Abstract
The leukotrienes are potent lipid mediators of inflammation formed by the 5-lipoxygenase-catalyzed oxidation of arachidonic acid. Although the effects of leukotrienes on neutrophil chemotaxis and activation have been established, their role in modulating innate host defense mechanisms is poorly understood. In a previous study (M. Bailie, T. Standiford, L. Laichalk, M. Coffey, R. Strieter, and M. Peters-Golden, J. Immunol. 157:5221–5224, 1996), we used 5-lipoxygenase knockout mice to establish a critical role for endogenous leukotrienes in pulmonary clearance and alveolar macrophage phagocytosis of Klebsiella pneumoniae. In the present study, we investigated the role of specific endogenous leukotrienes in phagocytosis of K. pneumoniae and explored the possibility that exogenous leukotrienes could restore phagocytosis in alveolar macrophages with endogenous leukotriene synthesis inhibition and enhance this process in leukotriene-competent cells. Rat alveolar macrophages produced leukotriene B4 (LTB4), LTC4, and 5-hydoxyeicosatetraenoic acid (5-HETE) during the process of phagocytosis, and the inhibition of endogenous leukotriene synthesis with zileuton and MK-886 dramatically attenuated phagocytosis. We also observed a reduction in phagocytosis when we treated alveolar macrophages with antagonists to the plasma membrane receptors for either LTB4, cysteinyl-leukotrienes, or both. In leukotriene-competent cells, LTC4 augmented phagocytosis to the greatest extent, followed by 5-HETE and LTB4. These 5-lipoxygenase reaction products demonstrated similar relative abilities to reconstitute phagocytosis in zileuton-treated rat alveolar macrophages and in alveolar macrophages from 5-lipoxygenase knockout mice. We conclude that endogenous synthesis of all major 5-lipoxygenase reaction products plays an essential role in phagocytosis. The restorative and pharmacologic effects of LTC4, LTB4, and 5-HETE may provide a basis for their exogenous administration as an adjunctive treatment for patients with gram-negative bacterial pneumonia.
Bacterial pneumonia is the leading cause of infectious death in industrialized nations (6), and its effective treatment grows increasingly more challenging due to the emergence of antibiotic-resistant strains and the increasing prevalence of immune suppression. The resident alveolar macrophage (AM) patrols the alveolar epithelial surface of the lung and maintains sterility by phagocytosing and killing microorganisms (21). If the microbial burden in the alveolar space overwhelms the ability of the AM to clear invading pathogens or when encapsulated gram-negative bacteria reach the alveolar surface, these resident cells secrete substances such as leukotriene B4 (LTB4), complement, and cytokines, which recruit neutrophils from the peripheral circulation to the alveolar focus of infection (24).
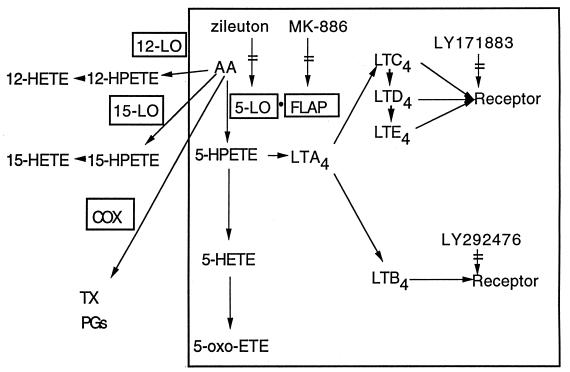
Leukotrienes (LTs) are potent lipid mediators of inflammation derived via the 5-lipoxygenase (5-LO) pathway of arachidonic acid (AA) metabolism (Fig. 1). In particular, the enzyme 5-LO, in concert with its helper protein 5-LO-activating protein (FLAP), can oxygenate AA to 5-hydroperoxyeicosatetraenoic acid (5-HPETE). This intermediate can either be dehydrated to LTA4 or reduced to 5-hydroxyeicosatetraenoic acid (5-HETE). 5-HETE can be further oxidized to 5-oxo-ETE, while LTA4 can be hydrolyzed to form LTB4 or conjugated with glutathione to form the cysteinyl-LTs (LTC4, LTD4, and LTE4). Alternative routes of metabolism found in certain cell types result in the synthesis of 12- and 15-HETE by 12- and 15-LO, respectively. In addition, metabolism via the cyclooxygenase pathway results in the formation of prostanoids and thromboxane.
FIG. 1.
Pathways for the oxidative metabolism of AA. The 5-LO pathway is within the box. COX, cyclooxygenase; TX, thromboxane; PG, prostaglandin.
Although the roles of LTs in neutrophil recruitment and cell activation are well established (20), their role in host defense is poorly understood. In addition to their role in neutrophil recruitment, LTs might promote host defense by virtue of their abilities to augment microbial phagocytosis and killing both in vitro and in vivo (1, 12). Evidence that the 5-LO pathway is activated during the course of lower respiratory tract infection includes the presence of elevated LTB4 levels in the bronchoalveolar lavage fluid of patients with bacterial pneumonia and elevated LTB4 and LTC4 levels in lung homogenates in animal models of bacterial pneumonia (4, 16). An important role for endogenously produced LTs in the host response to pneumonia was established by our recent report that 5-LO knockout (KO) mice exhibited enhanced mortality and reduced bacterial clearance compared with their wild-type (WT) counterparts following intratracheal administration of the gram-negative bacterium Klebsiella pneumoniae (1). This in vivo defect in bacterial clearance was associated with reduced phagocytosis and killing of K. pneumoniae in in vitro studies with AMs from 5-LO KO mice, compared with WT mice. Interestingly, phagocytosis could be enhanced in AMs from 5-LO KO mice by the addition of exogenous LTB4.
