ± 5.4* | ||
| V̇o2peak, mL/min | 2,322 ± 544 | 2,498 ± 589* | 2,452 ± 600 | 2,750 ± 638* | ||
| Plasma glucose, mM | 5.0 ± 0.4 | 4.9 ± 0.4 | 5.0 ± 0.5 | 4.8 ± 0.4 | 5.0 ± 0.4 | 4.9 ± 0.4 |
| Plasma insulin, µU/mL | 18.0 ± 9.0 | 17.5 ± 6.3 | 18.8 ± 7.8 | 16.0 ± 10.0 | 15.4 ± 14.6 | 15.3 ± 7.6 |
| Plasma NEFA, µM | 391 ± 159 | 440 ± 125 | 408 ± 118 | 433 ± 156 | 413 ± 123 | 387 ± 122 |
| Plasma triacylglycerol, mM | 1.25 ± 0.7 | 0.99 ± 0.4 | 1.10 ± 0.6 | 0.95 ± 0.64 | 0.96 ± 0.6 | 0.91 ± 0.69 |
Data are means ± SD. *Significant main effect of training status (P < 0.05), and post hoc analysis identifying significant difference from Pretraining. BMI, body mass index; f, female; HIIT, high-intensity interval training; m, male; MICT, moderate-intensity continuous training; NEFA, nonesterified fatty acid; V̇o2peak, peak oxygen uptake rate.
Figure 1.
Overall study design and clinical trial schematic. A: overall study design. A total of 48 sessions were completed during the 12-wk training intervention (4 days/wk). Clinical trials were completed once before training (Clinical Trial #1: Pre), 1 day after the last exercise session after the training period (Clinical Trial #2: 1d PostEx), and 4 days after the last exercise session (Clinical Trial #3: 4d PostEx). B: clinical trial schematic. Participants arrived to the Clinical Research Unit at 1730 h the day before metabolic testing and were provided a standardized meal. For Clinical Trial #2 only, the respective exercise session was completed at ∼1800 h. Muscle biopsies were obtained at ∼0815 h. *Nutritional supplement kcals were estimated to match energy expenditure from the previous exercise session. Created with BioRender.com.
Exercise Training Interventions
Participants in both groups exercised 4 days/wk for 12 wk. The exercise sessions for MICT consisted of 45-min continuous exercise at 70% HRmax. For the HIIT group, each training session consisted of 3-min warm up (65% HRmax), followed by 10 × 1-min intervals at 90% HRmax, with 1-min active recovery between intervals at ∼65% HRmax. Participants self-selected their mode of exercise (e.g., stationary bike, treadmill, elliptical, or rowing machines), and the mode of exercise selected by our participants was very similar between MICT and HIIT (cycling: n = 7 vs. n = 8; treadmill/elliptical: n = 10 vs. n = 11; rowing: n = 0 vs. n = 1 for MICT and HIIT, respectively). Our objective in this study was to compare HIIT and MICT programs commonly prescribed for improving fitness and health (33)—rather than attempting to match the exercise interventions for duration and/or energy expenditure. As such, HIIT sessions were considerably shorter than MICT (25 vs. 45 min), and energy expenditure for HIIT was less than MICT (∼150 vs. ∼250 kcals).
Training Familiarization, Exercise Adherence, and Weight Maintenance Monitoring
Participants were exposed to a progressive increase in exercise intensity and duration during the first 2 wk of exercise training. By the beginning of week 3, participants completed their prescribed exercise programs for MICT and HIIT. During the 2-wk familiarization period, research staff monitored all training sessions (4 sessions/wk). After completing the exercise familiarization, participants were still required to complete four exercise sessions per week, but only two of these sessions were required to be supervised directly by research staff. For the unsupervised session, participants wore downloadable telemetry heart rate devices (Polar; Kempele, Finland) that were monitored remotely by research staff to confirm exercise adherence and compliance. Adherence to this exercise training program was similar between MICT and HIIT, in which subjects completed 95% of the schedule exercise training sessions, as we reported previously (29). Body weight was also closely monitored throughout the training intervention, and participants were required to maintain their weight throughout the training intervention. Our research dietician consulted participants with modest dietary changes if their weight deviated ∼1%–2% pretraining weight.
Clinical Trials and Skeletal Muscle Biopsy Collection
During each of the three clinical trials, participants arrived to the Clinical Research Unit at 1730 h the evening before each clinical trial (Fig. 1B). The protocols for all three clinical trials were identical, with the exception of the 1d PostEx visit, during which participants completed their prescribed exercise sessions at 1800 h and then consumed a nutritional supplement (BoostPlus, Nestle; 360 kcals; 50% carbohydrate, 35% fat, and 15% protein) to match the estimated energy expended during the exercise session. For all three trials, participants consumed a standardized meal at 1900 h (30% estimated daily energy expenditure) and a snack (10% estimated daily energy expenditure) at 2200 h and slept in the Clinical Research Unit overnight.
The next morning, a fasting blood sample was obtained at 0730 h, and a skeletal muscle sample was collected from the vastus lateralis muscle at 0815 h. For the muscle samples to be used for microscopy analysis, visible connective tissue was removed under magnification, muscle fibers were oriented in parallel, and the sample was fixed in optimal cutting temperature (OCT) compound and flash frozen in isopentane chilled over liquid nitrogen. The remaining muscle samples were rinsed with saline, blotted dry, and flash frozen in liquid nitrogen for immunoblot analysis.
Measurement of Intramyocellular LD Content and Characteristics
Skeletal muscle samples frozen in OCT were cut into 5 µm sections at −20°C and mounted onto an ethanol-cleaned glass slide. Sections were fixed in 4% paraformaldehyde for 1 h, and permeabilized for 5 min in 0.5% Triton X-100. Sections were then incubated with primary antibodies for myosin heavy chain type 1 (MHC-1) at 1:200 for 1 h at 37°C, followed by 30-min secondary antibody incubation: AlexaFluor 647, goat anti-mouse IgG2b at 1:200. Afterward, sections were stained with bodipy 493/503 at 1:100 for 20 min, followed by 20-min incubation with anti-wheat germ agglutinin (WGA) conjugated with AlexaFluor 555 at 1:200 to identify muscle fiber borders. Sections were mounted with ProLong Gold Antifade Mountant, covered with No. 1 coverslips (2975-245, Corning; Corning, NY) and stored in the dark until imaging. See Supplemental Table S1 for complete list of reagents (https://doi.org/10.6084/m9.figshare.22341004).
