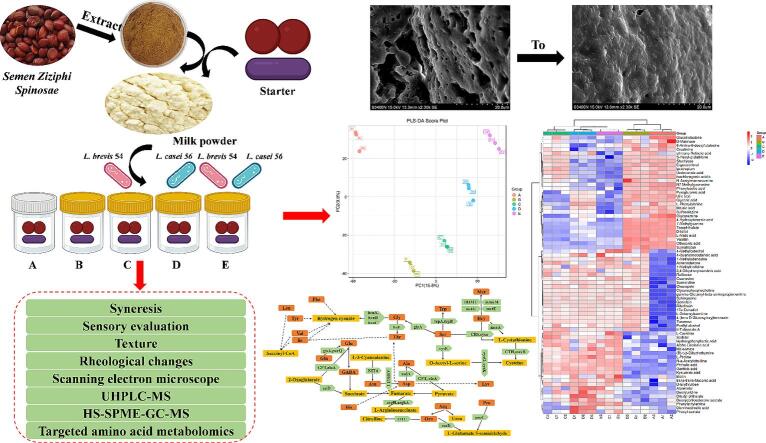
Graphical abstract
Effects of SZSE and binary probiotics on the gel structure and amino acid-like flavor compounds of yogurt and their molecular mechanisms. A simplified version of the experiment conducted in this study.
Keywords: Yogurt, Lactic acid bacteria, HPLC-MS, HS-SPME-GC-MS, Targeted metabolomics, Flavor compounds
Highlights
-
•
0.5% (w/v) Semen Ziziphi Spinosae extract can improved the gel structure of yogurt.
-
•
Synergistic fermentation by binary probiotics results in a dense gel structure.
-
•
Amino acid-derived metabolites and their metabolic pathways play a key role.
-
•
Hexanoic acid, 2-heptanone and 2-nonanone are responsible for the high odor score.
Abstract
The study aimed to investigate the impact of water-soluble extract from Semen Ziziphi Spinosae (SZSE) on yogurt quality and understand the underlying mechanism. The results demonstrated that adding 0.5% (w/v) SZSE had a significant effect on reducing yogurt syneresis and resulted in a more compact and uniform casein gel. Notably, the co-fermented yogurt with binary probiotics (Lacticaseibacillus casei CGMCC1.5956 and Levilactobacillus brevis CGMCC1.5954) along with SZSE led to increased viable probiotics and a higher odor score (23.23). This effect might be attributed to the increased amino acid utilization by binary probiotics through biosynthesis of valine, leucine and isoleucine, metabolic pathways, and amino acid biosynthesis to produce amino acid derivatives such as N5-(l-1-carboxyethyl)-l-ornithine and diaminopyrimidine acid. The yogurt contained 79 volatile flavor compounds, with hexanoic acid, 2-heptanone, and 2-nonanone potentially contributing to the high odor scores. These findings have strategic implications for developing yogurt with high gel characteristics and distinctive flavor.
1. Introduction
Yogurt, known for its distinctive flavor and nutritional benefits, is produced by fermenting cow’s milk with a starter culture consisting of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (Routray & Mishra, 2011). However, plain yogurt faces challenges such as whey separation, weak gelling properties, and limited flavor options (Rashwan, Osman, & Chen, 2023). Therefore, numerous researchers have employed lactic acid bacteria (LAB) with probiotic properties for fermentation. For instance, the inclusion of Lacticaseibacillus rhamnosus in yogurt significantly increased the levels of 2-heptanone and 2-nonanone (Innocente et al., 2016). Additionally, compared to using a single strain, the use of multiple probiotics strains for yogurt fermentation may result in improved texture and nutritional value (Wang et al., 2021). For instance, co-fermenting yogurt with Lacticaseibacillus casei Zhang and Bifidobacterium lactis V9 significantly promoted B. lactis V9 growth and increased acetate, caproic acid, butyric acid, and valeric acid levels, surpassing the effects of using a single probiotic strain (Peng et al., 2022). Nevertheless, the impacts of co-fermenting multiple probiotic strains on yogurt quality, especially its gel structure and flavor, as well as the underlying mechanisms of their combination effects, remain inadequately understood.
Scientific reports have emphasized the frequent utilization of natural derived plant-based ingredients in yogurt as colorants, stabilizers, flavor enhancers, and nutritional fortifiers (Fan et al., 2022). A recent study by Wang, Kristo, and LaPointe (2020) demonstrated that adding apple pomace to stirred yogurt modified its structure, increasing firmness, cohesiveness, higher viscosity, and reduced whey release during refrigeration. Similarly, Dong et al. (2022) discovered that incorporating carrot soluble dietary fiber into yogurt led to a more stable structure, increasing viscosity and consistency index, elevating pH, and reducing flow index, titratable acidity, and dehydration rate. Furthermore, plain yogurt lacks phenols, flavonoids, anthocyanins, and iron, which are associated with anti-diabetic and anti-cancer effects (Rashwan et al., 2023). Therefore, we anticipate enhancing yogurt flavor, gel strength, and nutritional value with natural plant-based water-soluble extracts.
Contrarily, Semen Ziziphi Spinosae (SZS) is a plant with both medicinal and dietary properties, containing a range of substances such as SZS oil, flavonoids, SZS saponins, alkaloids, amino acids, and polysaccharides. These components are known for their calming effects, relieving tension, anxiety, and depression (Cao et al., 2010, Dong et al., 2021). However, direct utilization of SZS in food fermentation is limited due to its antibacterial properties attributed to flavonoids, saponins, and alkaloids. Additionally, we have pre-screened two LAB strains with probiotic functions: Levilactobacillus brevis CGMCC1.5954 (L. brevis 54, which produces high levels of γ-aminobutyric acid and alleviates depression in mice) and L. casei 56 (exhibiting selenium-enriched capacity and prevention of cognitive dysfunction in rats) (Wu et al., 2021a, Wu et al., 2021b). Therefore, we hypothesized that co-fermentation of yogurt with SZS water-soluble extract and these binary probiotics could overcome the challenges of low viable bacterial count and poor gel strength caused by antibacterial substances in the SZS extract (SZSE).
This study aimed to investigate the effects of SZSE and binary probiotics on the quality of yogurt, as well as their underlying molecular mechanisms. Initially, the water-soluble SZSE was extracted from SZS to evaluate its impact on yogurt syneresis. Subsequently, the effects of combination fermentation of binary probiotics with SZSE on the gel structure and flavor compounds of yogurt were examined using scanning electron microscopy (SEM), DHR-2 rheometer, colorimetry, sensory evaluation, high-performance liquid chromatography-mass spectrometry (HPLC-MS), headspace solid-phase microextraction-gas chromatograph-mass spectrometry (HS-SPME-GC–MS), and targeted amino acid metabolomics and their mechanisms. The findings highlight the SZSE potential as a natural yogurt stabilizer, offering strategic guidance for developing yogurt with enhanced gel strength and amino acid-like flavors.
2. Materials and methods
2.1. Materials
Lactobacillus delbrueckii subsp. bulgaricus ATCC BAA-365 and Streptococcus thermophilus JIM 8232 were obtained from the ATCC. Additionally, our laboratory identified L. casei 56 and L. brevis 54, which were stored at the CGMCC and assigned accession numbers CGMCC1.5956 and CGMCC1.5954, respectively. SZS, whole milk powder, and sucrose were procured from Xi'an Safe & Austrian Biotechnology Co Ltd (Xi'an, China), Anchor (Auckland, New Zealand), and Anqi Yeast Co Ltd (Hubei, China) respectively. Acetonitrile (≥99.9 %; Thermo, Massachusetts, USA), formic acid (LC-MS grade; TCI, Shanghai, China), ammonium formate (≥99.9 %; Sigma, Shanghai, China), and amino acid standards (Sinopharm, Beijing, China) were used.