In the present study, we sought to extend our previous work on 5-LO products and AM phagocytosis. First, we wanted to explore the roles of both endogenously produced and exogenously added 5-LO metabolites in phagocytosis. Second, we wished to delineate the individual effects of all of the major 5-LO metabolites. Our data support an important role for endogenous 5-LO metabolites synthesized during the process of phagocytosis, as well as a pharmacologic effect of exogenously added products, in promoting AM phagocytosis of these relevant gram-negative bacteria.
MATERIALS AND METHODS
Cell isolation and culture.
Animals included pathogen-free 125- to 150-g female Wistar rats (Charles River Laboratories, Portage, Mich.), 5-LO KO mice, and their WT controls (129/SvEv) (The Jackson Laboratory, Bar Harbor, Maine). Resident AMs were obtained by lung lavage as previously described (1, 29). Ninety-six percent of the cells obtained from lavage have been identified as macrophages by use of a modified Wright-Giemsa stain (Diff-Quik; American Scientific Products, McGraw Park, Ill.) (29). Following centrifugation of lavage fluid at 200 × g for 5 min at 4°C, the pellet was resuspended in Hanks’ balanced salt solution (HBSS) and the cells were enumerated with a hemocytometer. The cells were centrifuged a second time and resuspended in RPMI 1640 (Gibco, Grand Island, N.Y.) to a final concentration of 5 × 105 cells/ml.
K. pneumoniae preparation.
K. pneumoniae 43816, serotype 2, was obtained from the American Type Culture Collection (Rockville, Md.), and aliquots were grown in tryptic soy broth (Difco, Detroit, Mich.) for 18 h at 37°C. The concentration of bacteria in culture was determined spectrophotometrically at 600 nm by using a standard curve to calculate the inoculum concentration (15).
Specific immune serum preparation and opsonization.
K. pneumoniae was suspended in phosphate-buffered saline–1% formalin for 24 h. The bacteria were then pelleted by centrifugation at 400 × g, and washed three times with saline, and 0.1 ml of the bacterial suspension (containing 108 bacteria) was administered to female Wistar rats via an intraperitoneal injection. The formalin-killed bacterial suspension was then frozen at −80°C. At 10 days after the primary inoculum, a second intraperitoneal injection was given; 7 days thereafter, the rats were anesthetized with sodium pentobarbital and blood was collected and allowed to clot at room temperature for 4 h. The blood was refrigerated overnight to allow the clot to retract. The clotted blood was centrifuged at 1,900 × g, and the serum was aliquoted and stored at −80°C for future experiments. Before each experiment, 20 × 108 K. pneumoniae cells were suspended in HBSS in a 5-ml polypropylene snap-cap tube (Sarstedt, Hayward, Calif.) and opsonized by mixing the bacterial suspension with 1% specific immune serum for 15 min at 37°C on an Adams rotating platform (Clay Adams, Parsippany, N.J.).
Analysis of AA metabolism.
AMs (106/well) were allowed to adhere for 1 h in 24-well culture plates (Falcon, Becton Dickson, Franklin Lakes, N.J.) and washed with HBSS to remove nonadherent cells. The AM monolayers were then covered with 1 ml of RPMI 1640 containing 5% fetal calf serum and 0.5 μCi of [3H]AA (specific activity, 76 to 100 Ci/mmol) (Dupont NEN, Boston, Mass.) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air overnight to allow the incorporation of the radiolabeled fatty acid into membrane phospholipids. Before stimulation, unincorporated label was removed by washing the cell monolayers three times with phosphate-buffered saline containing 0.1% fatty acid-free bovine serum albumin. [3H]AA incorporation was determined by scraping AM monolayers in methanol and scintillation counting. Incorporation averaged ∼95,000 dpm per well. After overnight incubation, the cell culture medium was replaced with fresh RPMI 1640, and the AM monolayers were incubated for 1 h at 37°C with or without K. pneumoniae (107 bacteria) opsonized with 1% specific immune serum. Cell culture medium from triplicate wells was then pooled for metabolite (eicosanoid) analysis. Eicosanoids were extracted from cell culture medium with C18 Sep-Pak cartridges (Waters Associates, Milford, Mass.) as previously described (28). The extracts were evaporated to dryness under nitrogen and stored at −80°C. The radiolabeled metabolites were separated by reverse-phase high-pressure liquid chromatography (HPLC) on a Waters μBondapak C18 column with a mobile phase of acetonitrile-water-trifluoroacetic acid at a flow rate of 1 ml/min as previously described (28). Eicosanoids were identified by coelution with authentic standards (Cayman Chemical, Ann Arbor, Mich.) and quantified with an on-line radioactivity detector (Radiomatic model 515; Packard, Downers Grove, IL). The released disintegrations per minute corresponding to a given eicosanoid were expressed as the percentage of radioactivity incorporated into the cell before incubation with or without K. pneumoniae.
Phagocytosis assay.
