Abstract
The objective of this study was to assess the effects of phosphate solubilizing rhizo-microbes inoculants on nutrient balance, physiological adaptation, growth characteristics, and rhizome yield traits as well as curcuminoids yield at the secondary-rhizome initiation stage of turmeric plants, subsequently subjected to water-deficit (WD) stress. Phosphorus contents in the leaf tissues of Talaromyces aff. macrosporus and Burkholderia sp. (Bruk) inoculated plants peaked at 0.33 and 0.29 mg g−1 DW, respectively, under well-watered (WW) conditions; however, phosphorus contents declined when subjected to WD conditions (p ≤ 0.05). Similarly, potassium and calcium contents reached their maximum values at 5.33 and 3.47 mg g−1 DW, respectively, in Burk inoculated plants under WW conditions, which contributed to sustained rhizome fresh weight even when exposed to WD conditions (p ≤ 0.05). There was an increase in free proline content in T. aff. macrosporus and Burk inoculated plants under WD conditions, which played a crucial role in controlling leaf osmotic potential, thereby stabilizing leaf greenness and maximum quantum yield of PSII. As indicators of drought stress, there were noticeable restrictions in stomatal gas exchange parameters, including net photosynthetic rate, stomatal conductance, and transpiration rate, accompanied by an increase in leaf temperature. These changes resulted in reduced total soluble sugar levels. Interestingly, total curcuminoids and curcuminoids yield in Burk inoculated plants under WD conditions were retained, especially in relation to rhizome biomass. Burk inoculation in turmeric plants is recommended as a promising technique as it alleviates water-deficit stress, sustains rhizome biomass, and stabilizes curcuminoids yield.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-024-03922-x.
Keywords: Burkholderia, Drought stress, Phosphate-solubilizing bacteria/rhizobacteria, Pseudomonas, Talaromyces aff. macrosporus, Total curcuminoids
Introduction
Drought stress is one of the prominent abiotic stresses, with its prevalence increasing year-by-year around the world, primarily linked to global climate change, especially the rise in global temperatures (Hermans and McLeman 2020; Philip et al. 2020; Pokhrel et al. 2021; Raza et al. 2023a, b). Plant growth, development, and productivity have been reported to be sensitive to prolonged drought conditions, resulting in food and nutrition insecurity and poor livelihood conditions (Kogan et al. 2019). It has been mentioned that an estimated 10 million people (~ 0.13% of the world’s population) suffered from hunger in 2015 due to drought (Kogan et al. 2020; Mansoor et al. 2022). There are several strategies to mitigate the impact of drought in crop production, such as (i) enhancing crop drought tolerance through a breeding program, (ii) selecting suitable crops having higher water productivity, (iii) implementing improved agricultural management practices, and (iv) employing short-gun technology, such as the exogenous application of drought-mitigating stimulants (both chemical and biological stimulants) (Patni et al. 2019; Ullah et al. 2019, 2021; Ingrisch et al. 2023). Recently, organic farming systems have been characterized as consumer-friendly and environmentally sustainable systems that requires minimal inputs for crop production (Eyhorn et al. 2019; Smith et al. 2019). In organic farming of medicinal plants, chemical residue-free production is desired, which necessitates the use of biofertilizers, plant growth-promoting rhizo-microbes, biopesticides, and bioelicitors for bioactive compound enrichment as normal practices (Gupta et al. 2020; Santos and Olivares 2021; Elhaissoufi et al. 2022; Suman et al. 2022).
Bio-inoculants have been extensively explored for their positive roles in stimulating plant growth, enhancing biotic and abiotic defense responses for plant growth and development, and promoting overall productivity (Kour et al. 2020a; He et al. 2021; Pal et al. 2022). Symbiotic rhizobacteria (Bacillus, Pseudomonas, Burkholderia, Enterobacter, among others) and fungi (Trichoderma, Rhizoctonia, Fusarium, Penicillium, Talaromyces, among others), residing in the root zone of target plants, have been reported as potential candidates for collection and identification of activities, such as phosphate solubilization, indole butyric acid (IBA)/gibberellic acid (GA3) production, and plant growth stimulation (Rawat et al. 2021; Li et al. 2022a; Majeed et al. 2022). To date, the utilization of microbial inoculants has been reported as economically beneficial and eco-friendly approach for the farmers, which provides several advantages in terms of solubilizing phosphorus (P) and potassium (K) (biofertilizer), enriching water-holding capacity, enhancing plant growth regulators (such as phytohormone), improving overall growth and yield, and ameliorating the quality of bioactive ingredients (as bioelicitor) (Pal et al. 2022; Shaffique et al. 2022; Lone et al. 2023; Sodhi and Saxena 2023). Plant growth-promoting rhizobacteria (PGPR)-mediated regulation on drought defense mechanisms, including osmotic adjustments, at the cellular level, physiological adaptations, and retention of plant biomass has been well established (Oleńska et al. 2020; Sati et al. 2023). There is a paucity of literature exploring the effects of phosphate-solubilizing bacteria in regulating belowground plant parts, especially in relation to rhizome yield traits under drought stress (Bargaz et al. 2021). In addition, the long growth duration (9 − 11 months) of rhizome crops, including turmeric (Curcuma longa L.) plant, could encounter significant challenges when subjected to water shortage, especially in rainfed regions without a structured irrigation schedule (Chintakovid et al. 2022; Praseartkul et al. 2023).
Turmeric, a member of the Zingiberaceae family, is a reliable source of supplementary food for both humans and animals. In addition, its rhizome contains curcuminoids, which possess pharmacological properties beneficial for preventing various diseases (Sultana et al. 2021; Fuloria et al. 2022; Jyotirmayee and Mahalik 2022). The elite turmeric genotypes, characterized by their giant rhizome and high curcuminoids content, have been identified and selected as good master-stock resources for large-scale production by farmers (Pal et al. 2020; Dudekula et al. 2022; Praseartkul et al. 2023). Likewise, efficient cultivation management practices, including irrigation scheduling, fertilization, elicitation techniques, and environmental regulations, have been reported as key factors for maximizing rhizome biomass and curcuminoids yield in turmeric plants (Tripathi et al. 2021; El Sherif et al. 2022; Thakur et al. 2022; Khattab et al. 2023). Recently, a significant attention has been directed toward implementing low input strategies for turmeric production, such as the use of biofertilization, optimized water management aligned with crop water requirements, and diseases prevention through biocontrol products (Kumar et al. 2017; Chandrashekhar and Hore 2019). One of the promising approaches within the realm of organic farming for turmeric production involves the inoculation of plant growth-promoting rhizo-microbes and phosphate solubilizing rhizo-microbes (PSR). This approach fulfills the requirements of using a plant species for medicinal purposes and minimizes chemical residue contamination, especially in turmeric plants (Dutta and Neog 2016, 2017; Kumar et al. 2016; Dinesh et al. 2022; Jagtep et al. 2023). The existing literature does not adequately address the improvement of turmeric plants grown under water-deficit stress using inoculation of PSR. It was hypothesized that the solubilization of phosphate in the soil solution might be regulated by candidate PSR in belowground root zone environments. The solubilized phosphate would then be taken up by the plant’s roots and translocate to the whole plant, which subsequently would alleviate water-deficit stress and retain the growth performance and yield attributes. The objective of this study was to evaluate the efficiency of PSR in mitigating the adverse impacts of water-deficit stress in turmeric plants in terms of nutrient balance, physiological adaptations, growth characteristics, rhizome yield traits, and curcuminoids yield.
Materials and methods
Plant materials, phosphate solubilizing rhizo-microbes inoculation, and water‑deficit treatments
The initial rhizome materials were collected from Surat Thani province, Southern region of Thailand. These rhizomes belong to a local variety known for its high curcuminoids content. The rhizomes were surface cleaned using sodium hypochlorite (0.2% active ingredient; Clorox®) to disinfect any bacterial and fungal contamination. The new shoots that sprouted from the rhizome were dissected, sterilized using 10% Clorox® solution, and proliferated through in vitro micropropagation. Subsequently, a single shoot from in vitro-cultured turmeric plant was separated and only meristematic tissues (0.5 − 1.0 mm in size from the shoot tip) were dissected under a compound microscope and then cultured on the slant agar medium. Following the successful regeneration of green plantlets, which were free from fungal and bacterial diseases, these plantlets were tested using tetrazolium media (chemical assay). Their disease-free status was further confirmed by PCR (polymerase chain reaction)-based assay. These disease-free plantlets were clonally propagated using the plant tissue culture technique. The resulting turmeric plantlets were used as starting materials for the experiment. These plantlets were directly transplanted into peat moss as the growing medium and were acclimatized under the greenhouse conditions (80 ± 10% relative humidity, 30 ± 2 °C air temperature, 700 ± 100 μmol m−2 s−1 photosynthetic photon flux density) at the Thailand Science Park, Pathum Thani, Thailand (latitude 14°04′51.6"N; longitude 100°36′09.1"E; 2.5 m above mean sea level) for one month. An individual acclimatized plantlet with uniform plant height and number of leaves was transferred into each plastic bag (width × length × height: 15 cm × 30 cm × 30 cm) containing gardening soil (EC = 2.7 dS m−1, pH = 5.5, organic matter = 10.4%, total N = 0.17%, total P = 0.07%, total K = 1.19%). Slow-release fertilizer (Osmocote®; N:P:K = 13:13:13) was applied at the rate of 10 g plant−1. Plants were irrigated daily, and weeds were manually removed when required. Turmeric plants were divided into five groups and grown for a period of five months in gardening soil under greenhouse conditions (80 ± 10% relative humidity, 30 ± 2 °C air temperature, and 700 ± 100 μmol m−2 s−1 photosynthetic photon flux density): (i) Negative control (without rock phosphate [RP] and PSR inoculation), (ii) + RP (control without PSR + 2.5% RP [30% active ingredient P2O5; Chewachem Ltd., Bangkok, Thailand]), (iii) T. aff. macrosporus (1.5 × 109 colony-forming unit [CFU] bag−1 of Talaromyces aff. macrosporus fungi [T. aff. macrosporus; Department of Agriculture, Ministry of Agriculture and Cooperatives, Thailand] inoculation + 2.5% RP), (iv) MMO1 (soil drenching with mixed phosphate solubilizing Pseudomonas and Bacillus strains [Institute of Product Quality and Standardization, Maejo University, Chaing Mai, Thailand] at the rate of 100 mL of 1:250 dilute liquid solution + 2.5% RP), and (v) Burk (Burkholderia sp. [a phosphate solubilizing bacterium at the rate of 35.14 g bag−1; Tumdin™, Chatuchak, Bangkok, Thailand] + 2.5% RP). Subsequently, after five months, these groups were divided into two irrigation regimes: (i) well-watered (WW; 100% field capacity [FC])— daily irrigated with 400 mL plant−1 and (ii) water-deficit (WD; 71% FC)— withholding water for 45 days (Fig. S1).
