Abstract
Introduction and importance
The rising incidence of sickle-cell disease in European countries has led to an increase in associated complications. Osteomyelitis, a rare complication in non-traumatic adult cases, poses diagnostic challenges and presents treatment difficulties due to limited cases and studies.
Case presentation
A 23-year-old woman diagnosed with sickle-cell disease presented with a six-day fever and painful swelling in the left upper extremity persisting for a fortnight. She had no history of trauma but had experienced a previous episode of bacteremia due to Salmonella, four years prior. Magnetic resonance imaging revealed an intramedullary bone injury with cortical rupture extending into soft tissues, forming a collection that raised clinical suspicion of osteomyelitis, despite negative blood and aspirate cultures. Empiric antibiotic therapy was initiated, followed by surgical debridement of infected tissues. The resulting dead space was filled with antibiotic-coated calcium phosphate beads and tissue grafting. Anatomopathological studies confirmed findings consistent with chronic osteomyelitis. Stabilization of the arm was achieved with an orthopedic brace, and antibiotic administration continued for 6 weeks post-surgery. The injury consolidated 4 months after treatment, and nearly two years later she has not suffered a recurrence.
Clinical discussion
The scarcity of literature implies the absence of clinical guidelines for treating osteomyelitis in these patients. Empirical antibiotic therapy combined with surgery when there are abscesses that need debridement can be an effective approach.
Conclusion
Humeral osteomyelitis in sickle-cell disease patients can be effectively managed using a pharmaco-surgical strategy, but it should be tailored to the patient's needs.
Keywords: Sickle-cell disease, Osteomyelitis, Empiric antibiotic therapy, Surgical debridement, Case report
Highlights
-
•
Humeral osteomyelitis is a rare complication in sickle-cell disease patients.
-
•
Empiric antibiotic therapy should be a first option when the condition is suspected in these patients.
-
•
A combined strategy with a surgical approach is necessary when abscess formation occurs.
-
•
External fixation may not be necessary when there is sufficient bone quality and structural integrity.
1. Introduction
Sickle cell disease (SCD) stands out as the most prevalent autosomal recessive structural hemoglobinopathy globally. While it is particularly common in regions such as Sub-Saharan Africa, tropical areas of Asia, and South and Central America, current migratory patterns have facilitated its spread to new countries [1]. The pathology is distinguished by the characteristic sickle-like shape assumed by erythrocytes. This morphology significantly heightens the likelihood of red blood cells becoming trapped in slow-flowing blood vessels, resulting in vaso-occlusion. This results in a functionally asplenic condition, increasing the risk of infections [2].
This disease can have a detrimental impact in bone tissue, leading to complications such as osteomyelitis (OM), septic arthritis and osteonecrosis [3]. The similarity in symptoms among these conditions, including vague bone pain, soft tissue edema, skin erythema and localized tenderness to palpation, poses a challenge for differentiation and accurate differential diagnosis [2]. A study conducted in France between 2015 and 2017 on osteoarticular complications in patients with SCD identified osteoarticular pain as the primary reason for consultation (83.8 %). While these complications predominantly affect children and lower extremities, with the knee (16.2 %) and hip (10.8 %) as the most frequently affected joints, they can manifest in individuals of all ages and in diverse locations [4].
OM represents a significant complication in patients with SCD. The geographical location has a dual impact, affecting both its incidence, with a higher rate in countries facing challenges in maintaining hygienic conditions, and the causative organisms [5]. In the United States and Europe gram-negative bacteria, especially Salmonella and Escherichia coli, predominate, while Staphylococcus aureus is the most common pathogen in sub-Saharan Africa and the Middle East [1,2]. Although it may be resolved with antibiotic therapy alone, unresponsiveness to it or the presence of soft tissue abscesses or necrotic bone could lead to a combined strategy involving surgical debridement [3].
Despite being a condition with low frequency in our clinical setting, there is a pressing need for more in-depth clinical and research attention, given the anticipated increase in cases in the coming years. This article has been reported in line with the SCARE criteria [6].
2. Presentation of case
This report describes the case of a 23-year-old woman from Ghana who presented to the emergency room with a six-day fever and painful swelling in the left upper extremity. Her discomfort had begun two weeks before, with slight pain and mild joint swelling, which had progressively increased in size and intensity since its onset. She denied any history of trauma, and the examination revealed no injuries, but there was an increase in local temperature. She had a prior diagnosis of SCD, which was under control with treatment, and she had presented an episode of bacteremia due to Salmonella in 2019. She reported no known drug allergies or toxic habits.
Upon admission, a routine blood test was conducted, revealing an elevated level of C-Reactive Protein (CRP) at 46.8 mg/L. Empiric antibiotic therapy was initiated with 2 g of ceftriaxone every 24 h while awaiting the results of a blood culture, which turned out to inconclusive. Given the clinical suspicion of bacterial OM, the treatment regimen was supplemented with 600 mg of clindamycin every 8 h, along with analgesics to address symptoms. This pharmacological protocol was administered during 27 days.