Total lipid content (% stained), LD number (number of LDs/µm2), and median LD area per muscle fiber (µm2) were measured using images captured on a Keyence BZ-X710 fluorescence microscope (Keyence; Osaka, Japan) with a ×20 N.A. = 0.45 objective lens using 1,440 × 1,080 pixels (533 µm × 400 µm) field of view. After image acquisition, LD characteristics and distribution were calculated using MATLAB R2021a (MathWorks, Inc., Natick, MA). Briefly, ×20 images were converted to grayscale and rescaled to accommodate for image variability between samples. Individual fibers were identified and labeled using a custom ridge detection algorithm and watershed segmentation to complete noncontinuous cell borders and completely identify cells. Type I fibers were identified based on positive MHC type I-positive stain, and nonstained fibers were considered type II fibers. Fiber types were partitioned into type I or type II fibers dependent on MHC type I signal intensity by k-means clustering (k = 2). Lipid droplets identified in the SS region were contained within ∼3 µm of the peripheral border of each fiber (10-pixel width), whereas LDs in the IMF region were all remaining LDs in the center of each fiber (∼91% of cross-sectional myocyte area). Lipid droplets were detected as the mean area of puncta, and a top-hat filter was used to accommodate for image variability, background, and light scatter produced by the fluorescence microscope for each sample. Lipid droplets identified and included for analysis for each muscle fiber were within a normal Gaussian distribution. In total, ∼3 fields of view were obtained per participant for each clinical trial, resulting in the analysis of 165 ± 71 (median = 147) muscle fibers per participant for each clinical visit.
Measurement of LD Colocalization with ER and Autophagosome Proteins to Estimate LD Biogenesis and Lipophagy
Skeletal muscle samples frozen in OCT compound were cut into 5-µm sections at −20°C onto an ethanol-cleaned glass slide, then fixed in 4% paraformaldehyde for 30 min, followed by 5-min permeabilization in 0.3% Triton X-100, and blocked for 1 h in 5% normal goat serum. For each muscle sample, one cross section was placed onto a glass slide incubated overnight at 4°C with primary antibodies of the LD marker, PLIN2 (1:100 in 0.1% BSA, 0.1% Triton X-100, PBS), and the ER marker, KDEL (1:100 in 0.1% BSA, 0.1% Triton X-100, PBS). Because LDs are synthesized in the ER, the colocalization of these proteins was used as an estimate of de novo LD biogenesis. In addition, a separate section from the same muscle sample was placed on another glass slide and incubated at 4°C overnight with primary antibodies of PLIN2 and the autophagosome marker, microtubule-associated protein 1A/1B light chain 3, which is specific to the LC3B isoform (LC3; 1:50 in 0.1% BSA, 0.3% Triton X-100, PBS). The colocalization of these proteins was used as an index of lipophagy, as previously reported (34). The next day, samples were incubated with secondary antibodies Alexa Fluor 647, goat anti-mouse IgG1 (specificity to PLIN2) at 1:200 and Alexa Fluor 488, goat anti-rabbit IgG (specificity to KDEL and LC3) at 1:200 for 1 h. Samples were then incubated with anti-WGA conjugated with AlexaFluor 555 at 1:200 for 20 min, mounted with ProLong Gold Antifade Mountant, and covered with No. 1 coverslips (Corning), and stored in the dark until imaging.
Colocalization analysis was performed separately by PLIN2 colocalization with KDEL (de novo LD biogenesis) and PLIN2 colocalization with LC3 (lipophagy). Images were captured using Keyence BZ-X710 with a ×40 N.A. = 0.60 objective lens using 1,440 × 1,080 pixels (362 µm × 273 µm). Following image acquisition, an intensity threshold was selected uniformly for each target of interest to identify positive signals and remove background intensities. Quality controls for nonspecific secondary antibody binding were completed and verified before colocalization analysis was conducted. Colocalization between PLIN2-KDEL and PLIN2-LC3 was quantified by Pearson’s and Mander’s (M1) overlap coefficient using the Coloc2 plug-in with FIJI (NIH Image J) (35).
Skeletal Muscle Lysate Preparation and Immunoblot
Frozen muscle samples were weighed (∼25 mg) and transferred into prechilled microcentrifuge tubes containing 1-mL ice-cold lysis buffer [radioimmunoprecipitation assay (RIPA) buffer: 20 mM Tris-HCl-pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin (9086, Cell Signaling Technology)], 1% phosphatase inhibitor cocktail No. 1 and No. 2 (P2850, P5726, Sigma-Aldrich), 1% protease inhibitor cocktail (P8340, Sigma-Aldrich), and two steel ball bearings per sample. Samples were homogenized for 30 s at 45 Hz using a Qiagen TissueLyser II, then solubilized for 60 min by inverted end-over-end rotation at 50 rpm in the cold (4°C). Afterward, samples were centrifuged at 15,000 g for 15 min at 4°C, and the pellet was discarded. Protein concentration was determined by bicinchoninic acid assay method (BCA; 23225, Pierce Thermo-Fisher), and samples were diluted in 4× Laemmli buffer and RIPA buffer to achieve 1 µg/µL concentration and heated for 7 min at 95°C. Proteins were separated by SDS-PAGE (8%–15% acrylamide gels), then transferred to nitrocellulose membranes, and probed by the primary antibodies listed in Supplemental Table S1 (https://doi.org/10.6084/m9.figshare.22341004). To account for loading variability, membranes were stained and normalized to total protein abundance by Memcode (24580, Pierce Thermo-Fisher) and an internal standard sample (i.e., homogenate of pooled muscle samples collected from eight individuals with obesity) that was included on all gels to account for gel-to-gel variability.
Statistical Analysis
Normality was tested using the Shapiro–Wilk test, and data not normally distributed were log transformed before analysis. When missing data for a specific measurement was present and unavoidable (e.g., poor frozen section or inability to obtain a sample), data for that specific measurement was excluded for all trials for the participant, and the specific sample size was reported in the figure legend where data were missing. For immunoblot and LD colocalization analysis, linear mixed models were used to determine the main effect of group (MICT vs. HIIT), visit (pre, 1d PostEx, and 4d PostEx), and group × visit interaction. For outcomes with significant main effects for time, then post hoc comparisons for visit was completed (e.g., Pre, 1d PostEx, and 4d PostEx) in both HIIT and MICT. For LD histochemistry analysis, measurements of LD area, LD number, and LD size were segregated by fiber type (type I and type II fibers) and regional distribution (IMF vs. SS region). Linear mixed models were then used on the segregated outcomes with the same main effects as previously described (group × visit). Data are displayed as means ± SD, and P ≤ 0.05 was considered statistically significant. Statistical analysis and figure generation were completed using R version 4.1.0 (36).
RESULTS
Participant Characteristics at Baseline and in Response to Training
Table 1 provides the physical characteristics and fasting plasma glucose, insulin, and lipid concentrations before and after training in the 36 participants who completed the 12-wk exercise training intervention (MICT = 17, HIIT = 19). MICT and HIIT both significantly increased aerobic capacity (P < 0.01). The trend for a greater increase in V̇o2peak after HIIT versus MICT did not reach statistical significance (P = 0.1). Although body weight did not change after the 12 wk training interventions in either group, the slight but consistent reduction in fat mass after training (∼0.5 kg) was found to be statistically significant (P = 0.02).
