Abstract
Mice rendered deficient in interleukin-10 (IL-10) by gene targeting (IL-10−/− mice) develop chronic enterocolitis resembling human inflammatory bowel disease (IBD) when maintained in conventional animal facilities. However, they display a minimal and delayed intestinal inflammatory response when reared under specific-pathogen-free (SPF) conditions, suggesting the involvement of a microbial component in pathogenesis. We show here that experimental infection with a single bacterial agent, Helicobacter hepaticus, induces chronic colitis in SPF-reared IL-10−/− mice and that the disease is accompanied by a type 1 cytokine response (gamma interferon [IFN-γ], tumor necrosis factor alpha, and nitric oxide) detected by restimulation of spleen and mesenteric lymph node cells with a soluble H. hepaticus antigen (Ag) preparation. In contrast, wild-type (WT) animals infected with the same bacteria did not develop disease and produced IL-10 as the dominant cytokine in response to Helicobacter Ag. Strong H. hepaticus-reactive antibody responses as measured by Ag-specific total immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, IgG3, and IgA were observed in both WT and IL-10−/− mice. In vivo neutralization of IFN-γ or IL-12 resulted in a significant reduction of intestinal inflammation in H. hepaticus-infected IL-10−/− mice, suggesting an important role for these cytokines in the development of colitis in the model. Taken together, these microbial reconstitution experiments formally establish that a defined bacterial agent can serve as the immunological target in the development of large bowel inflammation in IL-10−/− mice and argue that in nonimmunocompromised hosts IL-10 stimulated in response to intestinal flora is important in preventing IBD.
Inflammatory bowel disease (IBD) is thought to be the consequence of an aberrant mucosal immune response which damages tissues of the intestinal tract (22, 30, 34). It is not clear, however, whether this response is directed against self antigens (Ag) or gut-dwelling flora. In the case of both forms of IBD, ulcerative colitis and Crohn’s disease, studies in experimental animals have suggested that disease may be triggered by a cytokine imbalance. Thus, a variety of different genetically immunodeficient mice have been shown to develop chronic inflammation of the colon, and in many of the strains the observed tissue damage is associated with alterations in systemic and/or local cytokine production (8, 16, 19, 29). This association is perhaps clearest in mice deficient for interleukin-10 (IL-10), a major down-regulator of both macrophage and T-cell function. These animals spontaneously develop chronic inflammation of the lower intestinal tract, and this IBD-like syndrome has been shown to depend on excess gamma interferon (IFN-γ) production by CD4+ T cells generated in the absence of IL-10 suppression (2, 14). An important aspect of the intestinal disease occurring in IL-10-deficient (IL-10−/−) mice is that it is less severe and delayed in onset when the animals are bred and maintained under specific-pathogen-free (SPF) conditions. In that situation, the inflammation is initially limited to the proximal colon (14), but in mice with the appropriate genetic backgrounds it can eventually involve all regions of the large bowel (2).
The markedly diminished enterocolitis seen in SPF-reared IL-10−/− mice suggests that components of the intestinal flora may promote or be the targets of the dysregulated inflammatory response encountered in conventionally maintained animals of this strain. A common gut pathogen encountered in animal facilities is Helicobacter hepaticus. This urease-producing Helicobacter species colonizes the large bowel of mice and in some inbred strains is also found in the gallbladder and liver, where it is frequently associated with chronic active hepatitis and liver tumors. The organism was originally isolated from A/JCr mice (9, 35, 37) and subsequently found to be associated with inflammatory large bowel disease in immunodeficient nude and scid mice (36). Some mouse strains such as C57BL/6 can serve as carriers of the infection but are resistant to clinical disease or histological lesions (35–37). H. hepaticus has been cultured from several different immunodeficient animals, including IL-10−/− mice (34a), and has also been found in inflammatory bowel lesions of mice lacking the common cytokine receptor γ chain (4).
In the present study, we show that SPF-reared IL-10−/− mice experimentally infected with H. hepaticus develop an intestinal inflammatory disease which in part resembles that seen in conventionally maintained IL-10-deficient animals. The observed pathology is accompanied by an excessive type 1 cytokine response which is lacking in wild-type (WT [IL-10-sufficient]) mice receiving similar inocula. Together the data support the concept that IBD is elicited in response to specific agents in gut flora and establish a defined animal model for studying the role of the microbial component in its pathogenesis.
MATERIALS AND METHODS
Experimental animals and infections.
Six- to nine-week-old, SPF IL-10−/− mice backcrossed to C57BL/10SgSnAi and WT C57BL/10SgSnAi mice, both free of all Helicobacter species, were obtained from Taconic Farms (Germantown, N.Y.). The IL-10−/− animals (originally obtained from R. Kühn and W. Müller, University of Cologne, Cologne, Germany) (14) used in our experiments were from the 7th and 10th backcross generations. No differences were apparent in the results obtained from the mice in the two backcross generations. Initial experiments were performed with male and female mice. As similar results were obtained for both sexes, female animals were used in the subsequent experiments. The animals were housed in sterile microisolator cages with autoclaved bedding, food, and water at the animal facility of the National Institute of Allergy and Infectious Diseases in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (20a) under an animal study proposal approved by the NIAID Animal Care and Use Committee. In a separate experiment, animals were housed in a sterile biobubble (flexible film isolator; Harlan Isotec, Blackthorn, England). Before use, the biobubble was decontaminated by paraformaldehyde gas. All supplies going into the unit were autoclaved prior to entry, with autoclave efficacy assessed by using sterilization monitors. These supplies, as well as SPF mice arriving directly from Taconic Farms, were allowed to enter the biobubble through a transfer cylinder.
Mice were inoculated intraperitoneally (i.p.) or intragastrically (i.g.) with 0.5 ml of an H. hepaticus suspension (standard Frederick isolate 1A [9, 37]) prepared to a McFarland turbidity standard of 1.0 in phosphate-buffered saline (PBS), representing 2.45 × 109 CFU/ml. The bacteria were confirmed to be H. hepaticus by PCR before infection (1), and viability of the inoculum was determined pre- and postinjection by culture. Age- and sex-matched uninfected animals were included as controls. At different time points after infection, mice were sacrificed, spleens and mesenteric lymph nodes (MLN) were collected for in vitro culture, and stomach, intestine, and liver tissues were analyzed for gross and histopathological changes.
Microbiology.
Fecal and cecal samples were collected aseptically in brain heart infusion broth with horse serum and yeast extract (Remel Laboratories, Lenexa, Kans.). Suspensions of these materials were passed through a 0.45-μm-pore-size filter. Filtered material was spread over brucella agar with horse blood, trimethoprim, vancomycin, and polymyxin (Remel Laboratories) and incubated at 37°C under microaerophilic conditions for up to 5 days. Observed growth was tested for oxidase and rapid urease (selective rapid urease test; Remel Laboratories) reactions.