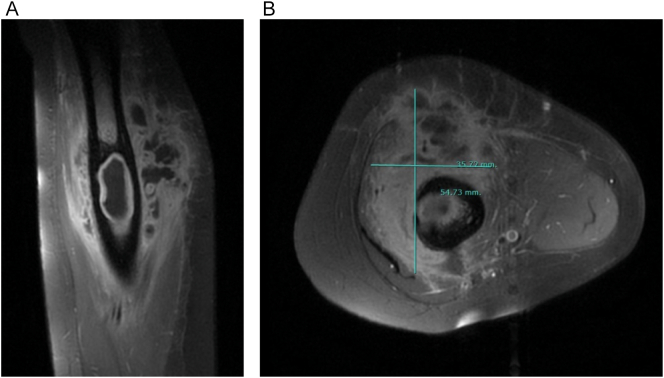
An X-ray of the left humerus (Fig. 1) revealed a lytic lesion in the middle third of the diaphysis. Subsequently, an ultrasound of the left upper extremity was conducted, indicating a collection suggestive of abscess versus hematoma. During this procedure, a fine-needle aspirated puncture was carried out under ultrasound guidance, yielding non-diagnostic results. Thus, the study was extended with an MRI, which showed an intramedullary bone injury with cortical rupture, extending into soft tissues and forming a collection indicative of a substantial area of OM and its extraosseous extension (Fig. 2).
Fig. 1.
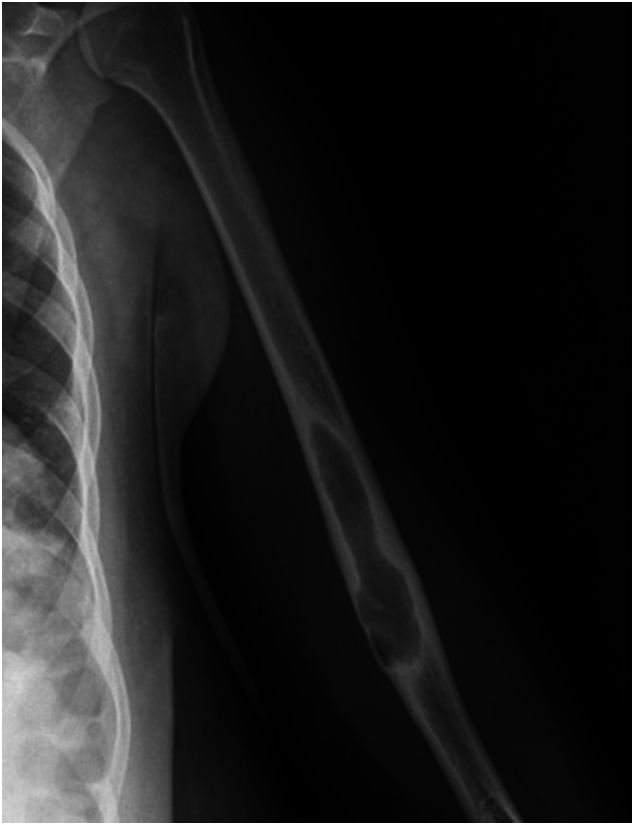
Initial radiograph showing lysis in the middle third of the humeral diaphysis.
Fig. 2.
Humeral MRI. A) Sagittal slice revealing intraosseous and soft tissue collection. B) Axial slice with the measurement (mm) of soft tissue collection.
Based on these findings, a surgical procedure was deemed necessary. Considering the location of the lesion, and to achieve proper exposure of the posterior and lateral surfaces of the humerus, as well as good visualization of the radial nerve, a lateral approach to the brachial region was performed. Both the radial and posterior antebrachial cutaneous nerves were identified to prevent iatrogenic injuries, and the condition of the surrounding soft tissue was assessed in situ (Fig. 3) [7]. The soft tissue abscess was debrided, and the humeral bone was exposed (Fig. 4). Then, an osteotomy was performed with the opening of the bone cavity, during which part of the affected bone tissue was removed. Endomedullary curettage, followed by thorough washing, was carried out until all infected tissue was resected (Fig. 5). The bone defect was filled with calcium phosphate beads (Stimulan, Biocomposites Ltd., Keele, UK) impregnated with vancomycin and gentamycin for local antibiotic administration, that were also placed in the surrounding soft tissues (Fig. 6). To conclude, a tissue graft was applied in the area, and after skin closure, a plaster splint was placed. Although microbiological analyses of intraoperatively collected osseous and soft tissues samples yielded negative results, their anatomopathological study indicated findings consistent with chronic OM, which ultimately allowed us to reach the diagnosis.
Fig. 3.
