Abstract
Characterizing bedside oculomotor deficits is a critical factor in defining the clinical presentation of hereditary ataxias. Quantitative assessments are increasingly available and have significant advantages, including comparability over time, reduced examiner dependency, and sensitivity to subtle changes. To delineate the potential of quantitative oculomotor assessments as digital-motor outcome measures for clinical trials in ataxia, we searched MEDLINE for articles reporting on quantitative eye movement recordings in genetically confirmed or suspected hereditary ataxias, asking which paradigms are most promising for capturing disease progression and treatment response. Eighty-nine manuscripts identified reported on 1541 patients, including spinocerebellar ataxias (SCA2, n = 421), SCA3 (n = 268), SCA6 (n = 117), other SCAs (n = 97), Friedreich ataxia (FRDA, n = 178), Niemann-Pick disease type C (NPC, n = 57), and ataxia-telangiectasia (n = 85) as largest cohorts. Whereas most studies reported discriminatory power of oculomotor assessments in diagnostics, few explored their value for monitoring genotype-specific disease progression (n = 2; SCA2) or treatment response (n = 8; SCA2, FRDA, NPC, ataxia-telangiectasia, episodic-ataxia 4). Oculomotor parameters correlated with disease severity measures including clinical scores (n = 18 studies (SARA: n = 9)), chronological measures (e.g., age, disease duration, time-to-symptom onset; n = 17), genetic stratification (n = 9), and imaging measures of atrophy (n = 5). Recurrent correlations across many ataxias (SCA2/3/17, FRDA, NPC) suggest saccadic eye movements as potentially generic quantitative oculomotor outcome. Recommendation of other paradigms was limited by the scarcity of cross-validating correlations, except saccadic intrusions (FRDA), pursuit eye movements (SCA17), and quantitative head-impulse testing (SCA3/6). This work aids in understanding the current knowledge of quantitative oculomotor parameters in hereditary ataxias, and identifies gaps for validation as potential trial outcome measures in specific ataxia genotypes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12311-023-01514-8.
Keywords: Oculomotor, Vestibular, Eye movement recordings, Hereditary ataxia, Systematic review, Recommendations
Introduction
Eye movement abnormalities are common in hereditary ataxia [1–3]. The characterization of oculomotor and vestibular deficits has contributed significantly to disease and gene discovery by contributing to the delineation of novel disease phenotypes, which has in turn informed our understanding of the clinical presentations of specific hereditary ataxias [4–7]. While oculomotor testing may be performed through expert visual interpretation at the bedside, objective and quantitative assessments have significant advantages, including comparability over time, reduced examiner dependency, and inter-/intra-individual variability along with increased sensitivity. Accordingly, quantitative oculomotor assessments have been sensitive enough to capture within-subject longitudinal disease progression and early signs of efficacy for future interventions [8] and alterations in at-risk carriers at the pre-ataxia stage [9] and to distinguish between ataxia diagnoses [10]. Together with the increasing availability of video-oculographic (VOG) devices for multicenter applicability, this sensitivity of quantitative oculomotor assessments makes them promising digital motor biomarkers for emerging treatment trials in ataxia.
Among the broad range of oculomotor alterations in ataxia, some are generic for cerebellar ataxia (e.g., slow saccades) [11], while others are more specific for particular hereditary ataxias (e.g., an impaired vestibulo-ocular reflex in RFC1-ataxia [12] or SCA6 [13]). An increasing number of studies are investigating quantitative oculomotor parameters in ataxia, and their findings have been partially reviewed at the level of individual genetic ataxias [14], or at a high level across large diagnostic groups [15]. With mechanistic treatment trials on the horizon, their validation as potential disease-specific outcome measures now requires the implementation of quantitative oculomotor assessments in genetically stratified longitudinal natural history studies and eventually in parallel with clinical outcome assessments and other biomarker modalities.
In the companion paper, we reviewed the published spectrum of quantitative oculomotor assessments, and have provided consensus recommendations and technical guidelines for a core set of oculomotor paradigms and parameters for validation in future clinical studies, across ataxia genotypes (Garces et al. 2022a submitted). This study is a complementary review, focusing on studies reporting on quantitative oculomotor assessments for specific ataxia genotypes. It provides disease-specific recommendations for selecting the most promising quantitative oculomotor parameters as of yet, and integrates available cross-validations with other measures of disease severity to facilitate future validation in genotype-specific natural history or interventional studies. To facilitate this validation process, we systematically review the patterns of oculomotor and vestibular abnormalities across hereditary ataxias, and how they relate to other measures of disease severity (such as clinical scores, disease duration, and/or imaging). A particular focus for this work is on the responsiveness to disease progression and treatment effects.