Leaf mineral nutrient analysis
Samples of the second fully-expanded leaf without leaf veins were collected and washed in two steps: (i) dipping two times in distilled water and then (ii) rinsing by deionized water to remove any surface ion contamination. Subsequently, the leaf samples were analyzed for their P, K, magnesium (Mg), and calcium (Ca) contents. The leaf samples were collected and dried in a hot air oven at 60 °C for three days and then stored inside a deep freezer at − 20 °C prior to further analysis. Dried samples were finely ground into powder in a mortar using liquid nitrogen. Subsequently, they underwent digestion with nitric acid according to modified United States Environmental Protection Agency (USEPA) Method 3050B (Phukunkamkaew et al. 2021). Fifty milligrams of powder sample were mixed with 1 mL of dilute 69% nitric acid: deionized water (1:1, v/v). This mixture was then heated to 100 ± 5 °C for 15 min using heat block digestion, and subsequently 0.5 mL of 69% nitric acid was added and then reheated at 100 ± 5 °C for 2 h. After cooling, the extracted solution was diluted with deionized water to reach a final volume of 10 mL. Digested solutions were filtered using a 0.45-µm pore size PTFE filter (polytetrafuoroethylene membrane). The contents of P, K, Mg, and Ca were analyzed using an inductively coupled plasma-optical emission spectroscope (ICP-OES; PerkinElmer Avio 200). The P, K, Mg, and Ca standard elements were calibrated on ICP-OES using 100 mg L−1 multi-element calibration standard in 5% nitric acid.
Plant growth characteristics and rhizome attributes
The aboveground traits, such as pseudostem height (cm), leaf length (cm), leaf width (cm), number of tillers, number of roots, pseudostem fresh (g plant−1) and dry weights (g plant−1), and leaf fresh (g plant−1) and dry weights (g plant−1) were measured. The following belowground traits were also measured: root length (cm), number of roots, root fresh (g plant−1) and dry weights (g plant−1), rhizome width (cm), rhizome length (cm), rhizome fresh (g plant−1) and dry weights (g plant−1), number of primary rhizomes, and number of secondary rhizomes. For dry weight calculations, the harvested materials (leaf, pseudostem, root, and rhizome) from each treatment were washed and chopped into small pieces, and then dried in a hot air oven at 50 °C until a stable weight was achieved, and subsequently dry weight was recorded.
Plant biochemical analysis
The second fully-expanded leaf was used to extract and analyze free proline. A total of 50 mg of freeze-dried samples was first ground into powder in a mortar with liquid nitrogen. Following that, 1 mL of aqueous sulfosalicylic acid (3%, w/v) was added into homogenate powder and the mixture was then filtered through a filter paper (Whatman™ No.1). Subsequently, an equal volume of glacial acetic acid and ninhydrin reagent (1.25 mg ninhydrin in 30 mL glacial acetic acid and 20 mL 6 M H3PO4) was combined with the extracted solution and incubated in a water bath at 95 °C for 1 h. The reaction was terminated by placing the container in an ice bath. Finally, the reaction mixture was vigorously vibrated with 2 mL of toluene. After cooling to 25 °C, the chromophore in the upper layer was collected and measured at 520 nm using an ultraviolet–visible spectrophotometer against the L-proline (0–100 μmol mL−1) calibration curve.
The sucrose, glucose, and fructose concentrations in both the rhizome and second fully-expanded leaf were determined using a high-performance liquid chromatography (HPLC). The process involved harvesting mature rhizome and leaf samples, chopping them into small pieces, freeze-drying, and then grounding them into fine powder using a mortar with liquid nitrogen. The water-soluble sugar components were extracted from 50 mg of the powder using 1 mL nanopure water, followed by vigorous vortexing for 15 s, sonication for 15 min using ultrasonic apparatus, and subsequent centrifugation at 12,000 × g for 15 min. The resulting supernatant was collected, diluted 10 folds, and purified through a 0.45 m membrane filter (VertiPure™, Vertical®, Thailand). The extracted solution (20 μL) was directly injected into the HPLC injection loop. The analysis was performed using the Waters 600 HPLC system (Waters Associates, Milford, MA, USA), equipped with Waters 410 differential refractometer detector and MetaCarb 87 °C column (7.8 × 300 mm; Agilent Technologies, Munich, Germany). The detector temperature was set at 40 °C, while the column and guard column were equilibrated at 85 °C using a column heater. The deionized water with a flow rate of 0.5 mL min−1 was used as the mobile phase. Glucose, fructose, and sucrose (Fluka® USA) standards were injected as a standard curve for the quantification of each sugar class.
For the curcuminoids assay, the dry-harvested rhizomes were cleaned thoroughly with tap water, sliced into small pieces, and allowed to dry in a hot air oven at 50 °C for 96 h. The pieces were then transformed into powder using Moulinex™ Blender (Groupe SEB, France). For the extraction of curcuminoids, a total of 50 mg of dried powder was transferred into a vial, followed by the addition of 5 mL methanol. The resulting mixture was vigorously agitated using a vortex, sonicated for 30 min, then the supernatant was filtered through a filter paper (Whatman™ No.1). The extracted solution was then dried and stored in a deep freezer (− 20 °C) prior to curcuminoids assay.
For curcuminoids analysis, dried extracted samples were suspended in 1 mL methanol and then filtrated through a 0.45 μm pore size (Millipore™ nylon filter). A total of 10 µL of sample was injected into an injection loop and analyzed using a HPLC (Waters Associates, Milford, MA, USA) equipped with a Water 2998 photodiode array detector at 425 nm. Bisdemethoxycurcumin (BIS), demethoxycurcumin (DEM), and curcumin (CUR) were separated using C18 (Vertisep™ UPS) column maintained at 25 °C. The mobile phase consisted of acetonitrile (100% HPLC grade) and acetic acid (0.25%, v/v). The elution was carried out using a gradient with a flow rate of 0.8 mL min−1. The solvent gradient sequence was: 50% acetonitrile up to 8 min, 50 to 40% acetonitrile from 8 to 10 min, 40% acetonitrile constant from 10 to 15 min, and 40 to 50% acetonitrile from 15 to 16 min.
Plant physiological measurements
Leaf greenness (SPAD value) was measured from the second fully-expanded leaf (average of five points per leaf) using a Chlorophyll Meter (SPAD-520Plus, Konica Minolta, Osaka, Japan). The data pertaining to the maximum quantum yield of PSII (Fv/Fm) and photon yield of PSII (ΦPSII) were collected from the adaxial surface of the second fully-expanded leaf using a Fluorescence Monitoring System (FMS 2; Hansatech Instruments Ltd., Norfolk, UK) in the pulse amplitude modulation mode. Net photosynthetic rate (Pn; µmol CO2 m−2 s−1), stomatal conductance (gs; mmol H2O m−2 s−1), and transpiration rate (E; mmol H2O m−2 s−1) were collected using a portable photosynthesis system (LI 6400XT, LI-COR, Lincoln, NE, USA).
A forward-looking infrared (FLIR) camera (spectral range 7.5–14.0 μm and resolution of 240 × 180 pixels) positioned 0.9 m above the top of the turmeric canopy was used. An emissivity value of 0.95 was considered. The wet reference (Twet) and the dry reference (Tdry) were recorded with wet-water cotton wool and black paper, respectively. Image analysis was carried out using FLIR Tool 5.1 software (https://flir-tools.software.informer.com/5.0/). Consequently, crop water stress index (CWSI) was derived from the thermal images using Eq. 1. CWSI indicates the water stress level in the plant and ranges between 0 and 1, in which 1 indicates the maximum degree of stress and 0 indicates the absence of stress.
| 1 |
where Tleaf is the mean leaf temperature, Tdry is the dry reference temperature obtained by black paper, and Twet is the wet reference temperature obtained by wet cotton wool.
Leaf osmotic potential was measured using an Osmometer (model 5600 Vapro®, Wescor Utah, USA). The fresh leaf sample was squeezed by a glass rod in a 1.5 mL plastic tube. Thereafter, 10 µL of the extracted leaf solution was collected and dropped onto a filter paper in an osmometer chamber, and the data were noted. The osmolarity (mmol kg−1) was converted to osmotic potential (ψs; MPa) using a conversion factor of osmotic potential measurement (Eq. 2):
| 2 |
where c is an osmolarity.
Experimental layout and statistical analysis
The experiment was set up as a 5 × 2 factorial design in a completely randomized layout with five replications of each treatment. Two-way analysis of variance (ANOVA) was performed for each parameter using the Statistical Package for the Social Sciences (SPSS) software (ver. 11.5 for Window®). The mean values obtained were compared using Tukey’s honest significant difference test at p ≤ 0.05.
Results
Leaf mineral nutrient content
Under well-watered (WW) conditions, P content in the leaf tissues of turmeric plants was very low under the negative control (0.24 mg g−1 DW), + RP (0.25 mg g−1 DW), and MMO1 (0.21 mg g−1 DW) treatments (Fig. 1; Table S1; p ≤ 0.05). However, P content was significantly increased with T. aff. macrosporus (0.33 mg g−1 DW) and Burk (0.29 mg g−1 DW) inoculations under WW conditions. Conversely, under water-deficit (WD) conditions, a substantial reduction in P content was observed under the negative control (0.12 mg g−1 DW or 51% reduction compared with WW), + RP (0.14 mg g−1 DW or 45% reduction compared with WW), and T. aff. macrosporus (0.16 mg g−1 DW or 50% reduction compared with WW) treatments (Fig. 1; Table S1; p ≤ 0.05). Moreover, the reduction in P content was relatively lesser under MMO1 [0.15 mg g−1 DW or 29% reduction compared with WW (0.21 mg g−1 DW)] and Burk inoculation [0.18 mg g−1 DW or 38% reduction compared with WW (0.29 mg g−1 DW)] (Fig. 1). Leaf K content of WW-turmeric plants under Burk inoculation was the highest [5.33 mg g−1 DW or 41% greater than the negative control (3.78 mg g−1 DW)] (p ≤ 0.01) (Table 1). Leaf K content was highly sensitive to WD conditions, which was decreased by 40% (2.27 mg g−1 DW), 51% (2.38 mg g−1 DW), 46% (2.76 mg g−1 DW), 37% (2.79 mg g−1 DW), and 43% (3.03 mg g−1 DW) compared with WW conditions under the negative control, + RP, T. aff. macrosporus, MMO1, and Burk inoculation, respectively. Leaf Mg content of plants grown with T. aff. macrosporus inoculation under WW conditions was the highest [0.83 mg g−1 DW or 43% greater than the negative control (0.58 mg g−1 DW)] (Table 1). Under WD conditions, Mg content was found stable under the negative control (0.41 mg g−1 DW), MMO1 (0.44 mg g−1 DW), and Burk inoculation (0.53 mg g−1 DW), whereas it was significantly reduced under + RP (0.38 mg g−1 DW or 43% reduction) and T. aff. macrosporus (0.41 mg g−1 DW or 51% reduction) inoculations. Interestingly, Ca content in the leaf tissues of plants under WW conditions was significantly increased under + RP treatment [2.81 mg g−1 DW or 66% increase compared with the negative control (1.69 mg g−1 DW)], and reached its peak under T. aff. macrosporus inoculation (3.29 mg g−1 DW or 95% increase compared with the negative control) and under Burk inoculation (3.47 mg g−1 DW or 105% increase compared with the negative control) (Table 1). However, Ca content under WD conditions remained unchanged under the negative control (0.84 mg g−1 DW) and MMO1 (1.88 mg g−1 DW) treatments, while it was sharply declined under + RP, T. aff. macrosporus, and Burk inoculation by 59% (1.15 mg g−1 DW), 59% (1.35 mg g−1 DW), and 65% (1.22 mg g−1 DW) compared with WW conditions, respectively.