AMs (105) were allowed to adhere to glass eight-well Labtek slides (Nunc, Naperville, Ill.) for 1 h in RPMI 1640. Following adherence, the RPMI 1640 was removed and replaced with fresh medium. In some experiments, cells were pretreated for 15 min with the 5-LO enzyme inhibitor zileuton (10 μM) (Abbott Laboratories, Chicago, Ill.), the FLAP inhibitor MK-886 (1 μM) (Merck-Frosst, Montreal, Canada), the cysteinyl-LT receptor antagonist LY171883 (1 μM), or the LTB4 receptor antagonist LY292476 (1 μM) (both from Eli Lilly, Indianapolis, Ind.), each diluted in HBSS. Where appropriate, LO metabolites (Cayman Chemical, Ann Arbor, Mich.) were added in HBSS to the AM monolayers 5 to 10 min before to the addition of 106 K. pneumoniae organisms opsonized with 1% specific immune serum. The culture contents were mixed for 1 min with a plate shaker (Hoefer Instruments, San Francisco, Calif.). The AM cultures were then incubated for 30 min at 37°C. After the incubation period, the extracellular bacteria were removed by three washes with HBSS. After being dried overnight, the plastic well chambers were removed and the slides were stained with Diff-Quik. For each slide, a standard pattern of high-power fields was examined by light microscopy (magnification, ×1,000) to enumerate 100 cells. The phagocytic index was determined by multiplying the percentage of cells containing at least one bacterium by the number of bacteria per positive cell. In rat AMs under control conditions, the phagocytic index was 38 ± 9 (mean ± standard error [SE]). The ability of this microscopic method to distinguish intracellular from surface-associated bacteria was verified by comparing the phagocytic index of untreated AM monolayers with that of monolayers with cytochalasin D (5 μg/ml), which has been shown to completely inhibit the internalization of adherent particles by inhibiting actin polymerization (25). The phagocytic index of AMs treated with cytochalasin D was determined to be 10% of the value obtained without the drug, indicating that 90% of the cell-associated bacteria in these experiments were actually internalized.
Statistical analysis.
A minimum of four replicate wells per condition were studied in each experiment, and the number of individual experiments is indicated in the figure legends. Data are expressed as the mean ± SE. Where appropriate, values were analyzed by using a one-way analysis of variance (ANOVA) and the Bonferroni test for mean separation. In all cases, P < 0.05 was considered significant.
RESULTS
AM phagocytosis of opsonized K. pneumoniae induces LT synthesis.
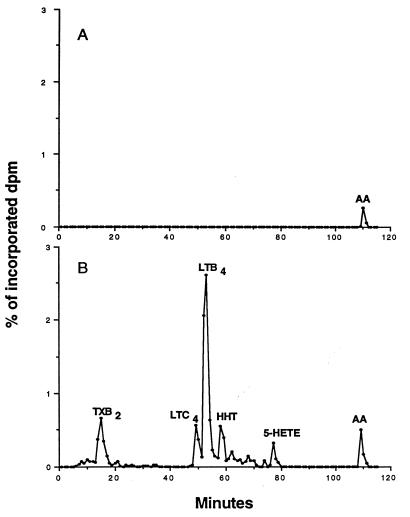
To determine if AM phagocytosis of opsonized K. pneumoniae results in the synthesis of LTs, medium with or without opsonized K. pneumoniae was incubated with AMs that had been prelabeled with [3H]AA for 1 h. Radioactive eicosanoids released into cell culture medium after incubation were separated by reverse-phase HPLC. As indicated by the HPLC chromatogram in Fig. 2A, control cultures contained a small amount of unmetabolized free [3H]AA but no eicosanoid metabolites. However, when the AMs were incubated with opsonized K. pneumoniae, radioactive metabolites of AA were detected. As shown by the HPLC chromatogram in Fig. 2B, LTB4 was the principle eicosanoid produced, with lower levels of the other 5-LO products, LTC4 and 5-HETE. The cyclooxygenase products thromboxane B2 (a nonenzymatic metabolite of thromboxane A2) and 12-hydroxyheptadecatrienoic acid were also detected. These results indicate that the 5-LO pathway in AMs is activated during phagocytosis of opsonized K. pneumoniae.
FIG. 2.
Representative radioactive HPLC elution profiles from prelabeled AMs incubated in the absence (A) or presence (B) of opsonized K. pneumoniae for 1 h. Eicosanoids were extracted from the medium before separation by HPLC. The radioactivity in each fraction was expressed as a percentage of the total radioactivity incorporated into cells. Peaks are designated on the basis of coelution with authentic standards. TXB2, thromboxane B2; HHT, 12-hydroxyheptadecatrienoic acid.
Inhibition of endogenous LT synthesis reduces AM phagocytosis of K. pneumoniae.
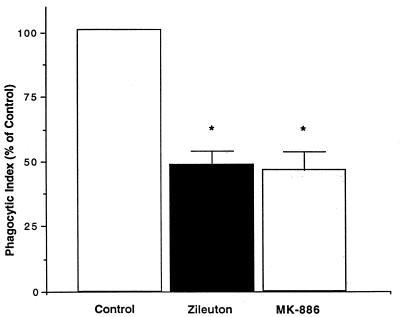
To determine if the synthesis of endogenous LTs plays an important functional role in AM phagocytosis of opsonized K. pneumoniae, we determined the effect on K. pneumoniae phagocytosis of two different types of LT synthesis inhibitors: zileuton, which inhibits LT synthesis by complexing with an iron atom in the active site of the 5-LO enzyme (5), and MK-886, which inhibits LT synthesis by binding to FLAP and interfering with its presentation of AA to 5-LO (33) (Fig. 1). In comparison to the control condition, both zileuton (10 μM) and MK-886 (1 μM), each at levels known to inhibit LT synthesis (3, 36), significantly attenuated AM phagocytosis of opsonized K. pneumoniae by approximately 50% (Fig. 3). The attenuation of phagocytosis by the inhibition of endogenous LT production indicates that LTs play a significant role in bacterial phagocytosis.