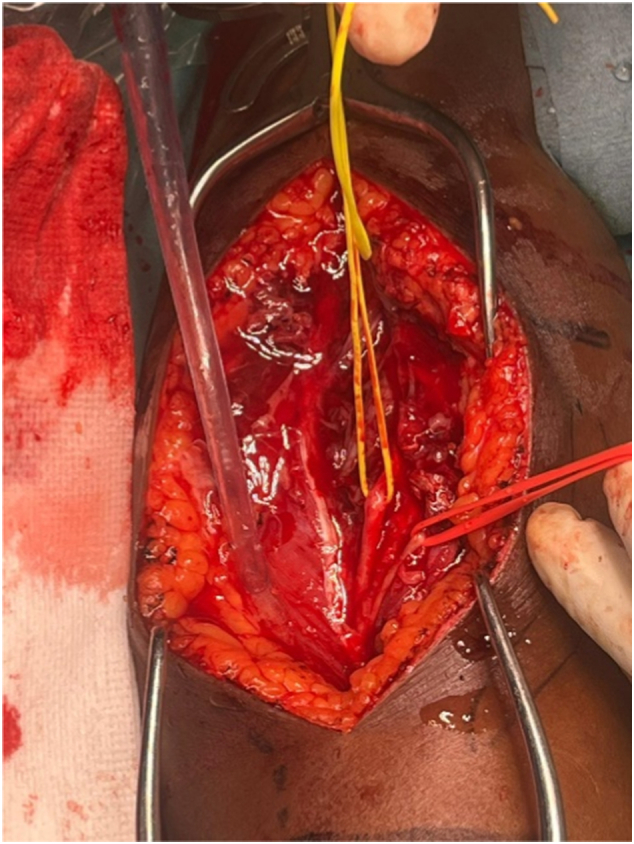
Identification of nerve structures during boarding. The condition of the soft tissues due to OM can be observed.
Fig. 4.
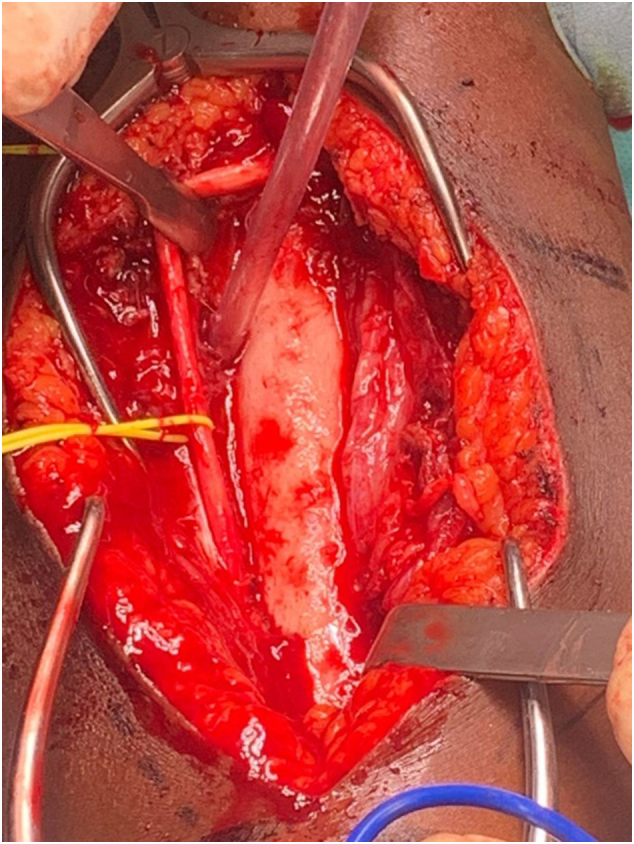
Exposure of the humeral bone following soft tissue debridement.
Fig. 5.
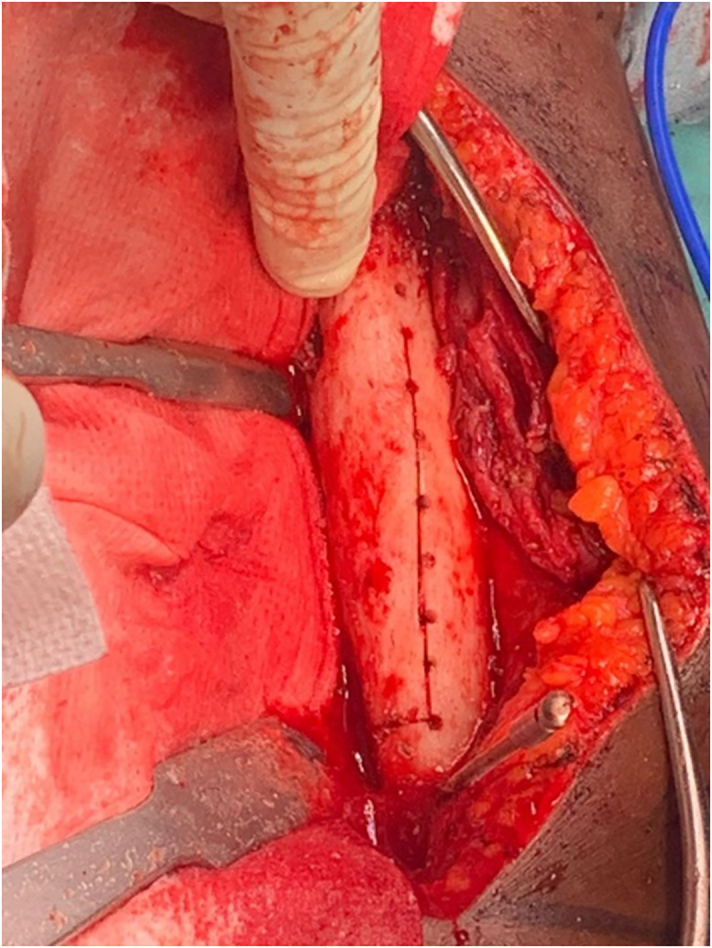
Humeral osteotomy after soft tissue debridement.
