Abstract
While walking humans generally plan foot placement two steps in advance. However, it is often necessary to rapidly alter foot placement position just before stepping due to the appearance of a new obstacle. While humans are quite capable of rapidly altering foot placement position, such changes can have major effects on centre of mass dynamics. We investigated how rapid changes to planned foot placement impact centre of mass dynamics, and how such changes influence the control of balance and forward progress, during both straight- and turning-gait. Thirteen young adults walked along a virtually projected walkway with precision footholds oriented either in a straight line or with a single 60°, 90° or 120° turn. On a subset of trials, participants were required to rapidly avoid stepping on select footholds. We found that if the centre of mass was disrupted such that it interfered with task success (i.e. staying upright and continuing along the planned path), walkers were more likely to sacrifice forward progress than the upright stability. Further, walkers appear to control centre of mass dynamics differently following inhibited steps during step turns than during spin turns, which may reflect a larger threat to task success when spin turns are interrupted.
Keywords: stability, forward progress, locomotor control, foot placement
1. Introduction
Human walking is often viewed as a cyclical and automated behaviour [1–3]. However, walking involves active control of gait, especially in dynamic environments regular to everyday life. It is not difficult to think of scenarios where other humans, animals or environmental features are identified in the desired path of travel and must be rapidly avoided. To successfully navigate such environments, humans must have flexible control strategies that allow them to anticipate obstacles and, when anticipation is not possible, quickly react to unexpected perturbations. Specifically, humans must be capable of rapidly adjusting foot placement and joint torque throughout gait to control the relationship between the centre of mass (CoM) and centre of pressure (CoP) [2,4,5].
Humans primarily control CoM trajectory through foot placement [2,4,5], and generally plan foot placement locations 2–3 steps in advance when walking over complex terrain [6–8]. Doing so allows walking to be a largely passive task where only foot placement needs to be updated to ensure the CoM travels along its anticipated ballistic trajectory (i.e. planned forward progress) [1,9,10]. While advantageous in environments devoid of obstacles, a wholly anticipatory strategy is susceptible to obstacles that can perturb planned gait. When new obstacles are identified in anticipated stepping locations with more than one step of warning, humans update their plan for CoM trajectory by altering upcoming foot placement and modifying the push-off force in the trailing limb [1,9,11]. However, as the amount of time to prepare for a new obstacle approaches one step or less, a person's ability to drastically alter their CoM trajectory decreases [12]. In such circumstances, individuals may still avoid stepping on the obstacle but be forced to accommodate the consequences of an unanticipated step.
Tasks that demand a rapid change to foot placement between planning and execution due to a new obstacle in a stepping location—referred to here as ‘stepping inhibition’—have been studied previously in straight-line walking [11,13–21]. Multiple investigations by Patla and Moraes [22] showed that walkers adopt stereotyped alternative foot placement strategies that favour longer or wider steps when performing stepping inhibition tasks. Many of these studies used alternative foot placement as the primary outcome measure and concluded that walkers select alternative foot placement location to prioritize either forward progress [12] or stability [14], by selecting longer steps or wider steps, respectively. Further, they concluded that the selection of foot placement is dependent on the task constraints, such as available reaction time or obstacle position. While these studies were informative, foot placement strategy alone is not sufficient when drawing conclusion for task prioritization because (1) interpretations will vary based on the theoretical model being used and (2) foot placement is not exclusively tied to forward progress or stability.
Results from the previous stepping inhibition studies may be interpreted in the opposite direction—that walkers prioritize mediolateral stability over forward progress—if they were interpreted within uncontrolled manifold [23] or optimal control [24,25] frameworks. Uncontrolled manifold theory and optimal control theory both suggest that when humans are performing a given motor task they allow variability in task-irrelevant dimensions, and tightly regulate variability in task-relevant dimensions [24,25]. When walking, movement along the desired path is the task-relevant dimension associated with forward progress, and orthogonal to the path is the task-relevant dimension associated with stability. Considering the existing stepping inhibition research in this framework, a longer step would not represent a prioritization of forward progress, as Patla originally suggested [12]. Instead, a longer step would indicate prioritization of stability because variability in foot placement is allowed in the dimension associated with forward progress (step length) but variability is constrained in the dimension associated with stability (step width). Vice versa, a wider step would represent a prioritization of forward progress because variability of foot position is allowed to vary in the task-irrelevant dimension, while control in the dimension of progression is unchanged. Further, the assumption that forward progress and stability are independent is only valid for straight-line gait tasks. When turning, body orientation and body trajectory fall out of alignment, and the desired path of travel constantly shifts from the original trajectory to a new trajectory. A change to foot placement in any direction during a turn likely impacts both forward progress and stability simultaneously [26]. There are also different types of turns which may have unique implications for stability and forward progress. Step turns (e.g. a reorientation of the body into a new direction of travel while on the outside limb of a turn) have vastly different dynamics from spin turns (e.g. a reorientation of the body into a new direction of travel while on the inside limb of a turn). Specifically, spin turns feature an excursion of the CoM beyond the lateral edge of the base of support that is not present during step turns. Consequently, some have proposed that spin turns are less stable than step turns [27]. Outcomes that are directly tied to task success, such as the ability to stay upright and the ability to achieve defined forward progress, may be more appropriate for determining task prioritization during such tasks.
When walking, the specific goal of stability is to avoid falls, and the specific goal of forward progress is to execute planned steps to move toward a goal destination. Adjusting foot placement is only one of several strategies humans use to support these goals. In the event of foot placement error, humans regularly draw from a wide array of stance-phase strategies (e.g. ankle strategy, hip strategy, push-off strategy, and arm-swing) that update CoM trajectory and still ensure task success for both goals [1,4,5,28]. If a walker suddenly lengthened their step the primary impact to planned gait would be a reduction of forward momentum [1,12]. Yet, this reduction in forward momentum could easily be overcome with an increase to push-off force in the trailing limb [1,11]. Considering the numerous strategies that complement foot placement, it is very difficult to conclude whether a task was prioritized just by examining foot placement alone, and using measures of body dynamics in conjunction with foot placement error (i.e. the distance from a planned stepping location) may provide a more detailed picture of motor organization [29].
Whole-body angular momentum (WBAM) is a valid measure of locomotor control that provides a snapshot of the complete rigid-body dynamics from each body segment, rather than simply looking at CoM translations alone. Humans tightly regulate WBAM to stay upright when walking [30,31]. Further, perturbations that impact frontal-plane WBAM, but not sagittal-plane WBAM, influence the placement of subsequent steps [32]. This association between WBAM and foot placement in the frontal plane is likely a necessary action to limit instability [32]. In the case of an inhibited step, a sudden change to foot placement would likely disrupt anticipated trajectory and WBAM. But, the effect of an inhibited step on WBAM is self-driven; the walker can select whether the disruption to WBAM occurs in the frontal or sagittal planes (i.e. the walker can choose a long/short or wide/narrow alternative foot placement). Given that the effect of the inhibited foot placement is up to the walker, it seems unlikely that they would choose forward progress over stability because (1) instability is dangerous and (2) any failure of upright stability results in a failure to reach the target destination. Within an optimal control framework, a person prioritizing stability would select a stepping strategy that reduces variance in frontal-plane WBAM (a longer or shorter step) while a person prioritizing forward progress would select a strategy that reduces variance in sagittal-plane WBAM (a wider or narrower step).
