Author's summary
Cone reconstruction is a standard treatment for Ebstein’s anomaly. It involves suturing the displaced tricuspid valve (TV) annulus to the anatomical TV annulus, resulting in a smaller annulus and improved coaptation. Concerns arise when applying this technique to pediatric patients, as TV annular growth may not occur with somatic growth. Additionally, Ebstein's anomaly often involves left ventricular (LV) dysfunction, and it is crucial to ensure long-term restoration of LV function and reverse remodeling. This study demonstrates that modified cone reconstruction provides durable tricuspid valve repair, appropriate annular growth, and possible progressive LV reverse remodeling in children with Ebstein’s anomaly.
Keywords: Ebstein anomaly, Tricuspid regurgitation, Left ventricular remodeling
Abstract
Background and Objective
We aimed to investigate long-term clinical and echocardiographic outcomes, including tricuspid valve durability, annular growth, and left ventricular reverse remodeling, after modified cone reconstruction in patients with Ebstein’s anomaly.
Methods
This was a retrospective analysis of all pediatric patients who underwent modified cone reconstruction for Ebstein’s anomaly at a single tertiary center between January 2005 and June 2021.
Results
A total of 14 pediatric patients underwent modified cone reconstruction for Ebstein’s anomaly; the median age was 5.8 years (range, 0.01–16.6). There were three patients (21.4%) with Carpentier type B, ten patients with Carpentier type C (71.4%), and one patient with Carpentier type D (7.1%). There was no early or late mortality, arrhythmia, or readmission for heart failure at a 10-year follow-up. There were no cases of more than mild tricuspid stenosis or more than moderate tricuspid regurgitation during the study period, except for one patient with severe tricuspid regurgitation who underwent reoperation. The z value for tricuspid valve annular size significantly decreased immediately after the operation (2.46 vs. −1.15, p<0.001). However, from 1 year to 7 years after surgery, the z values were maintained between −1 and +1. Left ventricular end-systolic volume, end-diastolic volume, and stroke volume increased after surgery and remained elevated until seven years postoperatively.
Conclusions
Ebstein’s anomaly in children can be repaired by modified cone reconstruction with low mortality and morbidity, good tricuspid valve durability, and annular growth relative to somatic growth.
Graphical Abstract
INTRODUCTION
Ebstein’s anomaly is a rare congenital heart disorder characterized by malformation of the tricuspid valve (TV) and right ventricle (RV). The condition has a diverse morphologic spectrum ranging from adequate true RV with a competent anterior leaflet to complete atrialization of the ventricle with a dysplastic anterior leaflet.1),2) Cone reconstruction of Ebstein’s anomaly was introduced by da Silva and colleagues,3) and has since been applied as a standard treatment for this condition with low rates of recurrent tricuspid regurgitation (TR) and reoperation, including in neonates.3),4),5),6),7),8),9),10)
Compared to other previous TV repair techniques, which are based on a monocuspid mechanism of TV closure with partial coaptation,2),11) the cone reconstruction sutures the cone base to the entire circumference of the anatomic TV annulus and achieves complete coaptation between valvular tissues, which makes the TV annulus smaller than that in the previous techniques.3) With the expansion of surgical indications to include younger patients, including neonates,6),9),10),12) there are concerns about TV annular growth and tricuspid stenosis (TS) according to the somatic growth of pediatric patients.3)
Since Ebstein’s anomaly is often associated with interventricular septal thinning and left ventricular (LV) dysfunction or dysmorphology,1),13),14),15) it is important to confirm adequate restoration of LV function and occurrence of LV reverse remodeling in the long-term after cone reconstruction. Therefore, in this study, we aimed to investigate the long-term clinical and echocardiographic outcomes, including TV durability, TV annular growth, and LV reverse remodeling, after modified cone reconstruction in patients with Ebstein’s anomaly by serial echocardiography.
METHODS
Ethical statement
The Institutional Review Board of the Samsung Medical Center approved this retrospective observational study (SMC 2022-03-015, date of approval: March 23, 2022). The Institutional Review Board waived the requirement of informed consent of individual patients because this retrospective study had minimal risk for patients.
