Abstract
Abstract
Aureobasidium is omnipresent and can be isolated from air, water bodies, soil, wood, and other plant materials, as well as inorganic materials such as rocks and marble. A total of 32 species of this fungal genus have been identified at the level of DNA, of which Aureobasidium pullulans is best known. Aureobasidium is of interest for a sustainable economy because it can be used to produce a wide variety of compounds, including enzymes, polysaccharides, and biosurfactants. Moreover, it can be used to promote plant growth and protect wood and crops. To this end, Aureobasidium cells adhere to wood or plants by producing extracellular polysaccharides, thereby forming a biofilm. This biofilm provides a sustainable alternative to petrol-based coatings and toxic chemicals. This and the fact that Aureobasidium biofilms have the potential of self-repair make them a potential engineered living material avant la lettre.
Key points
•Aureobasidium produces products of interest to the industry
•Aureobasidium can stimulate plant growth and protect crops
•Biofinish of A. pullulans is a sustainable alternative to petrol-based coatings
•Aureobasidium biofilms have the potential to function as engineered living materials
Keywords: Aureobasidium, Fungus, Biofilm, Coating, Wood protection, Engineered living material
Introduction
The growing world population causes environmental problems such as resource depletion, biodiversity loss, and pollution (Geissdoerfer et al. 2017; Maja and Ayano 2021). For instance, it drives the expansion of agricultural activity (Toop et al. 2017) and the building industry. The latter consumes large volumes of natural resources (e.g., sand, gravel, and oil) and energy and produces high amounts of CO2 and solid waste (Benachio et al. 2020). Therefore, there is a huge urgency to shift to a sustainable economy to reduce human impact on the environment.
The building industry relies on coatings to prevent the breakdown of building materials. These coatings are often based on non-renewable oil and contain toxic chemicals such as chromated copper arsenate, creosote, pentachlorophenol, or heavy metal combinations (Morrell 2017). Similarly, food products are often treated with chemicals such as copper-based fungicides to prevent colonization with pathogens or spoilage organisms (Lamichhane et al. 2018). The fungus Aureobasidium can contribute to the transition to a sustainable economy by providing a sustainable coating for wood and food products. Also, Aureobasidium can be used as a cell factory for the production of enzymes and other natural compounds that can replace non-sustainable chemicals.
This review describes the diversity and ecological niches of Aureobasidium. Moreover, it describes its life cycle, its (potential) use to protect products against deterioration, and as a (potential) source for of biobased molecules such as enzymes, biosurfactants, melanin, siderophores, gluconic acid, and the polysaccharides pullulan and β-glucan (Brumano et al. 2017; Canete-Rodriguez et al. 2016; Wang et al. 2022b). Furthermore, we describe the perspective of exploiting and expanding Aureobasidium as a component of a sustainable engineered living material with its biofilm on wood as a first example.
The genus Aureobasidium
Aureobasidium belongs to the phylum Ascomycota and the order Dothideales (Thambugala et al. 2014). Previously, Aureobasidium belonged to the Dothideaceae family (Schoch et al. 2006) but was reclassified in 2014 to belong to the Aureobasidiaceae. The latter family also includes the genera Kabatiella, Pseudoseptoria, Saccothecium, and the species Selenophoma mahoniae and Columnosphaeria fagi (Thambugala et al. 2014). Aureobasidium is described as mildew or blue or black stain (de Hoog 1993) and is popularly known as black yeast (Singh et al. 2015). Species of this genus can be found on all continents (Loque et al. 2010; Merín et al. 2011; Onetto et al. 2020; Peterson et al. 2013; van Nieuwenhuijzen et al. 2016; Woody et al. 2003) and have been isolated from air, water, and diverse (in)organic outdoor and indoor materials such as soil, phylloplanes, wood, rocks, marble, dishwashers, washing machines, house dust, and food (Babič et al. 2015; Humphries et al. 2017; Jiang et al. 2018; Li et al. 2015; van Nieuwenhuijzen 2014; Wang et al. 2022a; Zupancic et al. 2016). Currently, 32 DNA-identified Aureobasidium species are known (Table 1). These species include the best known Aureobasidium species, Aureobasidium pullulans, as well as the most recently identified species Aureobasidium insectorum, Aureobasidium planticola, Aureobasidium motuoense, and Aureobasidium intercalariosporum (Arnaud 1918; Arzanlou and Khodaei 2012; Ashish and Pratibha 2018; Barr 2001; Bills et al. 2012; Ciferri et al. 1957; Cooke 1962; Crous et al. 2021, 2011; de Hoog and Hermanides-Nijhof 1977a, 1977b; Gostinčar et al. 2014; Inamdar et al. 2019; Jia et al. 2019; Jiang et al. 2021, 2019; Lee et al. 2021; Nasr et al. 2018; Onetto et al. 2020; Peterson et al. 2013; Ramaley 1992; Wang et al. 2022a; Wu et al. 2023). An additional 15 species have been identified based on morphology (Table 2) (Cooke 1962; Crisan and Hodisan 1964; Della Torre 1963; de Hoog and Hermanides-Nijhof 1977a; Pande and Ghate 1985; Richardson and Pitkäranta 2011). Various loci (i.e., the internal transcribed spacer (ITS) rDNA, intergenic spacer 1, translation elongation factor-1α, β-tubulin, large ribosomal subunit (LSU), and RNA polymerase II) have been used for the phylogeny of Aureobasidium (Crous et al. 2011; Gostinčar et al. 2014; Manitchotpisit et al. 2009; Peterson et al. 2013; Wang et al. 2022a; Zalar et al. 2008). Kabatiella and Aureobasidium are closely related based on morphology and DNA sequences, which makes them difficult to distinguish (Bills et al. 2012; Crous et al. 2011). In fact, Kabatiella lini is now proposed to be part of the Aureobasidium clade (Thambugala et al. 2014). The same holds for Selenophoma mahoniae and Columnosphaeria fagi.
Table 1.
DNA-identified Aureobasidium species
| Species name | Synonym | Effective publication (according to the reference or MycoBank on 1 February 2023) | Source | Location | Mycobank, CBS, strain number | Reference | |
|---|---|---|---|---|---|---|---|
| Name | Year | ||||||
| Aureobasidium acericola | D. Hyeon Lee, J.Y. Oh, and Jong Kyu Lee | 2021 | Leaves of Acer pseudosieboldianum | Korea | MB836925 | Lee et al. (2021) | |
| Aureobasidium aerium | N. Jiang | 2022 | Air | Beijing, China | MB843527, CFCC 50324 | Wang et al. (2022a) | |
| Kabatiella bupleuri | Aureobasidium bupleuri | (Bills) Haelewaters and Aime | 2021 | Dead Bupleurum gibraltarium flower rachises | Spain | MB835676, CBS 131304 | Bills et al. (2012) |
| Aureobasidium castaneae | C.M. Tian and N. Jiang | 2021 | Castanea henryi leaves | China | MB838314JJ7-3 | Jiang et al. (2021) | |
| Kabatiella caulivora | Aureobasidium caulivorum | (Kirchn.) W.B. Cooke | 1962 | Trifolium incarnatum | USA | MB326817, CBS 242.64 | Cooke (1962) |
| Aureobasidium hainanensis | Aureobasidium pullulans strain P6 | S-L. Jia, Y. Ma, Z. Chi, G-L. Liu, Z. Hu, and Z-M. Chi | 2019 | Kandelia candel leaf | China | RZIQ01000000 | Jia et al. (2019) |
| Kabatiella harpospora | Aureobasidium harposporum | (Bres. and Sacc.) Hermanides-Nijhof | 1977 | Viscum album | Madrid, Spain | MB309380, CBS 122914 | de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium insectorum | Q.M. Wang, F. Wu, and M.M. Wang | 2023 | Spittle insects | China | OP856707, OP857208 | Wu et al. (2023) | |
| Aureobasidium intercalariosporum | Q.M. Wang, F. Wu, and M.M. Wang | 2023 | Leaf | China | OP856703, OP857205 | Wu et al. (2023) | |
| Aureobasidium iranianum | Arzanlou and Khodaei | 2012 | Bamboo | Iran | MB800705, CCTU 268 | Arzanlou and Khodaei (2012) | |
| Aureobasidium khasianum | J. Pratibha and Prabhug | 2018 | Decaying leaves of Wightia speciosissima | India | MB828278AVP 109 | Ashish and Pratibha (2018) | |
| Aureobasidium leucospermi | Crous | 2011 | Leucospermum conocarpodendron leaves | Stellenbosch, South Africa | MB560556, CBS 130593 | Crous et al. (2011) | |
| Kabatiella lini | Aureobasidium lini | (Laff.) Hermanides-Nijhof | 1977 | Linum usitatissimum | UK | MB283371, CBS 125.21 | de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium mangrovei | S. Nasr | 2018 | Healthy Avicennia marina plant | Qeshm Island, Iran | MB823444, IBRC M 30265 | Nasr et al. (2018) | |
| Aureobasidium melanogenum | (Hermanides-Nijhof) Zalar, Gostincar, and Gunde-Cimerman | 2014 | N/A | N/A | MB807698, CBS 105.22 | Gostinčar et al. (2014) | |
| Aureobasidium confer (cf.) melanogenum | Aureobasidium melanogenum | (de Bary) G. Arnaud | 1918 | Public fountain | Bangkok, Thailand | CBS 110374 | Arnaud (1918) and van Nieuwenhuijzen et al. (2016) |
| Aureobasidium microtermitis | S. Tiwari and A. Baghela | 2021 | Microtermes sp. Termite gut | Gujarat, Rajpipla district, India | MB839078, GTS2.7 | Crous et al. (2021) | |
| Aureobasidium motuoense | Q.M. Wang, F. Wu, and M.M. Wang | 2023 | Leaf | China | OP856710, OP857211 | Wu et al. (2023) | |
| Aureobasidium mustum | C. Onetto, S. Schmidt, M. Roach, and A. Borneman | 2020 | Fresh grape juice | South Australia | MB836845 | Onetto et al. (2020) | |
| Aureobasidium namibiae | (Zalar, de Hoog and Gunde-Cimerman) Zalar, Gostincar, and Gunde-Cimerman | 2014 | Dolomitic marble | Namib Desert, Namibia | MB807701, CBS 147.97 | Gostinčar et al. (2014) | |
| Aureobasidium pini | C.M. Tian and N. Jiang | 2019 | Pine needle | China | MB828664, CFCC 52778 | Jiang et al. (2019) | |
| Aureobasidium planticola | Q.M. Wang, F. Wu, and M.M. Wang | 2023 | Leaf | China | OP856711, OP857212 | Wu et al. (2023) | |
| Aureobasidium proteae | (Joanne E. Taylor and Crous) Joanne E. Taylor and Crous | 2011 | Leaves of Protea cv. ‘Sylvia’ | South Africa | MB560557, CBS 114273 | Crous et al. (2011) | |
| Aureobasidium pullulans | (De Bary) G. Arnaud ex Cif., Ribaldi, and Corte | 1957 | Vitis vinifera, fruit | Beaujolais, Beaujeu, France | MB508998, CBS 584.75 | Ciferri et al. (1957) and Gostinčar et al. (2014) | |
| Aureobasidium subglaciale | (Zalar, de Hoog and Gunde-Cimerman) Zalar, Gostincar, and Gunde-Cimerman | 2014 | Subglacial ice from sea water | Kongsvegen, Svalbard, Norway | MB807700, CBS 123387 | Gostinčar et al. (2014) | |
| Aureobasidium thailandense | S. W. Peterson, Manitchotpisit, and Leathers | 2013 | Wood surface | Prachuapkhirikhan, Thailand | MB801148, NRRL 58543 | Peterson et al. (2013) | |
| Aureobasidium tremulum | Inamdar, Roh. Sharma, and Adhapure | 2019 | Culture contaminant in a laboratory | Aurangabad, Maharashtra, India | MB829941, ATS4.2 | Inamdar et al. (2019) | |
| Aureobasidium uvarum | C. Onetto, S. Schmidt, M. Roach, and A. Borneman | 2020 | Fresh grape juice | South Australia | MB836846 | Onetto et al. (2020) | |
| Aureobasidium vineae | C. Onetto, S. Schmidt, M. Roach, and A. Borneman | 2020 | Fresh grape juice | South Australia | MB836849 | Onetto et al. (2020) | |
| Kabatiella zeae | Aureobasidium zeae | (Narita and Y. Hirats.) Dingley | 1973 | Leaf of Zea mays | Kiel-Kitzeberg, Germany | MB283372, CBS 767.71 | (de Hoog and Hermanides-Nijhof 1977b) |
| Columnosphaeria fagi | (H.J. Huds.) M.E. Barr | 2001 | Leaf of Populus sp. | UK | MB489000, CBS 171.93 | Barr (2001) | |
| Selenophoma mahoniae | A.W. Ramaley | 1992 | Leaf of Mahonia repens | USA | MB355521, CBS 388.92 | Ramaley (1992) | |
N/A, not available
Table 2.
