Abstract
Following infection or vaccination, early-minted antibody secreting cells (ASC) or plasmablasts appear in circulation transiently, and a small fraction migrates to the spleen or bone marrow (BM) to mature into long-lived plasma cells (LLPC). While LLPC, by definition, are quiescent or non-dividing, the majority of blood ASC are thought to be “blasting” or proliferative. In this study, we find > 95% nascent blood ASC in culture express Ki-67 but only 6–12% incorporate BrdU after 4 h or 24 h labeling. In contrast, < 5% BM LLPC in culture are Ki-67+ with no BrdU uptake. Due to limitations of traditional flow cytometry, we utilized a novel optofluidic technology to evaluate cell division with simultaneous functional IgG secretion. We find 11% early-minted blood ASC undergo division, and none of the terminally differentiated BM LLPC (CD19−CD38hiCD138+) divide during the 7–21 days in culture. While BM LLPC undergo complete cell cycle arrest, the process of differentiation into an ASC or plasmablasts also discourages entry into S phase. Since the majority of Ki-67+ nascent blood ASC have exited cell cycle and are no longer actively “blasting”, the term “plasmablast”, which traditionally refers to an ASC that still has the capacity to divide, may probably be a misnomer.
Subject terms: Cell biology, Immunology
Introduction
During infection or vaccination, B cells undergo proliferation and differentiation to generate protective antibody responses. Activation of the B cell receptors (BCR) following antigen encounter triggers a cascade of signaling events that lead to proliferation of B cells1. In addition to BCR and toll-like receptors (TLR), cytokine receptors and TNF family receptors, such as CD402,3, also enhance B cell proliferation. Upon induction, the first division of a naïve B cell requires 24 h (h), and the cells continue to divide every 6-8 h thereafter prior to differentiating into antibody secreting cells (ASC) or plasmablasts4,5. The term “plasmablast” has traditionally been used interchangeably with an early-minted ASC that has proliferative potential and the capacity for ongoing cell division6–11. While the majority of ASC undergo apoptosis, a small fraction migrates to bone marrow (BM) and further matures into long-lived plasma cells (LLPC)12,13. These BM LLPC have astonishing longevity and provide durable humoral protection against reinfection for a lifetime14,15. By this definition, BM LLPC are non-dividing, terminally differentiated cells16.
Commonly used methods for measuring cell proliferation include Ki-67, a nuclear protein strongly expressed in proliferating cells (although its function is unclear), and incorporation of thymidine analogs (BrdU) into the DNA of actively dividing cells. Studies interrogating intracellular Ki-67 expression or BrdU incorporation have suggested human BM LLPC do not divide while ASC in the blood are proliferative15,17,18. In non-human primates, BrdU+ BM LLPC can be found a decade after BrdU administration19, suggesting persistence for years in the BM of the same ASC (i.e. without division). In contrast, the majority (> 70–98%) of nascent blood ASC in vaccinated individuals are Ki-67+15,17,20. Whether these early-minted ASC have recently divided or if they are still actively cycling upon entering the bloodstream remains incompletely understood.
In this study, we overcome limitations in ASC survival ex vivo with a new human in vitro plasma cell survival system (PCSS)21,22. These culture systems allow us to visualize single ASC proliferation using a novel optofluidic platform to measure cell division simultaneously with ongoing Ig secretion. Results show that 90% of early-minted blood ASC stop dividing and that BM LLPC remain in a state of permanent cell cycle arrest. These results emphasize the induction of networks that promote cell cycle arrest in early ASC differentiation.
Results
Few blood ASC incorporate BrdU or undergo proliferation
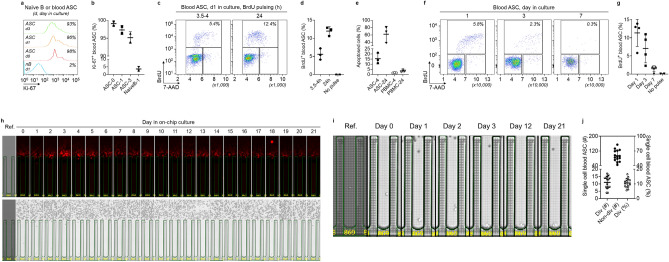
Human early-minted ASC (CD19loCD27hiCD38hi) were isolated from the blood by FACS sorting from healthy adults between 5 and 7 days after vaccination (since days 5–7 post-vaccination reveal the peak window for antigen-specific ASC circulation23–27). As previously shown15,17,20, the majority of blood ASC are Ki-67+ even when cultured in PCSS. Figure 1a,b shows that blood ASC express Ki-67 as high as 99% (99.1 ± 0.8%), 97% (97.3 ± 1.3%), and 95% (95.1 ± 1.7%) on day 0, 1, and 3, respectively. For non-proliferative controls, we used unstimulated blood naive B cells. These cultured ASC also secrete IgG when assayed by bulk ELISpots (Suppl. Fig. S1a,b).