2.2. Preparation of SZS water-soluble extract
The dried SZS was washed thrice with sterile water, powdered using a 2500C multifunctional pulverizer (Red Sun Group Co., Ltd., Nanjing, China) at 550 W for 5 min, and oven-dried at 65 °C for 24 h. Subsequently, the extract was obtained with double distilled water at a 1:10 (w/v) ratio and 60 °C for 6 h, repeated thrice. The collected extracts were combined and concentrated using a rotary evaporator (65 °C) and then autoclaved at 135 °C for 8 s. The dried water-soluble SZSE was obtained after freeze-drying the extracts and passing them through a 100-mesh sieve (Djeuzong et al., 2021).
2.3. Preparation of yogurt
S. thermophilus (42 °C, 16 h, M17), L. bulgaricus (37 °C, 24 h, MRS), L. casei 56 (37 °C, 12 h, MRS) and L. brevis 54 (37 °C, 16 h, MRS) were cultured for three consecutive generations. LAB cultured to the logarithmic phase were inoculated into blank MRS medium and incubated at 37 °C until the OD600 nm of the organisms to 1.0. Then, the number of viable LAB bacteria in the culture solution at an OD600 nm of 1.0 was determined using the 10-fold serial dilution smear plate method. Finally, based on the number of viable bacteria contained in each milliliter of the bacterial solution at this point, the culture solution was diluted using saline at a volumetric ratio to obtain a seed solution with 1 × 109 CFU/mL. The organisms were washed thrice with sterile saline, centrifuged at 5000 × g for 5 min at 4 °C each time, and kept as a seed solution. A mixture of 11 % (w/v) whole milk powder and 6.5 % (w/v) sucrose in sterile water was homogenized at 65 °C for 25 s at 17 MPa, based on the conditions reported by Peng et al. (2022) with minor revisions. The mixture was then sterilized at 95 °C for 10 min and cooled in a water bath at 42 °C. The recovery milk was inoculated with the seed solution at 6.5 × 106 CFU/mL and fermented in an incubator at 42 °C for 6 h. Following fermentation, the seed solution was post-matured in a refrigerator at 4 °C for 12 h (Fan et al., 2023).
2.4. SZSE effect on yogurt syneresis
The recovered milk, containing varying proportions of SZSE and whole milk powder, was homogenized, sterilized, and cooled to 42 °C following Section 2.3. SZSE was added at concentrations of 0, 0.125 %, 0.25 %, 0.5 %, 1 %, 2 % and 3 % (w/v), while whole milk powder was added at concentrations of 3 %, 5 %, 7 %, 9 %, and 11 %, respectively. Only S. thermophilus and L. bulgaricus were used for fermentation. After fermentation, the syneresis of yogurt samples was determined with slight modifications to the previously described method (Wang et al., 2019). For syneresis calculation, a 20 g yogurt sample was centrifuged at 222 × g and 10 °C for 10 min, and the weight of the resulting supernatant was measured and used in the following equation:
| (1) |
Where, m1 represents the weight of the initial yogurt sample, while m2 represents the weight of the resulting yogurt supernatant.
2.5. Effect of SZSE and binary probiotics on the viable LAB, pH, and titratable acidity (TA) of yogurt
Five different yogurt groups were prepared: group A (starter: S. thermophilus + L. bulgaricus); group B (starter + 0.5 % (w/v) SZSE); group C (starter + 0.5 % (w/v) SZSE + L. brevis 54); group D (starter + 0.5 % (w/v) SZSE + L. casei 56); and group E (starter + 0.5 % (w/v) SZSE + L. brevis 54 + L. casei 56). The viable content of the four LAB in yogurt was determined following the method of Peng et al. (2022) and Fan et al. (2022) with slight modifications. Additionally, the pH and titratable acidity (TA) of each yogurt group were measured using a pH meter and an alkaline titration method.
2.6. Effect on chromatic aberration and sensory evaluation
Twenty grams of yogurt was plated in a Petri dish, and a colorimeter (Wilford Optoelectronics Technology Co., Ltd., Guangdong, China) was used to measure the color difference of the five yogurt samples. The colorimeter assessed brightness (L*), redness (r), and yellowness (y). Additionally, we recruited 30 trained volunteers (15 males and 15 females) to evaluate the color (25 points), texture (25 points), odor (25 points), and taste (25 points) of the five yogurts. The sensory evaluation of the yogurts followed the scale established by Fan et al. (2023) and received approval by Ningbo University Institutional Review Board (NU-IRB, NBU20210024). The tested products were confirmed as safe for consumption, and the volunteers provided informed consent and had the option to withdraw from the survey without explanation.
2.7. Texture
The texture properties of the yogurt, including hardness, consistency, cohesiveness, gumminess, and chewiness, were evaluated using a texture property analyzer (Stable Micro System, UK) with slight modifications to the method described by Wang et al., 2022, Jiang et al., 2022. The analysis involved using a 12.7 mm column probe with pre-measurement, measurement, and post-measurement speeds set at 1.0, 1.0, and 2.0 mm/s, respectively. The compression distance and measurement temperature were set at 20 mm and 15 °C, respectively.
2.8. Rheological changes
The rheological properties of the yogurt were determined using the DHR-2 rheometer (TA-WATERS ltd, USA), following the method described by Wang et al., 2022, Jiang et al., 2022 with slight modifications. The test was conducted at 25 °C with a fixed frequency of 1 Hz and a strain range of 0–50 % (cross-section 0.5 %). The sample was subjected to shear mode, with the shear rate increasing from 0 to 500 s−1 and then decreasing from 500 to 0 s−1 (scan time of 360 s). The scanning frequency was set at 0.5 %, covering a frequency range of 0.1–10 Hz (Ren et al., 2021).
2.9. Microstructure
The central block of yogurt, measuring approximately 1.5 × 1.5 × 1.5 cm, was carefully extracted and placed in a 2.5 % glutaraldehyde solution. It was then fixed at 4 °C for 12 h (Wang et al., 2019). After fixation, the block was rinsed twice with sterile saline for 15 min each. It underwent cleaning and dehydration using an ethanol gradient (50 %, 60 %, 70 %, 80 %, and 90 %) for 10 min at each concentration. Afterward, the block was washed thrice with 100 % ethanol for 10 min each. Subsequently, the samples were immersed in tert-butanol for 10 min and pre-cooled at −80 °C for 24 h. Freeze-drying was then conducted for 12 h. Finally, the resulting samples were gold-sprayed and examined for microstructures using a Hitachi S-3400 SEM (Hitachi Limited, Tokyo, Japan).
2.10. Nonvolatile flavors compounds
For the analysis of nonvolatile flavors compounds, 100 mg of yogurt sample was vigorously agitated with 200 µL of methanol and methyl tert-butyl ether in a 2 mL sterile centrifuge tube for 60 s. The mixture was then centrifuged at 12,000 × g for 10 min at 4 °C, and the resulting solution was filtered through a 0.22 µm filter membrane. The filtrate obtained was utilized for HPLC-MS analysis, following the protocol recommended by Peng et al. (2022).
2.11. Volatile flavors compounds
To analyze the volatile flavors compounds, a 5 g yogurt sample was placed in an extraction vial, and the volatile flavor compounds were extracted through headspace at 55 °C and 350 rpm for 1 h (Fan et al., 2023). GC–MS analysis was performed using helium (He) as the carrier gas at a 1.0 mL/min flow rate, with an inlet temperature set at 250 °C. The temperature program started at 35 °C and held for 5 min, then gradually increased to 140 °C at a 5 °C/min rate, with a 2 min held. Subsequently, the temperature was ramped up to 250 °C at a 10 °C/min rate and held for 3 min.
2.12. Targeted amino acid metabolomics
To begin with, the amino acid standards were prepared by weighing them and creating separate master mixes using methanol. From each master batch, an appropriate amount was measured to form the mixed standard. This mixed standard was then diluted to the desired concentration using a 1:1 ratio of 10 % formic acid–methanol–water. Simultaneously, the internal standard master mix was prepared at a concentration of ng/mL, using a 1:1 ratio of 10 % formic acid in methanol–water. The metabolites were extracted, and the resulting supernatant was combined with 100 μL of Trp-d3 internal standard at a concentration of 10 ng/mL. After vortexing for 30 s, the solution was filtered through a 0.22 μm membrane and transferred to the assay bottle for further testing. The supernatant underwent another filtration step before being added to the test bottle through a 0.22 μm filter membrane. The HPLC-MS analysis was conducted using equipment from Shanghai Able Caisse Analytical Instruments Trading Co., Ltd., Shanghai, China, with assistance from Panomic Biomedical Technology Co (Suzhou, China).