Pathology.
Mice were necropsied, and cecum, colon, rectum, and liver tissues were fixed in Bouin’s fixative or 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) or with modified Steiner’s silver stain (11). Selected tissues fixed in Bouin’s fixative were used for immunohistochemical staining of T and B cells with a polyclonal rabbit anti-human CD3 antibody (Ab) (cross-reacts with mouse CD3; DAKO Corp., Carpinteria, Calif.) and an anti-mouse B220 monoclonal Ab (MAb) (PharMingen, San Diego, Calif.), respectively, and an ABC Vectastain Elite kit (Vector Laboratories Inc., Burlingame, Calif.).
Ag.
Soluble Helicobacter Ag (SHelAg) was prepared from cultures of H. hepaticus. The organisms were harvested and washed extensively in PBS, and the resulting suspension was sonicated at 4°C to lyse the bacteria. Cell debris was removed by centrifugation at 8,000 × g (Sorvall RC2-B, SS-34 rotor) for 30 min at 4°C. The supernatant was sterile filtered (0.22-μm-pore-size filter), protein content determined by Bradford (Pierce, Rockford, Ill.), and the Ag was stored at −40°C until use.
Cell preparations.
Single-cell suspensions were prepared from spleens and MLN, splenic erythrocytes were lysed by osmotic treatment, and cells were resuspended in tissue culture medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, 20 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 50 μM 2-mercaptoethanol). Experiments were performed with splenocyte suspensions from either individual mice or pools obtained by mixing equal numbers of cells from each animal within a group and with MLN cells pooled from two to four mice per group.
T-cell-depleted populations were prepared by treating MLN cells (6 × 106/ml) with anti-Thy1.2 (1:500 dilution of ascites fluid; Cedarlane Laboratories, Hornby, Ontario, Canada) and complement (Cedarlane).
Flow cytometric analysis.
MLN samples were stained with phycoerythrin-conjugated anti-CD4 MAb RM4-5 and fluorescein-isothiocyanate-conjugated anti-CD44 (Pgp-1) MAb IM7 (both from PharMingen). After addition of propidium iodide to exclude dead cells, lymphocytes were analyzed on a FACScan flow cytometer (Becton Dickinson).
Culture conditions.
To measure proliferative responses, splenocytes (2.5 × 106/ml) and MLN cells (1.5 × 106/ml) were cultured in medium alone or with titrating amounts of SHelAg in flat-bottomed 96-well plates in a total volume of 0.2 ml/well at 37°C and 5% CO2. Cultures were pulsed with [3H]thymidine (1 μCi/well; specific activity, 2 Ci/mmol; New England Nuclear Corp., Boston, Mass.) after 48 h of incubation and harvested 18 h later to determine incorporated [3H]thymidine.
To measure cytokine responses, spleen cells (5 × 106/ml) and total and T-cell-depleted MLN cell populations (3 × 106/ml, based on cell counts before T-cell depletion) were cultured in medium alone or with 0.3 to 1 μg of SHelAg per ml in flat-bottomed 24-well plates in a total volume of 1 to 1.2 ml/well, and supernatants were collected after 72 h.
Cytokine assays.
IFN-γ and IL-10 were measured by two-site enzyme-linked immunosorbent assay (ELISA) (20, 31). The amount of cytokine was quantitated by comparison to standard curves of recombinant IFN-γ (rIFN-γ; Genzyme, Cambridge, Mass.) and rIL-10 (PharMingen), respectively. Tumor necrosis factor alpha (TNF-α) was measured with an ELISA kit purchased from Genzyme.
Nitric oxide (NO) measurements.
Nitrate (NO2−) levels were used as an indicator of reactive nitrogen intermediates in samples and were measured by Griess assay (12). Briefly, 50-μl aliquots of supernatant were added in duplicate to 96-well plates followed by 50 μl of a 1:1 mixture of 1% sulfanilamide dihydrochloride (Sigma, St. Louis, Mo.) in 2.5% H3PO4 and 0.1% naphthylenediamide dihydrochloride (Sigma) in 2.5% H3PO4. After a 10-min incubation at room temperature, the absorbance of the samples (550 nm) was read spectrophotometrically, and amounts of nitrate were determined by comparison with a standard curve generated with sodium nitrate (NaNO2; Sigma).
Ab measurements.
Abs reactive to Helicobacter Ag were measured by ELISA. Briefly, 96-well Immunolon 2 plates (Dynex Technologies Inc., Chantilly, Va.) were coated with SHelAg (8 μg/ml; 50 μl/well) in 0.05 M sodium carbonate (pH 9.6) at 4°C overnight. After a 1-h blocking step with 5% milk at 37°C, sera were added to the wells at different dilutions and the plates were incubated at 4°C overnight. Thereafter, peroxidase-conjugated rabbit Ab specific for mouse immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, IgG3, or IgA (all from Zymed Laboratories, Inc., San Francisco, Calif.) was added for 3 h at 37°C. Color reactions were developed by addition of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Kirkegaard & Perry, Inc., Gaithersburg, Md.), and optical density was measured at 405 nm with an ELISA reader (Molecular Devices, Menlo Park, Calif.).
In vivo MAb treatment.
To analyze the effect of in vivo neutralization of IFN-γ or IL-12, IL-10−/− mice were injected i.p. with 1 mg of MAb XMG-6 (anti-IFN-γ) (5), MAb C17.8 (anti-IL-12, generated in the laboratory of G. Trinchieri) (38), or control MAb GL113 (anti-β-galactosidase) in 0.5 ml of PBS on days −1, 2, 5, 8, 12, 15, 19, 22, and 26. Mice were inoculated i.p. with H. hepaticus on day 0 as described above, and tissues were processed for histology 4 weeks later.
Statistics.
Data were analyzed by Student’s two-tailed t test, and differences with P values of <0.05 were considered significant.
RESULTS
Bacterial infections.
Wild-type C57BL/10 and IL-10−/− mice inoculated i.p. or i.g. with H. hepaticus developed large bowel infections as determined by fecal culture 14 days later. The infections persisted in both strains of mice, as H. hepaticus could be grown from fecal samples collected at later time points as well as from cecal contents obtained at the time of necropsy (up to 16 weeks after bacterial inoculation). Uninfected mice were negative for H. hepaticus throughout the experiments.
Intestinal histopathology of H. hepaticus-infected WT and uninfected IL-10-deficient mice.
WT mice inoculated i.p. with H. hepaticus showed minimal histopathological changes when necropsied at 2 to 16 weeks. These consisted of small lymphocytic foci in the cecum (Fig. 1A and B; Table 1). A similar pattern was seen when WT mice inoculated i.g. were examined at 4 and 11 weeks postinfection (not shown).
