Abstract
Global warming has direct and indirect effects, as well as short- and long-term impacts on the respiratory and skin barriers. Extreme temperature directly affects the airway epithelial barrier by disrupting the structural proteins and by triggering airway inflammation and hyperreactivity. It enhances tidal volume and respiratory rate by affecting the thermoregulatory system, causing specific airway resistance and reflex bronchoconstriction via activation of bronchopulmonary vagal C fibers and upregulation of transient receptor potential vanilloid (TRPV) 1 and TRPV4. Heat shock proteins are activated under heat stress and contribute to both epithelial barrier dysfunction and airway inflammation. Accordingly, the frequency and severity of allergic rhinitis and asthma have been increasing. Heat activates TRPV3 in keratinocytes, causing the secretion of inflammatory mediators and eventually pruritus. Exposure to air pollutants alters the expression of genes that control skin barrier integrity and triggers an immune response, increasing the incidence and prevalence of atopic dermatitis. There is evidence that extreme temperature, heavy rains and floods, air pollution, and wildfires increase atopic dermatitis flares. In this narrative review, focused on the last 3 years of literature, we explore the effects of global warming on respiratory and skin barrier and their clinical consequences.
Keywords: Climate change, global warming, extreme temperature, pollution, epithelial barrier, allergic diseases, asthma, allergic rhinitis, atopic dermatitis
Humanity has been facing an accelerating increase in global temperatures and global warming since the mid-19th century. After the industrial revolution, greenhouse gases such as carbon dioxide (CO2), methane, nitrous oxide, and fluorinated gases have increased as a result of anthropogenic activities and have caused global warming by trapping excessive heat in the atmosphere.1,2 Temperatures are now about 1°C above preindustrial levels, and they continue to rise.3 When high temperatures are combined with high humidity, evaporation decreases and the temperature feels higher than it is—the so-called wet-bulb temperature.4,5 A wet-bulb temperature of 35°C is theorized as the threshold for human survival. At wet-bulb temperatures above35°C, the human body is unable to adapt, causing an increase in both mortality and morbidity.4,6 Recent evidence in young, healthy adults suggests that this threshold may be lower, especially in hot and dry climates.7
Over the years, heat waves are becoming more common, intense, and prolonged in almost all regions.6 Climate change in Asia, Europe, Africa, Australia, and South America is thought to be responsible for regional trends in extreme temperatures.4,8,9 The effects of heat waves might be milder for people living in warmer climates because these populations are more acclimatized to higher temperatures, but more frequent extremes in these regions and in densely populated areas pose a serious problem.8 On the one hand, more hot and dry conditions cause heat waves, droughts, wildfires, and sandstorms. On the other hand, rising temperatures hold more moisture, changing precipitation patterns and causing heavy rains, floods, and thunderstorms.1,2,10
All these changes pose a risk for respiratory and skin allergies from the prenatal period to advanced age. Increasing temperatures can affect and disrupt the epithelial barriers of the skin and respiratory tracts, directly or indirectly through climatic exposures. Damaged epithelial cells release alarmins, activating the type 2 response and resulting in inflammation. In addition, cholinergic responses are stimulated by activated cytokines and chaperones. As a result of environmental and immunologic interactions, asthma, allergic rhinitis (AR), and atopic dermatitis (AD) are increasingly emerging or exacerbated.11,12 Evidence of the direct effects of extreme temperature on epithelial barriers is scarce. Most of the data are provided from in vitro studies, cell culture, and mouse models, along with a few human models.
In this narrative review, we highlight the recent and more resounding publications on the effects of climate change, particularly extreme heat and the climatic exposures due to increased temperature, such as heavy rains and floods, air pollution, and wildfires, on the respiratory and skin epithelium. For the airway section, in vitro studies, animal models, and human studies with strong and reliable results and highly cited review papers were included by scanning the publications in PubMed, Web of Science, Scopus, and Embase from the last 3 years. For the dermatologic section, most studies were epidemiologic in nature. We searched PubMed for the terms “atopic dermatitis” and any of the terms “climate change,” “extreme heat,” “heavy rains,” “floods,” “air pollution,” or “wild fires” from 2020 to early 2023 and cross-referenced articles. The introduction and conclusion used a combination of the abovementioned approaches to select references.
RESPIRATORY ALLERGIC DISEASES
Composition of respiratory epithelium
The respiratory epithelial barrier, one of the surfaces of the human body in contact with the external environment, is very thin and acts as a physical barrier with mucociliary cleansing and antibacterial functions. The anterior part of the nasal cavity forms a portal for gas exchange with the external environment and is lined with stratified squamous epithelium.13 The conductive part from the nasal cavity to the bronchi is lined with pseudostratified columnar ciliary epithelium, goblet cells, and basal cells; terminal bronchi are covered with nonciliary cubic cells (Clara cells), and the alveoli providing gas exchange are lined with thin, flattened simple squamous (type 1 pneumocyte) and cubic (type 2 pneumocyte) epithelium. Goblet cells are the main secretory cells that adhere to each other by tight junctions (TJ), joining between ciliated cells to form a physical barrier against external contaminants.13 Neighboring pseudostratified columnar ciliated airway cells are interconnected by TJs. Along with adherens junctions below them, they constitute the zonula adherens and mediate the cephalic propulsion of tracheobronchial secretions by the coordinated action of cilia.13,14 Desmosomes on the lateral borders of the cells connect one cell to another. The respiratory epithelium sits on the basal membrane and strongly binds to the underlying extracellular matrix that is formed by the lamina propria.15 Solitary chemosensory cells such as tuft cells in turbinated tissues of the upper airways, trachea, and proximal airways are close to the nerve fiber, acting as epithelial guards and initiating the productive immune response.16 Neuronal transient receptor potentials (TRPs) are expressed in epithelial cells and regulate the permeability of barriers. Increased expression of TRP vanilloid 1 (TRPV1) disrupts the epithelial barrier integrity in AR.16 Moreover, Lee and colleagues have suggested that TRPV4 plays a role in epithelial barrier disruption (Fig 1).17
FIG 1.
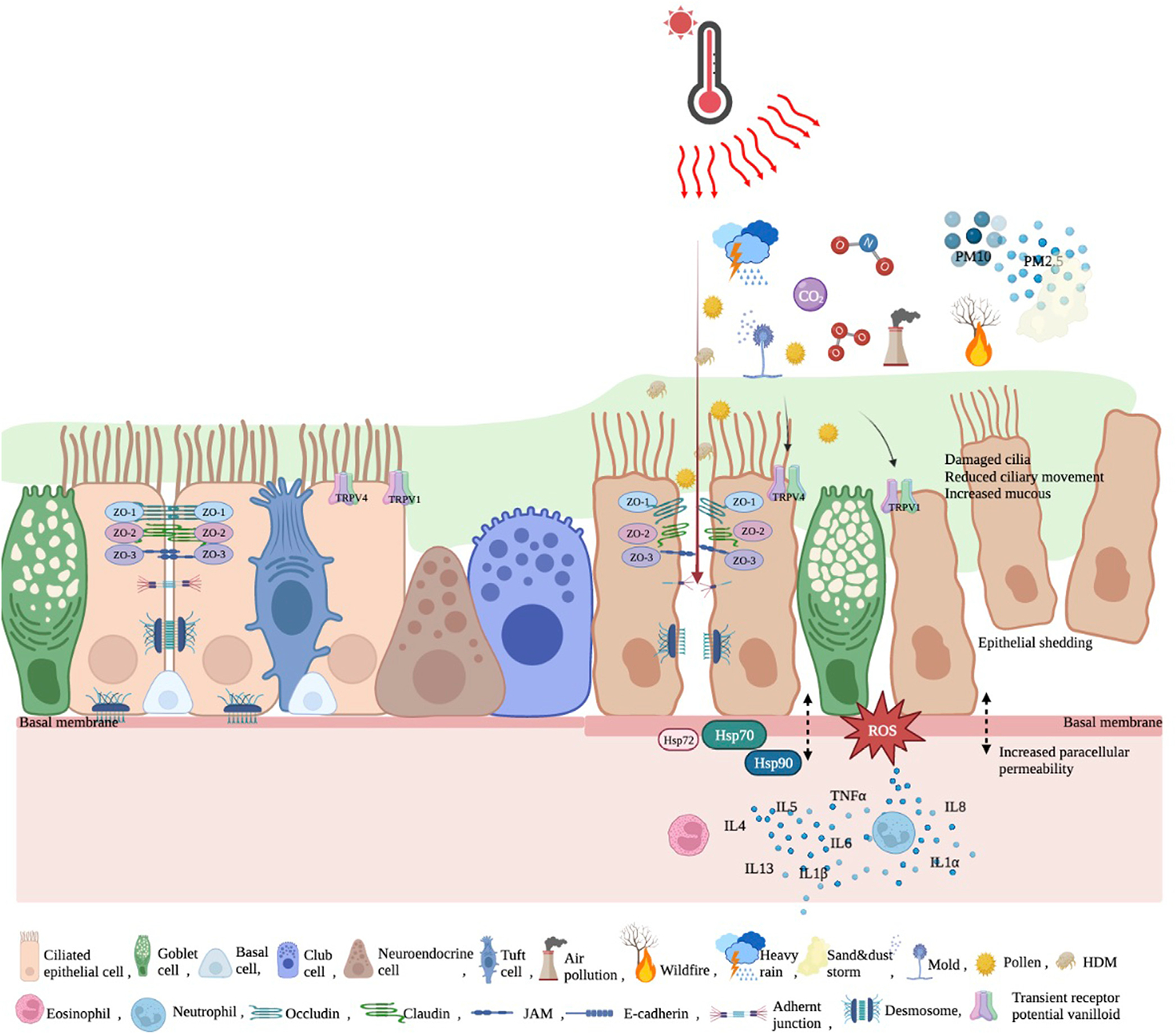
Healthy and impaired respiratory epithelial barrier. Adjacent pseudostratified columnar ciliated airway cells are interconnected by TJ, adherens junctions, and desmosomes. Respiratory epithelium sits on basal membrane, attached to lamina propria. Neuronal TRPs are expressed in epithelial cells and regulate permeability of barriers. When disrupted by increased temperature, airborne allergens, and pollution, TJ proteins such as zonula occludens–associated occludin, claudin, and JAM are disrupted and epithelial junctions open. TRPV1 and TRPV4 are upregulated; HSP-70, HSP-90, and HSP-72 are increased, and ROS are formed. All these cause increased inflammation, increased paracellular permeability, increased mucus, reduced ciliary movement, damaged cilia, and epithelial shedding. JAM, Junctional adhesion molecules; ROS, reactive oxygen species.