Fig. 1.
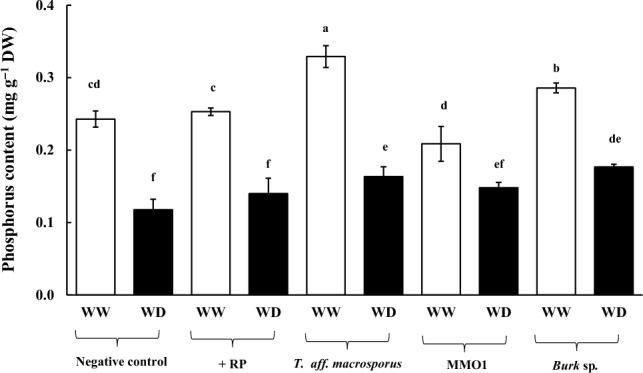
Phosphorus content in leaf tissues of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days. Data are presented as means of five replications ± standard errors. Different letters in each bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Table 1.
Potassium, magnesium, and calcium contents in the leaf tissues of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days
| Inoculation | Water regime | Mineral nutrient (mg g−1 DW) | ||
|---|---|---|---|---|
| Potassium | Magnesium | Calcium | ||
| Negative control | WW | 3.78 ± 0.12bcd | 0.58 ± 0.03bcd | 1.69 ± 0.17 cd |
| WD | 2.27 ± 0.20e (40%) | 0.41 ± 0.04 cd | 0.84 ± 0.09d | |
| + RP | WW | 4.82 ± 0.31ab | 0.67 ± 0.08abc | 2.81 ± 0.19ab |
| WD | 2.38 ± 0.42de (51%) | 0.38 ± 0.04d (43%) | 1.15 ± 0.13d (59%) | |
| T. aff. macrosporus | WW | 5.08 ± 0.30ab | 0.83 ± 0.08a | 3.29 ± 0.29a |
| WD | 2.76 ± 0.15de (46%) | 0.41 ± 0.05 cd (51%) | 1.35 ± 0.22d (59%) | |
| MMO1 | WW | 4.46 ± 0.63abc | 0.68 ± 0.09abc | 2.50 ± 0.45abc |
| WD | 2.79 ± 0.16de (37%) | 0.44 ± 0.01 cd | 1.88 ± 0.17bcd | |
| Burk sp. | WW | 5.33 ± 0.36a | 0.79 ± 0.12ab | 3.47 ± 0.21a |
| WD | 3.03 ± 0.05cde (43%) | 0.53 ± 0.01bcd | 1.22 ± 0.07d (65%) | |
| Significance level | ||||
| Inoculation | ** | ** | ** | |
| Water regime | ** | ** | ** | |
| Inoculation × Water regime | ns | Ns | ** | |
Different letters in each column represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05. ns indicates not significant and **indicates highly significant at p ≤ 0.01. Reduction percentage was calculated based on Eq.: [(WW–WD)/WW] × 100, and is presented in parenthesis
Data are presented as means of five replications ± standard errors
Plant growth performance and rhizome yield traits
Plant morphology and rhizome traits were collected from each treatment (Fig. 2). Under WD conditions, leaf chlorosis and tip burn were evident, especially under the negative control and + RP treatments, which resulted in the production of smaller rhizomes. Pseudostem height and leaf width of turmeric plants in each treatment remained unchanged (Table S2). Leaf length of turmeric plants in + RP treatment under WW conditions was the maximum (17.1 cm), which was significantly decreased by 29% when exposed to WD conditions (12.1 cm; p ≤ 0.05). The number of tillers was the maximum in the negative control (4.0 tillers plant−1) and + RP treatments (3.8 tillers plant−1) under WW conditions, which was sharply decreased (≥ 40%) when exposed to WD conditions (p ≤ 0.05). Under WW conditions, aboveground traits, such as number of leaves (16.2 leaves plant−1), pseudostem fresh weight (305 g plant−1), pseudostem dry weight (24.6 g plant−1), leaf fresh weight (109 g plant−1), and leaf dry weight (20.6 g plant−1), were notably favorable in turmeric plants treated with Burk inoculation (p ≤ 0.01) (Table 2). Under WD conditions, number of leaves per plant was significantly reduced in + RP treatment (6.0 leaves plant−1) by 63% compared with WW conditions (16.0 leaves plant−1), whereas it remained unchanged in other treatments. Pseudostem fresh weight under WD conditions, regardless of inoculations, decreased significantly by over 35% compared with WW conditions. The reduction in pseudostem fresh weight was larger under the negative control (51% or 137 g plant−1) and + RP (46% or 155 g plant−1) treatments compared with PSR-inoculated plants under WD conditions. Interestingly, pseudostem dry weight under WD conditions was significantly improved under MMO1 treatment (16.6 g plant−1), whereas it was declined by over 30% in other treatments compared with WW conditions. Leaf fresh weight and leaf dry weight in plants under WD conditions also declined by over 35% compared with WW conditions (p ≤ 0.01) (Table 2).
Fig. 2.
Morphological characteristics of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days
Table 2.
Number of leaves (NL), pseudostem fresh weight (PSFW), pseudostem dry weight (PSDW), leaf fresh weight (LFW), and leaf dry weight (LDW) of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days
| Inoculation | Water regime | NL | PSFW (g plant−1) | PSDW (g plant−1) | LFW (g plant−1) | LDW (g plant−1) |
|---|---|---|---|---|---|---|
| Negative control | WW | 14.2 ± 1.0ab | 278 ± 14abc | 21.8 ± 1.0ab | 104.6 ± 3.0a | 15.8 ± 0.1b |
| WD | 8.4 ± 1.4bc | 137 ± 16d (51%) | 11.8 ± 1.1d (46%) | 46.4 ± 2.9c (56%) | 8.3 ± 0.6c (48%) | |
| + RP | WW | 16.0 ± 0.8a | 287 ± 16ab | 21.2 ± 0.8abc | 110.5 ± 4.9a | 18.3 ± 0.7ab |
| WD | 6.0 ± 0.8c (62.5%) | 155 ± 24d (46%) | 13.9 ± 1.2d (34%) | 45.4 ± 9.7c (59%) | 8.5 ± 0.9c (54%) | |
| T. aff. macrosporus | WW | 10.6 ± 1.1abc | 320 ± 25a | 24.9 ± 1.7a | 108.2 ± 6.3a | 21.3 ± 1.0a |
| WD | 10.8 ± 1.0abc | 201 ± 9bcd (37%) | 17.1 ± 1.0bcd (31%) | 56.6 ± 2.7c (48%) | 10.7 ± 0.4c (50%) | |
| MMO1 | WW | 8.2 ± 4.1bc | 270 ± 24abc | 22.1 ± 2.1ab | 85.7 ± 9.3b | 16.5 ± 1.8b |
| WD | 6.2 ± 1.2c | 190 ± 9 cd | 16.6 ± 0.8bcd | 51.2 ± 6.2c (40%) | 10.8 ± 0.5c (35%) | |
| Burk sp. | WW | 16.2 ± 0.5a | 305 ± 29a | 24.6 ± 2.0a | 109.0 ± 6.6a | 20.6 ± 0.5a |
| WD | 10.0 ± 1.3abc | 192 ± 4 cd (37%) | 15.4 ± 0.2 cd (37%) | 65.9 ± 1.9bc (40%) | 11.4 ± 0.3c (45%) | |
| Significance level | ||||||
| Inoculation | ** | * | ** | * | ** | |
| Water regime | * | ** | ** | ** | ** | |
| Water regime × Inoculation | ** | ** | ns | ** | ** |
Different letters in each column represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05. ns, *, and **indicate not significant, significant at p ≤ 0.05, and highly significant at p ≤ 0.01, respectively. Reduction percentage was calculated based on Eq.: [(WW–WD)/WW] × 100, and is presented in parenthesis
Data are presented as means of five replications ± standard errors
The belowground root and rhizome traits of turmeric plants were determined at the harvesting stage. In plants under + RP treatment in WW conditions, a shorter root was evident (52.8 cm) compared with the negative control (78.5 cm), T. aff. macrosporus (78.9 cm), and Burk inoculation (72.1 cm) (p ≤ 0.01) (Table 3). Root length was retarded by 26% (48.8 cm) and 29% (51.1 cm) under WD conditions compared with WW conditions in MMO1 (65.9 cm) and Burk (72.1 cm) inoculated plants, respectively. However, root length remained stable in other treatments. Interestingly, the number of roots per plant under WD conditions was significantly decreased (p ≤ 0.05) under T. aff. macrosporus inoculation by 34% (31.0 roots plant−1), which was similar to 28% reduction (37.2 roots plant−1) under the negative control (Table S2). On the other hand, root fresh weight (38.7 g plant−1) and root dry weight (3.3 g plant−1) in MMO1 inoculated plants were the lowest, declined by more than 50% compared with the negative control (63.7 and 6.2 g plant−1, respectively) (Table 3; p ≤ 0.05). However, these parameters remained consistent when exposed to WD conditions under other treatments. Rhizome width in Burk inoculated plants under WW conditions was the maximum (24.4 cm), which was 26% larger compared with the + RP inoculated plants (19.4 cm; p ≤ 0.05) (Table 3). Rhizome length remained consistent across most treatments, except in Burk inoculated plants under WD conditions (15.2 cm) [declined by 17% compared with WW conditions (18.4 cm; p ≤ 0.05)] (Table 3). The maximum number of primary (4.4 rhizomes plant−1) and secondary rhizomes (19.4 rhizomes plant−1) was observed in + RP inoculation under WW conditions (Table 3; p ≤ 0.01). The number of primary rhizomes in MMO1 inoculated plants under WW conditions (1.6 rhizomes plant−1) was the lowest (64% lower compared with + RP treatment under WW conditions), whereas the number of primary rhizomes remained unaffected under WD conditions in MMO1 inoculated plants. Under WD conditions, the number of secondary rhizomes significantly declined in + RP (12.6 rhizomes plant−1) and T. aff. macrosporus inoculated plants (10.0 rhizomes plant−1) by 35% and 30%, respectively, compared with WW conditions (Table 3; p ≤ 0.01). A positive relationship was observed between pseudostem fresh weight and rhizome fresh weight (R2 = 0.84; Fig. 3a). Under WW conditions, rhizome fresh weight in T. aff. macrosporus inoculated plants was the highest (675 g plant−1) (Fig. 3b). However, under WD conditions, rhizome fresh weight was decreased by 39% (352 g plant−1), 27% (354 g plant−1), 38% (419 g plant−1), and 41% (319 g plant−1) in the negative control, + RP, T. aff. macrosporus, and MMO1 inoculated plants, respectively, compared with WW conditions (p ≤ 0.01; Table S1), whereas rhizome fresh weight was retained in Burk inoculated plants (Fig. 3b). A positive relationship was observed between pseudostem dry weight and rhizome dry weight (R2 = 0.76; Fig. 3c). The maximum rhizome dry weight (71.1 g plant−1) was observed in Burk inoculated plants under WW conditions (p ≤ 0.05) (Fig. 3d). Under WD conditions, rhizome dry weight was decreased by 35% (38.9 g plant−1), 42% (40.3 g plant−1), and 40% (42.5 g plant−1) in the negative control, T. aff. macrosporus, and Burk inoculated plants, respectively, compared with WW conditions (p ≤ 0.01; Table S1). However, rhizome dry weight was retained in + RP and MMO1 inoculated plants under WD conditions.