Figure 1.
Very few early-minted ASC (plasmablasts) divide. (a) Representative flow cytometric analysis of Ki-67 expression in blood ASC at day 0, 1, and 3 in culture (with unstimulated naïve B cells at day 1 as a non-proliferative control). The numbers indicate the percentages of the cells positive for Ki-67. (b) Quantitation of Ki-67+ blood ASC (n = 2 independent biological replicates). 0, 1, 3, day in culture. (c) Representative flow cytometric analysis of BrdU incorporation in blood ASC at day 1 in culture after 3.5–4 h or 24 h pulsing. (d) Quantitation of BrdU+ blood ASC (n = 3 independent biological replicates). h, hours BrdU pulsed. All cells were assayed at day 1 in culture. (e) Quantitation of apoptosed blood ASC after 3.5–4 h or 24 h BrdU pulsing (n = 3 independent biological replicates). PBMC were used as control populations. 4, 24, hours BrdU pulsed. All cells were assayed at day 1 in culture. (f) Representative flow cytometric analysis of BrdU incorporation in blood ASC at day 1, 3, and 7 in culture after 3.5–4 h pulsing (with apoptotic cells excluded). (g) Quantitation of BrdU+ blood ASC (n = 4 independent biological replicates). All 3.5–4 h BrdU pulsed. In (c,f): Individual cell cycle phases (S, BrdU+; G0/G1, 2N and BrdU−; and G2/M, 4N and BrdU−) were indicated and the percentage of ASC in S phase was estimated by the amount of BrdU detected. Apoptotic cells were excluded from the analysis. (h) Image series displaying a single ASC with ongoing Ig secretion showing evidence of division (bright field) from day 0 to 21. (i) Zoomed in bright-field image series for direct visualization of the dividing cells from (h) on select days in culture. (j) Quantitation (left y axis) and frequency (right y axis) of dividing and non-dividing single cell blood ASC across 15 independent experiments. Div, dividing; Non-div, no dividing. In (h,i): Ref., reference image for visualization of the number of cells penned at loading.
To assess the phases of cell cycle, we employed flow cytometric analysis of BrdU incorporation in blood ASC (Suppl. Fig. S1c). After a 3.5–4 h BrdU pulse label of blood ASC, we show 6% (5.6 ± 1.6%) the cells were in S phase on day 1 of culture (Fig. 1c, left panel, and Fig. 1d). In an attempt to capture asynchronous cells beyond the 4 h pulse, we labeled for 24 h and found 12% (12 ± 1.2%) of cells in S phase (Fig. 1c, right panel and Fig. 1d). Notably, the portion of cell death was substantially higher when pulsing for 24 h (61%; 61.4 ± 21.1%) compared to that of 4 h (15%; 15.5 ± 5.2%) (Fig. 1e). These results suggest longer BrdU labeling times may be toxic to blood ASC in culture with potential overestimation of labeled cells due to excessive cell death. Ultimately, longer labeling may lead to less predictable cell recovery and viability.
Since short pulses appeared to be sufficient to balance BrdU incorporation and toxicity for early-minted blood ASC, we used 4 h purses for all subsequent BrdU experiments. We found that the frequency of blood ASC in S phase decreased from 11% (11.4 ± 3.7%) on day 1–7% (7.1 ± 3.9%) on day 3, and further dropped to 1% (1.5 ± 0.8%) by day 7 (Fig. 1f,g). By day 22, S phase entry was not detected in blood ASC despite functional IgG secretion (Suppl. Fig. S1d–f), indicating that nascent ASC in long-term culture stop proliferating. These data suggest 90% early-minted ASC in the culture exit cell cycle early (at days 1–3) and nearly all are in complete cell cycle arrest from day 7 onwards.
Due to cell toxicity with longer pulses, BrdU labeling was not ideal to assess proliferation rates of ASC accurately. To overcome these issues, we evaluated ASC cell division coupled with ongoing IgG secretion by single cell analysis. We loaded early-minted blood ASC onto the optofluidic Lightning platform (Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.) and measured IgG blooms and counted cell numbers by bright field each day for 7–21 days in PCSS cultures (Suppl. Fig. S2). We observed by day 15, the majority of ongoing IgG secreting cells (IgG-blooming pens) had not divided (Suppl. Fig. S3a). Only 11% (10.5 ± 3.1%) had undergone cell division (Fig. 1h–j). While some division could occur as early as 8 h (Suppl. Fig. S3b), division typically occurred within 24-48 h if the cell indeed divided (Fig. 1h,i and Suppl. Fig. S3c). Even when followed for 21 days, no further divisions were seen (Fig. 1h). Importantly, during these periods, proliferation was not accompanied by cell death, since intact cells were visualized daily with effective ongoing IgG blooms up to day 21 (Fig. 1h). These results demonstrate that only 11% of bona fide IgG ASC in culture undergo proliferation over 21 days.