Fig. 6.
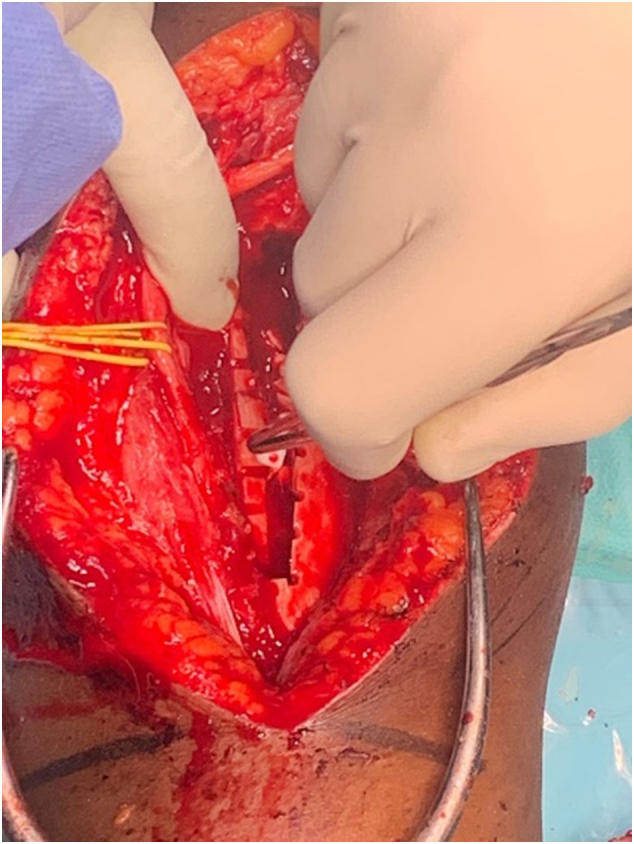
Filling of the bone defect with calcium phosphate beads impregnated with vancomycin and gentamycin for local antibiotic administration.
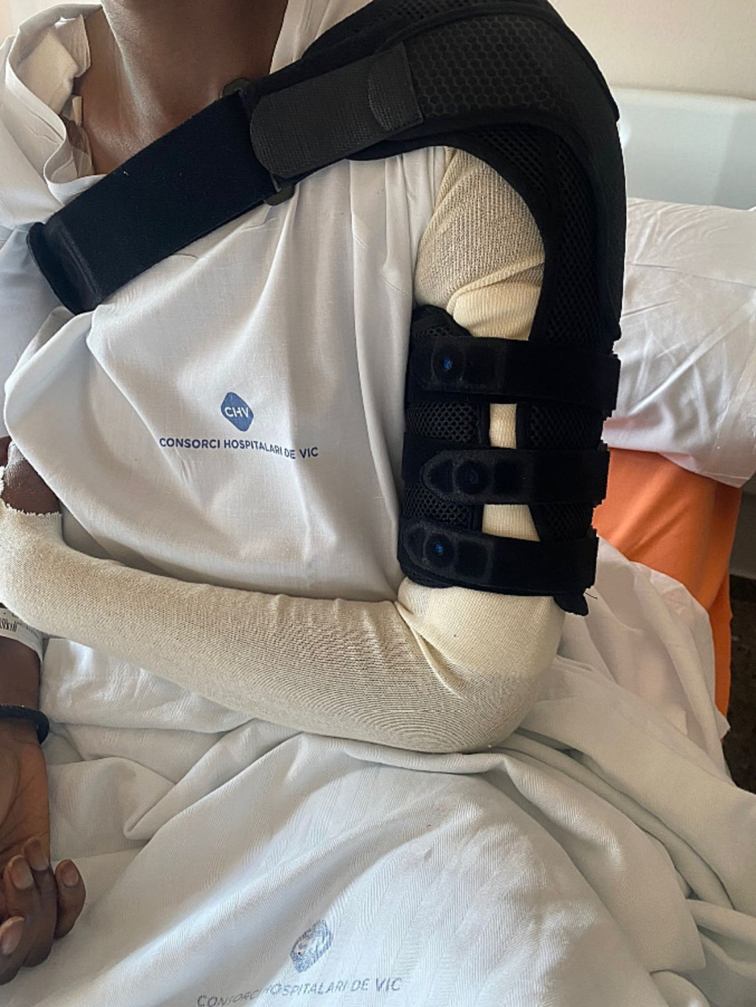
On the seventh day post-surgery the splint was removed for wound inspection that revealed satisfactory progress. The neurovascular state was also examined revealing its preservation except for a mild paresthesia around the posterior antebrachial cutaneous nerve. Four days later, the plaster splint was exchanged for an orthopedic brace (Fig. 7).
Fig. 7.
Definitive humerus immobilization with an orthopedic brace.
The patient was discharged on the twelfth postoperative day due to her favorable clinical and radiological progress (Fig. 8). She was prescribed with oral antibiotic therapy (750 mg of levofloxacin every 24 h for eight weeks) and scheduled for outpatient follow-up. Depending on her ongoing progress, she could be submitted for a second surgical intervention.