The aim of this study was to investigate how humans prioritize the goals of stability (staying upright) and forward progress (executing already planned steps accurately) when presented with an inhibited stepping task during both straight- and turning-gait. Specifically, we examined how sudden changes to foot placement following an inhibited step influence frontal, sagittal and transverse WBAM, and how those changes to WBAM associate with the ability to continue executing planned steps. We hypothesized that participants would prioritize stability over forward progress, and that greater disruptions to WBAM from inhibited steps would associate with greater step error on the first recovery step (i.e. that participants would abandon the goal of stepping accurately in recovery to limit instability).
2. Material and methods
2.1. Participants
Thirteen healthy young adult volunteers (7 female, 13 right limb dominant, mean (s.d.) age = 27.2 (3.5) years, mean (s.d.) mass = 73.8 (15.6) kg, mean (s.d.) height = 172.8 (7.7) cm) were recruited and screened from the University of Utah community. All participants provided written informed consent before participation in this University of Utah IRB-approved study. Participants in this study had to be between 18 and 35 years of age to be included. Exclusion criteria included (1) a history of neuromotor pathology, (2) a history of musculoskeletal injuries that could impact balance, (3) any reconstructive surgery of the lower extremities, and (4) any current medications that could contribute to balance impairments.
2.2. Instrumentation and experimental setup
A 3D motion capture system (Nexus 2.12, VICON, Oxford, UK) surrounded the walking environment to capture positional marker data at 200 Hz. Participants were outfitted with a custom marker set that featured 41 individual markers and six clusters. Markers were placed on the front of the head (L/R), back of the head (L/R), ears (L/R), acromion process (L/R), lateral epicondyle of the humerus (L/R), forearm (L/R), radial styloid process (L/R), ulnar styloid process (L/R), right shoulder, sternal notch, xiphoid process, C7, T10, posterior superior iliac spine (L/R), iliac crest (L/R), anterior superior iliac spine (L/R), greater trochanter (L/R), lateral epicondyle of the femur (L/R), lateral malleolus (L/R), first, second and fifth metatarsal heads (L/R), and calcaneus (L/R). Marker clusters were used bilaterally on the shank, thigh, and humerus segments. The marker set was designed such that a full-body 13-segment model could be obtained using the head, upper arms, forearms, trunk, pelvis, thighs, lower legs and feet. Segment lengths, CoM, anatomical orientations and inertial tensors were defined using the normative anthropometric data reported by Dumas et al. [33]. Two projectors (Optoma GT5600; 3600 ANSI lumens, 1080p resolution) projected a custom walking environment on the laboratory floor with circular stepping targets (radius: 50 mm). A virtually projected square was used to define the origin and global coordinate system to ensure the projectors and motion capture environment were aligned. Positions of the stepping targets were recorded before testing began and checked throughout testing to ensure that the projected walkway and motion capture system were aligned throughout the duration of the testing session (figure 1).
Figure 1.
Experimental setup and methods. Participants walked along precision stepping targets projected on the ground (a,b). To account for the anatomical reference frame falling out of alignment with the global reference frame, all data were rotated into a trajectory-based reference frame that aligned with the participant’s instantaneous velocity throughout the trial (a). Calculation of angular momentum was performed such that positive frontal momentum was associated with rotations about the centre of mass to the participant's right-hand side (c). Positive sagittal momentum was assocaited with rotations about the centre of mass backwards. (d) A sample trial with a participant walking along the precision stepping targets.
A custom walking environment was built in Unity (v2019.4.36f1) and displayed six semi-randomly distributed stepping targets oriented in a straight path (0°), or a path featuring a single turn with an angle of 60°, 90° or 120° to the right. Stepping targets were presented to elicit either step turns or spin turns (figure 1b). Since turns were always to the right, this means that the left foot was always disrupted for trials with an inhibited step turn, and the right foot placement was always disrupted for trials with an inhibited spin turn. For straight line trials (0° turns), there were an equal number of disruptions to the left foot (0° step turn) as there were the right foot (0° spin turn). The distribution of stepping targets was determined by the stepping pattern of the experimenter walking without visual targets. To scale the walking path to each participant’s step width and length, the distance between stepping targets was adjusted by the ratio of the participant’s leg length to the experimenter's (N.K.'s) leg length. Once scaled to leg length, stepping targets one, two, three and six were randomly jittered within a 150 mm × 150 mm box centred on the respective stepping target position. Stepping targets four and five were held to a constant position in the global space for ease of calculating stepping accuracy and strategy for the statistical analysis. Unity and Vicon Nexus programs were synchronized through a custom code written in C# so that live marker data could be used to trigger inhibited steps during selected trials. During trials with an inhibited step, the inhibited stepping target (step n) changed colour from black to red when the reflective marker on the second metatarsal head of the uninhibited limb entered within a one-step radius of the marker of the inhibited step (i.e. early stance phase of step n − 1). The timing of the colour change approximately coincides with toe-off of step n − 2, allowing walkers the entirety of swing phase to alter foot placement position (see electronic supplementary material, video). All participants walked barefoot to avoid the influence of differing shoe styles between participants [10].
2.3. Experimental protocol
Participants were asked to walk along the path of projected stepping targets from a start position to an end position (taped on the laboratory floor). Participants were instructed to walk at a brisk pace, as if ‘they were trying to catch the bus' [34] to ensure that gait was continuous and each step was not a unique motor event, and also to reduce the likelihood of participants slowing to anticipate a perturbation. Participants were also asked to ‘step on each projected stepping target accurately’, so that the reflective marker on their second metatarsophalangeal joint landed near the centre of each stepping target. To ensure that participants were able to walk briskly and accurately, each participant initially completed a series of practice trials. During these practice trials, targets would change from black to green if participants stepped accurately on the target; targets would remain black for inaccurate steps. Participants were deemed ‘familiar’ with the stepping behaviour when they could complete three consecutive practice trials with 100% accuracy while walking at the instructed brisk pace.
Once familiar with the task of walking briskly and accurately, participants were instructed about the remaining trials. Participants were informed that the remaining trials required them to walk along paths with the precision stepping targets oriented in either a straight path, or a path with a single turn to the right. Participants were then informed that on a random subset of trials, stepping targets in the path may change colour from black to red, and that red stepping targets should be avoided by stepping anywhere else in the environment. Before each trial, participants were instructed to stand in a starting box taped on the floor. Once in the starting box, we projected the entire path of precision stepping targets for the participant to walk along. Participants started the trial when the experimenter said ‘begin’. Each trial ended when participants were standing with both feet in the box taped at the end of the walkway, after which participants were instructed to return to the starting box.
