Abstract
Background:
Outcomes for children with relapsed/refractory (R/R) acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are poor and new therapies are needed. Pevonedistat is an inhibitor of the NEDD-8 activating enzyme, a key regulator of the ubiquitin proteasome system that is responsible for protein turnover, with protein degradation regulating cell growth and survival.
Procedure:
We evaluated the feasibility, toxicity, and pharmacokinetics (PK) of pevonedistat (20 mg/m2 days 1, 3, 5) in combination with azacitidine, fludarabine, cytarabine (aza-FLA) in children with R/R AML and MDS (NCT03813147). Twelve patients were enrolled, median age was 13 years (range 1-21). Median number of prior chemotherapeutic regimens was 2 (range 1-5) and 3 (25%) patients had prior hematopoietic cell transplantation. Diagnoses were AML NOS (n=10, 83%), acute monocytic leukemia (n=1), and therapy related AML (n=1).
Results:
Overall, 3/12 (25%) patients experienced DLTs. The day 1 mean±SD (n=12) Cmax, VSS, T1/2, and CL were 223±91 ng/mL, 104±53.8 L/m2, 4.3±1.2 hours, and 23.2±6.9 L/hr/m2, respectively. T1/2, VSS, and Cmax but not CL were significantly different between age groups. The overall response rate was 25%, with n=3 patients achieving a complete remission with incomplete hematologic recovery (CRi).
Conclusions:
Pevonedistat 20 mg/m2 combined with Aza-FLA was tolerable in children with R/R AML with similar toxicity profile to other intensive AML regimens. However, within the confines of a phase 1 study, we did not observe that the pevonedistat + Aza-FLA combination demonstrated significant anti-leukemic activity.
Keywords: AML, Pevonedistat, Relapse
Introduction
Supportive care and intensification of therapy for children with acute myeloid leukemia (AML) have resulted in improved survival rates over the last several decades, although approximately 40-50 % of children treated with intensive therapy at initial diagnosis, and in many cases stem cell transplant, will still experience relapse.1,2 For patients who experience relapse or are refractory to initial therapy, the long term survival rates are dismal. Many high-risk subgroups also have a very poor prognosis.3,4 Many children experience significant toxicities from AML therapies, especially cardiac toxicity from anthracycline treatment. Thus, new therapies are urgently needed, especially those that target novel pathways.5,6
Pevonedistat (TAK-924, MLN4924) is a small molecule inhibitor of the NEDD8-activating enzyme (NAE). The NEDD8 conjugation pathway is involved in the ubiquitin proteasome system (UPS), which is responsible for protein turnover within cells, and ultimately helps regulate key cellular processes including cell cycle progression, signal transduction, and cell death. The NAE is an essential component of the NEDD8 conjugation pathway and processes NEDD8 for binding to target substrates, specifically the E3 ubiquitin ligases or culling ring ligases, which function as control points of ubiquitination process and direct the degradation of substrates through the proteasome.7,8 Dysregulation of the UPS system has been implicated in the development of many cancers, including hematologic malignancies, and inhibition of NEDD8 has been associated with anti-cancer activity.8-12 Preclinical studies have demonstrated that, exposure to pevonedistat results in increased levels of E3 cullin ring ligase (CRL) substrates and induces apoptosis.13 Combination studies of pevonedistat and cytarabine (ara-C) in AML cells and primary patient samples have shown increased incorporation of ara-C into the DNA cells leading to increased DNA damage and the combination led to prolonged survival in xenografts.14 Additionally, the combination of pevonedistat and azacitdine (aza) demonstrated synergistic effects in AML cell lines and xenografts.15
Early phase studies in adults with R/R AML and MDS demonstrated the safety and tolerability of pevonedistat.16 A phase Ib study of pevonedistat administered on days 1,3,5 in combination with aza x 7 days in adults with AML demonstrated the safety and feasibility of the combination and a higher overall response rate (ORR) and complete remission (CR) rate compared to historical controls with aza alone.17 Children with R/R AML and MDS are treated with intensive, multiagent regimens with high dose cytarabine and fludarabine among the most commonly utilized backbone agents.4,18,19 Azacitidine can be safely added to an intensive fludarabine and cytarabine regimen in children with relapsed AML.20 These preclinical and clinical trial data led us to evaluate the combination pevonedistat added to aza, fludarabine, and araC (aza-FLA) in pediatric patients with R/R AML and MDS. The primary objectives of this study were to determine the safety and tolerability of this regimen and to characterize the pharmacokinetics (PK) of pevonedistat in children with R/R AML and MDS.
