Summary
Background
Concurrent chemoradiotherapy is the standard nonoperative treatment for locally advanced esophageal squamous cell carcinoma. However, local recurrence is still the main failure pattern, accounting for more than half of all treatment failures, indicating that the sensitivity of radiotherapy still needs to be improved. This trial aimed at demonstrating whether PD-1 inhibitors followed by chemoradiotherapy could promote esophageal tumor vascular normalization, alleviate hypoxia, and thus enhance radiosensitivity and improve local control.
Methods
We did a multicenter, single-arm, phase 2 trial in China. Patients with locally advanced esophageal cancer were enrolled in this study. In induction phase, patients received two cycles of sintilimab, paclitaxel and carboplatin once per 21 days. In concurrent phase, patients were treated with five cycles of carboplatin and paclitaxel once per week concurrent with radiotherapy of 50.4Gy delivered in 28 fractions. The primary endpoint was 2-year local control rate. Hypoxia and vessel normalization was assessed before and after induction phase using immunofluorescence and perfusion CT. This trial is registered with ClinicalTrials.gov (NCT03985046).
Findings
Seventy-five patients with esophageal cancer were enrolled in this study between October 2019 and April 2021. The median follow-up of surviving patients was 33.6 months (IQR 29.3–35.7). The 2-year local control rate was 81.7% (95% confidence interval, 72.7%–90.7%), which was much higher than that in concurrent chemoradiation only (71.3%) in previous studies. Vascular normalization and hypoxia alleviation were observed in both biopsy specimens and perfusion CT.
Interpretation
The addition of induction immunotherapy to standard concurrent chemoradiotherapy could improve radiosensitivity for locally advanced esophageal cancer as non-surgical treatment. New treatment combination led to higher local control rate through promoting vascular normalization and alleviating hypoxia. Our findings suggest that induction immunotherapy followed by concurrent chemoradiotherapy could be a potential option in future treatment.
Funding
National Natural Science Foundation of China and Shanghai Rising-Star Program.
Keywords: Esophageal cancer, Chemoradiotherapy, Immunotherapy, Radiosensitivity
Research in context.
Evidence before this study
Since Tian et al. proposed in Nature that PD-1 inhibitors could promote vascular normalization and thereby overcoming hypoxia and improving radiosensitivity, immunotherapy followed by chemoradiotherapy has becoming a promising approach in the locally advanced esophageal cancer. We searched PubMed for studies published from database inception until May 15, 2023, with the search terms ((esophageal squamous cell carcinoma) AND (PD-1 or PD-L1)) AND (chemoradiotherapy). The search was restricted to clinical trials, with no language restrictions. Our search yielded seven studies, including three ongoing trials with a study protocol and four trials with published results. Three of the four published trials assessed the efficacy and feasibility of definitive chemoradiotherapy concurrent with immunotherapy in patients with locally advanced esophageal cancer and tried to clarify potential biomarkers. The fourth trial was a randomized, double-blind, phase II study that failed to demonstrate that adjuvant durvalumab improved survival after neoadjuvant chemoradiation in patients with esophageal cancer.
Added value of this study
To the best of our knowledge, this study is the first published trial of adding induction immunotherapy to standard concurrent chemoradiotherapy to improve radiosensitivity for locally advanced esophageal cancer as non-surgical treatment. This study showed the addition of induction immunotherapy to standard concurrent chemoradiotherapy could improve radiosensitivity for locally advanced esophageal cancer as non-surgical treatment. New treatment combination led to higher local control rate through promoting vascular normalization and alleviating hypoxia.
Implications of all the available evidence
Our results, together with existing evidence from basic studies, suggest that induction immunotherapy followed by concurrent chemoradiotherapy could be a potential option in future treatment in patients with locally advanced esophageal squamous cell carcinoma. Phase 3 randomized trials are needed to confirm the clinical benefits of this treatment pattern.
Introduction
Esophageal cancer is one of the most common cancers and was responsible for an estimated 604 100 new cases and 544,076 deaths in 2020.1 Concurrent chemoradiotherapy is the standard nonoperative treatment for locally advanced esophageal squamous cell carcinoma.2 However, the survival has not substantially improved since the establishment of RTOG-8501 study more than 20 years ago. Researchers made various researches in different perspectives, including different chemotherapy regimens,3,4 different radiation doses and fractionation,5 or adding targeted therapy during chemoradiotherapy6 and very few attempts showed improvements in survival. Local recurrence was still the main failure pattern, accounting for more than half of all treatment failures,7 indicating that the sensitivity of radiotherapy still needs to be improved.