2.13. Statistical analysis
The experiments were performed thrice, and Duncan's multiple range analysis was employed to assess sample variations. Simca software (MKS Data Analytics Solutions) was used to generate Principal component analysis (PCA) plots and heat maps. The metabolic pathways were obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG). A P < 0.05 was considered statistically significant.
3. Results and discussion
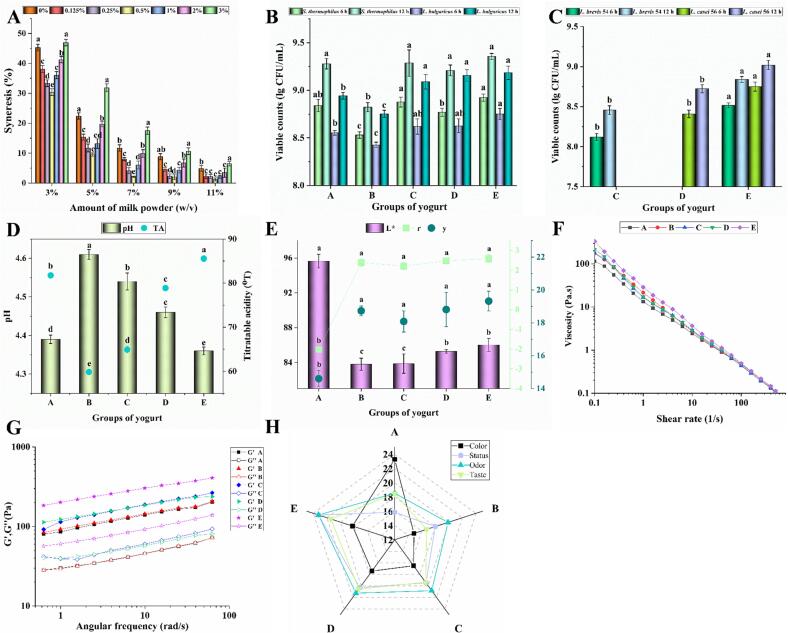
3.1. Effect of SZSE on syneresis of yogurt
Syneresis, the separation of whey on the surface of yogurt, is an undesirable occurrence that can greatly affect the quality of yogurt and consumer satisfaction. This whey separation can be visually unappealing (Sah, Vasiljevic, McKechnie, & Donkor, 2016). Fig. 1-A illustrates the effect of different concentrations of SZSE on yogurt syneresis at various levels of dairy powder: low concentrations of SESE (0–0.5 %) significantly reduced syneresis, while higher concentrations (0.5–3 %) had the opposite effect. For example, when using 7 % (w/v) of whole milk powder, yogurt syneresis without SZSE (0 % w/v) reached 11.73 %, which was significantly (P < 0.05) reduced to 2.12 % (w/v) with 0.5 % (w/v) SZSE. This demonstrated a significant improvement in yogurt syneresis at low concentrations of SZS water-soluble extract. This improvement might be attributed to the presence of abundant water-soluble polysaccharides and vegetable oils in the extract. This finding aligns with the combined effect of water-soluble polysaccharides on oleogels derived from oil-absorbing cryogels, as reported by Jiang et al., 2022, Wang et al., 2022. Additionally, creep recovery tests employing fractional order calculus analysis by Park, Campanella, & Maleky (2022) supported the notion that whey protein-oleogel networks exhibit higher strength than hydrogels. However, the negative impact observed at higher concentrations might be due to the presence of flavonoids, polyphenols, saponins, and alkaloids in the SZS water-soluble extract. These compounds have been found to inhibit the growth and acid production capacity of LAB in yogurt (Zhang et al., 2016). Therefore, 0.5 % (w/v) of the SZS water-soluble extract was chosen for subsequent experiments.
Fig. 1.
Effect of different SZSE additions on yogurt syneresis, pH, TA, viable bacterial count, chromatic aberration, rheological properties, and sensory evaluation. Effect on (A) Yogurt syneresis; (B) Viable S. thermophilus and L. bulgaricus; (C) Viable L. brevis 54 and L. casei 56; (D) pH and TA; (E) Chromatic aberration; (F) Apparent viscosity; (G) Frequency scanning; and (H) Sensory evaluation. The capital letters “A, B, C, D, and E” stand for yogurts fermented under different treatment conditions, where “A” represents the blank group without SZSE and probiotic; “B” represents the group only with SZSE; “C” represents the group with SZSE and L. brevis 54; “D” represents the group with SZSE and L. casei 56; “E” represents the group with SZSE, L. brevis 54, and L. casei 56.
3.2. Effect of SZSE and binary probiotics on the viable LAB
The viable LAB quantity is crucial in determining yogurt quality, as it is essential for conferring probiotic benefits to the host (Peng et al., 2022). Fig. 1-B illustrates that the addition of SZSE (group B) significantly reduced the levels of S. thermophilus and L. bulgaricus in yogurt at 6 h of fermentation and 12 h of post-maturation compared to the blank group (group A). Conversely, the addition of probiotics (groups C-E) significantly improved this phenomenon. This effect might be attributed to the presence of flavonoids and saponins in SZS, with crude saponins exhibiting potent antibacterial activity, particularly against Gram-positive bacteria. Furthermore, adding probiotic LAB facilitates the hydrolysis of casein and SZS polysaccharides (composed of galacturonic acid, arabinose, and galactose) into amino acids, formic acid, folic acid salt, and CO2, which are important for S. thermophilus and L. bulgaricus growth (Zou et al., 2022).
Fig. 1-C reveals that at 12 h of post-maturation, the binary probiotic group (group E) significantly increased L. casei 56 (9.02 lg (CFU/mL)) and L. brevis 54 (8.84 lg (CFU/mL)) compared to group D (L. casei 56, 8.72 lg (CFU/mL)) and group C (L. brevis 54, 8.45 lg (CFU/mL)), respectively. This may be due to the greater ability of L. casei 56 to hydrolyse casein, producing amino acid-like substances that further promote the growth of probiotics.
3.3. Effect on pH and TA
During fermentation, LAB metabolizes lactose to produce organic acids such as lactic acid, which lowers the pH of the yogurt. This acidification causes the conversion of colloidal calcium phosphate into soluble calcium phosphate in casein micelles, resulting in casein aggregation and precipitation around pH 4.6, known as the isoelectric point (Wang et al., 2019). The addition of SZSE (group B, pH 4.61) to the yogurt significantly elevated its pH than to the control group (group A, pH 4.39) (Fig. 1-D). This effect could be attributed to anthocyanins, flavonoids, polyphenols, and saponins in the SZSE, impeding LAB growth and acidogenesis in the yogurt (Li et al., 2022). Compare to group B, both group C (containing L. brevis 54, pH 4.64) and group D (containing L. casei 56, pH 4.46) significantly decreased pH. This could be attributed to the prsA gene in L. casei, which is crucial in the protein hydrolysis system, resulting in increased protein degradation during fermentation (Liu et al., 2010). The yogurt fermented with binary probiotics (group E) had a lower pH (4.36). This observation aligns with the findings in section 3.2, and this suggesting a synergistic effect between L. casei 56 and L. brevis 54, promoting their growth and acid production. The titratable acidity (TA) of the yogurt exhibited a consistent trend corresponding to its pH levels.
3.4. Chromatic aberration
The effect of SZSE addition and binary probiotics on yogurt color was determined using a colorimeter, with L*, r, and y representing brightness, redness, and yellowness, respectively (Wang et al., 2019). The addition of SZSE significantly changed the yogurt color, reducing its brightness and giving it a tawny appearance (Fig. 1-E). This change might be mainly attributed to anthocyanins, including carotenoids, in SZSE (Jiang et al., 2022, Wang et al., 2022). This finding was consistent with a study by Baria et al. (2021), which evaluated the color-enhancing potential of anthocyanin-enriched black carrot concentrates for yogurt and found that sensory evaluations indicated an acceptable color within 15 days.