Patient populations
Patients who underwent surgery for Ebstein’s anomaly at a single large tertiary center between January 2005 and June 2021 were enrolled. Patients aged >18 and those who underwent Starnes procedure or TV repair other than the modified cone technique were excluded (Figure 1A).
Figure 1. Study flow diagram and the distribution of age at operation. (A) Study flow diagram. Fourteen pediatric patients who underwent modified cone reconstruction at a single tertiary center were enrolled. (B) The age at the time of operation consistently decreased during the study period.
TV = tricuspid valve.
Data collection and clinical follow-up
The baseline demographic, operative, echocardiographic, laboratory, and follow-up clinical outcomes data were retrospectively collected through medical record review. Our research coordinators and physicians revalidated extracted data for clarity.
The primary outcome was the occurrence of reoperation after the modified cone reconstruction. The secondary endpoints included all-cause death, TR more than moderate, TS more than mild, and readmission for heart failure. The mortality data for patients lost to follow-up were confirmed using the National Death Records.
Modified cone reconstruction
After median sternotomy, extracorporeal circulation with bicaval cannulation was established. After antegrade blood cardioplegia was achieved, an oblique right atriotomy was performed. As the first step of the modified cone reconstruction, after evaluating TV leaflet mobility by saline test, the anterior and posterior leaflets were incised from the functional annulus and delaminated from their abnormal attachments in the RV as a single piece. At this time, unnecessary papillary muscles attached to valve leaflets were also divided, while care was taken to preserve the central and apical papillary muscles. As a modification of the cone reconstruction, we did not use the septal leaflet for the cone reconstruction. We removed most of the septal leaflet except for a very small portion near the apex of the Koch’s triangle. We utilized this portion for the attachment of the posterior leaflet. Next, the atrialized RV was plicated vertically while taking care not to injure the coronary artery by external inspection of the RV. Then, the detached anterior and posterior leaflets were rotated clockwise and reattached to the reduced anatomic annulus after vertical plication. To prevent atrioventricular (AV) block, leaflet tissue reattachment in the conduction area was avoided. In the case of residual TR, especially in anteroseptal commissure, where the two edges were approximated, additional anteroseptal commissuroplasty was performed.
Conventional echocardiographic evaluation
Comprehensive transthoracic echocardiography was performed using 2.5 MHz transducers (Acuson 512 [Siemens Medical Solutions, Mountain View, CA, USA], or Vivid 7 [GE Medical Systems, Milwaukee, WI, USA], or Sonos 5500 [Philips Medical System, Andover, MA, USA]). Standard M-mode, 2-dimensional, and color Doppler imaging scans were performed in parasternal, suprasternal, substernal, and apical views with positional adjustment of the patients. RV and LV function was quantified using the following parameters, which were obtained according to guidelines of the American Society of Echocardiography and indexed for body surface area when appropriate: RV end-systolic area index (ESAi, in cm2/m2); RV end-diastolic area index (EDAi, in cm2/m2); Z value for tricuspid annular plane systolic excursion; LV end-diastolic volume index (EDVi, in mL/m2); LV end-systolic volume index (ESVi, in mL/m2); LV ejection fraction (EF, in percent); LV stroke volume index (SVi, in mL/m2); and indexed LV muscle mass (g/m2).
Statistical analysis
Descriptive statistics for categorical variables were reported as frequency and percentage, whereas continuous variables were reported as median (interquartile range). The correlation between the age of the patient and the year of surgery was assessed using the Spearman’s rank correlation coefficient. All statistical tests were two-sided, with an alpha level of 0.05. The Kaplan–Meier method was used to estimate event-free survival. A linear mixed model was used to determine whether there is a linear trend over time in the repeated measurements and compare measurements at different time points. Statistical analysis was performed using SPSS software (version 25.0; SPSS, Chicago, IL, USA) and R statistical software (version 4.0.2; R Foundation of Statistical Computing, Vienna, Austria).
