Abstract
Background
Non‐small cell lung cancer (NSCLC) is often diagnosed at an advanced stage. Clinical trials have demonstrated that first‐line immunotherapy alone or in combination with chemotherapy improves overall survival. However, reports of survival outcomes in real‐world settings are limited. We assessed survival in advanced NSCLC patients treated with immunotherapy alone or in combination with chemotherapy in first‐ or second‐line at the Windsor Regional Cancer Program (WRCP) and compared it to existing literature.
Methods
We included patients diagnosed with stage IV NSCLC from January 2015 to December 2020 and treated with first‐line chemoimmunotherapy (ChemoImmuno1), chemotherapy followed by immunotherapy (Chemo1), or immunotherapy followed by chemotherapy (Immno1) in our survival analysis. Patients with oncogene‐addicted mutations were excluded.
Results
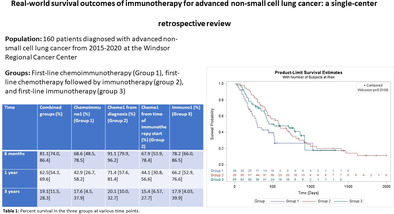
There were 160 patients of which 41.5% were female. Mean age was 68 years. Median overall survival from time of diagnosis was 474 days (95% CI: 249, 949) with an estimated 5‐year survival of 11.1% (95% CI: 4.5, 21.3). Median OS in ChemoImmuno1 was 9.6 months, in Chemo1 was 19.2 months from time of diagnosis and 10.5 months from time of initiation of immunotherapy, and in Immuno1 was 18.4 months, respectively. Estimated survival at three years from time of diagnosis for ChemoImmuno1 was 17.6% and for Immuno1 was 17.9%. For Chemo1, from diagnosis it was 20.1% and from second‐line therapy it was 15.4%. Survival outcomes were comparable to clinical trials and other studies.
Conclusion
Real‐world survival outcomes of immunotherapy for advanced NSCLC are comparable to the existing literature in this single center study.
Keywords: immunotherapy, lung cancer, NSCLC
We retrospectively reviewed patients diagnosed with stage IV non‐small cell lung cancer from 2015 to 2020 at the Windsor Regional Cancer Program treated with first or second‐line immunotherapy alone or in combination with chemotherapy. Median overall survival (OS) in the first‐line chemoimmunotherapy group was 9.6 months, 19.2 months in the first‐line chemotherapy groups from time of diagnosis and 10.5 months from time of initiation of second‐line immunotherapy, and 18.4 months in the first‐line immunotherapy group. Our results were comparable to landmark trials and other retrospective studies.
INTRODUCTION
Lung cancer (LC) is the most common cancer‐related death globally and is classified as small cell lung cancer (SCLC) or non‐small cell lung cancer (NSCLC) based on the 2015 World Health Organization classification system. 1 , 2 , 3 , 4 NSCLC comprises of 85% of LC cases, and can be further divided into adenocarcinoma and squamous cell carcinoma (SCC), the first and second most common types, respectively. 5 NSCLC is often diagnosed at advanced metastatic and unresectable stages, and thus has a poor estimated five‐year survival as low as 5%. 4 , 6 , 7
Many therapies exist for NSCLC, including surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. The established first‐line treatment for advanced NSCLC without targetable mutation is platinum‐based two‐drug chemotherapy; however, the identification of commonly mutated genes, such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), ROS‐1, and expression levels of programmed death ligand 1 (PD‐L1) has led to the development of targeted therapies and subsequent standard routine testing for targets in NSCLC patients which has improved overall NSCLC survival outcomes. 4 , 8 , 9 Several of these therapies, such as geftinib and afatinib, were approved for first‐line use in 2015 by the US Food and Drug Administration (FDA), the US‐based regulatory body for drug products, for the treatment of EGFR mutated adenocarcinomas after several trials demonstrated superior outcomes improved progression‐free survival, and reduced risk of disease progression and death compared to previous standard‐of‐care chemotherapy. 4 , 10 , 11
Despite advancements in targeted therapies, many lung cancer patients do not have targetable mutations, and treatment in these patients remains a major challenge. Platinum‐based chemotherapy is currently the first‐line treatment but has high toxicity. 12 During recent years, immunotherapy alone or in combination with chemotherapy in the first‐ or second‐line has been shown to improve overall survival (OS) in stage IV NSCLC and have a lower toxicity profile than chemotherapy. 7 , 12 Immunotherapy targets and prevents specific receptor‐ligand interactions at immune checkpoints, allowing for specific T cells to be activated and recognize tumor cells that would normally evade detection by the immune system. Specifically, the programmed cell death (PD‐1 and PD‐L1) and cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) pathways have been identified as targets for immunotherapy. The PD‐1 receptor is expressed on T cells, B cells, and NK cells involved in immune response while 51%–87% of NSCLC tumors express CTLA‐4. 5