Material and Methods
Data Sources and Searches
We searched MEDLINE (via PubMed) for articles using text words and controlled vocabulary terms related to research studies reporting on oculomotor and/or vestibular function in hereditary ataxia. A detailed description of the search strategy can be found in Appendix 1. Our search was updated through May 13, 2021.
Study Selection and Quality Rating
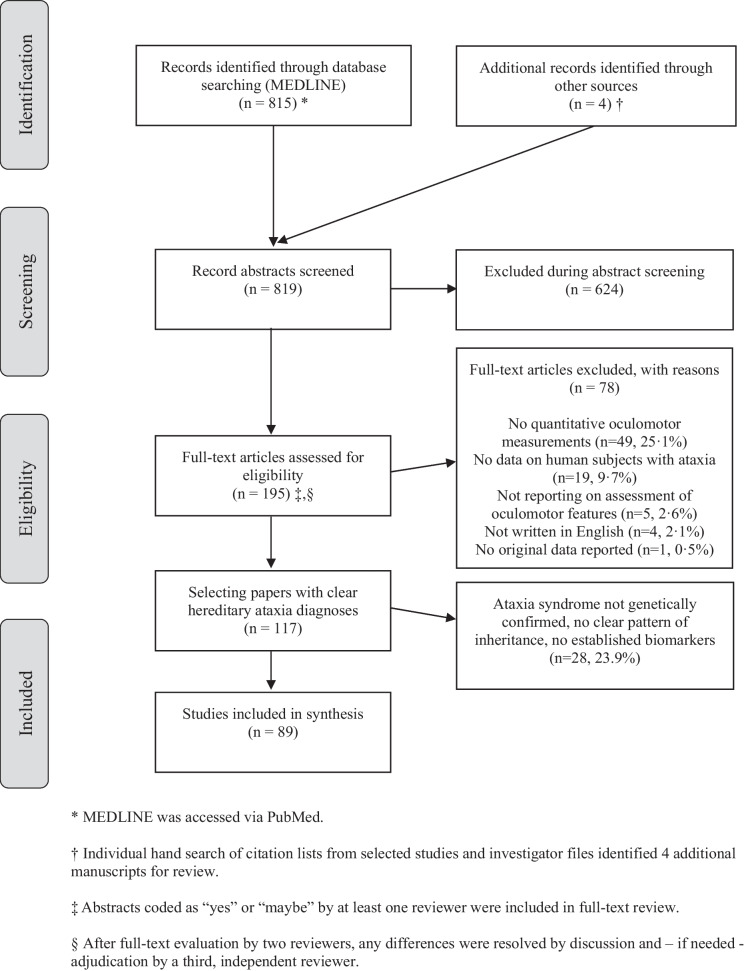
Articles were selected by two independent raters (PG and AAT) using pre-determined inclusion criteria and a structured protocol (see Appendix 1). Our focus was on studies reporting on quantitative oculomotor and/or vestibular testing in hereditary ataxia. For this review, we included only studies with well-defined patient cohorts: (a) with genetically confirmed hereditary ataxia or (b) (if no genetic testing was available) ataxias with either a positive family history with a clear pattern of inheritance (autosomal dominant, autosomal recessive, X-linked recessive) or (c) with established and specific diagnostic biomarkers (as e.g., alpha-fetoprotein (AFP) in ataxia telangiectasia and ataxia with ocular motor apraxia 2 [16]), as illustrated in the PRISMA flow chart (Fig. 1). We calculated inter-rater agreement on full-text inclusion using Cohen’s kappa.
Fig. 1.
Citation search and selection flow diagram
A quality rating of included studies was performed based on eight predefined quality criteria covering items related to (i) study cohort, (ii) data acquisition, and (iii) data analysis (see Appendix 2). An overall study quality rating (high, moderate, low) was derived from this quality assessment.
Data Extraction, Data Synthesis, and Statistical Analysis
Information extracted from each eligible article included the type of study conducted, sample size and disease cohort studied, the oculomotor paradigms applied, and the recording device(s) used. We also searched for correlations between oculomotor parameters and anchor measures of disease severity such as clinical parameters (clinical scales, various questionnaires, disease duration, age of onset), biological determinants (e.g., CAG repeat length in polyQ diseases), and/or (MR) imaging markers. This study reports in accordance with PRISMA guidelines [17].