Fig. 8.
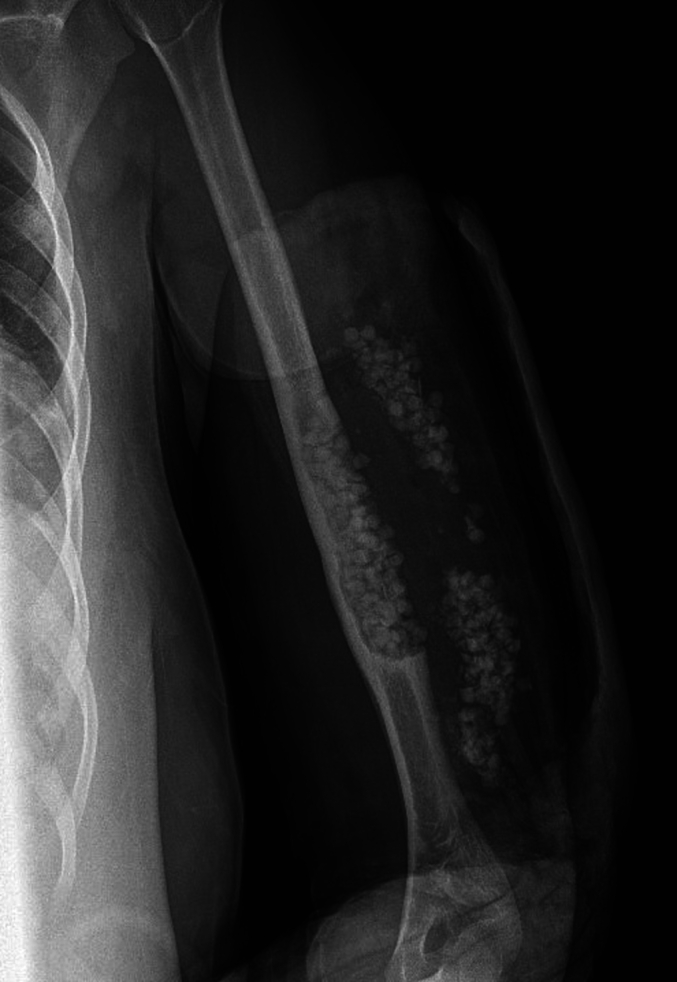
Postoperative radiograph. Calcium phosphate beads can be observed filling the bone defect and around soft tissues.
During her hospitalization and preceding the surgery, the patient was transfused on two occasions totaling four units of red blood cell concentrates due to anemia secondary to her underlying pathology. In the postoperative period, no additional transfusions were necessary.
At 4 months post-intervention, the injury had consolidated, and by 6 months, the patient reported complete resolution of symptoms and a full recovery of functionality and joint balance. However, this outcome was not clinically verified using official questionnaires, as they are not part of our clinical practice. At 22 months after being treated in our center, the patient has not experienced any recurrence, indicating a complete resolution of her OM.
3. Discussion
In 2021, around 515,000 people were born with SCD, accounting for 0.0036 % of an estimated 140 million births [8,9]. In Spain, we have observed a surge in cases of SCD, which is linked to the rising immigration from Africa and South America, with an estimated 1200 cases in our country last year [10]. Although the susceptibility of these patients to bacterial infections is higher than that of the rest of the population, OM in SCD individuals is considered a rare complication, with between 0.5 and 16 % of them experiencing it throughout their lives [2,[11], [12], [13]]. Our case, the first to be treated in our center, presented a diagnostic and therapeutic challenge from the outset due to its low prevalence, associated with the scarcity of specific literature and its occurrence in an unusual anatomical region.
The differential diagnosis of OM compared to other osteoarticular conditions with similar symptoms can be very challenging. Blood cultures are often inconclusive, while detection solely through imaging tests can be imprecise, and there is a low correlation between both diagnostic batteries. Since the gold standard for OM diagnosis is the histopathological study of the affected tissue, patients are frequently diagnosed well after undergoing surgical treatment. Pathologies considered plausible at the onset of this case included – in addition to OM – bone infarction, bone neoplasia, and chronic nonbacterial osteomyelitis (CNO) [2,13,14].
Both clinical evaluations and microbiological analyses of blood cultures did not enable us to rule out any pathology, a common occurrence in this condition, as reported in studies such as the pediatric series of Kao et al. (2020) [15]. They reported 20 OM cases among over 3000 SCD patients in a decade, with 6 of them presenting uni- or multifocal involvement in the humerus. The causative agent of the infection could only be determined in two cases.
Some authors assert that lytic changes resulting from OM can be observed in the bone through radiograph from two weeks after the onset of infection, and ultrasound allows for the detection of acute extraosseous infection signs. Indeed, our patient's X-ray revealed a lytic lesion, and the ultrasound indicated an abscess, but they did not allow us to define a differential diagnosis. Therefore, we turned to MRI, which, with a sensitivity close to 100 %, allowed us to approach the presumptive diagnosis of bacterial OM due to findings compatible with a large osteomyelitic area with extraosseous extension [3,14,16,17].