3.5. Texture
The texture of yogurt is critical for consumer acceptance (Wang et al., 2022, Jiang et al., 2022). The addition of SZSE improved the hardness, adhesiveness, and gumminess of the yogurt (Table 1). Wang et al. (2020) also found that adding apple pomace lyophilized powder altered the yogurt structure, increasing its firmness, cohesiveness, and viscosity. Compared to single probiotic groups C (L. brevis 54) and D (L. casei 56), the binary probiotic group E (L. brevis 54 and L. casei 56) significantly enhanced the hardness, adhesiveness, gumminess, and chewiness of yogurt. However, no significant effects were observed regarding springiness, cohesiveness, and resilience. During yogurt fermentation, LAB could generate extracellular polysaccharides (including galactose, mannose, arabinose, and xylose), contributing to an increased viscosity (Innocente et al., 2016).
Table 1.
The texture of yogurt. (A) Blank group without SZSE and probiotics; (B) with SZSE and without probiotics; (C) with SZSE and L. brevis 54; (D) with SZSE and L. casei 56; and (E) with SZSE, L. brevis 54, and L. casei 56.
| Groups | Hardness | Adhesiveness | Springiness | Cohesiveness | Gumminess | Chewiness | Resilience |
|---|---|---|---|---|---|---|---|
| A | 195.25 ± 0.23d | −323.59 ± 7.20a | 0.1680 ± 0.0004a | 0.072 ± 0.002a | 14.0110 ± 0.316c | 2.3580 ± 0.0550b | 0.0095 ± 0.0045a |
| B | 201.05 ± 2.83c | −362.65 ± 13.33b | 0.1685 ± 0.0005a | 0.075 ± 0.002a | 14.665 ± 0.392b | 2.4655 ± 0.0585b | 0.0083 ± 0.0054a |
| C | 212.69 ± 5.07b | −379.67 ± 13.79b | 0.1690 ± 0.0002a | 0.071 ± 0.001a | 15.102 ± 0.778b | 2.5475 ± 0.1315b | 0.0086 ± 0.0047a |
| D | 213.00 ± 3.66b | −467.19 ± 11.99c | 0.1680 ± 0.0001a | 0.071 ± 0.001a | 15.124 ± 0.335b | 2.4965 ± 0.0435b | 0.0081 ± 0.0065a |
| E | 267.40 ± 3.58a | −592.51 ± 14.53d | 0.1675 ± 0.0005a | 0.070 ± 0.001a | 18.730 ± 0.363a | 3.1295 ± 0.0515a | 0.0077 ± 0.0063a |
3.6. Rheological changes
As the shear rate increased, the apparent viscosity of the five yogurt groups gradually decreased (Fig. 1-F). This decrease might be attributed to the progressive alignment of protein molecules in the same direction, resulting in reduced forces of interaction between them. Such alignment could break chemical bonds, including hydrogen bonds and protein dissociation, triggered by a higher in shear rate (Sah et al., 2016). Adding SZSE significantly increased (P < 0.05) the apparent viscosity of the yogurt compared to the control group A. This increase could be attributed to the formation of super-aggregates between SZS particles and protein aggregates. These super-aggregates subsequently disintegrate into smaller aggregates under intensified shear rates, reducing the apparent viscosity of the yogurt (O’shea et al., 2015). Consistent with our findings, Fu et al. (2018) reported that the Salecan addition to yogurt increased its apparent viscosity and water retention capacity. The binary probiotics (group E) demonstrated a significant increase (P < 0.05) in the apparent viscosity of the yogurt compared to groups C (L. brevis 54) and D (L. casei 56). This increase could be attributed to the high acid production capacity of the binary probiotics, which induce the gelling of methoxypectin in SZSE at acidic pH levels. Another study found that the addition of anthocyanin-rich extracts (black soybean seed coat extract) reduced the size of soy protein aggregates, promoted bead string structures in the gel network, and limited the migration of bound and free water within the gel (Ren, Quan, & Li, 2022).
The observation of G' > G'' in all five yogurt samples indicated their significant colloidal viscoelasticity, with the elastic behavior surpassing the viscous behavior (Fig. 1-G). Adding SZSE and binary probiotics to the yogurt formulation significantly enhanced (P < 0.05) its elastic behavior. SZS, a polysaccharide-rich source, can interact with proteins to create food-grade Pickering emulsions (Ren et al., 2021, Zhang et al., 2016).
3.7. Sensory evaluation
Extensive research has investigated the influence of yogurt structure and composition on its flavor, as it affects the transport of aromatic compounds into the headspace (Innocente et al., 2016). The addition of SZSE significantly reduced the color score of the yogurt compared to the control group A (Fig. 1-H), which aligns with the results obtained from colorimeter measurement. The tissue status score increased from 15.86 (group A) to 23.21 (binary probiotic group E), indicating that the combined fermentation of SZSE and binary probiotics improved the texture of the yogurt, consistent with our findings on texture and rheology. Furthermore, there was a significant difference in odor score between the control group A (18.52) and the group B (19.87) with SZSE addition. The binary probiotic group E showed a significantly higher score of 23.23, indicating the presence of customary dairy flavor along with fragrant and fruity notes, potentially resulting from the complete release of aromatic compounds through the fermentation process by binary probiotics (Fan et al., 2022). However, regarding taste scores, group E demonstrated improvement, although the difference among the five yogurts were not statistically insignificant. This could be largely attributed to the diverse taste preferences within the test population (Brückner-Gühmann, Benthin, & Drusch, 2019).
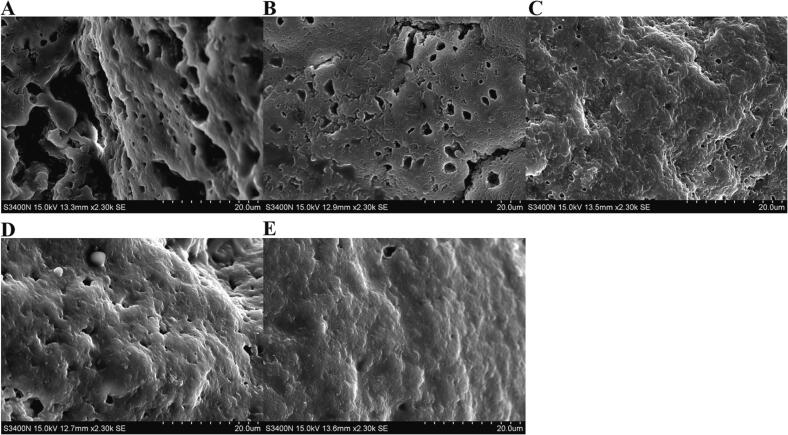
3.8. Microstructure
Microstructure analysis provides comprehensive insights into gel formation, shedding light on the underlying mechanisms behind physicochemical, rheological, and textural properties. The control yogurt (group A) exhibited a dense, low-connectivity protein network structure with wide pores and a rough surface (Fig. 2-A), consistent with previous findings (Dong et al., 2022). The addition of SZSE to the yogurt resulted in a rough surface but a more compact and homogeneous protein mass with fewer pores (Fig. 2-B), potentially contributing to its low syneresis. Incorporating a single probiotic (Fig. 2-C, D) led to in a smooth yogurt surface with a significant reduction in the number and sizes of pores in the protein network. This improvement in the protein network structure, texture, and rheological properties of soymilk was attributed to the extracellular polysaccharides produced by LAB, aligning with our result (Li et al., 2014). Contrarily, the yogurt fermented through the combination fermentation of binary probiotics and SZSE displayed a smooth and shiny surface, a dense protein network, and narrow and loose pores.
Fig. 2.
Microstructure. (A) A blank group without SZSE and probiotics; (B) With SZSE and without probiotics; (C) With SZSE and L. brevis 54; (D) With SZSE and L. casei 56; and (E) With SZSE, L. brevis 54, and L. casei 56.