FIG. 3.
Effect of endogenous LT synthesis inhibition on AM phagocytosis of K. pneumoniae. AMs were pretreated with medium alone, zileuton (10 μM), or MK-886 (1 μM) for 15 min before the addition of opsonized K. pneumoniae. Data are expressed as the mean phagocytic index (percentage of control) ± SE (n = 4). ∗, P < 0.05 with respect to the control by ANOVA.
Inhibition of phagocytosis by antagonists to plasma membrane receptors for LTs.
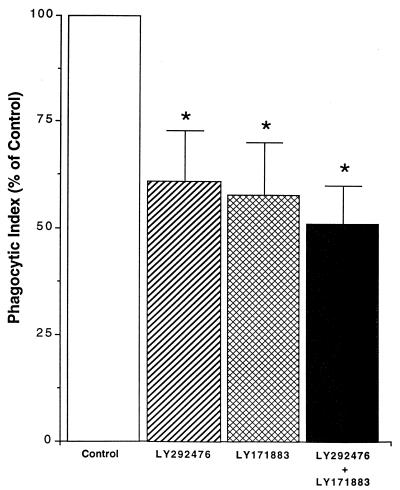
The preceding experiments established a role for a product(s) of the 5-LO pathway but did not identify the responsible endogenous metabolite. Since it is known that AMs express separate plasma membrane receptors for LTB4 and the cysteinyl-LTs and since both were produced during phagocytosis (Fig. 2B), we used a strategy involving selective receptor antagonists to determine the separate contributions of these two specific 5-LO metabolites (Fig. 1). AMs were treated with medium alone or medium containing LTB4 receptor antagonist (LY292476) (1 μM), cysteinyl-LT receptor antagonist (LY171883) (1 μM), or both for 15 min before the addition of opsonized K. pneumoniae. As shown in Fig. 4, preincubation of AMs with LY292476 or LY171883 reduced phagocytosis to approximately 60% of control level whereas the combination of the two receptor antagonists led to a further reduction in the phagocytic index to about 50% of the control level. These data indicate that both endogenously produced LTB4 and cysteinyl-LTs enhance phagocytosis and do so, at least in part, by moving out of the cell and activating their plasma membrane receptors.
FIG. 4.
Effect of LT receptor antagonists on AM phagocytosis of K. pneumoniae. AMs were treated with LTB4 receptor antagonist LY292476) (1 μM), cysteinyl-LT receptor antagonist LY171883) (1 μM), or both for 15 min before the addition of opsonized K. pneumoniae. Data are expressed as the mean phagocytic index (percentage of control) ± SE (n = 4). ∗, P < 0.05 with respect to the control by ANOVA.
Exogenous LTs exert pharmacologic effects on AM phagocytosis.
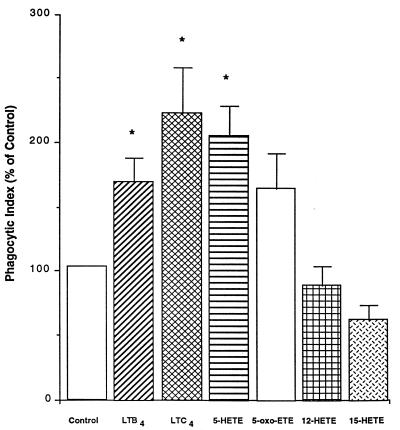
In the previous experiments, we established the importance of endogenous LTs as modulators of AM phagocytosis. We next addressed the possibility that 5-LO metabolites have pharmacologic effects on AM phagocytosis of K. pneumoniae. In these experiments, we used four products of the 5-LO pathway (LTB4, LTC4, 5-HETE, and 5-oxo-ETE) and, for comparison, the alternative lipoxygenase products 12-HETE and 15-HETE (Fig. 1). Each was used at 1 nM because preliminary dose-response experiments (results not shown) with 5-LO products in the range of 10−13 to 10−8 M showed this dose to be optimal. Exogenous lipids were coincubated with AMs 5 to 10 min before the addition of opsonized K. pneumoniae. As shown in Fig. 5, phagocytosis was significantly enhanced by exogenous LTC4 (approximately 220% of the control level), 5-HETE (200% of the control level), and LTB4 (175% of the control level). The effect of 5-oxo-ETE, which is known to have effects on neutrophils (30), was comparable to that of LTB4, and was nearly statistically significant (P = 0.10). 12-HETE had no effect on phagocytosis, whereas 15-HETE, a known inhibitor of leukocyte function (18), reduced phagocytosis slightly but not significantly.
FIG. 5.
Effects of exogenous lipids on phagocytosis of K. pneumoniae by LT-competent cells. AMs were incubated with lipids for 5 to 10 min before the addition of opsonized K. pneumoniae. Data are expressed as the mean phagocytic index (percentage of control) ± SE (n = 4). ∗, P < 0.05 with respect to the control by ANOVA.
The ability of exogenous LTs to enhance phagocytosis is mediated largely via interactions with plasma membrane receptors.
