Abstract
Mixed parasitic infections are common in many parts of the world. However, little is known about how concurrent infections affect the immunity to and/or pathogenesis of each other. Protection and elimination of blood-stage Plasmodium chabaudi chabaudi AS in resistant mice are characterized by a sequential activation of CD4+ Th1 and Th2 cells. The patent egg-laying stage of the murine model of Schistosoma mansoni is associated with a strong Th2 response to both Schistosoma and unrelated antigens. In this study, we investigated how infection of mice with S. mansoni would affect the immune response to and pathogenesis of a P. chabaudi infection. C57BL/6 mice infected with S. mansoni for 8 weeks were infected with blood-stage P. chabaudi. Malaria parasitemias were significantly higher in these mice than in mice infected with P. chabaudi only. In doubly infected mice, both spleen cell proliferative and Th2 responses to S. mansoni soluble egg antigen (SEA) or anti-CD3 were suppressed up to 1 month after the malaria infection. Findings for SEA-specific immunoglobulin M (IgM) and IgG serum antibody levels were similar. No significant effects were seen on P. chabaudi-induced gamma interferon responses. However, tumor necrosis factor alpha (TNF-α) production was significantly lower in double-infected mice. Thus, a defect in TNF-α production might contribute to the increased malaria parasitemias seen in S. mansoni-P. chabaudi-infected mice. Taken together, our data show that schistosoma and malaria infections profoundly affect each other, findings which might have implications for the development of vaccines.
Polyparasitism is common in human populations living in malaria-endemic areas. However, little is known about how concurrent infections affect the immune responses against malaria and in general. Such knowledge is of importance for the rational design and optimization of vaccination protocols and treatment programs. The immune response to the intraerythrocytic stages of malarial parasites has been best characterized in the rodent model of Plasmodium chabaudi chabaudi. In recent years, several investigators have provided evidence that cell-mediated immunity and humoral immunity act in concert or sequentially to control and clear a blood-stage malaria infection (24, 38, 40). During the early phase of infection, both reactive nitrogen and reactive oxygen metabolites produced by nonspecific immune cells such as neutrophils and macrophages are believed to participate in controlling the primary parasitemia (32, 37). This initial CD4+ Th1 cell response is followed by a switch to Th2 cytokine production, stimulating antibody-dependent mechanisms involved in the final control and clearance of the parasite (24, 38, 40).
Experimental Schistosoma mansoni infections are known to induce a strong Th2 type of response, as evidenced by the occurrence of high immunoglobulin E (IgE) and eosinophil levels. At the time point of egg production, approximately 5 weeks after infection, a Th2 response is seen in the host, with increased production of Th2 cytokines (interleukin-4 [IL-4], IL-5, and IL-10) and a concomitant downregulation in the secretion of Th1 cytokines (IL-2 and gamma interferon [IFN-γ]) (15, 31, 34). Importantly, infected mice immunized with nonschistosomal antigens also exhibit downregulated Th1 cytokines and increased IL-4 secretion, indicating that the shift toward Th2 also extends to foreign antigens (2, 23). The shift toward Th2 is believed to downmodulate the inflammatory response, induced by egg deposition, mainly in the liver, causing granuloma formation and tissue damage (13, 33).
This study was performed to examine if the S. mansoni-induced Th2 type of response would prevent or inhibit the Th1 response required for the clearance of P. chabaudi, thus giving rise to a more severe disease and/or mortality, or alternatively whether the P. chabaudi-induced Th1 response would downregulate the response to S. mansoni.
Our data demonstrate that mice with a patent S. mansoni infection developed higher malaria parasitemias following infection with blood-stage P. chabaudi AS. In vitro stimulation experiments using spleen cells showed that tumor necrosis factor alpha (TNF-α) production from mice with concurrent S. mansoni-P. chabaudi infection is impaired during the first week of the malaria infection. In contrast, the P. chabaudi-induced Th1 type of response, as measured by IFN-γ production, was not affected by the S. mansoni infection. Thus, it is likely that the suppressed TNF-α production is a defect at the macrophage level. In addition, our data show that 6 days after P. chabaudi infection, both the proliferative and Th2-type cytokine responses to S. mansoni soluble egg antigen (SEA) as well as to anti-CD3 stimulation were suppressed and that the suppression was sustained up to 1 month after P. chabaudi infection. Similarly, the levels of anti-SEA antibodies were also significantly reduced during the course of the malaria infection.
MATERIALS AND METHODS
Animals and experimental infections.
Female, 6- to 10-week-old C57BL/6 mice were purchased from B&K Universal (Sollentuna, Sweden). The animals were maintained in the animal facility at Stockholm University and supplied with food and water ad libitum. S. mansoni (Puerto Rican strain) cercariae were obtained from the Laboratory of Parasitology, Smittskyddsinstitutet (Stockholm, Sweden).
Blood-stage infection with P. chabaudi AS (kindly provided by D. Walliker, Edinburgh, United Kingdom) was maintained by weekly passages in naive mice. Experimental groups of mice were infected with 50 S. mansoni cercariae by percutaneous infection of abdominal skin (group A), 1 million P. chabaudi-infected erythrocytes intraperitoneally (group B), or S. mansoni plus P. chabaudi (group C). The S. mansoni infection was initiated 8 weeks before the P. chabaudi infection. Malaria parasitemia was monitored daily by examination of Giemsa (BDH, Poole, United Kingdom)-stained thin blood smears made from tail snips. Erythrocyte (RBC) counts were performed with a hematocytometer. Groups of mice were bled and killed at days 3, 6, 9, 12, 15, 22, and 30 after P. chabaudi infection for further examination.
Cell preparations and culture conditions.
Single-cell suspensions from individual spleens were prepared at days 3, 6, 9, 12, 15, 22, and 30 after P. chabaudi infection from P. chabaudi- or P. chabaudi-S. mansoni-infected mice. Spleens from controls infected with S. mansoni only were also prepared at each time point.
