Abstract
A 75-year-old man with severe bilateral pleural thickening and dense soft tissue masses surrounding the abdominal aorta on computed tomography was diagnosed with IgG4-related disease (IgG4-RD) as a complication of lung cancer. He was started on nivolumab as second-line therapy along with low-dose prednisolone. Nivolumab was administered for 15 months until disease progression, during which time IgG4-RD did not relapse, and no problematic immune-related adverse events occurred. These results suggest that anti-programmed cell death protein-1 antibody may be used safely in lung cancer associated with IgG4-RD concomitantly with low-dose steroids.
Keywords: IgG4-related disease, lung cancer, anti-programmed cell death protein-1 antibody, nivolumab, low-dose steroid
Introduction
IgG4-related disease (IgG4-RD) is characterized by elevated serum IgG4 concentrations and pathological findings of lymphoplasmacytic infiltration of IgG4-positive plasma cells with storiform fibrosis and obliterative phlebitis in various organs (1). Although the pathogenesis of IgG4-RD remains unknown, T helper 2 (Th2)-dominated cytokine production and immune responses by regulatory T cells (Tregs) play an important role in its development (2). In addition, evidence of an autoimmune basis for the etiology of IgG-RD is growing.
Furthermore, IgG4-RD is often associated with malignant tumors including those found in lung cancer. In a previous study, Yamamoto et al. examined 105 patients with IgG4-RD and reported that patients with IgG4-RD had an increased risk of malignancies, including lung cancer, pancreatic cancer, colon cancer, and malignant lymphoma (3). In a previous study, Asano et al. reported that 34 malignancies were found in 158 patients with IgG4-RD over approximately 6 years of follow-up, with the most common malignancy being lung cancer (4). In addition, other case reports of the coexistence of IgG4-RD and lung cancer have been reported (5,6).
The anti-programmed cell death 1 (PD-1) antibody nivolumab is currently the standard treatment for non-small-cell lung cancer (NSCLC). Several retrospective analyses have demonstrated the safety of using immune checkpoint inhibitors (ICIs) in patients complicated with autoimmune diseases (AIDs) (7,8). However, these findings may not necessarily be directly applicable to IgG4-RD cases, as IgG4-RD patients were not enrolled in these studies, and IgG4-RD is not fully considered an AID due to the complexity of the disease etiology.
We herein report a rare case of squamous cell carcinoma (SCC) of the lung complicated by IgG4-RD and treated with nivolumab. To our knowledge, this is the first report of the use of ICIs for lung cancer complicated by IgG4-RD.
Case Report
A 75-year-old man with a history of smoking was referred to our hospital in December 2015 for a thorough examination of his abnormal chest findings. He had a history of bronchial asthma, chronic obstructive pulmonary disease, and high blood pressure. He had worked in the foundry business for 17 years since 25 years old. Chest radiography and computed tomography (CT) showed bilateral pleural thickening. Because this thickening was mild, and no specific abnormalities were found on an examination, the patient was scheduled for periodic follow-up, including CT scans.
Follow-up CT in August 2016 showed marked worsening of the bilateral pleural thickening as well as mediastinal lymph node swelling (Fig. 1A). On a physical examination, the patient's temperature was 36.3°C, his oxygen saturation was 88% on room air, and coarse crackles were heard in the anterior chest wall. The laboratory findings were as follows: 5,500 /μL white blood cells, 11.1 g/dL hemoglobin, and 2.40 mg/dL C-reactive protein. In addition, the rheumatoid factor level was elevated to 53 IU/mL, and the serum IgG level was 2,916 mg/dL. Pulmonary function testing yielded the following results: vital capacity, 44.3% of the predicted value; forced expiratory volume 1.0%, 86.7%.