Methods
Patients
The trial (NCT03813147) was open to accrual from May 2019 through February 2021. Data for all patients were current through 2/19/2021. Patients were between ≥ 1 month and ≤ 21 years of age at the time of enrollment. Eligible patients had recurrent or refractory AML, with recurrence defined as ≥ 1st relapse with either ≥5% blasts in the bone marrow or ≥ 0.1% blasts as detected by flow cytometry or evidence recurrent cytogenetic or molecular abnormalities. Refractory AML was defined as ≥5% blasts in the bone marrow after ≥2 induction attempts. Patients with or without extramedullary disease were eligible. Patients with MDS that had advanced to AML, relapsed, or refractory to ≥1 cycle of induction chemotherapy were eligible. Patients were required to have normal hepatic function defined as bilirubin ≤ upper limit of normal (ULN) for age and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) < 2.5 X ULN for age with normal defined as 45 U/L. Other requirements included adequate renal, cardiac, and performance status. Patients with acute promyelocytic leukemia, Down syndrome or juvenile myelomonocytic leukemia were not eligible. The National Cancer Institute Pediatric Central Institutional Review Board (PCIRB) approved the study and patients and their parents signed informed consent according to the Declaration of Helsinki and local institutional regulatory policies.
Treatment Regimen
Patients received pevonedistat (20 mg/m2/dose IV on days 1, 3, and 5), azacitidine 75 mg/m2/dose IV on days 1-5), fludarabine (30 mg/m2/dose IV on days 6-10), and cytarabine (2000 mg/m2/dose IV on days 6-10). All patients received intrathecal (IT) cytarabine dosed according to age and patients with CNS2/3 disease could receive either IT cytarabine or triple intrathecal chemotherapy (cytarabine, methotrexate, hydrocortisone) according to treating physician on a twice weekly schedule until clearance of CNS blasts on 2 successive lumbar punctures. Patients could receive a second cycle if had stable disease or better response and without evidence of significant treatment related toxicity. Patients could receive up to 2 cycles of protocol therapy.
Pharmacokinetics
Serial blood samples to measure pevonedistat concentrations were obtained during Cycle 1, before the pevonedistat infusion, at the end of infusion and 1, 2, 4, 6-8, and 24 hours following infusion on days 1 and 5. Additional blood samples were collected before the infusion on Day 3 and 48-72 hours after the Day 5 infusion. The 1- and 4- hour post-infusion blood samples were omitted for children <10 kg. Blood samples (2-3 mL) were collected into chilled K2 EDTA tubes. After gentle inversion 8 to 10 times, plasma was separated by centrifugation in a refrigerated centrifuge (10 minutes, 1100-1300 x g, 4°C). Immediately following centrifugation, plasma was transferred into two 2.0 mL cryogenic vials. Pevonedistat concentrations were determined by a validated liquid chromatography/tandem mass spectrometry (LC-MS/MS) assay. Noncompartmental PK analyses were performed using WinNonlin Version 6.4 (Pharsight, Mountain View, CA). Standard descriptive statistics were used to summarize plasma pevonedistat PK parameters including maximum observed concentration, (Cmax), the area under the plasma concentration time curve from 0-24 hours post dose (AUC0-24h), volume of distribution (VSS), clearance (CL) and terminal disposition phase half-life (T1/2).