Morphology-identified Aureobasidium species
| Species name | Synonym | Name of effective publication | Year of effective publication | Source | Location | Mycobank MB No | Reference |
|---|---|---|---|---|---|---|---|
| Aureobasidium aleuritis | Kabatiella aleuritis | (Vassiljevsky) Hermanides-Nijhof | 1977 | Dying Aleurites fordii leaves | Russia | 309377 | Cooke (1962) and de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium apocryptum | Gloeosporium apocryptum | (Ellis and Everh.) Hermanides-Nijhof | 1977 | Leaves of Acer dasycarpum, A. negundo, A. saccharinum, A. saccharum, A. tataricum, and A. platanoides | USA and Russia | 309378 | Cooke (1962) and de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium australiense | McAlpine | 1896 | N/A | N/A | 501787 | Richardson and Pitkäranta (2011) | |
| Aureobasidium dalgeri | Kabatiella dalgeri | (M. Morelet) Hermanides-Nijhof | 1977 | Dead Eucalyptus leaves | Saroula, Tunesia | 309379 | de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium indicum | A. Pande and Ghate | 1985 | N/A | N/A | 103074 | Pande and Ghate (1985) and Richardson and Pitkäranta (2011) | |
| Aureobasidium lilii | Crisan and Hodisan | 1964 | Plant | N/A | 326819 | Crisan and Hodisan (1964) and Richardson and Pitkäranta (2011) | |
| Aureobasidium microstromoides | Gloeosporium microstromoides | (Moesz) W.B. Cooke | 1962 | Catalpa bignonioides capsules | Hungary | 326822 | Cooke (1962) |
| Aureobasidium nigricans | Kabatiella nigricans | (G.F. Atk. and Edgerton) W.B. Cooke | 1962 | Vicia sativa | N/A | 326823 | Cooke (1962) |
| Aureobasidium nigrum | Torula dematia | (Marpmann) Cif. and Dalla Torre | 1963 | N/A | N/A | 326824 | Della Torre (1963) and Richardson and Pitkäranta (2011) |
| Aureobasidium prunicola | (Ellis and Everh.) Hermanides-Nijhof | 1977 | Prunus virginiana leaves | Racine, Wisconsin, USA | 309382 | de Hoog and Hermanides-Nijhof (1977a) | |
| Aureobasidium ribis | Kabatiella ribis | (Vassiljevsky) Hermanides-Nijhof | 1977 | Ribes nigrum leaves | N/A | 309384 | de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium sanguinariae | Gloeosporium sanguinariae | (Ellis and Everh.) Hermanides-Nijhof | 1977 | Sanguinaria canadensis leaf | Nuttalburg, W-Virginia, USA | 309386 | de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium thujae-plicatae | M. Morelet | 1978 | Plant | N/A | 309387 | Richardson and Pitkäranta (2011) | |
| Aureobasidium umbellulariae | Kabatiella phoradendri | (Harv.) Hermanides-Nijhof | 1977 | Umbellularia californica leaves | Alpine Dam, Marine County, California, USA | 309388 | de Hoog and Hermanides-Nijhof (1977a) |
| Aureobasidium vaccinii | Richiteanu and Teodoru | 1989 | Plant | N/A | 126507 | Richardson and Pitkäranta (2011) |
N/A, not available
Colonies of Aureobasidium that grow on malt extract agar (MEA) are initially yellow, creamy, light pink, or light brown. After a day to a few weeks, colonies become dark brown/black due to melanin-like pigments (Li et al. 2015; van Nieuwenhuijzen 2014). The hyphae and chlamydospores are the main cause of the dark pigmentation (Zalar et al. 2008). ‘Color variants’ of Aureobasidium, which usually have been isolated from tropical regions, produce red, yellow, orange, or purple pigments (Leathers 1986; Wickerham and Kurtzman 1975).
Aureobasidium species can tolerate extreme environmental conditions (Gostinčar et al. 2019) (Table 3). For instance, A. subglaciale shows superior resistance to UV light and heavy metals compared to other yeasts and bacteria (Liu et al. 2017). A. pullulans and Aureobasidium mangrovei are salt-resistant, the latter being able to resist salt levels up to 17% (Gunde-Cimerman et al. 2000; Zalar et al. 2008), while 20% of the cells of A. melanogenum survives 200 mM H2O2 (Jiang et al. 2016).
Table 3.
Growth conditions of Aureobasidium species
| Species name | Optimum temperature (°C) | Temperature range (°C) | Optimum pH | Survival to UV radiation (%) | Survival at 200 mM H2O2 (%) | Maximum tolerated concentration: | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salt (%) | Ni2+ (mg/L) | Cd2+ (mg/L) | Cr2+ (mg/L) | Cu2+ (mg/L) | CO2+ (mg/L) | Pb2+ (mg/L) | Hg2+ (mg/L) | |||||||
| A. iranianum | 25 | 15–34 | nd | nd | 10 | nd | nd | nd | nd | nd | nd | nd | Nasr et al. (2018) | |
| A. mangrovei | 25 | 15–37 | nd | nd | Melanized cells: 20.2, non-melanized cells: 6.0 | 15 | nd | nd | nd | nd | nd | nd | nd | Nasr et al. (2018) |
| A. melanogenum | 30 | 10–40 | nd | 61.2 ± 5.1 at UV-A, 30 W for 5 min | 10 | nd | nd | nd | nd | nd | nd | nd | Jiang et al. (2016), Jiang et al. (2019), and Zalar et al. (2008) | |
| A. namibiae | 25 | 10–30 | nd | nd | 10 | nd | nd | nd | nd | nd | nd | nd | Zalar et al. (2008) | |
| A. pullulans | 25 | 4–30 | nd | nd | 17 | nd | nd | nd | nd | nd | nd | nd | Gunde-Cimerman et al. (2000), Torzilli (1997), and Zalar et al. (2008) | |
| A. subglaciale | 25 | 4–25 | nd | 0.8–1 at UV-B, 250 J/m2 for 15 min | 10 | 250 | 50–100 | 400–450 | 400–450 | 300 | 1200 | 650–700 | Liu et al. (2017) and Zalar et al. (2008) | |
| A. thailandense | 25–28 | nd | nd | nd | 10 | nd | nd | nd | nd | nd | nd | nd | Peterson et al. (2013) | |
| A. zeae (K. zeae) | 25 | 15–30 | 7 | nd | nd | nd | nd | nd | nd | nd | nd | nd | Sun et al. (2015) | |
Nd, not determined
Aureobasidium comprises dimorphic species that grow vegetatively by forming yeast cells and hyphae. The mode of growth depends on the species and environmental conditions (van Nieuwenhuijzen 2014). Hyphal growth of A. pullulans is more abundant at low cell density, while yeast cells are more abundant at high cell density (Finlay 1987; Park 1984). Hyphae of A. pullulans have an average width of 2–16 µm, are hyaline, smooth- and thin-walled, and become dark-brown and thick-walled when grown on MEA for a longer period (de Hoog et al. 2000; Samson et al. 2019). Yeast cells result from the division of conidia (see below), and therefore both names are used for these types of cells.
Although Dothideales are known to reproduce sexually, no sexual reproduction has been reported for A. pullulans (Humphries et al. 2017) and other Aureobasidium species. The high abundance of diploid strains in A. melanogenum is not considered indicative of sexual reproduction in nature. Rather, diploidy is the result of intraspecific hybridization events of haploids not being followed by meiosis or haploidization (Gostinčar et al. 2022). Aureobasidium does form asexual spores known as endoconidia, blastoconidia, arthroconidia, swollen cells, and chlamydospores (Figs. 1 and 2). The former four cell types are often collectively described as conidia. The formation of asexual spores depends on the species and environmental conditions (van Nieuwenhuijzen 2014). For instance, the formation of chlamydospores in A. pullulans is observed in a glucose medium (3% (w/v)) with a limiting nitrogen source at a low pH (Bermejo et al. 1981a; Liu et al. 2021). pH is, in fact, the key factor regulating cell morphogenesis of A. pullulans (Bermejo et al. 1981b; Li et al. 2009). At pH < 3 chlamydospores are produced by swollen cells (Li et al. 2009). On the other hand, blastoconidia transform into swollen cells at pH ~ 4.5, while they are stable at pH 6. These spores bud from short lateral branches of hyphae and conidiophore-like structures (van Nieuwenhuijzen 2014; Zalar et al. 2008). They are ellipsoidal, spindle-like, cylindrical, or lemon-like in shape with a size of 9–11 × 3–6.5 µm (de Hoog and Hermanides-Nijhof 1977a; Kocková-Kratochvílová et al. 1980). Swollen cells have been described to develop from blastoconidia by enlarging and by producing a thick cell wall (Campbell et al. 2004; Li et al. 2009; Pechak and Crang 1977). This cell type is globular and ellipsoid in shape and can be either septated or not, with an average size of 15 × 11 µm and 12 × 9 µm, respectively. Swollen cells not only produce blastoconidia, other (septated) swollen cells, and chlamydospores (Pechak and Crang 1977), but also germ tubes (Campbell et al. 2004). The cylindrical-shaped arthroconidia are generally bigger than blastoconidia with a length and width of 6.5–22 and 4.5–13 µm, respectively. They form through fragmentation of hyphae (Samson et al. 2019). Endoconidia are less frequently observed. They are about 6–8 × 3 µm in size and are present in intercalary cells (Zalar et al. 2008). Chlamydospores are formed from swollen cells or by hyphae and can be found in chains, as free cells, or firmly attached to hyphae (Brown et al. 1973; Dominguez et al. 1978; Pechak and Crang 1977). They have thick and melanized cell walls with single or numerous septa (Kocková-Kratochvílová et al. 1980; van Nieuwenhuijzen 2014) with an average size of 13 × 12 µm (Fig. 1). The life cycle of A. pullulans proposed by Ramos and García Acha (1975) consists of six subcycles, their occurrence depending on the environmental conditions (Fig. 2). In these sub-cycles, blastoconidia produce other blastoconidia or form a pseudomycelium. This pseudomycelium is formed because the daughter cells of the blastoconidia do not separate from the mother cell and continue budding, creating an aseptate chain of this type of spores. Blastoconidia can also differentiate into (non-)septated, swollen cells. These swollen cells can bud off blastoconidia, differentiate into chlamydospores, or produce germ tubes, giving rise to a septate mycelium or endoconidia. The chlamydospores can give rise to a mycelium, which in turn can produce blastoconidia and chlamydospores.
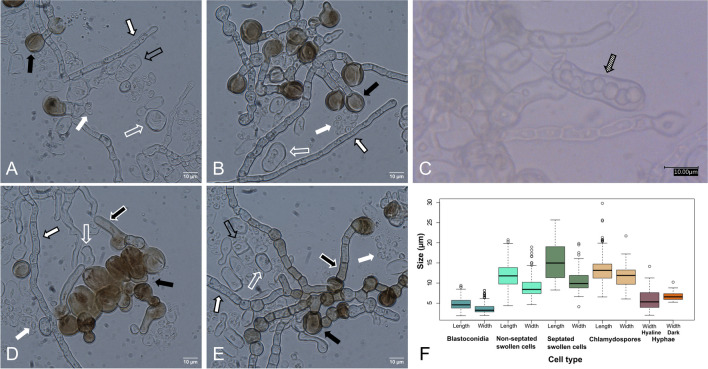
Fig. 1.
Schematic overview of cells in A. pullulans strain CBS 584.75 (A–E) and their dimensions (F). Blastoconidia or yeast cells (white arrow), non-septated swollen cells (white open arrow), septated swollen cells (black open arrow), chlamydospores (black arrow), hyaline hyphae (black lined, white filled arrow), dark hyphae (white lined, black filled arrow), and endoconidia (black striped arrow) (A–E) are the cell types distinguished in this fungal species. Arthroconidia (that could not be distinguished in liquid cultures) are also formed in this fungal species. C Adapted with contrast + 90 and brightness − 30
Fig. 2.
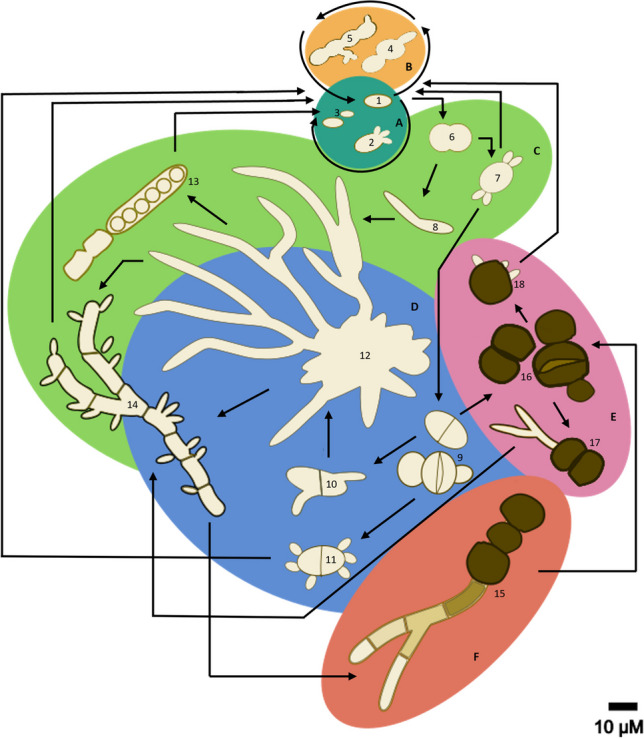
Aureobasidium pullulans life cycle with blastoconidia (1, 3) and blastoconidia producing other blastoconidia (2) in section A; pseudomycelium (4, 5) in section B; non-septated swollen cells (6), non-septated swollen cells giving rise to blastoconidia (7) and hyphae (8), mycelium (12), endoconidia (13), and septate mycelium producing other blastoconidia (14) in section C; septated swollen cells (9), septated swollen cells producing germ-tubes (10) or blastoconidia (11), mycelium (12), and septate mycelium producing blastoconidia (14) in section D; chlamydospores (16), chlamydospores producing germ-tubes (17), or blastoconidia (18) in section E; and chlamydospores growing on dark mycelium (15) in section F.