BM LLPC do not undergo cell division
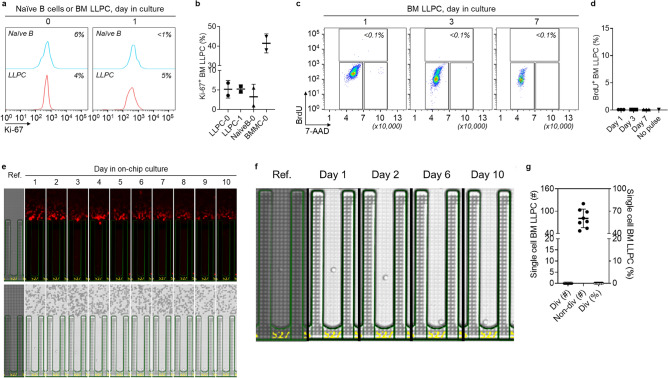
In direct contrast to the blood ASC, human BM LLPC, defined as CD19−CD38+CD138+ ASC (PopD)12,15 which retain long-lived antigen specificities from exposures over 40 years and were shown to be non-dividing by Ki-67 expression15,17,18. Similar to previous studies, BM LLPC demonstrate < 5% Ki-67 staining (Fig. 2a,b and Suppl. Fig. S4a). For non-proliferative and proliferative controls, we used unstimulated naive B cells and BMMC, respectively. We further confirmed that BM LLPC cultured in PCSS were indeed functionally secreting IgG (Suppl. Fig. S4b,c) despite minimal intracellular Ki-67 expression: 5.2% (5.2 ± 2.26%) and 5.1% (5.1 ± 1.03%) on day 0 and 1, respectively. To assess the phases of cell cycle, we employed flow cytometric analysis of BrdU incorporation in BM LLPC (Suppl. Fig. S4d). After BrdU pulse label for 4 h on day 1, 3, and 7 in culture; we observed < 0.1% BrdU incorporation at all time points (Fig. 2c,d). Finally, to assess if BM LLPC in culture could undergo division beyond day 7, we bulk-cultured BM LLPC for 56 days and found negligible Ki-67 expression or BrdU incorporation (Suppl. Fig. S4e,f), despite ongoing ability to secrete IgG (Suppl. Fig. S4g,h).
Figure 2.
BM LLPC do not divide. (a) Representative flow cytometric analysis of Ki-67 expression in BM LLPC at day 0 and 1 in culture (with unstimulated naïve B cells as a non-proliferative control). The numbers indicate the percentages of the cells positive for Ki-67. (b) Quantitation of Ki-67+ blood ASC (n = 2 independent biological replicates). BMMC were used for Ki-67 flow cytometry assay controls (see Suppl. Fig. S4a). BMMC, BM mononuclear cells. 0, 1, day in culture. (c) Representative flow cytometric analysis of BrdU incorporation in BM LLPC at day 1, 3, and 7 in culture after 3.5–4 h pulsing (with apoptotic cells excluded). (d) Quantitation of BrdU+ BM LLPC pulsed for 3.5–4 h (n = 3 independent biological replicates). In (c): Individual cell cycle phases (S, BrdU+; G0/G1, 2N and BrdU−; and G2/M, 4N and BrdU−) were indicated and the percentage of ASC in S phase was estimated by the amount of BrdU detected. Apoptotic cells were excluded from the analysis. (e) Image series displaying a single BM LLPC with ongoing Ig secretion and no evidence of division ((bright field). (f) Zoomed in bright-field image series for direct visualization of the non-dividing cell from (e) on select days in culture. (g) Quantitation (left y axis) and frequency (right y axis) of dividing and non-dividing single cell BM LLPC across 8 independent experiments. Div dividing, Non-div no dividing. In (e,f): Ref: reference images for visualization of single cell penned at loading.
To assess the proliferation potential of BM LLPC in culture by single-cell analysis similar to the blood ASC, BM LLPC were loaded onto the Lightning platform and IgG blooms were captured daily from each single cell over 10–14 days in the PCSS cultures. Not one IgG secreting BM LLPC divided when visualized daily for 2 weeks in culture (Fig. 2e-g and Suppl. Fig. S5a). Even an immature BM ASC subset, defined as CD19+CD38+CD138+ (PopB)12,15 also showed no evidence of proliferation potential in culture by day 4–14 (Suppl. Fig. S5b,c). These results confirm human BM CD138+ ASC which include the LLPC (PopD) and a less mature BM-resident ASC subset (PopB) do not divide and remain in permanent cell cycle arrest.