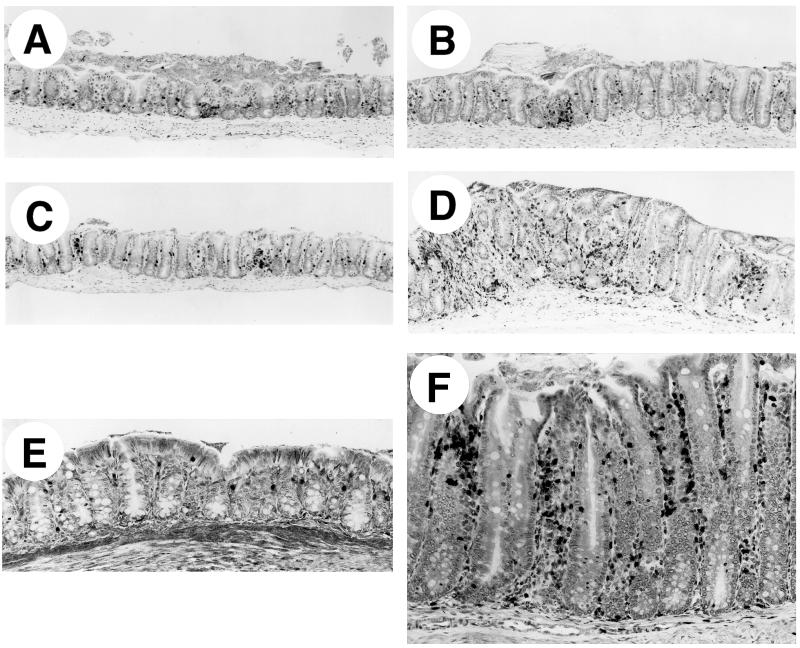
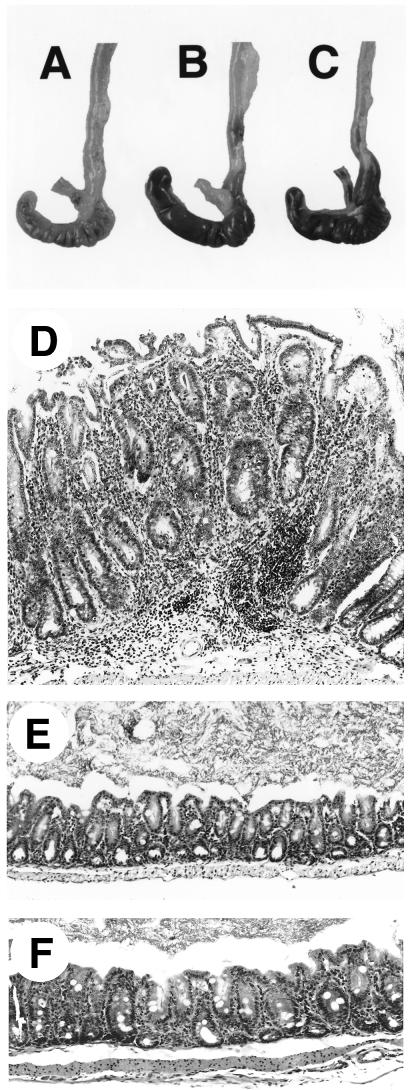
FIG. 1.
Immunostaining for CD3+ cells of Bouin’s-fixed cecum and colon of IL-10−/− and WT mice, all with hematoxylin counterstain. (A to C) Ceca of representative uninfected WT (A), infected WT (B), and uninfected IL-10−/− (C) mice at 4 weeks, showing few CD3+ cells. (D) Cecum of infected IL-10−/− mouse at 4 weeks, with many CD3+ cells and marked thickening (hyperplasia) of the epithelium. (E) Colon of uninfected IL-10−/− mouse at 4 weeks, with few CD3+ cells in the lamina propria. (F) Colon of infected IL-10−/− mouse at 4 weeks, with many CD3+ cells in lamina propria and marked diffuse epithelial hyperplasia. Magnifications: (A to D) ×75, (E and F) ×150.
TABLE 1.
Histopathological changes in the large intestine after H. hepaticus infection in WT and IL-10−/− animals
| Time (wk)a | Scoreb (no. of mice with lesions/no. examined)
|
||
|---|---|---|---|
| Cecum | Colon | Rectum | |
| H. hepaticus-infected WT | |||
| 2 | ± (1/6) | − (0/6) | ND |
| 4–5 | ± (7/20) | − (0/14) | − (0/6) |
| 9–11 | ± (4/11) | − (0/11) | − (0/6) |
| 16 | + (4/6) | + (2/6) | + (2/6) |
| Uninfected IL-10−/− | |||
| 2 | − (0/3) | − (0/3) | ND |
| 4–5 | ± (3/14) | − (0/8) | − (0/6) |
| 9–11 | ± (5/9) | − (0/9) | ± (1/6) |
| 16 | − (0/6) | − (0/6) | − (0/6) |
| H. hepaticus-infected IL-10−/− | |||
| 2 | +/++ (4/4) | +/++ (4/4) | ND |
| 4–5 | +/++ (17/17) | ±/+ (14/14) | + (6/6) |
| 9–11 | ++ (12/12) | + (11/12) | + (6/6) |
| 16 | ++ (5/5) | +/++ (5/5) | +/++ (5/5) |
Data pooled from two experiments at 2 weeks, four experiments at 4 to 5 weeks, three experiments at 9 to 11 weeks, and one experiment at 16 weeks. Uninfected WT controls displayed none of the inflammatory or hyperplastic lesions in cecum, colon, and rectum found in infected mice.
−, no lesions; ±, minimal degree of lesions described in text; +, mild degree of lesions; ++, moderate degree of lesions, with some mice having severe degree of lesions; ND, not determined.
Although the original generations of IL-10−/− mice introduced into our conventional animal facility developed severe intestinal lesions including colitis and rectal prolapse by 14 weeks of age (32a), the same pathologic syndrome was not observed after caesarean rederivation of the strain into an SPF facility (Taconic Farms) and further backcrossing. The SPF-reared IL-10−/− animals used as uninfected controls (Fig. 1C; Table 1) showed only minimal differences from uninfected WT mice when examined at 2 to 16 weeks after initiation of the experiment. Some uninfected IL-10−/− mice showed a small increase in CD3+ lymphocytes in the lamina propria of the cecum, although to levels far less than those observed in infected WT animals.
Exacerbated inflammatory response of IL-10-deficient mice to H. hepaticus.
IL-10-deficient mice infected i.p. with H. hepaticus showed gross large bowel lesions as early as 2 weeks after bacterial inoculation. In particular, the ceca of infected IL-10−/− mice were obviously smaller and paler (due to thickening of the intestinal wall) than those of uninfected IL-10-deficient or infected WT animals (Fig. 2A to C).