Each testing session consisted of 200 experimental trials and 20 catch trials. The 200 experimental trials were split evenly between turn types (100 trials each for step and spin turns), and also turn angle (50 trials for each of the four turn angles). Trials were distributed evenly into ten blocks, and two catch trials were added to each block. Of the 200 experimental trials, 120 were normal, with no steps being inhibited and 80 trials involved a single stepping inhibition task on the fourth step of the walking trial. Catch trials were included to prevent participants from focusing solely on the middle step of each trial and involved a single stepping inhibition task on the precision stepping target immediately before (n − 1) or after (n + 1) the normally inhibited target (n) in the middle of the turn. Each block had five trials of each turn angle (three normal trials and two inhibited trials). Within each block, the first catch trial always occurred randomly between the fourth and eighth trial of the block and the second catch trial always occurred between the 15th and 19th trials of the block (figure 2).
Figure 2.
Trial layout and outcome timing. (a) Participants completed 10 blocks of 22 trials. Five blocks had a 3 : 2 ratio of step turns while the remaining five blocks had a 3 : 2 ratio of spin turns. Two of the trials within each block were catch trials to prevent participants from expecting the central dot being inhibited. Twelve of the trials within each block were uninhibited trials and eight were inhibited. Trial order was evenly split between the four turn angles and trials were randomized within blocks. (b) A walker performing the task and the time-frames of the different outcome measures. WBAM was collected in the steps before and after the inhibited step (step N). iWBAM and ΔiWBAM were calculated for only between steps N and N + 1. Step error was measured at step N + 1.
2.4. Processing
All marker data were labelled and gap-filled using Vicon Nexus (v.2.12). All outcome measures were calculated using custom code written in Matlab (v. R2020b, The MathWorks Inc., Natick MA, USA). Raw kinematic data were filtered using a 4th order low-pass Butterworth filter with a cutoff frequency of 6 Hz. Foot strike and toe-off events were determined using a maximal displacement algorithm corrected for turning gait [35,36].
The primary outcome measures used in this study were WBAM [30] for the stride surrounding the inhibited step (step n − 1 through step n + 1), integrated WBAM (iWBAM) between the inhibited step and first recovery step (step n through step n + 1), and step error on the first recovery step (step n + 1). Step error was defined using the magnitude of the vector connecting the centre of the fifth stepping target and the position of the marker on the second metatarsal head at mid-stance of the first recovery step after an inhibited step. Any step error that surpassed 75 mm from the centre of the precision stepping target was considered an inaccurate step. The 75 mm threshold was selected as a distance where the entirety of a participant's foot would completely miss the stepping target, a clearly perceptible error to both the walker and the observer. WBAM was calculated using the following formula (equation (2.1)):
| 2.1 |
where is the position of the ith segment's CoM, is the position of the whole-body CoM, m is the mass of the ith segment, is the velocity of the ith segment, is the velocity of the whole-body CoM, is the inertial tensor of the ith segment (3 × 3 matrix), and is the angular velocity of the ith segment (3 × 1 matrix). WBAM was normalized by participant mass, velocity, and height to make it dimensionless [30]. Since turns cause the local reference frame to fall out of alignment with the global reference frame, all angular momentum data were rotated after being calculated in the global frame so that the anteroposterior (AP) axis was defined by the whole-body centre of mass velocity, the vertical axis was aligned with the vertical axis of the laboratory reference frame, and the mediolateral (ML) axis was orthogonal to the other two vectors, with the positive direction pointing to the participant's right [37–39].
The integral of WBAM (iWBAM) over the course of a step represents the total rotation of the body with respect to the gait cycle (i.e. total pitch, roll, or yaw over the course of a step) [32,40]. iWBAM was integrated after time-normalization and is with respect to the step cycle. To determine the change in iWBAM (ΔiWBAM) between uninhibited and inhibited trials for each participant we calculated the mean and standard deviation of iWBAM for each uninhibited condition. For each inhibited trial, the trial iWBAM was subtracted from the mean iWBAM of the corresponding uninhibited condition then divided by the standard deviation of iWBAM from the corresponding uninhibited condition [32]. The resulting value is the trial-level change in iWBAM between the uninhibited and inhibited trials, in units of standard deviations from the uninhibited condition mean.
2.5. Primary statistical analysis
Linear regression models assessed the association between frontal, sagittal, and transverse ΔiWBAM following the inhibited step and stepping error on the first recovery step. Separate models were used for step and spin turns. To examine how changes to WBAM relate to stepping accuracy on the recovery step more closely, we separated accurate steps (foot placement less than 50 mm from centre of stepping target) and inaccurate steps (foot placement greater than 75 mm from centre of stepping target). We performed two-sample Welch's t-tests to compare the means and distributions of ΔiWBAM between accurate and inaccurate steps in both the frontal and sagittal planes. Significance was assessed at α = 0.05.
2.6. Exploratory statistical analysis
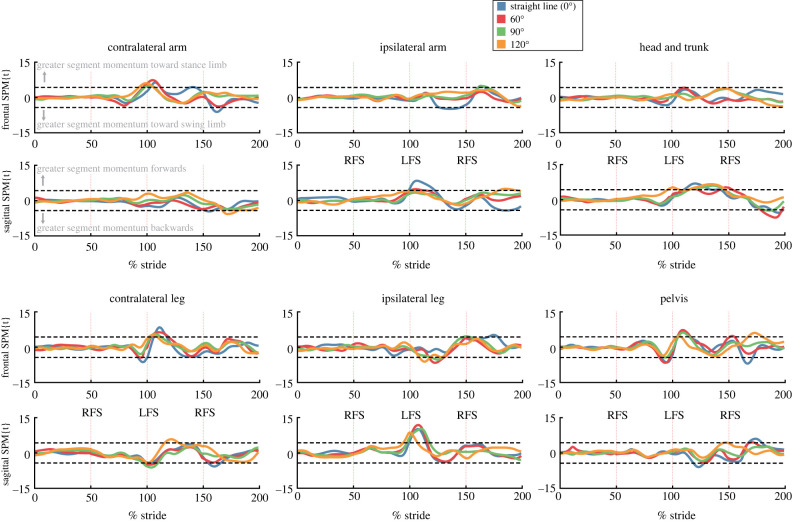
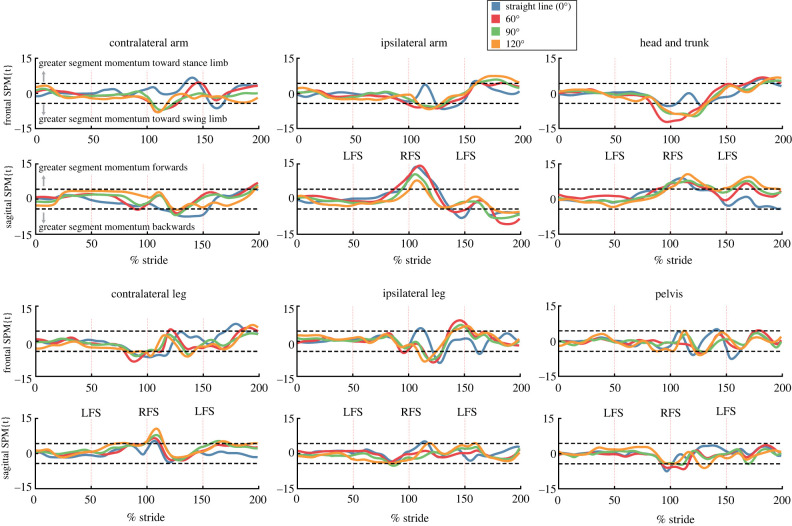
To understand the strategies that participants used to maintain accurate foot placement despite the inhibited step perturbation, we further broke down the strategies involved in maintaining stability and forward progress on the trials with accurate recovery steps. We calculated step time as a measure of temporal changes between inhibited and uninhibited steps. We investigated WBAM and the angular momentum of the head and trunk, pelvis, ipsilateral arm, ipsilateral leg, contralateral arm, and contralateral leg segments between the foot strike when the inhibited step was triggered (step n − 1), and the foot strike of the first recovery step (step n + 1). For each step in each trial the angular momentum data were time normalized to 100 samples using spline interpolation. Time-normalized WBAM data and time-normalized segment angular momentum data were compared using one-dimensional statistical parametric mapping (1dSPM) to determine the differences between uninhibited and inhibited trials within each condition [41]. Specifically, a SPM two-tailed independent sample t-test was used. The SPM{t} statistic was calculated at each individual time node of the time-normalized data, and interpretation is similar to scalar t-tests, where if the test statistic passes the critical threshold the null hypothesis is rejected. Due to the 48 distinct 1dSPM tests being performed (four turn angles, six body segments, two planes of motion) we determined significance at a threshold of α = 0.001 (i.e. α = 0.05/48). Therefore, for each significant event in the 1dSPM analyses, the probability that the difference between uninhibited and inhibited trials would be observed in repeated random samplings is one in one thousand.