Adapted from Ramos and García Acha (1975)
The genomes of A. pullulans, A. melanogenum, A. subglaciale, and A. namibiae contain genes that are implicated in stress tolerance, including genes encoding aquaporins, aquaglyceroporins, and alkali-metal cation transporters, proteins for the synthesis of compatible solutes and melanin, as well as bacteriorhodopsin-like proteins (Gostinčar et al. 2014). Moreover, genes encoding stress signaling pathways are present in Aureobasidium, including the cell wall integrity (CWI) signaling pathway, the target of rapamycin complex 1 (TORC1) signaling pathway, the high glycerol osmotic 1 (HOG1) signaling pathway, and the heat shock factor 1 (HSF1) signaling pathway (Chi et al. 2022). However, little is known about the stress resistance of most Aureobasidium cell types. The chlamydospores are considered resistant to desiccation and ultraviolet irradiation (Pechak and Crang 1977). It is believed that the resistant nature of chlamydospores is due to the melanin in the cell wall as well as other molecules (known as electron-dense granular material) that are present in the outermost wall layer and cross walls (Brown et al. 1973).
Bioproducts from Aureobasidium
Aureobasidium produces many products of interest for the industry, including pullulan, β-glucan, polymalic and malic acids, melanin, lipids, biosurfactants, and liamocins. These molecules are used in agriculture, food, cosmetics, water treatment, and as pharmaceuticals and biofuels (Kim et al. 2022; Wang et al. 2022b).
Polysaccharides
The exopolysaccharide (EPS) pullulan is produced commercially using different species of Aureobasidium, in particular, A. pullulans (Wang et al. 2022b). Pullulan consists of maltotriose units that are attached to each other via α-(1 → 6) glycosidic bonds (Singh et al. 2021). Yields up to 125.7 g l−1 have been achieved using agro-industrial waste (e.g., beet molasses, coconut, potato starch, and soybean) (An et al. 2017; Göksungur et al. 2004; Sheoran et al. 2012; Singh et al. 2019; Thirumavalavan et al. 2009). The highest pullulan production (162.3 g l−1), however, has been achieved with glucose as a carbon source (Li et al. 2023). Pullulan is a white, water-soluble, tasteless, and odorless biological binder mainly used as a food additive, functioning as a thickener, stabilizer, filler, gelling agent, and/or adhesive (Muthusamy et al. 2022; Prajapati et al. 2013). For example, it is used as a substitute for gelatin, starch, or wheat flour. Moreover, it is used in food packaging materials to prevent the oxidation of food. The viscosity of pullulan in water is not affected over a wide range of pH (2–11) and remains stable in the presence of most metal ions (Jindal and Khattar 2018; Tsujisaka and Mitsuhashi 1993). With its unique structure and being non-toxic and non-irritant to the human body, it is a potent candidate for pharmaceutical and cosmeceutical applications (Singh et al. 2023). Pullulan can be used as a carrier for the controlled release of compounds into the environment. In particular, pullulan-based conjugates have been developed for prolonged intravitreal drug release (Lu et al. 2009; Singh et al. 2023; Zhang et al. 2011). Also, active ingredients used in cosmetics and lotions can be targeted to site-specific skin layers (Nakashio et al. 1976; Singh et al. 2008). Recently, special focus has been directed toward pullulan-based biomaterials as wound dressings and skin tissue engineering scaffolds. Pullulan composites combined with other biopolymers, such as chitin, gelatin, collagen, and chitosan, are considered ideal wound dressing materials (Elangwe et al. 2023).
Along with pullulan, A. pullulans is known to synthesize aubasidan-like EPS. This glucan contains a core of β-1,3-linked glucopyranosyl residues, to which side chains of α-1,4-glucosyl residues are attached through β-1,6-glucosidic bonds (Singh and Saini 2012; Yurlova and De Hoog 1997). Aubasidan-like β-glucans are also produced by two non-pullulan-producing strains of A. pullulans (NRRL 58539 and NRRL 58543) as well as by Aureobasidium thailandense (Kayanna et al. 2022; Lotrakul et al. 2013). The latter produces 37.73 g l−1 of this polysaccharide. Adding this polysaccharide to gummy jellies results in increased color intensity, hardness, gumminess, and chewiness, as well as a decrease in springiness and cohesiveness. These properties suggest a great potential for A. thailandense β-glucan in the food industry (Kayanna et al. 2022). Another β-glucan formed by A. pullulans consists of a main chain of (1 → 3)-β-glucan and four β-(1 → 6)-d-glucosyl side chains linked to the backbone via β-(1 → 6)-glycosidic bonds every six glucose residues (Kono et al. 2017). Yields of 2.5 g l−1 are obtained with A. pullulans IMS822 KCTC 11179BP wild type (Kang et al. 2010), while 9.2 g l−1 is obtained with the UV mutant strain A. pullulans M-2 (Moriya et al. 2013). In general, β-glucans exhibit properties such as anticancer and anti-inflammatory activity, dermal wound healing, and enhancement of intestinal immune function in mice (Hu et al. 2023; Kim et al. 2023; No et al. 2021; Tanioka et al. 2013; Yun et al. 2015). Of interest, Byun et al. (2008) developed a gamma irradiation-based treatment for Aureobasidium β-glucan that reduces its high viscosity and poor water solubility by reducing its molecular weight.
(Poly)organic acids
Polymalic acid (PMA) and malic acid are produced at an industrial scale by Aureobasidium, in particular by A. pullulans (Zou et al. 2013). PMA was first isolated from Physarum polycephalum as a compound that functions in coordination with DNA replication (Fischer et al. 1989). The α-, β-, and γ-types are distinguished, with the β-type being primarily found in Aureobasidium (Nagata et al. 1993; Zou et al. 2019). β-PMA is formed by ester bonds between l-malic acid (2-hydroxybutanedioic acid) monomers (Qi et al. 2021). Aureobasidium sp. P6 synthesizes 100.7 g l−1 PMA (Ma et al. 2013), while A. pullulans var. pullulans MCW produces even 152.5 g l−1 of this polymer (Wang et al. 2015). Obviously, these amounts are higher than those produced by P. polycephalum (2.7 g l−1) (Lee and Holler 1999). PMA has broad potential in applications such as drug delivery, biomaterials, and biodegradable plastics because of its water solubility, biodegradability, and biocompatibility (Qi et al. 2021). It can also be used in materials with controllable shape memory based on cross-linked PMA with reconfigurable permanent shapes due to further crosslinking during heat treatment (Qiu et al. 2019). Malic acid can be produced from polymalic acid by acid hydrolysis (Zou et al. 2013). It is frequently used in the food industry as an acidulant and flavor enhancer (Reddy et al. 2016). Additionally, it is applied in metal cleaning, textile finishing, and pharmaceuticals (Chi et al. 2016).
Melanin
Many fungi synthesize melanin. These pigments are classified as 1,8-dihydroxy naphthalene (DHN) melanin, eumelanin, pyomelanin, pheomelanin, and 4-glutaminyl hydroxybenzene (GHB) melanin (Liu et al. 2022). A. pullulans and A. melanogenum mainly produce DHN melanin (Jiang et al. 2016). A total of 3.71 g l−1 (0.19 g l−1 intracellular and 3.52 g l−1 extracellular) of this pigment is produced by A. pullulans NBRC 100716 (Mujdeci 2021). It is widely applied in areas such as optical biomimetics, UV-protective lenses, food colorants, material coatings, and biomedical applications due to its functional properties related to photosensitivity, acting as a UV-light barrier, its free radical scavenging ability, antioxidant activity, and ability as a reducing and capping agent for metal nanoparticles (Campana et al. 2022; Roy and Rhim 2022).
Fatty acids and surfactants
Fatty acids are highly produced by A. melanogenum P10 with a yield of 66.3 g of oil per 100 g of cell dry weight. Their composition consists of 26.7% C16:0, 1.7% C16:1, 6.1% C18:0, 44.5% C18:1, and 21.0% C18:2 (Wang et al. 2014). The transformation of corncob-derived xylose into intracellular lipid by the engineered P10 strain shows even better properties than the standard US and EU biodiesels (ASTM D6751 and EN 14214) caused by the higher cetane and lower iodine numbers (Song et al. 2022).
Surfactants have large industrial applications for their ability to lower the surface tension of water. The fact that they are produced from petroleum has triggered interest in biosurfactants (Holmberg 2001). A. thailandese LB01 produces a biosurfactant with a yield of 139 mg l−1. The biosurfactant, which has a chemical structure similar to lauric acid ester, reduces the surface tension of water from 67 mN m−1 to as low as 31.2 mN m−1. The ability of this biosurfactant to disperse crude oil highlights its potential in bioremediation (Meneses et al. 2017). A. pullulans is even more promising as a cell factory for biosurfactants by producing pullusurfactans F and G as well as liamocins (Brumano et al. 2017; Garay et al. 2018; Kim et al. 2022). Liamocins, also described as extracellular heavy oils, are polyol lipids belonging to the fungal glycolipid biosurfactants (Garay et al. 2018). Diverse structures of liamocins can be produced by A. pullulans depending on the strain and culture conditions (Leathers et al. 2015; Price et al. 2017). These glycolipids are composed of a single headgroup (d-mannitol, d- and l-arabitol, d-xylitol, l-threitol, d-sorbitol, d-galactitol, and glycerol) that is linked to three, four, or six 3,5-dihydroxy decanoic ester tail groups (Kang et al. 2022; Leathers et al. 2018). A. pullulans NRRL 50380 produces up to 4.4 g l−1 liamocins when grown on sugars and polyols (Price et al. 2017). Notably, a melanin-free derivative of strain NRRL 50384 (B46p14KO1) even gives a yield of 22 g l−1 (Leathers et al. 2018). The saturated aqueous solution of liamocins from A. pullulans strain CU 43 exhibits a surface tension of 27 mN m−1, implying these oils may have solubilizing or emulsifying properties (Manitchotpisit et al. 2011). Liamocins exhibit anticancer and antibacterial activity (Kang et al. 2022; Sałek et al. 2022) and inhibit the biofilm formation of oral streptococcal biofilms by Streptococcus mutans and Streptococcus sobrinus (Leathers et al. 2019). After hydrolysis, liamocins release 3,5-dihydroxy decanoic acids, which can be transformed into massoia lactones (Kang et al. 2022). Massoia lactones are 10, 12, and 14 carbon chain compounds (also referred to as C-10, C-12, and C-14 massoia lactones). The α,β-unsaturated δ-lactone moieties of massoia lactones are substituted at the C6 position by an alkyl chain with a variable length containing five, seven, or nine carbons (Kang et al. 2022; Rali et al. 2007). These compounds show anticancer, anti-viral, anti-inflammatory, and anti-fungal activities. Therefore, it can be used as a fungicide or pesticide in agriculture (Kang et al. 2022). For instance, massoia lactone is active against the wheat pathogen Fusarium graminearum (Zhang et al. 2021).
Aureobasidium as plant growth promotor and biocontrol agent
Global warming results in drought and increased salinity of soils. This results in major agricultural losses (Cominelli and Tonelli 2010). Although Aureobasidium species are described as plant or human pathogens (Crous et al. 2011; Lee et al. 2019; Nasr et al. 2018), they are also known as plant growth promotors and biocontrol agents in various crops and fruits such as grapes, berries, apples, pears, citrus, tomato, peaches, and strawberries (Adikaram et al. 2002; Di Francesco et al. 2017; Ferreira-Pinto et al. 2006; Galli et al. 2021; Klein and Kupper 2018; Mari et al. 2012a, 2012b; Schena et al. 1999; Zajc et al. 2020). Non-volatile metabolites produced by A. pullulans inhibit the growth of the plant pathogen Rhizoctonia solani by 87.9%. Biofilm formation by A. pullulans strains L1 and L8 at the bean and soybean plant roots is a key factor in virulence control and plant growth stimulation (Di Francesco et al. 2021).
Aureobasidium species belong to the third-most common group of endophytes in desert plants. These endophytes have the capacity to increase nutrient uptake by the plant and promote resistance of the plant to pathogens and to drought, heat, and salt stress (Zhang and White 2021). The 3′-phosphoadenosine-5′-phosphatase (PAP) phosphatase ApHal2 confers resistance to sodium in A. pullulans (Gašparič et al. 2013). The 3′-phosphoadenosine-5′-phosphatase motif sequence META from this protein is believed to be responsible for the high tolerance to NaCl. The homolog of this protein in Arabidopsis thaliana, SAL1, lacks this motif. Therefore, a region of the ApHal2 enzyme, including the META motif, was inserted into SAL1 of Arabidopsis thaliana (Gašparič et al. 2013). Overexpression of this modified SAL1 (mSAL1) improves the salt tolerance of the plant compared to wild-type Arabidopsis and plants overexpressing native SAL1. The msal1 plants show longer roots and larger leaf surfaces at elevated salt concentrations when compared to the sal1 and wild-type plants. Also, the wild-type plants decrease in dry weight when exposed to moderate drought stress, while the msal1 and sal1 plants even show increased dry weight in comparison with plants that are watered normally (Gašparič et al. 2013). However, severe drought results in lower dry weights in all genotypes. Also, the sal1 plants show lower revitalization ability compared with the msal1 and wild-type plants (Gašparič et al. 2013). Together, overexpression of native SAL1 results in resistance to moderate drought stress but decreases the revitalization rate after severe drought stress. By contrast, overexpression of mSAL1 improves salt and drought tolerance without affecting revitalization at high drought stress. These results show that A. pullulans or other Aureobasidium species can be interesting gene donors to improve the stress tolerance of plants. It is not known yet if such genes, in particular in the case of ApHal2, have the same effect on stress resistance when present in A. pullulans endophytes.