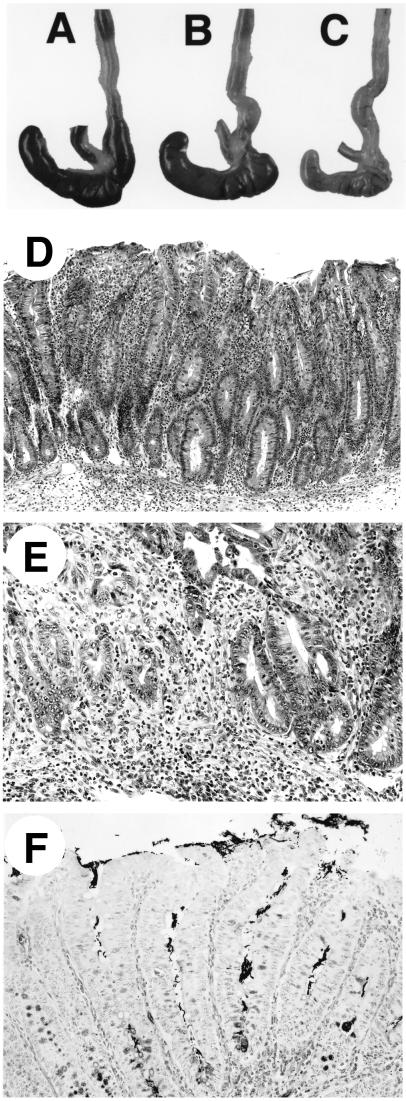
FIG. 2.
Gross and microscopic intestinal pathology of H. hepaticus-infected mice. (A to C) Gross pathology of portions of proximal large bowels of 4-week-infected WT (A), uninfected IL-10−/− (B), and 4-week-infected IL-10−/− (C) mice. Note the smaller, paler cecum of the infected IL-10−/− mouse; histologically, the mouse had typhlitis and diffuse hyperplasia. (D) Diffuse cecal hyperplasia in infected IL-10−/− mouse at 9 weeks, showing marked inflammation in lamina propria and submucosa. H&E stain. (E) Severe focal atypical hyperplasia in cecum of infected IL-10−/− mouse at 16 weeks. The muscularis mucosa is disrupted by atypical hyperplastic cells. H&E stain. (F) Steiner stain of cecum from infected IL-10−/− mouse at 4 weeks, showing numerous bacteria within epithelial crypts. Magnifications, ×75 (D) and ×150 (E and F).
When examined at the microscopic level, all of the infected IL-10−/− animals showed histopathological changes including moderate to severe inflammation of the cecum and mild inflammation of the colon and rectum (Table 1). The cecal lesions consisted of infiltration of lymphocytes, neutrophils, and macrophages into the lamina propria, with most of the lymphocytes staining positive for the pan-T-cell marker CD3 (Fig. 1D). Characteristic of an acute inflammatory response, lesions in 2-week-infected IL-10−/− mice had the highest numbers of neutrophils, while the proportion of these cells decreased at later time points. Plasma cells and focal collections of B220+ B lymphocytes were first noted in lesions at 4 weeks and increased in number as the infection proceeded into the chronic phase. The inflammation extended into the submucosa in mice infected for ≥9 weeks. Diffuse hyperplasia of the cecal epithelium (Fig. 2D) was observed from 2 weeks after infection, while more focal hyperplasia was first seen after 4 weeks, being more widespread and severe in 9- to 16-week-infected mice. The focal hyperplasia was often atypical, with hyperchromatic epithelium, many mitotic figures, and disruption of the muscularis mucosa (Fig. 2E).
Colonic and rectal lesions were always much less severe than those in the cecum and consisted of diffuse and focal epithelial hyperplasia along with accumulations of inflammatory cells in the lamina propria (Fig. 1F). Such changes were usually absent in equivalent sections from uninfected IL-10−/− (Fig. 1E) or infected WT (not shown) animals. Although not systematically surveyed, gastric and small intestinal lesions were not observed in any of the mice as determined by both gross and histological examination.
Examination of Steiner-stained sections from infected IL-10−/− mice revealed numerous helical bacteria within hyperplastic crypts of cecum (Fig. 2F) and fewer in colon and rectum. Bacteria were also observed within cecal crypts of infected WT mice but not of uninfected IL-10−/− or WT animals (not shown).
Liver lesions (focal inflammation, granulomas, and occasional necrosis) were found often in infected IL-10−/− and WT mice but rarely in uninfected controls. The lesions were observed after i.p. but not i.g. inoculation of the bacteria. No progressive hepatitis was seen, and Steiner-stained sections revealed no bacteria within hepatic lesions.
In the experiments described above, the bacteria were inoculated i.p. and were presumably transported to the intestine via the biliary tract. To confirm that the observed large bowel lesions in infected IL-10−/− mice were not dependent on the i.p. route of bacterial inoculation, H. hepaticus infections were initiated by i.g. administration. At 4 and 11 weeks postinoculation, these i.g.-infected IL-10−/− animals displayed the same intestinal histological changes as IL-10−/− mice infected in parallel by the i.p. route (data not shown). Thus, for convenience, i.p. inoculation was used for routine infections of mice.
While the IL-10−/− and WT mice were bred in an SPF facility, H. hepaticus infections were performed in a conventional animal room where other pathogens might be present. In an attempt to rule out the possible involvement of contaminating microbial agents in the induction of large bowel lesions by H. hepaticus, a separate i.p. infection experiment was performed in a sterile biobubble. Intestinal inflammatory changes indistinguishable from those documented in IL-10−/− mice infected in parallel outside the biobubble were observed at 4 and 10 weeks postinfection in these animals (data not shown).
H. hepaticus-infected WT and IL-10-deficient mice display T-cell proliferative and Ab responses to a soluble bacterial Ag preparation.
To study the cellular immune response to H. hepaticus, spleen and MLN cells from infected WT and IL-10−/− mice were stimulated in vitro with SHelAg, a soluble Ag preparation prepared from the same bacterial strain. The results of a representative experiment in which proliferation to increasing doses of SHelAg was assayed at 4 weeks after bacterial infection are shown in Fig. 3. Ag dose-dependent proliferative responses were observed with spleen cells from both WT and IL-10−/− mice, with no significant differences (Fig. 3A). Spleen cells from uninfected animals showed only marginal background proliferation to SHelAg, and this only at the highest Ag concentrations. In contrast, MLN cells from 4-week H. hepaticus-infected IL-10−/− mice displayed a stronger proliferative response than equivalent populations from WT animals (Fig. 3B). Although MLN of infected IL-10−/− animals were larger than those of infected WT mice, the higher proliferative capacity in vitro did not reflect an increased percentage of activated T cells. Thus, the percentages of both CD4+ and CD4+ CD44+ cells were comparable in MLN of 4- to 5-week-infected WT versus IL-10−/− animals (CD4+ cells, mean ± standard deviation [SD] = 37.8% ± 1.4% versus 34.8% ± 2.9%, respectively; CD4+ CD44+ cells, 4.0% ± 0.5% versus 4.0% ± 0.6%, respectively) (data pooled from three independent experiments). No significant SHelAg-induced proliferation was observed with MLN cells from uninfected controls.