The micrographs revealed that the void region surrounding the casein aggregates could potentially be filled with SZSE particles. These particles bind to the aqueous serum pockets around the aggregates, impeding whey separation and reducing syneresis (Wang et al., 2020). Moreover, the SZSE not only interacts electrostatically with anionic hydrocolloids from casein micelles (similarly to the interaction between extracellular polysaccharides and proteins), but also contains soluble fibers with a porous structure and absorbent components that can compact the casein aggregates by absorbing separated whey. Additionally, the combination fermentation of SZSE by binary probiotics may enhance the release of extracellular polysaccharides by LAB and increase the concentration of SZSE particles embedded in the protein network (Peng et al., 2022). Thus, the combination fermentation of binary probiotics and SZSE effectively controls the separation of whey and strengthens the protein network structure.
3.9. Nonvolatile flavors compounds
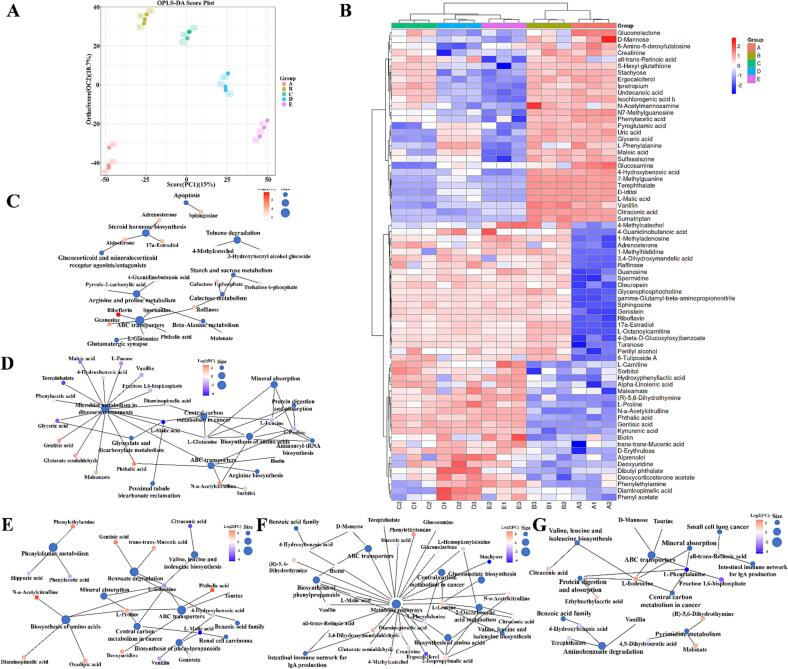
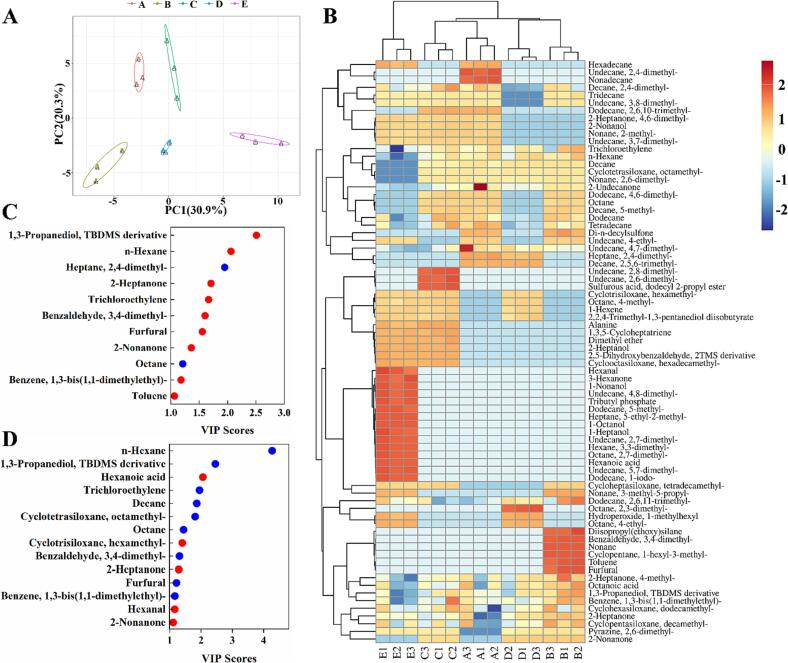
Based on the above results, the fermentation of binary probiotics and SZSE significantly impacted the structure and odor of the yogurt gel. Therefore, we conducted further research to explore the underlying mechanism and key flavor compounds that are responsible for these effects using HPLC-MS combined with metabolomics. According to Peng et al. (2022), potential biomarkers were selected based on criteria including VIP ≥1, p-value ≤0.05, and fold change ≥1.5 or ≤0.667. Fig. 3-A illustrates the raw data distribution, where the smaller distances between points within the same group indicate excellent reproducibility, while longer distances between points from different groups suggest significant differences among the five yogurt groups (Peng et al., 2022). Demonstrates the reliability of the data. The PCA analysis revealed a distinct separation of the five yogurts along PCA1 (15 %) and OC2 (10.7 %). A graph of PCA scores comparing the two groups is shown in Supplementary Fig. 1.
Fig. 3.
Metabolomic analysis. (A) PCA score; (B) A heatmap of nonvolatile metabolites; Comparison of differential metabolites and their metabolic pathway association analysis in (C) Group B and A; (D) Group C and B; (E) Group D and B; (F) Group E and C; (G) Group E and D. The capital letters “A, B, C, D, and E” stand for yogurts fermented under different treatment conditions, where “A” represents the blank group without SZSE and probiotic; “B” represents the group only with SZSE; “C” represents the group with SZSE and L. brevis 54; “D” represents the group with SZSE and L. casei 56; “E” represents the group with SZSE, L. brevis 54, and L. casei 56.
To identify the distinct key metabolites present in the five yogurt groups, we constructed a heatmap (Fig. 3-B). A total of 33 significant differential metabolites were identified when comparing group B (with the addition of SZSE) to group A (blank group), with 29 showing significant upregulation and 4 showing significant downregulation. Notably, the major components of SZSE, including riboflavin, l-octanoylcarnitine, 4-(beta-d-glucosyloxy) benzoate, guanosine, turanose, raffinose, 6-tuliposide A, sphingosine glycerophosphocholine, l-fucose, 3,4-dihydroxymandelic acid, and oleuropein, exhibited remarkable upregulated with fold changes of 2782.16, 111.05, 110.2, 40.08, 13.81, 11.81, 11.04, 10.42, 9.85, 4.64, 3.8 and 3.8-folds, respectively. The relevant metabolic pathways were shown in Fig. 3-C. Riboflavin, a vitamin B, plays a critical role in various metabolic processes, including amino acids and lipids oxidation, purine bases conversion to uric acid, aromatic compound hydroxylation, and the metabolism of folic acid, pyridoxal, and nicotinic acid (You et al., 2021). Katsuta, Nishimura, and Miura (1992), through their investigation of the effect of monosaccharides and disaccharides on rice starch gelation using iatrogenic kinetics, found that the gelation capacity of rice starch increased with the average number of e-OH groups and the dynamic hydration number of sugar molecules. Therefore, l-fucose (rare monosaccharide), turanose (disaccharide), and raffinose (trisaccharide) in SZSE may be responsible for the improved gel structure and reduced hardness of the yogurt. In addition, l-octanoylcarnitine (alkaloid), 6-tuliposide A, sphingosine, genistein (soybean flavonoids), 3,4-dihydroxymandelic acid, and oleuropein have been reported to possess antibacterial effects, which could be responsible for reducing viable bacteria in the yogurt after SZSE addition (Sharifi-Rad et al., 2021).