3. Results
3.1. Step error and relationship with iWBAM
Participants stepped within the precision stepping target of the recovery step on 74% of step turns and 73% of spin turns. Participants exhibited a medium stepping error (greater than 75 mm away from centre of stepping target) on 7% of step turns and 14% of spin turns, and large stepping error (greater than 100 mm away from centre of stepping target) on 3% of step turns and 10% of spin turns. Participants stepped accurately on 83% of uninhibited step turns (mean (SD) step error = 27 (12) mm) and 90% of spin turns (mean (SD) step error = 25 (12) mm). Frontal ΔiWBAM was not a significant predictor of step error on the first recovery step for either step or spin turns. The ΔiWBAM in the frontal plane explained less than 1% of variance in step error on the recovery step during step turns (r2 < 0.01, p = 0.873) and spin turns (r2 = 0.01, p = 0.057). Sagittal ΔiWBAM explained 2% of variance in recovery step error on the recovery step for step turns (r2 = 0.02, p = 0.004) and 13% of the variance in recovery step error for spin turns (r2 = 0.132, p < 0.001) (figure 3).
Figure 3.
Scatter plots for ΔiWBAM and step error. Plots show the relationship between ΔiWBAM and step error in the frontal plane (top row), sagittal plane (middle row) and transverse plane (bottom row) for both step turns (left) and spin turns (right).
In the frontal plane the independent sample Welch's t-test revealed no significant differences between ΔiWBAM on accurate (step turns: mean (SD) = 0.05 (2.49); spin turns: mean (SD) = −1.42 (2.44)) and ΔiWBAM on inaccurate (step turns: mean (SD) = −0.61 (2.94); spin turns: mean (SD) = −1.97 (3.37)) recovery steps for either step turns (p = 0.189) or spin turns (p = 0.187). In the sagittal plane the independent sample Welch's t-tests revealed significant differences between ΔiWBAM on accurate (step turns: mean (SD) = −0.89 (2.26); spin turns: mean (SD) = −1.35 (2.59)) and ΔiWBAM on inaccurate steps (step turns: mean (SD) = −2.61 (5.42); spin turns: mean (SD) = −3.06 (3.78)) for spin turns (p < 0.001) but not step turns (p = 0.063). Specifically, inaccurate steps featured a more negative ΔiWBAM in the sagittal plane than accurate recovery steps. In the transverse plane the independent sample t-tests did not indicate a significant difference between ΔiWBAM on accurate (step turns: mean (SD) = 0.55 (2.21); spin turns: mean (SD) = 0.46 (2.18)) and ΔiWBAM on inaccurate steps (step turns: mean (SD) = 0.35 (2.71); spin turns: mean (SD) = 2.03 (3.13)) for step turns (p = 0.664), but did indicate a significant difference for spin turns (p < 0.001). Specifically, inhibited spin turns with inaccurate recovery steps featured a more positive ΔiWBAM in the transverse plane than inhibited spin turns with accurate recovery steps (figure 4). Across all turn angles and both turn types, trials with an accurate first recovery step featured a 2.8% reduction in step time relative to uninhibited trials, while trials with an inaccurate first recovery step featured a 9.6% reduction in step time of the first recovery step. Participants predominantly stepped short of the target when they failed to step accurately after inhibited spin turns. For inhibited step turns participants predominantly stepped long and medial to the target (figure 5).
Figure 4.
Distribution of ΔiWBAM for accurate and inaccurate steps. The kernel density plots display the distributions for accurate steps (step error less than 50 mm, blue/solid) and inaccurate steps (step error greater than 75 mm, red/dashed) for step turns (left) and spin turns (right). Data are shown in the frontal (top row), sagittal (middle row), and transverse planes (bottom row).
Figure 5.
Magnitude and position of inaccurate recovery steps. Individual data points show the position of inaccurate foot strikes for both step turns (left) and spin turns (right) relative to the precision stepping target (black central dot). Each ring of the polar plot represents 200 mm and each spoke represents a 30° change around the arc.
3.2. WBAM control during inhibited stepping trials with accurate recovery
During step turns the mean WBAM was highly similar between uninhibited and inhibited trials throughout the two strides surrounding inhibited foot placement. Participants rarely exhibited significant changes to mean WBAM in frontal plane during the step immediately preceding or following inhibited foot placement (figure 6). In the sagittal plane participants exhibited a more negative WBAM in the latter half of the first recovery step (figure 6: 101–150% stride cycle) for straight, 90°, and 120° conditions. When sagittal plane WBAM is more negative it is indicative of quicker rotation of the body about the CoM in the forward direction. In the transverse plane WBAM was significantly more positive (p < 0.001) immediately following inhibited foot placement for all turn angles. A positive shift in transverse-plane WBAM indicates either a greater rotation to the left, or reduction in rotation to the right.
Figure 6.
Time-normalized whole-body angular momentum (WBAM) for two strides surrounding the inhibited step turns. Mean WBAM is shown for uninhibited (dashed-black) and inhibited (solid-coloured) trials with accurate recovery steps. 0° (blue, equivalent to straight gait), 60° turns (red), 90° turns (green) and 120° turns (yellow) are shown in the frontal (top), sagittal (middle), and transverse (bottom) planes. Subplots below each WBAM time-series depict the respective SPM{t} statistic from the 1dSPM analysis. Regions of the SPM{t} signal that cross the horizontal dashed lines indicate a significant difference between inhibited and uninhibited trials at α = 0.001. Vertical dashed lines display foot strike events and are labelled for left foot strikes (LFS) and right foot strikes (RFS).