Training Effects on Skeletal Muscle Lipid Content and LD Distribution
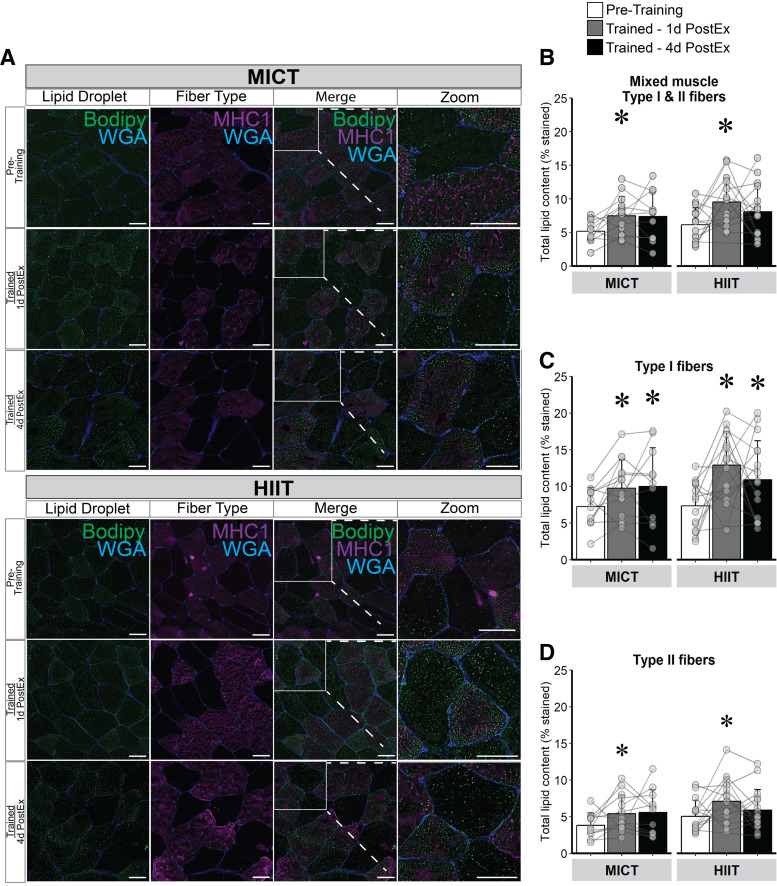
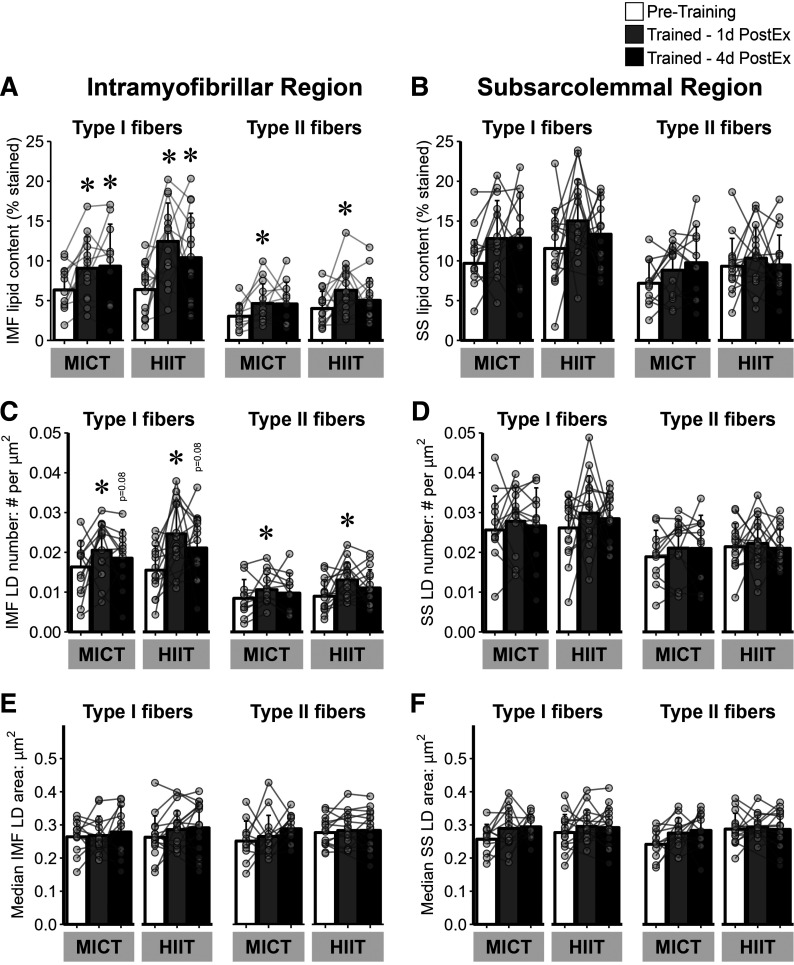
Twelve weeks of exercise training significantly increased total muscle lipid content, with no significant difference between MICT and HIIT (Fig. 2, A and B). The increase in muscle lipid content was found in both type I (Fig. 2C) and type II (Fig. 2D) muscle fibers (P ≤ 0.01 for both), with the increase in lipid content persisting 4 days after the last exercise session only in type I fibers. Moreover, the increase in muscle lipid content after training was specific to the IMF region of both type I and type II fibers (P < 0.01), and again, the responses were similar between MICT and HIIT (Fig. 3A). In contrast to the IMF region, lipid content in the SS region was not affected by training (Fig. 3B). The greater lipid content in the IMF region after training was attributed to an increase in the number of intramyocellular LDs (number of LD/µm2) in both type I and type II fibers (both P ≤ 0.02; Fig. 3C), but not an increase in individual LD area (Fig. 3E). There were no training-induced changes observed in LD number or LD area in the SS region of the muscle samples (Fig. 3, D and F).
Figure 2.
Lipid content in mixed skeletal muscle, type I, and II muscle fibers in response to training. A: representative image: samples were stained with bodipy 493/503 (LD, green), myofiber stain for myosin heavy chain I (MHC1; type I fibers, purple) and wheat germ agglutinin (WGA; membranes, blue). The regions inside the white boxes are enlarged for a clearer view. Representative images for each time point were obtained from the same participant in each group. Magnification, ×20. Scale bar, 100 µm for all images. B: lipid content represented as % stained in mixed skeletal muscle (type I and II fibers). C: lipid content in type I muscle fibers (MHC1+). D: lipid content in type II muscle fibers (MHC1−). MICT, n = 14; HIIT, n = 17. *Main effect for visit (P < 0.05) and post hoc analysis identifying significant difference from pretraining. Data are expressed as means ± SD. HIIT, high-intensity interval training; LD, lipid droplet; MICT, moderate-intensity continuous training.
Figure 3.
LD characteristics within the IMF and SS region of type I and II muscle fibers in response to training. A: lipid content (% stained) in the IMF region of type I and II muscle fibers. B: lipid content in the SS region of type I and II muscle fibers. C: LD number (number of LDs/µm2) in the IMF region of type I and II muscle fibers. D: LD number in the SS region of type I and II muscle fibers. E: LD median area (µm2) per fiber in the IMF region of type I and II muscle fibers. F: LD median area per fiber in the SS region of type I and II muscle fibers. MICT, n = 14; HIIT, n = 17. *Main effect for visit (P < 0.05), and post hoc analysis identifying significant difference from pretraining. Data are expressed as means ± SD. HIIT, high-intensity interval training; IMF, intramyofibrillar region; LD, lipid droplet; MICT, moderate-intensity continuous training; SS, subsarcolemmal region.