Table 3.
Root length (RTL), root fresh weight (RTFW), root dry weight (RTDW), rhizome width (RhW), rhizome length (RhL), number of primary rhizomes (NPRh), and number of secondary rhizomes (NSRh) of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days
| Inoculation | Water regime | RTL (cm) | RTFW (g plant−1) | RTDW (g plant−1) | RhW (cm) | RhL (cm) | NPRh | NSRh | |
|---|---|---|---|---|---|---|---|---|---|
| Negative control | WW | 78.5 ± 8.6a | 63.7 ± 6.2a | 6.2 ± 0.6ab | 20.6 ± 1.0ab | 18.6 ± 0.8a | 3.6 ± 0.2ab | 14.0 ± 1.6b | |
| WD | 80.2 ± 4.1a | 61.5 ± 6.5a | 7.9 ± 0.7a | 19.6 ± 0.8b | 17.3 ± 1.2ab | 2.0 ± 0.6ab | 12.0 ± 1.5bc | ||
| + RP | WW | 52.8 ± 7.0b | 50.0 ± 10.2ab | 4.1 ± 0.7b | 19.4 ± 0.6b | 16.6 ± 0.7ab | 4.4 ± 0.5a | 19.4 ± 2.3a | |
| WD | 52.3 ± 2.8b | 54.9 ± 12.3ab | 6.0 ± 1.3ab | 20.7 ± 0.7ab | 15.5 ± 0.6b | 2.0 ± 0.3ab | 12.6 ± 2.1bc (35%) | ||
| T. aff. macrosporus | WW | 78.9 ± 6.6a | 50.1 ± 4.8ab | 4.5 ± 0.6ab | 22.3 ± 0.7ab | 17.0 ± 0.7ab | 2.0 ± 0.3ab | 14.2 ± 1.9b | |
| WD | 75.0 ± 7.1a | 55.2 ± 5.7ab | 6.6 ± 0.8ab | 19.8 ± 1.0b | 17.1 ± 1.1ab | 2.8 ± 0.5ab | 10.0 ± 0.6c (30%) | ||
| MMO1 | WW | 65.9 ± 7.8ab | 38.7 ± 6.1b | 3.3 ± 0.4b | 22.9 ± 0.9b | 16.6 ± 2.2ab | 1.6 ± 0.4b | 11.4 ± 1.5bc | |
| WD | 48.8 ± 4.7c (26%) | 32.9 ± 6.7b | 4.2 ± 0.9ab | 19.5 ± 0.8ab | 14.3 ± 0.2b | 1.2 ± 0.2b | 8.0 ± 0.6c | ||
| Burk sp. | WW | 72.1 ± 9.1a | 57.3 ± 6.2a | 5.1 ± 0.5ab | 24.4 ± 1.1a | 18.4 ± 0.7a | 3.2 ± 1.3ab | 13.0 ± 3.1b | |
| WD | 51.1 ± 3.3bc (29%) | 52.7 ± 9.5ab | 5.7 ± 1.1ab | 20.4 ± 0.7ab | 15.2 ± 0.8b (17%) | 2.8 ± 0.5ab | 11.8 ± 1.5bc | ||
| Significance level | |||||||||
| Water regime | * | ns | * | * | * | * | ** | ||
| Inoculation | ** | * | ** | * | * | ** | ** | ||
| Inoculation × Water regime | ns | ns | ns | ** | ns | ns | ns | ||
Different letters in each column represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05. ns, *, and **indicate not significant, significant at p ≤ 0.05, and highly significant at p ≤ 0.01, respectively. Reduction percentage was calculated based on Eq.: [(WW–WD)/WW] × 100, and is presented in parenthesis
Data are presented as means of five replications ± standard errors
Fig. 3.
Relationship between pseudostem fresh weight and rhizome fresh weight (a), rhizome fresh weight (b), relationship between pseudostem dry weight and rhizome dry weight (c), and rhizome dry weight (d) of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days. Data are presented as means of five replications ± standard errors. Different letters in each bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Physiological and biochemical changes
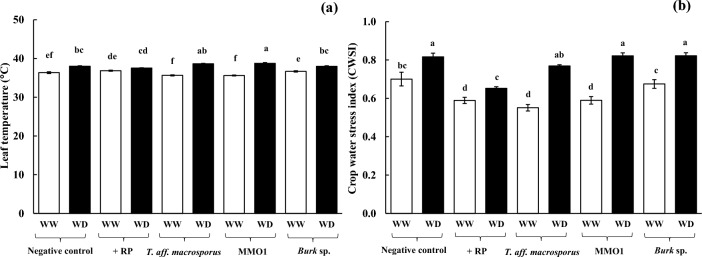
Free proline content was very low (6.8 − 19.7 g g−1 DW) in the leaf tissues of turmeric plants under WW conditions (Fig. 4a). Under WD conditions, free proline was enriched by 316% (28.06 g g−1 DW), 374% (60.75 g g−1 DW), 241% (60.90 g g−1 DW), and 251% (60.90 g g−1 DW) in the negative control, + RP, T. aff. macrosporus, and Burk inoculated plants, respectively, compared with WW conditions (p ≤ 0.01; Table S1). A negative relationship was evident between free proline enrichment and leaf ψs (R2 = 0.67; Fig. 4b). Leaf ψs was decreased by 35.6% (− 1.287 MPa), 17.3% (− 1.100 MPa), and 25% (− 1.224 MPa) in the negative control, + RP, and T. aff. macrosporus inoculated plants, respectively, under WD conditions, compared with WW conditions (p ≤ 0.01), whereas leaf ψs remained unchanged in MMO1 and Burk inoculated plants (Fig. 4c). Interestingly, leaf greenness (SPAD value) was significantly decreased by 28% (23.8 SPAD unit) and 27% (26.04 SPAD unit) in the negative control and + RP treated plants, respectively, under WD conditions compared with WW conditions (p ≤ 0.01). However, leaf greenness was stable in all PSR-inoculated plants (Fig. 4d). Under WD conditions, maximum quantum yield of PSII (Fv/Fm) was slightly decreased by 2% (0.821), 6% (0.786), and 8% (0.779) in the negative control, + RP and T. aff. macrosporus inoculated plants, respectively, compared with WW conditions (p ≤ 0.01), while Fv/Fm remained unaffected in MMO1 and Burk inoculated plants (Fig. 5a). Net photosynthetic rate (Pn) peaked at 6.53 mol m−2 s−1 in T. aff. macrosporus inoculated plants under WW conditions; however, it was found sensitive to WD conditions (more than 84% reduction compared with WW conditions; p ≤ 0.01) (Fig. 5b). Under WD conditions, Pn was dropped sharply by 84% (0.64 mol m−2 s−1), 86% (0.77 mol m−2 s−1), 90% (0.63 mol m−2 s−1), 87% (0.61 mol m−2 s−1), and 90% (0.58 mol m− s−1) in the negative control, + RP, T. aff. macrosporus, MMO1, and Burk inoculated plants, respectively, compared with WW conditions. A positive relationship was also found between Pn and total soluble sugar (R2 = 0.90; Fig. 5c). Consequently, a reduction of more than 50% in total soluble sugar in turmeric plants was observed under WD conditions (p ≤ 0.01) (Fig. 5d). In addition, stomatal conductance (gs) and transpiration rate (E) were found highly sensitive to WD conditions, leading to their decrease by more than 70% compared with WW conditions (p ≤ 0.01) (Fig. 5e–f). Leaf temperature under WD conditions was also increased (1.3 − 3.2 °C compared with WW conditions), leading to an increase in CWSI (> 0.6), which serves as an indicator of drought stress severity (Fig. 6a–b).
Fig. 4.
Free proline content (a), relationship between free proline content and leaf osmotic potential (b), leaf osmotic potential (c), and leaf greenness (d) of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days. Data are presented as means of five replications ± standard errors. Different letters in each bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Fig. 5.
Maximum quantum yield of PSII (a), net photosynthetic rate (b), relationship between net photosynthetic rate and total soluble sugar (c), total soluble sugar (d), stomatal conductance (d) and transpiration rate (e) of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days. Data are presented as means of five replications ± standard errors. Different letters in each bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Fig. 6.
Leaf temperature a and crop water stress index b of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days. Data are presented as means of five replications ± standard errors. Different letters in each bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Soluble sugar and curcuminoids
Sucrose content in the leaf tissues of turmeric plants under WD conditions was significantly dropped by 77% (6.0 mg g−1 DW), 77% (7.8 mg g−1 DW), 78% (6.8 mg g−1 DW), 74% (7.5 mg g−1 DW), and 87% (4.3 mg g−1 DW) in the negative control, + RP, T. aff. macrosporus, MMO1, and Burk inoculation, respectively, compared with WW conditions (p ≤ 0.01) (Table 4). A similar trend was observed in glucose content, which showed a reduction of 68% (3.8 mg g−1 DW) in the negative control, 60% (4.2 mg g−1 DW) in + RP, 55% (4.3 mg g−1 DW) in T. aff. macrosporus, and 42% (4.7 mg g−1 DW) in Burk inoculation when subjected to WD conditions (p ≤ 0.01). In contrast, fructose content was retained under WD conditions, especially in + RP, MMO1, and Burk inoculated plants (Table 4). In the rhizome, sucrose content was sharply declined by 79% (8.9 mg g−1 DW), 43% (33.7 mg g−1 DW), and 50% (30.7 mg g−1 DW) in the negative control, MMO1, and Burk inoculated plants, respectively, under WD conditions compared with WW conditions (p ≤ 0.01) (Table 4). However, sucrose content remained unaffected in + RP and T. aff. macrosporus treatments. Similarly, glucose content dropped substantially in the negative control (88%) and + RP (72%) treated plants under WD conditions, whereas it gradually decreased (decreased by 60 − 65%) in PSR-inoculated plants (p ≤ 0.01). Similarly, fructose content was markedly declined in the negative control (97% or 0.8 mg g−1 DW) and + RP (90% or 2.0 mg g−1 DW) treated plants under WD conditions (p ≤ 0.01), but it showed improvement under PSR inoculation.