Splenic RBC were lysed in an ammonium chloride-potassium carbonate buffer, and splenocytes were washed three times and resuspended in culture medium. In all cases, tissue culture medium was RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, HEPES (20 mM), l-glutamine (2 mM), 2-mercaptoethanol (0.05 mM; Life Technologies, Paisley, United Kingdom), and gentamicin (25 μg/ml; Sigma, St. Louis, Mo.). Spleen cells were cultured at 37°C and 5% CO2 in flat-bottomed 96-well microtiter plates (Costar, Cambridge, Mass.) at 5 × 106/ml in a final volume of 0.2 ml/well for cytokine measurement and at 2.5 × 106/ml (in 0.2 ml/well) for measurement of proliferation. Cells were stimulated with plate-bound anti-CD3 monoclonal antibody (MAb) 145-2C11 (50 μl/well of 10 μg/ml in phosphate-buffered saline; American Tissue Culture Collection [ATCC]), live P. chabaudi-parasitized RBC (pRBC) or normal RBC (nRBC) at a concentration of 106/ml, or S. mansoni SEA (22) at a final concentration of 10 μg/ml. All cultures were performed in triplicate. Proliferation was measured by pulsing 72-h cultures with [3H]thymidine (1 μCi/well; Amersham, Little Chalfont, United Kingdom) during the last 12 h of culture. The cells were then harvested onto glass fiber filter papers, and [3H]thymidine incorporation was determined by liquid scintillation counting. Supernatants (SN) were collected after 72 h for measurement of IFN-γ, IL-4, IL-5, or TNF-α.
T-cell depletion experiments were carried out at days 6, 9, 12, 15, 22, and 30, by complement-mediated lysis using a cocktail of anti-Thy.1.2 (Cedarlane, Hornby, Ontario, Canada), anti-CD4 (MAb RL 172.4; ATCC), and anti-CD8 (MAb 3.155; ATCC) antibodies followed by complement treatment (Low-tox M baby rabbit complement; Cedarlane) and washings to remove cell debris. The efficiency of the depletion was 90 to 99%, as determined by flow cytometric analysis with phycoerythrin-labelled anti-CD3 (Pharmingen, San Diego, Calif.) antibody (data not shown).
Cytokine assays.
IL-4 was measured in culture SN using the IL-4-sensitive cell line CT.4S (18), using 5,000 CT.4S cells per well. Proliferation was measured after 48 h by [3H]thymidine incorporation for 12 h, and the amount of IL-4 was quantitated by comparison to proliferation induced by known amounts of IL-4 obtained from an IL-4-transfected cell line. Calibration of the IL-4 standard has been described elsewhere (22). The sensitivity cutoff of the assay was between 4 and 8 U of IL-4 per ml in all experiments. Addition of anti-IL-4 MAb 11B11 (ATCC) to SN completely abrogated the proliferation of the CT.4S cells, demonstrating that the response was due to IL-4 (not shown).
IFN-γ was measured by a two-site enzyme-linked immunosorbent assay (ELISA) using MAb HB170 (ATCC) as catcher antibody and a polyclonal rabbit anti-mouse IFN-γ antibody (kindly provided by Alan Sher, National Institutes of Health) followed by a peroxidase-conjugated donkey anti-rabbit IgG (Jackson Immune Research Laboratories, West Grove, Pa.). The amount of cytokine was quantitated by comparison with standard curves of known amounts of recombinant IFN-γ (rIFN-γ; Genzyme, Cambridge, Mass.). The sensitivity cutoff for the ELISA was 0.05 ng/ml. TNF-α and IL-5 were measured by two-site ELISAs. TNF-α was analyzed with a Genzyme Duo-Set, and IL-5 was analyzed by using MAb TRFK-5 together with biotinylated TRFK-4 (ImmunoKontakt, Bioggio, Switzerland). rTNF-α and rIL-5 (Genzyme) were used as standards. The sensitivity cutoffs were 0.1 ng/ml for the IL-5 assay and 30 pg/ml for the TNF-α assay.
Parasite-specific IgM, IgG1, and IgG2a ELISAs.
ELISA plates (Costar) were coated with S. mansoni SEA (5 μg/ml) or P. chabaudi freeze-thawed crude lysate (16) in carbonate buffer (pH 9.6). The plates were coated overnight at 4°C, and the wells were blocked with 10% fetal calf serum in phosphate-buffered saline for 3 h at 37°C. Each serum was assayed in a series of dilutions from 1:100 to 1:1,000,000 and incubated on the plates in duplicate overnight at 4°C. Antibody binding was detected by using alkaline phosphatase-conjugated anti-murine IgM (μ specific; Sigma) or IgG1 or IgG2a (γ1 or γ2a specific; Southern Biotech, Birmingham, Ala.). The optical density (OD) values were plotted for each individual serum and found to be on a linear slope for all sera in each assay when dilutions of 1:1,000 for SEA-specific IgG1 or IgG2a, 1:10,000 for SEA-specific IgM, and 1:1,000 for P. chabaudi-specific IgG1, IgG2a, or IgM antibodies were used. No cross-reactivity between SEA and P. chabaudi antigen was observed when sera from S. mansoni-only-infected mice were analyzed with P. chabaudi antigen, and vice versa (data not shown).
Statistical analysis.
Student’s t test was used for assessing statistical significance. A probability of less than 0.05 was considered significant in comparisons of results between P. chabaudi-infected and P. chabaudi-S. mansoni-infected mice or between S. mansoni-infected and P. chabaudi-S. mansoni-infected mice.
RESULTS
P. chabaudi-S. mansoni-coinfected mice develop higher P. chabaudi parasitemia and exhibit lower RBC counts during malaria infection.