Given the known challenge of identifying the causative bacterial agent of OM, its optimal treatment involves a combination of drugs that offer appropriate antimicrobial coverage. Although the existing literature on this matter is somewhat limited, there appears to be a consensus on the choice of empirical therapy, starting as soon as possible to prevent bone sequelae, using drugs effective against S. aureus, Salmonella, and other gram-negative bacilli [2,3,13,18].
Isolated pharmacological treatment is indicated in cases of acute hematogenous OM before abscess formation, but later stages of the pathology may require a combined strategy with surgery. This was the case with our patient, for whom we initially prescribed a first-line antibiotic considering her previous Salmonella infection and underlying condition. The regimen turned broad-spectrum when we had a clinical suspicion of OM, initiating empiric antibiotic therapy before obtaining adequate culture samples, a practice that some authors do not recommend. Despite this, our patient's evolution was satisfactory [3,19,20].
While the surgical treatment strategy for the condition in SCD patients is not standardized, it is generally accepted that OM treatment in long bones should include debridement of the affected tissues, obliteration of the resulting dead space, and the maintenance of anatomical stability [19]. Thus, we implemented this strategy adapting it to the specific needs of our patient.
The goal of debridement is to achieve a viable vascularized environment by eliminating sequestration and resecting infected bone and soft tissues, which act as foreign material. Some surgeons recommend performing debridement until healthy bone is obtained, while others suggest carrying out an osteotomy of the entire affected area. In our patient, we performed debridement of the soft tissues combined with an osteotomy to ensure the complete removal of diseased tissues [21].
Proper debridement can result in a large dead space that must be managed to prevent recurrence and significant bone loss, potentially leading to instability; conversely, insufficient debridement is associated with high recurrence rates. Various strategies, including bone grafts, pedunculated muscle flap grafts with skin grafting, bone transport techniques, and more recently the Masquelet technique – which has showed promising results – can be employed to fill the new space. In our patient's case, absorbable calcium phosphate beads coated with antibiotics were utilized due to their local antibacterial effect in both bone and soft tissues, coupled with their efficacy in defect filling, building upon the positive clinical outcomes observed by the surgical team in previous cases [3,22,23].
To maintain stability, some authors propose the use of external fixators as a fundamental component of treatment [24]. Despite being part of our preoperative plan, this was dismissed due to the satisfactory bone quality and sufficient integrity of the humeral structure following curettage, and it was considered that the remaining healthy bone tissue was sufficient to allow immobilized management of a plaster orthosis.
Humeral OM cases in patients with SCD are extremely limited in the literature, making it challenging to establish uniform guidelines for their clinical treatment. As far as the authors could ascertain, only two previous case reports detail therapeutic strategies in SCD patients with a primary diagnosis of OM in the humerus. Ghazanfar et al. (2021) [25] treated a 28-year-old woman with involvement of both proximal and distal humerus, while Saturveithan et al. (2014) [26] treated a one-year-old child with involvement of the proximal humerus, both with undetectable infection in synovial samples. In both cases, antibiotic treatment began empirically due to fever or clinical suspicion of OM after surgical lavage, and it was later adjusted to ceftriaxone and ampicillin upon the isolation of Salmonella in blood culture and intraoperative samples, respectively. The favorable outcome for both cases followed two arthroscopic drainage and debridement procedures, accompanied by six weeks of antibiotic therapy. Our therapeutic strategy followed the same principles as theirs, saving the differences related to each case.
Up until this date, our patient has been recovering satisfactorily. Clinical follow-up examinations will be closely monitored in case of progression of the pathology.
4. Conclusion
We presented a rare of humeral osteomyelitis in a sickle-cell disease patient, which we managed with favorable outcomes using a pharmaco-surgical strategy adapted to the needs of our patient.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Considering that the article is a clinical case, not classified as research under Spanish data protection regulations, permission from the Ethics Committee of the centre was not sought. The case report includes only retrospectively collected information about the medical team's standard clinical practice. The patient did provide informed consent for the publication of this article.
Funding
None.
Author contribution
Pablo Viñuales: Methodology, Investigation, Data curation, Writing – Original draft. Paola Andrea Hortua: Methodology, Investigation, Data curation, Writing – Original draft. Jordi Zafra: Investigation, Data curation, Writing - Original draft. Ramón Clos: Conceptualization, Methodology. Jordi Villalba: Writing – Review & Editing, Supervision. All authors have read and approved the final manuscript.
Guarantor
Jordi Villalba.
Declaration of competing interest
The authors report no Conflict of Interest Statement.
Acknowledgements
We would like to express our appreciation to Dr. Xavier Gimeno for his dedication, both in the treatment of the patient and in providing assistance for the completion of this work. We also extend our gratitude to Mr. Ramón Anfruns for generously providing most of the surgical images. Finally, we thank María Rabanal and Pablo Roza of the MBA Institute and the MBA Institute Medical and Biomechanical Research Chair at the University of Oviedo, for their technical support in formatting and reviewing the manuscript.
Contributor Information
Pablo Viñuales, Email: pvinualesn@chv.cat.
Paola Andrea Hortua, Email: phortua@chv.cat.