After environmental epithelial damage, cross talk between nasal and bronchial epithelial cells and immune cells releases cytotoxic mediators, free oxygen radicals, and collagenases, leading to inflammation and activation of innate and adaptive immune cells.18 These type 1 and type 2 inflammatory responses open the bronchial epithelial barrier, resulting in increased epithelial permeability.15,18
Extreme temperature events
Extreme heat is roughly defined as a period of high heat in which temperatures are above normal average levels for at least 2 or 3 days.19 Extreme temperatures have been defined differently in different studies as being above the 90th, 95th, or 97.5th percentile cutoff points for the mean daily temperature.20,21 They are associated with more intense heat waves and increase the risk of desertification. The definition of a heat wave differs between regions, depending on how high the measured temperature is above the seasonal average and how many days it lasts.9 The probability of a heat wave occurring is 2.8 times greater now than in the preindustrial era. However, the increased risk is much higher in regions where heat waves are infrequent.6 For every Celsius degree increase in temperature, the risk of premature death from respiratory disease increases, primarily in vulnerable populations such as the elderly, pregnant persons, and children.2 A study of 5896 adults showed that there is a 20 mL decrease in the forced expiratory volume in 1 second (FEV1) for every 5°C increase in temperature, especially in the winter andspring.22 Urban areas likely have more propensity for extreme heat events compared to rural areas. By replacing natural land covers with closely packed buildings and paved surfaces, temperatures in urban areas are higher than in rural regions.2,23 This temperature gradient is a result of increased heat absorption, differences in convection efficiency, and evaporation in urban structures, termed the heat island effect. In addition, high pollution and high traffic-related air pollution levels in urban areas amplify the mortality and morbidity due to heat waves.
Direct effects
Studies about the direct effects of heat stress on respiratory system include mostly in vitro studies and animal models (Table I). According to a recent meta-analysis, extreme heat affects the thermoregulatory system; it increases tidal volume, respiratory rate, and systemic inflammatory response.24 In animal models, heat stress was found to directly disrupt the epithelial barrier of the intestine by enhancing TJ permeability, upregulating the structural protein occludin, and downregulating the functional protein zonula occludens 1.25 Because of these findings, we surmised that similar changes could be seen in the respiratory epithelial barrier under extreme heat. This process should be studied in animal and human models. Furthermore, high temperature may aggravate airway inflammation by increasing IL-4, IL-1β, IL-6, and TNF-α and by shifting the TH1/TH2 balance to TH2, altering mucus production, damaging the airway epithelium, and triggering airway hyperreactivity in mice.26 All these molecular effects detected in animal models give us an idea about the changes that can be seen in the respiratory epithelial barrier in humans under heat stress.
TABLE I.
Summary of studies on extreme heat exposures and airway outcomes
| Study | Study design | Study size | Main outcome/finding | Strengths | Limitations |
|---|---|---|---|---|---|
|
| |||||
| Rice (2019)22 | Cohort study | 5896 participants | • Each 5°C higher previous-week temperature was associated with 20 mL lower FEV1. • This association was present in winter or spring only. |
• Validated spatiotemporal temperature model using satellite surface temperature data was used to more precisely predict temperature. • Individual, neighborhood, and seasonal confounders were considered in measurements. |
• Includes only White, middle-age, and older people, so not representative. • Only 2 repeated measures. |
| Han (2022)24 | Meta-analysis | 111 studies | • Extreme heat increases tidal volume, respiratory rate, and SIR. • Extreme heat is an independent trigger of asthma exacerbations. • TRP receptors are activated by breathing hot air. |
• Systematically summarized studies with epidemiologic evidence and clinical implications. | • Lack of standardization across studies for meteorologic definitions, measurements, and exposure assessment. |
| Deng (2020)26 | Animal model | 60 mice | • High temperature caused bronchial epithelium thickness, subepithelial fibrosis and inflammatory cell infiltration around airways; triggered IL-4, IL-1β, IL-6, TNF-α releasing and shifted TH1/Th2 balance to TH2. • High temperatures aggravated airway wall remodeling. • TRPs are important in high temperature-induced allergic asthma. • TRPV1 expression was higher at 40°C. |
• Can be a predictor for human studies due to success of mouse model. | • Obscurity of way high temperature activates TRPs and changes at different humidity. |
| Nikolaizik (2020)27 | Experimental human study | 100 healthy adults | • Nasal ciliary beat frequency gradually increased at 25°C, 32°C, and 37°C. • Optimum temperature to measure ciliary beat frequency was 32°C. |
• Important to indicate physiologic temperature for nasal ciliary activity in humans. | • Effects of cofactors such as pH were not measured. • For ethical reasons, healthy children were not included in study. |
| Clary-Meinesz (1992)29 | Ex vivo and in vitro | 30 adults | • Cilia motility of nasal and bronchial mucosa reached normal level at 20–45°C and decreased above 45°C. | • Effect of a wide range of temperature on human cilia of both upper and lower respiratory tract was evaluated. | • Small sample size. |
| Harford (2021)31 | In vitro | Based on primary human bronchial epithelial cells derived from anonymous patient donors | • TRPV1 channels were overexpressed in airways of patients with asthma and activated by chemical mediators during chronic airway inflammation. | • First study to show increased basal and RSV-induced TRPV1 expression in lower airway epithelium of children with asthma. | • Performed in vitro, so cannot fully understand process in vivo. |
| Lu (2023)32 | In vivo/animal model | 90 Balb/c mice | TRPV1 upregulated after either separate or combined exposure to high temperature (35°C) and NO2. • Both separate and combined exposure to high temperature and NO2 aggravated AHR. • Total white blood cells, total IgE, IL-4, IL-4, IFN-γ increased after both separate and combined exposure to high temperature and NO2. |
• Supports epidemiologic evidence by revealing mechanism of heat-NO2-induced toxicity. | • Uncertainty of human health risk analysis through animal experimental studies. • Although effect of extreme heat alone was shown in study, its main purpose was to examine effect of high temperature-NO2 interaction on allergic asthma. |
| Bonvini (2020)34 | In vivo and in vitro/guinea pigs, human airway samples from donor tissue and cell culture | NA | • TRPV4 agonist caused contraction in vivo in guinea pig, and in human and guinea pig tracheal tissue. • TRPV4 activation increased intracellular Ca2+ and released ATP from ASM cells, triggering mast cell degranulation resulting in bronchoconstriction. |
• Revealed novel mast cell-ASM interaction and TRPV4 as driver of IgE-independent mast cell-dependent bronchospasm. | NA |
| Hayes (2012)35 | Experimental human study | 6 patients with asthma and 6 healthy volunteers | • Exposure to humidified air at high temperature (49°C) significantly increased specific airway resistance and triggered cough in patients with asthma. • Ipratropium pretreatment had a blocking effect on humidified hot air-induced bronchoconstriction in patients with asthma. • Humidified hot air-induced bronchoconstriction was mediated by cholinergic reflex pathway. |
• This rare human study shows that inhalation of warm, moist air causes cough and bronchoconstriction in patients with asthma and that airway constriction is mediated by cholinergic reflex. |
NA |
| Yombo (2019)37 | Mouse model | NA | • Lack of HSP-70 caused significant decrease in airway inflammation, goblet cell hyperplasia, IL-4, IL-5, and IL-13 in mouse model of SEA-induced airway inflammation. | • Identified pathogenic role of HSP-70 in allergic airway inflammation. • Indicated potential utility of targeting HSP-70 to reduce allergen-induced TH2 cytokines, goblet cell hyperplasia, and airway inflammation. |
NA |
| Hulina-Tomašković (2019)38 | Human bronchial epithelial cell culture | NA | • Recombinant human HSP-70 induced IL-6 and IL-8 secretion depending on concentration and time. • Rh HSP-70 suppressed caspase-3/7 activities. |
• Suggested proinflammatory effects of extracellular HSP-70 in chronic inflammation of human bronchial epithelium. | • Nature of study. |
| Nava (2020)39 | Meta-analysis | 12 studies including 118 human participants | • Heat acclimation increases HSP-70 protein and mRNA expression. • HSP-70 plays an important part in ability of cells become thermotolerant. |
• Notable in terms of showing effect of heat acclimation on HSP-70 induction in humans and indicating lack of work in field as well. | • Small number of studies. • High levels of statistical heterogeneity. • Failure to take into account systemic adaptation to heat exposure. |
| Ye (2019)41 | Mouse model | NA | • Secreted HSP-90a participated in epithelial barrier dysfunction of asthmatic mice. • Secreted HSP-90a promoted release of TH2 cytokines in asthmatic mice. • Neutralization of HSP-90α inhibited phosphorylation of AKT and ameliorated epithelial barrier dysfunction. |
• Well designed. • Can be a predictive model for human asthma and targeted therapy. |
• Need to be supported by human studies. |
| Carey (2022)42 | In vitro /cell culture | NA | • HSP-90 inhibition reduced T2R-stimulated NO production and ciliary beating. | • Important in demonstrating that HSP-90 plays important role in airway innate immunity. | • It is unclear how HSP-90 contributed to immune function performed by airway epithelial cells. |
| Bouchama (2017)43 | Experimental human study | 15 volunteers | • HSPA1A gene (encodes heat shock proteins HSP-70–1), upregulated immediately after heat stress. • Unfolded protein response was most significant pathway in early response to heat stress. • HSP90AB1 and HSPB11 genes (encode HSP-90 alpha family) were downregulated. • HSPB6 and HSPB8 were upregulated. • NF-κB pathways inhibited immediately after heat stress and 1 hour after heat stress. |
• First study to demonstrate human gene expression response to extreme heat. | • It is unclear whether gene changes observed in this study are generalizable to cell types other than peripheral blood mononuclear cells. |
AHR, Airway hyperresponsiveness; ASM, airway smooth muscle; ATP, adenosine triphosphate; NA, not applicable; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; RSV, respiratory syncytial virus; SEA, soluble egg antigen; SIR, systemic inflammatory response.