Immunotherapy has been supported by several landmark studies. The KEYNOTE‐024 study, a phase 3 open‐label trial, investigated stage IV NSCLC patients without targetable mutations, without any previous systemic therapy, and with a PD‐L1 ≥ 50% who were randomized to receive either first‐line pembrolizumab (PD‐l inhibitor) or a platinum‐based chemotherapy regimen. Results demonstrated survival at six months of 80% in the pembrolizumab group versus 72% in the chemotherapy group, and higher progression free survival in the immunotherapy group. 13 KEYNOTE‐042 then investigated pembrolizumab in the first‐line in those with PD‐L1 ≥1% and found longer OS in the pembrolizumab group regardless of level of PD‐L1 positivity. 14 IMpower130 compared atezolizumab with chemotherapy versus chemotherapy alone in the first‐line and found improved survival. 15 KEYNOTE‐010 assessed outcomes in previous chemotherapy‐treated NSCLC patients with PD‐L1 ≥ 1% who progressed on first‐line therapy, and then subsequently were randomized to receive either pembrolizumab or docetaxel, finding longer survival in the immunotherapy group. 16 Checkmate9LA assessed combined treatment with first‐line treatment of chemotherapy and two immunotherapy agents or chemotherapy alone, which improved survival. 17
In patients with advanced NSCLC with PD‐L1 tumor proportion score (TPS) ≥ 50%, pembrolizumab monotherapy is approved to be used as first‐line therapy. 6 In those with PD‐L1 >1%, it is second‐line therapy after failed chemotherapy. 5 Pembrolizumab in combination with chemotherapy for the treatment of metastatic NSCLC without EGFR or ALK mutations has been shown to improve overall survival compared to chemotherapy alone. 7 For the treatment of advanced SCC, the FDA approved nivolumab (PD‐1 inhibitor) in 2015 after it was shown to significantly reduce the risk of death and improve survival compared to docetaxel; the use of nivolumab was later expanded to include adenocarcinomas. 5 Pembrolizumab‐naive NSCLC patients with disease progression after treatment with first‐line chemotherapy are treated with nivolumab, pembrolizumab and atezolizumab due to improved survival and response compared to standard of care docetaxel. 18 Notably, population‐wide analysis of stage IV NSCLC patients receiving immunotherapy has shown better OS compared to chemotherapy. 7
Despite encouraging results, clinical trials have highly selective study populations which limits their validity and generalizability to heterogenous real‐world populations, and few studies have assessed the use of immunotherapy in real‐world settings. We conducted a retrospective chart review of patients diagnosed with stage IV NSCLC between January 1, 2015, and December 31, 2020, and treated them with immunotherapy in the first‐ or second‐line alone or in combination with chemotherapy in the Windsor Regional Hospital Cancer Program to investigate survival outcomes and compare the results to clinical trials and other real‐world studies.
METHODS
Data collection
We used data from the Windsor Regional Cancer Center (WRCC). A total of 522 patients underwent treatment for stage IV lung cancer at WRCC between January 2015 and December 2020. Charts were reviewed to determine patients that were (1) diagnosed with stage IV NSCLC during this time frame and (2) underwent treatment with immunotherapy alone or in combination with chemotherapy in the first or second lines of treatment. We retrospectively reviewed the charts of patients who met these criteria and extracted data on demographics characteristics such as sex, smoking status, and comorbidities, as well as disease‐specific data such as date of diagnosis, histology, stage at diagnosis, metastases (brain, liver, and/or bone), and presence of targetable mutations. We categorized PD‐L1 status as ≥50% (positive) or <50% (negative). We collected data on all treatment lines received, type of treatment, and start and end dates. These were categorized as chemotherapy, immunotherapy, chemoimmunotherapy or targeted therapy. Progression was categorized as imaging‐proven progression, inability to tolerate, symptomatic progression, or death on a line of treatment. Time to progression was calculated from first date of treatment to end of last treatment overall or for each respective line. The number of adverse events were also recorded on each line of treatment and defined as an event requiring presentation to hospital emergency department or admission to hospital. We also recorded if a patient received radiation at any point after diagnosis. This study was approved by the ethics board at the Windsor Regional Hospital.
Statistical analysis
We used descriptive statistics such as mean, standard deviation, median, range, and percent as appropriate. The survival endpoints were analyzed and compared by using Kaplan‐Meyer estimates and Wilcoxon's statistic. Multivariate analysis was used via Cox's proportional hazards model to adjust for relevant confounding factors. All analysis were performed by using the SAS and R software.