From each publication, we retrieved key information on the type of oculomotor paradigms performed and rated their potential responsiveness as outcome measures along four dimensions. Specifically, we assessed whether oculomotor parameters (i) discriminated significantly from healthy control groups, (ii) correlated to anchor measures of disease severity, (iii) captured progression over time (longitudinal observational studies), and/or (iv) captured modulation of progression by an intervention (clinical treatment trials). Correlations with anchor measures were classified for studies reporting Pearson correlation coefficients or Spearman correlation coefficients (strong: r ≥ 0.7; moderate: r ≥ 0.4 and r < 0.7; weak: r ≥ 0.1 and r < 0.4) [18]. For each oculomotor paradigm, a level of recommendation was graded according to the four criteria described above: Paradigms with high (or even disease-specific) discriminatory power and strong correlations with any measure of disease severity, or sensitivity to change (significant changes in observational or interventional studies) were recommended as “priority 1” for a given disease. Paradigms with low discriminatory power or—if any correlation analyses were performed—mild-to-moderate correlations with disease severity were considered “priority 2.” Paradigms without discriminatory power between patients and controls were “not recommended,” irrespective of correlations with disease severity measures. Correlations that were derived from pooled patient populations (i.e., merging several hereditary ataxias)—as reported in two publications [10, 19]—were not considered.
We focused on those five oculomotor domains that we have recommended for use in hereditary ataxia in the companion paper (Garces et al. 2022a, submitted), i.e., (i) pursuit eye movements (PEM), (ii) saccadic eye movements (SEM), (iii) fixation (including spontaneous nystagmus (SN) and saccadic intrusions (SI), (iv) eccentric gaze holding (looking for gaze-evoked nystagmus (GEN)), and (v) rotational vestibulo-ocular reflex (as measured with the quantitative head-impulse test (qHIT)).
Results
Overview of Studies
Our search identified 819 citations, of which 624 (76.2%) were excluded at the abstract level and 78 (9.5%) at the full-text manuscript level. From the 117 studies included after the full-text review, 89 studies (representing 10.9% of all manuscripts) reported on patients with either genetically confirmed hereditary ataxia or (if no genetic testing was available) ataxia with either a positive family history with a clear pattern of inheritance (autosomal dominant, autosomal recessive, X-linked recessive) or with established and specific biomarkers. Details on the study design and on specific disorders included are listed in Table 1. The overall study quality with respect to the predefined criteria (see Appendix 2) was judged “high” in 22 studies, “moderate” in 25 studies, and “low” in 42 studies (see Appendix 3, Table S1 for details).
Table 1.
Overview of study design and clinical population across studies
| Studies (n) | Patients (n) | |
|---|---|---|
| Gender | ||
| Female | 60 | 565 |
| Male | 59 | 614 |
| Unclear | 29 | 362 |
| Total | 89 | 1541 |
| Study design—timeline | ||
| Prospective | 84 | 1503 |
| Retrospective | 4 | 29 |
| Unclear | 1 | 9 |
| Study design—location | ||
| Monocentric | 83 | 1492 |
| Multicentric | 6 | 49 |
| Study type | ||
| Case series | 20 | 195 |
| Case–control studies | 57 | 1095 |
| Single-case reports | 2 | 2 |
| Observational studies | 2 | 112 |
| Randomized controlled treatment studies | 2 | 71 |
| Non-randomized treatment studies | 6 | 66 |
| Included disorders | Genetically confirmed | Based on positive family history/biochemical markers |
| Friedreich ataxia | 102 | 76 |
| SCA1 | 39 | NA |
| SCA2 | 421 | NA |
| SCA3 | 268 | NA |
| SCA6 | 117 | NA |
| ADCA others | 46§ | 51 |
| A-T | 43 | 42 |
| ATLD | 2 | NA |
| AOA1 | 12 | NA |
| AOA2 | 15 | NA |
| NPC | 45 | 12 |
| EA | 10 | NA |
| HSP | 2 | NA |
| RFC1-related ataxia | 11 | NA |
| ARCA | NA | 7 |
| Various | NA | 1# |
ADCA autosomal-dominant cerebellar ataxia, AOA ataxia with ocular motor apraxia, ARCA autosomal-recessive cerebellar ataxia, A-T ataxia telangiectasia, ATLD ataxia telangiectasia-like disease, EA episodic ataxia, HSP hereditary spastic paraparesis, NA not available, NPC Niemann-Pick disease type C, RFC-1 replication factor complex subunit 1, SCA spinocerebellar ataxia
§This included SCA7 (n = 5), SCA8 (n = 8), SCA17 (n = 15), SCA31 (n = 12), SCA37 (n = 2), and other SCAs not specified (n = 56 [65–72])
#One patient with “non-identified” genetic ataxia [73]
The studies included reported on a broad range of oculomotor paradigms that were obtained with a variety of recording techniques (see Appendix 3, Tables S2 and S4 and also the companion manuscript (Garces et al. 2022a, submitted) for details).