Siderophores can be used to stimulate plant growth (Di Francesco et al. 2022). These low-molecular-weight, iron-chelating compounds are produced by nearly all microbes to retrieve this metal from the environment (Chi et al. 2009; Johnson 2008). A. pullulans strain HN6.2 produces 1.1 g l−1 of siderophores (Wang et al. 2009). The siderophores of A. pullulans strain L1 not only increase the bioavailability of Fe in the soil but also that of Mn, Cu, and Zn by 50, 31.8, 38.4, and 27.1%, respectively, after 30 days of incubation with the fungus (Di Francesco et al. 2022). This is accompanied by an increased tomato root and stem diameter of 19.1 and 27.3%, respectively.
A. pullulans BSS6 improves heavy metal stress resistance and remediating mechanisms in cucumber (Cucumis sativus) (Ali et al. 2019). Cucumber plants inoculated with A. pullulans BSS6 and exposed to lead and cadmium show improved plant growth and a higher content of photosynthetic pigments (chlorophyll a and b and carotenoids) compared to non-inoculated plants. A. pullulans BSS6 causes enhanced antioxidant activities (catalase, peroxidase, and reduced glutathione) and inhibition of lipid peroxidation during stress conditions in plants. The inoculation of A. pullulans BSS6 also reduces metal accumulation and alleviates metal-induced stress in plants. Finally, when added to soil A. pullulans BSS6 reduces the availability of lead and cadmium. These findings indicate that treatment with A. pullulans BSS6 is a promising phytoremediation agent for crops growing in soils polluted with these metals (Ali et al. 2019).
Biofilms are differentiated accumulations of microorganisms that are formed on surfaces surrounded by an extracellular matrix consisting of EPS (Blankenship and Mitchell 2006). The application of A. pullulans biofilms on winter wheat spikes inhibits the growth of pseudomonads, Azotobacter bacteria, and filamentous fungal pathogens (Wachowska et al. 2016). Also, biofilm production by A. pullulans stimulates the biocontrol activity against Geotrichum citri-aurantii, the causal agent of sour rot in citrus fruits, as well as other, possibly plant pathogenic, microorganisms due to niche exclusion. Biofilm production can be stimulated by the addition of 1% ammonium sulfate, which increases the antagonistic activity against sour rot and allows for better survival of A. pullulans in wounded sites of citrus fruits (Klein and Kupper 2018). Notably, A. pullulans has been found to be one of the most active endophytes cultured from fruits. It produces the highest amount (9109.19 ± 146.02 μg/g) of indole-3-acetic acid (IAA), which induces plant growth (Kachalkin et al. 2022) and, thereby, could be a great characteristic in plant growth-promoting biofilms.
Aureobasidium as protective coatings in construction
In construction, the discoloration of wood due to mold growth is usually considered negative (Gobakken et al. 2010; Lie et al. 2019; Williams and Feist 1999). However, naturally grown biofilms of A. pullulans formed in combination with water-repellent linseed oil are, in fact, an attractive living protective wood layer (Sailer et al. 2010). Compared to traditional wood coatings, the A. pullulans linseed oil-based coating (from now on called Biofinish) has clear advantages in terms of sustainability and potential self-repairing abilities (Filippovych et al. 2015; 2016; Rensink et al. 2020; Sailer et al. 2010). In outdoor applications, natural Biofinish can be formed on vegetable oil-impregnated wood. This natural Biofinish consists of 26 to 34 fungal genera yet always contains species of Aureobasidium (van Nieuwenhuijzen et al. 2017). In terms of wood protection, liquid water uptake is prevented by the linseed oil, and together with the fungus, the wood is protected against wood-degrading microorganisms and UV light (Hernandez and Evans 2015). The latter can be explained by the high abundance of chlamydospores on the wood surface (Poohphajai et al. 2021). The industrial finish-coated wood showed superior aesthetic performance during a 3-month weathering study when compared to non-coated wood. Its surface roughness decreased, while it increased in non-coated wood. This can be explained by the local regrowth of the fungus to cover damaged spots and by the migration of the linseed oil to the wood surface, where it polymerizes (Poohphajai et al. 2021). Linseed oil consists of unsaturated linolenic (53.21%), oleic (18.51%), and linoleic (17.25%) acids, while the dominant saturated acids are palmitic (6.58%) and stearic (4.43%) acids (Gruia et al. 2012). The drying and hardening (i.e., polymerization) of linseed oil occur when the oil is exposed to air. It is a consequence of the high content of glycerol esters in linolenic acid that undergoes oxidation reactions (Juita et al. 2012). A. melanogenum can use linseed oil as a single carbon source (Peeters et al. 2018; van Nieuwenhuijzen et al. 2019), but not when it is cross-linked (Peeters et al. 2018). Specifically, the degree of cross-linking of the oil determines the growth of A. melanogenum. Assuming the same applies to A. pullulans, the growth of the fungus within Biofinish reduces, and eventually halts, upon cross-linking of the oil.
Adhesion is crucial for the colonization of Aureobasidium on any surface, including plant surfaces like wood, leaves, roots, and fruit (Blankenship and Mitchell 2006). EPS not only fill the space between cells in biofilms (Flemming et al. 2017), but these are also believed to attach cells to surfaces (Czaczyk and Myszka 2007). Adhesion of A. pullulans is controlled by the EPS uronic acid-based polymers and possibly pullulan as well (Bardage and Bjurman 1998; Pouliot et al. 2005). Cells harvested in the early-exponential growth phase show a lower density of uronic acid polymers but a higher adhesion to the AFM tip and a higher retention to quartz media when compared to late-exponential cells (Pouliot et al. 2005). Pullulan contributes to the adhesion of A. pullulans (De Bary) Arnaud blastospores on painted wood surfaces (Bardage and Bjurman 1998). In this case, early-exponential growth phase cells adhere better than late-exponential growth phase cultures of A. pullulans strain NRRL Y-2331–1, despite the fact that levels of pullulan are lower in the former cells. Notably, a pullulanase treatment has a minimal effect on the adhesion force, suggesting that pullulan is not involved in the adhesion of cells to silicon nitride and quartz (Pouliot et al. 2005). Unlike A. pullulans, A. thailandense is not producing pullulan. This species that is isolated from leaves and wooden surfaces (Peterson et al. 2013) may therefore produce other substances that are responsible for the adhesion to surfaces. For instance, hydrophobins could play such a role. These proteins function in hyphal attachment to hydrophobic surfaces such as those of plants. These small, cysteine-rich proteins are secreted by mycelial fungi and self-assemble at hydrophilic–hydrophobic interfaces (Wösten and Wessels 1997). For example, the hydrophobin SC3 of Schizophyllum commune mediates fungal attachment to hydrophobic surfaces such as Teflon. It does so by assembling into a highly surface-active protein film at the interface between the hydrophilic cell wall and the hydrophobic surface. A strain in which the sc3 gene is inactivated shows reduced but not abolished hyphal attachment to Teflon (Wösten et al. 1994). In the absence of SC3, the hydrophobin-like protein SC15 mediates the attachment of S. commune to hydrophobic surfaces (Lugones et al. 2004). The hydrophobin genes Aur1 and Aur2 have been identified in A. pullulans strain MUCL38722, while hfbA and hfbB have been identified in A. pullulans (De Bary) Arnaud P268. The amino acid sequences of aur1 and hfbA and aur2 and hfbB are about 90% identical, and their encoded hydrophobins are predicted to belong to the class II hydrophobins, with hfbB being closely related to the hydrophobins of Trichoderma (Stenbæk 2015). The class II hydrophobins HFBI and HFBII of Trichoderma reesei have different properties when compared to the class I hydrophobin SC3 of S. commune (Askolin et al. 2006). For example, (1) in contrast to SC3, self-assembly of HFBI and HFBII at the water–air interface is not accompanied by a change in secondary structure or in ultrastructure; (2) the maximal lowering of the water surface tension occurs much faster in the case of HFBI and HFBII (instantly to several minutes) compared to SC3 (several hours); and (3) the HFBI coating has lower resistance to a hot detergent treatment than the SC3 coating. It was also shown that oil emulsions prepared with HFBI and SC3 are more stable than those prepared with HFBII and that HFBI and SC3 interact more strongly with Teflon when compared to HFBII. Surface adhesion in Aureobasidium may also be mediated by other proteins. As mentioned above, SC15 can partially replace SC3 in S. commune (Lugones et al. 2004), while hydrophobin function has been (partially) replaced by repellents in Ustilago maydis (Teertstra et al. 2006). A variety of proteins are surface-active, explaining why non-related proteins can mediate attachment in fungi.
Perspective
Aureobasidium produces many products of interest for the industry, including enzymes, polysaccharides, and biosurfactants. These molecules have a wide range of applications. So far, the use of genetic modification has hardly been used to improve production yields. This is explained by the fact that, until recently, the efficiency of genetic modification of Aureobasidium was low. However, Zhang et al. (2019) developed an efficient CRISPR/Cas9-mediated genomic mutagenesis, which will be an important tool to improve the production levels of enzymes and other molecules in the future.
Apart from the use of Aureobasidium as a cell factory, it can also be used in coatings to protect crops or wood. For instance, A. pullulans is a key ingredient in commercial products. Blossom Protect (Nufarm) is used in the biological control of fire blight (Zeng et al. 2023), and Biofinish (Xylotrade) protects wood. These sustainable products replace non-sustainable chemicals and petrol-based coatings, respectively. Blossom Protect and Biofinish can be considered living materials. Nature produces a wide variety of “living” materials such as bone, wood, and tissue, while human society produces “non-living” materials like chemicals, fuels, and pharmaceuticals. Yet, researchers now start to produce living materials as well, often called engineered living materials (ELMs) (Srubar III 2021). ELMs are defined as engineered materials composed of living cells that form or assemble the material itself or modulate the functional performance of the material (Nguyen et al. 2018). These materials also contain scaffolding polymeric matrices (Rodrigo-Navarro et al. 2021). A key difference between ELMs and other biohybrid devices is that the living cells in ELMs act as material factories, whereby the cells use resources from their environment to create biopolymeric building blocks that direct and/or maintain the formation of the ELM (Nguyen et al. 2018). ELMs provide “smart” functionalities that exceed existing capabilities of conventional materials, including the adaptation to environmental conditions, different material states, and/or self-healing abilities (Nguyen et al. 2018). ELMs have been studied in the biomedical field with functions such as biosensing, wound healing, stem-cell-based tissue engineering, and drug delivery (Rodrigo-Navarro et al. 2021). Recently, ELMs have also been proposed to be implemented in the building industry to function as self-healing concrete, self-growing bricks, actuators, and energy generators, or as protective coatings and paints (Sandak 2023). As such, Aureobasidium biofilms can be considered a simple ELM. Its different cell types may provide different functions. For example, chlamydospores and dark hyphae produce protective melanin, while other cells provide adherence or the self-repair response. Also, certain types of cells may attract beneficial microbial partners or repel other microbes. Still, many aspects of A. pullulans biology have to be studied to develop this fungus as a fully functional ELM.
Concluding remarks
Aureobasidium is found in soil, water, wood, and other plant materials. Various species of this genus can play a role in the transition to a sustainable economy. Their enzymes and other molecules can, for instance, be used in agriculture, construction, food, health, cosmetics, biofuel, and bioremediation. When growing in biofilms, A. pullulans protects crops and wood with the potential of self-repair, thereby offering a sustainable living alternative to petrol-based coatings and toxic chemicals. It should be noted that some Aureobasidium species, such as A. melanogenum are opportunistic human pathogens (Černoša et al. 2021) and are therefore not suitable for applications such as in Biofinish. Previous publications reported pathogenic A. pullulans strains, but these strains were likely misclassified strains of A. melanogenum (Gostinčar et al. 2014). Clearly, only non-pathogenic Aureobasidium species such as A. pullulans should be used in applications. To improve the use of Aureobasidium in a sustainable economy, future research should address which of its cell types (i.e., hyphae, yeast cells, endoconidia, blastoconidia, arthroconidia, swollen cells, and chlamydospores) and underlying mechanisms contribute to the production of enzymes or other molecules or to the formation of biofilms and their performance as a functional coating.
Acknowledgements
The authors acknowledge Anna Sandak, Jakub Sandak, Faksawat Poohphajai, Karen Butina Ogorelec, and Ana Gubenšek (Innorenew CoE, Slovenia) for their help in taking the photo of endoconidia. The authors thank Collin Lutke Schipholt (Saxion) and Roos Demmers (Utrecht University) for their technical assistance. This work was supported by the Saxion Research and Graduate School of Saxion University of Applied Sciences, Enschede, The Netherlands.
Author contribution
SR and HW conceived and designed the manuscript. EN, CS, MS, and HW reviewed and edited the manuscript. All authors read and approved the manuscript.