Statistical Analysis
A rolling six design was utilized to estimate the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D) of the regimen.21 Once confirmed, an additional 6 patients could be enrolled to acquire additional PK data in young (< 12 years) and older (≥ 12 years) pediatric patients. Based on studies in adults, there was no planned escalation beyond dose level (DL) 1 (pevonedistat 20 mg/m2/dose). Given the novelty of the regimen, a conservative approach to enrollment was employed; if 1 dose limiting toxicity (DLT) was observed in the first 2 patients, a 30-day interval was required between cycle 1, day 1 in the 3rd patient and enrollment of the 4th patient. If requisite DLT’s per statistical plan were observed, then de-escalation to DL-1 (pevonedistat 15 mg/m2/dose) would occur. The observation period for DLTs was Cycle 1 of therapy. In brief, all ≥ Gr 3 toxicities not expected with this regimen or that were persistent as well as significantly prolonged count recovery were considered DLTs. A definition of all DLTs, including non-hematologic and hematologic are provided in supplementary material. The MTD/RP2D was defined as the maximum dose at which fewer than 1/3 of patients experienced a DLT during Cycle 1 of therapy. Patients who received 2/3 doses of pevonedistat during Cycle 1 or experienced a DLT were considered evaluable for toxicity assessment. Adverse events were graded using the NCI Common Toxicity Criteria (CTCAE) version 5.0.
Secondary objectives of the study included a descriptive analyses of PK parameters and disease response. Bone marrow aspirates and/or biopsies were performed at baseline and at the end of Cycle 1 for response evaluation. Responses were defined using standard criteria and included complete remission (CR), complete remission with incomplete blood count recovery (CRi), complete remission with incomplete platelet recovery (CRp), partial response (PR), and treatment failure (TF).22 Patients were considered unevaluable for response if they had an aplastic or severely hypocellular marrow.
Results
A total of 12 eligible patients were enrolled (Table 1). The median age of patients was 13 years (range 1-21 years). All patients had received prior chemotherapy with a median number of 2 prior cycles (range 1-9). Three patients (25%) had received prior hematopoietic stem cell transplant. Of 12 patients, one patient had therapy related AML (t-AML) and the remainder had AML in relapse.
Table 1:
Demographic and clinical summary of patients treated on ADVL1712
| Part | |||
|---|---|---|---|
| PK (N=6) |
Stratum 1 (N=6) |
Total (N=12) |
|
| Age | |||
| N | 6 | 6 | 12 |
| Median | 16.5 | 9.5 | 13.0 |
| Range | 1.0, 21.0 | 3.0, 17.0 | 1.0, 21.0 |
| Gender, n (%) | |||
| Male | 2 (33%) | 3 (50%) | 5 (42%) |
| Female | 4 (67%) | 3 (50%) | 7 (58%) |
| Race, n (%) | |||
| White | 5 (83%) | 2 (33%) | 7 (58%) |
| Multiple Races | 0 (0%) | 2 (33%) | 2 (17%) |
| Unknown | 1 (17%) | 2 (33%) | 3 (25%) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 3 (50%) | 1 (17%) | 4 (33%) |
| Not Hispanic or Latino | 3 (50%) | 5 (83%) | 8 (67%) |
| Diagnosis Disease, n (%) | |||
| Acute monocytic leukemia | 1 (17%) | 0 (0%) | 1 (8%) |
| Acute myeloid leukemia, NOS | 4 (67%) | 6 (100%) | 10 (84%) |
| Therapy-related acute myeloid leukemia, NOS | 1 (17%) | 0 (0%) | 1 (8%) |
| Prior Chemotherapy | |||
| N | 6 | 6 | 12 |
| Median | 2.0 | 3.0 | 2.0 |
| Range | 1.0, 4.0 | 2.0, 5.0 | 1.0, 5.0 |
| Prior Radiation | |||
| N | 1 | 1 | |
| Median | 1.0 | 1.0 | |
| Range | 1.0, 1.0 | 1.0, 1.0 | |
| Stem Cell Transplant, n (%) | |||
| No | 5 (83%) | 4 (67%) | 9 (75%) |
| Yes | 1 (17%) | 2 (33%) | 3 (25%) |
| 5% blasts in the bone marrow, n (%) | |||
| <=5% disease Baseline | 1 (17%) | 1 (17%) | 2 (17%) |
| > 5% disease Baseline | 5 (83%) | 5 (83%) | 10 (83%) |
| Response Status, n (%) | |||
| Complete Remission | 2 (33%) | 1 (17%) | 3 (25%) |
| Treatment Failure | 4 (67%) | 5* (83%) | 9 (75%) |
2 patients didn’t have marrow evaluation performed due to clinical status but did have circulating peripheral blasts at the time of evaluation and were determined to best classified as TF.