Jordi Zafra, Email: jzafra@chv.cat.
Ramón Clos, Email: rclos@chv.cat.
Jordi Villalba, Email: jvillalbamodol@gmail.com.
References
- 1.P. Reparaz, I. Serrano, R. Adan-Pedroso, I. Astigarraga, J. de Pedro Olabarri, A. Echebarria-Barona, M. Garcia-Ariza, R. Lopez-Almaraz, R.A. del Orbe-Barreto, M. Vara-Pampliega, P. Gonzalez-Urdiales, Clinical management of the acute complications of sickle cell anemia: 11 years of experience in a tertiary hospital, An. Pediatría (English Ed. 97 (2022) 4–11. doi: 10.1016/j.anpede.2022.06.002. [DOI] [PubMed]
- 2.M.R.D. Alex George, Michael R Debaun, Acute and chronic bone complications of sickle cell disease, (2022). https://www.uptodate.com/contents/acute-and-chronic-bone-complications-of-sickle-cell-disease?search=drepanocitosis y osteomielitis&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1#H2090111181.
- 3.Al Farii H., Zhou S., Albers A. Management of Osteomyelitis in sickle cell disease: review article. JAAOS Glob. Res. Rev. 2020;4 doi: 10.5435/JAAOSGlobal-D-20-00002. e20.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diakité S.M.A.A., Dembélé A., Cissé M.E., Kanté M., Coulibaly Y., Maïga B., Diakité F.L., Issa A., Doumbia A.K., Coulibaly O., Diall A., Togo P., Sacko K., Konaté D., Sanogo A., Traoré I., Doumbia A., Ahamadou I., Coulibaly Y.A., Dembélé G., Dicko F. To, Complications Ostéoarticulaires de la Drépanocytose au Département de Pédiatrie du CHU Gabriel Touré. Heal. Sci. Dis. 2019;20 https://www.hsd-fmsb.org/index.php/hsd/article/view/1474?articlesBySameAuthorPage=2 [Google Scholar]
- 5.Délicat-Loembet L.M., Baraïka M.A., Bougoudogo F., Diallo D.A. Bacterial infection in the sickle cell population: development and enabling factors. Microorganisms. 2023;11:859. doi: 10.3390/microorganisms11040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A., The S.C.A.R.E. Guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109(2023):1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassiouny Y., Elgohary H.A. Lateral approach to the humeral shaft: approach for special situations. Egypt. Orthop. J. 2016;51:180. doi: 10.4103/1110-1148.203153. [DOI] [Google Scholar]
- 8.Thomson A.M., McHugh T.A., Oron A.P., Teply C., Lonberg N., Tella V. Vilchis, Wilner L.B., Fuller K., Hagins H., Aboagye R.G., Aboye M.B., Abu-Gharbieh E., Abu-Zaid A., Addo I.Y., Ahinkorah B.O., Ahmad A., AlRyalat S.A.S., Amu H., Aravkin A.Y., Arulappan J., Atout M.M.W., Badiye A.D., Bagherieh S., Banach M., Banakar M., Bardhan M., Barrow A., Bedane D.A., Bensenor I.M., Bhagavathula A.S., Bhardwaj P., Bhardwaj P.V., Bhat A.N., Bhutta Z.A., Bilalaga M.M., Bishai J.D., Bitaraf S., Boloor A., Butt M.H., Chattu V.K., Chu D.-T., Dadras O., Dai X., Danaei B., Dang A.K., Demisse F.W., Dhimal M., Diaz D., Djalalinia S., Dongarwar D., Elhadi M., Elmonem M.A., Esezobor C.I., Etaee F., Eyawo O., Fagbamigbe A.F., Fatehizadeh A., Force L.M., Gardner W.M., Ghaffari K., Gill P.S., Golechha M., Goleij P., Gupta V.K., Hasani H., Hassan T.S., Hassen M.B., Ibitoye S.E., Ikiroma A.I., Iwu C.C.D., James P.B., Jayaram S., Jebai R., Jha R.P., Joseph N., Kalantar F., Kandel H., Karaye I.M., Kassahun W.D., Khan I.A., Khanmohammadi S., Kisa A., Kompani F., Krishan K., Landires I., Lim S.S., Mahajan P.B., Mahjoub S., Majeed A., Marasini B.P., Meresa H.A., Mestrovic T., Minhas S., Misganaw A., Mokdad A.H., Monasta L., Mustafa G., Nair T.S., Swamy S. Narasimha, Nassereldine H., Natto Z.S., Naveed M., Nayak B.P., Noubiap J.J., Noyes T., Nri-ezedi C.A., Nwatah V.E., Nzoputam C.I., Nzoputam O.J., Okonji O.C., Onikan A.O., Owolabi M.O., Patel J., Pati S., Pawar S., Petcu I.-R., Piel F.B., Qattea I., Rahimi M., Rahman M., Rawaf S., Redwan E.M.M., Rezaei N., Saddik B., Saeed U., Sharif-Askari F. Saheb, Samy A.M., Schumacher A.E., Shaker E., Shetty A., Sibhat M.M., Singh J.A., Suleman M., Sunuwar D.R., Szeto M.D., Tamuzi J.J.L., Tat N.Y., Taye B.T., Temsah M.-H., Umair M., Tahbaz S. Valadan, Wang C., Wickramasinghe N.D., Yigit A., Yiğit V., Yunusa I., Zaman B.A., Zangiabadian M., Zheng P., Hay S.I., Naghavi M., Murray C.J.L., Kassebaum N.J. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023;10:e585–e599. doi: 10.1016/S2352-3026(23)00118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statistics Times World death and birth rate. 2021. https://statisticstimes.com/demographics/world-death-and-birth-rate.php