Table 4.
Sucrose (Suc), glucose (Gluc), and fructose (Fruc) contents in the leaf and rhizome tissues of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days
| Inoculation | Water regime | Leaf | Rhizome | ||||
|---|---|---|---|---|---|---|---|
| Suc (mg g−1 DW) | Gluc (mg g−1 DW) | Fruc (mg g−1 DW) | Suc (mg g−1 DW) | Gluc (mg g−1 DW) | Fruc (mg g−1 DW) | ||
| Negative control | WW | 25.7 ± 1.4b | 11.9 ± 0.4a | 4.7 ± 1.4ab | 43.2 ± 5.5b | 24.2 ± 2.6a | 22.8 ± 3.2a |
| WD | 6.0 ± 0.2c (77%) | 3.8 ± 0.4d (68%) | 2.8 ± 0.4c (40%) | 8.9 ± 1.8c (79%) | 2.8 ± 0.3b (88%) | 0.8 ± 0.2c (97%) | |
| + RP | WW | 33.2 ± 2.1a | 10.6 ± 0.6ab | 4.7 ± 0.7ab | 41.2 ± 4.4b | 22.6 ± 2.5a | 19.8 ± 2.5a |
| WD | 7.8 ± 1.4c (77%) | 4.2 ± 0.5 cd (60%) | 4.3 ± 0.4b | 31.7 ± 7.2b | 6.3 ± 0.7b (72%) | 2.0 ± 0.6d (90%) | |
| T. aff. macrosporus | WW | 30.2 ± 1.7ab | 9.5 ± 0.5ab | 5.5 ± 1.2a | 50.6 ± 4.8ab | 21.1 ± 2.7a | 19.4 ± 3.4a |
| WD | 6.8 ± 0.8c (78%) | 4.3 ± 0.2 cd (55%) | 4.4 ± 0.4b (20%) | 36.4 ± 7.9b | 8.4 ± 1.6b (60%) | 7.2 ± 1.7bc (63%) | |
| MMO1 | WW | 28.9 ± 0.6ab | 7.2 ± 0.6bcd | 5.2 ± 1.42ab | 58.9 ± 8.0a | 18.5 ± 1.3a | 15.5 ± 1.7ab |
| WD | 7.5 ± 1.1c (74%) | 7.3 ± 1.9bcd | 5.2 ± 0.9ab | 33.7 ± 9.4b (43%) | 6.4 ± 0.7b (65%) | 5.2 ± 0.8c (67%) | |
| Burk sp. | WW | 32.1 ± 1.9a | 8.1 ± 1.67ab | 4.9 ± 0.4ab | 60.9 ± 5.3a | 21.5 ± 2.3a | 18.8 ± 3.4a |
| WD | 4.3 ± 0.5c (87%) |
4.7 ± 0.1 cd (42%) |
4.3 ± 0.7b | 30.7 ± 7.6b (50%) | 7.8 ± 0.7b (64%) | 7.8 ± 1.4c (59%) | |
| Significance level | |||||||
| Inoculation | ** | ns | ns | ** | ns | ns | |
| Water regime | ** | ** | * | ** | ** | ** | |
| Inoculation × Water regime | * | ** | ns | ns | ns | ns | |
Different letters in each column represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05. ns, *, and **indicate not significant, significant at p ≤ 0.05, and highly significant at p ≤ 0.01, respectively. Reduction percentage was calculated based on Eq.: [(WW–WD)/WW] × 100, and is presented in parenthesis
Data are presented as means of five replications ± standard errors
Bisdemethoxycurcumin (BIS), demethoxycurcumin (DEM), and curcumin (CUR) contents were increased by 37% (7.16 mg g−1 DW), 36% (7.38 mg g−1 DW), and 30% (12.52 mg g−1 DW), respectively, in the rhizomes of the negative control plants under WD conditions compared with WW conditions (p ≤ 0.01) (Table 5), which resulted in the maximum accumulation of total curcuminoids (28.46 mg g−1 DW) (Fig. 7a). Under WD conditions, these phytochemical compounds also exhibited an increase in + RP treated plants by 43% (4.46 mg g−1 DW), 34% (6.24 mg g−1 DW), and 26% (10.90 mg g−1 DW), respectively, compared with WW conditions. In contrast, curcuminoids content in the PSR-inoculated plants largely remained stable even when exposed to WD conditions. Notably, among the PSR-inoculated plants, only BIS level in MMO1-inoculated plants was decreased by 20% (5.38 mg g−1 DW) under WD conditions compared with WW conditions (6.74 mg g−1 DW) (Table 5). The maximum curcuminoids yield was observed in T. aff. macrosporus (1.65 g plant−1) and Burk (1.58 g plant−1) inoculated plants under WW conditions, which were greater than + RP by 79% and 72%, respectively (p ≤ 0.01) (Fig. 7b). However, under WD conditions, curcuminoids yield was decreased by 52% (0.79 g plant−1) and 46% (0.71 g plant−1) in T. aff. macrosporus and MMO1 inoculated plants, respectively, compared with WW conditions (p ≤ 0.01), whereas it remained unaffected in Burk inoculation.
Table 5.
Bisdemethoxycurcumin (BIS), demethoxycurcumin (DEM), and curcumin (CUR) contents in the rhizome of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days
| Inoculation | Water regime | BIS (mg g−1 DW) | DEM (mg g−1 DW) | CUR (mg g−1 DW) |
|---|---|---|---|---|
| Negative control | WW | 5.23 ± 0.54b | 5.43 ± 0.42bc | 9.60 ± 0.77bc |
| WD | 7.16 ± 0.70a (37%) | 7.38 ± 0.49a (36%) | 12.52 ± 0.54a (30%) | |
| + RP | WW | 4.52 ± 0.45b | 4.67 ± 0.38c | 8.63 ± 0.43c |
| WD | 6.46 ± 0.78a (43%) | 6.24 ± 0.60ab (34%) | 10.90 ± 0.60ab (26%) | |
| T. aff. macrosporus | WW | 6.03 ± 0.40ab | 6.13 ± 0.21ab | 11.44 ± 0.52ab |
| WD | 4.71 ± 0.32b | 4.95 ± 0.36bc | 9.55 ± 0.57bc | |
| MMO1 | WW | 6.74 ± 0.56a | 6.49 ± 0.41ab | 11.73 ± 0.64ab |
| WD | 5.38 ± 0.64b (20%) | 5.62 ± 0.46bc | 10.39 ± 0.60ab | |
| Burk sp. | WW | 6.39 ± 0.65a | 6.45 ± 0.37ab | 11.33 ± 0.49ab |
| WD | 6.79 ± 0.43a | 6.77 ± 0.35ab | 12.11 ± 0.38a | |
| Significance level | ||||
| Inoculation | ns | * | * | |
| Water regime | * | ns | ns | |
| Inoculation × Water regime | ** | ** | ** | |
Different letters in each column represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05. ns, *, and ** indicate not significant, significant at p ≤ 0.05, and highly significant at p ≤ 0.01, respectively
Data are presented as means of five replications ± standard errors
Fig. 7.
Total curcuminoids a and curcuminoids yield b of turmeric plants grown with phosphate solubilizing rhizo-microbes (PSR) and rock phosphate (RP) inoculation for 5 months, subsequently subjected to well-watered (WW) and water-deficit (WD) conditions under greenhouse for 45 days. Data are presented as means of five replications ± standard errors. Different letters in each bar represent significant difference based on Tukey’s honest significant difference test at p ≤ 0.05
Discussion
In the present study, P content in the leaf tissues of T. aff. macrosporus and Burkholderia sp. inoculated plants was the maximum under WW conditions compared with the negative control and + RP treatment. However, P content was significantly decreased when plants were subjected to WD conditions. Under WW conditions, P content in the shoots of PSR-consortium inoculated turmeric plants has been reported to be significantly increased by 3.6-fold over the uninoculated plants (Dutta and Neog 2016). The higher solubility of phosphate in the soil substrate when inoculated with PSR, including bacteria genus Bacillus, Burkholderia, and Pseudomonas, and fungi genus Thalaromyces, has been previously demonstrated in multiple studies (Kour et al. 2020b; Lotfi et al. 2022; Kaur and Saxena 2023). Interestingly, Ca content in the leaf tissues of + RP treated plants was significantly increased under WW conditions and was the maximum in Burkholderia sp. inoculated plants. Rock phosphate has been reported as a good source of essential plant nutrients, such as P and Ca, for promoting plant growth and development. However, it has a very low rate of solubilization when compared with other sources, such as single superphosphate, triple superphosphate, dicalcium phosphate, and tricalcium phosphate (Amarasinghe et al. 2022; Khan et al. 2022; Chatterjee and Margenot 2023; Nadia et al. 2023). An alternative approach to enhance the mineral nutrients’ solubility for economic crop production involves utilizing candidate microbes sourced from rock phosphate mines. These microbes can be used as biofertilizers to supply essential nutrients at a lower cost (Qarni et al. 2021; Azarmi-Atajan et al. 2022). Studies have revealed that PSR-inoculated plants exhibit increased uptake of mineral nutrients (especially P, K, Mg, and Ca) by roots and translocation to shoot, which are subsequently decreased when plants are subject to WD conditions (Rigi et al. 2023; Suhail et al. 2023). A possible strategy for reducing P level in the leaf tissues of drought-stressed plants involves limiting the rate of P uptake and transport across cell layers by root tissues. This nutrient can then be translocated to other organs via xylem vascular tissues, with a potential regulation by PSR inoculation (Bárzana and Carvajal 2020; Lopez-Zaplana et al. 2022). An accumulation of P in the leaf tissues of PSR-inoculated plants has been observed to fluctuate depending on some key factors, such as phosphate source, microbial genus used, degree of water shortage, and developmental stage of target plant species (Rotaru and Rîşnoveanu 2019; Kang et al. 2021; Nacoon et al. 2021). Under WD conditions, Mg and Ca contents in plants inoculated with MMO1 were sustained, similar to the findings in Bacillus subtilis inoculation (Halo et al. 2020; da Fonseca et al. 2022).
In the present study, under WW conditions, rhizome dry weight in T. aff. macrosporus and Burkholderia inoculated plants was the maximum and rhizome fresh weight was also sustained in Burkholderia inoculated plants even when subjected to WD conditions. Belowground rhizome in turmeric plants was regarded as the key target yield for harvesting, considering its importance for both food and medicinal purposes. Under WW conditions, rhizome yield of Bacillus safensis inoculated turmeric plants has been reported as the highest at 7.32 Mg ha−1 (2.5-fold over the control) (Dinesh et al. 2022). Similarly, various studies have reported that yield traits of tubers, bulbs, and rhizome, both in terms of fresh weight and dry weight, in PSR-inoculated plants have significantly elevated compared with the control (Nacoon et al. 2020; Tinna et al. 2020; Aallam et al. 2023). Under WD conditions, the overall growth performance in both aboveground (number of tillers, pseudostem fresh weight, and pseudostem dry weight) and root traits (number of roots) were sustained in MMO1 and Burkholderia inoculated plants. Previous studies have also reported an alleviation of WD stress and improvements in growth parameters of model plant species through PSR inoculation (Nacoon et al. 2021, 2022; Li et al. 2022b; Osman et al. 2022).