RESULTS
Baseline and operative characteristics
A total of 14 pediatric patients underwent modified cone reconstruction for Ebstein’s anomaly during the study reference period by a single surgeon (T.J.) with the same surgical principle (Figure 1A). The median age of patients at the time of modified cone reconstruction was 5.8 years (range, 0.01–16.6; Table 1). The age of patients at the time of operation showed a consistent decrease during the study period (p=0.0097). All patients operated since 2015 were under the age of 5 years (Figure 1B), and four patients underwent surgery within the first year of age (Supplementary Table 1).
Table 1. Baseline characteristics.
| Variable | Total patients (n=14) | ||
|---|---|---|---|
| Age (years) | 5.8 (1.3–10.4) | ||
| Age group | |||
| <1 month | 1 (7.1) | ||
| <1 year | 3 (21.4) | ||
| <4 years | 2 (14.3) | ||
| <18 years | 8 (57.1) | ||
| Male sex | 7 (50.0) | ||
| Weight (kg) | 20.0 (9.1–35.1) | ||
| Body surface area (m2) | 0.8 (0.4–1.2) | ||
| Indications for surgery | |||
| Exercise intolerance | 2 (14.3) | ||
| Cyanosis | 1 (7.1) | ||
| Fatigue | 1 (7.1) | ||
| Congestive heart failure | 2 (14.3) | ||
| Tachyarrhythmia | 2 (14.3) | ||
| Progressive RV dilatation without symptoms | 6 (42.9) | ||
| NYHA class | |||
| I | 8 (57.1) | ||
| II | 5 (35.7) | ||
| III | 0 (0.0) | ||
| IV | 1 (7.1) | ||
| Previous cardiac surgery | 0 (0.0) | ||
| Arrhythmia | |||
| WPW syndrome | 2 (14.3) | ||
| Cardiothoracic ratio on CXR | 0.6 (0.57–0.64) | ||
| Preoperative diagnosis | |||
| Ebstein’s anomaly | |||
| Carpentier type B | 3 (21.4) | ||
| Carpentier type C | 10 (71.4) | ||
| Carpentier type D | 1 (7.1) | ||
| Associated anomaly | |||
| VSD | 3 (21.4) | ||
| Tricuspid valve regurgitation | |||
| Moderate | 2 (14.3) | ||
| Moderate to severe | 2 (14.3) | ||
| Severe | 10 (71.4) | ||
Data presented as frequency (%) or median (interquartile range).
CXR = chest X-ray; NYHA = New York Heart Association RV = right ventricle; VSD = ventricular septal defect.
The primary indications for surgery included exercise intolerance (n=2, 14.3%), cyanosis (n=1, 7.1%), fatigue (n=1, 7.1%), congestive heart failure (n=2, 14.3%), tachyarrhythmia (n=2, 14.3%), and progressive RV dysfunction without any symptoms (n=6, 42.9%; Table 1). Preoperatively, 1 and 5 patients presented with New York Heart Association (NYHA) functional classes IV and II, respectively.
All patients had moderate or more than moderate TR on preoperative echocardiography. According to Carpentier’s classification, type C was present in 71.4% (n=10), type B in 21.4% (n=3), and type D in 7.1% (n=1). The median cardiothoracic ratio on preoperative chest x-ray was 0.6 (range, 0.46–0.75). Three patients (21.4%) had concomitant ventricular septal defect, and two patients (14.3%) had preoperative Wolff-Parkinson-White syndrome.
All patients except one had atrial reduction during the modified cone reconstruction, and nine (64.3%) underwent vertical RV plication (Table 2). Ring or DeVega annuloplasty was not performed in any of the patients. Ten patients (71.4%) underwent complete atrial septal defect (ASD)/patent foramen ovale (PFO) closure, and three patients (21.4%) underwent partial ASD/PFO closure. Concomitant procedures included anti-arrhythmia surgery with cryoablation (n=4, 28.6%), ventricular septal defect closure (n=3, 21.4%), and bidirectional cavopulmonary shunt (BCPS) (n=2, 14.3%). BCPS was performed in a patient with a small tricuspid annulus and a right atrial pressure higher than the left atrial pressure after repair. Furthermore, BCPS was performed on another patient who presented with severe right ventricular dysfunction after repair.