A study on the ciliated respiratory epithelial cells obtained by nasal brushing from 100 healthy individuals showed that rising temperatures increase the ciliary beat.27 Another study on ciliated airway cells obtained by brushing nasal and airway mucosa from 30 healthy adults showed that the cilia stabilizes after reaching normal motility between 20°Cand 45°C,and the motility declined above 45°C.28,29 Recently, a comprehensive meta-analysis reported that extreme heat plays a crucial and independent role in the pathogenesis of asthma flares (Figs 1 and 2).24
FIG 2.
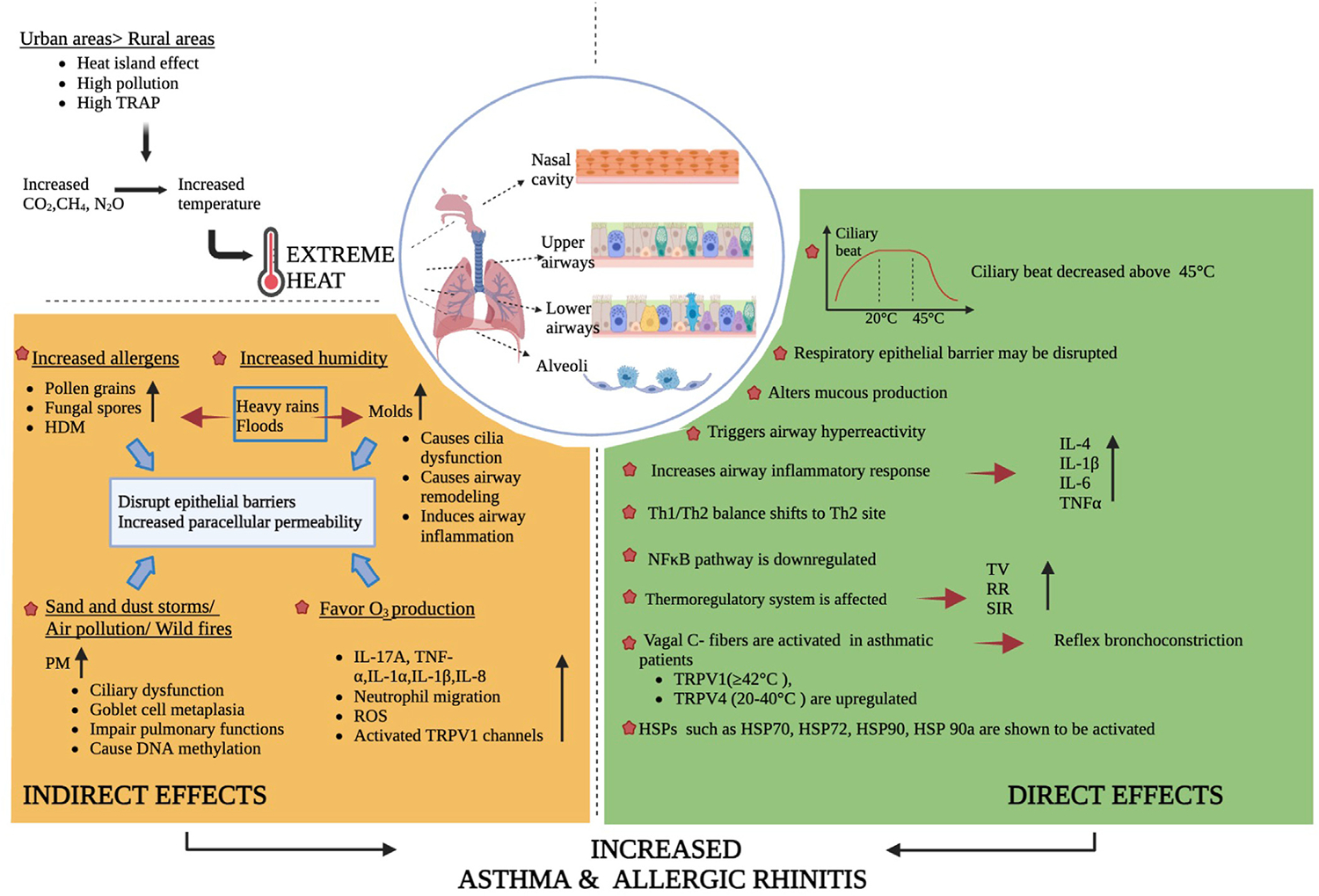
Direct and indirect effects of extreme heat on upper and lower respiratory epithelial barrier. Results are summarized of cell culture, animal models, and human studies; levels of evidence are indicated in text. HDM, House dust mite; ROS, reactive oxygen species; RR, respiratory rate; SIR, systemic inflammatory response; TRAP, traffic-related air pollution; TV, tidal volume.
From the larynx to the alveolar wall, the airway is innervated by vagal sensory nerves mostly composed of unmyelinated (C) afferent fibers expressing neural TRP channels. TRP receptors, especially TRPV1 and TRPV4, are sensitive to hot temperatures, >42°C and 22–40°C, respectively, and are activated by breathing hot air.24,30 TRPV1 channels are overexpressed in the airways of patients with refractory asthma and AR; they are activated by chemical mediators during chronic airway inflammation in vivo and in vitro.26,31 In addition to mice models showing that breathing hot air aggravates the airway inflammation, this hypothesis is supported by epidemiologic evidence of heat-induced asthma attacks.24,32 Breathing hot and humid air activates bronchopulmonary vagal C fibers, upregulating TRPV1 and TRPV4, increasing cholinergic responses, and increasing the specific airway resistance and reflex bronchoconstriction.24,33–35 There have been few human studies investigating the role of TRPV1 and TRPV4 in thermoregulation,35 and more research is needed. Future studies should investigate the effects of hot air inhalation on TRPV1 and TRPV4 activation in humans.