Six patients were initially treated during the safety phase at DL1: pevonedsitat (20 mg/m2/dose). There was 1 patient with DLTs in the safety phase, thus pevonedistat (20 mg/m2/dose) in combination with aza-FLA was deemed tolerable and additional 6 patients were enrolled in a dose expansion cohort to complete the safety and PK evaluation with 2 patients experiencing DLTs in this cohort. All 12 patients were evaluable for toxicity. The toxicities seen with the aza-FLA regimen were comparable to those expected with an intensive AML regimen. Hematologic toxicities of ≥ Gr3 were common as expected with myelosuppressive aza-FLA backbone. Other ≥ Gr3 toxicities included nausea, fever, fever/neutropenia, sepsis, hypertriglyceridemia, and electrolyte abnormalities (Table 2). Gastrointestinal AEs were also common but were mainly of lower grade. Based on prior studies in adults, liver toxicities were closely monitored. Elevations in liver enzymes were common, however, these were all generally mild and transient. Grade 1 and 2 transaminitis included alanine aminotransferase (ALT) elevations in 7 patients (58%), and aspartate aminotransferase (AST) elevations were seen in 7 patients (58%). Grade 1 or 2 bilirubin elevations were seen in 4 patients (33%). GGT elevations were seen in 2 patients (17%), with 1 being Gr 1 and the other being a Gr 4 DLT. Electrolyte abnormalities were common; however, all were correctable with appropriate supplementation and resolved within 72 hours. DLTs were observed in 3/12 patients (25%) at the 20 mg/m2 pevonedistat dose, 1 in stratum 1 and an additional 2 in the expansion phase . One patient experienced 3 DLTs (GGT increase, proteinuria, and hypertension). This patient had had multiorgan failure, including liver failure, and expired due to progressive AML after completing cycle 1. Other DLTs included one patient with Grade 3 weight loss that resolved with supplemental nutrition and one patient had Grade 3 hypoxia in the setting of bacteremia and sepsis that resolved in <24 hours with initiation of antibiotics.
TABLE 2.
≥ Grade 3 toxicities observed in all (N=12) evaluable patients
| # of Patients in Cycle 1 | |||
|---|---|---|---|
| By Maximum Grade | |||
| Grade 3 | Grade 4 | ||
| Hematologic Type | Toxicity Type | ||
| Hematologic | Anemia | 8 | |
| Lymphocyte count decreased | 1 | 8 | |
| Neutrophil count decreased | 1 | 4 | |
| Platelet count decreased | 5 | ||
| White blood cell decreased | 8 | ||
| Non-Hematologic | |||
| Anorexia | 1 | ||
| Febrile neutropenia | 2 | 1 | |
| Fever | 2 | ||
| GGT increased* | 1 | ||
| Hypertension* | 1 | ||
| Hypertriglyceridemia | 1 | ||
| Hypokalemia | 3 | 1 | |
| Hypophosphatemia | 2 | ||
| Hypoxia* | 1 | ||
| Nausea | 1 | ||
| Proteinuria* | 1 | ||
| Sepsis | 3 | ||
| Weight loss* | 1 | ||
dose limiting toxicity
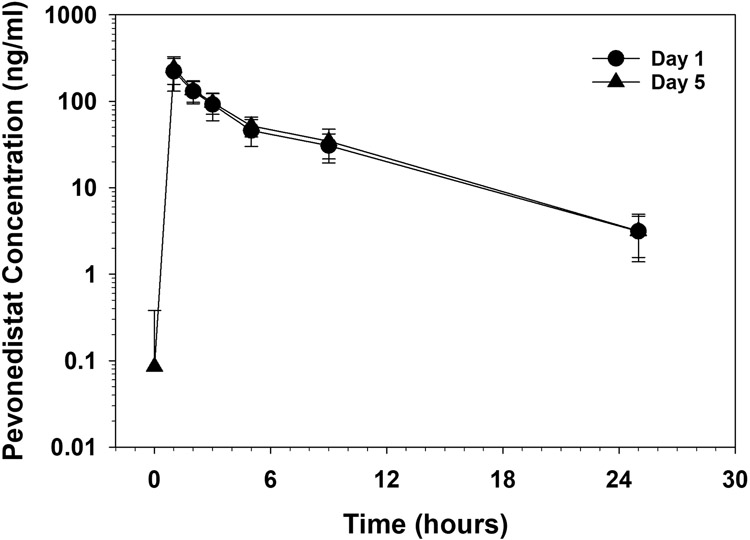