In previous studies, hypoxia is directly related to radiosensitivity. Rapid tumor growth and pathological angiogenesis result in hypoxia, providing a microenvironment favorable for radioresistance and tumor growth. Hypoxia and pathological angiogenesis create a vicious circle, making hypoxia a difficult problem in antitumor therapy.8,9
Anti-angiogenic drugs inhibit the transduction of angiogenic signals through the VEGF pathway, trim abnormal blood vessels and remodel the remaining blood vessels, normalize blood vessels, and therefore overcome hypoxia. However, this effect is temporary and high dose of anti-angiogenic drugs lead to a rapid decrease in tumor blood perfusion through excessive vascular pruning, and thus aggravate hypoxia, which lead to worse prognosis.10
Immune checkpoint inhibitors play a landmark role in tumor treatment. In addition to enhancing antitumor immunity by restoring the function of cytotoxic T cells, immune checkpoint inhibitors can also remodel the tumor microenvironment through immune-related pathways. Tian et al. proposed in Nature that PD-1 inhibitors could promote vascular normalization through activating CD4+ T lymphocytes and thereby overcoming hypoxia.11 However, whether the mechanism above could improve radiosensitivity and local control and thus survival in patients is still unknown. Therefore, we designed a single-arm, multicenter, phase II clinical proof-of-concept study to demonstrate whether PD-1 inhibitor followed by chemoradiotherapy could promote esophageal tumor vascular normalization, overcome hypoxia, and thus enhance radiosensitivity.
Methods
This multicenter, single-arm, phase 2 trial of inductive sintilimab, paclitaxel and carboplatin followed by concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma was performed at four sites in China.
Participants
Patients were eligible for enrollment if they were aged between 18 and 75 years and had histologically confirmed esophageal squamous cell carcinoma of stage II to IVA (American Joint Committee on Cancer, 8th edition) without prior treatment. All patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate organ functions. Patients were excluded if they were diagnosed esophageal perforation or had a history of autoimmune disease. Full inclusion and exclusion criteria are included in the trial protocol.
Procedures
In induction phase, patients received two cycles of sintilimab (200 mg), paclitaxel (135 mg/m2) and carboplatin (area under the curve [AUC] 5; 5 mg/mL/min) on day 1 per 21 days.
In concurrent chemoradiotherapy phase, patients received radiotherapy regimen with photons (≥6 MV) to a total dose of 50.4Gy in 28 fractions (5 days per week at 1.8Gy/d) to planning target volume (PTV). The gross target volume (GTV) was defined as all known involved field. The superior and inferior borders of the clinical target volume (CTV) were 3 cm beyond the primary tumor along the esophagus. The lateral, anterior and posterior borders of the field were the same as the GTV. All borders of PTV were 1 cm beyond the CTV. Intensity modulated radiotherapy (IMRT) was required for all patients. Patients were treated with 5 cycles of carboplatin (AUC 2; 2 mg/mL/min) and paclitaxel (50 mg/m2) on day 1 every week in concurrent chemoradiotherapy phase.
The primary endpoint was 2-year local control rate. We defined local control as no evidence of esophagus or regional lymph node progression from physical or image examinations. The key secondary endpoints were overall survival and progression-free survival. The exploratory endpoints were perfusion CT-based and pathological tumor vessel normalization and hypoxia alleviation. All the patients were monitored for adverse events, according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
All the patients underwent baseline tumor staging, including contrast-enhanced CT (or Positron emission tomography–computed tomography (PET–CT)), abdomen ultrasound, barium swallow and endoscopic ultrasound. These examinations (except for endoscopic ultrasound) were repeated before and after chemoradiotherapy phase and every 3 months afterwards for disease evaluation.
Biopsy specimens of esophagus were derived through gastroscopy before and after induction phase if additional informed consent was obtained from enrolled patients. The immunofluorescent staining was used to detect α-SMA, CD31 and HIF-1α expression. Whole tissue sections cut from formalin-fixed paraffin-embedded (FFPE) tissue blocks were deparaffinized and rehydrated with serial passage through changes of xylene and graded ethanols. Antigen retrieval was performed in Target Retrieval Solution (TRS) citrate buffer (pH 6.0) using a pressure cooker. The slides were blocked with 10% goat serum for 30 min. Primary antibodies were incubated overnight at 4 °C in the blocking solution. Secondary antibodies were added into the blocking solution and incubated for 1 h at room temperature. Nuclei were stained with 1 μg/mL DAPI (4,6-diamidino-2-phenylindole; Thermo Fisher), for 2 min at room temperature. Excess antibodies were removed by washing for 5 min in PBS.
Primary antibodies included: anti-α-SMA (GB111364 from Servicebio), anti-CD31 (ab9498 from Abcam), anti-HIF-1α (ab51608 from Abcam); Secondary antibodies included: alexa-fluor-488 goat anti-mouse, alexa-fluor-594 goat anti-mouse, alexa-fluor-647 goat anti-rabbit (both from Thermo Fisher). Stained sections were visualized by a Nikon Eclipse C1 microscope using ×10, ×20 or ×40 objectives and scanned with Nikon DS-U3. The whole-section imaging was performed by an independent researcher who was blind to sample group allocation.
Hypoxia was quantified as HIF-1α+ cells divided by the DAPI+ cells. For pericyte coverage quantification, vessels were counted manually in three independent ∼0.4 mm2 fields at ×20, and percentage of vessels covered by pericytes = pixels of CD31+ cells attached by α-SMA+ cells/total pixels of CD31+ cells.