Discussion
In this study, we show that the majority of bona fide blood ASC in PCSS culture have stopped dividing despite ongoing expression of Ki-67 and variable BrdU uptake. Using a novel optofluidic single cell analysis platform, we characterized single ASC by secretory function rather than surface markers and found only 11% blood ASC displayed evidence of proliferation. We saw no division after 3 days in culture. While ASC differentiation may be division linked, with upregulation of plasma cell programs especially PRDM-1 (the gene encoding for BLIMP-1), major hallmarks of cell cycle are downregulated. Although the “blasting” status of an ASC is traditionally used interchangeably with proliferative potential, in the case of early-minted ASC in the periphery, it should be interpreted with caution.
BCR activation leads to an increase in cell mass and metabolism as activated B cells prepare for proliferation. BCR crosslinking with TLR engagement synergizes to promote clonal expansion and class-switching of antigen-specific B cells28. Upregulation of BLIMP-1, a transcription factor that drives B cells to plasma cell differentiation, is known to repress c-myc, a transcription factor that drives functions needed for cell division, thus leading to cell cycle arrest upon ASC differentiation29. However, upstream to ASC differentiation, the DNA breaks involved in class-switching and somatic hypermutation in the germinal center (GC) are critical B cell functions with associated clonal proliferation. As ASC differentiate, the initiation of permanent cell cycle arrest likely protects from further malignant transformation.
Although ASC differentiation and maturation are ultimately linked to cell cycle arrest, the kinetics of an activated B cell in mitosis and initiation of secretory Ig synthesis are unclear. It was thought that increased Ig production by activated B cells and early ASC requires continuous cell division30,31, through which the cells remodel their ER and activate the UPR16. This mitosis-dependent remodeling is regulated by mTORC1, which controls biochemical pathways generating the cell biomass needed for division32. Indeed, during early induction, mTORC1 is crucial for the survival and proliferation of antigen-specific activated B cells in the GC33. However, recent reports provide a revised model where activated marginal zone B cells differentiate and adapt to increased Ig production in a cell division-independent process. These marginal zone B cells form ASC with rapid kinetics to generate timely responses to blood-borne pathogens34. This alternative model uncouples early ASC differentiation and cell division with mTORC1 proactively “notifying” the UPR34. Interestingly, following ASC differentiation, we also observe downregulation of mTORC1 pathways as nascent blood ASC mature into LLPC21.
In the present study, we confirmed that healthy BM LLPC do not divide for up to 10–14 days in culture. Perhaps, the lifelong survival potential remains benign in a BM LLPC because they engage in programs of cell cycle arrest, limiting malignant transformation. Survival without transformation is reminiscent of cellular senescence, which is a stress-induced persistent hypo-replicative state characterized by the expression of cell cycle inhibitors35. BM LLPC, which enter permanent cell cycle arrest are shown to become resistant to apoptosis13, engage in autophagy pathways15, and also upregulate cyclin inhibitors CDKN2A (p16) and CDKN2C (p18)42. Together, LLPC appear to undergo cellular senescence programs to prevent malignant conversion as the number of BM LLPC accumulate with age.
Proliferation status of human ASC has been difficult to infer due to three key technical challenges. First, the rapid death of ASC ex vivo limits investigation into their proliferation profiles. While the time to first division in naïve B cells is typically 24-36 h4,5,36,37, observation of ASC cell division in vitro requires viability for at least 48-72 h. This becomes a major problem with studying blood and BM ASC due to their rapid apoptosis within hours21,38–40. Second, blood and BM ASC are relatively rare. With each infection or vaccination, despite the addition of 10,000–100,000 newly-minted ASC12,16, only a small fraction undergoes maturation into LLPC13. Of the total BM nucleated cells, the ASC pool represents a tiny fraction (0.1%-0.3%) over a lifetime41. Thus, FACS sorting of rare ASC populations has rendered technical limitations for bulk proliferation studies due to a few contaminating B cells that may undergo proliferation. Lastly, although FACS sorting is widely used to enrich for ASC, it defines ASC only by surface phenotype and not Ig secretory function, which likely overestimates the number of authentic ASC.