RESULTS
Patient characteristics
There were 160 patients identified who met the inclusion criteria in the study. At the time of diagnosis for NSCLC, the mean age was 68 years (±7.93). About 40% of the patients were female. Most patients were former smokers (67.1%). About 50% of the patients had a history of hypertension while 30.8% had a history of chronic obstructive pulmonary disease. The most common type of NSCLC was adenocarcinoma (69.2%) followed by squamous cell carcinoma (17%). Metastases to the brain, liver, or bone at the time of diagnosis were in 23.9%, 13.8%, and 26.4% of patients, respectively. Additional characteristics are available in Table 1.
TABLE 1.
Demographic information of cohort and comparison of three subgroups.
| Variables | Results (N = 160) | p‐value comparison between 3 groups |
|---|---|---|
| Sex | 0.437 | |
| Female | 66 (41.5) | |
| Male | 93 (58.5) | |
| Unknown | 1 | |
| Age (years) | 0.829 | |
| Mean (std) | 68.06 (7.93) | |
| Smoking status | 0.923 | |
| Never | 5 (3.2) | |
| Current | 47 (29.7) | |
| Former | 106 (67.1) | |
| Comorbidities | ||
| Hypertension | 82 (51.6) | 0.293 |
| COPD | 49 (30.8) | 0.276 |
| Type 2 diabetes | 30 (18.9) | 0.319 |
| Previous history of cancer | 31 (19.5) | 0.108 |
| Family history of cancer | ||
| No | 33 (20.8) | 0.830 |
| First degree | 101 (63.5) | 0.781 |
| Second degree | 38 (23.9) | 0.247 |
| Yes, degree unknown | 2 (1.3) | 0.472 |
| Death at end of follow‐up | 0.011 | |
| Yes | 105 (66) | |
| Histology at diagnosis | ||
| Adenocarcinoma | 110 (69.2) | |
| Squamous cell carcinoma | 27 (17.0) | |
| Adenosquamous | 3 (1.9) | |
| Large cell carcinoma | 4 (2.5) | |
| NSCLC, NOS | 13 (8.2) | |
| Other | 2 (1.3) | |
| Metastatic sites at time of diagnosis | ||
| Brain | 38 (23.9) | 0.457 |
| Liver | 22 (13.8) | 0.796 |
| Bone | 42 (26.4) | 0.502 |
| PD‐L1 TPS >50 | <0.001 | |
| No | 57 (45.7) | |
| Yes | 63 (52.5) | |
| Unknown | 40 | |
| Group | ||
| ChemoImmuno1 | 39 (24.4) | |
| Chemo1 | 56 (35.0) | |
| Immuno1 | 65 (40.6) | |
| Number of radiation treatments received during course of treatment | 0.178 | |
| 0 | 46 (28.8) | |
| 1 | 72 (45.0) | |
| 2 | 26 (16.3) | |
| 3 | 7 (4.4) | |
| 4 | 9 (5.6) |
Abbreviation: COPD, chronic obstructive pulmonary disease; NOS, not otherwise specified; NSCLC, non‐small cell lung cancer.
Of the 160 patients, we identified 39 patients who received first‐line combination chemotherapy and immunotherapy (ChemoImmuno1), 56 patients who received second‐line immunotherapy (Chemo1) with chemotherapy in the first‐line, and 65 patients who received first‐line immunotherapy (Immuno1). Of those with known PD‐L1 status (n = 120), 52.5% were positive overall. In the ChemoImmuno1 and Chemo1 groups, about 12% had PD‐L1 ≥ 50% compared to 96.6% in those in the Immuno1 group. Radiation therapy between the groups was not significantly different; however, we found the 64.1% of patients in ChemoImmuno1 versus 62.5% of patients in Chemo1 versus 83.1% of patients in Immuno1 received at least one treatment of radiation at some point during their course of treatment. Comparison of baseline demographic between the three groups showed significant differences only in PD‐L1 status. Mean duration of line 1 in all patients was 230.5 days (7.57 months) and median was 129 days (4.24 months), in line 2 mean was 217.87 days (7.16 months) and median was 107 days (3.51 months), and in line 3 mean was 190.62 days (6.27 months) and median was 91.5 days (3.0 months), respectively.
Treatment groups
There were 39 patients in ChemoImmuno1. Mean duration of therapy on line 1 (N = 39) was 255.92 days (8.41 months) and median was 139 days (4.56 months), on line 2 (N = 10) was 81.6 days and 30 days, and on line 3 (N = 1) was 42 days and 42 days, respectively. A total of 41% of patients had imaging‐proven progression on the first‐line of ChemoImmuno1. An additional 23.1% of patients could not tolerate first‐line therapy. About 12% of patients died on the first‐line of therapy. At last follow‐up, 15% of patients were still on their first‐line of therapy. Ten patients went on to receive second‐line therapy in this group (25.6%).