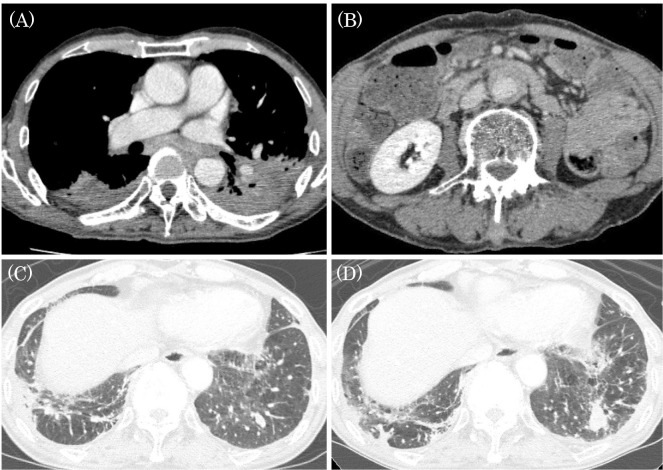
Figure 1.
(A) CT in August 2016 showed severe areas of bilateral pleural thickening and mediastinal lymph node swelling. (B) CT after the diagnosis of IgG4-RD showed a dense soft tissue mass surrounding the abdominal aorta (mantle sign). (C) In July 2015, CT showed a nodule in the left segment-9 (S9). (D) In August 2016, the nodule had grown. CT: computed tomography
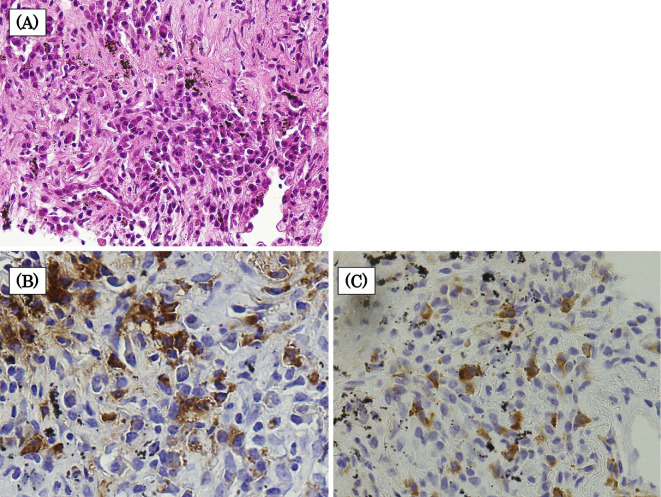
We suspected IgG4-related disease (IgG4-RD) and malignant pleural mesothelioma. Therefore, we performed a CT-guided percutaneous needle biopsy of the thickened pleura. A histological analysis of the corresponding biopsy specimen revealed a plasmacytic infiltration accompanied by prominent fibrosis. An immunohistochemical analysis showed a large number of IgG- (80.6 cells per high-power field) and IgG4-positive cells (39.4/high-power field). The IgG4/IgG cell ratio was 48.9%, exceeding 40% (Fig. 2). Combined with the high serum IgG4 level (677 mg/dL), these findings met the diagnostic criteria for IgG4-RD. Thus, the patient was diagnosed with IgG4-RD.
Figure 2.
Histopathological findings of the pleura. (A) A pleural biopsy revealed plasma cell infiltration accompanied by prominent fibrosis (Hematoxylin and Eosin staining, ×400). An immunohistochemical analysis showed a large number of IgG- (80.6 cells per high-power field) and IgG4-positive cells (39.4 cells per high-power field) (B) Immunohistochemical staining for IgG, ×400. (C) Immunohistochemical staining for IgG4, ×400.
In addition, another CT scan showed an irregular nodule in left segment-9 (S9) in July 2015, and by August 2016, the nodule had grown (Fig. 1C, D). A transbronchial biopsy later revealed SCC. Positron emission tomography-CT showed a high accumulation of 18F-fluorodeoxyglucose in the left pulmonary nodule, bilateral pleura, mediastinal lymph nodes, and around the abdominal aorta. Thickening of the anterolateral wall of the aorta (mantle sign; Fig. 1B) was also shown and thought to be periaortitis. Based on these results, the patient was ultimately diagnosed with IgG4-RD combined with SCC of the lower lobe of the left lung; however, the presence of IgG4-RD prevented the accurate staging of lung cancer.
For treatment, we first initiated systemic glucocorticoid therapy (prednisolone 25 mg/day) to improve lung function. The steroid dose was tapered to 10 mg/day because of a trend toward improvement in the pleural lesions and a reduction in mediastinal lymph node swelling. However, because the lung function did not improve sufficiently, we opted for chemotherapy after determining that surgery was not feasible.