Body perfusion CT scan was performed before and after the induction phase if additional informed consent was obtained from enrolled patients. CT scanning was first performed without intravenous contrast to localize the tumor, and eight adjacent 5 mm sections at the level of tumor were selected for the following studies. In dynamic study, a high-pressure syringe was used for rapid bolus injection of 60 mL ioversol (iodine 320 mg/mL) followed by 40 mL saline through the cubital vein at a flow rate of 5 mL/s. After a delay of 6 s, dynamic scanning was performed on the selected sections with a total scanning duration of 43.26 s. The third series was performed with 30 mL ioversol (iodine 320 mg/mL) rapid injection through the cubital vein at a flow rate of 1.5 mL/s and the scanning duration was 4.27 s. The images were uploaded to a Siemens workstation for data analysis using syngo.CT Body Perfusion software. After automatic correction and de-noising, in order to derive perfusion parameters, a region of interest (ROI) is delineated from the aorta to obtain an arterial time–density curve. If the tumor location was too high for us to obtain a ROI of the aorta, the ROI was delineated from the carotid artery. The ROI of the tumor was manually selected under the guidance of a radiologist with extensive experience in radiology, which included as much solid part of the tumor as possible, while avoiding the esophageal lumen and paraesophageal fat. The following parameters were generated from the software: blood flow (BF), blood volume (BV), and flow extraction product (FEP). The above perfusion parameters were calculated in mean values of tumor ROI.
Statistical analysis
The sample size was calculated using PASS 2008 software. The primary endpoint is 2-year local control rate. According to the results of previous research (ESO-Shanghai2), the 2-year local control rate of concurrent chemoradiotherapy for esophageal cancer was 71.3% (95% CI 66.2%–76.3%).4 In this trial, the target 2-year local control rate was 84%. A minimum of 67 patients will be necessary to warrant a power of 80% at a one-sided α level of 0.05 to demonstrate the superiority of induction sintilimab and chemotherapy followed by concurrent chemoradiotherapy, assuming an accrual period of 12 months, a minimum follow-up period of 24 months. Therefore, sample size was 75 with 10% dropout rate.
Statistical analysis was performed using SPSS 22.0 software. Survival-related indicators were assessed by the Kaplan–Meier method. Paired t-test was plan to be used for paired analyses and described in protocol, Section 8 Statistical methods. However, due to concerns about normal distribution in a small sample size, Wilcoxon matched-pairs signed–ranks test was used for vessel normalization and hypoxia analysis. P values lower than 0.05 were considered statistically significant. Survival curves were drawn using Graphpad Prism 9.0.
The trial protocol was approved by the institutional review boards at each site, and the trial was done in accordance with the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent to participate in accordance with the Declaration of Helsinki. This trial is registered with ClinicalTrials.gov (NCT03985046).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Characteristics of the patients
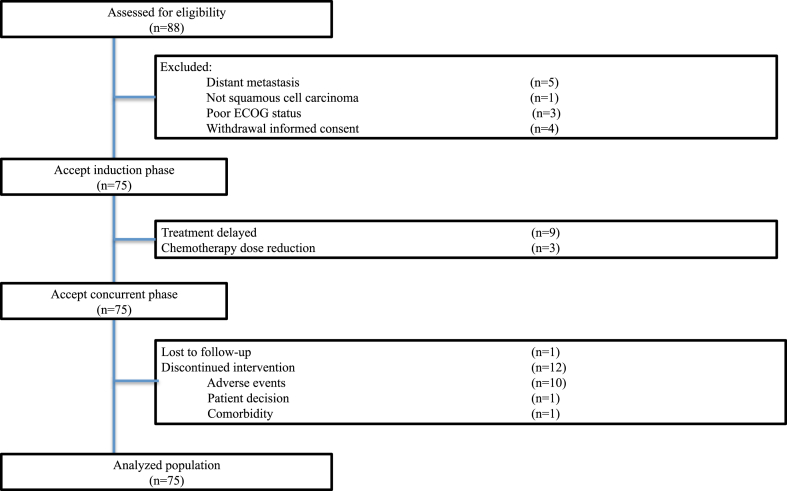
From October 2019 through April 2021, a total of 88 patients were assessed for eligibility, and 75 patients were enrolled at 4 centers (Fig. 1). Clinical characteristics were listed in Table 1. Most patients were male (74.7%) and median age of all patients was 65 years (IQR 60–69). The majority of patients were stage III and stage IVA (36.0% and 38.7%, respectively).
Fig. 1.
Trial profile. Eighty-eight patients with esophageal cancer were assessed for eligibility. Seventy-five patients were enrolled in this trial. Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 1.