Root length of plants treated with + RP under WW conditions was the shortest when compared with other treatments, which might be linked to alkali-soil pH (Soumare et al. 2021). Interestingly, root length of MMO1 and Burkholderia inoculated plants under WD conditions was significantly decreased, which is consistent with a previous finding in Bacillus-inoculated mustard plant (Suhail et al. 2023). This decrease has been attributed to high amount of ethylene production, which obstructs the elongation of root cell (Kumari et al. 2016; Zhang et al. 2020). Moreover, number of tillers, leaf length, and number of secondary rhizomes in plants treated with + RP under WD conditions were declined by more than 35% compared with WW conditions. In contrast, these parameters showed an improvement under MMO1 and Burkholderia inoculation. In the case of Aloe vera plants subjected to WD conditions, parameters, such as leaf biomass, leaf width, and gel fresh yield, were found to be sensitive under Pantoea agglomerans strain P5 inoculation. It has been reported that only a consortium of PSR and arbuscular mycorrhizal fungi (AMF) alleviates WD stress in Aloe vera plants (Khajeeyan et al. 2022).
Free proline content was found to be very low in turmeric plants under WW conditions. However, when these plants were subjected to WD conditions, there was a significant increase in free proline at the cellular level, serving as an indicator of osmotic stress. Remarkably, free proline played a key role as a major osmolyte in T. aff. macrosporus and Burkholderia inoculated plants under WD conditions. This helped in the regulation of leaf osmotic potential, which subsequently contributed to the stabilization of leaf greenness or chlorophyll photosynthetic pigment, better than the negative control and the plants treated with + RP. The regulation of pyrroline-5-carboxylate synthase (P5CR) enzyme in proline biosynthesis and proline enrichment have been observed in water deficit-stressed plants (Arora and Jha 2023). However, when PSR was inoculated, proline enrichment was repressed even when exposed to WD conditions (Azizi et al. 2021; Kaur and Saxena 2023). Under drought stress, co-suppression of polyamine biosynthesis with low levels of free proline and enchantment of γ-aminobutyric acid in plants inoculated with Pseudomonas putida has been reported (Sen and Mohapatra 2022; Nikhil et al. 2023), leading to the retention of chlorophyll pigment (Saglam et al. 2022). In addition, the maximum quantum yield of PSII of MMO1 and Burkholderia inoculated plants under WD conditions was also sustained. However, there have been also reports of a reduced electron transport rate in photosystem II of PSR-inoculated plants under WD conditions (Li et al. 2022b; Taghizadeh et al. 2023), leading to a marked decline in leaf gas exchange parameters, such as Pn, gs, and E. A dual inoculation of PSR (Pseudomonas) and AMF has been reported as a promising strategy for regulating plant physiological adaptation under drought stress (Azizi et al. 2021; Nacoon et al. 2022; Taghizadeh et al. 2023). It is well established that stomatal closure is regulated by abscisic acid under drought stress, and a decrease in abscisic acid content in the leaf tissues of PSR-inoculated plants has been observed to delay leaf senescence (Zhang et al. 2020; He et al. 2021; Arora and Jha 2023). In the case of stomatal closure, leaf temperature (Tleaf) and CWSI in plants under WD conditions increase, which serve as stress indicators (Chintakovid et al. 2022). Due to the reduction of photosynthesis under drought stress, total soluble sugar contents, especially sucrose and glucose in the leaf tissues, as well as glucose and fructose in the rhizome, showed a sharp decrease, regardless of PSR inoculation. This decline in total soluble sugar contents in leaf tissues is a consequence of decreased photosynthetic rate (sink organ) in leaves, and it limits the transfer rate to the storage organ, especially under WD conditions (Kumari et al. 2016; Taghizadeh et al. 2023).
Bisdemethoxycurcumin, demethoxycurcumin, and curcumin levels in the rhizomes of turmeric plants under WD conditions without PSR-inoculation were the maximum. This leads to the highest total curcuminoids when compared with the plants under T. aff. macrosporus and MMO1 inoculation. These bioactive compounds (total curcuminoids and curcuminoids yield per plant) were also sustained in plants under Burkholderia inoculation even when subjected to WD conditions. Interestingly, aloin content in the harvested leaves of Aloe vera has been reported to be significantly enhanced in plants subjected to 25% of plant water requirement treatment along with co-inoculation of PSR and AMF (biofertilizer) (Khajeeyan et al. 2022). Essential oil yield and total flavonoids have also been reported to elevate in dual inoculated (PGPR and AMF) common myrtle plants under WD conditions compared with WW conditions (Azizi et al. 2021). Furthermore, essential oil percentage, essential oil yield, oil content, and oil yield in Ajowan are highly sensitive to drought stress (threshold at < 75% field capacity), and these parameters improve through consortium inoculation (PSR and AMF) (Rezaei-Chiyaneh et al. 2021). Total flavonoids content in Bacillus inoculated plants also increases under drought stress compared with WW conditions (Kang et al. 2021; Suhail et al. 2023).
Conclusion
Phosphate-solubilizing rhizo-microbes (PSR) have shown great potential as biofertilizers, and they have demonstrated the capability to enhance phosphorus, potassium, and calcium in the leaf tissues of turmeric plants under well-watered (WW) conditions, especially in Burkholderia sp. and mixed phosphate solubilizing bacteria (MMO1) inoculated plants. Under water-deficit (WD) conditions, leaf osmotic potential of Burkholderia sp. inoculated plants was maintained through the enrichment of free proline. This preservation of leaf osmotic potential leads to the retention of chlorophyll pigment, maximum quantum yield of PSII, and rhizome fresh weight. Furthermore, the rhizomes of plants inoculated with PSR, especially Burkholderia sp., retained bisdemethoxycurcumin, demethoxycurcumin, curcumin, and total curcuminoids contents under WD conditions. This resulted in a high yield of curcuminoids relative to the substantial size of the rhizome, even when exposed to WD conditions. Burkholderia sp. inoculation in turmeric plants is recommended as a promising technique as it alleviates water-deficit stress, sustains rhizome biomass, and stabilizes curcuminoids yield. Further research and validation are required before advocating large-scale production of turmeric plants using Burkholderia sp. inoculation, particularly under WD conditions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Financial support received from the National Science and Technology Development Agency (Grant Number P-22-50096) is highly acknowledged.
Author contributions
The authors have made the following declarations about their contributions: conceived and designed the experiments: SC-U and AD. Performed the experiments: DC, RT, KS, and TS. Analyzed the data: RT, PP, and UP. Wrote the paper: SC-U, SKH, and AD.
Funding
The authors are thankful to the National Science and Technology Development Agency (Grant Number P-22-50096) for financial support.
Data availability
All data will be available after a reasonable request to the corresponding author.
Declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Aallam Y, Dhiba D, El Rasafi T, Abbas Y, Haddioui A, Tarkka M, Hamdali H. Assessment of two endemic rock phosphate solubilizing Streptomyces spp. on sugar beet (Beta vulgaris L.) growth under field conditions. Sci Hortic. 2023 doi: 10.1016/j.scienta.2023.112033. [DOI] [Google Scholar]
- Amarasinghe T, Madhusha C, Munaweera I, Kottegoda N. Review on mechanisms of phosphate solubilization in rock phosphate fertilizer. Comm Soil Sci Plant Anal. 2022;53:944–960. doi: 10.1080/00103624.2022.2034849. [DOI] [Google Scholar]
- Arora S, Jha PN. Drought-tolerant Enterobacter bugandensis WRS7 induces systemic tolerance in Triticum aestivum L. (wheat) under drought conditions. J Plant Growth Regul. 2023;42:7715–7730. doi: 10.1007/s00344-023-11044-6. [DOI] [Google Scholar]
- Azarmi-Atajan F, Sayyari-Zohan MH. Effect of phosphate solubilizing bacteria and triple superphosphate on the growth, physiological parameters and phosphorus uptake of pistachio seedlings. J Hortic Postharv Res. 2022;5:69–78. [Google Scholar]
- Azizi S, Kouchaksaraei MT, Hadian J, Abad ARFN, Sanavi SAMM, Ammer C, Bader MKF. Dual inoculations of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria boost drought resistance and essential oil yield of common myrtle. Forest Ecol Manag. 2021 doi: 10.1016/j.foreco.2021.119478. [DOI] [Google Scholar]
- Bargaz A, Elhaissoufi W, Khourchi S, Benmrid B, Borden KA, Rchiad Z. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol Res. 2021 doi: 10.1016/j.micres.2021.126842. [DOI] [PubMed] [Google Scholar]
- Bárzana G, Carvajal M. Genetic regulation of water and nutrient transport in water stress tolerance in roots. J Biotechnol. 2020;324:134–142. doi: 10.1016/j.jbiotec.2020.10.003. [DOI] [PubMed] [Google Scholar]
- Chandrashekhar G, Hore JK. Effect of growth and yield of the ginger with the combination of organic, inorganic and biofertilizers. J Pharmacog Phytochem. 2019;8:1174–1176. [Google Scholar]
- Chatterjee N, Margenot AJ. Crop growth is increased by arbuscular mycorrhizae for both phosphate rock and soluble phosphorus fertilizers, but fertilizer solubility primarily determines crop growth. Biol Fert Soil. 2023;59:843–862. doi: 10.1007/s00374-023-01751-3. [DOI] [Google Scholar]
- Chintakovid N, Tisarum R, Samphumphuang T, Sotesaritkul T, Cha-um S. Evaluation of curcuminoids, physiological adaptation, and growth of Curcuma longa under water deficit and controlled temperature. Protoplasma. 2022;259:301–315. doi: 10.1007/s00709-021-01670-w. [DOI] [PubMed] [Google Scholar]