Effect of Training on Estimates of De Novo LD Biogenesis
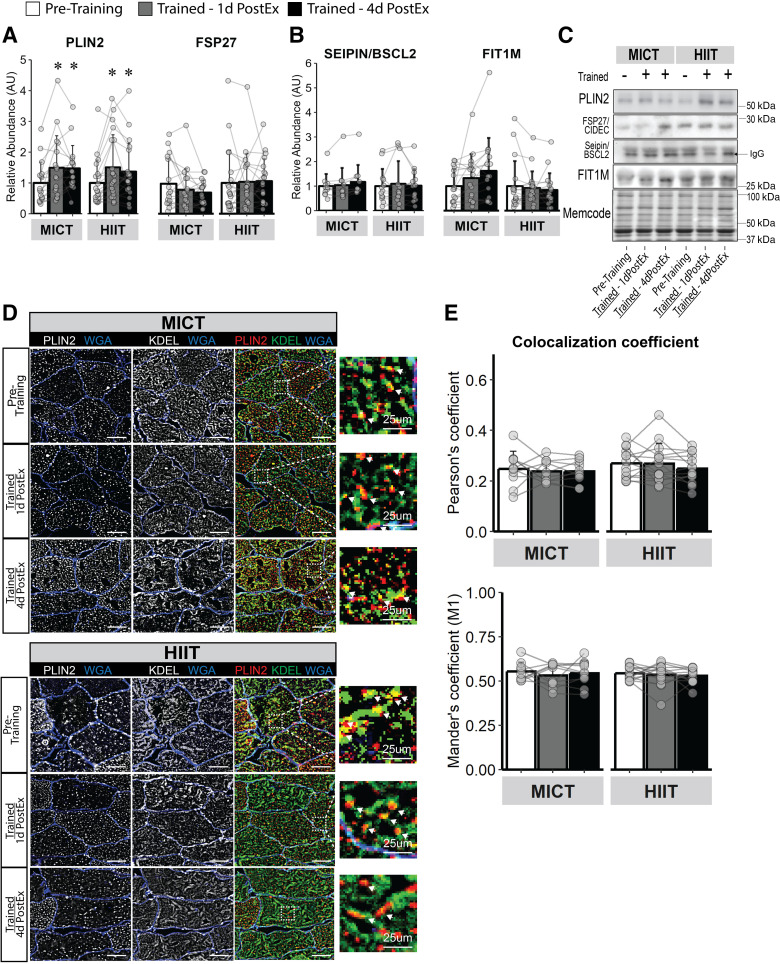
Both MICT and HIIT significantly increased whole muscle PLIN2 abundance (P < 0.01), a protein highly expressed on LD membranes involved in lipolytic regulation (37; Fig. 4, A and C). However, neither MICT nor HIIT altered the protein abundance of Seipin (BSCL2) or FIT1M (Fig. 4, B and C), which are proteins involved in LD stabilization and LD budding from the ER (38). Importantly, the absence of change in the abundance of proteins involved in LD formation from the ER cannot be interpreted to indicate that LD biogenesis is also unchanged with training. To examine this further, we measured the colocalization of PLIN2 (a constitutive lipid droplet protein), commonly used as a marker for lipid droplets (39) with the ER protein, KDEL, as a crude index of LD emergence from the ER (Fig. 4D provides a representative image of the colocalization experiment). There was no effect for training to modify PLIN2 and KDEL colocalization, measured by both Pearson’s and Mander’s colocalization coefficients (Fig. 4E).
Figure 4.
Skeletal muscle LD abundance and de novo LD biogenesis activity in response to training. Immunoblots for proteins corresponding to triacylglycerol storage and release localized to the LD membrane (PLIN2 and FSP27; A) and LD stabilization localized to the ER (Seipin/BSCL2 and FIT1M; B). C: representative images for immunoblots in A and B. D: representative immunofluorescence images for LD (PLIN2, red) colocalization with the ER (KDEL, green) as a measure of de novo LD biogenesis from the ER. Membranes are stained blue. Magnification, ×40. Scale bar, 100 µm for standard images and 25 µm for zoomed images. White arrows from zoom represent colocalization event. E: LD-ER colocalization measured by Pearson’s and Mander’s (M1) coefficient. MICT, n = 11; HIIT, n = 13. *Main effect for visit (P < 0.05) and post hoc analysis identifying significant difference from pretraining. Data are expressed as means ± SD. ER, endoplasmic reticulum; HIIT, high-intensity interval training; LD, lipid droplet; MICT, moderate-intensity continuous training.
Training Effects on Estimates of Autophagy-Mediated LD Degradation
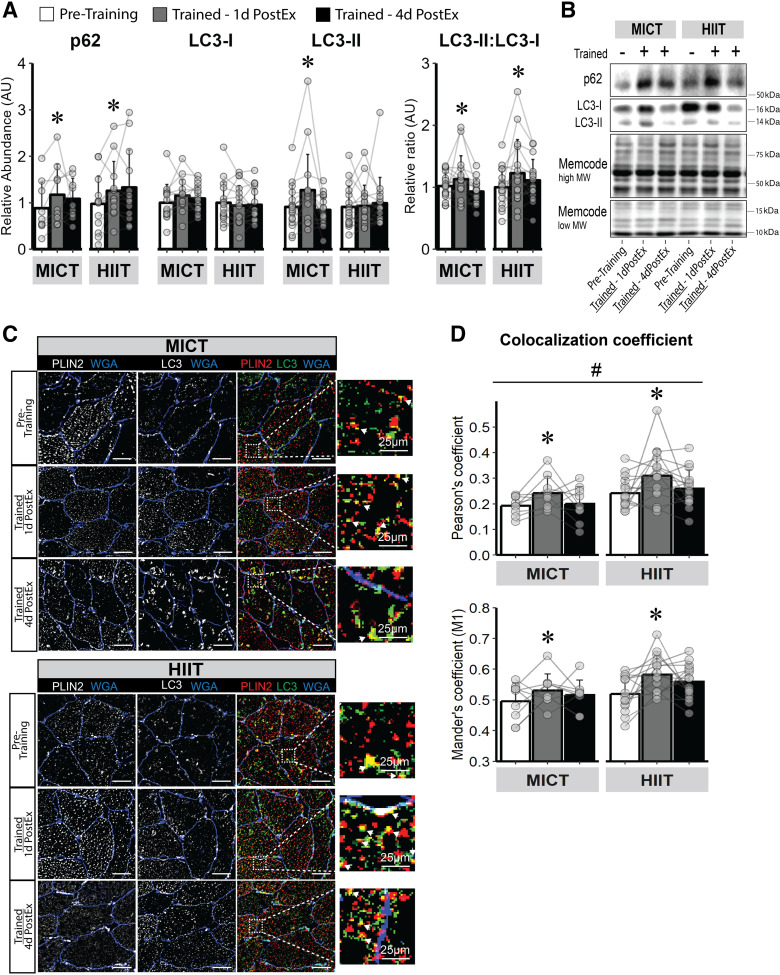
The abundance of p62, a marker of protein ubiquitination, was increased after training, and there were no differences between MICT and HIIT. However, p62 was only significantly increased 1 day after the last exercise session (P < 0.01) and was no longer significantly greater than pretraining abundance 4 days after the last exercise session (Fig. 5A). LC3-II, a protein marker specific to the autophagosome, increased only after MICT (P = 0.02; Fig. 5A). However, the ratio of LC3-II:LC3-I, which is reflective of LC3 activation, was significantly elevated after both MICT and HIIT 1 day after the last exercise session (P = 0.03; Fig. 5A), but returned toward pretraining levels 4 days after the last exercise session (Fig. 5A). We measured the colocalization of PLIN2 with LC3 as a crude index of lipophagy in skeletal muscle (Fig. 5C). Here, we found both MICT and HIIT increased LC3 colocalization with PLIN2 measured by both Pearson’s (main effect for visit, P = 0.04) and Mander’s (main effect for visit, P < 0.01) coefficients, with no significant difference in response between MICT and HIIT (Fig. 5D). Interestingly, LC3-PLIN2 colocalization was only significantly elevated 1 day after the last exercise session (Pearson’s, P = 0.03; Mander’s, P = 0.01) and returned toward pretraining levels 4 days after the last exercise session.