Patient characteristics.
| Characteristics | Patients (n = 75) |
|---|---|
| Sex | |
| Male | 56 (74.7%) |
| Female | 19 (25.3%) |
| Age | 65 (60–69) |
| T Stage | |
| T2 | 31 (41.3%) |
| T3 | 20 (26.7%) |
| T4a | 6 (8.0%) |
| T4b | 18 (24.0%) |
| N Stage | |
| N0 | 0 |
| N1 | 38 (50.7%) |
| N2 | 27 (36.0%) |
| N3 | 10 (13.3%) |
| Stage at Diagnosis | |
| II | 19 (25.3%) |
| III | 27 (36.0%) |
| IVA | 29 (38.7%) |
| Tumor Location | |
| Cervical | 5 (6.7%) |
| Upper thoracic | 33 (44.0%) |
| Middle thoracic | 26 (34.7%) |
| Lower thoracic | 10 (13.3%) |
| Multiple | 1 (1.3%) |
| Tumor Length (cm) | 5 (3–8) |
| Reasons for no surgery | |
| Local extent of disease | 48 (64.0%) |
| Surgical contraindication | 14 (18.7%) |
| Patient refusal | 13 (17.3%) |
| Smoking History | |
| Yes | 52 (69.3%) |
| No | 23 (30.7%) |
| Drinking History | |
| Yes | 53 (70.7%) |
| No | 22 (29.3%) |
Continuous variables were shown in median (IQR) and categorical variables in n (%).
Treatment and compliance
All patients finished induction phase with 3 patients (4.0%) experienced dose reduction in chemotherapy and 9 patients (12.0%) delayed treatment.
Most patients (71 cases, 94.7%) received all radiotherapy and a total of 63 patients (84.0%) completed 5 cycles of concurrent chemotherapy per protocol. Radiation interruption and chemotherapy delay occurred in 14 patients (18.7%) and 8 patients (10.7%) in concurrent phase, respectively.
Outcomes
At the time of analysis on May 1st, 2023, the median follow-up time was 33.6 months (IQR 29.3–35.7). A total of 18 patients experienced loco regional recurrence and 2-year local control rate was 81.7% (95% CI 72.7%–90.7%), which was much higher than that in concurrent chemoradiotherapy only (71.3%) in previous studies.4
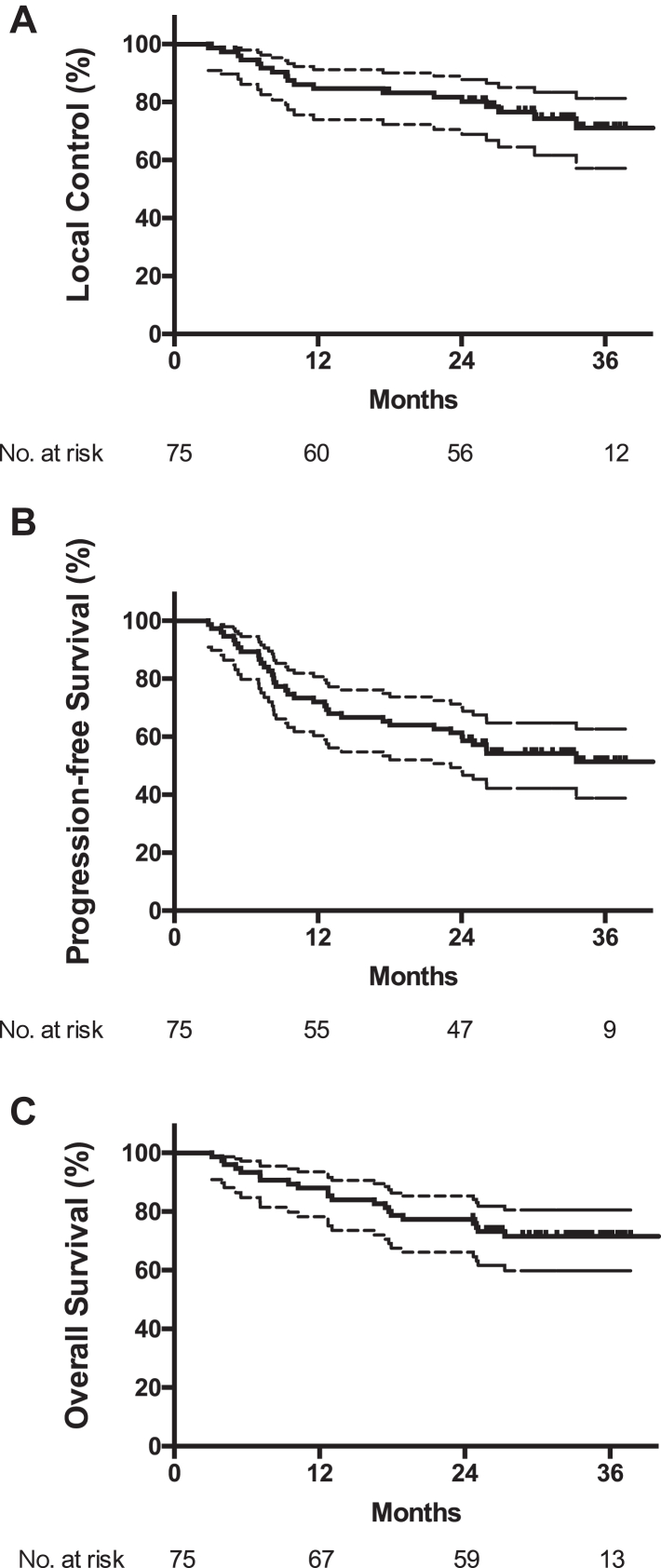
A total of 21 deaths (28.0%) were recorded and median overall survival was not reached. The 1- and 2-year OS rates were 88.0% (95% CI 80.6%–95.4%) and 77.3% (95% CI 67.9%–86.7%), respectively. The model-based estimation of median OS was 80.0 (95% CI 41.0–158.2) months. A total of 40 patients (53.3%) were alive without disease progression at the time of analysis and median PFS was not reached. The 1- and 2-year PFS rates were 72.0% (95% CI 61.8%–82.2%) and 61.3% (95% CI 50.3%–72.3%), respectively. The model-based estimation of median PFS was 33.7 (95% CI 22.9–48.3) months (Fig. 2). Patterns of treatment failure were shown in Table 2.