There were 56 patients in Chemo1. Mean duration of therapy on line 1 (N = 56) was 131.58 days (4.3 months) and median was 86 days (2.83 months), which was shorter than both ChemoImmuno1 and Immuno1 groups. Mean duration of line 2 (N = 56) was 257.53 (8.47 months) and median was 122 days (4.01 months), and on line 3 (N = 18) was mean 243.06 days (7.99 months) and median was 125 days (4.11 months). A total of 82.1% progressed on the first‐line, while 3.6% could not tolerate it. Because we were interested in second‐line immunotherapy, each patient in this group based on our inclusion and exclusion criteria went on to receive immunotherapy. Progression on second‐line therapy in the Chemo1 group demonstrated that 76.7% of patients had imaging‐confirmed progression, an additional 17.9% could not tolerate the therapy, and 1.8% symptomatically progressed. Eighteen patients (32.7%) went on to receive third‐line therapy.
There were 65 patients in Immuno1. Mean duration of therapy on line 1 (N = 65) was 298.95 days (9.83 months) and median was 191 days (6.28 months), on line 2 (N = 18) was 172.39 days (5.65 months) and 99.5 days (3.27 months), and on line 3 (N = 7) was 77 days (2.53 months) and 63 days (2.07 months), respectively. Forty‐six (46.2%) patients had imaging proven‐progression on the first‐line, while 7.7% could not tolerate it. About 12% of patients died on first‐line therapy, while at last‐follow‐up, about 19% of patients were still on the first‐line of therapy. Seven patients (10.8%) went on to receive third‐line therapy. Additional information is available in Appendix S1.
Survival
By the end of the longest follow‐up at 1995 days (5.47 years) restricted to the longest event time, 105 (65.6%) patients had died. In ChemoImmuno1, 66.7% died versus 80% in Chemo1 versus 53.8% in Immuno1 (p = 0.001). The median OS in all groups combined from time of diagnosis was 474 days (15.6 months, 95% CI: 249, 949) while the mean was 667.8 days (21.95 months). The estimated survival at five years was 11.1% (95% CI: 4.5, 21.3) (Figure 1).
FIGURE 1.
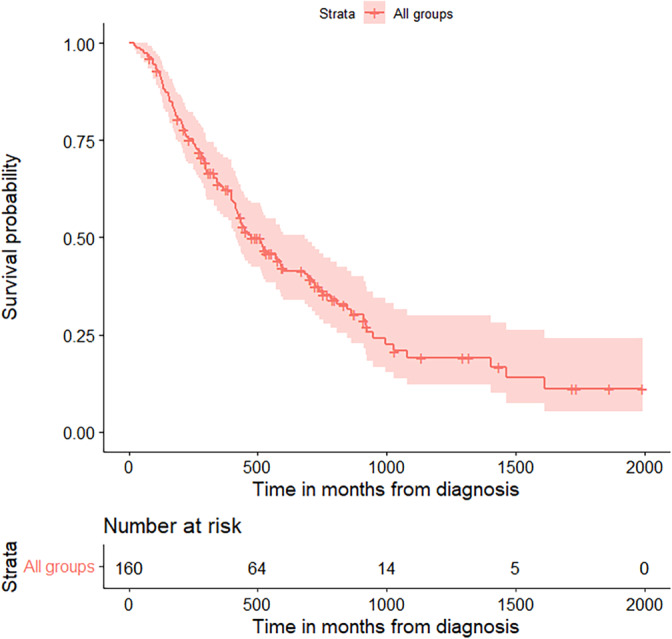
Overall survival. Overall survival of all patients with stage IV non‐small cell lung cancer (NSCLC) in our cohort who received immunotherapy in the first‐ or second‐line of treatment alone or in combination with chemotherapy.
When overall survival (OS) between the three groups was compared from time of diagnosis, we found it to be significantly different with a chi‐squared value of 8.17 (p = 0.017) (Figure 2). Pairwise comparisons of survival between the three groups showed that OS between ChemoImmuno1 and Chemo1 was significantly different with a chi‐squared value of 7.11 (p = 0.023), indicating that Chemo1 had better OS compared to ChemoImmuno1. There was no significant difference in OS between ChemoImmuno1 and Immuno1 or Chemo1 and Immuno1 in pairwise comparison (Table 2).
FIGURE 2.