- da Fonseca MC, Bossolani JW, de Oliveira SL, Moretti LG, Portugal JR, Scudeletti D, de Oliveira EF, Crusciol CAC. Bacillus subtilis inoculation improves nutrient uptake and physiological activity in sugarcane under drought stress. Microorganisms. 2022;10:809. doi: 10.3390/microorganisms10040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh R, Srinivasan V, Praveena R, Subila KP, George P, Das A, Shajina O, Anees K, Leela NK, Haritha P. Exploring the potential of P solubilizing rhizobacteria for enhanced yield and quality in turmeric (Curcuma longa L.) Ind Crops Prod. 2022 doi: 10.1016/j.indcrop.2022.115826. [DOI] [Google Scholar]
- Dudekula MV, Kandasamy V, Balaraman SS, Selvamani SB, Muthurajan R, Adhimoolam K, Manoharan B, Natesan S. Unlocking the genetic diversity of Indian turmeric (Curcuma longa L.) germplasm based on rhizome yield traits and curcuminoids. Front Plant Sci. 2022 doi: 10.3389/fpls.2022.1036592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SC, Neog B. Accumulation of secondary metabolites in response to antioxidant activity of turmeric rhizomes co-inoculated with native arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria. Sci Hortic. 2016;204:179–184. doi: 10.1016/j.scienta.2016.03.028. [DOI] [Google Scholar]
- Dutta SC, Neog B. Inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in modulating phosphorus dynamics in turmeric rhizosphere. Nat Acad Sci Lett. 2017;40:445–449. doi: 10.1007/s40009-017-0582-1. [DOI] [Google Scholar]
- El Sherif F, Alkuwayti MA, Khattab S. Foliar spraying of salicylic acid enhances growth, yield, and curcuminoid biosynthesis gene expression as well as curcuminoid accumulation in Curcuma longa. Horticulturae. 2022;8:417. doi: 10.3390/horticulturae8050417. [DOI] [Google Scholar]
- Elhaissoufi W, Ghoulam C, Barakat A, Zeroual Y, Bargaz A. Phosphate bacterial solubilization: a key rhizosphere driving force enabling higher P use efficiency and crop productivity. J Adv Res. 2022;38:13–28. doi: 10.1016/j.jare.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyhorn F, Muller A, Reganold JP, Frison E, Herren HR, Luttikholt L, Mueller A, Sanders J, Scialabba NEH, Seufert V, Smith P. Sustainability in global agriculture driven by organic farming. Nature Sustain. 2019;2:253–255. doi: 10.1038/s41893-019-0266-6. [DOI] [Google Scholar]
- Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Subramaniyan V, Chidambaram K, Thangavelu L, Nordin R, Wu YS, Sathasivam KV, Lum PT, Meenakshi DU, Kumarasamy V, Azad AK, Fuloria NK. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front Pharmacol. 2022 doi: 10.3389/fphar.2022.820806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C, Yadav MK, Meena V, Singh A, Singh HB, Sarma BK, Singh SP, Rakshit A. Bio-inoculants as prospective inputs for achieving sustainability: Indian story. Econ Affairs. 2020;65:31–41. doi: 10.30954/0424-2513.1.2020.5. [DOI] [Google Scholar]
- Halo BA, Al-Yahyai RA, Al-Sadi AM. An endophytic Talaromyces omanensis enhances reproductive, physiological and anatomical characteristics of drought-stressed tomato. J Plant Physiol. 2020 doi: 10.1016/j.jplph.2020.153163. [DOI] [PubMed] [Google Scholar]
- He A, Niu S, Yang D, Ren W, Zhao L, Sun Y, Meng L, Zhao Q, Paré PW, Zhang J. Two PGPR strains from the rhizosphere of Haloxylon ammodendron promoted growth and enhanced drought tolerance of ryegrass. Plant Physiol Biochem. 2021;161:74–85. doi: 10.1016/j.plaphy.2021.02.003. [DOI] [PubMed] [Google Scholar]
- Hermans K, McLeman R. Climate change, drought, land degradation and migration: exploring the linkages. Curr Opin Environ Sustain. 2021;50:236–244. doi: 10.1016/j.cosust.2021.04.013. [DOI] [Google Scholar]
- Ingrisch J, Umlauf N, Bahn M. Functional thresholds alter the relationship of plant resistance and recovery to drought. Ecology. 2023 doi: 10.1002/ecy.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap RR, Mali GV, Waghmare SR, Nadaf NH, Nimbalkar MS, Sonawane KD. Impact of plant growth promoting rhizobacteria Serratia nematodiphila RGK and Pseudomonas plecoglossicida RGK on secondary metabolites of turmeric rhizome. Biocat Agric Biotechnol. 2023 doi: 10.1016/j.bcab.2023.102622. [DOI] [Google Scholar]
- Jyotirmayee B, Mahalik G. A review on selected pharmacological activities of Curcuma longa L. Int J Food Proper. 2022;25:1377–1398. doi: 10.1080/10942912.2022.2082464. [DOI] [Google Scholar]
- Kang SM, Khan MA, Hamayun M, Kim LR, Kwon EH, Kang YS, Kim KY, Park JJ, Lee IJ. Phosphate-solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 rescues alfalfa’s growth under post-drought stress. Agriculture. 2021;11:485. doi: 10.3390/agriculture11060485. [DOI] [Google Scholar]
- Kaur R, Saxena S. Evaluation of drought-tolerant endophytic fungus Talaromyces purpureogenus as a bioinoculant for wheat seedlings under normal and drought-stressed circumstances. Folia Microbiol. 2023;68:781–799. doi: 10.1007/s12223-023-01051-1. [DOI] [PubMed] [Google Scholar]
- Khajeeyan R, Salehi A, Dehnavi MM, Farajee H, Kohanmoo MA. Growth parameters, water productivity and aloin content of Aloe vera affected by mycorrhiza and PGPR application under different irrigation regimes. S Afr J Bot. 2022;147:1188–1198. doi: 10.1016/j.sajb.2021.02.026. [DOI] [Google Scholar]
- Khan H, Akbar WA, Shah Z, Rahim HU, Taj A, Alatalo JM. Coupling phosphate-solubilizing bacteria (PSB) with inorganic phosphorus fertilizer improves mungbean (Vigna radiata) phosphorus acquisition, nitrogen fixation, and yield in alkaline-calcareous soil. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e09081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab S, Alkuwayti MA, Yap YK, Meligy AM, Bani Ismail M, El Sherif F. Foliar spraying of ZnO nanoparticals on Curcuma longa had increased growth, yield, expression of curcuminoid synthesis genes, and curcuminoid accumulation. Horticulturae. 2023;9:355. doi: 10.3390/horticulturae9030355. [DOI] [Google Scholar]
- Kogan F, Guo W, Yang W. Drought and food security prediction from NOAA new generation of operational satellites. Geomat Nat Hazards Risk. 2019;10:651–666. doi: 10.1080/19475705.2018.1541257. [DOI] [Google Scholar]
- Kogan F, Guo W, Yang W. Near 40-year drought trend during 1981–2019 earth warming and food security. Geomat Nat Hazards Risk. 2020;11:469–490. doi: 10.1080/19475705.2020.1730452. [DOI] [Google Scholar]
- Kour D, Rana KL, Kaur T, Sheikh I, Yadav AN, Kumar V, Dhaliwal HS, Saxena AK. Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocat Agric Biotechnol. 2020 doi: 10.1016/j.bcab.2020.101501. [DOI] [Google Scholar]
- Kour D, Rana KL, Sheikh I, Kumar V, Yadav AN, Dhaliwal HS, Saxena AK. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc Nat Acad Sci Sect B Biol Sci. 2020;90:785–795. doi: 10.1007/s40011-019-01151-4. [DOI] [Google Scholar]
- Kumar A, Singh M, Singh PP, Singh SK, Singh PK, Pandey KD. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocat Agric Biotechnol. 2016;8:1–7. doi: 10.1016/j.bcab.2016.07.002. [DOI] [Google Scholar]
- Kumar A, Singh AK, Kaushik MS, Mishra SK, Raj P, Singh PK, Pandey KD. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: a review. 3 Biotech. 2017;7:357. doi: 10.1007/s13205-017-0971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK. Induced drought tolerance through wild and mutant bacterial strain Pseudomonas simiae in mung bean (Vigna radiata L.) World J Microbiol Biotechnol. 2016;32:4. doi: 10.1007/s11274-015-1974-3. [DOI] [PubMed] [Google Scholar]
- Li J, Wang J, Liu H, Macdonald CA, Singh BK. Application of microbial inoculants significantly enhances crop productivity: a meta-analysis of studies from 2010 to 2020. J Sustain Agric Environ. 2022;1:216–225. doi: 10.1002/sae2.12028. [DOI] [Google Scholar]
- Li Q, Huang Z, Deng C, Lin KH, Hua S, Chen SP. Endophytic Klebsiella sp. San01 promotes growth performance and induces salinity and drought tolerance in sweet potato (Ipomoea batatas L.) J Plant Inter. 2022;17:608–619. doi: 10.1080/17429145.2022.2077464. [DOI] [Google Scholar]
- Lone R, Hassan N, Bashir B, Rohela GK, Malla NA. Role of growth elicitors and microbes in stress management and sustainable production of sorghum. Plant Stress. 2023 doi: 10.1016/j.stress.2023.100179. [DOI] [Google Scholar]
- Lopez-Zaplana A, Martinez-Garcia N, Carvajal M, Bárzana G. Relationships between aquaporins gene expression and nutrient concentrations in melon plants (Cucumis melo L.) during typical abiotic stresses. Environ Exp Bot. 2022 doi: 10.1016/j.envexpbot.2021.104759. [DOI] [Google Scholar]
- Lotfi N, Soleimani A, Çakmakçı R, Vahdati K, Mohammadi P. Characterization of plant growth-promoting rhizobacteria (PGPR) in Persian walnut associated with drought stress tolerance. Sci Rep. 2022;12:12725. doi: 10.1038/s41598-022-16852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed A, Farooq M, Naveed M, Hussain M. Combined application of inorganic and organic phosphorous with inoculation of phosphorus solubilizing bacteria improved productivity, grain quality and net economic returns of pearl millet (Pennisetum glaucum [L.] R. Br.) Agronomy. 2022;12:2412. doi: 10.3390/agronomy12102412. [DOI] [Google Scholar]
- Mansoor S, Khan T, Farooq I, Shah LR, Sharma V, Sonne C, Rinklebe J, Ahmad P. Drought and global hunger: biotechnological interventions in sustainability and management. Planta. 2022;256:97. doi: 10.1007/s00425-022-04006-x. [DOI] [PubMed] [Google Scholar]
- Nacoon S, Jogloy S, Riddech N, Mongkolthanaruk W, Kuyper TW, Boonlue S. Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Sci Rep. 2020;10:4916. doi: 10.1038/s41598-020-61846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacoon S, Jogloy S, Riddech N, Mongkolthanaruk W, Ekprasert J, Cooper J, Boonlue S. Combination of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria on growth and production of Helianthus tuberosus under field condition. Sci Rep. 2021;11:6501. doi: 10.1038/s41598-021-86042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacoon S, Seemakram W, Ekprasert J, Jogloy S, Kuyper TW, Mongkolthanaruk W, Ekprasert J, Cooper J, Boonlue S. Promoting growth and production of sunchoke (Helianthus tuberosus) by co-inoculation with phosphate solubilizing bacteria and arbuscular mycorrhizal fungi under drought. Front Plant Sci. 2022;13:1022319. doi: 10.3389/fpls.2022.1022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadia Amanullah Arif M, Muhammad D. Improvement in wheat productivity with integrated management of beneficial microbes along with organic and inorganic phosphorus sources. Agriculture. 2023;13:1118. doi: 10.3390/agriculture13061118. [DOI] [Google Scholar]