Pharmacokinetics were evaluable for all 12 patients enrolled in this study. The day 1 and day 5 plasma profiles for pevonedistat are illustrated in Figure 1. There was no difference in the mean ± standard deviation (SD) Cmax (223±91 and 243±85 ng/mL, P=0.14) and AUC0-24h (921±215 and 1018±238 ng•hr/mL, P=0.06) values following days 1 and 5, respectively, consistent with the short half-life after the first dose (Table 3). The day 1 T1/2 and BSA-adjusted CL were 4.3±1.2 hours and 23.2±6.9 L/hr/m2, respectively. The Cmax (253±75 versus 180±102 ng/mL, P=0.18) were higher and BSA-adjusted CL (19.8±2.2 hours versus 27.8±8.8 L/hr/m2, P=0.04) were lower in females as compared to males. When the data were analyzed according to age, Cmax was significantly higher (274±99 versus 172±46 ng/mL, P=0.04), T1/2 was significantly shorter (3.6±1.3 versus 5.1±0.3 hours; P=0.03), and volume of distribution (Vss) was significantly lower (120±94 hours versus 284±93 L, P=0.01) in the younger (< 12 years) as compared to the older (≥ 12 years) cohort. When VSS was adjusted for BSA, VSS remained lower for the younger cohort, but the difference was no longer statistically significant (129±76 versus 158±4L/m2, P=0.43). We also found higher clearance in older children who had higher body surface area (1.8±0.4 versus 0.9±0.4 m2, p<0.01) compared to younger children (38.4±10.4 versus 22.9±14.3 L/hr, P=0.0565).
Figure 1.
Pevonedistat concentration versus time following administration of a 20 mg/m2 dose on day 1 and 5.
TABLE 3.
Descriptive statistics of mean pevonedistat PK parameters in cycle 1
| Day 1 | Day 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) |
T1/2 (hrs) |
CL (L/hr/m2) |
VSS (L/m2) |
AUC0-24h (ng.hr/ml) |
Cmax (ng/ml) |
T1/2 (hrs) |
CL (L/hr/m2) |
VSS (L/m2) |
AUC0-24h (ng.hr/ml) |
|
| Mean | 223 | 4.3 | 23.2 | 104 | 921 | 243 | 4.7 | 20.5 | 96.7 | 1018 |
| Median | 215 | 4.8 | 21.4 | 93.2 | 935 | 213 | 4.7 | 19.1 | 91.0 | 1033 |
| Max | 381 | 5.6 | 40.3 | 190 | 1192 | 400 | 6.1 | 30.7 | 170 | 1381 |
| Min | 121 | 1.8 | 16.2 | 53.8 | 487 | 132 | 1.9 | 14.1 | 56.4 | 638 |
| SD | 90.6 | 1.2 | 6.9 | 41.9 | 215 | 85.4 | 1.1 | 5.4 | 35.6 | 238 |
Response evaluations were performed in 10 patients. There were 2 patients who did not undergo end of cycle 1 bone marrow evaluation due leukemic blasts present in peripheral blood prior to end of cycle 1. For response rate calculations the best possible response for these 2 patients was determined to be treatment failure (TF). Thus, the overall response rate was 25%, with 3 patients classified as achieving a CRi, with the other 9 patients (75%) experiencing TF (Table 1). Of the 3 patients who achieved a CRi, all 3 had ≥5% disease at the time of study entry. Among the patients who were classified as CRi, residual disease detection by flow cytometry was not available for further assessment.