Data Availability
Data will be made available on request.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Michael F. Sailer is co-owner of Xylotrade, which produces Biofinish. The other authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adikaram NKB, Joyce DC, Terryc LA. Biocontrol activity and induced resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australas Plant Path. 2002;31:223–229. doi: 10.1071/AP02017. [DOI] [Google Scholar]
- Ali A, Bilal S, Khan AL, Mabood F, Al-Harrasi A, Lee I-J. Endophytic Aureobasidium pullulans BSS6 assisted developments in phytoremediation potentials of Cucumis sativus under Cd and Pb stress. J Plant Interact. 2019;14(1):303–313. doi: 10.1080/17429145.2019.1633428. [DOI] [Google Scholar]
- An C, Ma S-j, Chang F, Xue W-j. Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz J Microbiol. 2017;48:180–185. doi: 10.1016/j.bjm.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud G. Les Astérinées. Annales De L’école Nationale D’agriculture De Montpellier. 1918;16(1–4):1–288. [Google Scholar]
- Arzanlou M, Khodaei S. Aureobasidium iranianum, a new species on bamboo from Iran. Mycosphere. 2012;3(4):404–408. doi: 10.5943/mycosphere/3/4/2. [DOI] [Google Scholar]
- Ashish P, Pratibha J. Aureobasidium khasianum (Aureobasidiaceae) a novel species with distinct morphology. Phytotaxa. 2018;374(3):257–262. doi: 10.11646/phytotaxa.374.3.7. [DOI] [Google Scholar]
- Askolin S, Linder M, Scholtmeijer K, Tenkanen M, Penttilä M, de Vocht ML, Wösten HAB. Interaction and comparison of a class I hydrophobin from Schizophyllum commune and class II hydrophobins from Trichoderma reesei. Biomacromol. 2006;7(4):1295–1301. doi: 10.1021/bm050676s. [DOI] [PubMed] [Google Scholar]
- Babič MN, Zalar P, Ženko B, Schroers H-J, Džeroski S, Gunde-Cimerman N. Candida and Fusarium species known as opportunistic human pathogens from customer-accessible parts of residential washing machines. Fungal Biol. 2015;119(2–3):95–113. doi: 10.1016/j.funbio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Bardage SL, Bjurman J. Isolation of an Aureobasidium pullulans polysaccharide that promotes adhesion of blastospores to water-borne paints. Can J Microbiol. 1998;44(10):954–958. doi: 10.1139/w98-091. [DOI] [Google Scholar]
- Barr ME. Revisionary studies on the Dothioraceae. Harvard Pap Bot. 2001;6(1):25–34. [Google Scholar]
- Benachio GLF, Freitas MdCD, Tavares SF. Circular economy in the construction industry: a systematic literature review. J Clean Prod. 2020;260:121046. doi: 10.1016/j.jclepro.2020.121046. [DOI] [Google Scholar]
- Bermejo J, Dominguez J, Goni F, Uruburu F. Influence of carbon and nitrogen sources on the transition from yeast-like cells to chlamydospores in Aureobasidium pullulans. Antonie Van Leeuwenhoek. 1981;47(2):107–119. doi: 10.1007/BF02342194. [DOI] [PubMed] [Google Scholar]
- Bermejo JM, Dominguez JB, Goni FM, Uruburu F. Influence of pH on the transition from yeast-like cells to chlamydospores in Aureobasidium pullulans. Antonie Van Leeuwenhoek. 1981;47:385–392. doi: 10.1007/BF00426000. [DOI] [PubMed] [Google Scholar]
- Bills GF, Menéndez VG, Platas G. Kabatiella bupleuri sp. Nov. (Dothideales), a pleomorphic epiphyte and endophyte of the Mediterranean plant Bupleurum gibraltarium (Apiaceae) Mycologia. 2012;104(4):962–973. doi: 10.3852/12-003. [DOI] [PubMed] [Google Scholar]
- Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9(6):588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Brown RG, Hanic LA, Hsiao M. Structure and chemical composition of yeast chlamydospores of Aureobasidium pullulans. Can J Microbiol. 1973;19(2):163–168. doi: 10.1139/m73-025. [DOI] [PubMed] [Google Scholar]
- Brumano LP, Antunes FAF, Souto SG, dos Santos JC, Venus J, Schneider R, da Silva SS. Biosurfactant production by Aureobasidium pullulans in stirred tank bioreactor: new approach to understand the influence of important variables in the process. Bioresour Technol. 2017;243:264–272. doi: 10.1016/j.biortech.2017.06.088. [DOI] [PubMed] [Google Scholar]
- Byun E-H, Kim J-H, Sung N-Y, Choi J-i, Lim S-T, Kim K-H, Yook H-S, Byun M-W, Lee J-W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat Phys Chem. 2008;77(6):781–786. doi: 10.1016/j.radphyschem.2007.12.008. [DOI] [Google Scholar]
- Campana R, Fanelli F, Sisti M. Role of melanin in the black yeast fungi Aureobasidium pullulans and Zalaria obscura in promoting tolerance to environmental stresses and to antimicrobial compounds. Fungal Biol. 2022;126(11–12):817–825. doi: 10.1016/j.funbio.2022.11.002. [DOI] [PubMed] [Google Scholar]
- Campbell BS, Siddique ABM, McDougall BM, Seviour RJ. Which morphological forms of the fungus Aureobasidium pullulans are responsible for pullulan production? FEMS Microbiol Lett. 2004;232(2):225–228. doi: 10.1016/s0378-1097(04)00076-x. [DOI] [PubMed] [Google Scholar]
- Canete-Rodriguez AM, Santos-Duenas IM, Jimenez-Hornero JE, Ehrenreich A, Liebl W, Garcia-Garcia I. Gluconic acid: properties, production methods and applications - an excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem. 2016;51(12):1891–1903. doi: 10.1016/j.procbio.2016.08.028. [DOI] [Google Scholar]
- Černoša A, Sun X, Gostinčar C, Fang C, Gunde-Cimerman N, Song Z. Virulence traits and population genomics of the black yeast Aureobasidium melanogenum. J Fungi. 2021;7(8):665. doi: 10.3390/jof7080665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Wang F, Chi Z, Yue L, Liu G, Zhang T. Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl Microbiol Biotechnol. 2009;82(5):793–804. doi: 10.1007/s00253-009-1882-2. [DOI] [PubMed] [Google Scholar]
- Chi Z, Wang Z-P, Wang G-Y, Khan I, Chi Z-M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit Rev Biotechnol. 2016;36(1):99–107. doi: 10.3109/07388551.2014.924474. [DOI] [PubMed] [Google Scholar]
- Chi Z, Kong C-C, Wang Z-Z, Wang Z, Liu G-L, Hu Z, Chi Z-M. The signaling pathways involved in metabolic regulation and stress responses of the yeast-like fungi Aureobasidium spp. Biotechnol Adv. 2022;55:107898. doi: 10.1016/j.biotechadv.2021.107898. [DOI] [PubMed] [Google Scholar]
- Ciferri R, Ribaldi M, Corte A. Revision of 23 strains of Aureobasidium pullulans (de B) Arn. (Pullularia pullulans) Atti Dell’istituto Botanico Della Università e Laboratorio Crittogamico Di Pavia. 1957;14:78–90. [Google Scholar]
- Cominelli E, Tonelli C. Transgenic crops coping with water scarcity. N Biotechnol. 2010;27(5):473–477. doi: 10.1016/j.nbt.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Cooke WB. A taxonomic study in the “black yeasts”. Mycopathol Mycol Appl. 1962;17(1):1–43. doi: 10.1007/BF02279261. [DOI] [PubMed] [Google Scholar]
- Crisan A, Hodisan I (1964) Contributii la cunoasterea florei micologice din Valea Fenesului (Raion Alba) I. Contributii Botanice Universitatea Cluj-Napoca Gradina Botanica 81–87
- Crous PW, Summerell BA, Swart L, Denman S, Taylor J, Bezuidenhout C, Palm ME, Marincowitz S, Groenewald JZ. Fungal pathogens of Proteaceae. Persoonia. 2011;27(1):20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck E, Jurjevi Ž, Boers J, Van Iperen A, Starink-Willemse M, Dima B, Balashov S, Bulgakov T, Johnston P, Morozova O, Pinruan U, Sommai S, Alvarado P, Decock C, Lebel T, McMullan-Fisher S, Moreno G, Shivas R, Zhao L, Abdollahzadeh J, Abrinbana M, Ageev D, Akhmetova G, Alexandrova A, Altés A, Amaral A, Angelini C, Antonín V, Arenas F, Asselman P, Badali F, Baghela A, Bañares A, Barreto R, Baseia I, Bellanger J-M, Berraf-Tebbal A, Yu Biketova A, Bukharova N, Burgess T, Cabero J, Câmara M, Cano-Lira J, Ceryngier P, Chávez R, Cowan D, de Lima A, Oliveira R, Denman S, Dang Q, Dovana F, Duarte I, Eichmeier A, Erhard A, Esteve-Raventós F, Fellin A, Ferisin G, Ferreira R, Ferrer A, Finy P, Gaya E, Geering A, Gil-Durán C, Glässnerová K, Glushakova A, Gramaje D, Guard F, Guarnizo A, Haelewaters D, Halling R, Hill R, Hirooka Y, Hubka V, Iliushin V, Ivanova D, Ivanushkina N, Jangsantear P, Justo A, Kachalkin A, Kato S, Khamsuntorn P, Kirtsideli I, Knapp D, Kochkina G, Koukol O, Kovács G, Kruse J, Kumar T, Kušan I, Læssøe T, Larsson E, Lebeuf R, Levicán G, Loizides M, Marinho P, Luangsa-ard J, Lukina E, Magaña-Dueñas V, Maggs-Kölling G, Malysheva E, Malysheva V, Martín B, Martín M, Matočec N, McTaggart A, Mehrabi-Koushki M, Mešić A, Miller A, Mironova P, Moreau P-A, Morte A, Müller K, Nagy L, Nanu S, Navarro-Ródenas A, Nel W, Nguyen T, Nóbrega T, Noordeloos M, Olariaga I, Overton B, Ozerskaya S, Palani P, Pancorbo F, Papp V, Pawłowska J, Pham T, Phosri C, Popov E, Portugal A, Pošta A, Reschke K, Reul M, Ricci G, Rodríguez A, Romanowski J, Ruchikachorn N, Saar I, Safi A, Sakolrak B, Salzmann F, Sandoval-Denis M, Sangwichein E, Sanhueza L, Sato T, Sastoque A, Senn-Irlet B, Shibata A, Siepe K, Somrithipol S, Spetik M, Sridhar P, Stchigel A, Stuskova K, Suwannasai N, Tan Y, Thangavel R, Tiago I, Tiwari S, Tkalčec Z, Tomashevskaya M, Tonegawa C, Tran H, Tran N, Trovão J, Trubitsyn V, Van Wyk J, Vieira W, Vila J, Visagie C, Vizzini A, Volobuev S, Vu D, Wangsawat N, Yaguchi T, Ercole E, Ferreira B, de Souza A, Vieira B, Groenewald J. Fungal planet description sheets: 1284–1382. Persoonia. 2021;47(1):178–374. doi: 10.3767/persoonia.2021.47.06. [DOI] [PubMed] [Google Scholar]
- Czaczyk K, Myszka K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol J Environ Stud. 2007;16(6):799–806. [Google Scholar]
- de Hoog GS. Evolution of black yeasts: possible adaptation to the human host. Antonie Van Leeuwenhoek. 1993;63(2):105–109. doi: 10.1007/BF00872386. [DOI] [PubMed] [Google Scholar]
- de Hoog GS, Hermanides-Nijhof EJ. Aureobasidium and allied genera. Stud Mycol. 1977;15:166–173. [Google Scholar]
- de Hoog GS, Hermanides-Nijhof EJ. The black yeasts and allied Hyphomycetes. Stud Mycol. 1977;15:222. [Google Scholar]
- de Hoog GS, Guarro J, Gene J, Figueras MJ (2000) Atlas of clinical fungi, 2nd edition. Amer Society for Microbiology, Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands, pp 1126
- Della Torre B. Tentativo di reidentificazione del Saccharomyces niger Marpmann (1886) Atti Del’istituto Botanico e Del Laboratorio Crittogamico Dell'università Di Pavia. 1963;20:208–234. [Google Scholar]
- Di Francesco A, Milella F, Mari M, Roberti R. A preliminary investigation into Aureobasidium pullulans as a potential biocontrol agent against Phytophthora infestans of tomato. Biol Control. 2017;114:144–149. doi: 10.1016/j.biocontrol.2017.08.010. [DOI] [Google Scholar]
- Di Francesco A, Di Foggia M, Corbetta M, Baldo D, Ratti C, Baraldi E. Biocontrol activity and plant growth promotion exerted by Aureobasidium pullulans strains. J Plant Growth Regul. 2021;40(3):1233–1244. doi: 10.1007/s00344-020-10184-3. [DOI] [Google Scholar]
- Di Francesco A, Sciubba L, Bencivenni M, Marzadori C, Baraldi E. Application of Aureobasidium pullulans in iron-poor soil. Can the production of siderophores improve iron bioavailability and yeast antagonistic activity? Ann Appl Biol. 2022;180(3):398–406. doi: 10.1111/aab.12742. [DOI] [Google Scholar]
- Dominguez JB, Goni FM, Uruburu F. The transition from yeast-like to chlamydospore cells in Pullularia pullulans. Microbiology. 1978;108(1):111–117. doi: 10.1099/00221287-108-1-111. [DOI] [PubMed] [Google Scholar]
- Elangwe CN, Morozkina SN, Olekhnovich RO, Polyakova VO, Krasichkov A, Yablonskiy PK, Uspenskaya MV. Pullulan-based hydrogels in wound healing and skin tissue engineering applications: a review. Int J Mol Sci. 2023;24(5):4962. doi: 10.3390/ijms24054962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Pinto MM, Moura-Guedes MC, Barreiro MG, Pais I, Santos MR, Silva MJ. Aureobasidium pullulans as a biocontrol agent of blue mold in “Rocha” pear. Commun Agric Appl Biol Sci. 2006;71(3b):973–978. [PubMed] [Google Scholar]