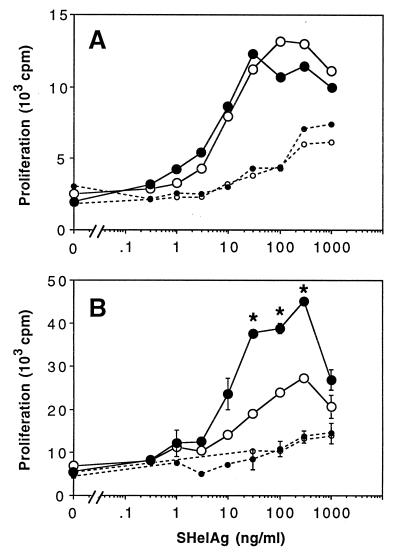
FIG. 3.
SHelAg-induced proliferative responses of cells from H. hepaticus-infected WT and IL-10−/− mice. Spleen cells (2.5 × 106/ml) (A) and MLN cells (1.5 × 106/ml) (B) from 4-week-infected (solid lines) and uninfected (dotted lines) WT (○) and IL-10−/− (•) mice were stimulated in vitro with various doses of SHelAg. Cultures were pulsed with [3H]thymidine after 48 h and harvested 18 h later. Spleen cell results represent the mean values for three individual infected and a pool of two uninfected mice per group. MLN results represent means ± SD of duplicate cultures of pools of the same mice shown in A. Data for one of six experiments (using 4-, 6-, 10-, or 11-week-infected mice) with similar results are shown. An asterisk indicates a significant difference (P < 0.05) in proliferative response between infected WT and IL-10−/− mice.
To compare levels of Helicobacter-reactive Ab in infected WT and IL-10−/− mice, animals were bled 16 weeks after H. hepaticus inoculation and sera were analyzed for total anti-SHelAg IgG by ELISA. Both strains of mice showed comparable high titers of anti-SHelAg IgG (Fig. 4). No SHelAg-reactive Ab was detected in sera from uninfected WT or IL-10−/− mice. Furthermore, comparable titers of SHelAg-specific IgG1, IgG2a, IgG2b, and IgG3 as well as Ag-specific IgA were found in infected IL-10−/− and WT animals, with only slightly higher levels of IgA in the IL-10-deficient mice (Fig. 4). Results were similar for a second experiment in which Ab were analyzed at 4 weeks postinfection (not shown).
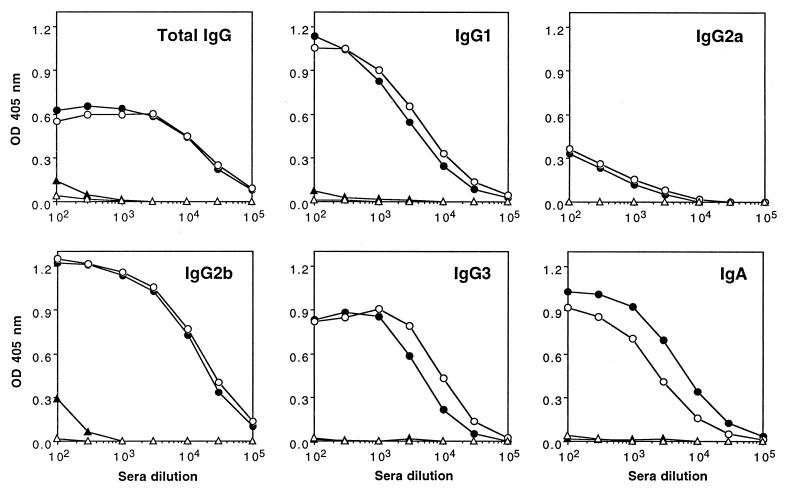
FIG. 4.
SHelAg-specific Ab in sera of H. hepaticus-infected WT and IL-10−/− mice. Sera from uninfected (▵, ▴) and 16-week-infected (○, •) WT (open symbols) and IL-10−/− (closed symbols) mice were analyzed for levels of Ab (total IgG, IgG1, IgG2a, IgG2b, IgG3, and IgA) reactive to SHelAg by ELISA as described in Materials and Methods. Each dot represents the mean optical density (OD) value obtained from two separate pools of sera per group tested in duplicate (three females and three males in each pool).
Altered cytokine response of IL-10-deficient mice to H. hepaticus.
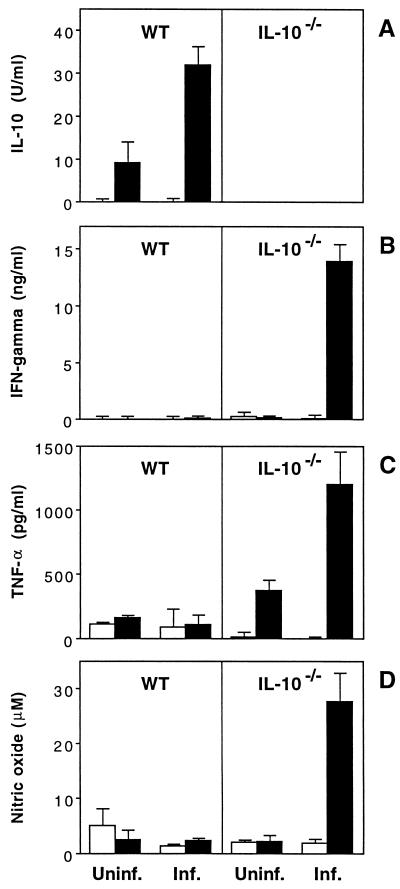
To characterize cytokine responses elicited in WT and IL-10−/− mice following H. hepaticus infection, an initial experiment was performed in which splenocytes were stimulated with SHelAg and 72-h supernatants were assayed for various lymphokines. Splenocytes from infected WT animals secreted substantial amounts of IL-10, but no significant IFN-γ, TNF-α, or NO, when stimulated with Ag (Fig. 5). In contrast, infected IL-10−/− mice mounted a strong type 1 response to SHelAg, as evidenced by spleen cell production of IFN-γ, TNF-α, and NO (Fig. 5B to D).
FIG. 5.