Spin turns featured more drastic changes to mean WBAM than step turns did. In the frontal plane there were significant differences between inhibited and uninhibited trials in the step immediately preceding inhibited foot placement (figure 7: 51–100% stride cycle), as well as the two steps following inhibited foot placement (101–200% stride cycle). Surrounding inhibited foot placement, frontal WBAM was significantly less positive during inhibited trials than uninhibited trials for all conditions (p < 0.001), indicating less frontal plane roll towards the stance (inhibited) limb. Sagittal WBAM was significantly more negative during inhibited trials across all conditions (p < 0.001), indicating that inhibited foot placement was accompanied by an increase to forward angular momentum. There were fewer changes in the transverse plane WBAM for spin turns than there was for step turns, with participants exhibiting a significant difference (p < 0.001) only for the 60° turning condition.
Figure 7.
Time-normalized whole-body angular momentum (WBAM) for two strides surrounding the inhibited spin turns. Mean WBAM is shown for uninhibited (dashed-black) and inhibited (solid-coloured) trials with accurate recovery steps. 0° (blue, equivalent to straight gait), 60° turns (red), 90° turns (green) and 120° turns (yellow) are shown in the frontal (top), sagittal (middle), and transverse (bottom) planes. Subplots below each WBAM time-series depict the respective SPM{t} statistic from the 1dSPM analysis. Regions of the SPM{t} signal that cross the horizontal dashed lines indicate a significant difference between inhibited and uninhibited trials at α = 0.001. Vertical dashed lines display foot strike events and are labelled for left foot strikes (LFS) and right foot strikes (RFS).
3.3. Segmental angular momentum control during inhibited steps
Many of the observed changes to segment momenta during inhibited steps were consistent across each turn angle (figure 8). In the frontal plane the contralateral arm, contralateral leg, and pelvis segments generated greater momentum (p < 0.001) towards the stance limb in the period immediately following inhibited foot placement (101–125% stride cycle). In the sagittal plane significant differences were observed in the contralateral leg, ipsilateral leg, and head and trunk segments (p < 0.001). The contralateral leg generated less forward momentum at the time of the alternative step (100% stride cycle) contributed. Opposite to the contralateral leg, the ipsilateral leg momentum generated more forward momentum immediately following inhibited foot placement (101–125% stride cycle). The head and trunk segment also exhibited significantly different sagittal plane momentum across all turn angles (p < 0.001). Relative to uninhibited step turns, the head and trunk segment generated significantly greater forward momentum over the course of the first recovery step (101–150% stride cycle).
Figure 8.
SPM{t} statistic comparing time-normalized segment angular momenta for uninhibited and inhibited step turns. For each segment, the top plot is the time series of the SPM{t} in the frontal plane while the bottom plot displays the SPM{t} in the sagittal plane. Two strides surrounding the inhibited step are presented, with the foot strike of the inhibited step always occuring at 100% of the stride cycle. Vertical dashed lines indicate foot strikes. Significant differences between uninhibited and inhibited trials (α = 0.001) occurred when the t-statistic crossed either of the horizontal dashed lines. Data presented in this figure are only for trials where participants accurately stepped on the first recovery target. Vertical dashed lines display foot strike events and are labelled for left foot strikes (LFS) and right foot strikes (RFS).
Changes to frontal and sagittal plane momentum in the two strides surrounding inhibited steps were much more extensive for spin turns than step turns (figure 9). Before inhibited foot placement even occurred during spin turns, the contralateral leg and head and trunk segments started generating momentum away from the inhibited stance limb (75–100% stride cycle). Following inhibited foot placement, both arm segments, both leg segments, the head and trunk segment, and the pelvis segment all generated momentum away from the stance limb (101–150% stride cycle). In the sagittal plane the ipsilateral arm segment and the head and trunk segment generated significantly greater forward momentum just before inhibited foot placement, and throughout the first recovery step (p < 0.001). The contralateral leg also contributed greater forward momentum immediately after inhibited foot placement. For each turn angle, the pelvis generated less forward momentum in the time just before inhibited foot placement (p < 0.001). The generation of momentum away from the stance limb and forward was observed across all turn angles, with slightly different temporal onsets depending on turn angle. Notably, significant deviations from uninhibited trials were observed earliest during 60° spin turns.
Figure 9.
SPM{t} statistic comparing time-normalized segment angular momenta for uninhibited and inhibited spin turns. For each segment, the top plot is the time series of the SPM{t} in the frontal plane while the bottom plot displays the SPM{t} in the sagittal plane. Two strides surrounding the inhibited step are presented, with foot strike of the inhibited step always occuring at 100% of the stride cycle. Vertical dashed lines indicate foot strikes. Significant differences between uninhibited and inhibited trials (α = 0.001) occurred when the t-statistic crossed either of the horizontal dashed lines. In this figure, frontal plane spin turn data were multiplied by −1 so that the positive direction was with respect to the right limb. Data presented in this figure are only for trials where participants accurately stepped on the first recovery target. Vertical dashed lines display foot strike events and are labelled for left foot strikes (LFS) and right foot strikes (RFS).
4. Discussion
This study aimed to investigate the effect of a stepping inhibition task on control of stability and forward progress during both straight- and turning-gait tasks. We defined stability as the ability to stay upright and forward progress as the ability to step accurately on precision stepping targets after an inhibited step. We predicted that a greater change in WBAM after an inhibited step would lead to less accurate recovery steps. Participants were able to stay upright and step accurately on approximately 74% of steps, and we found no association between ΔiWBAM and recovery step error in the frontal plane for both step and spin turns. However, we found that sagittal ΔiWBAM was a significant predictor of step error after inhibited steps for both step and spin turns. For trials with inaccurate recovery steps, we found that participants tightly regulated frontal ΔiWBAM but allowed changes in sagittal and transverse ΔiWBAM. To better understand the control strategies humans use to recover from inhibited steps, we further examined control of WBAM and segment angular momentum over the two strides surrounding the inhibited step and found distinct control strategies for step turns and spin turns respectively.
4.1. ΔiWBAM and recovery step accuracy
On the vast majority of trials participants had no problem with stepping accurately and remaining upright, despite some large variation in frontal and sagittal ΔiWBAM. Recovery steps fell within the precision stepping target on 74% of step turns and 73% of spin turns. This result is perhaps unsurprising since participants had approximately 400 ms between inhibited steps and recovery steps where they could select from the wide array of strategies to recover anticipated CoM trajectory [1,2,4,5,28,42]. Consequently, we observed a substantial floor effect (a numerical floor effect, not an effect of the walking surface) in the recovery step accuracy that limited the interpretation of its relationship with WBAM. Humans have excessive degrees of freedom when performing motor tasks and variability is allowed as long as it does not interfere with the task goal [23,25]. Since the task goal was to step within each precision stepping target, and not to step as close to the centre of the target as possible, participants may not have treated step error as a continuous value (i.e. a step error of 10 mm is likely equal to a step error of 45 mm since both are within the precision stepping target). As such, the variability in ΔiWBAM on trials where participants stepped accurately could be attributed to sensorimotor noise [43] or motor redundancy [24]. Alternatively, the variability could be a result of step-specific tradeoffs between stability and forward progress that contribute to task success [44]. Young adults likely have a solid grasp on the limits of their stability, and may be willing to allow more variability in stability if they are confident it can be corrected in future steps.