Figure 5.
Skeletal muscle autophagy induction and LD-targeted autophagy in response to training. A: immunoblot abundance for proteins corresponding to autophagy induction (p62), autophagosome activation (LC3-II), and LC3-II: LC3-I ratio—an index of active (LC3-II) to inactive (LC3-I) autophagosome abundance. B: representative images for immunoblots. C: representative immunofluorescence image for LD (PLIN2, red) colocalization with the autophagosome (LC3, green) as a measure of selective LD degradation (lipophagy). Membranes are stained blue. Magnification, ×40. Scale bar, 100 µm for standard images and 25 µm for zoomed images. White arrows from zoom represents colocalization event. D: LD-LC3 colocalization measured by Pearson’s and Mander’s (M1) coefficient. MICT, n = 9; HIIT, n = 13. *Main effect for visit (P < 0.05) and post hoc analysis identifying significant difference from pretraining. #Main effect for group (HIIT vs. MICT; P < 0.05). Data are expressed as means ± SD. HIIT, high-intensity interval training; LD, lipid droplet; MICT, moderate-intensity continuous training.
DISCUSSION
Endurance exercise training is often found to increase skeletal muscle lipid content (10–15). The current study found 12 wk of both MICT and HIIT increased intramyocellular lipid content in previously sedentary adults with obesity. One important finding from this study was that the increase in total lipid content in skeletal muscle after training was due to an increased number of intramyocellular LDs, but not increased LD size. Furthermore, the increased number of LDs was specific to the IMF region of muscle fibers, suggestive of an adaptive response to increase energy supply in close proximity to the site of high energy expenditure during muscle contraction. The mechanisms underlying the increased LD number within the IMF region are not clear, but de novo LD biogenesis from the ER, fission of preexisting LDs, and LD turnover by lipophagy are likely candidates. Another key finding from our study was that despite the robust differences in training intensity and energy expenditure between MICT and HIIT, their effects on lipid abundance and metabolism in skeletal muscle were remarkably similar.
Endurance exercise training is consistently found to increase muscle lipid content in lean participants (10, 11) but this is not always the case in adults with overweight and obesity (16–18, 40–42). The absence of a measurable increase in muscle lipid content after training in some studies may be a consequence of a very high muscle lipid abundance found in some sedentary obese adults (43), which may make it challenging to detect a relatively small exercise-induced increase in muscle lipid content. In addition, when an exercise training intervention is accompanied by weight loss, a training-induced increase in muscle lipid storage capacity may be countered by a reduction in lipid availability due to weight loss (4, 13, 44, 45). The observed increase in muscle lipid content in our study may be a result of the study population having relatively modest obesity (class 1 obesity) and the strict requirement for body weight maintenance during the training intervention. It is also important to consider that the effects of exercise on muscle lipid accumulation may largely be a consequence of the most recent session, or sessions of exercise, rather than an adaptive response to long-term training. For example, adipose tissue lipolysis is elevated after exercise, thereby increasing the availability of fatty acids to provide substrate for muscle lipid synthesis during the hours after a session of exercise (46, 47). This increase in muscle lipid synthesis and storage after exercise may be adaptive to support the metabolic demand of the working muscle for subsequent exercise sessions.
Muscle lipids are largely stored within LDs, which are intramyocellular organelles composed of a hydrophobic core surrounded by a phospholipid monolayer (48). Assessments of the number, size, and cellular distribution of LDs enhance our interpretation of intramyocellular lipid metabolism and storage after endurance training. Our finding that an increase in muscle lipid content after training was largely attributed to more LDs within muscle fibers, and not LD size, agrees with some previous studies (11, 14, 49). This adaptation to increase muscle lipid content, via a greater number of LDs after training, permits total lipid content expansion without accumulating larger-sized LDs. Relatively large LDs may be metabolically unfavorable due to their relatively low surface area-to-volume ratio, which can compromise lipolytic control of the lipids within the LD (50, 51). Interestingly, the accumulation of larger-sized LDs has been shown to inversely correlate with insulin sensitivity (19, 52), and the decrease in LD size after training has been proposed to contribute to the insulin-sensitizing effect of exercise (16–18, 52). Moreover, increasing muscle lipid content by increasing the number of LDs has been found to increase the physical interaction between the LDs and the mitochondria (14, 16, 41), which can further enhance coordination between fatty acid availability for muscle lipid oxidation during exercise.
The increase in lipid content specifically in the IMF region of the muscle fiber after training, as reported here by us and by others (16–18, 53), is also favorable for coordinating energy supply and demand during exercise. Skeletal muscle fibers are highly specialized, and different subcellular regions of the myocyte support different processes. The peripheral SS region is adjacent to the cell membrane, and components of the SS are largely involved in membrane function, signaling, and maintenance (23). In contrast, the centrally localized IMF region of the muscle fiber contains contractile proteins and is the site of greatest energy demand during exercise (23). Therefore, an increase in LDs in the IMF region after training will likely support energy required for muscle contraction (24). Some previous reports suggest exercise training may lead to a redistribution of LDs from the SS and into the IMF region of the muscle fiber (16–18, 53). However, this was not apparent after either MICT or HIIT in our study, because the increase in LDs in the IMF region was not accompanied by a reduction in SS LDs.
Other proposed mechanisms that may contribute to increased LD number within the IMF region after training include de novo LD biogenesis from the ER (38) and fragmentation (i.e., fission) of preexisting LDs (54, 55). De novo LD biogenesis involves fatty acid esterification into triacylglycerol within the ER leaflets, contributing to nascent LD growth and budding toward the cytosol (38). Here, immunofluorescence microscopy quantified LD colocalization with the ER as a rough index of de novo LD biogenesis, and we found exercise training did not affect our measure of LD colocalization with the ER. However, based on the somewhat limited resolution of the fluorescence colocalization approach we used to assess LD budding from the ER compared with confocal microscopy, we cannot rule out the possibility that exercise may impact LD biogenesis. In addition, it is possible our sampling timeline (samples collected ∼14 h after the last exercise session) was not optimal to capture an acute increase in de novo LD biogenesis. LD fission of preexisting LDs is also difficult to detect from human biopsies, but the observed increase in the number of LDs without a reduction in LD size could reflect LD fission coupled with increased intramyocellular triacylglycerol synthesis that is known to increase after exercise (3). Mechanisms contributing to LD fission are still unclear (38), but it is intriguing to consider that an increase triacylglycerol synthesis in the hours after a session of exercise may increase tension within the hydrophobic core of LDs, facilitating LD fission (54).