Fig. 2.
Local control rate (A), progression-free survival (B) and overall survival (C) in enrolled patients. Median overall survival and progression-free survival was not reached. Lower quartiles of progression-free survival and overall survival was 9.47 (95% CI 7.50–18.0) months and 25.0 (95% CI 12.7–NR) months, respectively.
Table 2.
Patterns of treatment failure.
| Type of Event | Patients (n = 75) |
|---|---|
| Live without treatment failure | 40 (53.3%) |
| Failure | 35 (46.7%) |
| Locoregional only | 15 (20.0%) |
| Distant only | 11 (14.7%) |
| Locoregional and distant | 3 (5.3%) |
| Second primary cancer | 1 (1.3%) |
| Toxicity-induced deatha | 4 (5.3%) |
| Sudden death for unknown reasonsb | 1 (1.3%) |
Data are n (%).
Two patients died of acute pneumonitis. One patient died of thrombocytopenia-induced hemorrhage. One patient died of laryngeal edema.
Sudden death without symptoms or image evidence of tumor progression or toxicities more than two years after the end of treatment.
Safety
All patients were included in safety analysis (Table 3). In induction phase, the most common treatment-related adverse events of any grade were anemia (45 cases, 60.0%), leukopenia (41 cases, 54.7%), neutropenia (36 cases, 48.0%) and thrombocytopenia (25 cases, 33.3%). The most common grade 3–5 adverse events were neutropenia (20 cases, 26.7%), leukopenia (11 cases, 14.7%), rash (3 cases, 4.0%), thrombocytopenia (2 cases, 2.7%) and anemia (1 case, 1.3%). In concurrent phase, the most common grade 3–5 treatment-related adverse events were also the hematologic toxic effects (leukopenia (35 cases, 46.7%), thrombocytopenia (21 cases, 28.0%), neutropenia (17 cases, 22.7%), and anemia (17 case, 22.7%)). Two patients died of acute pneumonitis. One patient died of thrombocytopenia-induced hemorrhage. One patient died of laryngeal edema.
Table 3.
Acute adverse events of patients by treatment phase.
| Induction Phase (n = 75) |
Concurrent Phase (n = 75) |
|||
|---|---|---|---|---|
| All grade | Grade 3–5 | All grade | Grade 3–5 | |
| Hematological | ||||
| Leukopenia | 41 (54.7%) | 11 (14.7%) | 69 (92.0%) | 35 (46.7%) |
| Neutropenia | 36 (48.0%) | 20 (26.7%) | 51 (68.0%) | 17 (22.7%) |
| Anemia | 45 (60.0%) | 1 (1.3%) | 72 (96.0%) | 17 (22.7%) |
| Thrombocytopenia | 25 (33.3%) | 2 (2.7%) | 67 (89.3%) | 21 (28.0%) |
| Nutritional | ||||
| Hypokalemia | 2 (2.7%) | 0 | 9 (12.0%) | 0 |
| Hyponatremia | 5 (6.7%) | 0 | 11 (14.7%) | 1 (1.3%) |
| Hypoalbuminemia | 3 (4.0%) | 0 | 17 (22.7%) | 0 |
| Gastrointestinal | ||||
| Nausea | 7 (9.3%) | 0 | 15 (20.0%) | 0 |
| Vomiting | 2 (2.7%) | 0 | 9 (12.0%) | 0 |
| Loss of appetite | 11 (14.7%) | 0 | 44 (58.7%) | 0 |
| Diarrhea | 1 (1.3%) | 0 | 0 | 0 |
| Constipation | 8 (10.7%) | 0 | 37 (49.3%) | 0 |
| Renal and hepatic | ||||
| AST increased | 3 (4.0%) | 0 | 4 (5.3%) | 0 |
| ALT increased | 6 (8.0%) | 0 | 6 (8.0%) | 0 |
| Total bilirubin increased | 1 (1.3%) | 0 | 4 (5.3%) | 0 |
| Creatine increased | 1 (1.3%) | 0 | 2 (2.7%) | 0 |
| Endocrinal | ||||
| Hyperthyroidism | 1 (1.3%) | 0 | 0 | 0 |
| Hypothyroidism | 2 (2.7%) | 0 | 0 | 0 |
| TSH increased | 6 (8.0%) | 0 | 0 | 0 |
| Mediastinal | ||||
| Hiccup | 0 | 0 | 20 (26.7%) | 0 |
| Hoarseness | 3 (4.0%) | 0 | 23 (30.6%) | 0 |