Diagnostic Discriminatory Power
Discriminatory power for each combination of oculomotor paradigm and ataxia diagnosis are summarized in Table 2, focusing on discrimination from healthy controls, which was the most common statistical comparison performed across studies. A succinct overview of the key oculomotor alterations for each genotype, what we refer to as their oculomotor “fingerprint,” is provided in Table 3.
Table 2.
Overview of discriminatory power across studies for the five selected oculomotor paradigms
| Pursuit eye movements (PEMs) | Saccadic eye movements (SEMs) | Saccadic intrusions (SIs) | Spontaneous nystagmus (SN) | GEN | qHIT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Velocity ↓ and sacc. intrusions | Velocity | Latency | Accuracy | MGS: sacc. latency/AS: prop. errors | SWJ (frequency) | OF (frequency) | Micro-opsoclonus (frequency) | Hor SN (presence) | Vertical SN (presence) | Torsional SN (presence) | GEN (presence) | qHIT gain | |
| FRDA [10, 19, 26, 33, 34, 44–49, 74, 75] | ↓/↓↓ | 82–100% | → /↓ | ↑ | ↓ | ↑ | + + | + + | ∅ | ∅-DBN (15–45%) | 15–100% | ↓↓↓ | ||
| SCA1 [10, 19, 70, 76–78] | ↓–↓↓ | ↓ | + | ∅ | ∅ | ∅ | 60–100% | → /↓ | ||||||
| SCA2 [8, 10, 20, 23, 37–40, 70, 77, 79, 80] | ↓↓↓ | ↑ | → /↓ | ↑ | 20–30% | ∅ | ∅ | ∅ | 20–40% | → | ||||
| SCA3 [9, 10, 19, 32, 70, 77, 81–87] | ↓ Vert/hor | 100% | → /↓ | → | ↓ | ↑, 43–64% | ∅ | + | ∅/ + | ∅/ + (DBN) | 66–100% | ↓↓ | ||
| SCA6 [41, 67, 70, 77, 78, 88–91] | ↓↓↓ | + + | → /↓ | ↓ | 80–100% | DBN (50–100%) | 100% | ↑/↓* | ||||||
| SCA17 [42] | ↓ | → | ↓ | ↑ | 33% | |||||||||
| A-T [16, 50–52, 92, 93] | ↓↓ | + + | ↑ | ↓ | ↑ | 31–85% | 50–69% | 38%, PAN (62%) | DBN (43–46%) | 100% | 50% | |||
| NPC [24, 43, 94, 95] | ↓↓ | ↓↓↓ (vert), ↓ (hor) | → | |||||||||||
| CANVAS/RFC-1 [6, 12, 96–98] | DBN (45%) | ↓↓/↓↓↓ | ||||||||||||
Symbol legend: ∅ = finding absent; ( +)/ + / + + / + + + = finding present rarely (( +))/sometimes ( +)/in a significant fraction (+ +)/frequently (+ + +); ↑/↑↑/↑↑↑ = value increased mildly (↑)/moderately (↑↑)/strongly (↑↑↑); → = value unchanged, ↓/↓↓/↓↓↓ = value decreased mildly (↓)/moderately (↓↓)/strongly (↓↓↓) compared to healthy controls
amp amplitude, A-T ataxia telangiectasia, CANVAS cerebellar ataxia-nystagmus-vestibular areflexia syndrome, DBN downbeat nystagmus, FRDA Friedreich ataxia, GEN gaze-evoked nystagmus, hor horizontal, lat latency, NPC Niemann-Pick disease type C, OF ocular flutter, qHIT quantitative head-impulse test, PAN periodic alternating nystagmus, RBN rebound nystagmus, RFC-1 replication factor complex subunit 1, sacc saccadic, SCA spinocerebellar ataxia, vert vertical, SN spontaneous nystagmus, SPV slow-phase velocity, SWJ square-wave jerks
*Increased qHIT gain in mild cases, decreased qHIT gain in more severe cases
Table 3.