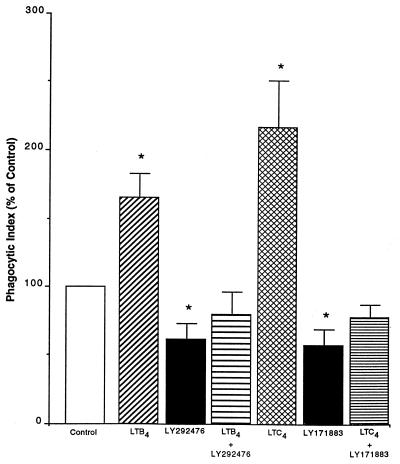
We next wished to determine if the positive effects of exogenous LTB4 and LTC4 (Fig. 5) were mediated by their interaction with surface receptors. As shown in Fig. 6, receptor antagonists completely prevented the increase in phagocytosis over baseline caused by exogenous LTs (1 nM) alone. However, the phagocytic index for exogenous LTs plus antagonist still slightly exceeded the value for the antagonist alone.
FIG. 6.
Effect of LT receptor antagonists on the augmentation of phagocytosis in response to exogenous LTB4 and LTC4. AMs were treated with exogenous LTs (LTB4 or LTC4) (1 nM), LTB4 receptor antagonist (LY292476) (1 μM), or cysteinyl-LT receptor antagonist (LY171883) (1 μM) or both before the addition of opsonized K. pneumoniae. Data are expressed as the mean phagocytic index (percentage of control) ± SE (n = 4). ∗, P < 0.05 with respect to the control by ANOVA.
The inhibitory effects on phagocytosis caused by inhibition of endogenous LT synthesis are overcome by the addition of exogenous LTs.
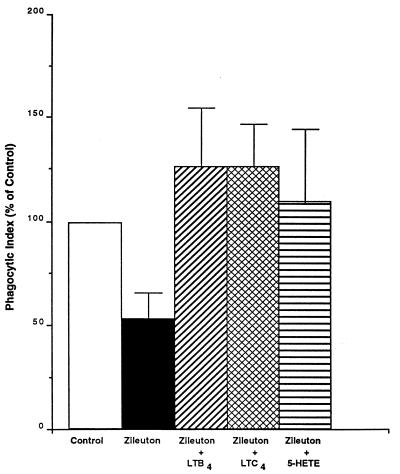
In the next set of experiments, we investigated whether it was possible to restore phagocytosis with exogenous LTs in AMs treated with inhibitors of endogenous LT synthesis. In these experiments, AMs were not treated or were treated with zileuton for 15 min before the addition of medium or exogenous LTB4, LTC4, or 5-HETE (1 nM). As shown in Fig. 7, when exogenous LTB4, LTC4, or 5-HETE was added to AMs treated with zileuton, the phagocytic response was increased to 125% (LTB4 and LTC4) and 110% (5-HETE) of control levels. These results indicate that exogenous LTB4, LTC4, and 5-HETE were each capable of completely overcoming the inhibitory effects of endogenous LT inhibition on phagocytosis.
FIG. 7.
Effect of exogenous LTB4, LTC4, or 5-HETE (1 nM) on phagocytosis of K. pneumoniae by AMs pretreated for 15 min with zileuton (10 μM). Data are expressed as the mean phagocytic index (percentage of control) ± SE of quadruplicate values from one experiment representative of a total of four.
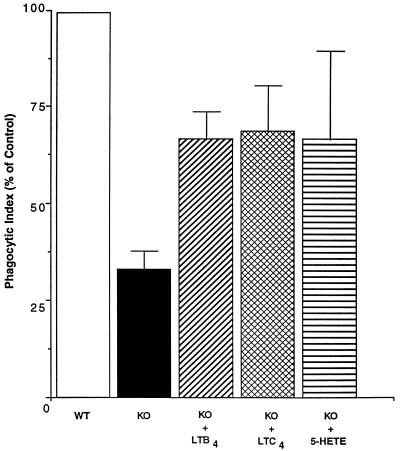
Exogenous LTs partially restore phagocytosis in AMs from 5-LO KO mice.
We previously reported (1) that AMs from 5-LO KO mice showed reduced phagocytosis of K. pneumoniae compared with cells from their WT counterparts. Furthermore, phagocytosis in these cells could be enhanced by the addition of exogenous LTB4. Since the experiments presented above indicated that exogenous LTC4 and 5-HETE, in addition to LTB4, enhanced phagocytosis in normal as well as zileuton-treated rat AMs, we asked if LTC4 and 5-HETE could likewise restore phagocytosis in AMs from 5-LO KO mice. The data in Fig. 8 confirm a profound defect in phagocytosis of K. pneumoniae in AMs from 5-LO KO mice compared with that in AMs from their WT counterparts. In addition, LTB4, LTC4, and 5-HETE each nearly restored the level of phagocytosis in AMs from 5-LO KO mice to that in cells from WT mice.
FIG. 8.
Ability of exogenous LTs to restore phagocytosis of K. pneumoniae in AMs from 5-LO KO mice. AMs from WT or 5-LO KO mice were treated with medium alone (control) or exogenous LTB4, LTC4, or 5-HETE (1 nM) for 5 to 10 min before the addition of opsonized K. pneumoniae. Data are expressed as the mean phagocytic index (percentage of control) ± SE of triplicate values. The phagocytic index for the control was 122 ± 16.