| Neurological | ||||
| Dizziness | 2 (2.7%) | 0 | 17 (22.7%) | 0 |
| Headache | 0 | 0 | 4 (5.3%) | 0 |
| Peripheral neuropathy | 1 (1.3%) | 0 | 16 (21.3%) | 0 |
| Arthralgia or myalgia | 9 (12.0%) | 0 | 4 (5.3%) | 0 |
| Pneumonitis | 3 (4.0%) | 0 | 40 (56.0%) | 3 (4.0%) |
| Radiation esophagitis | – | – | 67 (89.3%) | 4 (5.3%) |
| Radiation dermatitis | – | – | 37 (49.3%) | 0 |
| BNP increased | 21 (28.0%) | 0 | 17 (22.7%) | 0 |
| Fatigue | 9 (12.0%) | 0 | 36 (48.0%) | 1 (1.3%) |
| Hair loss | 17 (22.7%) | – | 51 (68.0%) | – |
| Rash | 11 (14.7%) | 3 (4.0%) | 0 | 0 |
Data are n (%).
Grade 5 toxicities were as follows:
Two patients died of acute pneumonitis.
One patient died of thrombocytopenia-induced hemorrhage.
One patient died of laryngeal edema.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; TSH, thyroid stimulating hormone; BNP, brain natriuretic peptide.
Pathological findings
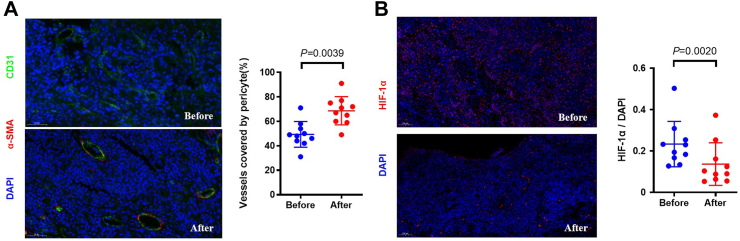
The vessel normalization and hypoxia were evaluated in 10 voluntary patients and results were shown in Fig. 3. After induction phase, the increased percentage of pericyte coverage was observed. (P = 0.0039) On the basis of tumor vessel normalization, hypoxia of tumor was also alleviated (P = 0.0020).
Fig. 3.
Induction immunotherapy increased vessel normalization and alleviated hypoxia. (A) Immunofluorescence quantification of vessel normalization with percentage endothelial cells (CD31+) attached by pericytes (α-SMA+) (B) Quantification of tumor hypoxia with HIF1α.
Functional imaging analysis
Before and after the induction phase, 10 voluntary patients underwent body perfusion CT evaluation on the basis of contrast-enhanced CT, and the results of PET-CT were also analyzed as follows.
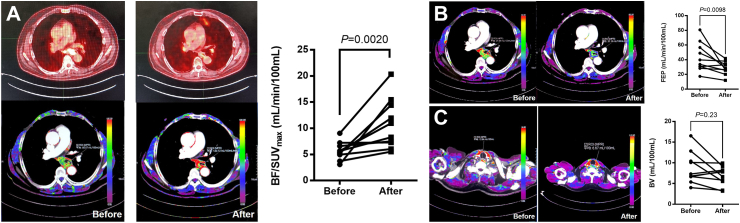
BF represented the volume of fresh blood through the tumor tissue in a unit of time, which could reflect the oxygen supply from vasculature to the tumor tissue. The standard uptake value (SUV) in PET-CT represented the metabolic intensity of the tumor and reflected the relative oxygen demand of the tumor. Here, we employed BF/SUVmax, which aimed to assess the improvement in the oxygen supply and demand balance. This indicator has been used in the previous literature and has a certain predictive effect on prognosis.12 In this study, BF/SUVmax increased significantly after immune induction (P = 0.0020), indicating that the supply and demand of oxygen in the tumor was significantly balanced (Fig. 4A).
Fig. 4.
Perfusion CT analysis. After induction immunotherapy, the supply and demand of oxygen in the tumor was significantly balanced (A) and the vessels in the tumor were significantly normalized (B) with not excessively pruned (C). Abbreviations: BF, blood flow; BV, blood volume; FEP, flow extraction product; SUV, standard uptake value.
FEP was the product of blood flow and extraction fraction, indicating the amount of contrast agent that reached a unit volume of tumor tissue in a unit of time and leaked into the extravascular space through the vasculature, which reflected the maturity of vessels. Compared with baseline, FEP showed significant decrease (P = 0.0098). The vessels in the tumor were significantly normalized after immune induction (Fig. 4B).