Oculomotor “fingerprint” for selected hereditary ataxias
| Disease | Domain | Key oculomotor changes |
|---|---|---|
| FRDA | PEM | • Moderately reduced velocity and gain |
| Gaze holding |
• Frequent saccadic intrusions (SWJ, OF) • Frequently impaired eccentric gaze holding (GEN) |
|
| qHIT | • Strongly reduced qHIT gain | |
| SCA1 | SEM | • Moderately reduced velocity |
| Gaze holding | • Frequently impaired eccentric gaze holding (GEN) | |
| SCA2 | SEM | • Strongly reduced velocity |
| qHIT | • Preserved qHIT gain | |
| SCA3 | PEM | • Frequently reduced velocity and saccadic intrusions |
| SEM | • Moderately reduced velocity of vertical saccades | |
| Gaze holding |
• Saccadic intrusions in a significant fraction (SWJ, no OF) • Frequently impaired eccentric gaze holding (GEN) |
|
| qHIT | • Moderately reduced qHIT gain | |
| SCA6 | PEM | • Frequent gain reductions |
| Gaze holding |
• Frequent saccadic intrusions (SWJ) and spontaneous nystagmus (DBN) • Frequently impaired eccentric gaze holding (GEN) |
|
| qHIT | • Markedly reduced qHIT gain | |
| A-T | PEM | • Moderately reduced gain, reduced velocity in a significant fraction |
| Gaze holding |
• Frequent saccadic intrusions (SWJ, OF) • Impaired straight-ahead fixation (SN) and eccentric gaze holding (GEN) in a significant fraction |
|
| NPC | PEM | • Moderately reduced gain |
| SEM | • Strongly reduced velocity for vertical saccades | |
| qHIT | • Preserved qHIT gain | |
| CANVAS/RFC1-related ataxia | qHIT | • Strongly reduced qHIT |
| Gaze holding | • Impaired straight-ahead fixation (DBN) in a significant fraction |
A-T ataxia telangiectasia, CANVAS cerebellar ataxia-nystagmus-vestibular areflexia syndrome, DBN downbeat nystagmus, FRDA Friedreich ataxia, GEN gaze-evoked nystagmus, HSN head-shaking nystagmus, NPC Niemann-Pick disease type C, OF ocular flutter, qHIT quantitative head-impulse test, SCA spinocerebellar ataxia, SEM saccadic eye movement, SN spontaneous nystagmus, PEM pursuit eye movement, SWJ square-wave jerks
Correlation with Disease Severity Measures
Twenty-seven studies reported on at least one correlation with a measure of disease severity for specific hereditary ataxias, most frequently using SEM paradigms (n = 21 studies), followed by head-impulse testing (n = 6 studies) and PEM (n = 5 studies; see Table 4 for all paradigms). Disease severity measures were most often clinical scores (n = 18 studies; SARA in 9 studies, for more detailed information, see Table 4) or chronological measures (such as age, disease duration, or time to (calculated or observed) symptom onset, n = 17 studies), followed by genetic stratification (usually repeat expansion size, n = 9 studies) and imaging measures of atrophy (including MR imaging in 5 studies) as summarized in Table 4. The overall study quality was high (n = 8) or moderate (n = 12) in most cases, with low study quality that was noted in a minority of studies (n = 7) only.
Table 4.