Table 2. Operative characteristics.
| Variable | Total patients (n=14) | |
|---|---|---|
| Procedure modification | ||
| Right atrial reduction | 13 (92.8) | |
| Right ventricle vertical plication | 9 (64.3) | |
| Ring or De Vega annuloplasty | 0 (0.0) | |
| Concomitant procedures | ||
| Complete ASD/PFO closure | 10 (71.4) | |
| Subtotal ASD/PFO closure | 3 (21.4) | |
| BCPS | 2 (14.3) | |
| VSD closure | 3 (21.4) | |
| Antiarrhythmic procedure with cryoablation | 4 (28.6) | |
| Cardiopulmonary bypass time (minutes) | 105.5 (99.8–122.3) | |
| Cross clamp time (minutes) | 79.5 (75.0–83.5) | |
Data presented as frequency (%) or median (interquartile range).
ASD = atrial septal defect; BCPS = bidirectional cavopulmonary shunt; PFO = patent foramen ovale; VSD = ventricular septal defect.
Clinical outcomes
During the early postoperative period, defined as within 30 days of surgery, there was no early mortality, postoperative cardiac arrest, mechanical circulatory support, postoperative AV block, or pacemaker implantation (Table 3). Postoperative pneumothorax, intracerebral hemorrhage, and chylothorax occurred in one case each.
Table 3. Clinical outcomes.
| Variable | Total patients (n=14) | ||
|---|---|---|---|
| Early outcomes (<30 days) | |||
| Hospital stay (days) | 7 (6–9) | ||
| Mortality | 0 (0.0) | ||
| Cardiac | |||
| Cardiac arrest or mechanical circulatory support | 0 (0.0) | ||
| Arrhythmia | 0 (0.0) | ||
| Pacemaker implantation | 0 (0.0) | ||
| Pulmonary | |||
| Pneumothorax | 1 (7.1) | ||
| Pneumonia | 0 (0.0) | ||
| Reintubation or tracheostomy | 0 (0.0) | ||
| Neurologic | |||
| Intracerebral hemorrhage | 1 (7.1) | ||
| Neurologic deficit at discharge | 0 (0.0) | ||
| Miscellaneous | |||
| Pericardial effusion | 1 (7.1) | ||
| Chylothorax | 1 (7.1) | ||
| Diaphragm palsy | 0 (0.0) | ||
| Vocal cord palsy | 0 (0.0) | ||
| Wound infection/dehiscence | 0 (0.0) | ||
| 10-year outcomes | |||
| Mortality | 0 (0.0) | ||
| Reoperation | 1 (9.1) | ||
| Readmission for heart failure | 0 (0.0) | ||
| Atrioventricular block | 0 (0.0) | ||
| PPM insertion | 0 (0.0) | ||
| TS more than mild | 0 (0.0) | ||
| TR more than moderate | 1 (9.1) | ||
| NYHA class | |||
| I | 13 (92.8) | ||
| II | 1 (7.1) | ||
| Median follow-up (years) | 7.6 (4.9–11.3) | ||
| Median number of echocardiographic studies during 10-year follow-up | 6.5 (4–7) | ||
Data presented as frequency (%) or median (interquartile range).
NYHA = New York Heart Association; PPM = permanent pacemaker; TR = tricuspid regurgitation; TS = tricuspid stenosis.
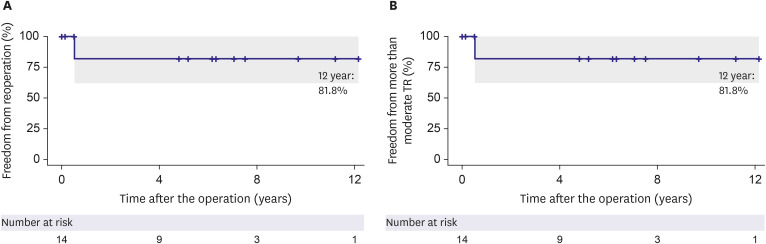
There was no mortality, atrioventricular block, or readmission for heart failure at ten years after the surgery. Thirteen patients (92.8%) presented with NYHA functional class I, and one (7.1%) with NYHA functional class II at the last follow-up. None of the patients had more than mild TS during follow-up (Table 4). None of the patients had more than moderate TR during follow-up except one patient who had a perforation near the newly developed septal annulus at 6 months postoperatively and underwent reoperation. (Figure 2, Table 3). The median follow-up duration was 7.6 years (interquartile range: 4.9–11.3). The follow-up completion rate was 92.8%.