Heat shock proteins (HSPs), chaperones that are activated in heat stress conditions, increase to protect cells from heat injury. Several in vivo and in vitro experiments have shown that the effects of HSP-70 on the respiratory epithelium are controversial.24,36 Traditionally, it is thought that intracellular HSP-70, a cytoprotective chaperone that has proinflammatory properties, acts as an alarmin when secreted into the extracellular space.36 Proinflammatory HSP-70 can support the allergic airway response and maintains increased levels of IL-4, IL-5, IL-13, and eosinophils.37 It also stimulates the secretion of IL-1, IL-6, and IL-8 by epithelial cells and alveolar macrophages, which amplifies the recruitment of neutrophils to the site.38 However, HSP-70 also has anti-inflammatory properties, preventing neutrophil hyperactivation, downregulating eosinophils, and suppressing allergic cytokines.36 Moreover, higher levels of HSP-70 in Caco-2 cell cultures have been shown to increase occludin levels and decrease epithelial permeability.39,40 This finding suggests that HSP-70, which is stimulated in the respiratory tract epithelium by heat, may have similar effects and may have protective effects for the airway epithelial barrier. Further research is needed. Both in vitro experiments and human studies showed that when activated by extreme heat, HSP-70/72 prevents cell apoptosis and plays a critical role in the ability of cells to become thermotolerant.39 HSP-90a, which plays a role in heat acclimation, also has an important role in airway barrier dysfunction and supports the development of asthma in house dust mite–induced asthmatic mice.41 In cell cultures, HSP-90 has been shown to regulate bitter taste receptor (T2R)-dependent nitric oxide(NO)pathways in primary sinonasal epithelial cells, and its inhibition reduces T2R-/NO-driven nasal ciliary beating.42 Consistent with a sustained inhibition of the inflammatory response, the nuclear factor kappa–light-chain enhancer of activated B cells (aka NF-κB) pathway, which influences both adaptive and innate immunity, has been shown to be downregulated by heat stress in a study population of young and healthy volunteers exposed to extreme heat for short periods in a sauna (Figs 1 and 2).39,43
Indirect effects
Heavy rains and floods.
Excessive evaporation due to increased air temperature causes heavy rains and floods, creating hot and humid conditions that contribute to increased indoor mold contamination.44 Molds disrupt the epithelial barrier by secreting proteases and reducing the transepithelial resistance, causing cilia dysfunction, and increasing barrier permeability.45,46 In addition, they induce airway inflammation and cause airway remodeling.45 Moreover, heavy rainfall increases the atmospheric concentration of allergenic particles such as pollen grains and fungal spores by mechanical impact and fractionation. Independent of humidity and pollution exposure, Nassikas and colleagues found that precipitation was associated with higher fractional exhaled nitric oxide among adolescents with asthma.47 In conjunction, multiple studies have demonstrated worsening of AR and asthma after thunderstorms.48,49
Airborne allergens.
Increasing temperature, humidity and high CO2 levels increase the concentration of airborne allergens (ie, pollens, mites, fungi), leading to a longer pollen season—and even driving the emergence of new allergenic species.50 In addition, air pollutants (ie, nitrogen dioxide [NO2] and ozone [O3]) can modify the chemical characteristics of airborne pollen and increase its allergenicity.2 Pollen with proteolytic activity such as molds and house dust mites disrupt the epithelial barrier integrity. This facilitates the recognition and presentation of allergens in the respiratory tract, initiating allergic inflammation and leading to the development of sensitivity to other allergens (Figs 1 and 2).51 Accordingly, a 25-year longitudinal study showed a significant association between increased temperatures and longer pollen seasons, increased peak pollen duration, more severe seasonal AR, and increased hospital admissions.50
Air pollution, sand and dust storms, and wildfires.
Climate change and air pollution affect the mortality and morbidity of allergic diseases. Increased solar activity, which amplifies the effects of particulate matter with a diameter of 2.5 mm or less (PM2.5), induces reductions in FEV1 and forced vital capacity.52 A recent large study from Europe showed that deaths from lung diseases increase much more when air pollution is combined with high temperatures.53 PM2.5 disrupts both the nasal and the respiratory tract epithelium by reducing TJ proteins, increasing paracellular permeability, causing goblet cell metaplasia, and leading to increased mucus secretion, ciliary loss, and dysfunction.16,54,55 On another note, sand and dust storms are an undeniable cause of air pollution with PM2.5, pollen, chemicals, and microbiologic elements such as bacteria and fungi. They induce neutrophilic and eosinophilic inflammation by either stimulating proinflammatory cytokines and mediators or via Toll-like receptor (TLR)-2– and TLR-4–mediated inflammatory response.56,57 PM2.5, particulate matter with a diameter of 10 mm or less, and dust storms have been shown to worsen both AR and asthma clinically.58,59 Recently a study from Turkey showed that dust storms increased the risk of mortality, emergency visits, and hospitalization in patients with asthma.60
Wildfires destroy ecosystems by absorbing CO2 from the atmosphere and creating particulate pollution such as dust storms. Zeglinski and colleagues demonstrated the disruptive effects of wildfires on the barrier dysfunction in a human airway epithelium model (Figs 1 and 2).61 Wildfires have caused specific DNA methylation patterns in nasal epithelial cells, affecting the TH1 and TH2 activation pathways.62 Clinically, in a Brazilian study involving more than 2 million patient records, wildfire-related PM2.5 was associated with a 23% increase in respiratory complaint–related hospital admissions.63
An increased frequency of heat waves leads to increased ground-level O3 production and exacerbation of surface O3 pollution events.64 Animal models have shown that exposure to O3 results in shedding of the ciliary epithelium, transcriptional changes in genes related to barrier function, and epithelial organization.65,66 O3 exposure augments TLR-2– and TLR-4–enhanced neutrophil migration, stimulates cytokines including IL-17A, TNF-α, IL-8, IL-1a, and IL-1β, activating TRPV1 channels, and forming reactive oxygen species (Figs 1 and 2).66,67 Consistent with these results, chronic exposure to O3 was associated with an increased risk of airway hyperresponsiveness, asthma flares, and even respiratory mortality.59,68
Implications for AR and asthma
In summary, recent reports have revealed the direct and indirect effects of extreme temperatures on the respiratory epithelial barrier. The evidence points to an increased prevalence and risk of AR and asthma exacerbations, hospitalizations, and mortality with climatic exposures. Further studies are needed to individually elucidate the potential treatment strategies to act on affected mediators, chaperones, receptors, and proteins. In addition, there is a need for translational studies investigating the validity in humans of results obtained in cell cultures or animal models, as well as epidemiologic studies to see the actual effects in populations.
DERMATOLOGIC ALLERGIC DISEASES
Composition of dermatologic epithelium
Skin is divided into 2 major layers, the epidermis and dermis (Fig 3). The epidermis is composed of 4 sublayers—the stratum corneum, stratum granulosum, stratum spinosum, and stratum basale—followed by the basement membrane.69 The stratum corneum is important in barrier formation; it is the outer layer containing enucleated keratinocytes called corneocytes. In addition to theepithelial cells, structural proteins, secreted epithelial products, and microbiota comprise the epidermis. Filaggrin, an important structural protein, aligns the keratin filaments, aiding in the barrier function. TJ prevent transepidermal water loss.70 The dermis, below the epidermis, is primarily composed of connective tissue. Fibroblasts function as the primary cells, providing resistance and elasticity through the secretion of collagen and elastin. Nerve endings, sebaceous glands, hair follicles, and blood and lymphatic vessels are embedded within this layer.
FIG 3.
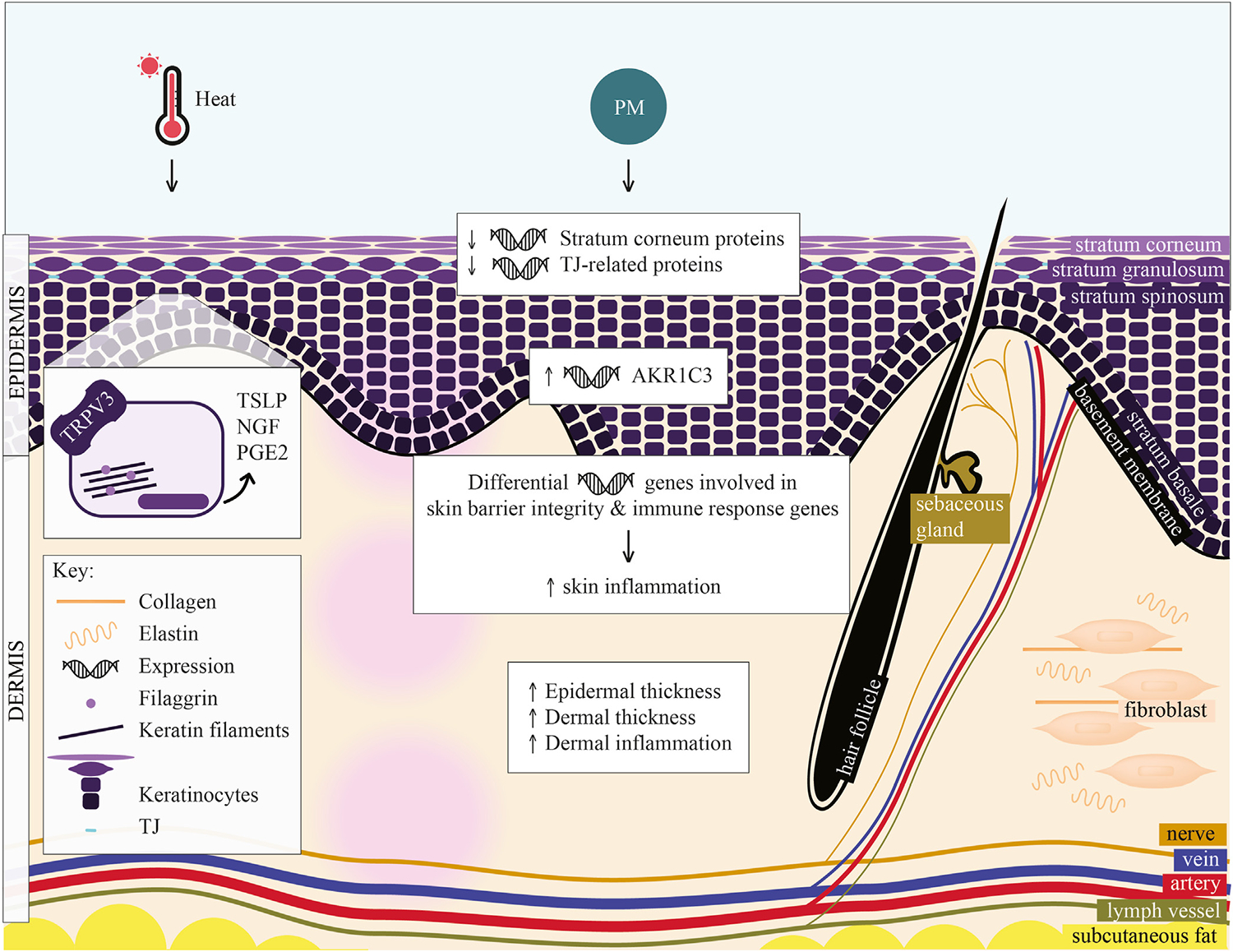
Hypothesized effects of heat and particulate matter on skin barrier structure. Skin is composed of epidermis and dermis; layers and components of these layers are labeled. Heat activates TRPV3 in keratinocytes, increasing TSLP, NGF, and PGE2. (TRPV3 expression and function is increased in AD.) PM has several hypothesized effects in AD: (1) decreases expression of stratum corneum and TJ-related proteins, (2) induces expression of AKR1C3 (AKR1C3 variant is overexpressed in AD), (3) leads to differential expression of genes controlling skin barrier integrity and immune response, increasing skin inflammation, and (4) results in increased epidermal and dermal thickness and increased dermal inflammation. NGF, Nerve growth factor; PGE2, prostaglandin E2; TSLP, thymic stromal lymphopoietin.