BV represented the blood volume in tumor tissue at a certain moment, which could reflect the proliferation status of the intratumoral vessels (whether normal or abnormal). In this study, BV did not change significantly (P = 0.23) after induction phase, which showed the vessels were not excessively pruned (Fig. 4C).
Discussion
In this study, with the application of PD-1 inhibitor, vascular normalization, hypoxia relief, and thus enhancing radiosensitivity were observed, and thus the locoregional failure rate was lower than that of chemoradiotherapy alone.
PD-1 inhibitors have now been widely used in advanced esophageal cancer improving the overall survival and progression-free survival of patients.13, 14, 15, 16, 17 Besides, more studies focused on neoadjuvant therapy, and the pathological complete response rates reported in various studies were inspiring18, 19, 20, 21, 22, 23 while CheckMate 577 study showed adjuvant immune checkpoint inhibitors led to longer disease-free survival in patients who had received neoadjuvant chemoradiotherapy.24 However, in non-surgical therapy for locally advanced esophageal cancer, the combination of immunotherapy and other treatment approaches is still being explored. Studies on concurrent immune-chemoradiotherapy or standard chemoradiotherapy followed by immunotherapy as maintenance therapy are in the enrollment, and the efficacy and safety remain to be followed up.25, 26, 27
As for the sequence of immunotherapy and chemoradiotherapy, researchers initiated some explorations. In Pacific study, immune checkpoint inhibitor maintenance therapy could improve progression-free survival and overall survival and thus immune checkpoint inhibitors became the standard maintenance treatment regimen after definitive chemoradiotherapy for non-small cell lung cancer patients.28 In terms of esophageal cancer, nivolumab adjuvant therapy also showed longer disease-free survival in patients with residue pathological disease after neoadjuvant chemoradiotherapy in CheckMate 577 study.24 However, the control groups were both placebo in Pacific study and CheckMate 577 study, which could only demonstrate the benefit of adding immune checkpoint inhibitors to definitive treatment rather than the sequence of immunotherapy and chemoradiotherapy. Moreover, the effect of immune checkpoint inhibitors rely on immune cells infiltration in the tumor microenvironment, but radiation exposure and surgical dissection both damage the tumor immune microenvironment and tumor-related lymph nodes. It is doubtful whether immune checkpoint inhibitors could play the best role with a decreased absolute number of immune cells and, therefore, whether immune checkpoint inhibitors maintenance therapy after definitive treatment is the best sequence is still debatable. Many studies changed the strategy and applied immune checkpoint inhibitors to neoadjuvant therapy followed by surgery.18, 19, 20, 21, 22, 23 However, there was no clinical study with immune checkpoint inhibitors as induction therapy followed by definitive chemoradiotherapy for esophageal cancer.
In pervious basic studies, activation of CD4+ T lymphocytes by immune checkpoint inhibitors increased vessel normalization through interferon-γ-related pathway and therefore hypoxia was reduced.11 Enhanced radiosensitivity was also observed after the application of immune checkpoint inhibitors.29 In this study, based on the results of the previous basic research, we tried to use immune checkpoint inhibitors as induction therapy to promote vascular normalization, relieve hypoxia, and enhancing radiosensitivity, thereby improving local control rates. The local control rate was higher than that of the concurrent chemoradiotherapy in previous studies. Although long-term survival was yet to be followed up, the new combination proposed a new approach to improve the radiosensitivity and reduce the local failure. In the mechanism explorations, the blood vessel normalization and the hypoxia relief suggested by basic experiments were verified in tumor tissues acquired before and after the application of immune checkpoint inhibitors from voluntary patients. At the same time, in order to avoid the under-representation problem caused by biopsy, we performed perfusion CT evaluation during the same period. The maturity of blood vessels has been observed, and the imbalance between the oxygen demand and supply has also been reversed. The relief of hypoxia laid the foundation for the improvement of local control rate.
In terms of the toxicity of this study, there was no immune-related grade 3 or above toxicity except for rash. The hematological toxicities were the most common grade 3–4 adverse events due to carboplatin application. Other regimens, FOLFOX or cisplatin/fluorouracil, could be considered in future exploration to avoid severe hematologic toxicity. In this study, two patients died of radiation pneumonitis. These grade 5 toxicities may be partly related to the addition of PD-1 inhibitors during the induction phase, which led to pulmonary interstitial changes and thus increased the incidence and severity of radiation pneumonitis. As for T4b patients, due to more adjacent structures invaded, the toxicities of radiotherapy tended to be more serious, which subsequently aggravated the toxicities of chemotherapy and immunotherapy in the combination as well. In the application of this new combination, it is necessary to be vigilant to the changes in the pulmonary interstitium during immunotherapy induction phase and following concurrent chemoradiotherapy phase, and more active monitoring and response are beneficial.