SHelAg-induced cytokine responses of spleen cells from H. hepaticus-infected WT and IL-10−/− mice. Spleen cells (5 × 106/ml) from uninfected (Uninf.) and 9-week-infected (Inf.) WT and IL-10−/− mice were stimulated with medium alone (□) or 1 μg of SHelAg per ml (■), and IL-10 (A), IFN-γ (B), TNF-α (C), and NO (D) were measured in 72-h supernatants. Bars represent means ± SD of values obtained from two separate pools of mice per group tested in duplicate (three females and three males in each pool). Data for one of seven experiments (using 4-, 6-, 9-, 10-, or 11-week-infected mice) with similar results are shown.
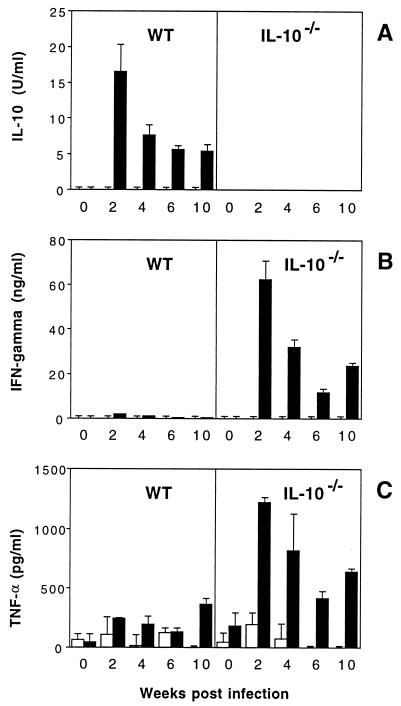
To better assess the local cytokine milieu at different time points following infection, we next examined lymphokine responses from MLN draining the intestinal tract of infected mice. As observed with the spleen cell cultures, MLN cells from infected WT mice secreted IL-10, whereas infected IL-10−/− mice produced IFN-γ and TNF-α in response to SHelAg (Fig. 6). In general, the cytokine secretion by MLN cells appeared to peak early during the infection, with highest lymphokine levels observed at the 2-week time point. In contrast to spleen cells, NO production by MLN cells was detected only at the 2-week time point and only at low levels (data not shown). Minimal or no SHelAg-induced IL-4 and IL-5 responses were detected in spleen and MLN cell cultures from either WT or IL-10−/− mice (data not shown).
FIG. 6.
SHelAg-induced cytokine responses of MLN cells from H. hepaticus-infected WT and IL-10−/− mice. MLN cells (3 × 106/ml) from uninfected and 2-, 4-, 6-, and 10-week-infected WT and IL-10−/− mice were stimulated with medium alone (□) or 1 μg of SHelAg per ml (■), and IL-10 (A), IFN-γ (B), and TNF-α (C) were measured in 72-h supernatants. Bars represent means ± SD of duplicate ELISA values from a pool of two to three infected WT and three to four infected IL-10−/− mice per time point. Cytokine levels for the 0-week postinfection time point have been calculated after pooling data from three separate determinations for uninfected mice assayed in parallel with the infected mice at 4, 6, and 10 weeks postinfection.
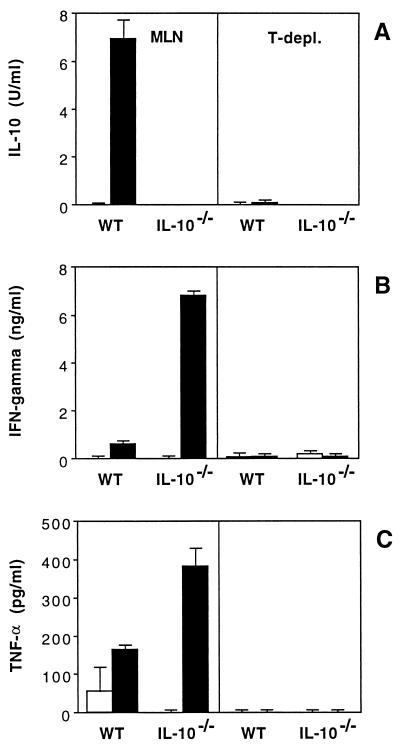
To analyze the cellular origin of the cytokines produced in response to SHelAg, MLN populations were depleted of T cells by treatment with anti-Thy1.2 MAb and complement. IL-10 production by MLN cells from infected WT as well as IFN-γ and TNF-α secretion by cells from infected IL-10−/− mice were totally abrogated by T-cell depletion, demonstrating a requirement for T cells in the production of these cytokines (Fig. 7).
FIG. 7.
T-cell depletion (T-depl.) of MLN populations from H. hepaticus-infected mice abolishes SHelAg-induced cytokine secretion. Total (left) and anti-Thy1.2-plus-complement-treated (right) MLN cells from 4-week-infected WT and IL-10−/− mice were cultured with medium alone (□) or 0.3 μg of SHelAg per ml (■), and IL-10 (A), IFN-γ (B), and TNF-α (C) were measured in 72-h supernatants. Bars represent means ± SD of duplicate ELISA values from a pool of three mice per group.
Anti-IFN-γ or anti-IL-12 treatment inhibits H. hepaticus-induced IBD in IL-10-deficient mice.
To examine the role of IFN-γ and IL-12 in the development of large bowel lesions following H. hepaticus infection of SPF-reared IL-10−/− mice, animals were treated with neutralizing MAb to either cytokine or with a control MAb every 3 to 4 days starting 1 day prior to bacterial inoculation. After 4 weeks, markedly reduced gross and microscopic lesions were evident in the ceca and colons of mice treated with anti-IFN-γ or anti-IL-12 compared to animals receiving control MAb (Fig. 8). Thus, while the latter animals showed inflammatory lesions and hyperplasia indistinguishable from that observed in mice receiving no MAb, anti-cytokine MAb-treated groups displayed reduced large bowel lesions with fewer inflammatory cells in the lamina propria and diminished hyperplasia (Fig. 8D to F). When SHelAg-induced cytokine responses were analyzed, MLN from anti-IL-12-treated mice secreted reduced amounts of IFN-γ compared to animals receiving control MAb (1.9 ± 0.9 ng/ml versus 7.3 ± 1.3 ng/ml). In contrast, levels of SHelAg-induced IFN-γ in MLN cultures from mice treated with anti-IFN-γ were comparable to or up to sixfold higher than those of control MAb-treated mice.
FIG. 8.
Treatment with anti-IFN-γ or anti-IL-12 inhibits development of H. hepaticus-induced colitis in IL-10−/− mice. IL-10−/− mice were treated every 3 to 4 days with 1 mg of neutralizing MAb to IFN-γ or IL-12 or with a control MAb starting 1 day prior to H. hepaticus inoculation, and tissues were examined 4 weeks later. Shown are gross pathology (A to C) and histology (D to F) of ceca from 4-week-infected IL-10−/− mice treated with control (A and D), anti-IFN-γ (B and E), or anti-IL-12 (C and F) MAb. Note the small, pale cecum from the mouse treated with control MAb (A) and much less inflammation and epithelial hyperplasia in mice receiving anticytokine MAb (E to F). (D to F) Formalin-fixed tissues stained with H&E; magnification, ×75.