Mice with and without an 8-week S. mansoni infection were infected with 106 blood-stage P. chabaudi parasites, and the malaria parasitemia was monitored on Giemsa-stained thin blood smears. Mice with a concurrent S. mansoni infection developed a significantly higher malaria parasitemia than mice infected with P. chabaudi alone (Fig. 1A). Parasitemia developed faster in the coinfected mice, being 12.5% ± 0.5% (mean ± standard error of the mean [SEM]) on day 5, compared to 3.09% ± 0.3% in the P. chabaudi-only-infected group (P = 0.0001). The levels of parasitemia in double-infected and malaria-only-infected groups at day 6 were 42.7% ± 2.5% and 23.0% ± 1.6%, respectively (P = 0.0001). The parasitemia peaked at day 7 for both groups but was significantly higher in the coinfected mice (48.0% ± 3.2% versus 42.1% ± 1.1%; P = 0.04). Thereafter, the malaria parasitemia slowly declined, remaining higher in the double-infected group until becoming undetectable on day 12 after P. chabaudi infection for both groups (P = 0.01 for days 8, 9, and 10). Some of the mice with concurrent S. mansoni-P. chabaudi infection died during the course of the malaria infection. The deaths occurred at peak parasitemia or shortly thereafter. The frequencies of deaths were 13.3% (2 of 15), 10% (2 of 20), and 7.5% (3 of 40) in three separate experiments, while none of the P. chabaudi-only-infected mice died. The double-infected mice showed significantly lower RBC counts during and after peak parasitemia than the group with malaria only (Fig. 1B; P < 0.05 for days 5, 8, and 10). There was no significant difference in RBC counts between mice with and without S. mansoni infection at the initiation of P. chabaudi infection (Fig. 1B, day 0).
FIG. 1.
Malaria parasitemia (A) and RBC counts (B) in peripheral blood of mice infected with P. chabaudi AS alone (•) or concomitantly with S. mansoni (○). Each value represents the mean ± SEM for 20 to 40 mice. ∗, statistically significant difference (P < 0.05) between mice with P. chabaudi infection only and mice with concurrent S. mansoni-P. chabaudi infection. Solid lines represent mean RBC counts from S. mansoni-only-infected control animals ± SEM (−−−).
P. chabaudi infection suppresses anti-SEA antibody responses in S. mansoni-infected mice.
To analyze the effect of malaria challenge on antibody production in the S. mansoni-P. chabaudi-infected mice, sera were collected from mice in the experimental groups at various time points and analyzed individually in ELISA against SEA or P. chabaudi antigen.
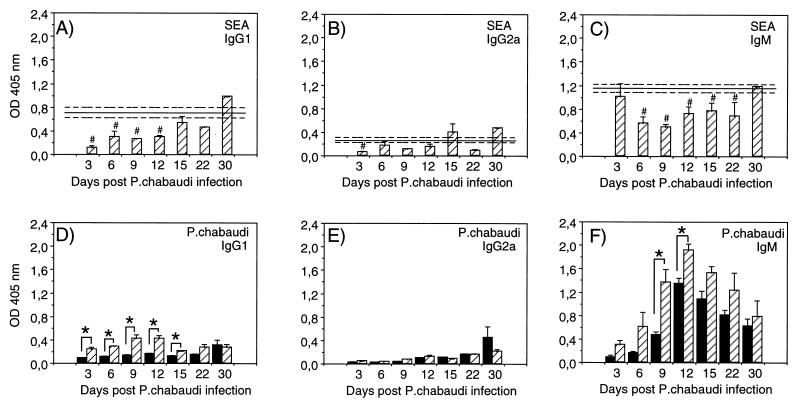
As can be seen in Fig. 2, both IgG and IgM antibody responses to SEA were lower in mice with concurrent S. mansoni-P. chabaudi infection than in mice infected with S. mansoni only. The IgG1 levels were significantly reduced at day 3 after P. chabaudi infection and remained lower than in the S. mansoni-only-infected group up to 15 days after malaria infection (Fig. 2A; P < 0.05 for days 3 through 12). The IgG2a levels, normally low during S. mansoni infections, were reduced or unaffected (Fig. 2B). The IgM levels were normal at day 3 after malaria infection but then declined and remained significantly lower until day 30 postinfection (Fig. 2C; P < 0.05 for days 6 through 22).
FIG. 2.
SEA- and P. chabaudi-specific antibodies in serum samples from mice with P. chabaudi infection only (■) or with concurrent S. mansoni-P. chabaudi infection ( ). Anti-SEA (A to C) and anti-P. chabaudi (D to F) antibodies were measured by ELISA. IgG1 (A and D), IgG2a (B and E), and IgM (C and F) levels were determined and are presented as absorbance (OD) at 405 nm. Results shown were obtained from serum dilutions of 1:1,000 for SEA-specific IgG1 and IgG2a, 1:10,000 for SEA-specific IgM, and 1:1,000 for P. chabaudi-specific IgG1, IgG2a, and IgM antibodies. Bars represent mean OD ± SEM for three to five mice, and solid lines represent pooled data from control animals infected with S. mansoni only assayed in parallel at each time point ± SEM (−−−). #, statistically significant differences (P < 0.05) between mice with S. mansoni infection only and mice with concurrent S. mansoni-P. chabaudi infection; ∗, statistically significant differences (P < 0.05) between mice with P. chabaudi infection only and mice with concurrent S. mansoni-P. chabaudi infection.
The antibody response to P. chabaudi is mainly of the IgM type during the early phase of the infection. As can be seen in Fig. 2F, the S. mansoni-P. chabaudi-infected mice generally showed higher levels of P. chabaudi-specific IgM antibodies throughout the experiment than the mice infected with P. chabaudi only (P = 0.02 on days 9 and 12). Also, the levels of IgG1 were significantly higher in the mice with concurrent infections during the first weeks of the malaria infection (Fig. 2D; P < 0.01 for days 3 through 15), while no obvious difference was observed for malaria-specific IgG2a (Fig. 2E). Sera from mice with S. mansoni infection only did not react with P. chabaudi antigen (data not shown).