DISCUSSION
The AM provides the first line of antibacterial defense in the distal lung and maintains the sterility of the alveolar surface by ingesting and killing bacteria (21). Among the various substances released by the AM during its encounter with bacteria are the LTs. We previously demonstrated that 5-LO KO mice exhibited impaired bacterial clearance of K. pneumoniae in vivo and an associated defect in phagocytosis in AMs in vitro (1). The present study was designed to further delineate the role of LTs in AM phagocytosis by distinguishing the contribution of individual endogenous 5-LO reaction products and determining whether exogenous LTs exert a pharmacologic effect on LT-competent cells.
The major findings of this study were as follows: (i) AM phagocytosis of K. pneumoniae induced LT synthesis; (ii) inhibition of endogenous LT synthesis reduced AM phagocytosis of K. pneumoniae; (iii) exogenous LTC4, LTB4, and 5-HETE have pharmacologic effects, since they enhanced the AM phagocytosis of K. pneumoniae in LT-competent AMs; (iv) endogenous and exogenous LTB4 and LTC4 augmented AM phagocytosis primarily via their plasma membrane receptors; and (v) the impairment in phagocytosis observed in cells rendered LT deficient by either pharmacologic or genetic means could be overcome by the addition of exogenous LTC4, LTB4, or 5-HETE.
If endogenous LTs played a physiologic role in supporting phagocytosis, one would expect their synthesis to be demonstrable during the encounter of the phagocyte with bacteria. The principal 5-LO reaction product recovered from the medium of AMs cultured with opsonized K. pneumoniae was LTB4 followed by LTC4 and 5-HETE. The LT profile observed in this experiment was similar to that seen with other phagocytic stimuli such as zymosan, in which the ratio of LTB4 to LTC4 is about 10:1 (29). In the present experiments, we opsonized the bacteria with specific immune serum, which would be expected to contain both anti-K. pneumoniae-specific antibodies and complement. Whether either or both of these opsonins are required for the synthesis of LTs is an issue for future investigation. However, other reports demonstrate LT generation by leukocytes incubated with both unopsonized (8) and serum-opsonized (7) bacteria.
In this study, we used a pharmacologic approach to demonstrate that endogenously generated LTs enhance phagocytosis. This conclusion is based on the observations that inhibition of LT synthesis by two inhibitors acting via distinct molecular mechanisms reduced phagocytosis. This finding (confirmed in Fig. 8) is in agreement with our previous report, which demonstrated that AMs from 5-LO KO mice exhibited defective phagocytosis compared with AMs from WT mice. Likewise, blocking the action of LT with receptor antagonists also attenuated phagocytosis. When antagonists to receptors for both LTB4 and cysteinyl-LTs were combined, phagocytosis was inhibited to a greater extent than with either alone and to an extent similar to that observed with synthesis inhibitors. Taken together, these results suggest that the modulatory actions of the two groups of LTs are not redundant.
Previously, we observed the ability of exogenous LTB4 to increase phagocytosis in AMs from 5-LO KO mice. We verified the role of LTB4 in phagocytosis in the present experiments by using both specific LT receptor antagonists and exogenous addition of LTB4. The positive effect of LTB4 on phagocytosis was not surprising, since there is ample evidence demonstrating that LTB4 activates many leukocyte functions, including the upregulation of complement receptors and increased calcium mobilization in human peripheral blood neutrophils (23) and phagocytosis in murine peritoneal macrophages (12). Likewise, the effects of exogenous 5-HETE and 5-oxo-ETE on phagocytosis were not surprising, since these eicosanoids activate certain leukocyte functions in a manner similar to that of LTB4 (30).
Since the cysteinyl-LTs are best known for their ability to elicit smooth muscle contraction (20), the effects of LTC4 on phagocytosis were somewhat surprising. These data suggest that cysteinyl-LTs possess important leukocyte-activating properties that have not been generally recognized. One mechanism by which they might augment phagocytosis relates to their ability to promote actin polymerization (26). Although we observed cysteinyl-LTs to augment phagocytosis within 30 min, it is possible that in vivo cysteinyl-LTs further enhance phagocytosis by also upregulating Fc receptor expression via de novo synthesis (31).
It is well known that macrophages express LTB4 (32) and cysteinyl-LT receptors (11) on their plasma membrane. We used LY292476 (a LTB4 receptor antagonist) and LY171883 (a cysteinyl-LT receptor antagonist) in our experiments to determine if LTs were acting through these receptors to enhance phagocytosis. Based on the data presented in Fig. 6, it appears that most, but perhaps not all, of the actions of LTB4 and LTC4 on phagocytosis are mediated via classical plasma membrane receptors. A precedent for LT actions via a novel intranuclear receptor has recently been presented (13). Further studies are necessary to elucidate the mechanism underlying any receptor-independent actions of LTs in this experimental system.
In addition to the 5-LO products mentioned above, 12- and 15-HETE, which are formed by 12- and 15-LO, respectively, were used as negative controls. The fact that these LO products failed to augment phagocytosis demonstrates that the positive effect of 5-LO products on phagocytosis was not due to a nonspecific physical interaction of lipids with K. pneumoniae and AMs. The inability of 12-HETE to influence phagocytosis is consistent with the work of Sun and Funk, who showed that Fc receptor-mediated phagocytosis was not significantly altered in macrophages from 12-LO KO mice (35). That 15-HETE tended to decrease phagocytosis fits well with the fact that it generally has suppressive effects on leukocyte functions (18). This may actually reflect the ability of 15-HETE to inhibit 5-LO metabolism (14).