Lipophagy is a specialized form of autophagy and is essential for the degradation of intracellular LDs by the lysosome (21). In skeletal muscle, lipophagy has been reported to help regulate LD turnover (56). Lipophagy appears to be regulated, at least in part, by the energy status within the cell, increasing in response to an energy deficit (21, 34). Autophagy is a cellular recycling process involving the ubiquitin-proteasome and the autophagosome-lysosome systems to promote cellular remodeling and has been shown to be activated after endurance exercise (57, 58). Perhaps, this process is stimulated by an exercise session to promote the homeostatic turnover of organelles and substrates for training-induced skeletal muscle remodeling (58). Our findings suggest skeletal muscle substrate remodeling by lipophagy may increase the day after the most recent exercise training session but may return to pretraining levels after abstaining from exercise for 4 days. The transient nature of this response may reflect the changes in intracellular energy flux occurring during the several hours of recovery after exercise, in effort to increase fatty acid availability as local lipid and carbohydrate (i.e., glycogen) stores are being replenished. However, we recognize the increased LC3-II/LC-I ratio we found after exercise provides a rough estimate of autophagosome activation and does not confirm the rate of autophagic flux is increased in skeletal muscle after exercise (59).
It is reasonable to surmise that an increase in lipophagy in skeletal muscle after exercise may coincide with a reduction in the number of LDs; our findings suggest the opposite (i.e., the number of muscle LDs increased). In agreement with our findings, it has been reported that an increase in lipophagy in response to energy deficit was accompanied by an increase in the number of LDs, especially an increase in LDs adjacent to the mitochondria (34). Therefore, perhaps an exercise-induced increase in lipophagy may serve as an adaptive response to increase LD turnover and redistribution within the IMF region of the muscle fiber, in effort to support fatty acid availability and oxidation for the working muscle, which has been shown to occur after exercise training (14, 16).
Excessive accumulation of lipid in skeletal muscle is commonly associated with insulin resistance (7, 9). In particular, the accumulation of lipid intermediates and metabolites (e.g., diacylglycerol, ceramides, and acylcarnitine) has been causally linked to impaired insulin signaling (60–62). The subcellular distribution of these lipids also appears to be important, whereby their accumulation specifically in the SS region of the myocyte has been linked with insulin resistance (18, 19, 52, 63, 64). The insulin desensitizing effects of an accumulation of these lipids specific to the SS region may be due largely to their proximity to the cell membrane, the site of important insulin signaling events. It has been proposed that exercise training may help alleviate insulin resistance in obesity, in part, by decreasing SS lipid content or SS LD size (16–19, 52). In contrast, however, we did not observe a reduction in SS lipid content or LD size after 12 wk of MICT or HIIT. Moreover, we recently reported that 12 wk of HIIT or MICT training did not improve insulin sensitivity when assessed 4 days after the final exercise bout (29). Perhaps, this was a consequence of our strict requirement for weight maintenance in all participants. Based on our findings, it is intriguing to consider that a reduction in SS lipid content after an exercise/lifestyle intervention may be very important for improving insulin sensitivity. Our findings that exercise training increased muscle lipid content specifically in the IMF region suggest the exercise-induced muscle lipid adaptations to MICT and HIIT in our participants may have occurred primarily to support oxidative metabolism, without compromising insulin sensitivity.
Surprisingly, the exercise-induced adaptations in muscle lipid outcomes measured in this study were very similar between MICT and HIIT, despite marked differences in exercise intensity, duration, and energy expenditure during each training session. Although this finding was contrary to our hypothesis, it does align with previous work in which similar increases in muscle lipid content were found in response to MICT versus sprint interval training, which represents an even greater disparity in intensity, duration, and energy expenditure than our MICT versus HIIT comparison (11). Reasons to explain the similar adaptive responses between these distinct exercise stimuli are not clear. It is possible that the adaptive response to the high-intensity stimuli of HIIT may have been quantitatively matched by the longer duration of muscle contraction and/or the higher energy requirements of MICT. It is also possible that exercise during each session of both MICT and HIIT surpass a “threshold” stimuli required for the observed adaptations in lipid abundance and localization. Alternatively, similar increases in the number of LDs in muscle after MICT and HIIT could derive from very different mechanisms; one training program may increase the rate of LD biogenesis whereas the other training program may reduce the rate of LD degradation. But this possibility seems unlikely and is not supported by our assessments of LD biogenesis and lipophagy in this study. Regardless of the mechanisms involved to explain the similar responses between MICT and HIIT, the observation that these two exercise programs both increased the number of LDs in the IMF region of muscle fibers suggests prioritization for adaptive responses to exercise training to provide energy-rich substrate near the site of highest energy requirements in the cell.
Overall, our findings shed new light on the effects of MICT and HIIT on muscle lipid accumulation; however, we acknowledge there are some limitations that may impact interpretation. For example, although participants were instructed to maintain their habitual dietary behaviors during the intervention, we did not strictly control for macronutrient composition, which can affect muscle lipid content (47). In addition, regarding the comparisons between MICT and HIIT, our study was designed to address an applied comparison between these two commonly implemented training prescriptions. As a result, each session of MICT was longer than HIIT (45 vs. 25 min), and estimated energy expenditure was also higher in MICT than HIIT (∼250 vs. ∼150 kcals/session), so we were not able to distinguish the effects of exercise intensity, duration, or energy expenditure. However, an important outcome from our study was how remarkably similar the training adaptations were to MICT and HIIT despite the very large differences in intensity, duration, and energy expended during each exercise session. It is also important to acknowledge that our study was not powered to assess sex differences, and although we did not observe any obvious trends for different responses in our male and female participants, sex differences in skeletal muscle lipid content and metabolism have been reported previously (14, 65). In addition, the fluorescence microscopy images used for our analyses of LD size, density, and localization were captured at a lower resolution compared with some other studies (47, 52, 66). The lower resolution limited our ability to specifically quantify the number of LDs directly underneath the sarcolemma, which may have somewhat overestimated the number of SS LDs (and underestimated the number of IMF LDs) we reported here. However, this limitation should not impact our interpretation that exercise training (both MICT and HIIT) increased the number of LDs in the more central IMF region of the myocyte. The lower resolution may have also limited the accuracy of our measurements of LD size because the number of pixels allocated for the LD-positive area was rather small (2–10 pixels per LD). Furthermore, light emitted from excited pixels in the fluorescence microscope may have created false-positive LD pixels in raw image acquisition. To compensate for this limitation, we implemented a top-hat filter in our image processing script in MatLab to limit the number of false-positive pixels and subset the light-scattered pixels into an LD-negative pixel. Finally, our measures of LD colocalization with the ER and the autophagosome provide only a crude index of LD biogenesis and degradation. For example, our interpretation that exercise increased the colocalization of LDs with the autophagosome can be confounded because the lipidation of LC3-I to LC3-II cannot be distinguished by microscopy techniques in the current study. As such, we cannot confirm the observed increased in PLIN2 colocalization with LC3 was indeed due to an increased colocalization of PLIN2 with the autophagosome-specific isoform, LC3-II, or the cytosolic isoform LC3-I. Immunoblot analysis can distinguish between these isoforms, so to complement our immunohistochemistry analysis, we used an immunoblot approach to quantify the LC3-II/LC3-I ratio (often used as a marker of autophagosome activation; 59). Our finding that LC3-II/LC3-I ratio increased similarly to the observed increase PLIN2-LC3 interaction measured by microscopy supports the notion that exercise may increase LD-autophagosome interaction. Future studies using alternative analytical techniques, such as transmission electron microscopy, may more effectively identify LD interaction with the autophagosome and key organelles regulating LD turnover. In addition, further work is warranted to confirm this dynamic and multistep process of lipophagy, including monitoring the rate of autophagic flux through lysosomes, and tracking the subsequent release of breakdown products, as explained by Klionsky et al. (59).