Overview of the number of studies reporting on correlation analyses between the five selected oculomotor parameters and other clinical information
| Studies (n) | Subjects (n) | |
|---|---|---|
| Any correlation analysis performed | ||
| Yes | 27 | 671 |
| No | 62 | 870 |
| Oculomotor parameters considered for correlation analyses | ||
| Pursuit EM | 5 | 187 |
| Saccadic EM | 21 | 636 |
| Saccadic intrusions | 3 | 89 |
| Spontaneous nystagmus | 1 | 73 |
| Gaze-evoked nystagmus | 2 | 129 |
| Head-impulse test | 6 | 126 |
| Oher variables used in the correlations | ||
| Clinical scores | ||
| SARA | 9 | 270 |
| ICARS | 4 | 114 |
| FARS | 4 | 33 |
| A-T index | 1 | 33 |
| SLCLC | 2 | 33 |
| Others§ | 2 | 155 |
| Any clinical score | 18 | 459 |
| Patient-reported outcomes | ||
| VISATAX questionnaire | 1 | 21 |
| Other clinical information | ||
| Disease duration | 11 | 254 |
| Age at symptom onset | 7 | 194 |
| Current age | 7 | 189 |
| Time to manifestation | 3 | 164 |
| Any other clinical parameter | 17 | 519 |
| Imaging parameters | ||
| MRI-based | 5 | 97 |
| Optical coherence tomography (OCT) | 1 | 31 |
| Laboratory parameters | ||
| Genetic testing (CAG-repeat length) | 9 | 318 |
A-T ataxia telangiectasia, EM eye movement, ICARS International Cooperative Ataxia Rating Scale, FARS Friedreich Ataxia Rating Scale, SARA Scale for the Assessment and Rating of Ataxia, SLCLC Sloan Low-Contrast Letter Chart
§Other clinical scores included SCAFI/CCFS/NESSCA/INAScout [9] and a clinical ataxia score not further specified [23]
Overall, the majority of correlations identified were related to spinocerebellar ataxia (SCA) type 2 (SCA2), SCA3, or Friedreich ataxia (FRDA) (see Appendix 3, supplementary table S5 for all 105 significant correlations found in 20 studies), and the strength of correlations was high (n = 36) to moderate (n = 41) in most cases (see Table 5 and Appendix 3, supplementary table S6 for details). Disease severity measures were most frequently correlated with parameters of saccadic eye movements (including latency, peak velocity, accuracy, and the regression slope of saccadic peak duration vs. amplitude; 67/105 correlations (64%)), to parameters of pursuit eye movements (focusing on PEM gain in most cases, 11/105 correlations (10%)), to the gain obtained in quantitative head-impulse testing (qHIT; 8/105 correlations (8%)), and to the slow-phase velocity of gaze-evoked nystagmus (11/105 (10%)). Correlations taking fixation stabilities (SI or SN) into account were infrequently found (8/105 (8%)).
Table 5.
Number of studies reporting significant correlations between oculomotor parameters and other clinical information for specific hereditary ataxias
| PEM | SEM | SI | SN | GEN | qHIT | |
|---|---|---|---|---|---|---|
| Correlation with clinical scores | ||||||
| SARA | 3 (SCA3 (2 ×), SCA2) [9, 32, 37] | 2 (SCA3 (2 ×)) [9, 32] | 1 (SCA3) [9] | |||
| ICARS | 2 (SCA3/SCA17) [9, 42] | 2 (SCA3, SCA2) [9, 42] | 1 (SCA3) [9] | 1 (SCA3) [9] | 2 (SCA3, SCA6) [9, 41] | |
| FARS | 4 (FRDA) [33–36] | |||||
| A-T index | 1 (A-T) [52] | |||||
| SLCLC | 2 (FRDA) [33, 35] | 1 (FRDA) [34] | ||||
| Others§ | 2 (SCA3, SCA2) [9, 23] | 1 (SCA3) [9] | 1 (SCA3) [9] | 1 (SCA3) [9] | ||
| Correlation with other clinical parameters | ||||||
| Disease duration | 2 (SCA3, SCA17) [32, 42] | 4 (FRDA, SCA17, SCA2, SCA3) [23, 32, 33, 42] | 1 (SCA3) [32] | |||
| Age at symptom onset | 2 (both SCA2) [23, 37] | 1 (FRDA) [34] | ||||
| Current age | 1 (A-T) [52] | |||||
| Time to manifestation | 3 (SCA3, SCA2 (2 ×)) [9, 39, 40] | 1 (SCA3) [9] | 1 (SCA3) [9] | |||
| Correlation with imaging parameters | ||||||
| MRI-based | 3 (SCA2, NPC (2 ×)) [20–22] | |||||
| OCT | 1 (NPC) [43] | |||||
| Correlation with laboratory parameters | ||||||
| Genetic testing | 6 (FRDA, SCA2 (4 ×), SCA3) [8, 23, 32, 33, 37, 39] | |||||
A total of 20 studies were included in this table. The ataxia diagnosis investigated in each study is indicated in brackets. A summary of correlations identified for additional oculomotor paradigms is provided in Appendix 3, supplementary Table S3. Two studies reported on correlations after pooling genetically confirmed hereditary ataxia syndromes only, and thus were not considered here [10, 19]
A-T ataxia telangiectasia, FARS Friedreich Ataxia Rating Scale, FRDA Friedreich ataxia, GEN gaze-evoked nystagmus, HSN head-shaking nystagmus, ICARS International Cooperative Ataxia Rating Scale, NPC Niemann-Pick disease type C, OCT optical coherence tomography, OKN optokinetic nystagmus, qHIT quantitative head-impulse test, rVOR rotational VOR, SARA Scale for the Assessment and Rating of Ataxia, SCA spinocerebellar ataxia, SEM saccadic eye movements, SI saccadic intrusions, SLCLC Sloan Low-Contrast Letter Chart, SN spontaneous nystagmus, PEM pursuit eye movement, SWJ square-wave jerks, VOR vestibulo-ocular reflex, VVOR visually enhanced VOR, VORS vestibulo-ocular reflex suppression
§Other clinical scores included SCAFI/CCFS/NESSCA/INAScout [9] and a clinical ataxia score not further specified [23]
On MR imaging, SEM (peak velocity, gain) correlated with pontine volume/anterior–posterior pontine diameter in pre-symptomatic and symptomatic SCA2 [20] and with total cerebellar volume/gray matter [21] and various brainstem measurements (midbrain midsagittal area, pontine-midbrain ratio) in NPC [22]. For other oculomotor paradigms, no correlations with (MR) imaging were identified.