- Filippovych K, Huinink H, van der Ven L, Adan OCG. Self healing biofilms for wood protection. In: van der Zwaag S, Brinkman E, editors. Self healing materials - pioneering research in the Netherlands. IOS Press; 2015. pp. 181–185. [Google Scholar]
- Filippovych K, Huinink H, van der Ven L, Adan OCG (2016) Dynamics of biofilm formation on wood impregnated with vegetable oils. Paper presented at the International Research Group on Wood Protection (IRG-WP), Lisbon, Portugal, IRG/WP 16–40769, pp 14
- Finlay AR. Cellular communication and control of dimorphic behaviour in Aureobasidium pullulans. Trans Br Mycol Soc. 1987;89(2):227–233. doi: 10.1016/S0007-1536(87)80157-2. [DOI] [Google Scholar]
- Fischer H, Erdmann S, Holler E. An unusual polyanion from Physarum polycephalum that inhibits homologous DNA-polymerase α in vitro. Biochemistry. 1989;28(12):5219–5226. doi: 10.1021/bi00438a045. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Neu TR, Wingender J. The perfect slime: microbial extracellular polymeric substances (EPS) London, United Kingdom: IWA Publishing; 2017. [Google Scholar]
- Galli V, Romboli Y, Barbato D, Mari E, Venturi M, Guerrini S, Granchi L. Indigenous Aureobasidium pullulans strains as biocontrol agents of botrytis cinerea on grape berries. Sustainability. 2021;13(16):9389. doi: 10.3390/su13169389. [DOI] [Google Scholar]
- Garay LA, Sitepu IR, Cajka T, Xu J, Teh HE, German JB, Pan Z, Dungan SR, Block DE, Boundy-Mills KL. Extracellular fungal polyol lipids: a new class of potential high value lipids. Biotechnol Adv. 2018;36(2):397–414. doi: 10.1016/j.biotechadv.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Gašparič MB, Lenassi M, Gostinčar C, Rotter A, Plemenitaš A, Gunde-Cimerman N, Gruden K, Žel J. Insertion of a specific fungal 3′-phosphoadenosine-5′-phosphatase motif into a plant homologue improves halotolerance and drought tolerance of plants. PLoS ONE. 2013;8(12):e81872. doi: 10.1371/journal.pone.0081872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissdoerfer M, Savaget P, Bocken NMP, Hultink EJ. The circular economy - a new sustainability paradigm? J Clean Prod. 2017;143:757–768. doi: 10.1016/j.jclepro.2016.12.048. [DOI] [Google Scholar]
- Gobakken LR, Høibø OA, Solheim H. Mould growth on paints with different surface structures when applied on wooden claddings exposed outdoors. Int Biodeterior Biodegradation. 2010;64(5):339–345. doi: 10.1016/j.ibiod.2009.11.005. [DOI] [Google Scholar]
- Göksungur Y, Uçan A, GÜvenÇ U. Production of pullulan from beet molasses and synthetic medium by Aureobasidium pullulans. Turk J Biol. 2004;28(1):23–30. [Google Scholar]
- Gostinčar C, Ohm RA, Kogej T, Sonjak S, Turk M, Zajc J, Zalar P, Grube M, Sun H, Han J, Sharma A, Chiniquy J, Ngan C, Lipzen A, Barry K, Grigoriev I, Gunde-Cimerman N. Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genom. 2014;15:549. doi: 10.1186/1471-2164-15-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostinčar C, Turk M, Zajc J, Gunde-Cimerman N. Fifty Aureobasidium pullulans genomes reveal a recombining polyextremotolerant generalist. Environ Microbiol. 2019;21(10):3638–3652. doi: 10.1111/1462-2920.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostinčar C, Sun X, Černoša A, Fang C, Gunde-Cimerman N, Song Z. Clonality, inbreeding, and hybridization in two extremotolerant black yeasts. GigaScience. 2022;11:giac095. doi: 10.1093/gigascience/giac095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruia A, Raba DN, Dumbrava D, Moldovan C, Bordean D, Mateescu C. Fatty acids composition and oil characteristics of linseed (Linum Usitatissimum L.) from Romania. J Agroaliment Processes Technol. 2012;18(2):136–40. [Google Scholar]
- Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitaš A. Hypersaline waters in salterns - natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol. 2000;32(3):235–240. doi: 10.1111/j.1574-6941.2000.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Hernandez VA, Evans PD. Melanization of the wood-staining fungus Aureobasidium pullulans in response to UV radiation. Wood Fiber Sci. 2015;47(1):120–124. [Google Scholar]
- Holmberg K. Natural surfactants. Curr Opin Colloid Interface Sci. 2001;6(2):148–159. doi: 10.1016/S1359-0294(01)00074-7. [DOI] [Google Scholar]
- Hu X, Shui Y, Hirano H, Kusano K, Guo W-Z, Fujino M, Li X-K. PD-L1 antibody enhanced β-glucan antitumor effects via blockade of the immune checkpoints in a melanoma model. Cancer Immunol Immunother. 2023;72(3):719–731. doi: 10.1007/s00262-022-03276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries Z, Seifert KA, Hirooka Y, Visagie CM. A new family and genus in Dothideales for Aureobasidium-like species isolated from house dust. IMA Fungus. 2017;8(2):299–315. doi: 10.5598/imafungus.2017.08.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar A, Adhapure NN, Sharma R, Sonawane M. Aureobasidium tremulum sp. nov.-Fungal Planet 907. Persoonia. 2019;42:330–331. [Google Scholar]
- Jia S-L, Ma Y, Chi Z, Liu G-L, Hu Z, Chi Z-M. Genome sequencing of a yeast-like fungal strain P6, a novel species of Aureobasidium spp.: insights into its taxonomy, evolution, and biotechnological potentials. Ann Microbiol. 2019;69(13):1475–1488. doi: 10.1007/s13213-019-01531-1. [DOI] [Google Scholar]
- Jiang H, Liu N-N, Liu G-L, Chi Z, Wang J-M, Zhang L-L, Chi Z-M. Melanin production by a yeast strain XJ5-1 of Aureobasidium melanogenum isolated from the Taklimakan desert and its role in the yeast survival in stress environments. Extremophiles. 2016;20(4):567–577. doi: 10.1007/s00792-016-0843-9. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xue SJ, Li YF, Liu GL, Chi ZM, Hu Z, Chi Z. Efficient transformation of sucrose into high pullulan concentrations by Aureobasidium melanogenum TN1-2 isolated from a natural honey. Food Chem. 2018;257:29–35. doi: 10.1016/j.foodchem.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Jiang N, Liang Y-M, Tian C-M. Aureobasidium pini sp nov from pine needle in China. Phytotaxa. 2019;402(4):199–206. doi: 10.11646/phytotaxa.00.0.0. [DOI] [Google Scholar]
- Jiang N, Fan X, Tian C. Identification and characterization of leaf-inhabiting fungi from Castanea plantations in China. J Fungi. 2021;7(1):64. doi: 10.3390/jof7010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal N, Khattar JS. Chapter 4 - Microbial polysaccharides in food industry. In: Grumezescu AM, Holban AM, editors. Handbook of food bioengineering, biopolymers for food design. Academic Press; 2018. pp. 95–123. [Google Scholar]
- Johnson L. Iron and siderophores in fungal host interactions. Mycol Res. 2008;112(2):170–183. doi: 10.1016/j.mycres.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Juita J, Dlugogorski BZ, Kennedy EM, Mackie JC. Low temperature oxidation of linseed oil: a review. Fire Sci Rev. 2012;1:3. doi: 10.1186/2193-0414-1-3. [DOI] [Google Scholar]
- Kachalkin A, Glushakova A, Streletskii R. Diversity of endophytic yeasts from agricultural fruits positive for phytohormone IAA production. Biotech. 2022;11(3):38. doi: 10.3390/biotech11030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B-K, Yang H-J, Choi N-S, Ahn K-H, Park C-S, Yoon B-D, Kim M-S. Production of pure β-glucan by Aureobasidium pullulans after pullulan synthetase gene disruption. Biotechnol Lett. 2010;32:137–142. doi: 10.1007/s10529-009-0127-x. [DOI] [PubMed] [Google Scholar]
- Kang X-X, Jia S-L, Wei X, Zhang M, Liu G-L, Hu Z, Chi Z, Chi Z-M. Liamocins biosynthesis, its regulation in Aureobasidium spp., and their bioactivities. Crit Rev Biotechnol. 2022;42(1):93–105. doi: 10.1080/07388551.2021.1931017. [DOI] [PubMed] [Google Scholar]
- Kayanna N, Suppavorasatit I, Bankeeree W, Lotrakul P, Punnapayak H, Prasongsuk S. Production of prebiotic aubasidan-like β-glucan from Aureobasidium thailandense NRRL 58543 and its potential as a functional food additive in gummy jelly. LWT. 2022;163:113617. doi: 10.1016/j.lwt.2022.113617. [DOI] [Google Scholar]
- Kim J-S, Ki D-W, Lee I-K, Yun B-S. Two novel biosurfactants produced by Aureobasidium pullulans A11211–4–57 from a fleabane, Erigeron annus (L) pers. J Antibiot. 2022;75(10):589–592. doi: 10.1038/s41429-022-00556-0. [DOI] [PubMed] [Google Scholar]
- Kim JH, Seo J, No H, Kuge T, Mori T, Kimoto H, Kim J-K. Low-molecular-weight β-1, 3–1, 6-glucan derived from Aureobasidium pullulans exhibits anticancer activity by inducing apoptosis in colorectal cancer cells. Biomedicines. 2023;11(2):529. doi: 10.3390/biomedicines11020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MN, Kupper KC. Biofilm production by Aureobasidium pullulans improves biocontrol against sour rot in citrus. Food Microbiol. 2018;69:1–10. doi: 10.1016/j.fm.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Kocková-Kratochvílová A, Černáková M, Sláviková E. Morphological changes during the life cycle of Aureobasidium pullulans (de Bary) Arnaud. Folia Microbiol. 1980;25(1):56–67. doi: 10.1007/BF02876398. [DOI] [PubMed] [Google Scholar]
- Kono H, Kondo N, Hirabayashi K, Ogata M, Totani K, Ikematsu S, Osada M. NMR spectroscopic structural characterization of a water-soluble β-(1→ 3, 1→ 6)-glucan from Aureobasidium pullulans. Carbohydr Polym. 2017;174:876–886. doi: 10.1016/j.carbpol.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Lamichhane JR, Osdaghi E, Behlau F, Köhl J, Jones JB, Aubertot J-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A Review Agron Sustain Dev. 2018;38:28. doi: 10.1007/s13593-018-0503-9. [DOI] [Google Scholar]
- Leathers TD. Color variants of Aureobasidium pullulans overproduce xylanase with extremely high specific activity. Appl Environ Microbiol. 1986;52(5):1026–1030. doi: 10.1128/aem.52.5.1026-1030.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers TD, Price NPJ, Bischoff KM, Manitchotpisit P, Skory CD. Production of novel types of antibacterial liamocins by diverse strains of Aureobasidium pullulans grown on different culture media. Biotechnol Lett. 2015;37(10):2075–2081. doi: 10.1007/s10529-015-1892-3. [DOI] [PubMed] [Google Scholar]
- Leathers TD, Skory CD, Price NPJ, Nunnally MS. Medium optimization for production of anti-streptococcal liamocins by Aureobasidium pullulans. Biocatal Agric Biotechnol. 2018;13:53–57. doi: 10.1016/j.bcab.2017.11.008. [DOI] [Google Scholar]
- Leathers TD, Rich JO, Bischoff KM, Skory CD, Nunnally MS. Inhibition of Streptococcus mutans and S. sobrinus biofilms by liamocins from Aureobasidium pullulans. Biotechnol Rep. 2019;21:e00300. doi: 10.1016/j.btre.2018.e00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BS, Holler E. Effects of culture conditions on β-poly (L-malate) production by Physarum polycephalum. Appl Microbiol Biotechnol. 1999;51:647–652. doi: 10.1007/s002530051445. [DOI] [Google Scholar]
- Lee D-H, Cho S-E, Park J-H, Seo S-T, Lee S-H, Lee SK. First report of Aureobasidium pullulans causing anthracnose on Paeonia suffruticosa in Korea. J Plant Pathol. 2019;101:1255. doi: 10.1007/s42161-019-00315-5. [DOI] [Google Scholar]
- Lee D-H, Cho S-E, Oh JY, Cho E-J, Kwon S. A novel species of Aureobasidium (Dothioraceae) recovered from Acer pseudosieboldianum in Korea. J Asia Pac Biodivers. 2021;14(4):657–661. doi: 10.1016/j.japb.2021.08.005. [DOI] [Google Scholar]