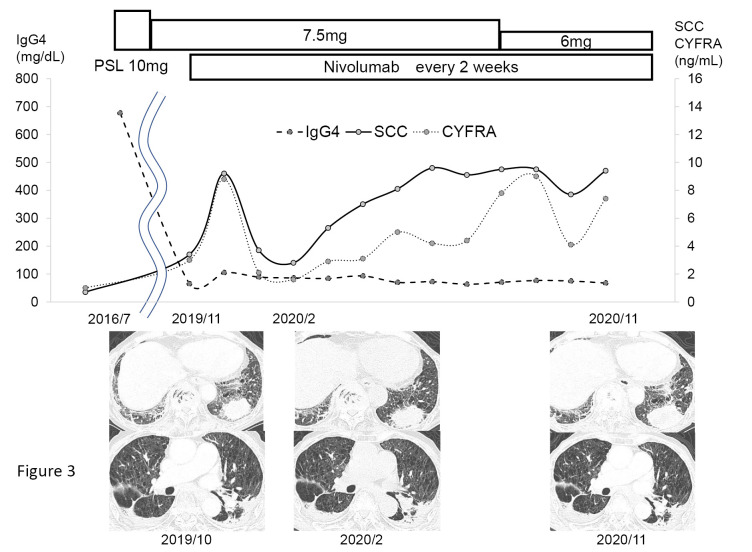
In January 2017, carboplatin+nab-paclitaxel was begun as the first-line therapy. For approximately two years after five cycles of chemotherapy, CT continued to show that the nodule in the left lower lobe had maintained its size reduction; however, in July 2019, we observed an obvious increase in the nodules and mediastinal lymph nodes. In November 2019, a bronchoscopic re-biopsy of a nodule in the left lower lobe was performed, and pathology again confirmed SCC. An immunohistochemical analysis revealed a programmed cell death ligand 1 (PD-L1) tumor proportion score of 65%. After determining that the patient had a progressive disease and obtaining fully informed consent, nivolumab was initiated as the second-line therapy in November 2019. Thereafter, the pharmacokinetics of prednisolone (PSL) dose was tapered from 10 to 6 mg/day. The patient had no IgG4-RD relapse or problematic immune-related adverse events (irAEs) for 15 months before nivolumab treatment was ultimately discontinued due to disease progression (Fig. 3). Seventeen months later, the patient died of progressive cancer.
Figure 3.
Clinical course. Nivolumab was initiated as second-line therapy in November 2019. Thereafter, PSL was tapered from 10 mg/day to 6 mg/day. Tumor markers had nearly normalized early after the start of treatment but then showed a tendency to gradually re-increase. The images also showed an increasing trend in the primary tumor after about one year. The patient had no recurrence of pleural thickening. PSL: prednisolone
Discussion
To our knowledge, there have been no previous reports on the safety of ICI treatment for lung cancer complicated by IgG4-RD. This was a rare case of a patient with IgG4-RD and lung cancer who was treated with nivolumab without any adverse events or exacerbation of IgG4-RD.
A previous report described a case of IgG4-related pleural disease after treatment with durvalumab, an anti-PD-L1 antibody, suggesting that IgG4-related pleural disease may occur as an irAE (9).
As ICI administration did not aggravate IgG4-RD in the present case, we will now discuss the pathogenesis of IgG4-RD and the mechanism of action of ICI. We will first touch on the pathogenesis of IgG-RD from an immunological perspective. Since Th2 (IL-4, IL-5, and IL-13) and Treg cytokines (IL-10) induce B cells to class-switch to IgG4, it is clear that immune responses by Th2 cells and Tregs play an important role in the pathogenesis of IgG4-RD. Recently, IL-21 produced by follicular helper T cells (fTh cells) was also thought to enhance the IgG4 class-switch in B cells, resulting in high IgG4 levels. Furthermore, Tregs not only promote IgG4 class-switch in B cells but also promote the collagen fiber production and accumulation by supplying profibrotic cytokines, which induce tissue fibrosis (10).