Importantly, for a number of combinations of genotypes and oculomotor paradigms, no data was available from the literature review, and therefore, no recommendations could be provided.
Sensitivity to Disease Progression and Treatment Effects
In contrast to discriminatory or correlative cross-sectional studies, longitudinal studies monitoring disease progression (n = 2, both SCA2 [8, 23]) or treatment responses (n = 8, NPC, autosomal-dominant cerebellar ataxia (ADCA), episodic ataxia (EA) 4, A-T (2x), SCA2 (2x), FRDA) [24–31]) were rare. The two observational studies in SCA2 monitored horizontal visually guided saccades over variable/different timescales, demonstrating a significant decrease in saccade peak velocity and saccade accuracy over a period of 5 years [8], whereas saccade latency increased significantly over a 5-year period [8], but not over a shorter follow-up period of 1 year as reported in a different study [23].
Saccadic intrusions were reduced in peak velocity, frequency, and amplitude after memantine treatment in ADCA [28]; PEM and gaze holding were improved after application of gabapentin in EA4 [25]; vestibulo-ocular reflex time constant (VOR Tc) and SN slow-phase velocity was decreased after treatment with 4-aminopyridine in A-T patients [29], and saccade latencies were reduced after zinc sulphate supplementation in SCA2 [30]. In contrast, no positive effect of idebenone on saccadic intrusions was noted in FRDA [26], as well as for neurorehabilitation [27] or lisuride [31] on SEM in SCA2.
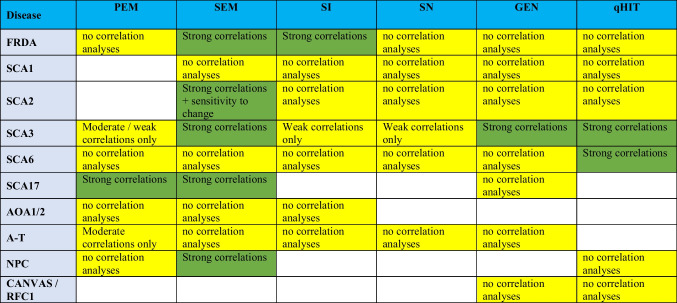
Genotype-Specific Recommendations for Further Validation
To provide guidance in selecting suitable oculomotor paradigms for validation in genetically stratified natural history studies, we prioritized each paradigm per ataxia genotype, based on its discriminatory power, correlations with parameters of disease severity, and sensitivity to change (see “Material and Methods”). Overall, recommendations strongly depended on the specific oculomotor paradigm and disorder (Table 6). While SEM yielded generic oculomotor parameters across most ataxia types, recommendation of other paradigms was limited by the scarcity of cross-validating correlation analyses in most genotypes, even in more frequent ataxias such as SCA1, RFC1, or AOA1 and 2. SCA3 presents the most widely explored ataxia, with studies substantiating the recommendation of SEM, GEN, and qHIT [9, 32]. A broad range of parameters have also been assessed in both symptomatic and even pre-symptomatic SCA3 patients, with significant correlations for SEM, PEM, SN, gaze holding, and head-impulse testing [9]. Other recommended oculomotor paradigms were SEM [33–36] and SI for FRDA [34], SEM for SCA2 [8, 20, 23, 37–40], qHIT for SCA6 [41], SEM and PEM for SCA17 [42], and SEM for NPC [21, 22, 43]. Focusing on observational studies, faster progression rates of saccadic slowing were associated with higher trinucleotide CAG repeat expansions in SCA2 patients [8].