In summary, 12 wk of exercise training (both MICT and HIIT) in adults with obesity increased muscle lipid abundance via an increase in the number of LDs in the more centrally located IMF region of the myocyte. This adaptation to exercise training results in an enhanced energy supply for mitochondrial respiration in the region of highest energy requirements during muscle contraction. Our findings also suggest an exercise-induced increase in lipophagy may underlie changes in LD turnover and redistribution within the myocyte. Interestingly, the adaptive responses to MICT and HIIT were surprisingly similar despite the considerable differences in exercise intensity, duration, and energy expenditure between these exercise programs. Overall, our findings indicated that 12 wk of exercise training in adults with obesity (both MICT and HIIT) led to a greater number of LDs in the IMF region of the muscle cell, thereby increasing lipid availability to support oxidative metabolism near the site of highest energy expenditure during exercise.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22341004.
GRANTS
This work was supported by National Institutes of Health (R01DK077966, P30DK089503, U24DK097153, T32DK007245, F32DK117522, R50CA221807) and the Canadian Institutes of Health Research (338735 and 146190).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.W.S., C.A., B.J.R., J.B.G., A.C.L., D.W.V.P., L.M.P., S.M.H., C.F.B., and J.F.H. conceived and designed research; M.W.S., C.A., B.J.R., O.K.C., A.T.L., T.Z., T.R., and J.F.H. performed experiments; M.W.S. and K.E.L. analyzed data; M.W.S., C.A., B.J.R., O.K.C., A.T.L., K.E.L., J.B.G., A.C.L., and J.F.H. interpreted results of experiments; M.W.S. prepared figures; M.W.S. and J.F.H. drafted manuscript; M.W.S., C.A., B.J.R., J.B.G., A.C.L., L.M.P., and J.F.H. edited and revised manuscript; M.W.S., C.A., B.J.R., O.K.C., A.T.L., K.E.L., J.B.G., A.C.L., D.W.V.P., L.M.P., T.Z., T.R., S.M.H., C.F.B., and J.F.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study participants for their efforts; Dr. Benjamin Carr, Dr. Jacob Haus, Jeffrey Wysocki, RN; the staff at the Michigan Clinical Research Unit; and all the members of the Substrate Metabolism Lab for study assistance. Figure 1 and Graphical Abstract created with BioRender and published with permission.
REFERENCES
- 1. Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Physiol 265: E380–E391, 1993. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 2. van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553: 611–625, 2003. doi: 10.1113/jphysiol.2003.052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 5. Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 6. Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol Endocrinol Physiol 276: E278–E284, 1999. doi: 10.1152/ajpendo.1999.276.2.E278. [DOI] [PubMed] [Google Scholar]
- 7. Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42: 113–116, 1999. [Erratum in Diabetologia 42: 386, 1999]. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 8. Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48: 1600–1606, 1999. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 9. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 10. Schrauwen-Hinderling VB, Schrauwen P, Hesselink MK, van Engelshoven JM, Nicolay K, Saris WH, Kessels AG, Kooi ME. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab 88: 1610–1616, 2003. doi: 10.1210/jc.2002-021464. [DOI] [PubMed] [Google Scholar]
- 11. Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, Wagenmakers AJ, Shaw CS. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol 591: 657–675, 2013. doi: 10.1113/jphysiol.2012.240952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haus JM, Solomon TP, Lu L, Jesberger JA, Barkoukis H, Flask CA, Kirwan JP. Intramyocellular lipid content and insulin sensitivity are increased following a short-term low-glycemic index diet and exercise intervention. Am J Physiol Endocrinol Physiol 301: E511–E516, 2011. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubé JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, Coen P, Goodpaster BH. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54: 1147–1156, 2011. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol 292: R1271–R1278, 2007. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 15. Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Physiol 287: E857–E862, 2004. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 16. Devries MC, Samjoo IA, Hamadeh MJ, McCready C, Raha S, Watt MJ, Steinberg GR, Tarnopolsky MA. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. J Clin Endocrinol Metab 98: 4852–4862, 2013. doi: 10.1210/jc.2013-2044. [DOI] [PubMed] [Google Scholar]
- 17. Koh HE, Ørtenblad N, Winding KM, Hellsten Y, Mortensen SP, Nielsen J. High-intensity interval, but not endurance, training induces muscle fiber type-specific subsarcolemmal lipid droplet size reduction in type 2 diabetic patients. Am J Physiol Endocrinol Physiol 315: E872–E884, 2018. doi: 10.1152/ajpendo.00161.2018. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen J, Mogensen M, Vind BF, Sahlin K, Højlund K, Schrøder HD, Ortenblad N. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Physiol 298: E706–E713, 2010. doi: 10.1152/ajpendo.00692.2009. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen J, Christensen AE, Nellemann B, Christensen B. Lipid droplet size and location in human skeletal muscle fibers are associated with insulin sensitivity. Am J Physiol Endocrinol Physiol 313: E721–E730, 2017. doi: 10.1152/ajpendo.00062.2017. [DOI] [PubMed] [Google Scholar]
- 20. Walther TC, Chung J, Farese RV Jr.. Lipid droplet biogenesis. Annu Rev Cell Dev Biol 33: 491–510, 2017. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature 458: 1131–1135, 2009. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012: 282041, 2012. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferreira R, Vitorino R, Alves RM, Appell HJ, Powers SK, Duarte JA, Amado F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10: 3142–3154, 2010. doi: 10.1002/pmic.201000173. [DOI] [PubMed] [Google Scholar]
- 24. Shaw CS, Jones DA, Wagenmakers AJ. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol 129: 65–72, 2008. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 25. Shaw CS, Swinton C, Morales-Scholz MG, McRae N, Erftemeyer T, Aldous A, Murphy RM, Howlett KF. Impact of exercise training status on the fiber type-specific abundance of proteins regulating intramuscular lipid metabolism. J Appl Physiol (1985) 128: 379–389, 2020. doi: 10.1152/japplphysiol.00797.2019. [DOI] [PubMed] [Google Scholar]
- 26. Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol (1985) 98: 1985–1990, 2005. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 27. Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity (Silver Spring) 21: 2249–2255, 2013. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- 28. Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol (1985) 111: 1554–1560, 2011. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 29. Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, Van Pelt DW, Pitchford LM, Chenevert TL, Gioscia-Ryan RA, Howton SM, Rode T, Hummel SL, Burant CF, Little JP, Horowitz JF. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab 105: e2941–e2959, 2020. doi: 10.1210/clinem/dgaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahn C, Ryan BJ, Schleh MW, Varshney P, Ludzki AC, Gillen JB, Van Pelt DW, Pitchford LM, Howton SM, Rode T, Hummel SL, Burant CF, Little JP, Horowitz JF. Exercise training remodels subcutaneous adipose tissue in adults with obesity even without weight loss. J Physiol 600: 2127–2146, 2022. doi: 10.1113/JP282371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heath GW, Gavin JR 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol Respir Environ Exerc Physiol 55: 512–517, 1983. doi: 10.1152/jappl.1983.55.2.512. [DOI] [PubMed] [Google Scholar]