Comparing group C (containing L. brevis 54) with group B, revealed 27 significantly differential metabolites, with 10 exhibiting significant upregulated and 17 showing significant downregulated. Notably, phthalic acid, kynurenic acid, N-a-acetylcitrulline, Alpha-linolenic acid, gentisic acid, and maleamate were upregulated by a factor of 6.34, 4.76, 4.16, 3.98, 3.87, and 3.07, respectively, which may account for the observed lower pH in the yogurt. These findings were primarily attributed to microbial metabolism in various environments, biosynthesis of amino acids, and protein digestion and absorption metabolic pathways (Fig. 3-D). Furthermore, comparing group D (containing L. casei 56) with group B revealed 39 significantly differential metabolites, with 14 showing upregulation and 25 showing downregulation. The production of N-a-acetylcitrulline, phthalic acid, kynurenic acid, gentisic acid, trans–trans-muconic acid, oxoadipic acid, diaminopimelic acid, and l-proline was upregulated by 5.92, 5.3, 5.03, 4.2, 2.66, 2.53, 2.19, and 1.82 folds, respectively. This was consistent with the previous findings in section 3.3, which highlighted the high acid production capacity of L. casei 56. These effects were mainly associated with phenylalanine metabolism, biosynthesis of amino acids, valine, leucine and isoleucine biosynthesis, and the ABC transporters pathway (Fig. 3-D).
When comparing group E (containing L. casei 56 and L. brevis 54) with group C (containing L. brevis 54), 20 significantly differential metabolites was discovered. Among them, 9 showed an increase in production while 11 showed a decrease. Specifically, the production of phenylethylamine, 3,4-dihydroxymandelaldehyde, l-leucine, N5-(l-1-carboxyethyl)-l-ornithine, diaminopimelic acid, 2-isopropylmalic acid, 4-Methylcatechol, and N-a-acetylcitrulline was upregulated by 6.23, 3.87, 2.86, 2.75, 1.91, 1.78, 1.75, and 1.56 folds, respectively. Wu et al. (2023) obtained similar findings in their result, which demonstrated that higher levels of p-hydroxybenzoic acid, p-coumaric acid, ferulic acid, and sinapic acid in yogurt fermented with rice bran led to increase pH, denser gel formation, and reduced hardness. Phenylethylamine, is known for its neurotransmitter functions and role in regulating mood, stress, and concentration, is a brain chemical that produces feelings of well-being and love (Marcobal, De Las Rivas, Landete, Tabera, & Muñoz, 2012). Additionally, yogurt fermented with binary probiotics and SZSE has shown to have a synergistic effect on nutritional aspects. The formation of these metabolites was mainly related to the valine, leucine and isoleucine biosynthesis, metabolic pathways, and biosynthesis of amino acids (Fig. 3-F).
Comparison groups E and D (containing L. casei 56) revealed 22 significantly different metabolites, with 8 showing upregulation and 14 showing downregulation. Notably, epinephrine, l-isoleucine, malonate, (9E)-octadecenoic acid, 4-methylcatechol, and citraconic acid were upregulated by 4.61, 2.11, 1.88, 1.79, 1.67 and 1.58-folds, respectively. Their metabolic pathway was shown in Fig. 3-G. A 4-methylcatechol can bind to cysteine residues on proteins, forming cysteine-4-methylcatechol adducts, increasing the addition of larger protein polymers (Zainudin, Jongberg, & Lund, 2021). This substance was also found to be upregulated when compared to group C, indicating that it was a key substance in the improvement of the gel structure of group E yogurt. Prag et al. (2022) found the protective effects of malonate on the hearts of mice with cardiac ischemia. They found that the degree of cardiac uptake of malonate was proportional to the ischemia duration.
3.10. Volatile flavors compounds
As described in section 3.7, the addition of SZSE and binary probiotics had a significant impact on the flavor score of the yogurt. Therefore, HS-SPME-GC–MS was used to characterize the volatile flavor substances. Fig. 4-A illustrates the greater distances between groups A, B, and D, indicating a higher flavor variability, while shorter distances between groups C, D, and E, indicating a lower flavor variability, showing consistency with the odor scores obtained during sensory evaluation (Wang et al., 2021). The PCA scores of volatile flavor compounds between the two groups are plotted in Supplementary Fig. 2. Total 79 volatile flavor compounds were found in the yogurt, encompassing carbonyl compounds, alcohols, aldehydes, acids, esters, and aromatic compounds (Fig. 4-B). Among these compounds, 1,3-propanediol, TBDMS derivative (odorless), hexane (alkane), 2-heptanone (pearly fruit flavor), trichloroethylene (slightly sweet odor), benzaldehyde, 3,4-dimethyl- (almond flavor), furfural (mildly sweet, bitter flavor), 2-nonanone (fruit, floral, oil and herbal flavor), benzene, 1,3-bis(1,1-dimethylethyl)- (sweet flavor), and toluene (paint) were upregulated, while heptane, 2,4-dimethyl- (alkane) and octane (alkane) were downregulated (Peng et al., 2022). Similarly, 54 volatile flavor compounds were identified in group E, with hexanoic acid (cheese and coconut flavor), cyclotetrasiloxane, octamethyl-(alkane), 2-heptanone (pearly fruit flavor), and 2-nonanone (fruit. floral, oil, and herbal flavor) showing significant upregulation (Fig. 4-D). These findings might be attributed to the high odor scores of yogurts fermented by binary probiotics and SZSE (Rashwan, Osman, & Chen, 2023).
Fig. 4.
Volatile flavors compounds. (A) PCA analysis. (B) A heatmap of volatile flavor compounds. (C) Comparison of groups B and A yielded important variables for changes in yogurt volatile flavor compounds resulting from SZSE addition: (D) Comparison of groups E and B yielded important variables for changes in yogurt volatile flavor compounds resulting from binary probiotic (L. brevis 54 and L. casei 56) addition. The capital letters “A, B, C, D, and E” stand for yogurts fermented under different treatment conditions, where “A” represents the blank group without SZSE and probiotic; “B” represents the group only with SZSE; “C” represents the group with SZSE and L. brevis 54; “D” represents the group with SZSE and L. casei 56; “E” represents the group with SZSE, L. brevis 54, and L. casei 56.
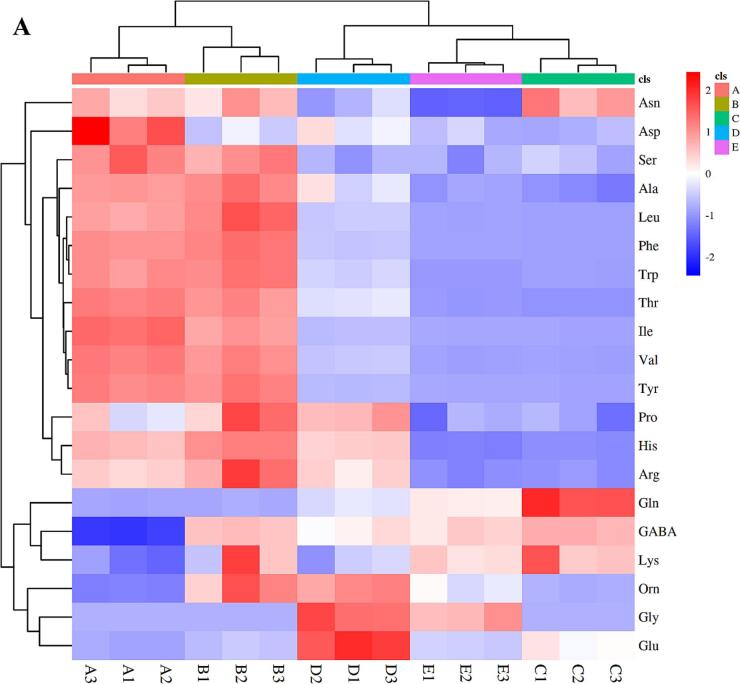
3.11. Targeted amino acid metabolomics
The analysis of the above results revealed the significant role of amino acids in the fermentation process of binary probiotics. Therefore, targeted amino acid metabolomics was performed to elucidate the underlying mechanism. Fig. 5 illustrates that the SZSE addition increased the levels of γ-aminobutyric acid (GABA), lysine (Lys), and ornithine (Orn) in the yogurt compared to group A. Group E exhibited a significant reduction in amino acid content compared to the other 4 groups. Newstead (2019) highlighted the preference for l-type amino acids by living organisms due to their enhanced digestion and absorption. Additionally, certain amino acids, including arginine (Arg), leucine (Leu), tryptophan (Trp), and tyrosine (Tyr), have been found to impact LAB growth positively, as noted by Yonezawa et al. (2010). This could be attributed to the higher probiotic content observed in the yogurt synergistic fermentation with binary probiotics and SZSE.