Table 4. Echocardiographic and clinical outcomes at various time points.
| Variable | Preoperative (n=14) | Immediate postoperative (n=13) | 1-year follow-up (n=12) | 3-year follow-up (n=10) | 5-year follow-up (n=10) | 7-year follow-up (n=8) | p value* | |
|---|---|---|---|---|---|---|---|---|
| NYHA class | I (I–II) | I (I–I) | I (I–I) | I (I–I) | I (I–II) | I (I–I) | 0.139 | |
| TS | ||||||||
| TS more than mild | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| TV annulus | ||||||||
| TR more than moderate | 12 (85.7) | 0 (0) | 1 (9.1) | 1 (9.1) | 1 (9.1) | 1 (9.1) | ||
| Size, mm | 35.9 (24.7, 39.7) | 15.0 (12.5, 19.3) | 17.4 (13.8, 24.8) | 20.1 (17.0, 22.5) | 23.0 (19.7, 25.7) | 23.0 (20.4, 29.4) | 0.169 | |
| Z value | 2.91 (1.7, 3.7) | −1.4 (−1.92, −0.43) | −0.82 (−1.11, 0.14) | −0.4 (−1.2, 0.15) | 0.15 (−0.2, −0.39) | −0.39 (−0.49, −0.7) | 0.040 | |
| RA-ESAi (cm2/m2) | 29.5 (25.5, 40.5) | 14.8 (13.3, 17.9) | 13.8 (11.8, 15.6) | 12.9 (12.0, 13.9) | 11.8 (11.0, 12.8) | 11.5 (10.8, 12.8) | <0.001 | |
| RV-EDAi (cm2/m2) | 14.5 (10.2, 20.9) | 16.8 (13.5, 23.1) | 15.3 (13.0, 19.8) | 16.3 (13.3, 18.4) | 15.4 (13.1, 18.6) | 14.6 (13.4, 16.7) | 0.293 | |
| TAPSE, z value | −4.49 (−5.4, −2.67) | −4.89 (−6.5, −2.8) | −4.65 (−7.5, −3.97) | −4.53 (−5.3, −4.0) | 0.507 | |||
| LV-EDVi† (mL/m2) | 37.6 (28.9, 44.1) | 40.2 (36.8, 53.6) | 55.3 (41.6, 59.2) | 60.6 (56.5, 70.9) | 49.4 (42.9, 52.7) | 53.3 (44.1, 67.5) | 0.008 | |
| LV-ESVi† (mL/m2) | 10.4 (9.1, 12.8) | 13.2 (6.7, 14.6) | 16.9 (13.2, 23.6) | 17.2 (12.6, 23.4) | 13.9 (11.7, 16.2) | 17.6 (13.1, 19.4) | 0.013 | |
| LV EF† (%) | 68.5 (66.8, 74.5) | 72.0 (70.4, 79.3) | 63.4 (58.9, 70.4) | 74.4 (61.4, 77.2) | 71.2 (64.5, 73.7) | 69.4 (67.7, 73.2) | 0.513 | |
| LV-SVi† (mL/m2) | 25.2 (20.9, 34.2) | 32.8 (27.9, 37.4) | 32.3 (30.3, 34.8) | 47.2 (32.9, 51.6) | 34.2 (26.8, 43.5) | 35.0 (31.0, 43.8) | 0.018 | |
| LV muscle mass index† (g/m2) | 42.9 (38.8, 52.8) | 48.2 (43.1, 60.8) | 51.3 (49.8, 59.7) | 66.0 (55.3, 86.6) | 59.0 (52.8, 62.2) | 52.2 (44.9, 56.2) | 0.196 | |
Data presented as frequency (%) or median (interquartile range).