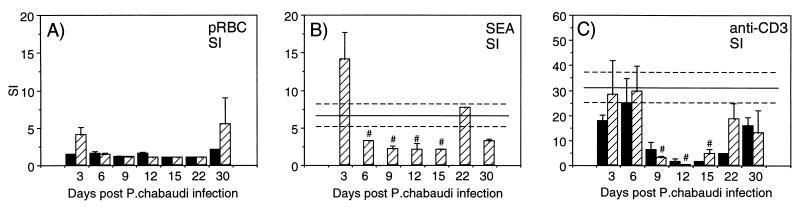
S. mansoni-infected mice show suppressed SEA- and anti-CD3-induced proliferation after challenge with P. chabaudi.
Spleen cell cultures from individual mice were analyzed for proliferative capacity at various time points during malaria infection. The cultures were stimulated with pRBC, nRBC, SEA, or plate-bound anti-CD3. The background proliferation in medium alone was between 800 and 2,000 cpm for all cultures. The data presented in Fig. 3A show that the proliferative response to pRBC was generally low or nonexistent during the first 30 days of P. chabaudi infection and that there was no significant difference between the S. mansoni-P. chabaudi-infected and P. chabaudi-only-infected mice. The response to SEA by cells from double-infected mice (Fig. 3B) was slightly elevated on day 3 after malaria infection but then significantly lower during the course of malaria infection than in mice infected with S. mansoni only (P < 0.05 for days 6 through 15). Spleen cells from mice with only P. chabaudi infection did not respond to SEA stimulation, nor did they respond to nRBC (data not shown). When anti-CD3 antibodies were used as a measure of total T-cell proliferation, a clear malaria-induced immunosuppression was seen (Fig. 3C). Both mice with concurrent infection as well as mice infected only with P. chabaudi showed reduced proliferative responses during days 9, 12, and 15. Restoration of proliferative capacity was detected at days 22 and 30, in concordance with results published by others (3).
FIG. 3.
Stimulation indices (SI) in spleen cell cultures from
mice with P. chabaudi infection only (■) or from
mice with concurrent S. mansoni-P. chabaudi
infection
( ).
Cultures were stimulated with pRBC (A), SEA (B), or anti-CD3 (C). Bars
represent mean SI for three to five mice ± SEM, and solid lines
represent pooled data from S. mansoni-only-infected
control animals assayed in parallel at each time point ± SEM
(−−−). Note the different scales on the y axes. #,
statistically significant differences (P < 0.05)
between mice with S. mansoni infection only and mice
with concurrent S. mansoni-P. chabaudi
infection.
).
Cultures were stimulated with pRBC (A), SEA (B), or anti-CD3 (C). Bars
represent mean SI for three to five mice ± SEM, and solid lines
represent pooled data from S. mansoni-only-infected
control animals assayed in parallel at each time point ± SEM
(−−−). Note the different scales on the y axes. #,
statistically significant differences (P < 0.05)
between mice with S. mansoni infection only and mice
with concurrent S. mansoni-P. chabaudi
infection.
S. mansoni-induced Th2 cytokine production is suppressed after P. chabaudi challenge.
To investigate if the malaria-induced suppressed proliferative responses seen in the S. mansoni-P. chabaudi-infected mice was also reflected in cytokine production, we analyzed the in vitro production of IL-4 and IL-5 in 72-h SN from spleen cell cultures stimulated with pRBC, SEA, or plate-bound anti-CD3. As shown in Fig. 4, both IL-4 production and IL-5 production in response to SEA were significantly reduced in S. mansoni-P. chabaudi-infected mice (P < 0.05 for IL-4 at days 6 and 9; P < 0.05 for IL-5 at day 9) (Fig. 4A and C). In response to anti-CD3, the IL-4 levels were not affected whereas the levels of IL-5 were severely reduced (P < 0.05 at days 6 to 15). No IL-4 or IL-5 was detected in cultures of T-depleted spleen cells, indicating that the cytokine responses were T cell dependent (data not shown). Only very low levels of IL-4 were detected in response to pRBC in both groups of mice, and no IL-5 in response to pRBC could be detected at any time point (data not shown).
FIG. 4.
Th2 cytokine responses in in vitro-stimulated spleen
cell cultures from mice with P. chabaudi infection
only (■) or from mice with concurrent S. mansoni-P.
chabaudi infection
( ). IL-4
(A and B) and IL-5 (C and D) were measured in the SN from 72-h spleen
cell cultures stimulated with SEA (A and C) or anti-CD3 (B and D). Bars
represent mean values from three to five mice ± SEM, and solid
lines represent pooled data from S.
mansoni-only-infected control animals assayed in parallel at each
time point ± SEM (−−−). Note the different scales on the
y axes of panels C and D. #, statistically significant
differences (P < 0.05) between mice with S.
mansoni infection only and mice with concurrent S.
mansoni-P. chabaudi infection.
). IL-4
(A and B) and IL-5 (C and D) were measured in the SN from 72-h spleen
cell cultures stimulated with SEA (A and C) or anti-CD3 (B and D). Bars
represent mean values from three to five mice ± SEM, and solid
lines represent pooled data from S.
mansoni-only-infected control animals assayed in parallel at each
time point ± SEM (−−−). Note the different scales on the
y axes of panels C and D. #, statistically significant
differences (P < 0.05) between mice with S.
mansoni infection only and mice with concurrent S.
mansoni-P. chabaudi infection.