DISCUSSION
A number of animal models of spontaneous intestinal inflammation have now been described, and it is striking that nearly all of these involve mice with dysregulated immunological functions (6, 15, 19, 28, 29, 33). A second major feature of the disease observed in many of these models is that mice maintained in SPF or germfree conditions develop only minimal inflammatory changes (19, 29). The role of both pathogenic components is clearly evidenced in the enterocolitis developed by IL-10-deficient mice in that they lack a critical immunoregulatory cytokine and spontaneously develop a generalized enterocolitis starting around 3 weeks of age, a disease which is delayed in onset and less severe when the animals are reared in an SPF environment (2, 14).
In the present study, we have formally demonstrated that experimental infection of SPF-reared IL-10-deficient mice with a single pathogen can partially reconstitute the pathological response in the intestinal tract. The organism used was the gram-negative bacterium H. hepaticus (9, 35, 37), a common gut pathogen in mouse colonies (32). The choice of this microbe was based on previous evidence suggesting its involvement in the development of intestinal inflammation in certain naturally infected immunodeficient animals (36). In addition, H. hepaticus has been shown to induce enterocolitis when administered to germfree outbred Swiss Webster mice as well as to scid mice reconstituted with defined CD4+ effector T-cell populations (3, 10). However, it is important to note that in most immunocompetent mouse strains, including those with the C57BL background studied here, H. hepaticus fails to induce significant disease (35–37).
IL-10−/− mice raised under conventional conditions suffer from chronic enterocolitis affecting the duodenum, adjoining jejunum, and colon (14). In contrast, SPF-reared IL-10−/− mice develop only a local inflammation limited to the proximal colon (14). However, with time, the disease becomes more severe also in the SPF IL-10−/− mice, affecting the cecum, colon, and rectum as well as the duodenum (2). Importantly, the genetic background appears to affect the development of enterocolitis in these SPF-reared mice. Thus, IL-10-deficient animals on a C57BL/6 background display minimal disease compared to 129/SvEv or BALB/c IL-10−/− mice (2). The above observations suggest that the severe spontaneous enterocolitis originally described for conventionally reared, partially backcrossed 129/Ola IL-10−/− mice may have been due to their mixed genetic background as well as the intestinal flora to which the mice were exposed. For the experiments performed here, we used SPF-reared IL-10-deficient mice backcrossed to C57BL/10 for 7 to 10 generations, and, consistent with the findings of Berg et al. (2), found minimal lesions (in the form of minor lymphocytic infiltration) during the 5- to 6-month period of examination. As all of the animals were negative for H. hepaticus as well as other Helicobacter species upon cecal culture, it is unlikely that this background pathology resulted from contaminating infections with such bacteria.
In striking contrast, SPF-reared IL-10−/− mice experimentally infected with H. hepaticus developed severe large bowel lesions as early as 2 weeks after bacterial inoculation. These lesions occurred primarily in the cecum, the ascending colon, and to some extent the rectum, increasing in severity with time. Nevertheless, the pathology induced by H. hepaticus in IL-10−/− mice clearly differs from that originally described for conventional, partially backcrossed IL-10-deficient animals in that the small intestine appears to be largely unaffected and large bowel lesions are more severe in the natural disease. The reason for this difference is not clear. One possibility is that other microbial components in the intestinal flora of the conventionally reared animals triggered the small intestinal lesions observed, and H. hepaticus is not pathogenic in that site. Alternatively, as alluded to above, the contaminating 129/Ola genes present in the originally characterized IL-10−/− mice may have predisposed these animals to inflammation of the small intestine.
One possible explanation for the increased severity of disease in H. hepaticus-infected IL-10−/− mice is that these animals are more susceptible to infection with the bacteria and/or fail to control bacterial growth. This interpretation of the data seems unlikely based on several considerations. First, bacteria were observed in crypts and cecal contents of both WT and IL-10−/− mice at all time points analyzed (up to 4 months postinfection). Second, comparable levels of H. hepaticus-reactive Ab were found in sera of WT and IL-10-deficient animals at 4 months postinfection, arguing for similar levels of infection. Finally, previously published studies have failed to reveal a direct correlation between bacterial load and disease severity in murine H. felis infections (17, 18). Although accurate quantitation of H. hepaticus in the intestine is difficult (the bacteria tend to spread out and do not form colonies easily), we are currently in the process of estimating bacterial loads in cecal contents from infected WT and IL-10−/− mice.
The enhanced intestinal disease observed in H. hepaticus-infected IL-10-deficient mice was found to correlate with augmented T-cell proliferative and altered cytokine responses to a soluble extract of the bacteria (SHelAg), whereas Ab responses to the same preparation were unaltered. In the case of lymphocyte proliferation, the increase in response was evident in MLN but not spleen cell populations and was observed only in mice infected for longer than 3 weeks. More pronounced differences between the two strains of mice were observed for cytokine responses. Thus, H. hepaticus-infected IL-10−/− mice mounted a strong type 1 response as measured by IFN-γ, TNF-α, and NO production after in vitro stimulation of spleen and MLN cells with SHelAg, whereas IL-10 was the dominant cytokine secreted by cells from infected WT mice. Considering the high levels of H. hepaticus-reactive Ab of all isotypes in both WT and IL-10−/− mice, it was somewhat surprising to find minimal or no detectable IL-4 and IL-5 responses to SHelAg from these mice. However, our results are in agreement with the report by Mohammadi et al., who found that neither spleen cells nor gastric lamina propria lymphocytes from H. felis-infected mice produced IL-4 or IL-5 in response to bacterial Ag (17).
The cytokine pattern observed in the IL-10−/− mice undergoing H. hepaticus-induced colitis is very similar to that reported to be associated with the late-arising spontaneous enterocolitis occurring in SPF-reared, partially backcrossed IL-10-deficient mice (2), the intestinal inflammation induced by transferred CD45RBhigh CD4+ T cells into scid recipients (26), and the gastric inflammation induced by H. felis infection in C57BL/6 mice (17), suggesting that a common type 1 cytokine-dependent disease mechanism may be involved. Previous studies on this mechanism in the above as well as other models involving chemically induced colitis (21) have suggested that it is dependent on both IL-12 and IFN-γ (2, 24, 26, 27). Consistent with the latter observations, IL-10−/− animals treated with anti-IL-12 or anti-IFN-γ MAb during the course of H. hepaticus infection displayed reduced intestinal inflammation (Fig. 8). As SHelAg-induced IFN-γ secretion measured in vitro was reduced in anti-IL-12-treated mice but unaltered or elevated in anti-IFN-γ-treated mice, the mechanisms by which these two reagents diminished disease appear to be distinct. While anti-IL-12 treatment is likely to function by inhibiting the generation of Th1-type cells, neutralization of IFN-γ probably acts directly on the effector arm of the immune response, which triggers tissue inflammation. An important issue which we are currently examining is whether treatment with these anti-cytokine MAbs will reduce previously established H. hepaticus-induced intestinal inflammation. In the case of the late spontaneous enterocolitis developed by partially backcrossed SPF-reared IL-10−/− mice, anti-IFN-γ treatment fails to affect established disease (27), whereas in the chronic inflammation triggered by contact sensitization with the hapten trinitrobenzene sulfonic acid, administration of anti-IL-12 MAb has been shown to abrogate previously induced colitis (21).