- 32. King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol (1985) 64: 1942–1946, 1988. doi: 10.1152/jappl.1988.64.5.1942. [DOI] [PubMed] [Google Scholar]
- 33. Gibala MJ. Interval training for cardiometabolic health: why such a HIIT? Curr Sports Med Rep 17: 148–150, 2018. doi: 10.1249/JSR.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 34. Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 32: 678–692, 2015. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org/. [Google Scholar]
- 37. Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263, 1997. doi: 10.1016/S0022-2275(20)34939-7. [DOI] [PubMed] [Google Scholar]
- 38. Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20: 137–155, 2019. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai TH, Chen E, Li L, Saha P, Lee HJ, Huang LS, Shelness GS, Chan L, Chang BH. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 13: 1130–1144, 2017. doi: 10.1080/15548627.2017.1319544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, van de Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shepherd SO, Cocks M, Meikle PJ, Mellett NA, Ranasinghe AM, Barker TA, Wagenmakers AJM, Shaw CS. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int J Obes (Lond) 41: 1745–1754, 2017. doi: 10.1038/ijo.2017.170. [DOI] [PubMed] [Google Scholar]
- 42. He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res 12: 761–769, 2004. doi: 10.1038/oby.2004.92. [DOI] [PubMed] [Google Scholar]
- 43. Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Physiol 284: E741–E747, 2003. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- 44. Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol 587: 4949–4961, 2009. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Helge JW, Stallknecht B, Drachmann T, Hellgren LI, Jiménez-Jiménez R, Andersen JL, Richelsen B, Bruun JM. Improved glucose tolerance after intensive life style intervention occurs without changes in muscle ceramide or triacylglycerol in morbidly obese subjects. Acta Physiol (Oxf) 201: 357–364, 2011. doi: 10.1111/j.1748-1716.2010.02180.x. [DOI] [PubMed] [Google Scholar]
- 46. Mulla NA, Simonsen L, Bülow J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: the effects of exercise intensity. J Physiol 524: 919–928, 2000. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Daemen S, van Polanen N, Bilet L, Phielix E, Moonen-Kornips E, Schrauwen-Hinderling VB, Schrauwen P, Hesselink MKC. Postexercise changes in myocellular lipid droplet characteristics of young lean individuals are affected by circulatory nonesterified fatty acids. Am J Physiol Endocrinol Physiol 321: E453–E463, 2021. doi: 10.1152/ajpendo.00654.2020. [DOI] [PubMed] [Google Scholar]
- 48. Walther TC, Farese RV Jr.. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 81: 687–714, 2012. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Physiol 287: E558–E565, 2004. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 50. Thiam AR, Beller M. The why, when and how of lipid droplet diversity. J Cell Sci 130: 315–324, 2017. doi: 10.1242/jcs.192021. [DOI] [PubMed] [Google Scholar]
- 51. Hesselink MK, Mensink M, Schrauwen P. Intramyocellular lipids and insulin sensitivity: does size really matter? Obes Res 12: 741–742, 2004. doi: 10.1038/oby.2004.88. [DOI] [PubMed] [Google Scholar]
- 52. Daemen S, Gemmink A, Brouwers B, Meex RCR, Huntjens PR, Schaart G, Moonen-Kornips E, Jörgensen J, Hoeks J, Schrauwen P, Hesselink MKC. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete's paradox. Mol Metab 17: 71–81, 2018. doi: 10.1016/j.molmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y, Lee S, Langleite T, Norheim F, Pourteymour S, Jensen J, Stadheim HK, Storås TH, Davanger S, Gulseth HL, Birkeland KI, Drevon CA, Holen T. Subsarcolemmal lipid droplet responses to a combined endurance and strength exercise intervention. Physiol Rep 2: e12187, 2014. doi: 10.14814/phy2.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Long AP, Manneschmidt AK, VerBrugge B, Dortch MR, Minkin SC, Prater KE, Biggerstaff JP, Dunlap JR, Dalhaimer P. Lipid droplet de novo formation and fission are linked to the cell cycle in fission yeast. Traffic 13: 705–714, 2012. doi: 10.1111/j.1600-0854.2012.01339.x. [DOI] [PubMed] [Google Scholar]
- 55. Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem 281: 11901–11909, 2006. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- 56. Lam T, Harmancey R, Vasquez H, Gilbert B, Patel N, Hariharan V, Covey M, Taegtmeyer H. Reversal of intramyocellular lipid accumulation by lipophagy and a p62-mediated pathway. Cell Death Discov 2: 16061, 2016. doi: 10.1038/cddiscovery.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481: 511–515, 2012. [Erratum in Nature 503: 146, 2013]. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saleem A, Carter HN, Hood DA. p53 is necessary for the adaptive changes in cellular milieu subsequent to an acute bout of endurance exercise. Am J Physiol Cell Physiol 306: C241–C249, 2014. doi: 10.1152/ajpcell.00270.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17: 1–382, 2021. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes 49: 1353–1358, 2000. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- 61. Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 62. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 63. Chee C, Shannon CE, Burns A, Selby AL, Wilkinson D, Smith K, Greenhaff PL, Stephens FB. Relative contribution of intramyocellular lipid to whole-body fat oxidation is reduced with age but subsarcolemmal lipid accumulation and insulin resistance are only associated with overweight individuals. Diabetes 65: 840–850, 2016. doi: 10.2337/db15-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perreault L, Newsom SA, Strauss A, Kerege A, Kahn DE, Harrison KA, Snell-Bergeon JK, Nemkov T, D'Alessandro A, Jackman MR, MacLean PS, Bergman BC. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight 3: e96805, 2018. doi: 10.1172/jci.insight.96805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Devries MC, Lowther SA, Glover AW, Hamadeh MJ, Tarnopolsky MA. IMCL area density, but not IMCL utilization, is higher in women during moderate-intensity endurance exercise, compared with men. Am J Physiol Regul Integr Comp Physiol 293: R2336–R2342, 2007. doi: 10.1152/ajpregu.00510.2007. [DOI] [PubMed] [Google Scholar]
- 66. Shaw CS, Shepherd SO, Wagenmakers AJ, Hansen D, Dendale P, van Loon LJ. Prolonged exercise training increases intramuscular lipid content and perilipin 2 expression in type I muscle fibers of patients with type 2 diabetes. Am J Physiol Endocrinol Physiol 303: E1158–E1165, 2012. doi: 10.1152/ajpendo.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.22341004.
Data Availability Statement
Data will be made available upon reasonable request.