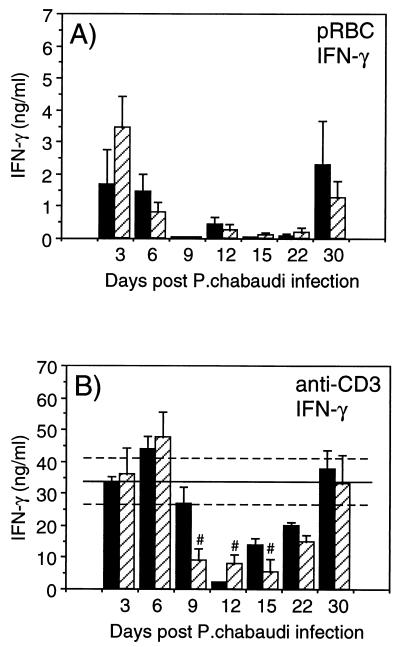
P. chabaudi-induced Th1 cytokine production is not affected in S. mansoni-infected mice.
A P. chabaudi infection is characterized by an early Th1 type of response (IFN-γ production). To investigate if the dominant Th2 response in mice with patent S. mansoni infection would affect the P. chabaudi-induced Th1 response, we analyzed IFN-γ in 72-h SN from spleen cell cultures stimulated with pRBC, nRBC, SEA, or plate-bound anti-CD3. As can be seen in Fig. 5A, during the course of malaria infection, similar levels of IFN-γ in response to pRBC were seen in mice with concomitant infection and in mice with P. chabaudi infection only. The IFN-γ responses were also similar in the two groups when anti-CD3 was used for stimulation. The malaria-induced immunosuppression was evident on days 9, 12, and 15 compared to the S. mansoni-only-infected controls (Fig. 5B). No IFN-γ production in response to nRBC was detected at any time, and the levels of SEA-induced IFN-γ in S. mansoni-infected mice were very low and not affected by a concurrent P. chabaudi infection (data not shown).
FIG. 5.
IFN-γ responses in in vitro-stimulated spleen cell
cultures from mice with P. chabaudi infection only
(■) or from mice with concurrent S. mansoni-P.
chabaudi infection
( ).
IFN-γ was measured in the SN from 72-h spleen cell cultures
stimulated with pRBC (A) or anti-CD3 (B). Bars represent mean levels
for three to five mice ± SEM, and solid lines represent pooled
data from S. mansoni-only-infected control animals
assayed in parallel at each time point ± SEM (−−−). Note the
different scales on the y axes. #, statistically significant
differences (P < 0.05) between mice with S.
mansoni infection only and mice with concurrent S.
mansoni-P. chabaudi infection.
).
IFN-γ was measured in the SN from 72-h spleen cell cultures
stimulated with pRBC (A) or anti-CD3 (B). Bars represent mean levels
for three to five mice ± SEM, and solid lines represent pooled
data from S. mansoni-only-infected control animals
assayed in parallel at each time point ± SEM (−−−). Note the
different scales on the y axes. #, statistically significant
differences (P < 0.05) between mice with S.
mansoni infection only and mice with concurrent S.
mansoni-P. chabaudi infection.
No IFN-γ was detected in the culture SN when T-depleted spleen cells were used at days 6, 9, 12, 15, 22, and 30, indicating that the cytokine was T cell dependent at these time points (data not shown).
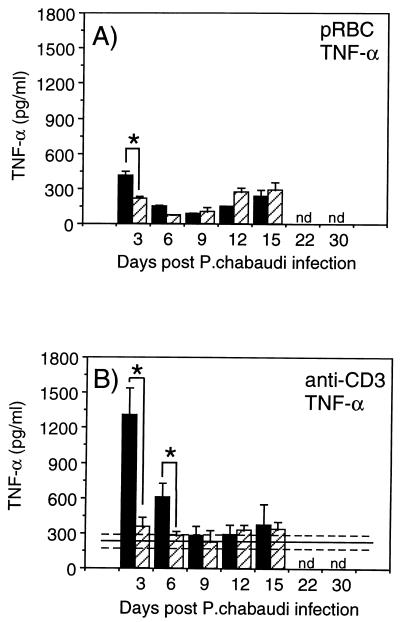
Reduced TNF-α production in mice with concomitant S. mansoni-P. chabaudi infections.
TNF-α, probably produced by malarial antigen-activated macrophages, has been shown to reduce parasitemia and protect mice against lethal malaria infection (5, 21). Therefore, we investigated the levels of TNF-α in spleen cell supernatants after stimulation with pRBC, nRBC, and anti-CD3.
The data revealed significantly lower TNF-α production in response to both pRBC as well as anti-CD3 stimulation in the mice with concomitant S. mansoni-P. chabaudi infection compared to the malaria-only-infected mice at days 3 and 6 after P. chabaudi infection (Fig. 6). At day 3, the response to pRBC was two times higher in the P. chabaudi-only-infected group (414 ± 31 pg/ml versus 213 ± 23 pg/ml; P < 0.05). No TNF-α was detected in response to nRBC at any time (data not shown). The response to anti-CD3 was fourfold higher at day 3 (1,310 ± 450 pg/ml versus 352 ± 81 pg/ml) and twofold higher at day 6 (605 ± 121 pg/ml versus 278 ± 35 pg/ml) in the P. chabaudi-only-infected mice than in the S. mansoni-P. chabaudi-infected group (P < 0.05 for both time points) (Fig. 6). After day 6, levels of TNF-α were again comparable in the two groups (Fig. 6).
FIG. 6.
TNF-α responses in in vitro-stimulated spleen cell
cultures from mice with P. chabaudi infection only
(■) or from mice with concurrent S. mansoni-P.
chabaudi infection
( ).
Cultures were stimulated with pRBC (A) or anti-CD3 (B). Bars represent
mean levels for three to five mice ± SEM, and solid lines
represent pooled data from S. mansoni-only-infected
control animals assayed in parallel at each time point ± SEM
(−−−). ∗, represents statistically significant differences
(P < 0.05) between mice with P.
chabaudi infection only and mice with concurrent S.
mansoni-P. chabaudi infection. nd, not determined.