These results raise the possibility that the inhibitory effects of zileuton or MK-886 on AM phagocytosis reflect increased 15-HETE synthesis due to the shunting of AA from the inhibited 5-LO pathway to the uninhibited 15-LO pathway. However, we have never observed such shunting in HPLC chromatograms when using these LT synthesis inhibitors in AMs (10, 27). Furthermore, the LT receptor antagonist experiments provide convincing evidence that it is the 5-LO metabolites themselves which are responsible for the augmentation of AM phagocytosis.
The abilities of exogenous LTB4, LTC4, and 5-HETE to largely or completely restore phagocytosis in AMs with pharmacologic and genetic LT synthesis inhibition may have far-reaching implications for the treatment of patients with bacterial pneumonia. Leukocytes obtained from certain patient populations particularly susceptible to bacterial pneumonia (AIDS patients [9], protein-calorie malnourished individuals [34], newborns [22], diabetics [17], and smokers [2, 19]) have been demonstrated to manifest reduced LT-synthetic capacity. These patients may ultimately benefit from the intrapulmonary administration of LTs as adjunctive immunomodulatory treatment for bacterial pneumonia. Certainly, additional research is first needed to explore the effects of such an approach in appropriate animal models of bacterial pneumonia.
In summary, we have shown that endogenously produced LTs play an essential role in AM phagocytosis of K. pneumoniae. We have also provided evidence that LTC4, LTB4, and 5-HETE, in addition to enhancing phagocytosis in AMs with absent LT synthetic capacity, possess the ability to enhance phagocytosis in normal AMs. Future investigations will determine whether these effects extend to other microbes and to other populations of phagocytic cells and will explore the molecular mechanisms by which LTs augment phagocytosis.
ACKNOWLEDGMENTS
We thank Rob McNish for technical assistance and Tom Brock, Marc Jacobs, and Michael Coffey for helpful discussions.
This work was supported by National Heart Lung, and Blood Institute grant RO1-HL58897. Support for Peter Mancuso was provided by National Institutes of Health Training Grant T32 HL07749.
REFERENCES
- 1.Bailie M, Standiford T, Laichalk L, Coffey M, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumoniae in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 2.Balter M, Toews G, Peters-Golden M. Multiple defects in arachidonate metabolism in alveolar macrophages from young asymptomatic smokers. J Lab Clin Med. 1989;114:662–673. [PubMed] [Google Scholar]
- 3.Brock T, McNish R, Peters-Golden M. Capacity for repeatable leukotriene generation after transient stimulation of mast cells and macrophages. Biochem J. 1998;329:519–525. doi: 10.1042/bj3290519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buret A, Dunkley M, Clancy R, Cripps A. Effector mechanisms of intestinally induced immunity to Pseudomonas aeruginosa in the rat lung: role of neutrophils and leukotriene B4. Infect Immun. 1993;61:671–679. doi: 10.1128/iai.61.2.671-679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter G, Young P, Albert D, Bouska J, Dyer R, Bell R, Summers J, Brooks D. 5-Lipoxygenase inhibitory activity of zileuton. J Pharmacol Exp Ther. 1991;256:929–937. [PubMed] [Google Scholar]
- 6.Centers for Disease Control. CDC surveillance summaries: surveillance of major causes of hospitalization among the elderly, United States, 1988. Morbid Mortal Weekly Rep. 1991;40:7SS–15SS. [PubMed] [Google Scholar]
- 7.Claesson H, Lindgren J, Gustafsson B. Opsonized bacteria stimulate leukotriene synthesis in human leukocytes. Biochim Biophys Acta. 1985;836:361–367. doi: 10.1016/0005-2760(85)90140-7. [DOI] [PubMed] [Google Scholar]
- 8.Clementsen P, Bisgaard H, Pedersen M, Permin H, Struve-Christensen E, Milman N, Nuchel-Petersen B, Norn S. Staphylococcus aureus and influenza A virus stimulate human bronchalveolar cells to release histamine and leukotrienes. Agents Actions. 1989;27:107–109. doi: 10.1007/BF02222212. [DOI] [PubMed] [Google Scholar]
- 9.Coffey M, Phare S, Kazanjian P, Peters-Golden M. 5-Lipoxygenase metabolism in alveolar macrophages from subjects infected with the human immunodeficiency virus. J Immunol. 1996;157:393–399. [PubMed] [Google Scholar]
- 10.Coffey M J, Wilcoxen S E, Peters-Golden M. Increases in 5-lipoxygenase activating protein expression account for enhanced capacity for 5-lipoxygenase metabolism which accompanies differentiation of peripheral blood monocytes into alveolar macrophages. Am J Respir Cell Mol Biol. 1994;11:153–158. doi: 10.1165/ajrcmb.11.2.8049076. [DOI] [PubMed] [Google Scholar]
- 11.de Brum-Fernandes A, Guillemette G, Sirois P. Leukotriene B4 binding sites in guinea-pig alveolar macrophages. Prostaglandins. 1990;40:515–527. doi: 10.1016/0090-6980(90)90113-a. [DOI] [PubMed] [Google Scholar]