EDAi = end-diastolic area indexed to body surface area; EDVi = end-diastolic volume indexed to body surface area; EF = ejection fraction; ESAi = end-systolic area indexed to body surface area; ESVi = end-systolic volume indexed to body surface area; LV = left ventricle; NA = not applicable; NYHA = New York Heart Association; RA = right atrium; RV = right ventricle; SVi = stroke volume indexed to body surface area; TR = tricuspid regurgitation; TS = tricuspid stenosis; TV = tricuspid valve; TAPSE = tricuspid annular plane systolic excursion.
*The p-values were evaluated using a linear mixed model to determine whether a linear trend existed over time.
†Patients who underwent one and a half repair (n=2) were excluded when evaluating values related to LV.
Figure 2. Time to event curves for clinical outcomes. The rate of freedom from reoperation (A) and freedom from more than moderate tricuspid regurgitation (B) in total patients.
Echocardiographic outcomes
The mean size of the TV annulus significantly decreased immediately after the operation (34.58 vs. 15.69 mm, p<0.001). However, the annulus size increased during the follow-up period, and the mean size of TV annulus at 1-, 3-, 5-, and 7-year follow-up were 18.72, 20.28, 23.01, and 23.88 mm, respectively (Figure 3A, Table 4). The z value for TV annulus size (Figure 3B, Table 4) also showed a significant decrease immediately after the operation (2.46 vs. −1.15, p<0.001). However, from 1 year to 7 years after surgery, the z values were maintained between −1 and +1.
Figure 3. Temporal changes of measures related to the right atrium and right ventricle. Serial changes in (A) tricuspid valve annulus size, (B) z value for tricuspid valve annulus size, (C) body surface area-indexed RA-ESA, (D) body surface area-indexed RV-EDA, (E) z value for TAPSE, and (F) cardiothoracic ratio in chest X-ray are presented. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively; and the top and bottom ends of the line mark the 90th and 10th percentiles.
EDA = end-diastolic area; ESA = end-systolic area; RA = right atrium; RV = right ventricle; TAPSE = tricuspid annular plane systolic excursion.
A linear mixed model was used to determine whether there is a linear trend over time in the repeated measurements (expressed as the p-value) and compare measurements at different time points (expressed as asterisks). *p<0.05, while **p<0.005.
The z-value for TAPSE could not be measured before surgery, but at one year after surgery, it had a median value of −4.49, and at 7 years after surgery, it remained unchanged with a median value of −4.53, showing no significant change (p=0.507; Figure 3B, Table 4). Right atrial ESAi showed a decrease in median value from 29.5 cm2/m2 before surgery to 14.8 cm2/m2 immediately after surgery, and this reduction persisted at 11.5 cm2/m2 at 7 years after surgery (p<0.001; Figure 3C, Table 4). However, RV-EDAi demonstrated no significant change, with a median value of 14.5 cm2/m2 before surgery and 14.6 cm2/m2 at 7 years after surgery (p=0.293; Figure 3D, Table 4).
LV-ESVi showed a significant increase in median value from 10.1 mL/m2 before surgery to 13.2 mL/m2 immediately post-surgery and further to 17.6 mL/m2 at 7 years after surgery (p=0.013; Figure 4A, Table 4). Similarly, LV-EDVi increased significantly from 37.6 mL/m2 before surgery to 40.2 mL/m2 immediately post-surgery and reached 53.3 mL/m2 at 7 years after surgery (p=0.008; Figure 4B, Table 4). However, LVEF exhibited no significant differences, with a median value of 68.5% before surgery, 72% immediately after surgery, and 69.4% at 7 years after surgery (p=0.513; Figure 4C, Table 4).
Figure 4. Temporal changes of measures related to the left ventricle. Serial changes in (A) body surface area-indexed LV-ESV, (B) body surface area-indexed LV-EDV, (C) LVEF, (D) body surface area-indexed LV-SV, and (E) body surface area-indexed LV muscle mass are presented. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively; and the top and bottom ends of the line mark the 90th and 10th percentiles.
EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; LV = left ventricular; SV = stroke volume.
A linear mixed model was used to determine whether there is a linear trend over time in the repeated measurements (expressed as the p-value) and compare measurements at different time points (expressed as asterisks). *p<0.05, **p<0.005.