Trials where participants failed at the task of forward progress (i.e. they failed to step accurately in recovery) or stability (i.e. they fell) are likely more useful for examining how forward progress and stability are organized. Trials with inaccurate steps and/or falls represent situations where variability in the motor system led to task failure. Since no participants fell in this experiment, we can only use trials with inaccurate recovery steps. When inaccurate recovery steps occurred, sagittal and transverse ΔiWBAM deviated from the strategies observed during trials with accurate recovery steps, but frontal ΔiWBAM remained consistent. These results show that even when the task of forward progress was seriously threatened, participants limited variability in frontal ΔiWBAM, and prioritized control in that plane. Within an uncontrolled manifold framework, the shift in sagittal and transverse ΔiWBAM suggests that when a gait-related task must be sacrificed due to perturbation, forward movement (sagittal plane) and reorientation of CoM trajectory (transverse plane) are sacrificed before upright stability (frontal plane). Overall, our data appear to support a control structure where within-step dynamics and step-to-step dynamics are balanced so long as it does not threaten overarching task success. However, if the overarching goal-directed walking task is threatened, forward progress is sacrificed before stability.
4.2. WBAM during anticipatory and reactive steps
While foot placement strategy was very similar between step turns and spin turns, the effects of the inhibited step on the control of WBAM appear to be very different. Across all step turns, there was a consistent change in transverse WBAM just after the foot-strike of the inhibited step, followed by a mid- and late-stance change to sagittal WBAM. For spin turns the difference between normal and inhibited WBAM was more pronounced. There are multiple explanations for these differences between step and spin turns. First, the difference could be a function of step distance. For step turns the distance for the unperturbed foot to move from the n − 1 step to the n + 1 step decreases as the turn angle gets sharper. For spin turns the opposite is true because the unperturbed limb is exterior to the turn [27,45,46]. The greater distance the foot has to travel around the body during spin turns may necessitate an earlier response to ensure that the foot can reach the subsequent stepping target in time. Alternatively, the differences between turn types may be associated with how participants perceived threats to stability. Spin turns have been suggested to be less stable because the CoM moves beyond the base of support [46]. Our data seem to support this as spin turns were treated with greater urgency by adjusting frontal and sagittal WBAM in anticipation of the inhibited step, while the strategy we observed for inhibited step turns appeared to be a ‘wait and see’ approach that is similar to expected underfoot perturbations in other gait tasks [47].
4.3. Segmental contributions to WBAM
Changes to individual segment momenta during step turns were primarily observed in the sagittal plane. We found significant differences in the contralateral limb and pelvis around the time of inhibited foot placement. These changes may have been driven by changes to push-off force in the trailing limb when the inhibited step was performed. Similar results have been observed in the support-limb in studies with WBAM and tripping, where the support-limb helps provide time and clearance for the contralateral limb to recover by reducing forward WBAM [48,49]. While the healthy controls in this study appeared to treat these trials with ease, controlling push-off force may be difficult for individuals with peripheral motor disorders or injuries [50,51], including general decline with ageing [19]. While we lack empirical data on these groups, an inability to alter push-off force may contribute to the higher frequency of falls observed in such groups. For instance, individuals with lower-limb amputation may be unable to use such a strategy on the prosthetic limb and may have to resort to a less appropriate recovery strategy. Future work should prioritize understanding the neurophysiological mechanisms and biomechanical contributions of a push-off strategy during inhibited stepping and developing assistive devices that are resistant to real-world obstacles for such groups.
Compared to step turns, we observed an earlier, more complex, whole-body change in angular momentum during spin turns that featured a significant change from normal in every segment at some point over the preparatory or recovery steps. When compared with the time-series of WBAM, it appears that each segment was part of a coordinated effort around the time of inhibited foot placement to generate frontal-plane momentum away from the inhibited stepping limb, which is the interior limb of the turn. Large effects were seen in the head and trunk segment before inhibited foot placement, and also each arm immediately after foot placement. The use of upper body segments to control frontal-plane stability is usually reserved for larger disturbances that ankle torque cannot sufficiently address [42,52], and suggests that inhibited spin turns were perceived as a more threatening task. We also observed greater generation of forward momentum in the sagittal plane, which may seem counterintuitive at first. The excessive forward momentum observed in the head and trunk segment is likely a function of the short stepping strategy we observed in a companion study [53]. Meanwhile, the shift to sagittal momentum in the ipsilateral arm segment may be explained by the use of the arms for reactive balance control. During normal gait arm swing contributes to momentum in the sagittal plane; however, if the arms are outstretched due to an unexpected threat to stability, this is no longer the case.
Several limitations should be considered when interpreting the results of this study. First, participants only turned in one direction. Turn direction was always to the right to limit the number of walking trials participants performed during the testing session. However, the single direction of turn means that the spin-turn and step-turn behaviour may have been related to limb dominance. Second, the frontal and sagittal planes were defined by instantaneous trajectory. This method has commonly used in previous turning research [38,39], but it should be noted that the method may mis-represent holonomic movements where trajectory is not aligned with anatomical orientation. For example, sideways walking is an extreme example of holonomic movement, and in this circumstance greater frontal-plane momentum in the anatomical frame of reference would be greater sagittal plane momentum in a trajectory reference frame. As direction of travel and anatomical orientation fall further out of alignment, the frame of reference in which the data are analysed could lead to drastically different interpretations [37]. The frequency of inhibited steps at the middle of the turn may have also led participants to anticipate inhibited steps. While the proportion of total trials that featured an inhibited step in the middle (approx. 36%) is similar to previous research [9,12] it was likely more predictable than inhibited steps in the real world. Future projects should incorporate eye-tracking to help determine whether specific footholds attract heightened attentional focus. Finally, participants walked barefoot, which may have impacted foot contact posture, and walking speed may have fluctuated between trials. Both of these factors are known to impact whole-body dynamics during gait tasks and likely influenced WBAM results between participants or between conditions.
5. Conclusion
We found that when healthy young adults perform an inhibited stepping task they are remarkably capable of correcting for unplanned shifts to CoM dynamics, caused by inhibited foot placement, and returning to planned trajectory. However, when the effect of an inhibited step threatens overarching task success, walkers are more likely to sacrifice forward progress to ensure upright stability. Further, participants appear to control CoM dynamics differently following inhibited steps during step turns than during spin turns. Specifically, step turns are treated with less caution, waiting for the effect of inhibited foot placement on CoM dynamics to occur before making corrections. On the other hand, spin turns are corrected proactively, showing changes to CoM dynamics before the foot placement of the inhibited step even occurs.