Discussion
In this phase 1 study we demonstrate that pevonedistat 20 mg/m2/dose on days 1,3, and 5 is the RP2D and can be feasibly combined with an aza-FLA backbone. Toxicities are consistent with intensive AML re-induction regimens. While many PK parameters were similar between younger and older children and males vs. females, T1/2, VSS, and Cmax were significantly different between the two age groups. In general, the pevonedistat clearance and exposure in this study were similar to those reported in adults.16,17,23,24 However, a population PK analysis of data from six adult phase 1 trials with pevonedistat identified a significant effect of body surface area on pevonedistat clearance and volume of distribution.23 Consistent with this observation, we found higher clearance in older children who had higher BSA compared to younger children. Furthermore, we found lower VSS, higher peak concentrations and shorter T1/2 in the younger cohort of pediatric patients. These data are consistent with lipophilicity of pevonedistat and higher fraction of body water in younger children. Thus, younger children with a smaller body size would have lower volume of distribution with a concomitant increase in Cmax. The reasons for the difference in half-life are less clear. However, albumin concentrations had a significant effect on tissue distribution in adult patients. While albumin values for children were not available for this study, many patients had already undergone intensive therapies. Differences in albumin concentrations between adults and this small cohort of children might contribute to differences in the half-life. Interestingly, that population PK analysis showed a lower clearance in female patients but the difference was not significant.23 Our data are consistent with this observation, and the finding of a significant difference may be due to patient selection and the small cohort size. Given these potential differences in PK between adults and children, it is important to fully characterize drug PK in pediatric studies.
Regimens for patients with relapsed AML that contain anthracyclines have demonstrated efficacy, with the most recent example being CPX-351 for patients with first relapse.4,25 However, given the significant acute and late cardiac toxicity risks it is important to evaluate non-anthracycline re-induction regimens. This is especially important in the setting of second or greater relapse where FLA or aza-FLA regimens may be an appropriate choice. In this study, we show that novel agents can be added to this backbone without significant increases in toxicity. In early adult studies, higher doses of pevonedistat were associated with hepatic toxicity, namely transient transaminitis. This pediatric clinical trial did not demonstrate any significant (≥ Grade 3 transaminitis, even when combined with chemotherapeutic agents that can be hepatotoxic.16,26 This is in line with recent studies of pevonedistat + azacitidine combinations in MDS and AML that have not showed any signal of increased hepatotoxicity of the regimen.27 The one case of significantly elevated GGT observed on study was also in the setting of significant disease progression and leukemic involvement and leukemic associated liver dysfunction liver.
Based on the early signal of the efficacy of aza in combination with pevonedistat in adults, a subsequent randomized phase 2b trial of aza vs. aza in combination with pevonedistat in adults with MDS, chronic myelomonocytic leukemia (CMML) and AML was conducted. While trends toward improved responses and outcomes were seen in some subgroups, including patients with higher risk MDS, there was no significant difference in overall or event free survival.27 This lack of a definitive efficacy signal aligns with our observation of limited activity of the combination. It is important to acknowledge the significant differences in underlying biology between pediatric and adult AML, and particularly adult MDS.28,29 These differences can impact response to therapy and thus it is essential to evaluate agents specifically in the pediatric population as some agents in adult AML/MDS may lack efficacy in pediatric disease.
One limitation of our study was that pharmacodynamic studies (PD) were not performed, thus a target/response association could not be demonstrated. Prior PD studies in adults with AML/MDS demonstrated induction of expression of 8 target genes known to be induced by pevonedistat-mediated NAE inhibition across a range of dose levels tested, as well as tissue accumulation of the CRL substrate CDT1, although the lowest dose tested was 25 mg/m2.16 One possible reason for the lack of efficacy signal may have been due to lower levels of NAE inhibition. Additional studies would need to be performed to understand PD parameters and give an accurate response assessment in our population to determine if this led to the low response rate.