Since ASC rapidly die ex vivo in conventional cultures, here we used a novel in vitro PCSS for both bulk and single-cell experiments. The question arises whether the limited proliferation potential of early-minted blood ASC is acquired due to intrinsic characteristics of the cells themselves or heavily influenced by the experimentally induced PCSS cultures. We favor the former for several reasons. First, the relationship between PRDM1 and c-myc and our recent bulk transcriptomics revealed that ASC undergo cell cycle arrest as they mature13. Second, our more recent single-cell studies with ASC expressing MKI67 (the gene encoding for Ki-67) and genes involved in cell cycle arrest42. In Duan et al., we show that early BM resident ASC with MKI67 expression have already upregulated cyclin inhibitors CDKN2A (p16) and CDKN2C (p18) without the use of PCSS42. The expression of these genes was found in the same cell in this single-cell analysis. Despite MKI67 expression, it appears that terminal ASC differentiation by its intrinsic nature simultaneously limits further entry into cell cycle.
Assays with Ki-67 and BrdU are often used to infer the proliferative status of mammalian cells. The former is used to assess cells as they exit M phase43, while BrdU is used to assess S phase entry and cellular commitment to enter a new cell cycle. In the present study, we used both Ki-67 and BrdU for a more comprehensive examination of the ASC proliferation status. However, technically, the persistence of Ki-67 proteins after S phase and increased cellular toxicity with longer BrdU labeling led to inconsistent conclusions of the number of proliferating early-minted Ig secreting cells. To our knowledge, the present study is the first to directly visualize both ongoing Ig secretion and cell division of bona fide ASC at the single cell level with the use of the novel optofluidic Lightning platform.
In the absence of antigen, in vitro generated effector ASC require cell division to become Ig producing cells31,44,45. Earlier work established that the majority (80–85%) of in vitro differentiating B cells enter the cell cycle by day 3 and do not undergo cell cycle arrest until day 630,31. Interestingly, these studies also showed that in vitro-generated ASC must first exit the cell cycle for terminal differentiation30,45. This study is the first to validate these in vitro models by demonstrating that primary ex vivo human early-minted ASC or plasmblasts after vaccination also exit cell cycle after terminal differentiation.
In all, the present study provides the first single-cell visualization of bona fide IgG secreting ASC together with direct cell division in culture. Since our ex vivo experiments require the PCSS, it may not reflect the behavior of the cells in vivo and thus a limitation of the study. We also did not assess other antibody isotypes (such as IgA and IgM). In addition, the newly introduced optofluidic platform (the Lightning system) captures Ig secretion over 80 min, while ELISpots can capture Ig secretion over 18–24 h to enhance sensitivity of extremely low level Ig secreting cells, such as ARH-77, a myeloma cell line (Suppl. Fig. S6a,b). Nevertheless, engagement of plasma cell transcriptional programs results in many pathway changes in cellular structure, processes, and metabolism but must exit out of cell cycle to commence IgG secretion and to mature into a LLPC.
Methods
Human subjects
Peripheral blood samples were obtained from 66 healthy subjects, 47 of which received vaccines for Tdap, influenza, shingles, meningitis, hepatitis A, hepatitis B, yellow fever, or COVID-19 (the 3rd, 4th, or 5th dose) at 5-7d prior to sample collection. 17 healthy BM aspirate and 21 femoral head samples were also obtained. The age ranges of subjects that provided blood, bone marrow, and femoral head samples are 18–73, 24–65, and 54–89 years, respectively. There were no subjects who provided both bone marrow and matching blood samples. Informed consent was obtained from all subjects. All studies were approved by the Emory University Institutional Review Board Committee. All methods were performed in accordance with the relevant guidelines and regulations and in accordance with the Declaration of Helsinki.
Purification and bulk-culture of blood and BM ASC and ELISpot assays
PBMC/BMMC isolation was performed as previously described21. Fresh blood ASC, BM PopB, and BM LLPC (PopD) were purified using FACS-based sorting, as previously described15,21. All sorted blood ASC populations were 88–98% pure. Blood ASC were bulk-cultured in PCSS, which is a culture system that consists of mesenchymal stem cells (MSC) secretome supplemented with APRIL (200 ng/mL) and in hypoxic conditions (2.5% O2) at 37 °C (36 °C if for single cell on-chip culture), as previously described21,22. BM PopB and BM LLPC (PopD) were bulk-cultured in the same conditions except that no APRIL was supplemented (since exogenous APRIL provides no advantages for survival and secretory functions of human BM ASC in culture)46. IgG secretion of cultured ASC was assessed by ELISpot assays, which used goat anti-human IgG to capture secreted IgG and alkaline phosphatase-conjugated goat anti-human IgG to detect, and were performed as previously described21.
Human multiple myeloma (MM) cell line
The ARH-77 human MM cell line (ATCC) was used and handled according to the provider’s recommendations. ELISpot assays on those cells were performed as described above21.