Patients with AD have a disrupted skin barrier, thereby allowing allergens and air pollutants entry into the body, triggering a type 2 immune response.71 Recent reviews offer comprehensive summaries of mechanistic and epidemiologic studies relating to climate change and AD.72,73 Much of the data are epidemiologic in nature and provide interesting hypotheses that should be explored further.
Direct effects
Extreme temperature events.
One feature of climate change is extreme temperature, especially, as mentioned above, extreme heat events.74 Limited data exist on the effect of extreme heat on the skin epithelium. However, heat can trigger itch in AD.75 One study examined the role of TRPV3 in heat-induced itch in AD.76 TRPV3, a thermosensitive TRP channel, is expressed in keratinocytes and activated by heat. Keratinocytes from patients with AD had increased expression and hyperactive function of TRPV3. TRPV3 activation led to increased secretion of thymic stromal lymphopoietin, nerve growth factor, and prostaglandin E2, suggesting that TRPV3 is a therapeutic target for heat-induced itch (Fig 3).
The data on the effect of sweat on the epithelial function in the setting of extreme heat is limited and over a decade old,77–79 comprising a mix of in vitro and in vivo studies. We know that ambient temperature, humidity, and climate affect sweat glandactivity.80 However, while sweat gland activity increases with increasing temperatures, it is unclear whether skin sweat glands are constitutively active or activated at a certain temperature.77–79,81 Furthermore, increased transepidermal water loss, a measure of a disturbed or disrupted skin barrier, is associated with increasing temperature and decreasing relative humidity, although it is more strongly correlated with temperature than humidity.77 Studies examining the role of sweat on epithelial barrier function in current climate patterns are needed.
The clinical evidence for the effect of temperature on AD has been inconclusive. A time series analysis found that both low and high temperatures were associated with increased AD outpatient visits, especially in the pediatric age group of 14 years old and younger.82 Another time series analysis explored the synergistic effect of heat with pollution.83 This study examined the association between multiple meteorologic factors and over 2 million clinical visits for childhood allergic diseases in 2 large pediatric hospitals in Shanghai, China, over a decade (2007 to 2017); it found that low temperatures and high levels of NO2, among other factors, were associated with increased clinical visits for childhood AD. A smaller longitudinal study found that higher indoor temperatures and higher relative humidity contributed to higher indoor formaldehyde levels.84 The extent to which formaldehyde exposure increased AD symptoms varied according to child age and indoor temperature. Taken together, these data suggest that extreme temperatures may contribute to AD flares, with limited data suggesting TRPV3 to be a potential therapeutic target in heat-triggered AD (Fig 3).
Indirect effects
Heavy rains and floods.
Two recent clinical studies have assessed the effect of heavy rains and floods on skin conditions.85,86 The first was a retrospective population-based study using a time-stratified matched-period case-crossover design of children aged 0 to 12 years in Taiwan.85 There were increased odds of childhood AD visits on weeks with floods compared to weeks without floods. The second study was a retrospective cross-sectional study of pediatric patients in the Houston area before and after Hurricane Harvey.86 Dermatologic complaints were more commonly seen in the 30 days after the hurricane compared to the same period a year before. These time series studies provide initial data supporting an association between floods and AD flares, but more data are needed.
Air pollution.
Recent studies have explored the association between air pollution and AD, including 3 mechanistic studies examining the effects of particulate matter (PM) (Fig 3).87–89 Using a mouse model, Woo and colleagues found that exposure to PM with diameter 10 mm or less induces and worsens skin inflammation via the differential expression of genes controlling skin barrier integrity and immune response.88 Next, using a mouse model of oxazolone-induced AD-like skin and human keratinocyte cells, Bae and colleagues found that PM downregulates the expression levels of several stratum corneum and TJ-related proteins.87 The epidermal and dermal thickness in an AD-like mouse model was significantly increased, and dermal inflammation was prominent in the PM-treated, AD-like skin. Finally, Vogeley and colleagues explored whether genetic susceptibilities modified the effect to which air pollution affects AD.89 Their group specifically looked at a gene variant of aldo-keto reductase 1C3 (AKR1C3), which is highly expressed in AD skin, in the GINIplus/LISA birth cohort. PM exposure inducedAKR1C3 expression inhuman skin. With chronic PM exposure, each rs12529 single nucleotide polymorphism allele of this gene increased the chance of developing AD by 37% to 38%, suggesting that certain populations may be more susceptible to the effects of pollution on AD.
A wealth of recent epidemiologic information adds to these mechanistic studies by showing an association between air pollution and AD.90–99 We highlight recent longitudinal, time series, and machine learning studies assessing this topic. Several longitudinal studies made up of several thousand participants each have found an association between pollution and AD incidence and symptoms.94–96 Using the Korean National Health Insurance Service–National Sample Cohort database from 2008 to 2013, this study found that long-term exposure to air pollutants, including sulfur dioxide, NO2, carbon monoxide, and PM, was an independent risk factor for developing AD.94 Another study used 13 years of the Taiwan National Health Insurance research database linked to the Taiwan Air Quality Monitoring Database and similarly found that exposure to higher concentrations of total hydrocarbons and methane were associated with an increased risk of childhood AD.95 Both studies used more than 5 years’ worth of national health insurance data with more than 3000 subjects and independently found an association of pollution with AD incidence. An additional longitudinal study found that indoor concentrations of PM, CO2, and volatile organic compounds were lower in energy-efficient homes after controlling for seasonality.96 CO2 was associated with daily risk of AD symptoms. Finally, Huang and colleagues used machine learning in a longitudinal birth cohort and found that prenatal air quality was important to predict childhood AD development.90 Four time series studies found that various air pollutants, including PM, sulfur dioxide, NO2, carbon monoxide, and O3, were associated with an increase in AD visits.97–100 This effect was stronger in the cool season and among children and the elderly.98,100 Interestingly, a recent study explored the effect of air pollution on patients with moderate-to-severe AD on dupilumab and found a dose-dependent relationship between air pollutants and risk of an AD flare.101 These data are limited as a result of the lack of a control group (ie, patients not receiving dupilumab therapy),so we cannot assess whether dupilumab might provide some protective effect, given its positive effect on restoring the skin barrier.102 In summary, there is recent robust clinical evidence showing an association between air pollution and an increase in the incidence, prevalence, symptoms, and severity of AD. Next steps should attempt to bridge these studies by providing translational human mechanistic studies of patients affected by air pollution.
Wildfires.