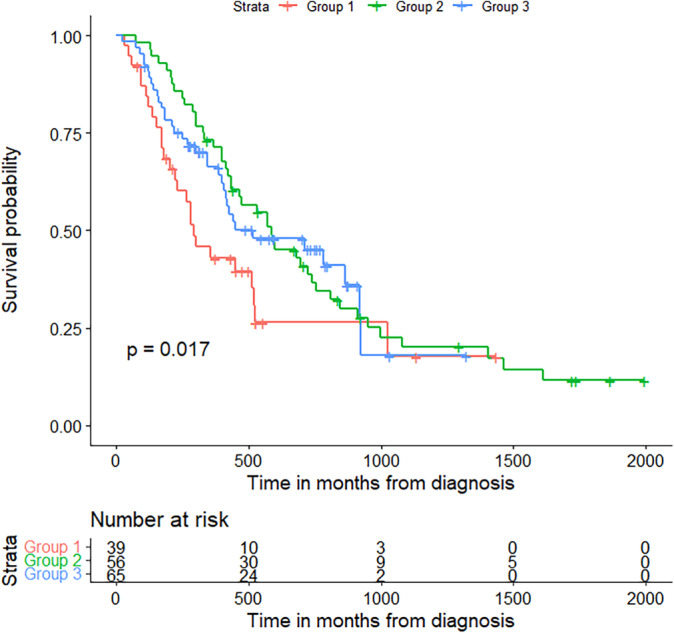
Overall survival of each treatment group from time of diagnosis. Overall survival of ChemoImmuno1 (blue), Chemo1 (red), and Immuno1 (green) groups from the time of diagnosis. Survival was similar between the three groups, with a trend for worse early survival in the ChemoImmuno1 group.
TABLE 2.
Survival at 6 months, one year and three years from time of treatment initiation in all groups combined and subgroups, including Chemo1 from time of immunotherapy initiation (line 2).
| Time | Combined groups | ChemoImmuno1 | Chemo1 from diagnosis | Chemo1 from time of immunotherapy start | Immuno1 |
|---|---|---|---|---|---|
| 6 months | 81.1 (74.0, 86.4) | 68.6 (48.5, 78.5) | 91.1 (79.9, 96.2) | 67.9 (53.9, 78.4) | 78.2 (66.0, 86.5) |
| 1 year | 62.5 (54.3, 69.6) | 42.9 (26.7, 58.2) | 71.4 (57.6, 81.4) | 44.1 (30.8, 56.6) | 66.2 (52.9, 76.6) |
| 3 years | 19.1 (11.5, 28.3) | 17.6 (4.5, 37.9) | 20.1 (10.0, 32.7) | 15.4 (6.57, 27.7) | 17.9 (4.03, 39.9) |
In ChemoImmuno1, the longest follow‐up was 1435 days (47.2 months) with 17.6% survival by end of follow‐up. Median OS was 292 days (9.6 months, 95% CI: 170, 1027) and mean survival was 459.9 days (15.12 months). In Chemo1 from time of diagnosis, longest follow‐up was 1995 days (65.6 months). Median survival was 585 days (19.2 months) and mean survival was 721.5 days (23.7 months). We also looked at Chemo1 from time of initiation for immunotherapy (line 2) since we were most interested in the survival after immunotherapy. The longest follow‐up was 1835 days (60.3 months). Median survival was 318 days (10.5 months, 95% CI: 204, 568) and mean survival was 491.8 days (16.2 months) from time of start of immunotherapy. In Immuno1, 53.8% of the patients died with longest follow‐up of 1321 days (43.4 months). Median survival was 516 days (18.44 months, 95% CI: 249, 516) while mean survival was 567.7 days (18.66 months). (Figure 2).
We estimated survival at six months, one and three years from time of diagnosis for each group. We also included survival from Chemo1 from time of starting immunotherapy (line 2) given that survival in those who received second‐line chemotherapy has been investigated in clinical trials. Estimated survival in all groups at 6 months was predicted as 81.1%, at 1 year was 62.5%, and at 3 years was 19.1% (Table 2).
We also assessed survival between patients based on PDL1 status and found no significant difference in survival (chi‐squared = 0.0704, p = 0.79) (Figure 3). Of the 57 patients who were PD‐L1 negative, 68.4% died by a follow‐up of 1866 days. Median overall survival was 474 days (15.6 months, 95% CI: 219, 846) while mean survival was 528.6 days (17.4 months). There were 63 patients in the PD‐L1 positive group, of which 34 (54%) died by a follow‐up of 1453 days. Median overall survival was 450 days (14.8 months, 95% CI: 211, 924) and mean survival was 554.2 days (18.2 months).
FIGURE 3.