- Nikhil PT, Faiz U, Mohapatra S. The drought-tolerant rhizobacterium, Pseudomonas putida AKMP7, suppresses polyamine accumulation under well-watered conditions and diverts putrescine into GABA under water-stress, in Oryza sativa. Environ Exp Bot. 2023 doi: 10.1016/j.envexpbot.2023.105377. [DOI] [Google Scholar]
- Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140682. [DOI] [PubMed] [Google Scholar]
- Osman HS, Rady AM, Awadalla A, Omara AED, Hafez EM. Improving the antioxidants system, growth, and sugar beet quality subjected to long-term osmotic stress by phosphate solubilizing bacteria and compost tea. Int J Plant Prod. 2022;16:119–135. doi: 10.1007/s42106-021-00176-y. [DOI] [Google Scholar]
- Pal K, Chowdhury S, Dutta SK, Chakraborty S, Chakraborty M, Pandit GK, Dutta S, Paul PK, Chaouhury A, Majumder B, Sahana N, Mandal S. Analysis of rhizome colour content, bioactive compound profiling and ex-situ conservation of turmeric genotypes (Curcuma longa L.) from sub-Himalayan terai region of India. Ind Crops Prod. 2020 doi: 10.1016/j.indcrop.2020.112401. [DOI] [Google Scholar]
- Pal G, Saxena S, Kumar K, Verma A, Sahu PK, Pandey A, Sahu PK, Pandey A, White JF, Verma SK. Endophytic Burkholderia: multifunctional roles in plant growth promotion and stress tolerance. Microbiol Res. 2022 doi: 10.1016/j.micres.2022.127201. [DOI] [PubMed] [Google Scholar]
- Patni B, Ansari S. Role of exogenous application of salicylic acid on medicinal plants under drought stress: a review. J Stress Physiol Biochem. 2019;15:76–85. [Google Scholar]
- Philip SY, Kew SF, van der Wiel K, Wanders N, van Oldenborgh GJ. Regional differentiation in climate change induced drought trends in the Netherlands. Environ Res Lett. 2020 doi: 10.1088/1748-9326/ab97ca. [DOI] [Google Scholar]
- Phukunkamkaew S, Tisarum R, Pipatsitee P, Samphumphuang T, Maksup S, Cha-um S. Morpho-physiological responses of indica rice (Oryza sativa sub. indica) to aluminum toxicity at seedling stage. Environ Sci Pollut Res. 2021;28:29321–29331. doi: 10.1007/s11356-021-12804-1. [DOI] [PubMed] [Google Scholar]
- Pokhrel Y, Felfelani F, Satoh Y, Boulange J, Burek P, Gädeke A, Gerten D, Gosling SN, Grillakis M, Gudmundsson L, Hanasaki N, Kim H, Koutroulis A, Liu J, Papadimitriou L, Schewe J, Schmied HM, Stacke T, Telteu CE, Thiery W, Veldkamp T, Zhao F, Wada Y. Global terrestrial water storage and drought severity under climate change. Nat Clim Change. 2021;11:226–233. doi: 10.1038/s41558-020-00972-w. [DOI] [Google Scholar]
- Praseartkul P, Taota K, Pipatsitee P, Tisarum R, Sakulleerungroj K, Sotesaritkul T, Himanshu SK, Datta A, Cha-um S. Unmanned aerial vehicle-based vegetation monitoring of aboveground and belowground traits of the turmeric plant (Curcuma longa L.) Int J Environ Sci Technol. 2023;20:8673–8686. doi: 10.1007/s13762-022-04545-6. [DOI] [Google Scholar]
- Qarni A, Billah M, Hussain K, Shah SH, Ahmed W, Alam S, Sheikh AA, Jafri L, Munir A, Malik KM, Khan N. Isolation and characterization of phosphate solubilizing microbes from rock phosphate mines and their potential effect for sustainable agriculture. Sustainability. 2021;13:2151. doi: 10.3390/su13042151. [DOI] [Google Scholar]
- Rawat P, Das S, Shankhdhar D, Shankhdhar SC. Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nutr. 2021;21:49–68. doi: 10.1007/s42729-020-00342-7. [DOI] [Google Scholar]
- Raza A, Charagh S, Salehi H, Abbas S, Saeed F, Poinern GE, Siddique KHM, Varshney RK. Nano-enabled stress-smart agriculture: can nanotechnology deliver drought and salinity-smart crops? J Sustain Agric Environ. 2023;2:189–214. doi: 10.1002/sae2.12061. [DOI] [Google Scholar]
- Raza A, Mubarik MS, Sharif R, Habib M, Jabeen W, Zhang C, Chen H, Chen ZH, Siddique KHM, Zhuang W, Varshney RK. Developing drought-smart, ready-to-grow future crops. Plant Genom. 2023 doi: 10.1002/tpg2.20279. [DOI] [PubMed] [Google Scholar]
- Rezaei-Chiyaneh E, Mahdavikia H, Subramanian S, Alipour H, Siddique KH, Smith DL. Co-inoculation of phosphate-solubilizing bacteria and mycorrhizal fungi: effect on seed yield, physiological variables, and fixed oil and essential oil productivity of Ajowan (Carum copticum L.) under water deficit. J Soil Sci Plant Nutr. 2021;21:3159–3179. doi: 10.1007/s42729-021-00596-9. [DOI] [Google Scholar]
- Rigi F, Saberi M, Ebrahimi M. Improved drought tolerance in Festuca ovina L. using plant growth promoting bacteria. J Arid Land. 2023;15:740–755. doi: 10.1007/s40333-023-0015-6. [DOI] [Google Scholar]
- Rotaru V, Rîşnoveanu L. Interactive effects of plant growth-promoting rhizobacteria and phosphates sources on growth and phosphorus nutrition of soybean under moderate drought. Not Bot Horti Agrobo Cluj-Napoca. 2019;47:872–880. [Google Scholar]
- Saglam A, Demiralay M, Colak DN, Gedik NP, Basok O, Kadioglu A. Pseudomonas putida KT2440 induces drought tolerance during fruit ripening in tomato. Bioagro. 2022;34:139–150. doi: 10.51372/bioagro342.4. [DOI] [Google Scholar]
- Santos LF, Olivares FL. Plant microbiome structure and benefits for sustainable agriculture. Curr Plant Biol. 2021 doi: 10.1016/j.cpb.2021.100198. [DOI] [Google Scholar]
- Sati D, Pande V, Pandey SC, Samant M. Recent advances in PGPR and molecular mechanisms involved in drought stress resistance. J Soil Sci Plant Nutr. 2023;23:106–124. doi: 10.1007/s42729-021-00724-5. [DOI] [Google Scholar]
- Sen S, Mohapatra S. Drought-mitigating Pseudomonas putida strain modulates polyamine catabolism in Arabidopsis thaliana. J Plant Growth Regul. 2022;41:300–313. doi: 10.1007/s00344-021-10297-3. [DOI] [Google Scholar]
- Shaffique S, Khan MA, Imran M, Kang SM, Park YS, Wani SH, Lee IJ. Research progress in the field of microbial mitigation of drought stress in plants. Front Plant Sci. 2022 doi: 10.3389/fpls.2022.870626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith OM, Cohen AL, Rieser CJ, Davis AG, Taylor JM, Adesanya AW, Jones MS, Meier AR, Reganold JP, Orpet RJ, Northfield TD, Crowder DW. Organic farming provides reliable environmental benefits but increases variability in crop yields: A global meta-analysis. Front Sustain Food Syst. 2019;3:82. doi: 10.3389/fsufs.2019.00082. [DOI] [Google Scholar]
- Sodhi GK, Saxena S. Plant growth promotion and abiotic stress mitigation in rice using endophytic fungi: advances made in the last decade. Environ Exp Bot. 2023 doi: 10.1016/j.envexpbot.2023.105312. [DOI] [Google Scholar]
- Soumare A, Boubekri K, Lyamlouli K, Hafidi M, Ouhdouch Y, Kouisni L. Efficacy of phosphate solubilizing Actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere. 2021 doi: 10.1016/j.rhisph.2020.100284. [DOI] [Google Scholar]
- Suhail F, Afzal A, Naseer L, Pervaiz A, Ikram M, Shaheen S, Islam Y, Khan N. Influence of phosphate solubilizing bacteria on the growth of mustard grown under drought stress conditions. Agric Res. 2023;12:375–386. doi: 10.1007/s40003-023-00656-9. [DOI] [Google Scholar]
- Sultana S, Munir N, Mahmood Z, Riaz M, Akram M, Rebezov M, Kuderinova N, Moldabayeva Z, Shariati MA, Rauf A, Rengasamy KR. Molecular targets for the management of cancer using Curcuma longa Linn. phytoconstituents: a review. Biomed Pharmacother. 2021 doi: 10.1016/j.biopha.2020.111078. [DOI] [PubMed] [Google Scholar]
- Suman A, Govindasamy V, Ramakrishnan B, Aswini K, SaiPrasad J, Sharma P, Pathak D, Annapurna K. Microbial community and function-based synthetic bioinoculants: a perspective for sustainable agriculture. Front Microbiol. 2022 doi: 10.3389/fmicb.2021.805498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh MH, Farzam M, Nabati J. Rhizobacteria facilitate physiological and biochemical drought tolerance of Halimodendron halodendron (Pall.) Voss. J Arid Land. 2023;15:205–217. doi: 10.1007/s40333-023-0092-6. [DOI] [Google Scholar]
- Thakur N, Sharma P, Govender PP, Shukla SK. Analysis and extraction of curcumin at mid and late phase harvested Curcuma longa samples collected from Western Himalayan regions. Chem Afr. 2022;5:1733–1742. doi: 10.1007/s42250-022-00451-z. [DOI] [Google Scholar]
- Tinna D, Garg N, Sharma S, Pandove G, Chawla N. Utilization of plant growth promoting rhizobacteria as root dipping of seedlings for improving bulb yield and curtailing mineral fertilizer use in onion under field conditions. Sci Hortic. 2020 doi: 10.1016/j.scienta.2020.109432. [DOI] [Google Scholar]
- Tripathi SK, Sharma B, Kumari P, Deb P, Ray R, Denis AF. Evaluation of productivity, quality and economics of turmeric under different moisture regime and integrated nutrient management at Eastern Indo-Gangetic plains, India. Agric Res. 2021;10:601–612. doi: 10.1007/s40003-020-00524-w. [DOI] [Google Scholar]
- Ullah A, Nisar M, Ali H, Hazrat A, Hayat K, Keerio AA, Ihsan M, Laiq M, Ullah S, Fahad S, Khan A, Khan AH, Akbar A, Yang X. Drought tolerance improvement in plants: an endophytic bacterial approach. Appl Microbiol Biotechnol. 2019;103:7385–7397. doi: 10.1007/s00253-019-10045-4. [DOI] [PubMed] [Google Scholar]
- Ullah N, Ditta A, Imtiaz M, Li X, Jan AU, Mehmood S, Rizwan MS, Rizwan M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: a review. J Agron Crop Sci. 2021;207:783–802. doi: 10.1111/jac.12502. [DOI] [Google Scholar]
- Zhang M, Yang L, Hao R, Bai X, Wang Y, Yu X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil. 2020;452:423–440. doi: 10.1007/s11104-020-04582-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be available after a reasonable request to the corresponding author.