DISCUSSION
The current study has three key findings: 1) Early TV repair with modified cone reconstruction in children showed good long-term outcomes with no late mortality or AV block and low rates of reoperation or recurrence of TR. 2) TV annulus was maintained at an adequate size according to the growth of pediatric patients in the long term. 3) LV function was also improved with progressive reverse remodeling during long-term follow-up.
Despite the diverse morphologic spectrum of Ebstein’s anomaly, cone reconstruction can be used as the standard approach in most patients.3),7),10),16) Contemporary studies of cone reconstruction have shown excellent rates of freedom from more than moderate TR and reoperation.8),9) However, the results vary depending on the age at the time of correction and the severity of malformation.9) In the current study, except for one patient who underwent reoperation for perforation near the valve annulus, no other patient required reoperation or developed more than moderate TR in the long term.
We refer to our technique as modified cone repair because we did not incorporate a septal leaflet during the procedure. Most cases of Ebstein’s anomaly have small and dysmorphic septal leaflets. The septal leaflet is almost absent in many cases of Carpentier type C Ebstein’s anomaly. It is very cumbersome and difficult to include the small and dysmorphic septal leaflet in the newly repaired TV, securing competent TV function. One potential drawback of this modified cone repair is the possibility of a small size of the valve annulus after repair.
Therefore, there are concerns about the growth of TV annulus and the development of TS when patients grow up,3) not only because of the surgical modification described above but also because the age of correction has been continuously lowered with the advancement of operative techniques and the expansion of surgical indications.6),17) Compared with other reconstructive techniques for Ebstein’s anomaly, cone reconstruction achieves 360° cone-shaped TV reconstruction with complete coaptation between valvular tissues, which may reduce the TV annulus considerably.3) However, as the technique uses only native tissues and no artificial materials, it may offer sufficient growth potential for TV annulus. In the current study, the growth of the TV annulus size was appropriate according to the somatic growth of pediatric patients, and none of the patients developed significant TS in the long term.
Abnormalities of LV morphology and function are common in Ebstein’s anomaly.1),13),14),15) These include LV systolic or diastolic dysfunction, LV dilatation, and LV myocardial changes resembling noncompaction, occurring in up to 39% of patients.1),15) In the current study, the LV volume increased consistently immediately after the surgery and over approximately three years postoperatively. Moreover, the LVEF and LV muscle mass were also maintained in the long term. This may suggest the potential for LV remodeling in patients with preoperative LV abnormality. Future research through magnetic resonance imaging (MRI) studies will be necessary to investigate long-term LV volume and function improvements.
Some limitations of our study should be acknowledged. First, this was a single-center study with a small number of pediatric patients with various Carpentier types. However, during the study period, all patients underwent cone reconstruction by a single surgeon (T.J.) using the same surgical principle and strategies related to perioperative management. A more extensive study is required to provide more definitive evidence of the long-term growth of the TV annulus and biventricular reverse remodeling. Second, the absence of MRI data made it impossible to compare and analyze ventricular sizes and functions before and after surgery. Although long-term follow-up of most patients in the same center with frequent assessment by serial echocardiography is a strength of our study, additional MRI research is necessary to assess long-term changes in ventricular volumes and functions.
In conclusion, Ebstein’s anomaly in children can be repaired by modified cone reconstruction with low mortality and morbidity, good TV durability, and annular growth relative to somatic growth. Early surgery can restore and improve LV function with progressive reverse remodeling in the long term.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: The authors have no financial conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding author upon reasonable request.
- Conceptualization: Park I, Jun TG, Yang JH, Kang IS, Huh J, Song J, Lee OJ.
- Data curation: Park I, Jun TG, Yang JH, Kang IS, Huh J, Song J, Lee OJ.
- Formal analysis: Park I, Jun TG, Yang JH, Kang IS, Huh J, Lee OJ.
- Investigation: Park I, Jun TG, Yang JH, Kang IS, Huh J, Song J.
- Methodology: Park I, Yang JH, Huh J, Song J, Lee OJ.
- Software: Park I.
- Supervision: Park I.
- Validation: Park I.
- Writing - original draft: Park I.
- Writing - review & editing: Park I.