).
Cultures were stimulated with pRBC (A) or anti-CD3 (B). Bars represent
mean levels for three to five mice ± SEM, and solid lines
represent pooled data from S. mansoni-only-infected
control animals assayed in parallel at each time point ± SEM
(−−−). ∗, represents statistically significant differences
(P < 0.05) between mice with P.
chabaudi infection only and mice with concurrent S.
mansoni-P. chabaudi infection. nd, not determined.
DISCUSSION
The effects of concomitant infections on the development as well as the maintenance of an immune response remain largely unknown. The murine models of P. chabaudi AS and S. mansoni have been well studied and characterized in terms of both cell-mediated immunity and humoral immunity. Thus, the two models should be ideal for studying the interaction between two different parasitic infections with regard to parasite-specific immune responses. In this report, we demonstrate that concurrent infection with two parasites, a Th2-inducing helminth and a Th1-inducing protozoan, severely alters the development of an immune response as well as affecting already established responses.
Recent studies have demonstrated that Trichuris muris-susceptible mice can be made resistant by a concurrent S. mansoni infection (6). Little information regarding concurrent S. mansoni and malaria infections has been published. Some years ago, Lewinsohn (27) demonstrated no effect on the percentages of malaria parasitemia or the development of anemia in mice infected with both S. mansoni and P. berghei. Lwin et al. (28) used several different malaria strains and isolates and obtained variable results, depending on the strains used. No information is available with regard to cytokine or antibody analysis in mixed infections involving Plasmodium species.
Blood-stage P. chabaudi AS infection is normally nonlethal in C57BL/6 mice, with a peak parasitemia of approximately 30 to 40% around days 7 to 9 when an infectious inoculum of 106 pRBC is used. In resistant mice, the early acute stage is accompanied by the activation of inflammatory CD4+ T cells, which induces macrophages to produce reactive oxygen and nitrogen and leads to enhanced phagocytosis involved in the elimination of intraerythrocytic parasites (20, 36, 39). In both P. chabaudi and P. yoelii, spleen macrophages have been shown to exhibit increased oxidative activity during the early phase of infection, indicating the importance for macrophage activation in murine malaria (10, 19, 43). The inflammatory response is then replaced by a Th2 type of response, as evidenced by production of IL-4 and a subsequent activation of B cells and antibody production necessary for complete clearance of the parasites (24, 25, 40).
The immune response to patent S. mansoni infection is generally directed into a systemic Th2 type of response at the onset of egg production, with elevated production of IL-4, IL-5, and IL-10 in response to SEA as well as nonparasite antigens (15, 23, 31, 34). The present study was undertaken to investigate if an S. mansoni-directed Th2 response would affect the development of the protective Th1 immune response to P. chabaudi in resistant mice, and vice versa.
Thus, 8-week S. mansoni-infected mice were challenged with P. chabaudi, and cellular and humoral responses were monitored during the development and clearance of the malaria infection. Mice with a patent S. mansoni infection normally exhibit high serum levels of SEA-specific IgG1 and low levels of IgG2a antibodies, consistent with the Th2-type response (11, 23). In the present study, serum antibody analysis revealed a striking effect of the malaria infection on the levels of anti-SEA antibodies. Thus, the levels of SEA-specific IgG1, IgG2a, and IgM were decreased as early as 3 days after P. chabaudi infection and during the following 2 to 3 weeks. IgM levels were still normal at this time point but decreased from day 6 onward, time points that coincide with the strong immune suppression seen during the malaria infection. The decrease in circulating SEA-specific antibodies may be due to IFN-γ production, known to influence B-cell differentiation and Ig production (11, 35), or an increased turnover of plasma cells or antibodies. However, mice with concurrent infections exhibited higher levels of circulating anti-P. chabaudi IgM and IgG1 antibodies. This finding indicates that there is no general decrease in B-cell activity; rather, the B-cell repertoire is focused on the acute malaria infection and not on the patent S. mansoni infection. The rapid decrease in anti-SEA IgG antibodies during the early inflammatory response could be due to a malaria-induced increase in Fc-γ receptor expression, involved in the elimination of the antibodies from the circulation. Increased Fc-γ receptor expression has been found in mice with bacterial, viral, and parasitic infections (41), in humans with P. falciparum malaria (17, 42), and in mice with P. chabaudi adami infection (26). An alternative explanation could be a defect at the T-cell level. Our in vitro experiments showed reduced T-cell responses in mice with concurrent S. mansoni-P. chabaudi infection with regard to both proliferation and IL-4 and IL-5 production during weeks 1 and 2 of the malaria infection. The suppression was evident also in anti-CD3-stimulated cultures except for the IL-4 production that was not suppressed in the double-infected mice. The differences seen in IL-4 and IL-5 production might indicate distinct cellular sources for production of these cytokines.
Our data demonstrate that a murine P. chabaudi malaria infection clearly affects the in vivo antibody levels as well as the in vitro cytokine response to S. mansoni antigen in mice with patent S. mansoni infection. Since we did not examine S. mansoni pathology during these experiments, we do not know if the changed immune response affected the pathogenesis of S. mansoni. Interestingly, P. yoelii infections have been reported to reduce granuloma formation in the lungs of mice injected with S. mansoni eggs, indicating that the malaria infection may influence granuloma formation in vivo (1). Further studies on granuloma size and worm burden will be needed to establish the effects of concomitant S. mansoni-malaria infection on schistosomal pathology.