There are also limited recent mechanistic studies assessing the effect of wildfires on AD. We reviewed recent epidemiologic studies, which found an association between wildfires and AD flares. Two studies of the 2018 California Camp Fire highlight key findings about the relationship between wildfires and AD.103,104 The authors evaluated associations between wildfire air pollution and (1) clinic visits for AD or itch and (2) prescribed medications for AD management.103 This time series study found an increased rate of AD and itch visits for both pediatric and adult patients on weeks with wildfires compared to weeks without wildfires. There was a statistically significant increase in the rate of systemic medication prescription during wildfire weeks among adults with AD. Among adults aged 65 years or older, there were higher rates of clinic visits for itch on weeks with a wildfire.104 For both this age group and the younger adult age group of 18- to 64-year-olds, there was a significant increase in AD visits on wildfire weeks. Smoke plume density from satellite imaging was also associated with a higher rate of clinic visits for AD in both age groups and for pruritus in the older age group. Future work on the effect of wildfires on AD may focus on elucidating mechanistic pathways through animal or human models.
Implications for AD
Recent studies provide evidence of the effects of climate change—specifically of extreme temperature, heavy rains and floods, air pollution, and wildfires—on AD. Some of these associations need to be studied further to account for confounders and to further elucidate the biological mechanisms, ultimately to better understand how to manage climate change–associated AD exacerbations.
Conclusion
In this review article, we discuss the effects of extreme temperatures resulting from the deterioration of the climate system. Extreme temperatures adversely affect respiratory and skin barriers by acting directly, or by causing extreme weather events such as heavy rain, floods, dust storms, wildfires, and air pollution. While evidence is limited, these changes appear to disrupt structural proteins that make up the epithelial barriers and the development of inflammation and the activation of cholinergic pathways, contributing to increased frequency and severity of AR, asthma, and AD. Future studies should aim to fill our knowledge gaps of the effects of climate change on atopic disease (Table II).
TABLE II.
What remains unknown?
| Characteristic | Biological mechanism | Epidemiologic trends |
|---|---|---|
|
| ||
| Global warming effect—Direct, extreme temperature; indirect, (1) heavy rains and floods, (2) wildfires, and (3) air pollution | • What are the biological mechanisms in humans of the direct and indirect effects of global warming on asthma, AR, and AD? • What are the long-term impacts on the epithelial barrier of the direct and indirect effect of global warming in asthma, AR, and AD? • Are airway inflammation and structural changes in the epithelial barrier caused by heavy rains and floods, air pollution, sand and dust storms and wildfires reversible if necessary precautions are taken? • What is the role of sweat in epithelial barrier function in different climates and among different population groups? • What are the genetic factors that may increase susceptibility for developing allergic disease when exposed to global warming? |
• How do extreme temperature, heavy rains and floods, and wildfires affect the prevalence or incidence of AD? • What are the synergistic effects of climatic factors on the severity of asthma, AR, and AD? • How may climatic factors affect the burden of allergic diseases between low-resource and high-resource regions? |
| Implications for AR, asthma, and AD | • What is the efficacy and safety of individualized treatment strategies acting on affected mediators, chaperones, and TRPV channels in global warming-related AR and asthma? • What is the mechanistic effect of different treatment modalities, including biologics, on global warming-related AD? |
• What are the underlying risk factors for asthma, AR, and AD development due to global warming? • What are the best air pollution mitigation interventions to decrease the effects of climatic factors on asthma, AR, and AD severity? • Which medical interventions (eg, biologics) are more effective at reducing the burden of asthma and AD due to global warming? |
Individuals, providers, scientists, and legislators should act in unison to combat the climate change crisis and its effects on atopic disease. Future research will illuminate individualized management strategies against the damage caused by global warming. Together, we can all take steps to mitigating the legacy of climate change for future generations.
Abbreviations used
- AD
Atopic dermatitis
- AKR1C3
Aldo-keto reductase 1C3
- AR
Allergic rhinitis
- CO2
Carbon dioxide
- FEV1
Forced expiratory volume in 1 second
- HSP
Heat shock protein
- NO
Nitric oxide
- NO2
Nitrogen dioxide
- O3
Ozone
- PM
Particulate matter
- PM2.5
PM with diameter 2.5 μm or less
- T2R
Bitter taste receptors
- TJ
Tight junctions
- TLR
Toll-like receptor
- TRP
Transient receptor potential
- TRPV
TRP vanilloid
Footnotes
DISCLOSURE STATEMENT
E.R.T. is supported by the National Institutes of Health (NIH; grant 5T32AI007512). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. E.R.T. is funded in part by the Boston Children’s Hospital Pediatric Health Equity Fellowship.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figs 1 and 2 were created with BioRender.com. Fig 3 was created with Adobe Illustrator.
REFERENCES
- 1.Singh AB, Kumar P. Climate change and allergic diseases: an overview. Front Allergy 2022;3:964987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacheco SE, Guidos-Fogelbach G, Annesi-Maesano I, Pawankar R, d’Amato G, Latour-Staffeld P, et al. Climate change and global issues in allergy and immunology. J Allergy Clin Immunol 2021;148:1366–77. [DOI] [PubMed] [Google Scholar]
- 3.Global warming vs climate change, 2023. NASA global climate change, Available at: https://climate.nasa.gov/faq/12/whats-the-difference-between-climate-change-and-global-warming/. Accessed May 10, 2023.
- 4.Chen H, He W, Sun J, Chen L. Increases of extreme heat-humidity days endanger future populations living in China. Environ Res Lett 2022;17:064013. [Google Scholar]
- 5.Spangler KR, Liang S, Wellenius GA. Wet-bulb globe temperature, universal thermal climate index, and other heat metrics for US counties, 2000–2020. Sci Data 2022;9:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke B, Otto F, Stuart-Smith R, Harrington L. Extreme weather impacts of climate change: an attribution perspective. Environ Res Climate 2022;1:012001. [Google Scholar]
- 7.Vecellio DJ, Wolf ST, Cottle RM, Kenney WL. Evaluating the 35°C wet-bulb temperature adaptability threshold for young, healthy subjects (PSU HEAT Project). J Appl Physiol (1985) 2022;132:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrova A, Ingole V, Basagaña X, Ranzani O, Milà C, Ballester J, et al. Association between ambient temperature and heat waves with mortality in South Asia: systematic review and meta-analysis. Environ Int 2021;146:106170. [DOI] [PubMed] [Google Scholar]
- 9.Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, editors. Climate change 2021: the physical science basis. Summary for policymakers. Working Group I contribution to the sixth assessment report of the Intergovernmental Panel on Climate Change. Interlaken (Switzerland): IPCC; 2021. Available at: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM_final.pdf. [Google Scholar]
- 10.Prunicki MM, Dant CC, Cao S, Maecker H, Haddad F, Kim JB, et al. Immunologic effects of forest fire exposure show increases in IL-1β and CRP. Allergy 2020;75:2356–8. [DOI] [PubMed] [Google Scholar]
- 11.Luschkova D, Traidl-Hoffmann C, Ludwig A. Climate change and allergies. Allergo J Int 2022;31:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celebi Sozener Z, Ozdel Ozturk B, Cerci P, Turk M, Gorgulu Akin B, Akdis M, et al. Epithelial barrier hypothesis: effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022;77:1418–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik MS, Chakraborty S, Veleri S, Kateriya S. Mucociliary respiratory epithelium integrity in molecular defense and susceptibility to pulmonary viral infections. Biology 2021;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nawroth JC, van der Does AM, Ryan Firth A, Kanso E. Multiscale mechanics of mucociliary clearance in the lung. Philos Trans R Soc Lond B Biol Sci 2020;375:20190160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Dutta J, Ray A, Karmakar A, Mabalirajan U. Airway epithelium: a neglected but crucial cell type in asthma pathobiology. Diagnostics (Basel) 2023;13:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nur Husna SM, Tan HTT, Md Shukri N, Mohd Ashari NS, Wong KK. Nasal epithelial barrier integrity and tight junctions disruption in allergic rhinitis: overview and pathogenic insights. Front Immunol 2021;12:663626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, Byun J, Kim B, Yeon J, Tai J, Lee SH, et al. TRPV4-mediated epithelial junction disruption in allergic rhinitis triggered by house dust mites. Am J Rhinol Allergy 2021;35:432–40. [DOI] [PubMed] [Google Scholar]
- 18.Cecchi L, Vaghi A, Bini F, Martini M, Musarra A, Bilo MB. From triggers to asthma: a narrative review on epithelium dysfunction. Eur Ann Allergy Clin Immunol 2022;54:247–57. [DOI] [PubMed] [Google Scholar]
- 19.McElroy S, Schwarz L, Green H, Corcos I, Guirguis K, Gershunov A, et al. Defining heat waves and extreme heat events using sub-regional meteorological data to maximize benefits of early warning systems to population health. Sci Total Environ 2020;721:137678. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Pan J, Xu R, Lu W, Wang Y, Liu T, et al. Asthma mortality attributable to ambient temperatures: a case-crossover study in China. Environ Res 2022;214:114116. [DOI] [PubMed] [Google Scholar]
- 21.Sohail H, Kollanus V, Tiittanen P, Schneider A, Lanki T. Heat, Heatwaves and cardiorespiratory hospital admissions in Helsinki, Finland. Int J Environ Res Public Health 2020;17:7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice MB, Li W, Wilker EH, Gold DR, Schwartz J, Zanobetti A, et al. Association of outdoor temperature with lung function in a temperate climate. Eur Respir J 2019;53:1800612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jay O, Capon A, Berry P, Broderick C, de Dear R, Havenith G, et al. Reducing the health effects of hot weather and heat extremes: from personal cooling strategies to green cities. Lancet 2021;398:709–24. [DOI] [PubMed] [Google Scholar]
- 24.Han A, Deng S, Yu J, Zhang Y, Jalaludin B, Huang C. Asthma triggered by extreme temperatures: from epidemiological evidence to biological plausibility. Environ Res 2023;216(pt 2):114489. [DOI] [PubMed] [Google Scholar]