Phase-flow BrdU cell proliferation and flow analysis of Ki-67 intracellular expression
Proliferation of blood ASC and BM LLPC was evaluated on the basis of bromodeoxyuridine (BrdU) incorporation using a phase-flow BrdU commercial kit (BioLegend, Inc.) in accordance with the manufacturer’s recommendations. Briefly, d1, d3, and d7 PCSS-cultured blood ASC were pulsed with 10-15 uM BrdU for 3.5–4 h, then fixed and permeabilized, and were subsequently treated with DNAse for 1 h, followed by incubation with anti-BrdU-FITC antibody (to detect BrdU incorporation) and addition of 7-AAD (10 ug/mL; to determine cellular DNA contents; 7-AAD was incubated for 0.5–1.5 h prior to acquisition). Negative staining controls for sorted ASC were included to confirm specificity of BrdU (FITC) and 7-AAD staining. FACS-sorted naive B cells were included as non-proliferative controls to establish cell cycle gates. To assess the background binding of the specific anti-BrdU antibody, cultured cells that had not been pulsed with BrdU and were similarly treated were also included. Samples were run on an LSR-II or Symphony A3 flow cytometer and analyzed with the FlowJo v10.8 software (FlowJo, LLC). The percentage of ASC in S phase was estimated by the amount of BrdU-FITC detected.
For intracellular staining of Ki-67, cells were fixed and permeabilized, followed by incubated with anti-Ki-67 antibody or isotype controls. Cellular fluorescence data was acquired with a Symphony A3 flow cytometer and analyzed with the FlowJo v10.8 software (FlowJo, LLC).
Single cell Ig secretion and proliferation analysis
Lightning optofluidic system preparation. For functional single cell analysis, the Lightning system (Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.), equipped with the OEP technology which can identify single ASC and precisely position them individually into nanoliter-volume pens (i.e. a NanoPen chamber), was used. The system was sterilized by purging all fluidic lines before the initiation of the workflows.
Blood and BM ASC single cell imports
After sterilization, an OptoSelect 1500 chip was loaded, wetted, primed, and focus-calibrated using standard workflows. Immediately after FACS sorting, a volume of 10 uL cell suspension containing 3000–25,000 FACS-purified ASC in PCSS was imported into the chip channels through a customized Targeted Pen Selection (TPS) penning algorithm with a small volume import (SVI) operation – and with or without penning chip by multiple rounds of imports and/or by fields of view (FOV) indexing. Following equilibration, the system performed reference imaging by capturing the bright-field high-magnification (4× and 10×) images across the chips (as the post-penning background reference and the normalization reference for ASC quantity during on-chip culture). Single cell ASC were penned in fresh PCSS supplemented with Loading Reagent (Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.). Residual cells in the channels were subsequently flushed to a sterile PCSS collection tube for reuse.
On-chip blood and BM ASC single cell cultures
Following single cell deposition into NanoPens, ASC were cultured on-chip at 36 °C with constant perfusion of fresh PCSS at a rate of 0.01 μL/s and in hypoxic condition that was infused with a pre-analyzed gas mixture containing 2.5% O2, 5% CO2, and 92.5% N2 (AirGas). To keep track of penned ASC numbers, timelapse bright-field images were taken daily to keep track of cell growth for each colony. For blood ASC, PCSS was supplemented with APRIL (200 ng/mL); for BM ASC, all but one were without APRIL.
Bead-based, in-channel IgG capture assay
In-channel IgG capture assays were performed using single assay mixtures containing anti-human IgG-coated 6–8 μm beads (Spherotech) and fluorescently-labeled detection anti-IgG antibodies (2.5 μg/mL; Jackson ImmunoResearch). The mixtures were imported into the channels and floated above the pens throughout the course of the capture assay. IgG secreted by single cells contained in pens diffused into the channels and bound the beads. Accumulation of fluorescence from secondary antibodies on the IgG-coated bead upon secreted IgG binding led to the development of fluorescent halos—or blooms—in the channels adjacent to the pens. In this way, secreted IgG concentrations (which represent ASC secretion rate) could be semi-quantitatively assessed. IgG bloom development was time-dependent, with capture assays running for 60–80 min, and imaged at 10-min intervals using a TRED (AF-594), FITC (AF-488), or PE (AF-555) filter cube. Single, penned ASC were directly captured for functional Ig secretion (as “blooms”) and simultaneously enumerated for the quantity of cells by direct bright field microscopy. The chip was flushed extensively after each assay (to remove all bead assay mixture). Thus, the secreted IgG were captured at 10-min intervals over the entire time of the assay (i.e. 60–80 min). All assays were performed with PCSS.
Workflows and chip image analysis
All the workflow operations were controlled using the Cell Analysis Suite (CAS) software (v2.2.8.40; Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.). IgG blooms and ASC quantitation were determined using Image Analyzer (v2.2; Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.) and Assay Analyzer (v2.2; Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.), and subsequently confirmed through manual assessment.