- 10.y Hemoterapia S.E. de H. CONSALUD: Unas 1.200 personas en España sufren la enfermedad de células falciformes. 2022. https://www.sehh.es/noticias/101359-la-sehh-es-noticia/125222-consalud-unas-1-200-personas-en-espana-sufren-la-enfermedad-de-celulas-falciformes
- 11.Ochocinski D., Dalal M., Black L.V., Carr S., Lew J., Sullivan K., Kissoon N. Life-threatening infectious complications in sickle cell disease: a concise narrative review. Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Salem A.H., Ahmed H.A., Qaisaruddin S., Al-Jam’a A., Elbashier A.M., Al-Dabbous I. Osteomyelitis and septic arthritis in sickle cell disease in the eastern province of Saudi Arabia. Int. Orthop. 1992;16 doi: 10.1007/BF00189627. [DOI] [PubMed] [Google Scholar]
- 13.Rothman J.A. Osteomyelitis in sickle cell disease: you know it when you see it? Pediatr. Blood Cancer. 2020;67 doi: 10.1002/pbc.28585. [DOI] [PubMed] [Google Scholar]
- 14.Fritz J.M., McDonald J.R. Osteomyelitis: approach to diagnosis and treatment. Phys. Sportsmed. 2008;36 doi: 10.3810/psm.2008.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao C.M., Yee M.E., Maillis A., Lai K., Bakshi N., Rostad B.S., Jerris R.C., Lane P.A., Yildirim I. Microbiology and radiographic features of osteomyelitis in children and adolescents with sickle cell disease. Pediatr. Blood Cancer. 2020;67 doi: 10.1002/pbc.28517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delicat Loembet L.M., Mabiala A.D.N., Bisvigou U., Avoune E., Bongo S., N’Tchoreret A., Kouoyo A., Ondo A., Dokekias A., Koko J., Ategbo S. Diagnosis of sickle cell disease in Gabon using sickle SCAN®: a point-of-care blood test. Int. J. Transl. Med. Res. Public Heal. 2023;6 doi: 10.21106/ijtmrph.315. [DOI] [Google Scholar]
- 17.Lee Y.J., Sadigh S., Mankad K., Kapse N., Rajeswaran G. The imaging of osteomyelitis. Quant. Imaging Med. Surg. 2016;6:184–198. doi: 10.21037/qims.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martí-Carvajal A.J., Agreda-Pérez L.H. Antibiotics for treating osteomyelitis in people with sickle cell disease. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.CD007175.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenny J.-Y., Gaudias J. Principios del tratamiento de la infección ósea. EMC - Técnicas Quirúrgicas - Ortop. y Traumatol. 2013;5:1–10. doi: 10.1016/S2211-033X(13)65973-3. [DOI] [Google Scholar]
- 20.Larry M.T.V.-P., Bush M. Infecciones por Salmonella no tifoidea, Man. MSD. 2022. https://www.msdmanuals.com/es-es/professional/enfermedades-infecciosas/bacilos-gramnegativos/infecciones-por-salmonella-no-tifoidea
- 21.Gallego-Goyanes A., Caeiro-Rey J.R. Tratamiento de la osteomielitis crónica de tibia: a propósito de un caso y revisión bibliográfica. Rev. Colomb. Ortop. y Traumatol. 2017;31:41–45. doi: 10.1016/j.rccot.2017.01.005. [DOI] [Google Scholar]
- 22.Ilyas A.M., Mudgal C.S. Management of Medullary Osteomyelitis of the Humerus. Tech. Hand Up. Extrem. Surg. 2008;12:144–149. doi: 10.1097/BTH.0b013e31816d1fa5. [DOI] [PubMed] [Google Scholar]
- 23.Lew D.P., Waldvogel F.A. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 24.Mohd Yusof N., Saleh A.K., Abuomira I.E.A.A., Attallah A.A., Elshal E.A., Abdelhalem A., Khames A. Mono-lateral external fixation for treatment of femoral osteomyelitis. Orthop. Res. Rev. 2022;14:437–443. doi: 10.2147/ORR.S383863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghazanfar H., Nawaz I., Fortuzi K., Tieng A., Franchin G. A rare case of concomitant septic arthritis, osteomyelitis, and pyomyositis caused by Salmonella. Cureus. 2021 doi: 10.7759/cureus.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.C S., A A., G P., N S. Salmonella osteomyelitis in a one year old child without sickle cell disease: a case report. Malaysian Orthop. J. 2014;8:52–54. doi: 10.5704/MOJ.1407.005. [DOI] [PMC free article] [PubMed] [Google Scholar]