Mice carrying a patent S. mansoni infection and infected with blood-stage P. chabaudi parasites developed a more rapid and severe course of malaria, indicative of a defect in the initial control mechanism. A low but consistent number (around 10% in three separate experiments) of double-infected animals died during each experiment. The mice with concomitant infections showed significantly reduced RBC counts during the acute phase of malaria compared to mice with P. chabaudi only; thus, the deaths may have been due to severe anemia. The reasons for the reduced RBC counts are unknown but likely associated with the increased malaria parasitemia and subsequent destruction of RBC. The frequency of mortality is not striking, but it may be of interest to continue to study the effects of infection dose, inoculation route, etc., in order to elucidate this potentially important factor.
In resistant mice, blood-stage P. chabaudi infections are dependent on a Th1 type of response during the first phase of infection (24, 38, 40). Thus, the elevated parasitemias seen in S. mansoni-P. chabaudi-infected mice compared to P. chabaudi-only-infected mice could perhaps be explained by a Th2-mediated inhibition of the P. chabaudi-induced Th1 response. To investigate this possibility, we measured IFN-γ production from double- or P. chabaudi-only-infected mice. The results revealed that spleen cells from mice with concomitant S. mansoni-P. chabaudi infection produced similar levels of IFN-γ in response to both pRBC and anti-CD3 stimulation. Thus, there was no inhibition of the early P. chabaudi Th1 type of response in mice with patent S. mansoni infections.
Another explanation for the increased malaria parasitemia seen in the coinfected mice could be a defect in the capacity of the macrophages to produce TNF-α, known to reduce malaria parasitemia in both mice and humans and to enhance survival in mice (5, 20, 21). Our in vitro cytokine data demonstrated a reduced production of TNF-α in response to both pRBC and anti-CD3 in double-infected mice. Since macrophages are believed to be the major source for this early TNF-α production, the decreased levels are likely to represent a suppression at the macrophage level. We examined the TNF-α response after anti-CD3 stimulation because with this stimulation, the levels of IFN-γ were similar between the two experimental groups; hence, the levels of IFN-γ-induced TNF-α should be similar. However, despite elevated levels of IFN-γ, no increase in TNF-α was detected in the mice with concomitant infection. This suggests that the macrophages from S. mansoni-P. chabaudi-infected animals are nonresponsive to IFN-γ stimulation and may thus not be fully functional.
The downregulated IFN-γ responsiveness of macrophages in mice with concomitant S. mansoni-P. chabaudi infection is unknown but might be caused by Th2 cytokines such as IL-4, IL-10, and transforming growth factor β, all known to be potent macrophage suppressors. Modes of action have been shown to involve inhibition of the upregulation of costimulatory molecules such as B7 (9), downregulation of major histocompatibility complex class II molecules (8), or inhibition of macrophage-derived cytokine production (4, 7, 12). It has been shown that in S. mansoni infection, IL-10 inhibits IFN-γ-activated macrophage killing by blocking TNF-α production, necessary for the IFN-γ-induced activation (14, 30). This mechanism has been suggested to be an important strategy for the parasite to evade macrophage-mediated immune destruction.
Taken together, one likely explanation for the increased parasitemias seen in mice with concomitant S. mansoni-P. chabaudi infection might be an S. mansoni-induced suppression of macrophage activation, probably through IL-10 and possibly also IL-4 and/or transforming growth factor β (14, 29, 30). This would lead to an inability of the macrophages to respond to IFN-γ and thus a defect in their capacity to kill pRBC at an early stage. Attempts to analyze the macrophage-derived product nitric oxide (NO) in spleen cell culture supernatants revealed no differences in NO levels between single- and double-infected mice (data not shown). The reason for this is probably that in whole spleen cultures, cell types in addition to macrophages are responsible for the NO production. Thus, to determine NO production at the macrophage level, we are in the process of analyzing purified macrophage cultures.
In conclusion, our data show that two parasitic infections, Th2-dominated S. mansoni infection and acute Th1-dependent blood-stage P. chabaudi infection, when present concomitantly in experimental mice, severely affect the immune response to each other. (i) Blood-stage P. chabaudi malaria suppresses proliferative and Th2 cytokine responses to SEA as well as reducing the serum levels of SEA-specific IgG and IgM antibodies. (ii) Mice with concurrent S. mansoni-P. chabaudi infection develop a more rapid and higher-level malaria parasitemia in the blood. Patent S. mansoni infection does not inhibit the P. chabaudi Th1 response but renders macrophages nonresponsive to IFN-γ stimulation, as reflected by decreased TNF-α production in vitro. Further studies using purified macrophage cultures will provide more detailed information about the modes of action and the involvement of different cytokines in this important aspect of immune regulation of concomitant S. mansoni-P. chabaudi infection.
ACKNOWLEDGMENTS
We thank Ann Sjölund and Ingegärd Andersson for excellent technical assistance.
This work was supported by grants from the Swedish Agency for Research Cooperation with Developing Countries.