Acknowledgements
The authors would like to thank Cameron Jensen and Paula Kramer for their assistance with data collection on this project.
Ethics
This study was reviewed and approved by the institutional review board at the University of Utah (IRB_00158811). All participants signed informed consent documents prior to participation.
Data accessibility
Anonymized data are publicly available from the OSF repository: https://osf.io/cj7yu/ [54].
Supplementary material is available online [55].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
N.K.: conceptualization, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; P.C.F.: conceptualization, supervision, writing—review and editing.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This project was funded by the University of Utah Graduate Research Fellowship Award.
References
- 1.Bauby CE, Kuo AD. 2000. Active control of lateral balance in human walking. J. Biomech. 33, 1433-1440. ( 10.1016/S0021-9290(00)00101-9) [DOI] [PubMed] [Google Scholar]
- 2.Bruijn SM, Van Dieën JH. 2018. Control of human gait stability through foot placement. J. R. Soc. Interface 15, 20170806. ( 10.1098/rsif.2017.0816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGeer T. 1990. Passive dynamic walking. Int. J. Rob. Res. 9, 62-82. ( 10.1177/027836499000900206) [DOI] [Google Scholar]
- 4.Fettrow T, Reimann H, Grenet D, Thompson E, Crenshaw J, Higginson J, Jeka J. 2019. Interdependence of balance mechanisms during bipedal locomotion. PLoS ONE 14, e0225902. ( 10.1371/journal.pone.0225902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimann H, Fettrow T, Jeka JJ. 2018. Strategies for the control of balance during locomotion. Kinesiol. Rev. 7, 18-25. ( 10.1123/kr.2017-0053) [DOI] [Google Scholar]
- 6.Matthis JS, Yates JL, Hayhoe MM. 2018. Gaze and the control of foot placement when walking in natural terrain. Curr. Biol. 28, 1224-1233.e5. ( 10.1016/j.cub.2018.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthis JS, Barton SL, Fajen BR. 2017. The critical phase for visual control of human walking over complex terrain. Proc. Natl Acad. Sci. USA 114, E6720-E6729. ( 10.1073/pnas.1611699114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthis JS, Fajen BR. 2014. Visual control of foot placement when walking over complex terrain. J. Exp. Psychol. Hum. Percept. Perform. 40, 106-115. ( 10.1037/A0033101) [DOI] [PubMed] [Google Scholar]
- 9.Barton SL, Matthis JS, Fajen BR. 2019. Control strategies for rapid, visually guided adjustments of the foot during continuous walking. Exp. Brain Res. 237, 1673-1690. ( 10.1007/S00221-019-05538-7) [DOI] [PubMed] [Google Scholar]
- 10.Matthis JS, Fajen BR. 2013. Humans exploit the biomechanics of bipedal gait during visually guided walking over complex terrain. Proc. R. Soc. B 280, 20130700. ( 10.1098/rspb.2013.0700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moraes R, Allard F, Patla AE. 2007. Validating determinants for an alternate foot placement selection algorithm during human locomotion in cluttered terrain. J. Neurophysiol. 98, 1928-1940. ( 10.1152/jn.00044.2006) [DOI] [PubMed] [Google Scholar]
- 12.Patla AE, Prentice SD, Rietdyk S, Allard F, Martin C. 1999. What guides the selection of alternate foot placement during locomotion in humans. Exp. Brain Res. 128, 441-450. ( 10.1007/S002210050867) [DOI] [PubMed] [Google Scholar]
- 13.Moraes R, Lewis MA, Patla A. 2004. Strategies and determinants for selection of alternate foot placement during human locomotion: influence of spatial and temporal constraints. Exp. Brain Res. 159, 1-13. ( 10.1007/s00221-004-1888-z) [DOI] [PubMed] [Google Scholar]
- 14.Moraes R, Patla AE. 2006. Determinants guiding alternate foot placement selection and the behavioral responses are similar when avoiding a real or a virtual obstacle. Exp. Brain Res. 171, 497-510. ( 10.1007/s00221-005-0297-2) [DOI] [PubMed] [Google Scholar]
- 15.Patla AE, Adkin A, Ballard T. 1999. Online steering: coordination and control of body center of mass, head and body reorientation. Exp. Brain Res. 129, 0629-0634. ( 10.1007/s002210050932) [DOI] [PubMed] [Google Scholar]
- 16.Potocanac Z, Smulders E, Pijnappels M, Verschueren S, Duysens J. 2015. Response inhibition and avoidance of virtual obstacles during gait in healthy young and older adults. Hum. Mov. Sci. 39, 27-40. ( 10.1016/j.humov.2014.08.015) [DOI] [PubMed] [Google Scholar]
- 17.Potocanac Z, Hoogkamer W, Carpes FP, Pijnappels M, Verschueren SMP, Duysens J. 2014. Response inhibition during avoidance of virtual obstacles while walking. Gait Posture 39, 641-644. ( 10.1016/J.GAITPOST.2013.07.125) [DOI] [PubMed] [Google Scholar]
- 18.Potocanac Z, Duysens J. 2017. Online adjustments of leg movements in healthy young and old. Exp. Brain Res. 235, 2329-2348. ( 10.1007/s00221-017-4967-7) [DOI] [PubMed] [Google Scholar]
- 19.Weerdesteyn V, Nienhuis B, Mulder T, Duysens J. 2005. Older women strongly prefer stride lengthening to shortening in avoiding obstacles. Exp. Brain Res. 161, 39-46. ( 10.1007/S00221-004-2043-6) [DOI] [PubMed] [Google Scholar]
- 20.Weerdesteyn V, Nienhuis B, Hampsink B, Duysens J. 2004. Gait adjustments in response to an obstacle are faster than voluntary reactions. Hum. Mov. Sci. 23, 351-363. ( 10.1016/J.HUMOV.2004.08.011) [DOI] [PubMed] [Google Scholar]
- 21.Weerdesteyn V, Schillings AM, Van Galen GP, Duysens J. 2003. Distraction affects the performance of obstacle avoidance during walking. J. Mot. Behav. 35, 53-63. ( 10.1080/00222890309602121) [DOI] [PubMed] [Google Scholar]
- 22.Moraes R. 2014. A model for selecting alternate foot placement during human locomotion. Psychol. Neurosci. 7, 319-329. ( 10.3922/j.psns.2014.038) [DOI] [Google Scholar]
- 23.Scholz JP, Schöner G. 1999. The uncontrolled manifold concept: identifying control variables for a functional task. Exp. Brain Res. 126, 289-306. ( 10.1007/s002210050738) [DOI] [PubMed] [Google Scholar]
- 24.Todorov E, Jordan M. 2002. A minimal intervention principle for coordinated movement. Adv. Neural Inform. Process. Syst. 15, 27-34. [Google Scholar]
- 25.Todorov E, Jordan MI. 2002. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 5, 1226-1235. ( 10.1038/nn963) [DOI] [PubMed] [Google Scholar]
- 26.Hof AL, Gazendam MGJ, Sinke WE. 2005. The condition for dynamic stability. J. Biomech. 38, 1-8. ( 10.1016/j.jbiomech.2004.03.025) [DOI] [PubMed] [Google Scholar]