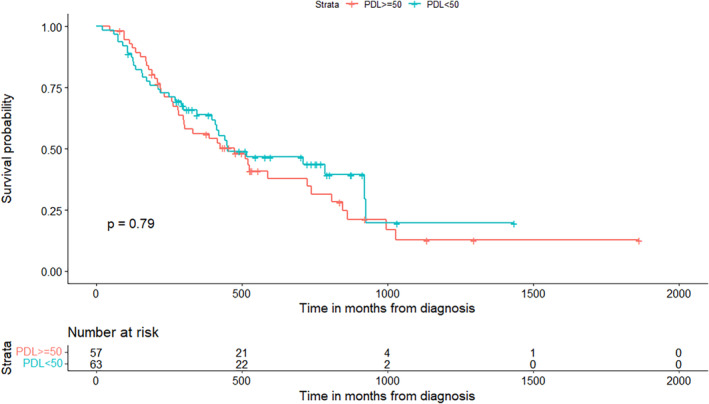
Survival by PD‐L1 status. Survival in those with available PD‐L1 status, where “Yes” are patients with PD‐L1 ≥ 50%, are positive PD‐L1 status in this analysis. PD‐L1 negative (blue) and positive (red) have similar survival.
On univariable analysis, we found that the only variables associated with overall survival were treatment group, hypertension, and progression on first‐line of therapy. On multivariable analysis, only progression on first‐line of therapy was associated with overall survival (chi‐squared = 7.72, p = 0.0055), with a hazard ratio of 0.425 indicating decreased survival in those that progressed on first‐line therapy. We did not find any significant difference in the number of adverse events between each treatment group or line of treatment within each group.
DISCUSSION
We investigated single center outcomes of immunotherapy in advanced NSCLC patients. We assessed three groups of patients: combination chemotherapy and immunotherapy in first‐line (ChemoImmuno1), first‐line chemotherapy followed by immunotherapy in the second‐line (Chemo1), and first‐line immunotherapy (Immuno1). We found an overall 5‐year survival of 11.1%. Median OS in months in ChemmoImmuno1 was 9.6 months, in Chemo1 was 19.2 months from time of diagnosis and 10.5 months from time of initiation of immunotherapy, and in Immuno1 was 18.4 months, respectively. ChemoImmuno1 had significantly lower OS compared to Chemo1 in pairwise comparison. In multivariate regression analysis, progression on first‐line therapy was associated with poorer OS. We found the ChemoImmuno1 had the lowest 1‐year survival, but 3‐year survival was similar between the three groups. We did not find PD‐L1 status to be associated with survival in patients in which it was available.
Various landmark trials have demonstrated benefit to the use of immunotherapy in the first‐or second‐line depending on patient characteristics and previous treatment received. KEYNOTE‐024 assessed pembrolizumab in the first‐line in untreated advanced NSCLC patients with PDL1≥ 50%, and found improved survival compared to platinum‐based chemotherapy, with a 6‐month survival of 80.2% in the pembrolizumab group compared to 72.4% in the chemotherapy group. In patients with PDL1 ≥ 1%, KEYNOTE‐042 found improved survival in those treated with pembrolizumab compared to paclitaxel or pemetrexed with carboplatin with a median OS of 20 months in the pembrolizumab group versus 12.2 in the chemotherapy group with PDL1 ≥ 50%, 17.7 versus 13 months in those with PDL1 ≥20%, and 16.7 versus 21.1 months in those with PDL1 ≥ 1%. 14 , 19 In our study, we found a median OS of 18.4 months and 6‐month survival of 78.2% in the first‐line immunotherapy group, comparable to these randomized controlled trials (RCT). 6 In previously treated advanced NSCLC patients, the KEYNOTE‐010 trial demonstrated improved OS with pembrolizumab compared to docetaxel PD‐L1≥ 1%, and a more pronounced improvement in the PDL1 ≥ 50% group. Three‐year survival outcomes were 34.5% in the PD‐L1 ≥ 50% group and 22.9% in the PDL1 ≥ 1% group. Median OS was 10.4 months in the lower dose pembrolizumab group and 12.7 months in the higher dose group. 16 , 20 Comparatively, we found lower 3‐year survival in the second‐line immunotherapy group at 20.1%; however, a similar median OS of 10.5 months from time of initiation of second‐line therapy.