SUPPLEMENTARY MATERIAL
Characteristics of patients under one year of age undergoing modified cone reconstruction
References
- 1.Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation. 2007;115:277–285. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 2.Carpentier A, Chauvaud S, Macé L, et al. A new reconstructive operation for Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1988;96:92–101. [PubMed] [Google Scholar]
- 3.da Silva JP, Baumgratz JF, da Fonseca L, et al. The cone reconstruction of the tricuspid valve in Ebstein’s anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg. 2007;133:215–223. doi: 10.1016/j.jtcvs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Dearani JA, Said SM, O’Leary PW, Burkhart HM, Barnes RD, Cetta F. Anatomic repair of Ebstein’s malformation: lessons learned with cone reconstruction. Ann Thorac Surg. 2013;95:220–226. doi: 10.1016/j.athoracsur.2012.04.146. [DOI] [PubMed] [Google Scholar]
- 5.Lange R, Burri M, Eschenbach LK, et al. Da Silva’s cone repair for Ebstein’s anomaly: effect on right ventricular size and function. Eur J Cardiothorac Surg. 2015;48:316–320. doi: 10.1093/ejcts/ezu472. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno M, Hoashi T, Sakaguchi H, et al. Application of cone reconstruction for neonatal Ebstein anomaly or tricuspid valve dysplasia. Ann Thorac Surg. 2016;101:1811–1817. doi: 10.1016/j.athoracsur.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Holst KA, Dearani JA, Said S, et al. Improving results of surgery for Ebstein anomaly: where are we after 235 cone repairs? Ann Thorac Surg. 2018;105:160–168. doi: 10.1016/j.athoracsur.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Burri M, Mrad Agua K, Cleuziou J, et al. Cone versus conventional repair for Ebstein’s anomaly. J Thorac Cardiovasc Surg. 2020;160:1545–1553. doi: 10.1016/j.jtcvs.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Schulz A, Marathe SP, Chávez M, et al. The association of age and repair modification with outcome after cone repair for Ebstein’s malformation. Semin Thorac Cardiovasc Surg. 2022;34:205–212. doi: 10.1053/j.semtcvs.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Huang SC, Wu ET, Chen SJ, et al. Surgical strategy toward biventricular repair for severe Ebstein anomaly in neonates and infancy. Ann Thorac Surg. 2017;104:917–925. doi: 10.1016/j.athoracsur.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 11.Danielson GK, Driscoll DJ, Mair DD, Warnes CA, Oliver WC., Jr Operative treatment of Ebstein’s anomaly. J Thorac Cardiovasc Surg. 1992;104:1195–1202. [PubMed] [Google Scholar]
- 12.Wackel PL, Dearani JA, Cetta F. Neonatal Ebstein repair-where are we now? Ann Transl Med. 2017;5:109. doi: 10.21037/atm.2017.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postma AV, van Engelen K, van de Meerakker J, et al. Mutations in the sarcomere gene MYH7 in Ebstein anomaly. Circ Cardiovasc Genet. 2011;4:43–50. doi: 10.1161/CIRCGENETICS.110.957985. [DOI] [PubMed] [Google Scholar]
- 14.Bagur RH, Lederlin M, Montaudon M, et al. Images in cardiovascular medicine. Ebstein anomaly associated with left ventricular noncompaction. Circulation. 2008;118:e662–e664. doi: 10.1161/CIRCULATIONAHA.108.767822. [DOI] [PubMed] [Google Scholar]
- 15.Attenhofer Jost CH, Connolly HM, O’Leary PW, Warnes CA, Tajik AJ, Seward JB. Left heart lesions in patients with Ebstein anomaly. Mayo Clin Proc. 2005;80:361–368. doi: 10.4065/80.3.361. [DOI] [PubMed] [Google Scholar]
- 16.Dearani JA. Ebstein repair: how I do it. JTCVS Tech. 2020;3:269–276. doi: 10.1016/j.xjtc.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar TK, Boston US, Knott-Craig CJ. Neonatal Ebstein anomaly. Semin Thorac Cardiovasc Surg. 2017;29:331–337. doi: 10.1053/j.semtcvs.2017.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of patients under one year of age undergoing modified cone reconstruction