Supplementary Information
Acknowledgements
We thank Drs. Jonathan Didier, Suhagi Baxi, and Eugene Gibbs of Berkeley Lights, Inc. (now Bruker Cellular Analysis, Inc.), and Shuya Kyu and Ariel Ley for technical assistance. We thank Robert E. Karaffa, Kametha T. Fife, and Sommer Durham of the Emory University School of Medicine Flow Cytometry Core (EFCC) for technical support. We also thank our team of clinical coordinators and donors who made this study possible.
Author contributions
D.C.N. and F.E.L. planned the research; D.C.N., C.S., I.T. H., M.C., and V.C. performed the experiments; J.A. and S.L. acquired healthy human BM aspirates; D.C.N. and C.S. analyzed the data; M.C.W. and I.S. provided important editorial input; F.E.L. and I.S. obtained funding and human samples; F.E.L. and I.S. supervised research. D.C.N. wrote the first draft of the paper. All authors have reviewed, edited, and approved the final manuscript.
Funding
This work was supported by the following grants: NIH/NIAID 1R01AI121252, 1P01AI125180, U01AI141993, U54CA260563, U19AI110483, T32AI070081 and the Bill & Melinda Gates Foundation Grant INV-002351.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
F.E.L. is the founder of Micro-Bplex, Inc., serves on the scientific board of Be Biopharma, is a recipient of grants from the BMGF and Genentech, Inc., and has served as a consultant for Astra Zeneca. I.S. has consulted for GSK, Pfizer, Kayverna, Johnson & Johnson, Celgene, Bristol Myer Squibb, and Visterra. F.E.L., D.N., and I.S. are inventors of the patents (assigned to Emory University) concerning the plasma cell survival media related to this work. The other authors declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-53977-2.
References
- 1.Donahue AC, Fruman DA. Proliferation and survival of activated B cells requires sustained antigen receptor engagement and phosphoinositide 3-kinase activation. J. Immunol. 2003;170:5851–5860. doi: 10.4049/jimmunol.170.12.5851. [DOI] [PubMed] [Google Scholar]
- 2.Hasbold J, Johnson-Leger C, Atkins CJ, Clark EA, Klaus GG. Properties of mouse CD40: Cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur. J. Immunol. 1994;24:1835–1842. doi: 10.1002/eji.1830240817. [DOI] [PubMed] [Google Scholar]
- 3.Thomas M, et al. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 4.Tangye SG, Hodgkin PD. Divide and conquer: The importance of cell division in regulating B-cell responses. Immunology. 2004;112:509–520. doi: 10.1111/j.1365-2567.2004.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins ED, et al. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat. Protoc. 2007;2:2057–2067. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- 6.Larsson LG, Schena M, Carlsson M, Sallstrom J, Nilsson K. Expression of the c-myc protein is down-regulated at the terminal stages during in vitro differentiation of B-type chronic lymphocytic leukemia cells. Blood. 1991;77:1025–1032. doi: 10.1182/blood.V77.5.1025.1025. [DOI] [PubMed] [Google Scholar]
- 7.Jego G, et al. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells. Blood. 1999;94:701–712. doi: 10.1182/blood.V94.2.701. [DOI] [PubMed] [Google Scholar]
- 8.Sze, D. M., Toellner, K. M., Garcia de Vinuesa, C., Taylor, D. R. & MacLennan, I. C. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med.192, 813–821 (2000). [DOI] [PMC free article] [PubMed]
- 9.Tarte K, Zhan F, De Vos J, Klein B, Shaughnessy J., Jr Gene expression profiling of plasma cells and plasmablasts: Toward a better understanding of the late stages of B-cell differentiation. Blood. 2003;102:592–600. doi: 10.1182/blood-2002-10-3161. [DOI] [PubMed] [Google Scholar]
- 10.Bromage ES, Kaattari IM, Zwollo P, Kaattari SL. Plasmablast and plasma cell production and distribution in trout immune tissues. J. Immunol. 2004;173:7317–7323. doi: 10.4049/jimmunol.173.12.7317. [DOI] [PubMed] [Google Scholar]
- 11.Tellier J, Nutt SL. Plasma cells: the programming of an antibody-secreting machine. Eur. J. Immunol. 2018 doi: 10.1002/eji.201847517. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen DC, Joyner CJ, Sanz I, Lee FE. Factors affecting early antibody secreting cell maturation into long-lived plasma cells. Front. Immunol. 2019;10:2138. doi: 10.3389/fimmu.2019.02138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyner, C. J. et al. Generation of human long-lived plasma cells by developmentally regulated epigenetic imprinting. Life Sci. Alliance5. 10.26508/lsa.202101285 (2022). [DOI] [PMC free article] [PubMed]
- 14.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 15.Halliley JL, et al. Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity. 2015;43:132–145. doi: 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen DC, et al. Plasma cell survival: The intrinsic drivers, migratory signals, and extrinsic regulators. Immunol. Rev. 2021;303:138–153. doi: 10.1111/imr.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garimalla, S. et al. Differential transcriptome and development of human peripheral plasma cell subsets. JCI Insight4, 1. 10.1172/jci.insight.126732 (2019). [DOI] [PMC free article] [PubMed]