Studies using several IBD animal models have demonstrated an important role for Th1-like CD4+ cells in mediating disease (7, 25, 27). In the case of the IL-10−/− mouse model, transfer of CD4+ CD8α− or CD4+ CD8α+ colonic T cells from IL-10-deficient mice developing spontaneous enterocolitis, but not WT mice, results in the induction of intestinal inflammatory disease in RAG-2−/− recipients (7). In the experiments reported here, SHelAg-induced production by MLN cells of IFN-γ and TNF-α, the two cytokines associated with disease pathogenesis, was shown to be both T-cell dependent (Fig. 7) and inhibitable by anti-CD4 MAb (not shown), suggesting that this lymphocyte subset will also be critical for the generation of H. hepaticus-induced colitis. A key issue which we hope to address in future experiments is whether the T cells which mediate inflammatory disease in the H. hepaticus-infected IL-10−/− mice are directed against specific bacterial components or host self Ag. This general question in IBD pathogenesis can be directly addressed in our model as a defined microbial agent is used for eliciting disease.
An important finding in the present study is the observation that H. hepaticus infection induces an IL-10-dominated cytokine response in WT animals not developing disease. Previous studies have indicated that repeated administration of rIL-10 into SPF-reared, partially backcrossed IL-10-deficient mice can transiently cure the spontaneous enterocolitis developing in these mice (27). Therefore it is probable that the bacterium-induced IL-10 in H. hepaticus-infected WT mice plays a critical role in protecting the animals against disease. The results of the in vitro stimulation experiments performed here (Fig. 7) suggest that T cells are the major source of the cytokine following bacterial Ag stimulation. As H. hepaticus did not induce a typical Th1 or Th2 response in the infected WT animals, it is tempting to speculate that the T cells involved may belong to the recently described IL-10- and transforming growth factor β (TGF-β)-producing regulatory CD4+ subset shown to protect scid mice against the intestinal inflammation induced by transferred CD45RBhigh lymphocytes (13). In this regard, it is interesting that the mechanism by which CD45RBlow CD4+ T cells transfer protection to IBD in scid mice cotransferred with CD45RBhigh cells is dependent on TGF-β (23). Thus, it is possible that the key cytokine which protects WT mice against H. hepaticus-induced damage is not IL-10 itself but rather TGF-β produced by an IL-10-dependent regulatory T-cell subset. In future experiments, we plan to define the IL-10-producing cell population in H. hepaticus-infected WT mice, study its mode of action, and identify the bacterial components which serve as its stimulus. The information gained from such studies may lead to strategies for treatment of IBD based on the induction of IL-10-producing regulatory T cells directed against specific Ag shared by those agents in gut flora associated with disease induction.
ACKNOWLEDGMENTS
We are grateful for the help of Dee Green, Gayle Krietz, Roberta Smith, and Barbara Kasprzak with mouse necropsy and Jane Battles and Kris Pike for PCR analysis. We also thank Warren Strober, Ivan Fuss, Brian Kelsall, and Tim Mosmann for their thoughtful advice and criticism during the course of this project. Lastly, we thank Warren Strober, Brian Kelsall, George Yap, and Marco Schito for critical reading of the manuscript.
This work was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000.
REFERENCES
- 1.Battles J K, Williamson J C, Pike K M, Gorelick P L, Ward J M, Gonda M A. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol. 1995;33:1344–1347. doi: 10.1128/jcm.33.5.1344-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg D J, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach M W, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Investig. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X, Shores E W, Hu-Li J, Anver M R, Kelsall B L, Russell S M, Drago J, Noguchi M, Grinberg A, Bloom E T, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 5.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong Y, Brandwein S L, McCabe R P, Lazenby A, Birkenmeier E H, Sundberg J P, Elson C O. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson N J, Leach M W, Fort M M, Thompson-Snipes L, Kühn R, Müller W, Berg D J, Rennick D M. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrhardt R O, Ludviksson B R, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol. 1997;158:566–573. [PubMed] [Google Scholar]
- 9.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garvey W, Fathi A, Bigelow F. Modified Steiner for the demonstration of spirochetes. J Histotechnol. 1985;8:15–17. [Google Scholar]
- 12.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 13.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 14.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni A B, Huh C G, Becker D, Geiser A, Lyght M, Flanders K C, Roberts A B, Sporn M B, Ward J M, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann G M, Tonegawa S, Nagler-Anderson C, Bhan A K. Cytokine imbalance and autoantibody production in T cell receptor-α mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 18.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P, Mizoguchi E, Grusby M J, Glimcher L H, Bhan A K, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T R, Schumacher J H, Fiorentino D F, Leverah J, Moore K W, Bond M W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–2945. [PubMed] [Google Scholar]
- 20a.National Institutes of Health. Guide for the care and use of laboratory animals. Bethesda, Md: National Institute of Allergy and Infectious Diseases, National Institutes of Health; 1996. [Google Scholar]
- 21.Neurath M F, Fuss I, Kelsall B L, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 23.Powrie F, Carlino J, Leach M W, Mauze S, Coffman R L. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powrie F, Correa-Oliveira R, Mauze S, Coffman R L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powrie F, Leach M W, Mauze S, Caddle L B, Coffman R L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 26.Powrie F, Leach M W, Mauze S, Menon S, Caddle L B, Coffman R L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 27.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph U, Finegold M J, Rich S S, Harriman G R, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 29.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 30.Sartor R B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 31.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shames B, Fox J G, Dewhirst F, Yan L, Shen Z, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Sher, A., S. Hieny, and J. Ward. Unpublished data.
- 33.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strober W, Ehrhardt R O. Chronic intestinal inflammation: an unexpected outcome in cytokine or T cell receptor mutant mice. Cell. 1993;75:203–205. doi: 10.1016/0092-8674(93)80062-j. [DOI] [PubMed] [Google Scholar]
- 34a.Ward, J. M., and P. Gorelick. Unpublished data.
- 35.Ward J M, Anver M R, Haines D C, Benveniste R E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 36.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 37.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 38.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]