While our findings of first‐ and second‐line immunotherapy are similar in the real‐world to RCTs, we found lower OS in the first‐line chemoimmunotherapy group. Several RCTs have assessed the use of chemoimmunotherapy, although the survival benefit in metastatic disease is unclear. IMpower130 found that in advanced no squamous NSCLC, atezolizumab with chemotherapy improved OS (18.5 months vs. 13.9 months) in the intention‐to‐treat (ITT) group, except for in those with liver metastasis or with targetable mutations. However, without ITT analysis, no improvement was found. 15 CheckMate 9LA demonstrated improved OS when two cycles of platinum‐doublet chemotherapy were used in combination with dual immunotherapy as first‐line treatment in advanced metastatic or recurrent NSCLC without targetable mutations; however, more serious adverse events were found in the combination therapy group. 17 We did not find any differences in adverse events. Our study found lower median OS of 9.6 months in the combination group; however, most patients in our combination group received carboplatin, pemetrexed, and pembrolizumab. With the addition of pembrolizumab to chemotherapy, Gandhi et al. found that in nonsquamous treatment‐naïve NSCLC patients without targetable mutations, estimated OS at 1‐year was 69.2% comparable to our study of 62.5%, Mean durations of treatment were also similar 7.4 months their study versus 8.41 months in our study. 21
In other real‐world studies, immunotherapy used in any line of treatment in advanced NSCLC has been shown to improve survival with an 80% increase in the unadjusted risk of death in the chemotherapy only group. Our 1‐ and 3‐year survival rates were similar to the 61.1% and 16.5% survival found in the immunotherapy group in a study on an Italian cohort, who found the survival benefit was predominantly in the first two years. 22 Improved OS was found in another study comparing immunotherapy in any line and chemotherapy in first‐line, with a median OS of 12.7 months in the immunotherapy group compared to our median OS of 15.6 months. First‐line immunotherapy median OS was reported as 19.9 months with 91% survival at 6 months, comparable to our 18.4 months OS. We found lower 6‐month survival at 78%. In those who received immunotherapy as second‐line treatment, we found a slightly lower OS (10.5 months) compared to the 12.17 months reported. 23
Studies have previously demonstrated that PDL1 positivity is a biomarker to predict better response to PD‐L1 immunotherapy, such as landmark trials described above, although tumors with low or negative PD‐L1 expression have still shown a response. However, overall, there is mixed evidence for the association between PD‐L1 status and prognosis. 24 We did not find any difference in survival based on PD‐L1 status overall. Due to low sample size, we were not able to compare PD‐L1 status within groups nor were we able to compare PD‐L1 divided into additional subgroups of degree of positivity as has been compared in other studies.
There were several limitations to our study. First, the retrospective nature of our study was subject to bias with treatment selection and data collection. Data collection was not a blinded process. We did not randomize patients to treatment groups nor did we perform a matched analysis. While most landmark studies cited in this paper analyzed treatment outcomes by specific therapy regimens, our treatment groups were heterogenous with regard to specific chemotherapy or immunotherapy used. We did not separately analyze specific immunotherapies or chemotherapies separately due to our lower center‐level sample size, therefore our results may not be directly comparable to the results of RCTs which is a limitation of this study. We did not exclude patients who received radiation therapy and not all patients received radiation therapy which may have influenced outcomes. Our data cannot be used to compare survival of first‐line chemotherapy to first‐line immunotherapy (Chemo1 vs. Immuno1 groups) because patients were only included in the Chemo1 group if they received second‐line chemotherapy. Therefore, patients whose disease was successfully managed on chemotherapy alone would not be included in our study. We did not separate patients by metastatic disease (brain, bone, or liver) and compare outcomes in each group due to the small sample size, which again limits the ability to generalize this data to specific patient populations who may have more aggressive disease. Lastly, several variables were not available widely in the retrospective dataset, such as the ECOG performance status or grade of adverse event, which may be important in determining factors associated with failure of treatment of immunotherapy.
In conclusion, our results from a single center community‐based study on the treatment of stage IV NSCLC are comparable to RCTs and similar real‐world studies, supporting the use of immunotherapy in the first‐ and second‐line treatments. Further studies should be done to assess the efficacy of chemoimmunotherapy in a real‐world setting, with particular focus on increased sample size and prospective data collection.
AUTHOR CONTRIBUTIONS
GP, AH, SK: Writing, review and editing, conceptualization and methodology. GP, SK: Data curation and project administration. AH: Formal analysis. GP, AH: Investigation and visualization. SK: Supervision and validation. GP: Writing – original draft preparation.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to declare.
Supporting information
Appendix S1: Duration and progression on treatment lines by subgroup, along with adverse events.
ACKNOWLEDGEMENTS
None.