- 12.Demitsu T, Katayama H, Saito-Taki T, Yaoita H, Nakano M. Phagocytosis and bactericidal action of mouse peritoneal macrophages treated with leukotriene B4. Int J Immunopharmacol. 1989;11:801–808. doi: 10.1016/0192-0561(89)90134-3. [DOI] [PubMed] [Google Scholar]
- 13.Devchand P, Keller H, Peters J, Vazquez M, Gonzalez F, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 14.Fischer D, Christman J, Badr K. Fifteen-S-hydroxyeicosatetraenoic acid (15-S-HETE) specifically antagonizes the chemotactic action and glomerular synthesis of leukotriene B4 in the rat. Kidney Int. 1992;41:1155–1160. doi: 10.1038/ki.1992.176. [DOI] [PubMed] [Google Scholar]
- 15.Greenberger M, Strieter R, Kunkel S, Danforth J, Goodman R, Standiford T. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 16.Hopkins H, Stull T, Von Essen S, Robbins R, Rennard S. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989;95:1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- 17.Jubiz W, Draper R, Gale J, Nolan G. Decreased leukotriene B4 synthesis by polymorphonuclear leukocytes from male patients with diabetes mellitus. Prostaglandins Leukotrienes Med. 1984;14:305–311. doi: 10.1016/0262-1746(84)90114-8. [DOI] [PubMed] [Google Scholar]
- 18.Kang L, Vanderhoek J. Characterization of specific subcellular 15-hydroxyeicosatetraenoic acid (15-HETE) binding sites on rat basophilic leukemia cells. Biochim Biophys Acta. 1995;1256:297–304. doi: 10.1016/0005-2760(95)00039-f. [DOI] [PubMed] [Google Scholar]
- 19.Laviolette M, Coulombe R, Picard S, Braquet P, Borgeat P. Decreased leukotriene B4 synthesis in smokers’ alveolar macrophages in vitro. J Clin Invest. 1986;77:54–60. doi: 10.1172/JCI112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis R A, Austen K F, Soberman R J. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human disease. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann-Matthes M, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 22.Lu M-C, Peters-Golden M, Hostetler D, Robinson N, Derksen F. Age-related enhancement of 5-lipoxygenase metabolic capacity in cattle alveolar macrophages. Am J Physiol. 1996;271:L547–L554. doi: 10.1152/ajplung.1996.271.4.L547. [DOI] [PubMed] [Google Scholar]
- 23.Marder P, Sawyer J, Froelich L, Mann L, Spaethe S. Blockade of human neutrophil activation by 2-[2-propyl-3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxphenoxy]propoxy]phenoxy]benzoic acid (LY29311), a novel leukotriene B4 receptor antagonist. Biochem Pharmocol. 1995;49:1683–1690. doi: 10.1016/0006-2952(95)00078-e. [DOI] [PubMed] [Google Scholar]
- 24.Nelson S, Mason C, Knolls J, Summer W. Pathophysiology of pneumonia. Clin Chest Med. 1995;16:1–12. [PubMed] [Google Scholar]
- 25.Newman S, Mikus L, Tucci M. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol. 1991;146:967–974. [PubMed] [Google Scholar]
- 26.Peppelenbosch M, Tertoolen L, Hage W, de Laat S. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- 27.Peters-Golden M, Feyssa A. Transcellular eicosanoid synthesis in cocultures of alveolar epithelial cells and macrophages. Am J Physiol. 1993;264:L438–L447. doi: 10.1152/ajplung.1993.264.5.L438. [DOI] [PubMed] [Google Scholar]
- 28.Peters-Golden M, McNish R W, Hyzy R, Shelly C, Toews G B. Alterations in the pattern of arachidonate metabolism accompany rat macrophage differentiation in the lung. J Immunol. 1990;144:263–270. [PubMed] [Google Scholar]
- 29.Peters-Golden M, Thebert P. Inhibition by methylprednisolone of zymosan-induced leukotriene synthesis in alveolar macrophages. Am Rev Respir Dis. 1987;135:1020–1026. doi: 10.1164/arrd.1987.135.5.1020. [DOI] [PubMed] [Google Scholar]
- 30.Powell W, Gravel S, MacLeod R, Mills E, Hashefi M. Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem. 1993;268:9280–9286. [PubMed] [Google Scholar]
- 31.Rhodes J, Salmon J, Wood J. Macrophage Fcγ2b receptor expression and receptor-mediated phospholipase activity: regulation by endogenous eicosanoids. Eur J Immunol. 1985;15:222–227. doi: 10.1002/eji.1830150304. [DOI] [PubMed] [Google Scholar]
- 32.Rochette C, Nicholson D, Metters K. Identification and target-size analysis of the leukotriene D4 receptor in the human THP-1 cell line. Biochim Biophys Acta. 1993;1177:283–290. doi: 10.1016/0167-4889(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 33.Rouzer C A, Ford-Hutchinson A W, Morton H E, Gillard J W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 34.Skerrett S, Henderson W, Martin T. Alveolar macrophage function in rats with severe protein calorie malnutrition: arachidonic acid metabolism, cytokine release, and antimicrobial activity. J Immunol. 1990;144:1052–1061. [PubMed] [Google Scholar]
- 35.Sun D, Funk C. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. J Biol Chem. 1996;271:24055–24062. [PubMed] [Google Scholar]
- 36.Woods J, Coffey M, Brock T, Singer I, Peters-Golden M. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J Clin Invest. 1995;95:2035–2040. doi: 10.1172/JCI117889. [DOI] [PMC free article] [PubMed] [Google Scholar]