- 18.Mei HE, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113:2461–2469. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- 19.Hammarlund E, et al. Plasma cell survival in the absence of B cell memory. Nat. Commun. 2017;8:1781. doi: 10.1038/s41467-017-01901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caraux, A. et al. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138+ plasma cells. Haematologica95, 1016–1020. 10.3324/haematol.2009.018689 (2010). [DOI] [PMC free article] [PubMed]
- 21.Nguyen DC, et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat. Commun. 2018;9:3698. doi: 10.1038/s41467-018-05853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodruff, M., Nguyen, DC, Faliti, CE, Saini, AS, Lee, FE, Sanz, I. Response under pressure: Deploying emerging technologies to understand B-cell-mediated immunity in COVID-19. Nat. Methods (accepted) (2022). [DOI] [PMC free article] [PubMed]
- 23.Halliley JL, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–3587. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee FE, et al. Circulating human antibody-secreting cells during vaccinations and respiratory viral infections are characterized by high specificity and lack of bystander effect. J. Immunol. 2011;186:5514–5521. doi: 10.4049/jimmunol.1002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrammert J, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 2012;86:2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odendahl M, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 27.Sanz I, et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol. 2019;10:2458. doi: 10.3389/fimmu.2019.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pone EJ, et al. Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: Relevance to microbial antibody responses. Crit. Rev. Immunol. 2010;30:1–29. doi: 10.1615/critrevimmunol.v30.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 30.Tourigny MR, et al. CDK inhibitor p18(INK4c) is required for the generation of functional plasma cells. Immunity. 2002;17:179–189. doi: 10.1016/s1074-7613(02)00364-3. [DOI] [PubMed] [Google Scholar]
- 31.Jelinek DF, Lipsky PE. The role of B cell proliferation in the generation of immunoglobulin-secreting cells in man. J. Immunol. 1983;130:2597–2604. doi: 10.4049/jimmunol.130.6.2597. [DOI] [PubMed] [Google Scholar]
- 32.Weber JD, Gutmann DH. Deconvoluting mTOR biology. Cell Cycle. 2012;11:236–248. doi: 10.4161/cc.11.2.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DD, et al. mTOR has distinct functions in generating versus sustaining humoral immunity. J. Clin. Invest. 2016;126:4250–4261. doi: 10.1172/JCI86504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaudette BT, Jones DD, Bortnick A, Argon Y, Allman D. mTORC1 coordinates an immediate unfolded protein response-related transcriptome in activated B cells preceding antibody secretion. Nat. Commun. 2020;11:723. doi: 10.1038/s41467-019-14032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberts B, J. A., & Lewis J. Molecular Biology of the Cell (2002).
- 37.Patterson DG, Kania AK, Zuo Z, Scharer CD, Boss JM. Epigenetic gene regulation in plasma cells. Immunol. Rev. 2021 doi: 10.1111/imr.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minges Wols, H. A., Underhill, G. H., Kansas, G. S. & Witte, P. L. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol.169, 4213–4221 (2002). [DOI] [PubMed]
- 39.Cassese G, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen DC, et al. Extracellular vesicles from bone marrow-derived mesenchymal stromal cells support ex vivo survival of human antibody secreting cells. J. Extracell Vesicles. 2018;7:1463778. doi: 10.1080/20013078.2018.1463778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terstappen LW, Johnsen S, Segers-Nolten IM, Loken MR. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood. 1990;76:1739–1747. doi: 10.1182/blood.V76.9.1739.1739. [DOI] [PubMed] [Google Scholar]
- 42.Duan M, et al. Understanding heterogeneity of human bone marrow plasma cell maturation and survival pathways by single-cell analyses. Cell Rep. 2023;42:112682. doi: 10.1016/j.celrep.2023.112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.O'Connor BP, Cascalho M, Noelle RJ. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 2002;195:737–745. doi: 10.1084/jem.20011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J. Immunol. 2003;170:261–269. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen DC, S. C., Sanz I, Lee FE. Human bone marrow plasma cell survival is independent of APRIL. J Immunol (2019) 202 (Suppl 1): 1816.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.