Punchhi G, Hussein A, Kulkarni S. Real‐world survival outcomes of immunotherapy for advanced non‐small cell lung cancer: A single‐center retrospective review. Thorac Cancer. 2024;15(5):394–401. 10.1111/1759-7714.15205
REFERENCES
- 1. Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. 2019;85(1):3–4. 10.5334/aogh.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ricciardi S, Tomao S, de Marinis F. Efficacy and safety of erlotinib in the treatment of metastatic non‐small‐cell lung cancer. Lung Cancer (Auckl). 2011;2:1–9. 10.2147/LCTT.S10167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddiqui F, Siddiqui AH. Lung Cancer. StatPearls. StatPearls Publishing Copyright © 2020. Treasure Island, FL: StatPearls Publishing LLC.; 2020. [Google Scholar]
- 4. Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non‐Small‐Cell Lung Cancer. N Engl J Med. 2017;377(9):849–861. 10.1056/NEJMra1703413 [DOI] [PubMed] [Google Scholar]
- 5. Vafadar S. Immunotherapy for non‐small cell lung cancer. JAAPA. 2019;32(9):37–42. 10.1097/01.JAA.0000569792.99069.e6 [DOI] [PubMed] [Google Scholar]
- 6. Kawachi H, Tamiya M, Tamiya A, et al. Association between metastatic sites and first‐line pembrolizumab treatment outcome for advanced non‐small cell lung cancer with high PD‐L1 expression: a retrospective multicenter cohort study. Invest New Drugs. 2020;38(1):211–218. 10.1007/s10637-019-00882-5 [DOI] [PubMed] [Google Scholar]
- 7. Foster CC, Sher DJ, Rusthoven CG, et al. Overall survival according to immunotherapy and radiation treatment for metastatic non‐small‐cell lung cancer: a National Cancer Database analysis. Radiat Oncol. 2019;14(1):18. 10.1186/s13014-019-1222-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Y, Ding VW, Zhang H, Zhang X, Jablons D, He B. Spotlight on afatinib and its potential in the treatment of squamous cell lung cancer: the evidence so far. Ther Clin Risk Manag. 2016;12:807–816. 10.2147/TCRM.S92996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137(6):828–860. 10.5858/arpa.2012-0720-OA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folch E, Costa DB, Wright J, VanderLaan PA. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Transl Lung Cancer Res. 2015;4(4):392–403. 10.3978/j.issn.2218-6751.2015.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR‐mutant lung cancer: A meta‐analysis. J Clin Oncol. 2015;33(17):1958–1965. 10.1200/JCO.2014.58.1736 [DOI] [PubMed] [Google Scholar]
- 12. Meng X, Liu Y, Zhang J, Teng F, Xing L, Yu J. PD‐1/PD‐L1 checkpoint blockades in non‐small cell lung cancer: New development and challenges. Cancer Lett. 2017;10(405):29–37. 10.1016/j.canlet.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 13. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD‐L1‐Positive Non‐Small‐Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 14. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. 10.1016/s0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 15. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. 10.1016/s1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 16. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. 10.1016/s0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 17. Paz‐Ares L, Ciuleanu TE, Cobo M, et al. First‐line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non‐small‐cell lung cancer (CheckMate 9LA): an international, randomised, open‐label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. 10.1016/s1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 18. Socinski MA, Obasaju C, Gandara D, et al. Current and Emergent Therapy Options for Advanced Squamous Cell Lung Cancer. J Thorac Oncol. 2018;13(2):165–183. 10.1016/j.jtho.2017.11.111 [DOI] [PubMed] [Google Scholar]
- 19. Shields MD, Marin‐Acevedo JA, Pellini B. Immunotherapy for Advanced Non‐Small Cell Lung Cancer: A Decade of Progress. Am Soc Clin Oncol Educ Book. 2021;41:1–23. 10.1200/edbk_321483 [DOI] [PubMed] [Google Scholar]
- 20. Herbst RS, Garon EB, Kim DW, et al. Long‐Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death‐Ligand 1–Positive, Advanced Non–Small‐Cell Lung Cancer in the KEYNOTE‐010 Study. J Clin Oncol. 2020;38(14):1580–1590. 10.1200/jco.19.02446 [DOI] [PubMed] [Google Scholar]
- 21. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non‐Small‐Cell Lung Cancer. N Engl J Med. 2018;378(22):2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 22. Andreano A, Bergamaschi W, Russo AG. Immune checkpoint inhibitors at any treatment line in advanced NSCLC: Real‐world overall survival in a large Italian cohort. Lung Cancer. 2021;159:145–152. 10.1016/j.lungcan.2021.06.019 [DOI] [PubMed] [Google Scholar]
- 23. Ruiz‐Patiño A, Arrieta O, Cardona AF, et al. Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non‐small cell lung cancer (NSCLC) compared with chemotherapy (Quijote‐CLICaP). Thorac Cancer. 2020;11(2):353–361. 10.1111/1759-7714.13272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD‐L1 Expression in Lung Cancer. J Thorac Oncol. 2016;11(7):964–975. 10.1016/j.jtho.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Duration and progression on treatment lines by subgroup, along with adverse events.