Our study does contribute to the data on the tolerability of the aza-FLA backbone in patients with relapsed/refractory AML. There may be interest in some subsets of pediatric myeloid leukemias of adding targeted agents to this backbone and our study provides additional information on the feasibility of this approach. Unfortunately, a significant anti-leukemic signal was not seen in the pevonedistat + aza-FLA combination in children and adolescents with AML and our study does not support further investigation of this agent in pediatric AML.
Supplementary Material
Acknowledgements:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
The research reported above is supported by the Children's Oncology Group, the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number UM1CA228823 and the Cookies for Kids’ Cancer Foundations. J.R. was supported in part by P30 CA015083 from the National Cancer Institute (NCI). E.I. was supported by National Institute of General Medical Sciences (NIGMS) grant T32GM 08685. TMH was supported by an NIH R01 CACA164024.
Abbreviations:
- AML
Acute myeloid leukemia
- MDS
Myelodysplastic syndrome
- Aza-FLA
Azacitidine + fludarabine and cytarabine
- R/R
Relapsed or refractory
- PK
Pharmacokinetics
- NAE
NEDD8 activating enzyme
- CRL
Cullin ring ligases
Footnotes
This article was previously presented in abstract form at the American Society of Clinical Oncology (ASCO) 2021 Annual Conference, June 2021.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: a report from the Children's Oncology Group. Haematologica. Feb 6 2020;doi: 10.3324/haematol.2019.220962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J Clin Oncol. Sep 20 2015;33(27):2949–62. doi: 10.1200/JCO.2015.62.8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasche M, Zimmermann M, Steidel E, et al. Survival Following Relapse in Children with Acute Myeloid Leukemia: A Report from AML-BFM and COG. Cancers (Basel). May 12 2021;13(10)doi: 10.3390/cancers13102336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. Feb 10 2013;31(5):599–607. doi: 10.1200/JCO.2012.43.7384 [DOI] [PubMed] [Google Scholar]
- 5.Getz KD, Sung L, Ky B, et al. Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report From the Children's Oncology Group. J Clin Oncol. Jan 1 2019;37(1):12–21. doi: 10.1200/JCO.18.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein K, de Haas V, Kaspers GJL. Clinical challenges in de novo pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. Mar 2018;18(3):277–293. doi: 10.1080/14737140.2018.1428091 [DOI] [PubMed] [Google Scholar]
- 7.Brownell JE, Sintchak MD, Gavin JM, et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. Jan 15 2010;37(1):102–11. doi: 10.1016/j.molcel.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 8.Soucy TA, Smith PG, Milhollen MA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. Apr 9 2009;458(7239):732–6. doi: 10.1038/nature07884 [DOI] [PubMed] [Google Scholar]
- 9.Leclerc GM, Zheng S, Leclerc GJ, DeSalvo J, Swords RT, Barredo JC. The NEDD8-activating enzyme inhibitor pevonedistat activates the eIF2alpha and mTOR pathways inducing UPR-mediated cell death in acute lymphoblastic leukemia. Leuk Res. Nov 2016;50:1–10. doi: 10.1016/j.leukres.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Guo N, Azadniv M, Coppage M, Nemer M, Mendler J, Becker M, Liesveld J. Effects of Neddylation and mTOR Inhibition in Acute Myelogenous Leukemia. Transl Oncol. Apr 2019;12(4):602–613. doi: 10.1016/j.tranon.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. Jun 15 2009;15(12):3912–6. doi: 10.1158/1078-0432.CCR-09-0343 [DOI] [PubMed] [Google Scholar]
- 12.Xie P, Zhang M, He S, et al. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat Commun. May 13 2014;5:3733. doi: 10.1038/ncomms4733 [DOI] [PubMed] [Google Scholar]
- 13.Swords RT, Kelly KR, Smith PG, et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. May 6 2010;115(18):3796–800. doi: 10.1182/blood-2009-11-254862 [DOI] [PubMed] [Google Scholar]