While the impact of anti-PD-1 antibodies on the pathogenesis of IgG4-RD remains unclear, multiple mechanisms suggest that IgG4-RD may be exacerbated, as discussed below. First, PD-1/PD-L2 interactions inhibit Th2 cells; therefore, blocking this connection may lead to Th2 inflammation and exacerbate the pathogenesis of IgG4-RD (11). Second, the PD-1/PD-L1 pathway contributes to the interaction between fTh cells and plasmablasts, since fTh cells express PD-1, and the PD-1/PD-L1 axis suppresses the development and function of fTh cells (12). Inhibition of the PD-1/PD-L1 pathway encourages fTh cells to promote the growth, differentiation, and class-switching of B cells to IgG4, which may exacerbate IgG4-RD (13). However, the PD-1/PD-L1 axis is complexly involved in the Treg development and function (14), and given these mechanisms, inhibition of the PD-1/PD-L1 axis may not exacerbate IgG4-RD.
The present patient was treated with nivolumab for about 15 months while maintaining PSL at 6.0-7.5 mg/day, and no worsening of IgG4-RD or appearance of irAE was observed. In a report by Leonardi et al. (7) on the safety of PD-1 or PD-L1 antibody treatment in 56 patients with NSCLC with an AID, the incidence of AID flare was 23%. The patients with AID symptoms at the time of PD-1 or PD-L1 antibody initiation had a significantly higher rate of AID flare than did the patients without AID symptoms [50% (5/10) vs. 18% (8/45); p=0.04]. In addition, the rate of AID flare was similar among patients who were and were not receiving immunosuppressive or immunomodulatory treatment at the time of PD-1 or PD-L1 antibody initiation [36% (4/11) vs. 20% (9/45), respectively; p=0.43]. These results suggest that the risk of AID flare is low in asymptomatic patients with AID. In the present case, the control of disease activity by low-dose steroids may have contributed to the prevention of IgG4-RD exacerbation.
To our knowledge, this is the first report of using an ICI to treat lung cancer complicated by IgG4-RD. We suggest that anti-PD-1 antibodies may be safely used in lung cancer complicated with IgG4-RD if IgG4-RD can be controlled with a low-dose steroid.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344: 732-738, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Moriyama M, Tanaka A, Maehara T, Furukawa S, Nakashima H, Nakamura S. T helper subsets in Sjögren's syndrome and IgG4-related dacryoadenitis and sialoadenitis: a critical review. J Autoimmun 51: 81-88, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol 22: 414-418, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Asano J, Watanabe T, Oguchi T, et al. Association between immunoglobulin G4-related disease and malignancy within 12 years after diagnosis: an analysis after long-term follow-up. J Rheumatol 42: 2135-2142, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Ishikawa H, Uruga H, Fujii T, et al. IgG4-related disease in the differential diagnosis of lung nodules. Respirol Case Rep 8: e00550, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue T, Hayama M, Kobayashi S, et al. Lung cancer complicated with IgG4-related disease of the lung. Ann Thorac Cardiovasc Surg 20: 474-477, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 36: 1905-1912, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cortellini A, Buti S, Santini D, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist 24: e327-e337, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terashima T, Iwami E, Shimada T, et al. IgG4-related pleural disease in a patient with pulmonary adenocarcinoma under durvalumab treatment: a case report. BMC Pulm Med 20: 104, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsieh SC, Shen CY, Liao HT, et al. The cellular and molecular bases of allergy, inflammation, and tissue fibrosis in patients with IgG4-related disease. Int J Mol Sci 21: 5082, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe H, Asada K, Shirai T, Torii H, Yoshimura K, Kusafuka K. Eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis during nivolumab and ipilimumab. Respirol Case Rep 8: e00638, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maehara T, Moriyama M, Nakamura S. Pathogenesis of IgG4-related disease: a critical review. Odontology 107: 127-132, 2019. [DOI] [PubMed] [Google Scholar]
- 13. Umehara H, Okazaki K, Kawano M, Tanaka Y. The front line of research into immunoglobulin G4-related disease - do autoantibodies cause immunoglobulin G4-related disease? Mod Rheumatol 29: 214-218, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Cai J, Wang D, Zhang G, Guo X. The role of PD-1/PD-L1 axis in Treg development and function: implications for cancer immunotherapy. OncoTargets Ther 12: 8437-8445, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]