REFERENCES
- 1.Abdel-Wahab M F, Powers K G, Mahmoud S S, Good W C. Suppression of schistosome granuloma formation by malaria in mice. Am J Trop Med Hyg. 1974;23:915–918. doi: 10.4269/ajtmh.1974.23.915. [DOI] [PubMed] [Google Scholar]
- 2.Actor J K, Shirai M, Kullberg M C, Buller M L, Sher A, Berzofsky J A. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahvazi B C, Jacobs P, Stevenson M M. Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria. J Leukoc Biol. 1995;58:23–31. doi: 10.1002/jlb.58.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark I A, Hunt N H, Butcher G A, Cowden W B. Inhibition of murine malaria (Plasmodium chabaudi) in vitro by recombinant interferon-γ or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987;139:3493–3496. [PubMed] [Google Scholar]
- 6.Curry A J, Else K J, Jones F, Bancroft A, Grencis R K, Dunne D W. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J Exp Med. 1995;181:769–774. doi: 10.1084/jem.181.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, Haanen J, Spits H, Roncarolo M-G, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding L, Linsley P S, Huang L, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 10.Dockrell H M, Alavi A, Playfair J H L. Changes in oxidative burst capacity during murine malaria and the effect of vaccination. Clin Exp Immunol. 1986;66:37–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelman F D, Holmes J, Katona I M, Urban J F, Beckman M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivoimmunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 13.Flores-Villanueva P O, Zheng X X, Strom T B, Stadecker M J. Recombinant IL-10 and IL-10/Fc treatment down-regulate egg antigen-specific delayed hypersensitivity reactions and egg granuloma formation in schistosomiasis. J Immunol. 1996;156:3315–3320. [PubMed] [Google Scholar]
- 14.Gazzinelli R T, Oswald I P, James S L, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 15.Grzych J-M, Pearce E J, Cheever A, Caulada Z A, Caspar P, Hieny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine Schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 16.Helmby H, Perlmann H, Troye-Blomberg M, Perlmann P. Immunoglobulin E elevation in Plasmodium chabaudimalaria. Infect Immun. 1996;64:1432–1433. doi: 10.1128/iai.64.4.1432-1433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho M, White N J, Looareesuwan S, Wattanagoon Y, Hee Lee S, Walport M J, Bunnag D, Harinasuta T. Splenic Fc receptor function in host defence and anemia in acute Plasmodium falciparummalaria. J Infect Dis. 1990;161:555–561. doi: 10.1093/infdis/161.3.555. [DOI] [PubMed] [Google Scholar]
- 18.Hu-Li J, Ohara J, Watson C, Tsang W, Paul W E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 19.Jacobs P, Radzioch D, Stevenson M M. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J Immunol. 1995;155:5306–5313. [PubMed] [Google Scholar]
- 20.Jacobs P, Radzioch D, Stevenson M M. In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood-stage malaria in mice. Infect Immun. 1996;64:44–49. doi: 10.1128/iai.64.1.44-49.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullberg M C, Berzofsky J A, Jankovic D L, Barbieri S, Williams M E, Perlmann P, Sher A, Troye-Blomberg M. T cell derived IL-3 induces the production of IL-4 by non-B, non-T cells to amplify the Th2-cytokine response to a non-parasite antigen in Schistosoma mansoni-infected mice. J Immunol. 1996;156:1482–1489. [PubMed] [Google Scholar]
- 23.Kullberg M C, Pearce E J, Hieny S E, Sher A, Berzofsky J A. Infection with Schistosoma mansonialters the Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–3270. [PubMed] [Google Scholar]
- 24.Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989;5:362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- 25.Langhorne J, Cross C, Seixas E, Li C, Von Der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langhorne J, Titus J A. Expression of Fc γ receptors on splenic T cells of mice infected with Plasmodium chabaudi adami. Eur J Immunol. 1988;18:1–6. doi: 10.1002/eji.1830180102. [DOI] [PubMed] [Google Scholar]
- 27.Lewinsohn R. Anemia in mice with concomitant Schistosoma mansoni and Plasmodium berghei yoeliiinfection. Trans R Soc Trop Med Hyg. 1975;69:51–56. doi: 10.1016/0035-9203(75)90010-3. [DOI] [PubMed] [Google Scholar]
- 28.Lwin M, Last C, Targett G A T, Doenhoff M J. Infection of mice concurrently with Schistosoma mansoni and rodent malarias: contrasting effects of patent S. mansoni infections on Plasmodium chabaudi, P. yoelii and P. berghei. Ann Trop Med Parasitol. 1982;76:265–273. doi: 10.1080/00034983.1982.11687541. [DOI] [PubMed] [Google Scholar]
- 29.Oswald I P, Gazzinelli R T, Sher A, James S L. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 30.Oswald I P, Wynn T A, Sher A, James S L. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor α required as a costimulatory factor for interferon γ-induced activation. Proc Natl Acad Sci USA. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce E J, Caspar P, Grzych J-M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Playfair J H L, Dockrell H, Taverne J. Macrophages as effector cells in immunity to malaria. Immunol Lett. 1985;11:233–237. doi: 10.1016/0165-2478(85)90173-7. [DOI] [PubMed] [Google Scholar]
- 33.Rosa Brunet L, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. IL-4 protects against TNF-α mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 34.Sher A, Fiorentino D, Caspar P, Pearce E J, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with the down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147:2713–2716. [PubMed] [Google Scholar]
- 35.Snapper C M, Paul W E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson M M, Ghadirian E, Pillips N C, Rae D, Podoba J E. Role of mononuclear phagocytes in elimination of Plasmodium chabaudiAS infection. Parasite Immunol. 1989;11:529–544. doi: 10.1111/j.1365-3024.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson M M, Huang D Y, Podoba J E, Nowotarski M E. Macrophage activation during Plasmodium chabaudiAS infection in resistant C57BL/6 and susceptible A/J mice. Infect Immun. 1992;60:1193–1201. doi: 10.1128/iai.60.3.1193-1201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson M M, Tam M-F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudiAS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taverne J, Treagust J D, Playfair J H L. Macrophage cytotoxicity in lethal and non-lethal murine malaria and the effect of vaccination. Clin Exp Immunol. 1986;66:44–51. [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of Th1 and Th2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 41.Titus J A, Finkelman F D, Stephany D A, Jones J F, Segal D M. Quantitative analysis of Fcγ receptors on murine spleen cell populations by using dual parameter flow cytometry. J Immunol. 1984;133:556–561. [PubMed] [Google Scholar]
- 42.Ward K N, Warrell M J, Rhodes J, Looareesuwan S, White N J. Altered expression of human monocyte Fc receptors in Plasmodium falciparummalaria. Infect Immun. 1984;44:623–626. doi: 10.1128/iai.44.3.623-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wozencraft A O, Croft S L, Sayers G. Oxygen radical release by adherent cell populations during the initial stages of a lethal rodent malarial infection. Immunology. 1985;56:523–531. [PMC free article] [PubMed] [Google Scholar]