Fig. 5.
Targeted amino acid metabolomics. Groups A-E correspond to the following conditions: (A) represents the blank group without SZSE and probiotic; (B) represents the group only with SZSE; (C) represents the group with SZSE and L. brevis 54; (D) represents the group with SZSE and L. casei 56; (E) represents the group with SZSE, L. brevis 54, and L. casei 56.
In addition to constituting proteins and oxidative breakdown, amino acids can be derived into a variety of active molecules, such as one-carbon units (groups containing one carbon atom, mainly methyl, methane and hydroxymethyl), hormones, neurotransmitters, etc. One-carbon units (derived from the oxidation of amino acid architectures, particularly glycine, to which both threonine and serine can be converted) are methyl donors and are associated with the biosynthesis of adrenaline, creatine, purines, etc., for which there is a high demand in rapidly growing cells (Scotton, Hill, Williams, & Barnes, 2019). Riboflavin (vitamin B2), which significantly increased by 2782.16 times in group B yogurt, is vital in converting and utilizing one-carbon units. Tyrosine is catalyzed by tyrosine hydroxylase (TH) to form dihydroxyphenylalanine, or dopa. Dopa decarboxylase (DDC) catalyzes the formation of dopamine, which is catalyzed by dopamine beta-hydroxylase (DBH) to form noradrenaline, which is then catalyzed by phenylethanolamine N-methyltransferase (PNMT) to accept the methyl group of S-adenosylmethionine (SAM) to form epinephrine, which possesses significant cardiovascular and neurological effects. Furthermore, the glutamate is decarboxylated to produce gamma-aminobutyric acid (GABA), a neurotransmitter with inhibitory effects (Wu et al., 2021b).
4. Conclusion
This study investigated the effects of binary probiotics and SZSE on the quality of yogurt and their underlying mechanisms. The results demonstrated that 0.5 % (w/v) of SZSE significantly reduced yogurt syneresis, increased apparent viscosity and tissue status score, and formed a more compact and homogeneous casein gel. This might be attributed to l-fucose, turanose, and raffinose in the SZSE. The yogurt co-fermented with binary probiotics and SZSE has an enhanced gel structure, optimal rheological properties, and an increased number of viable probiotics. Metabolomic indicated this might be mainly attributed to increased amino acid utilization by binary probiotics through the biosynthesis of valine, leucine and isoleucine, metabolic pathways, and amino acid biosynthesis to produce amino acid derivatives, including N5-(l-1-carboxyethyl)-l-ornithine, diaminopyrimidine acid, 2-isopropylmalic acid, N-a-acetylguanine, and 4-methylcatechol, resulting in increased protein polymers to improve yogurt gel strength. Moreover, the yogurt contained 79 volatile flavor compounds, with hexanoic acid, 2-heptanone, and 2-nonanone might attributed to the high odor scores of yogurt combination fermentation with binary probiotics and SZSE. These findings have strategic implications for developing yogurt with high gel characteristics and distinctive flavor. However, we acknowledge certain limitations in the experimental setup and data interpretation, such as shelf life and Pilot magnification, which we will further investigate in the next stage.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Xiankang Fan: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Ang Zhang: Methodology. Tao Zhang: Methodology, Validation. Maolin Tu: Conceptualization, Writing – review & editing. Qiwei Du: Data curation. Nan Ling: Resources. Jihuan Wu: Formal analysis. Xiaoqun Zeng: Conceptualization, Formal analysis. Zhen Wu: Formal analysis, Visualization, Resources, Methodology. Daodong Pan: Conceptualization, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Funding of China (32272339), National Key R&D Program of China (2021YFD2100104), Key Project of Ningbo Science and Technology (2023Z127), Open Fund for the State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products (2021DG700024-KF202423), and Ningbo University Postgraduate Research Innovation Fund (IF2023053).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101191.
Contributor Information
Tao Zhang, Email: zhangtao@nbu.edu.cn.
Daodong Pan, Email: daodongpan@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Baria B., Singh A.K., Panjagari N.R., Arora S., Minz P.S. Colouring properties and stability of black carrot anthocyanins in yoghurt. Journal of Food Science and Technology. 2021;58:3953–3962. doi: 10.1007/s13197-020-04858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner-Gühmann M., Benthin A., Drusch S. Enrichment of yoghurt with oat protein fractions: Structure formation, textural properties and sensory evaluation. Food Hydrocolloids. 2019;86:146–153. doi: 10.1016/j.foodhyd.2018.03.019. [DOI] [Google Scholar]
- Cao J.X., Zhang Q.Y., Cui S.Y., Cui X.Y., Zhang J., Zhang Y.H., Zhao Y.Y. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. Journal of Ethnopharmacology. 2010;130(1):163–166. doi: 10.1016/j.jep.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Djeuzong, E., Kandeda, A. K., Djiogue, S., Stéphanie, L., Nguedia, D., Ngueguim, F., & Dimo, T. (2021). Antiamnesic and neuroprotective effects of an aqueous extract of Ziziphus jujuba Mill.(Rhamnaceae) on scopolamine-induced cognitive impairments in rats. Evidence-Based Complementary and Alternative Medicine, 2021. 10.1155/2021/5577163. [DOI] [PMC free article] [PubMed]
- Dong R., Liao W., Xie J., Chen Y., Peng G., Xie J., Yu Q. Enrichment of yogurt with carrot soluble dietary fiber prepared by three physical modified treatments: Microstructure, rheology and storage stability. Innovative Food Science & Emerging Technologies. 2022;75 doi: 10.1016/j.ifset.2021.102901. [DOI] [Google Scholar]
- Dong Y.J., Jiang N.H., Zhan L.H., Teng X., Fang X., Lin M.Q., Chen S.H. Soporific effect of modified Suanzaoren Decoction on mice models of insomnia by regulating Orexin-A and HPA axis homeostasis. Biomedicine & Pharmacotherapy. 2021;143 doi: 10.1016/j.biopha.2021.112141. [DOI] [PubMed] [Google Scholar]
- Fan X., Li X., Du L., Li J., Xu J., Shi Z., Pan D. The effect of natural plant-based homogenates as additives on the quality of yogurt: A review. Food Bioscience. 2022 doi: 10.1016/j.fbio.2022.101953. [DOI] [Google Scholar]
- Fan X., Yu L., Shi Z., Li C., Zeng X., Wu Z., Pan D. Characterization of a novel flavored yogurt enriched in γ-aminobutyric acid fermented by Levilactobacillus brevis CGMCC1. 5954. Journal of Dairy Science. 2023;106(2):852–867. doi: 10.3168/jds.2022-22590. [DOI] [PubMed] [Google Scholar]
- Fu R., Li J., Zhang T., Zhu T., Cheng R., Wang S., Zhang J. Salecan stabilizes the microstructure and improves the rheological performance of yogurt. Food Hydrocolloids. 2018;81:474–480. doi: 10.1016/j.foodhyd.2018.03.034. [DOI] [Google Scholar]
- Innocente N., Biasutti M., Rita F., Brichese R., Comi G., Iacumin L. Effect of indigenous Lactobacillus rhamnosus isolated from bovine milk on microbiological characteristics and aromatic profile of traditional yogurt. LWT-Food Science and Technology. 2016;66:158–164. [Google Scholar]
- Jiang Q., Li P., Ji M., Du L., Li S., Liu Y., Meng Z. Synergetic effects of water-soluble polysaccharides for intensifying performances of oleogels fabricated by oil-absorbing cryogels. Food Chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131357. [DOI] [PubMed] [Google Scholar]