- 27.Hase K, Stein RB. 1999. Turning strategies during human walking. J. Neurophysiol. 81, 2914-2922. ( 10.1152/JN.1999.81.6.2914) [DOI] [PubMed] [Google Scholar]
- 28.Winter D. 1995. Human balance and posture control during standing and walking. Gait Posture 3, 193-214. ( 10.1016/0966-6362(96)82849-9) [DOI] [Google Scholar]
- 29.Patil NS, Dingwell JB, Cusumano JP. 2019. Correlations of pelvis state to foot placement do not imply within-step active control. J. Biomech. 97, 109375. ( 10.1016/j.jbiomech.2019.109375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herr H, Popovic M. 2008. Angular momentum in human walking. J. Exp. Biol. 211, 467-481. ( 10.1242/jeb.008573) [DOI] [PubMed] [Google Scholar]
- 31.Popovic M, Hofmann A, Herr H. 2004. Angular momentum regulation during human walking: biomechanics and control. In Proc. IEEE Int. Conf. on Robotics and Automation, New Orleans, LA, USA, 26 April–1 May 2004, pp. 2405-2411. ( 10.1109/ROBOT.2004.1307421) [DOI] [Google Scholar]
- 32.Leestma JK, Golyski PR, Smith CR, Sawicki GS, Young AJ. 2023. Linking whole-body angular momentum and step placement during perturbed human walking. J. Exp. Biol. 226, jeb244760. ( 10.1242/jeb.244760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumas R, Chèze L, Verriest JP. 2007. Adjustments to McConville et al. and Young et al. body segment inertial parameters. J. Biomech. 40, 543-553. ( 10.1016/J.JBIOMECH.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 34.Brinkerhoff SA, Murrah WM, Hutchison Z, Miller M, Roper JA. 2022. Words matter: instructions dictate ‘self-selected’ walking speed in young adults. Gait Posture 95, 223-226. ( 10.1016/j.gaitpost.2019.07.379) [DOI] [PubMed] [Google Scholar]
- 35.Ulrich B, Santos AN, Jolles BM, Benninger DH, Favre J. 2019. Gait events during turning can be detected using kinematic features originally proposed for the analysis of straight-line walking. J. Biomech. 91, 69-78. ( 10.1016/J.JBIOMECH.2019.05.006) [DOI] [PubMed] [Google Scholar]
- 36.Zeni JA, Richards JG, Higginson JS. 2008. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27, 710-714. ( 10.1016/J.GAITPOST.2007.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho TK, Kreter N, Jensen CB, Fino PC. 2023. The choice of reference frame alters interpretations of turning gait and stability. J. Biomech. 151, 111544. ( 10.1016/J.JBIOMECH.2023.111544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imai T, Moore ST, Raphan T, Cohen B. 2001. Interaction of the body, head, and eyes during walking and turning. Exp. Brain Res. 136, 1-18. ( 10.1007/s002210000533) [DOI] [PubMed] [Google Scholar]
- 39.Nolasco LA, Silverman AK, Gates DH. 2019. Whole-body and segment angular momentum during 90-degree turns. Gait Posture 70, 12-19. ( 10.1016/j.gaitpost.2019.02.003) [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Macedo LD, Finley JM. 2018. Conservation of reactive stabilization strategies in the presence of step length asymmetries during walking. Front. Hum. Neurosci. 12, 251. ( 10.3389/fnhum.2018.00251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pataky TC. 2010. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 43, 1976-1982. ( 10.1016/j.jbiomech.2010.03.008) [DOI] [PubMed] [Google Scholar]
- 42.Horak FB, Nashner LM. 1986. Central programming of postural movements: adaptation to altered support-surface configurations. J. Neurophysiol. 55, 1369-1381. ( 10.1152/JN.1986.55.6.1369) [DOI] [PubMed] [Google Scholar]
- 43.Faisal AA, Selen LPJ, Wolpert DM. 2008. Noise in the nervous system. Nat. Rev. Neurosci. 9, 292-303. ( 10.1038/nrn2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dingwell JB, Cusumano JP. 2019. Humans use multi-objective control to regulate lateral foot placement when walking. PLoS Comput. Biol. 15, e1006850. ( 10.1371/journal.pcbi.1006850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strike SC, Taylor MJD. 2009. The temporal-spatial and ground reaction impulses of turning gait: is turning symmetrical? Gait Posture 29, 597-602. ( 10.1016/j.gaitpost.2008.12.015) [DOI] [PubMed] [Google Scholar]
- 46.Taylor MJD, Dabnichki P, Strike SC. 2005. A three-dimensional biomechanical comparison between turning strategies during the stance phase of walking. Hum. Mov. Sci. 24, 558-573. ( 10.1016/J.HUMOV.2005.07.005) [DOI] [PubMed] [Google Scholar]
- 47.Kreter N, Lybbert C, Gordon KE, Fino PC. 2022. The effects of physical and temporal certainty on human locomotion with discrete underfoot perturbations. J. Exp. Biol. 225, jeb244509. ( 10.1242/jeb.244509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pijnappels M, Bobbert MF, van Dieën JH. 2005. How early reactions in the support limb contribute to balance recovery after tripping. J. Biomech. 38, 627-634. ( 10.1016/j.jbiomech.2004.03.029) [DOI] [PubMed] [Google Scholar]
- 49.Pijnappels M, Bobbert MF, van Dieën JH. 2004. Contribution of the support limb in control of angular momentum after tripping. J. Biomech. 37, 1811-1818. ( 10.1016/j.jbiomech.2004.02.038) [DOI] [PubMed] [Google Scholar]
- 50.Winter DA, Sienko SE. 1988. Biomechanics of below-knee amputee gait. J. Biomech. 21, 361-367. ( 10.1016/0021-9290(88)90142-X) [DOI] [PubMed] [Google Scholar]
- 51.Zelik KE, et al. 2011. Systematic variation of prosthetic foot spring affects center-of-mass mechanics and metabolic cost during walking. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 411-419. ( 10.1109/TNSRE.2011.2159018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Bogaart M, Bruijn SM, Spildooren J, van Dieën JH, Meyns P. 2022. The effect of constraining mediolateral ankle moments and foot placement on the use of the counter-rotation mechanism during walking. J. Biomech. 136, 111073. ( 10.1016/J.JBIOMECH.2022.111073) [DOI] [PubMed] [Google Scholar]
- 53.Kreter N, Fino PC. Submitted. Bases for the selection of alternate foot placement during straight- and turning-gait.
- 54.Kreter N, Fino PC. 2024. Consequences of changing planned foot placement on balance control and forward progress. OSF repository. (https://osf.io/cj7yu/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreter N, Fino PC. 2024. Consequences of changing planned foot placement on balance control and forward progress. Figshare. ( 10.6084/m9.figshare.c.7072513) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are publicly available from the OSF repository: https://osf.io/cj7yu/ [54].
Supplementary material is available online [55].