In this study, there are still some limitations. First, this study put forward the role of PD-1 inhibitors in promoting vascular normalization, relieving hypoxia, and thus enhancing radiosensitivity in humans. However, this study is a proof-of-concept single-arm study, and randomized studies are still needed. The primary endpoint was local control rate in this study to highlight the purpose of the study and long-term survival outcomes should be focused in future studies. Second, several fatal adverse events occurred in this study. In future studies, the selection of chemotherapy regimens and the prevention, monitoring and timely management of pneumonitis still need further improvement. Furthermore, only 10 patients performed vessel normalization and hypoxia analysis. We hope to enroll more samples for analysis in future research and further explore the mechanism.
To our knowledge, this study is the first published trial of adding induction immunotherapy to standard concurrent chemoradiotherapy to improve radiosensitivity for locally advanced esophageal cancer as non-surgical treatment. New treatment combination led to higher local control rate through promoting vascular normalization and relieving hypoxia. Our findings suggest that induction immunotherapy followed by concurrent chemoradiotherapy could be a potential option in future treatment.
Contributors
DA, SH, JY, and KZ were responsible for the conceptualization and design of the study. DA, SH and KZ were responsible for funding acquisition. DA, SH, WS, QW, YC, QL, JD, HZ, DG, YL, Zhi Zhang, GZ, Zhen Zhang, JY, and KZ enrolled and treated patients. DA, SH, WS, QW, SZ, KC, and JH collected data. DA, SH, WS, QW, MM, JY, and KZ were responsible for data analysis and interpretation. All authors were involved in writing and revising the manuscript. All authors participated in reviewing and editing of the manuscript, and approved the final version before submission. Four principal investigators (DA, SH, JY, and KZ) verified the raw data and had final responsibility for the decision to submit the manuscript for publication. DA and SH contributed equally.
Data sharing statement
Individual participant data are not publicly available because this requirement was not anticipated in the study protocol and sharing was not included in the ethical approvals.
Declaration of interests
We declare no competing interests.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82003033, 81872454 and U22A20326), and Shanghai Rising-Star Program (23YF1406700). We thank the patients and their families for making the study possible, the investigators, and the clinical study teams.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102471.
Contributor Information
Jinjun Ye, Email: jjye2004@163.com.
Kuaile Zhao, Email: kuaile_z@fudan.edu.cn.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Herskovic A., Martz K., al-Sarraf M., et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Ye J., Zhu Z., et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. J Clin Oncol. 2019;37:1695–1703. doi: 10.1200/JCO.18.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ai D., Ye J., Wei S., et al. Comparison of 3 paclitaxel-based chemoradiotherapy regimens for patients with locally advanced esophageal squamous cell cancer: a randomized clinical trial. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minsky B.D., Pajak T.F., Ginsberg R.J., et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 6.Crosby T., Hurt C.N., Falk S., et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 7.Cooper J.S., Guo M.D., Herskovic A., et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 8.Harris A.L. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 9.Viallard C., Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 10.Jain R.K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian L., Goldstein A., Wang H., et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh V., Engledow A., Rodriguez-Justo M., et al. The flow-metabolic phenotype of primary colorectal cancer: assessment by integrated 18F-FDG PET/perfusion CT with histopathologic correlation. J Nucl Med. 2012;53:687–692. doi: 10.2967/jnumed.111.098525. [DOI] [PubMed] [Google Scholar]
- 13.Kojima T., Shah M.A., Muro K., et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 14.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 15.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 16.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato K., Cho B.C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Zhao S., Zheng Y., et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1) Eur J Cancer. 2021;144:232–241. doi: 10.1016/j.ejca.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Yan X., Duan H., Ni Y., et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE) Int J Surg. 2022;103 doi: 10.1016/j.ijsu.2022.106680. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z., Ye J., Li H., et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: a prospective, single-arm, phase 2 trial. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1031171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan H., Shao C., Pan M., et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE) Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.849984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Yang Y., Liu Z., et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Ende T., de Clercq N.C., van Berge Henegouwen M.I., et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a single-arm phase II feasibility trial (PERFECT) Clin Cancer Res. 2021;27:3351–3359. doi: 10.1158/1078-0432.CCR-20-4443. [DOI] [PubMed] [Google Scholar]
- 24.Kelly R.J., Ajani J.A., Kuzdzal J., et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 25.Yu R., Wang W., Li T., et al. Rationale 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol. 2021;17:4081–4089. doi: 10.2217/fon-2021-0632. [DOI] [PubMed] [Google Scholar]
- 26.Shah M.A., Bennouna J., Doi T., et al. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol. 2021;17:1143–1153. doi: 10.2217/fon-2020-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bando H., Kotani D., Tsushima T., et al. TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer. 2020;20:336. doi: 10.1186/s12885-020-06716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 29.Hao S., Zhang X., Han L., et al. PD-1 inhibitor enhanced radiosensitivity by reactivating T cells and inducing G2/M phase arrest in esophageal squamous cell carcinoma. Radiat Res. 2022;198:458–466. doi: 10.1667/RADE-22-00061.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.