- 25.Lian P, Braber S, Garssen J, Wichers HJ, Folkerts G, Fink-Gremmels J, et al. Beyond heat stress: intestinal integrity disruption and mechanism-based intervention strategies. Nutrients 2020;12:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng L, Ma P, Wu Y, Ma Y, Yang X, Li Y, et al. High and low temperatures aggravate airway inflammation of asthma: evidence in a mouse model. Environ Pollut 2020;256:113433. [DOI] [PubMed] [Google Scholar]
- 27.Nikolaizik W, Hahn J, Bauck M, Weber S. Comparison of ciliary beat frequencies at different temperatures in young adults. ERJ Open Res 2020;6:00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaheen S, Maqbool K, Beg OA, Gul F. Thermal analysis of airway mucus clearance by ciliary activity in the presence of inertial forces. SN Appl Sci 2021;3:1–11. [Google Scholar]
- 29.Clary-Meinesz CF, Cosson J, Huitorel P, Blaive B. Temperature effect on the ciliary beat frequency of human nasal and tracheal ciliated cells. Biol Cell 1992;76:335–8. [DOI] [PubMed] [Google Scholar]
- 30.Silverman HA, Chen A, Kravatz NL, Chavan SS, Chang EH. Involvement of neural transient receptor potential channels in peripheral inflammation. Front Immunol 2020;11:590261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harford TJ, Grove L, Rezaee F, Scheraga R, Olman MA, Piedimonte G. RSV infection potentiates TRPV1-mediated calcium transport in bronchial epithelium of asthmatic children. Am J Physiol Lung Cell Mol Physiol 2021; 320:L1074–l84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu C, Liu Q, Deng M, Liao H, Yang X, Ma P. Interaction of high temperature and NO2 exposure on asthma risk: in vivo experimental evidence of inflammation and oxidative stress. Sci Total Environ 2023;869:161760. [DOI] [PubMed] [Google Scholar]
- 33.Dumitrache MD, Jieanu AS, Scheau C, Badarau IA, Popescu GDA, Caruntu A, et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (review). Exp Ther Med 2021;22:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonvini SJ, Birrell MA, Dubuis E, Adcock JJ, Wortley MA, Flajolet P, et al. Novel airway smooth muscle–mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur Respir J 2020;56:1901458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes D Jr, Collins PB, Khosravi M, Lin RL, Lee LY. Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med 2012;185:1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shevchenko M, Servuli E, Albakova Z, Kanevskiy L, Sapozhnikov A. The role of heat shock protein 70 kDa in asthma. J Asthma Allergy 2020;13:757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yombo DJK, Mentink-Kane MM, Wilson MS, Wynn TA, Madala SK. Heat shock protein 70 is a positive regulator of airway inflammation and goblet cell hyperplasia in a mouse model of allergic airway inflammation. J Biol Chem 2019; 294:15082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulina-Tomašković A, Grdić Rajković M, Jelić D, Bosnar M, Sladoljev L, Žanić Grubisić T, et al. Pro-inflammatory effects of extracellular Hsp70 on NCI-H292 human bronchial epithelial cell line. Int J Exp Pathol 2019;100:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nava R, Zuhl MN. Heat acclimation–induced intracellular HSP70 in humans: a meta-analysis. Cell Stress Chaperones 2020;25:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuhl MN, Lanphere KR, Kravitz L, Mermier CM, Schneider S, Dokladny K, et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol (1985) 2014; 116:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye C, Huang C, Zou M, Hu Y, Luo L, Wei Y, et al. The role of secreted Hsp90α in HDM-induced asthmatic airway epithelial barrier dysfunction. BMC Pulm Med 2019;19:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey RM, Hariri BM, Adappa ND, Palmer JN, Lee RJ. HSP90 modulates T2R bitter taste receptor nitric oxide production and innate immune responses in human airway epithelial cells and macrophages. Cells 2022;11:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouchama A, Aziz MA, Mahri SA, Gabere MN, Dlamy MA, Mohammad S, et al. A model of exposure to extreme environmental heat uncovers the human transcriptome to heat stress. Sci Rep 2017;7:9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du C, Li B, Yu W. Indoor mould exposure: characteristics, influences and corresponding associations with built environment—a review. J Build Eng 2021;35:101983. [Google Scholar]
- 45.Namvar S, Labram B, Rowley J, Herrick S. Aspergillus fumigatus–host interactions mediating airway wall remodelling in asthma. J Fungi 2022;8:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naeem M, Manzoor S, Abid MU, Tareen MBK, Asad M, Mushtaq S, et al. Fungal proteases as emerging biocatalysts to meet the current challenges and recent developments in biomedical therapies: an updated review. J Fungi (Basel) 2022;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nassikas NJ, Rifas-Shiman SL, Luttmann-Gibson H, Chen K, Blossom JC, Oken E, et al. Precipitation and adolescent respiratory health in the Northeast United States. Ann Am Thorac Soc 2023;20:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damialis A, Bayr D, Leier-Wirtz V, Kolek F, Plaza M, Kaschuba S, et al. Thunderstorm asthma: in search for relationships with airborne pollen and fungal spores from 23 sites in Bavaria, Germany. A rare incident or a common threat? J Allergy Clin Immunol 2020;145:AB336. [Google Scholar]
- 49.Park JH, Lee E, Fechter-Leggett ED, Williams E, Yadav S, Bakshi A, et al. Associations of emergency department visits for asthma with precipitation and temperature on thunderstorm days: a time-series analysis of data from Louisiana, USA, 2010–2012. Environ Health Perspect 2022;130:87003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schreurs W, Schermer TRJ, Akkermans RP, Bischoff E, Luijks HD. 25-year retrospective longitudinal study on seasonal allergic rhinitis associations with air temperature in general practice. NPJ Prim Care Respir Med 2022;32:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaspar R, de Matos MR, Cortes L, Nunes-Correia I, Todo-Bom A, Pires E, et al. Pollen proteases play multiple roles in allergic disorders. Int J Mol Sci 2020;21:3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anand K, Vieira CLZ, Garshick E, Wang V, Blomberg A, Gold DR, et al. Solar and geomagnetic activity reduces pulmonary function and enhances particulate pollution effects. Sci Total Environ 2022;838:156434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Exhaustion Project. We breathe climate change—climate change worsens the impacts of air pollution. December 19, 2022. Available at: https://www.exhaustion.eu/resources/we-breathe-climate-change2.
- 54.Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev 2022;31:210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sözener ZC, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol 2020;145:1517–28. [DOI] [PubMed] [Google Scholar]
- 56.Sadakane K, Ichinose T, Maki T, Nishikawa M. Co-exposure of peptidoglycan and heat-inactivated Asian sand dust exacerbates ovalbumin-induced allergic airway inflammation in mice. Inhal Toxicol 2022;34:231–43. [DOI] [PubMed] [Google Scholar]
- 57.Shin SH, Ye MK, Lee DW, Chae MH. Asian sand dust particles enhance the development of Aspergillus fumigatus biofilm on nasal epithelial cells. Int J Mol Sci 2022;23:3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geravandi S, Sicard P, Khaniabadi YO, De Marco A, Ghomeishi A, Goudarzi G, et al. A comparative study of hospital admissions for respiratory diseases during normal and dusty days in Iran. Environ Sci Pollut Res Int 2017;24:18152–9. [DOI] [PubMed] [Google Scholar]
- 59.Maio S, Fasola S, Marcon A, Angino A, Baldacci S, Bilo MB, et al. Relationship of long-term air pollution exposure with asthma and rhinitis in Italy: an innovative multipollutant approach. Environ Res 2023;224:115455. [DOI] [PubMed] [Google Scholar]
- 60.Boğan M, Kul S, Al B, Oktay MM, Akpinar Elçi M, Pinkerton KE, et al. Effect of desert dust storms and meteorological factors on respiratory diseases. Allergy 2022;77:2243–6. [DOI] [PubMed] [Google Scholar]
- 61.Zeglinski MR, Turner CT, Zeng R, Schwartz C, Santacruz S, Pawluk MA, et al. Soluble wood smoke extract promotes barrier dysfunction in alveolar epithelial cells through a MAPK signaling pathway. Sci Rep 2019;9:10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noah TL, Worden CP, Rebuli ME, Jaspers I. The effects of wildfire smoke on asthma and allergy. Curr Allergy Asthma Rep 2023;23:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Requia WJ, Amini H, Mukherjee R, Gold DR, Schwartz JD. Health impacts of wildfire-related air pollution in Brazil: a nationwide study of more than 2 million hospital admissions between 2008 and 2018. Nat Commun 2021;12:6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khomsi K, Chelhaoui Y, Alilou S, Souri R, Najmi H, Souhaili Z. Concurrent heat waves and extreme ozone (O3) episodes: combined atmospheric patterns and impact on human health. Int J Environ Res Public Health 2022;19:2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tovar A, Smith GJ, Thomas JM, Crouse WL, Harkema JR, Kelada SNP. Transcriptional profiling of the murine airway response to acute ozone exposure. Toxicol Sci 2020;173:114–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mumby S, Chung KF, Adcock IM. Transcriptional effects of ozone and impact on airway inflammation. Front Immunol 2019;10:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Müller I, Alt P, Rajan S, Schaller L, Geiger F, Dietrich A. Transient receptor potential (TRP) channels in airway toxicity and disease: an update. Cells 2022;11:2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SY, Kim E, Kim WJ. Health effects of ozone on respiratory diseases. Tuberc Respir Dis (Seoul) 2020;83:S6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitamura Y, Ogulur I, Pat Y, Rinaldi AO, Ardicli O, Cevhertas L, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis 2021;85:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dijkhoff IM, Drasler B, Karakocak BB, Petri-Fink A, Valacchi G, Eeman M, et al. Impact of airborne particulate matter on skin: a systematic review from epidemiology to in vitro studies. Part Fibre Toxicol 2020;17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Celebi Sozener Z Özbey Yücel Ü, Altiner S, Ozdel Oztürk B, Cerci P, Türk M, et al. The external exposome and allergies: from the perspective of the epithelial barrier hypothesis. Front Allergy 2022;3:887672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fadadu RP, Abuabara K, Balmes JR, Hanifin JM, Wei ML. Air pollution and atopic dermatitis, from molecular mechanisms to population-level evidence: a review. Int J Environ Res Public Health 2023;20:2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan Z, Dai Y, Akar-Ghibril N, Simpson J, Ren H, Zhang L, et al. Impact of air pollution on atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol 2023. [DOI] [PubMed] [Google Scholar]
- 74.United States Environmental Protection Agency (EPA), Climate change indicators: high and low temperatures, 2022, Available at: https://www.epa.gov/climate-indicators/climate-change-indicators-high-and-low-temperatures. Accessed May 10, 2023.