- Katsuta K., Nishimura A., Miura M. Effects of saccharides on stabilities of rice starch gels. 1. Mono-and disaccharides. Food Hydrocolloids. 1992;6(4):387–398. doi: 10.1016/S0268-005X(09)80006-4. [DOI] [Google Scholar]
- Li C., Li W., Chen X., Feng M., Rui X., Jiang M., Dong M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT-Food Science and Technology. 2014;57(2):477–485. doi: 10.1016/j.lwt.2014.02.025. [DOI] [Google Scholar]
- Li L., Zhou P., Wang Y., Pan Y., Chen M., Tian Y., Zheng J. Antimicrobial activity of cyanidin-3-O-glucoside–lauric acid ester against Staphylococcus aureus and Escherichia coli. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132410. [DOI] [PubMed] [Google Scholar]
- Liu M., Bayjanov J.R., Renckens B., Nauta A., Siezen R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genomics. 2010;11:1–15. doi: 10.1186/1471-2164-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A., De Las Rivas B., Landete J.M., Tabera L., Muñoz R. Tyramine and phenylethylamine biosynthesis by food bacteria. Critical Reviews in Food Science and Nutrition. 2012;52(5):448–467. doi: 10.1080/10408398.2010.500545. [DOI] [PubMed] [Google Scholar]
- Newstead S. Insights into L-type heteromeric amino acid transporters. Nature Structural & Molecular Biology. 2019;26(6):395–396. doi: 10.1038/s41594-019-0240-z. [DOI] [PubMed] [Google Scholar]
- O’shea N., Ktenioudaki A., Smyth T.P., McLoughlin P., Doran L., Auty M.A.E., Gallagher E. Physicochemical assessment of two fruit by-products as functional ingredients: Apple and orange pomace. Journal of Food Engineering. 2015;153:89–95. doi: 10.1016/j.jfoodeng.2014.12.014. [DOI] [Google Scholar]
- Park C., Campanella O., Maleky F. The effects of whey protein and oleogel interactions on mechanical properties of oleocolloids and hydro-oleocolloids matrices. Food Hydrocolloids. 2022;124 doi: 10.1016/j.foodhyd.2021.107285. [DOI] [Google Scholar]
- Peng C., Yao G., Sun Y., Guo S., Wang J., Mu X., Zhang H. Comparative effects of the single and binary probiotics of Lacticaseibacillus casei Zhang and Bifidobacterium lactis V9 on the growth and metabolomic profiles in yogurts. Food Research International. 2022;152 doi: 10.1016/j.foodres.2021.110603. [DOI] [PubMed] [Google Scholar]
- Prag H.A., Aksentijevic D., Dannhorn A., Giles A.V., Mulvey J.F., Sauchanka O., Krieg T. Ischemia-selective cardioprotection by malonate for ischemia/reperfusion injury. Circulation Research. 2022;131(6):528–541. doi: 10.1161/CIRCRESAHA.121.320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashwan A.K., Osman A.I., Chen W. Natural nutraceuticals for enhancing yogurt properties: A review. Environmental Chemistry Letters. 2023;1–25 doi: 10.1007/s10311-023-01588-0. [DOI] [Google Scholar]
- Ren C., Quan T., Li B. Understanding the effect of anthocyanin-rich extract on the gel and digestive properties of soy protein cold-set gels. Food Biophysics. 2022;1–10 doi: 10.1007/s11483-022-09765-4. [DOI] [Google Scholar]
- Ren Z., Li Z., Chen Z., Zhang Y., Lin X., Weng W., Li B. Characteristics and application of fish oil-in-water pickering emulsions structured with tea water-insoluble proteins/κ-carrageenan complexes. Food Hydrocolloids. 2021;114 doi: 10.1016/j.foodhyd.2020.106562. [DOI] [Google Scholar]
- Routray W., Mishra H.N. Scientific and technical aspects of yogurt aroma and taste: A review. Comprehensive Reviews in Food Science and Food Safety. 2011;10(4):208–220. [Google Scholar]
- Sah B.N.P., Vasiljevic T., McKechnie S., Donkor O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Science and technology. 2016;65:978–986. doi: 10.1016/j.lwt.2015.09.027. [DOI] [Google Scholar]
- Scotton W.J., Hill L.J., Williams A.C., Barnes N.M. Serotonin syndrome: Pathophysiology, clinical features, management, and potential future directions. International Journal of Tryptophan Research. 2019;12 doi: 10.1177/1178646919873925. 1178646919873925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Quispe C., Imran M., Rauf A., Nadeem M., Gondal T.A., Calina D. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Medicine and Cellular Longevity. 2021;2021 doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu B., Qi Y., Wu D., Liu X., Liu C., Min W. Impact of Auricularia cornea var. Li polysaccharides on the physicochemical, textual, flavor, and antioxidant properties of set yogurt. International Journal of Biological Macromolecules. 2022;206:148–158. doi: 10.1016/j.ijbiomac.2022.02.141. [DOI] [PubMed] [Google Scholar]
- Wang J., Sun H., Guo S., Sun Y., Kwok L.Y., Zhang H., Peng C. Comparison of the effects of single probiotic strains Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis Probio-M8 and their combination on volatile and nonvolatile metabolomic profiles of yogurt. Journal of Dairy Science. 2021;104(7):7509–7521. doi: 10.3168/jds.2020-20099. [DOI] [PubMed] [Google Scholar]
- Wang X., Kristo E., LaPointe G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocolloids. 2019;91:83–91. doi: 10.1016/j.foodhyd.2019.01.004. [DOI] [Google Scholar]
- Wang X., Kristo E., LaPointe G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocolloids. 2020;100 doi: 10.1016/j.foodhyd.2019.105453. [DOI] [Google Scholar]
- Wu T., Deng C., Luo S., Liu C., Hu X. Effect of rice bran on properties of yogurt: Comparison between addition of bran before fermentation and after fermentation. Food Hydrocolloids. 2023;135 doi: 10.1016/j.foodhyd.2022.108122. [DOI] [Google Scholar]
- Wu Z., Chen T., Pan D., Zeng X., Guo Y., Zhao G. Resveratrol and organic selenium-rich fermented milk reduces D-galactose-induced cognitive dysfunction in mice. Food & Function. 2021;12(3):1318–1326. doi: 10.1039/d0fo02029j. [DOI] [PubMed] [Google Scholar]
- Wu Z., Wang P., Pan D., Zeng X., Guo Y., Zhao G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. Journal of Dairy Science. 2021;104(1):78–91. doi: 10.3168/jds.2020-19154. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Xiao J.Z., Odamaki T., Ishida T., Miyaji K., Yamada A., Iwatsuki K. Improved growth of bifidobacteria by cocultivation with Lactococcus lactis subspecies lactis. Journal of Dairy Science. 2010;93(5):1815–1823. doi: 10.3168/jds.2009-2708. [DOI] [PubMed] [Google Scholar]
- You J., Pan X., Yang C., Du Y., Osire T., Yang T., Rao Z. Microbial production of riboflavin: Biotechnological advances and perspectives. Metabolic Engineering. 2021;68:46–58. doi: 10.1016/j.ymben.2021.08.009. [DOI] [PubMed] [Google Scholar]
- Zainudin M.A.M., Jongberg S., Lund M.N. Combination of light and oxygen accelerates formation of covalent protein-polyphenol bonding during chill storage of meat added 4-methyl catechol. Food Chemistry. 2021;334 doi: 10.1016/j.foodchem.2020.127611. [DOI] [PubMed] [Google Scholar]
- Zhang F.X., Li M., Qiao L.R., Yao Z.H., Li C., Shen X.Y., Dai Y. Rapid characterization of Ziziphi Spinosae Semen by UPLC/Qtof MS with novel informatics platform and its application in evaluation of two seeds from Ziziphus species. Journal of Pharmaceutical and Biomedical Analysis. 2016;122:59–80. doi: 10.1016/j.jpba.2016.01.047. [DOI] [PubMed] [Google Scholar]
- Zou X., Xiao J., Chi J., Zhang M., Zhang R., Jia X., Huang F. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. International Journal of Biological Macromolecules. 2022;223:663–672. doi: 10.1016/j.ijbiomac.2022.11.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.