- Li B-x, Zhang N, Peng Q, Yin T, Guan F-f, Wang G-l, Li Y. Production of pigment-free pullulan by swollen cell in Aureobasidium pullulans NG which cell differentiation was affected by pH and nutrition. Appl Microbiol Biotechnol. 2009;84(2):293–300. doi: 10.1007/s00253-009-1955-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Chi Z, Wang G-Y, Wang Z-P, Liu G-L, Lee C-F, Ma Z-C, Chi Z-M. Taxonomy of Aureobasidium spp and biosynthesis and regulation of their extracellular polymers. Crit Rev Microbiol. 2015;41(2):228–237. doi: 10.3109/1040841X.2013.826176. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao S, Chen L, Zhou Q, Qiu J, Xin X, Zhang Y, Yuan W, Tian C, Yang J. High-level production of pullulan from high concentration of glucose by mutagenesis and adaptive laboratory evolution of Aureobasidium pullulans. Carbohydr Polym. 2023;302:120426. doi: 10.1016/j.carbpol.2022.120426. [DOI] [PubMed] [Google Scholar]
- Lie SK, Vestøl GI, Høibø O, Gobakken LR. Visual appearance of unpainted wood: mould coverage, lightness and uniformity. Int Wood Prod J. 2019;10(1):9–15. doi: 10.1080/20426445.2019.1569299. [DOI] [Google Scholar]
- Liu T, Zhu L, Zhang Z, Huang H, Zhang Z, Jiang L. Protective role of trehalose during radiation and heavy metal stress in Aureobasidium subglaciale F134. Sci Rep. 2017;7(1):17586. doi: 10.1038/s41598-017-15489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Zhang J, Zhang L, Diao M, Ling P, Wang F. Correlation between the synthesis of pullulan and melanin in Aureobasidium pullulans. Int J Biol Macromol. 2021;177:252–260. doi: 10.1016/j.ijbiomac.2021.02.108. [DOI] [PubMed] [Google Scholar]
- Liu R, Meng X, Mo C, Wei X, Ma A. Melanin of fungi: from classification to application. World J Microbiol Biotechnol. 2022;38(12):228. doi: 10.1007/s11274-022-03415-0. [DOI] [PubMed] [Google Scholar]
- Loque CP, Medeiros AO, Pellizzari FM, Oliveira EC, Rosa CA, Rosa LH. Fungal community associated with marine macroalgae from Antarctica. Polar Biol. 2010;33:641–648. doi: 10.1007/s00300-009-0740-0. [DOI] [Google Scholar]
- Lotrakul P, Unhapattaratitikul P, Seelanan T, Prasongsuk S, Punnapayak H. An aubasidan-like β-glucan produced by Aureobasidium pullulans in Thailand. Sci Asia. 2013;39(4):363–368. doi: 10.2306/scienceasia1513-1874.2013.39.363. [DOI] [Google Scholar]
- Lu D, Wen X, Liang J, Gu Z, Zhang X, Fan Y. A pH-sensitive nano drug delivery system derived from pullulan/doxorubicin conjugate. J Biomed Mater Res. 2009;89B(1):177–183. doi: 10.1002/jbm.b.31203. [DOI] [PubMed] [Google Scholar]
- Lugones LG, De Jong JF, De Vries OMH, Jalving R, Dijksterhuis J, Wösten HAB. The SC15 protein of Schizophyllum commune mediates formation of aerial hyphae and attachment in the absence of the SC3 hydrophobin. Mol Microbiol. 2004;53(2):707–716. doi: 10.1111/j.1365-2958.2004.04187.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang G-Y, Liu G-L, Wang Z-P, Chi Z-M. Overproduction of poly (β-malic acid)(PMA) from glucose by a novel Aureobasidium sp. P6 strain isolated from mangrove system. Appl Microbiol Biotechnol. 2013;97:8931–8939. doi: 10.1007/s00253-013-5150-0. [DOI] [PubMed] [Google Scholar]
- Maja MM, Ayano SF. The impact of population growth on natural resources and farmers’ capacity to adapt to climate change in low-income countries. Earth Syst Environ. 2021;5(2):271–283. doi: 10.1007/s41748-021-00209-6. [DOI] [Google Scholar]
- Manitchotpisit P, Leathers TD, Peterson SW, Kurtzman CP, Li X-L, Eveleigh DE, Lotrakul P, Prasongsuk S, Dunlap CA, Vermillion KE, Punnapayak H. Multilocus phylogenetic analyses, pullulan production and xylanase activity of tropical isolates of Aureobasidium pullulans. Mycol Res. 2009;113(10):1107–1120. doi: 10.1016/j.mycres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Manitchotpisit P, Price NPJ, Leathers TD, Punnapayak H. Heavy oils produced by Aureobasidium pullulans. Biotechnol Lett. 2011;33:1151–1157. doi: 10.1007/s10529-011-0548-1. [DOI] [PubMed] [Google Scholar]
- Mari M, Martini C, Guidarelli M, Neri F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Aureobasidium pullulans strains. Biol Control. 2012;60(2):132–140. doi: 10.1016/j.biocontrol.2011.10.013. [DOI] [Google Scholar]
- Mari M, Martini C, Spadoni A, Rouissi W, Bertolini P. Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol Technol. 2012;73:56–62. doi: 10.1016/j.postharvbio.2012.05.014. [DOI] [Google Scholar]
- Meneses DP, Gudiña EJ, Fernandes F, Gonçalves LR, Rodrigues LR, Rodrigues S. The yeast-like fungus Aureobasidium thailandense LB01 produces a new biosurfactant using olive oil mill wastewater as an inducer. Microbiol Res. 2017;204:40–47. doi: 10.1016/j.micres.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Merín MG, Mendoza LM, Farías ME, De Ambrosini VIM. Isolation and selection of yeasts from wine grape ecosystem secreting cold-active pectinolytic activity. Int J Food Microbiol. 2011;147(2):144–148. doi: 10.1016/j.ijfoodmicro.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Moriya N, Moriya Y, Nomura H, Kusano K, Asada Y, Uchiyama H, Park EY, Okabe M. Improved β-glucan yield using an Aureobasidium pullulans M-2 mutant strain in a 200-L pilot scale fermentor targeting industrial mass production. Biotechnol Bioprocess Eng. 2013;18:1083–1089. doi: 10.1007/s12257-013-0516-9. [DOI] [Google Scholar]
- Morrell JJ. Protection of wood: a global perspective on the future. In: Pandey KK, Ramakantha V, Chauhan SS, Arun Kumar AN, editors. Wood is good. Singapore: Springer; 2017. pp. 213–226. [Google Scholar]
- Mujdeci GN. Natural melanin synthesized by Aureobasidium pullulans using food wastes and its characterization. Appl Food Biotechnol. 2021;8(4):307–318. doi: 10.22037/afb.v8i4.34599. [DOI] [Google Scholar]
- Muthusamy S, Anandharaj SJ, Kumar PS, Meganathan Y, Vo D-VN, Vaidyanathan VK, Muthusamy S. Microbial pullulan for food, biomedicine, cosmetic, and water treatment: a review. Environ Chem Lett. 2022;20:3199–3234. doi: 10.1007/s10311-022-01460-7. [DOI] [Google Scholar]
- Nagata N, Nakahara T, Tabuchi T, Morita R, Brewer JR, Fujishige S. Characterization of poly (β-L-malic acid) produced by Aureobasidium sp. A-91. Polym J. 1993;25:585–592. doi: 10.1295/polymj.25.585. [DOI] [Google Scholar]
- Nakashio S, Tsuji K, Toyota N, Fujita F (1976) Novel cosmetics containing pullulan. US Patent 3,972,997 (US3972997A)
- Nasr S, Mohammadimehr M, Geranpayeh Vaghei M, Amoozegar MA, Shahzadeh Fazeli SA. Aureobasidium mangrovei sp. nov., an ascomycetous species recovered from Hara protected forests in the Persian Gulf. Iran Antonie Van Leeuwenhoek. 2018;111:1697–1705. doi: 10.1007/s10482-018-1059-z. [DOI] [PubMed] [Google Scholar]
- Nguyen PQ, Courchesne NMD, Duraj-Thatte A, Praveschotinunt P, Joshi NS. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. Adv Mater. 2018;30(19):1704847. doi: 10.1002/adma.201704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No H, Kim J, Seo C-R, Kim JH, Kuge T, Mori T, Kimoto H, Kim J-K. Anti-inflammatory effects of β-1, 3–1, 6-glucan derived from black yeast Aureobasidium pullulans in RAW264.7 cells. Int J Biol Macromol. 2021;193A:592–600. doi: 10.1016/j.ijbiomac.2021.10.065. [DOI] [PubMed] [Google Scholar]
- Onetto CA, Schmidt SA, Roach MJ, Borneman AR. Comparative genome analysis proposes three new Aureobasidium species isolated from grape juice. FEMS Yeast Res. 2020;20(6):foaa052. doi: 10.1093/femsyr/foaa052. [DOI] [PubMed] [Google Scholar]
- Pande A, Ghate N. Three new Hyphomycetes from paper factory effluent. Biovigyanam J Life Sci. 1985;11(1):113–114. [Google Scholar]
- Park D. Population density and yeast mycelial dimorphism in Aureobasidium pullulans. Trans Br Mycol Soc. 1984;82(1):39–44. doi: 10.1016/S0007-1536(84)80209-0. [DOI] [Google Scholar]
- Pechak DG, Crang RE. An analysis of Aureobasidium pullulans developmental stages by means of scanning electron microscopy. Mycologia. 1977;69(4):783–792. doi: 10.1080/00275514.1977.12020123. [DOI] [Google Scholar]
- Peeters LHM, Huinink HP, Voogt B, Adan OCG. Oil type and cross-linking influence growth of Aureobasidium melanogenum on vegetable oils as a single carbon source. MicrobiologyOpen. 2018;7(6):e00605. doi: 10.1002/mbo3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SW, Manitchotpisit P, Leathers TD. Aureobasidium thailandense sp. nov. isolated from leaves and wooden surfaces. Int J Syst Evol Microbiol. 2013;63(Pt_2):790–795. doi: 10.1099/ijs.0.047613-0. [DOI] [PubMed] [Google Scholar]
- Poohphajai F, Sandak J, Sailer M, Rautkari L, Belt T, Sandak A. Bioinspired living coating system in service: evaluation of the wood protected with biofinish during one-year natural weathering. Coatings. 2021;11(6):701. doi: 10.3390/coatings11060701. [DOI] [Google Scholar]
- Pouliot JM, Walton I, Nolen-Parkhouse M, Abu-Lail LI, Camesano TA. Adhesion of Aureobasidium pullulans is controlled by uronic acid based polymers and pullulan. Biomacromol. 2005;6(2):1122–1131. doi: 10.1021/bm0492935. [DOI] [PubMed] [Google Scholar]
- Prajapati VD, Jani GK, Khanda SM. Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym. 2013;95(1):540–549. doi: 10.1016/j.carbpol.2013.02.082. [DOI] [PubMed] [Google Scholar]
- Price NPJ, Bischoff KM, Leathers TD, Cossé AA, Manitchotpisit P. Polyols, not sugars, determine the structural diversity of anti-streptococcal liamocins produced by Aureobasidium pullulans strain NRRL 50380. J Antibiot. 2017;70:136–141. doi: 10.1038/ja.2016.92. [DOI] [PubMed] [Google Scholar]
- Qi C-Y, Jia S-L, Liu G-L, Chen L, Wei X, Hu Z, Chi Z-M, Chi Z. Polymalate (PMA) biosynthesis and its molecular regulation in Aureobasidium spp. Int J Biol Macromol. 2021;174:512–518. doi: 10.1016/j.ijbiomac.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Wanyan Q, Xie W, Wang Z, Chen M, Wu D. Green and biomass-derived materials with controllable shape memory transition temperatures based on cross-linked Poly (l-malic acid) Polymer. 2019;180:121733. doi: 10.1016/j.polymer.2019.121733. [DOI] [Google Scholar]
- Rali T, Wossa SW, Leach DN. Comparative chemical analysis of the essential oil constituents in the bark, heartwood and fruits of Cryptocarya massoy (Oken) Kosterm. (Lauraceae) from Papua New Guinea. Molecules. 2007;12(2):149–154. doi: 10.3390/12020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley AW. Tectacervulus mahoniae, Kabatina mahoniae, and Selenophoma mahoniae, three new fungi on Mahonia repens. Mycotaxon. 1992;43:437–452. [Google Scholar]
- Ramos S, García Acha I. A vegetative cycle of Pullularia pullulans. Trans Br Mycol Soc. 1975;64(1):129–135. doi: 10.1016/S0007-1536(75)80083-0. [DOI] [Google Scholar]
- Reddy CK, Sivapriya TVS, Kumar UA, Ramalimgam C. Optimization of food acidulant to enhance the organoleptic property in fruit jellies. J Food Process Technol. 2016;7:1–3. doi: 10.4172/2157-7110.1000635. [DOI] [Google Scholar]
- Rensink S, Sailer MF, Bulthuis RJH, Oostra MAR (2020) Application of a bio-based coating for wood as a construction material: fire retardancy and impact on performance characteristics. In: Makovicka Osvaldova L, Markert F, Zelinka S (eds) Wood & Fire Safety (WFS). Springer, Cham, Switzerland, pp 90–96
- Richardson M, Pitkäranta M (2011) Chapter 6. Aureobasidium. In: Liu D (ed) Molecular detection of human fungal pathogens. CRC Press, Taylor & Francis Group, United States, pp 37–48
- Rodrigo-Navarro A, Sankaran S, Dalby MJ, del Campo A, Salmeron-Sanchez M. Engineered Living Biomaterials Nat Rev Mater. 2021;6(12):1175–1190. doi: 10.1038/s41578-021-00350-8. [DOI] [Google Scholar]
- Roy S, Rhim J-W. New insight into melanin for food packaging and biotechnology applications. Crit Rev Food Sci Nutr. 2022;62(17):4629–4655. doi: 10.1080/10408398.2021.1878097. [DOI] [PubMed] [Google Scholar]
- Sailer MF, van Nieuwenhuijzen EJ, Knol W. Forming of a functional biofilm on wood surfaces. Ecol Eng. 2010;36(2):163–167. doi: 10.1016/j.ecoleng.2009.02.004. [DOI] [Google Scholar]
- Sałek K, Euston SR, Janek T. Phase behaviour, functionality, and physicochemical characteristics of glycolipid surfactants of microbial origin. Front Bioeng Biotechnol. 2022;10:816613. doi: 10.3389/fbioe.2022.816613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2019) Food and indoor fungi. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands
- Sandak A. Engineered living materials for sustainable and resilient architecture. Nat Rev Mater. 2023;8:357–359. doi: 10.1038/s41578-023-00554-0. [DOI] [Google Scholar]
- Schena L, Ippolito A, Zahavi T, Cohen L, Nigro F, Droby S. Genetic diversity and biocontrol activity of Aureobasidium pullulans isolates against postharvest rots. Postharvest Biol Technol. 1999;17(3):189–199. doi: 10.1016/S0925-5214(99)00036-8. [DOI] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98(6):1041–1052. doi: 10.1080/15572536.2006.11832632. [DOI] [PubMed] [Google Scholar]
- Sheoran SK, Dubey KK, Tiwari DP, Singh BP. Directive production of pullulan by altering cheap source of carbons and nitrogen at 5 L bioreactor level. Int Sch Res Notices. 2012;2012:867198. doi: 10.5402/2012/867198. [DOI] [Google Scholar]
- Singh RS, Saini GK. Biosynthesis of pullulan and its applications in food and pharmaceutical industry. In: Satyanarayana T, Johri B, editors. Microorganisms in Sustainable Agriculture and Biotechnology. Dordrecht, The Netherlands: Springer; 2012. pp. 509–553. [Google Scholar]
- Singh RS, Saini GK, Kennedy JF. Pullulan: Microbial sources, production and applications. Carbohydr Polym. 2008;73(4):515–531. doi: 10.1016/j.carbpol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Singh R, Gaur R, Bansal S, Biswas P, Pandey PK, Jamal F, Tiwari S, Gaur MK. Aureobasidium pullulans - an industrially important pullulan producing black yeast. Int J Curr Microbiol Appl Sci. 2015;4(10):605–622. [Google Scholar]
- Singh RS, Kaur N, Kennedy JF. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr Polym. 2019;217:46–57. doi: 10.1016/j.carbpol.2019.04.050. [DOI] [PubMed] [Google Scholar]
- Singh RS, Kaur N, Hassan M, Kennedy JF. Pullulan in biomedical research and development - a review. Int J Biol Macromol. 2021;166:694–706. doi: 10.1016/j.ijbiomac.2020.10.227. [DOI] [PubMed] [Google Scholar]
- Singh RS, Kaur N, Singh D, Purewal SS, Kennedy JF. Pullulan in pharmaceutical and cosmeceutical formulations: a review. Int J Biol Macromol. 2023;231:123353. doi: 10.1016/j.ijbiomac.2023.123353. [DOI] [PubMed] [Google Scholar]
- Song S-S, Tian B-C, Chen H, Chi Z, Liu G-L, Chi Z-M. Transformation of corncob-derived xylose into intracellular lipid by engineered strain of Aureobasidium melanogenum P10 for biodiesel production. Renew Energ. 2022;200:1211–1222. doi: 10.1016/j.renene.2022.10.050. [DOI] [Google Scholar]
- Srubar WV., III Engineered living materials: taxonomies and emerging trends. Trends Biotechnol. 2021;39(6):574–583. doi: 10.1016/j.tibtech.2020.10.009. [DOI] [PubMed] [Google Scholar]
- Stenbæk J (2015) Bio-sustainable control of the blue stain fungi Aureobasidium pullulans on exterior wood coatings. Dissertation, University of Copenhagen
- Sun J, Xiao S, Lu Y, Tu G, Xue C, Chen J. Isolation, identification and biological characteristics of Aureobasidium zeae in Liaoning Province. Acta Phytophylacica Sinica. 2015;42(6):927–934. [Google Scholar]
- Tanioka A, Tanabe K, Hosono A, Kawakami H, Kaminogawa S, Tsubaki K, Hachimura S. Enhancement of intestinal immune function in mice by β-D-Glucan from Aureobasidium Pullulans ADK-34. Scand J Immunol. 2013;78(1):61–68. doi: 10.1111/sji.12067. [DOI] [PubMed] [Google Scholar]
- Teertstra WR, Deelstra HJ, Vranes M, Bohlmann R, Kahmann R, Kamper J, Wosten HAB. Repellents have functionally replaced hydrophobins in mediating attachment to a hydrophobic surface and in formation of hydrophobic aerial hyphae in Ustilago maydis. Microbiology. 2006;152(12):3607–3612. doi: 10.1099/mic.0.29034-0. [DOI] [PubMed] [Google Scholar]
- Thambugala KM, Ariyawansa HA, Li Y-M, Boonmee S, Hongsanan S, Tian Q, Singtripop C, Bhat DJ, Camporesi E, Jayawardena R. Dothideales Fungal Divers. 2014;68:105–158. doi: 10.1007/s13225-014-0303-8. [DOI] [Google Scholar]
- Thirumavalavan K, Manikkadan TR, Dhanasekar R. Pullulan production from coconut by-products by Aureobasidium pullulans. Afr J Biotechnol. 2009;8(2):254–258. [Google Scholar]
- Toop TA, Ward S, Oldfield T, Hull M, Kirby ME, Theodorou MK. AgroCycle - developing a circular economy in agriculture. Energy Procedia. 2017;123:76–80. doi: 10.1016/j.egypro.2017.07.269. [DOI] [Google Scholar]
- Torzilli AP. Tolerance to high temperature and salt stress by a salt marsh isolate of Aureobasidium pullulans. Mycologia. 1997;89(5):786–792. doi: 10.1080/00275514.1997.12026845. [DOI] [PubMed] [Google Scholar]
- Tsujisaka Y, Mitsuhashi M (1993) Chapter 16 - Pullulan. In: Whistler RL, BeMiller JN (eds) Industrial gums. Polysaccharides and their derivatives (3rd edition). Academic Press, San Diego, United States, 447–460
- van Nieuwenhuijzen EJ. Aureobasidium. In: Batt CA, Tortorello ML, editors. Encyclopedia of food microbiology. 2. Oxford, United Kingdom: Academic Press; 2014. pp. 105–109. [Google Scholar]
- van Nieuwenhuijzen EJ, Houbraken JAMP, Meijer M, Adan OCG, Samson RA. Aureobasidium melanogenum: a native of dark biofinishes on oil treated wood. Antonie Van Leeuwenhoek. 2016;109:661–683. doi: 10.1007/s10482-016-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhuijzen EJ, Houbraken JAMP, Punt PJ, Roeselers G, Adan OCG, Samson RA. The fungal composition of natural biofinishes on oil-treated wood. Fungal Biol Biotechnol. 2017;4:2. doi: 10.1186/s40694-017-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhuijzen EJ, Sailer MF, van den Heuvel ER, Rensink S, Adan OCG, Samson RA. Vegetable oils as carbon and energy source for Aureobasidium melanogenum in batch cultivation. MicrobiologyOpen. 2019;8(6):e00764. doi: 10.1002/mbo3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowska U, Głowacka K, Mikołajczyk W, Kucharska K. Biofilm of Aureobasidium pullulans var. pullulans on winter wheat kernels and its effect on other microorganisms. Microbiology. 2016;85:523–530. doi: 10.1134/S0026261716050192. [DOI] [Google Scholar]
- Wang WL, Chi ZM, Chi Z, Li J, Wang XH. Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresour Technol. 2009;100(9):2639–2641. doi: 10.1016/j.biortech.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Wang C-L, Li Y, Xin F-H, Liu Y-Y, Chi Z-M. Evaluation of single cell oil from Aureobasidium pullulans var melanogenum P10 isolated from mangrove ecosystems for biodiesel production. Process Biochem. 2014;49(5):725–731. doi: 10.1016/j.procbio.2014.02.017. [DOI] [Google Scholar]
- Wang Y-K, Chi Z, Zhou H-X, Liu G-L, Chi Z-M. Enhanced production of Ca 2+-polymalate (PMA) with high molecular mass by Aureobasidium pullulans var pullulans MCW. Microb Cell Factories. 2015;14:115. doi: 10.1186/s12934-015-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-B, Jiang N, Tu Y, Zhu Y-Q, Xue H, Li Y. Aureobasidium aerium (Saccotheciaceae, Dothideales), a new yeast-like fungus from the air in Beijing. China. Phytotaxa. 2022;544(2):185–192. doi: 10.11646/PHYTOTAXA.544.2.4. [DOI] [Google Scholar]
- Wang P, Jia S-L, Liu G-L, Chi Z, Chi Z-M. Aureobasidium spp. and their applications in biotechnology. Process Biochem. 2022;116:72–83. doi: 10.1016/j.procbio.2022.03.006. [DOI] [Google Scholar]
- Wickerham LJ, Kurtzman CP. Synergistic color variants of Aureobasidium pullulans. Mycologia. 1975;67(2):342–361. doi: 10.1080/00275514.1975.12019756. [DOI] [PubMed] [Google Scholar]
- Williams RS, Feist WC (1999) Water repellents and water-repellent preservatives for wood. General Technical Report FPL-GTR-109, Unites States Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, WI, USA, pp 12
- Woody ST, Spear RN, Nordheim EV, Ives AR, Andrews JH. Single-leaf resolution of the temporal population dynamics of Aureobasidium pullulans on apple leaves. Appl Environ Microbiol. 2003;69(8):4892–4900. doi: 10.1128/AEM.69.8.4892-4900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten HAB, Wessels JG. Hydrophobins, from molecular structure to multiple functions in fungal development. Mycoscience. 1997;38:363–374. doi: 10.1007/BF02464099. [DOI] [Google Scholar]
- Wösten HAB, Schuren FH, Wessels JG. Interfacial self-assembly of a hydrophobin into an amphipathic protein membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 1994;13:5848–5854. doi: 10.1002/j.1460-2075.1994.tb06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Feng Z, Wang M, Wang Q. Proposal of four new Aureobasidium species for exopolysaccharide production. J Fungi. 2023;9(4):447. doi: 10.3390/jof9040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Ku S-K, Kwon Y-S. Effect of β-glucan from Aureobasidium on dermal wound healing in diabetic C57BL/KsJ-db/db mouse model. J Biomed Transl Res. 2015;16(4):140–145. doi: 10.12729/jbr.2015.16.4.140. [DOI] [Google Scholar]
- Yurlova NA, De Hoog GS. A new variety of Aureobasidium pullulans characterized by exopolysaccharide structure, nutritional physiology and molecular features. Antonie Van Leeuwenhoek. 1997;72:141–147. doi: 10.1023/A:1000212003810. [DOI] [PubMed] [Google Scholar]
- Zajc J, Cernosa A, Di Francesco A, Casteria R, De Curtis F, Lima G, Badri H, Jijakli H, Ippolito A, Gostincar C, Zalar P, Gunde-Cimerman N, Wojciech JJ. Characterization of Aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genom Biol. 2020;10:163. doi: 10.35248/2165-8056.20.10.161. [DOI] [Google Scholar]
- Zalar P, Gostincar C, de Hoog GS, Ursic V, Sudhadham M, Gunde-Cimerman N. Redefinition of Aureobasidium pullulans and its varieties. Stud Mycol. 2008;61(1):21–38. doi: 10.3114/sim.2008.61.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Johnson KB, Mukhtar S, Nason S, Huntley R, Millet F, Yang C-H, Amine Hassani M, Zuverza-Mena N, Sundin GW. Aureobasidium pullulans from the fire blight biocontrol product, Blossom Protect, induces host resistance in apple flowers. Phytopathology. 2023;113(7):1192–1201. doi: 10.1094/PHYTO-12-22-0452-R. [DOI] [PubMed] [Google Scholar]
- Zhang Q, White JF. Bioprospecting desert plants for endophytic and biostimulant microbes: a strategy for enhancing agricultural production in a hotter, drier future. Biology. 2021;10(10):961. doi: 10.3390/biology10100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li F, Yi J, Gu C, Fan L, Qiao Y, Tao Y, Cheng C, Wu H. Folate-decorated maleilated pullulan–doxorubicin conjugate for active tumor-targeted drug delivery. Eur J Pharm Sci. 2011;42(5):517–526. doi: 10.1016/j.ejps.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng J, Wang P, Xia J, Li X, Zou X. CRISPR/Cas9-mediated efficient genome editing via protoplast-based transformation in yeast-like fungus Aureobasidium pullulans. Gene. 2019;709:8–16. doi: 10.1016/j.gene.2019.04.079. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gao Z-C, Chi Z, Wang Z, Liu G-L, Li X-F, Hu Z, Chi Z-M. Massoia lactone displays strong antifungal property against many crop pathogens and its potential application. Microb Ecol. 2021;84:376–390. doi: 10.1007/s00248-021-01885-7. [DOI] [PubMed] [Google Scholar]
- Zou X, Zhou Y, Yang ST. Production of polymalic acid and malic acid by Aureobasidium pullulans fermentation and acid hydrolysis. Biotechnol Bioeng. 2013;110(8):2105–2113. doi: 10.1002/bit.24876. [DOI] [PubMed] [Google Scholar]
- Zou X, Cheng C, Feng J, Song X, Lin M, Yang S-T. Biosynthesis of polymalic acid in fermentation: advances and prospects for industrial application. Crit Rev Biotechnol. 2019;39(3):408–421. doi: 10.1080/07388551.2019.1571008. [DOI] [PubMed] [Google Scholar]
- Zupancic J, Novak Babic M, Zalar P, Gunde-Cimerman N. The black yeast Exophiala dermatitidis and other selected opportunistic human fungal pathogens spread from dishwashers to kitchens. PLoS ONE. 2016;11(2):e0148166. doi: 10.1371/journal.pone.0148166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.