- 75.Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Association of itch triggers with atopic dermatitis severity and course in adults. Ann Allergy Asthma Immunol 2020;125:552–9.e2. [DOI] [PubMed] [Google Scholar]
- 76.Seo SH, Kim S, Kim SE, Chung S, Lee SE. Enhanced thermal sensitivity of TRPV3 in keratinocytes underlies heat-induced pruritogen release and pruritus in atopic dermatitis. J Invest Dermatol 2020;140:2199–209.e6. [DOI] [PubMed] [Google Scholar]
- 77.Cravello B, Ferri A. Relationships between skin properties and environmental parameters. Skin Res Technol 2008;14:180–6. [DOI] [PubMed] [Google Scholar]
- 78.Peng C, Hwang RL, Chang SY, Lu YT. Effects of temperature steps on human skin physiology and thermal sensation response. Fuel Energy Abstr 2011;46:2387–97. [Google Scholar]
- 79.Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990;22:164–78. [DOI] [PubMed] [Google Scholar]
- 80.Schwab H, Flora J, Mayrovitz HN. Impacts of skin eccrine glands on the measured values of transepidermal water loss. Cureus 2022;14:e32266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rieg S, Garbe C, Sauer B, Kalbacher H, Schittek B. Dermcidin is constitutively produced by eccrine sweat glands and is not induced in epidermal cells under inflammatory skin conditions. Br J Dermatol 2004;151:534–9. [DOI] [PubMed] [Google Scholar]
- 82.Wang F, Shi C, Dong J, Nie H. Association between ambient temperature and atopic dermatitis in Lanzhou, China: a time series analysis. Environ Sci Pollut Res Int 2021;28:67487–95. [DOI] [PubMed] [Google Scholar]
- 83.Hu Y, Xu Z, Jiang F, Li S, Liu S, Wu M, et al. Relative impact of meteorological factors and air pollutants on childhood allergic diseases in Shanghai, China. Sci Total Environ 2020;706:135975. [DOI] [PubMed] [Google Scholar]
- 84.Kim YM, Kim J, Ha SC, Ahn K. Harmful effect of indoor formaldehyde on atopic dermatitis in children: a longitudinal study. Allergy Asthma Immunol Res 2021; 13:468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen NT, Chen MJ, Wu CD, Guo YL. Emergency room visits for childhood atopic dermatitis are associated with floods? Sci Total Environ 2021;773:145435. [DOI] [PubMed] [Google Scholar]
- 86.Fanny SA, Kaziny BD, Cruz AT, Camp EA, Murray KO, Nichols TJ, et al. Pediatric emergency departments and urgent care visits in houston after Hurricane Harvey. West J Emerg Med 2021;22:763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bae YJ, Park KY, Han HS, Kim YS, Hong JY, Han TY, et al. Effects of particulate matter in a mouse model of oxazolone-induced atopic dermatitis. Ann Dermatol 2020;32:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woo YR, Park SY, Choi K, Hong ES, Kim S, Kim HS. Air pollution and atopic dermatitis (AD): the impact of particulate matter (PM10) on an AD mouse-model. Int J Mol Sci 2020;21:6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogeley C, Kress S, Lang D, Vogel CFA, Hartung F, Brenden H, et al. A gene variant of AKR1C3 contributes to interindividual susceptibilities to atopic dermatitis triggered by particulate air pollution. Allergy 2023;78:1372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Y, Wen HJ, Guo YL, Wei TY, Wang WC, Tsai SF, et al. Prenatal exposure to air pollutants and childhood atopic dermatitis and allergic rhinitis adopting machine learning approaches: 14-year follow-up birth cohort study. Sci Total Environ 2021;777:145982. [DOI] [PubMed] [Google Scholar]
- 91.Kim S, Carson KA, Chien AL. The association between urinary polycyclic aromatic hydrocarbon metabolites and atopic triad by age and body weight in the US population. J Dermatolog Treat 2022;33:2488–94. [DOI] [PubMed] [Google Scholar]
- 92.Lee J, Yun S, Oh I, Kim MH, Kim Y. Impact of environmental factors on the prevalence changes of allergic diseases in elementary school students in Ulsan, Korea: a longitudinal study. Int J Environ Res Public Health 2020;17:8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao M, Xiao Y, Li S, Su J, Li J, Zou B, et al. Synergistic effects between ambient air pollution and second-hand smoke on inflammatory skin diseases in Chinese adolescents. Int J Environ Res Public Health 2022;19:10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park SK, Kim JS, Seo HM. Exposure to air pollution and incidence of atopic dermatitis in the general population: a national population-based retrospective cohort study. J Am Acad Dermatol 2022;87:1321–7. [DOI] [PubMed] [Google Scholar]
- 95.Wang C, Wei CC, Wan L, Lin CL, Tsai JD. Association of exposure to hydrocarbon air pollution with the incidence of atopic dermatitis in children. Ital J Pediatr 2021;47:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim AY, Yoon M, Kim EH, Kim HA, Lee MJ, Cheong HK. Effects of mechanical ventilation on indoor air quality and occupant health status in energy-efficient homes: a longitudinal field study. Sci Total Environ 2021;785:147324. [DOI] [PubMed] [Google Scholar]
- 97.Luo P, Wang D, Luo J, Li S, Li MM, Chen H, et al. Relationship between air pollution and childhood atopic dermatitis in Chongqing, China: a time-series analysis. Front Public Health 2022;10:990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang HL, Sun J, Qian ZM, Gong YQ, Zhong JB, Yang RD, et al. Association between air pollution and atopic dermatitis in Guangzhou, China: modification by age and season. Br J Dermatol 2021;184:1068–76. [DOI] [PubMed] [Google Scholar]
- 99.Baek JO, Cho J, Roh JY. Associations between ambient air pollution and medical care visits for atopic dermatitis. Environ Res 2021;195:110153. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Yang Y, Fu L, Jing D, Sun B, Chen Y, et al. Short-term exposure of PM2.5 and PM10 increases the number of outpatients with eczema in Guangzhou: a time-series study. Front Public Health 2022;10:930545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bellinato F, Adami G, Furci A, Cattani G, Schena D, Girolomoni G, et al. Association between short-term exposure to environmental air pollution and atopic dermatitis flare in patients treated with dupilumab. JAAD Int 2023; 11:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Montero-Vilchez T, Rodriguez-Pozo JA, Diaz-Calvillo P, Salazar-Nievas M, Tercedor-Sanchez J, Molina-Leyva A, et al. Dupilumab improves skin barrier function in adults with atopic dermatitis: a prospective observational study. J Clin Med 2022;11:3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fadadu RP, Grimes B, Jewell NP, Vargo J, Young AT, Abuabara K, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol 2021;157:658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fadadu RP, Green M, Jewell NP, Grimes B, Vargo J, Wei ML. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open 2022;5:e2238594. [DOI] [PMC free article] [PubMed] [Google Scholar]