- 14.Nawrocki ST, Kelly KR, Smith PG, et al. The NEDD8-activating enzyme inhibitor MLN4924 disrupts nucleotide metabolism and augments the efficacy of cytarabine. Clin Cancer Res. Jan 15 2015;21(2):439–47. doi: 10.1158/1078-0432.CCR-14-1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visconte V, Nawrocki ST, Espitia CM, et al. Comprehensive quantitative proteomic profiling of the pharmacodynamic changes induced by MLN4924 in acute myeloid leukemia cells establishes rationale for its combination with azacitidine. Leukemia. May 2016;30(5):1190–4. doi: 10.1038/leu.2015.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swords RT, Erba HP, DeAngelo DJ, et al. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol. May 2015;169(4):534–43. doi: 10.1111/bjh.13323 [DOI] [PubMed] [Google Scholar]
- 17.Swords RT, Coutre S, Maris MB, et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood. Mar 29 2018;131(13):1415–1424. doi: 10.1182/blood-2017-09-805895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penel-Page M, Plesa A, Girard S, Marceau-Renaut A, Renard C, Bertrand Y. Association of fludarabin, cytarabine, and fractioned gemtuzumab followed by hematopoietic stem cell transplantation for first-line refractory acute myeloid leukemia in children: A single-center experience. Pediatr Blood Cancer. Jun 2020;67(6):e28305. doi: 10.1002/pbc.28305 [DOI] [PubMed] [Google Scholar]
- 19.Fleischhack G, Hasan C, Graf N, Mann G, Bode U. IDA-FLAG (idarubicin, fludarabine, cytarabine, G-CSF), an effective remission-induction therapy for poor-prognosis AML of childhood prior to allogeneic or autologous bone marrow transplantation: experiences of a phase II trial. Br J Haematol. Aug 1998;102(3):647–55. doi: 10.1046/j.1365-2141.1998.00836.x [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Triche T Jr., Malvar J, et al. A phase 1 study of azacitidine combined with chemotherapy in childhood leukemia: a report from the TACL consortium. Blood. Mar 8 2018;131(10):1145–1148. doi: 10.1182/blood-2017-09-803809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. Jan 10 2008;26(2):190–5. doi: 10.1200/JCO.2007.12.7712 [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. Dec 15 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036 [DOI] [PubMed] [Google Scholar]
- 23.Faessel HM, Mould DR, Zhou X, Faller DV, Sedarati F, Venkatakrishnan K. Population pharmacokinetics of pevonedistat alone or in combination with standard of care in patients with solid tumours or haematological malignancies. Br J Clin Pharmacol. Nov 2019;85(11):2568–2579. doi: 10.1111/bcp.14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah JJ, Jakubowiak AJ, O'Connor OA, et al. Phase I Study of the Novel Investigational NEDD8-Activating Enzyme Inhibitor Pevonedistat (MLN4924) in Patients with Relapsed/Refractory Multiple Myeloma or Lymphoma. Clin Cancer Res. Jan 1 2016;22(1):34–43. doi: 10.1158/1078-0432.CCR-15-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper TM, Absalon MJ, Alonzo TA, et al. Phase I/II Study of CPX-351 Followed by Fludarabine, Cytarabine, and Granulocyte-Colony Stimulating Factor for Children With Relapsed Acute Myeloid Leukemia: A Report From the Children's Oncology Group. J Clin Oncol. Jul 1 2020;38(19):2170–2177. doi: 10.1200/JCO.19.03306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarantopoulos J, Shapiro GI, Cohen RB, et al. Phase I Study of the Investigational NEDD8-Activating Enzyme Inhibitor Pevonedistat (TAK-924/MLN4924) in Patients with Advanced Solid Tumors. Clin Cancer Res. Feb 15 2016;22(4):847–57. doi: 10.1158/1078-0432.CCR-15-1338 [DOI] [PubMed] [Google Scholar]
- 27.Sekeres MA, Watts J, Radinoff A, et al. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia. Jul 2021;35(7):2119–2124. doi: 10.1038/s41375-021-01125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarlock K, Zhong S, He Y, et al. Distinct age-associated molecular profiles in acute myeloid leukemia defined by comprehensive clinical genomic profiling. Oncotarget. May 29 2018;9(41):26417–26430. doi: 10.18632/oncotarget.25443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolouri H, Farrar JE, Triche T Jr., et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. Jan 2018;24(1):103–112. doi: 10.1038/nm.4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.