Table 6.
Genotype-specific recommendation of paradigms
Priority 1 requires data supporting high discriminatory power and at least one confirmed strong correlation, or sensitivity to change in observational or interventional studies. Priority 2 indicates that studies have found moderate to low discriminatory power or—if performed—the level of significance of correlation analyses being moderate or low only. Parameters that had no discriminatory power are not recommended. Priority 1 paradigms are marked in dark green, whereas priority 2 paradigms are marked in light yellow. Areas of uncertainty (i.e., paradigms that have not been quantitatively assessed in specific diseases) are shown in white
AOA ataxia ocular motor apraxia, A-T ataxia telangiectasia, CANVAS cerebellar ataxia-nystagmus-vestibular areflexia syndrome, FRDA Friedreich ataxia, GEN gaze-evoked nystagmus, NPC Niemann-Pick disease type C, qHIT quantitative head-impulse test, SCA spinocerebellar ataxia, SEM saccadic eye movement, SN spontaneous nystagmus, PEM pursuit eye movement, RFC-1 replication factor complex subunit 1, SI saccadic intrusion
Noteworthy, in selected disorders, the fraction of patients that received a diagnosis based on either established biomarkers or a positive family history only was significant. Specifically, for FRDA (57%, 102/178 patients) and for A-T (51%, 43/85), only about half of patients had genetically confirmed hereditary ataxia, while in six studies that reported on FRDA patients [44–49] and in 3 studies on A-T patients [50–52], diagnosis was based on biomarkers/positive family history only. While this lack of genetically confirmed diagnosis increases the risk of misclassification due to overlapping phenotypes between distinct ataxias, all significant correlations reported for FRDA (see Table 5) were obtained from studies with genetically confirmed FRDA. For A-T, few correlations were reported (see Table 5), but originated from a study cohort that did not receive genetic testing [52].
Discussion
Given that oculomotor and vestibular abnormalities are increasingly identified in hereditary ataxias [1], quantitative assessments have been of growing interest over the last few years. In this systematic review, we have examined the potential of quantitative oculomotor parameters as digital-motor outcome measures in specific hereditary ataxias, and made a range of suggestions for future validation studies based on gaps in our present knowledge. Specifically, we have shown that characteristic oculomotor abnormalities can be quantified by the use of eye movement recordings in hereditary ataxias, and that they are not only useful in differentiating between distinct underlying disorders, but can, in some cases, also capture disease severity and other clinical characteristics. Importantly, the pattern of oculomotor and vestibular alterations depends on the patient population studied.
Disease-Specific Selection of Oculomotor Paradigms
Currently, our understanding of the value of a given oculomotor paradigm for specific hereditary ataxias with regards to its ability to discriminate ataxia patients from healthy controls, to capture progression over time or modulation of progression by an intervention and any correlations with other known measures of disease severity, is far from being complete. Historically, certain paradigms have been preferred based on hallmark signs identified by bedside testing as, e.g., very slow saccades in SCA2 [53] or saccadic intrusions in A-T [14]. This has significantly affected the selection of quantitative oculomotor parameters in previous studies. Whereas for certain disorders such as FRDA, SCA2, SCA3, SCA6, and A-T, we were able to retrieve descriptions of oculomotor abnormalities for a broad range of paradigms, in other disorders such as AOA1/2, NPC, or RFC1-related ataxia previous studies focused on only a limited number of paradigms. Table 6 may serve as a valuable starting point in guiding the choice of oculomotor paradigms for future validation in natural history studies, and eventually clinical trials, as it can help to prioritize across oculomotor paradigms in selecting those that are likely to be of most value, based on existing data. In designing future clinical trials, many aspects will affect the application of oculomotor testing, including competition with other domains to be assessed (e.g., appendicular motor or cognitive assessment), consideration of subject fatigue, stage of disease (e.g., assessments in non-ambulatory patients), and the infrastructure available at the various clinical sites. Furthermore, when selecting specific oculomotor paradigms, potential synergies with other measured information should be considered, as discussed in the next section.
Various Oculomotor Assessments Are Correlated with Other (Clinical) Parameters
Construct validity of any outcome assessment requires a correlation with other measures of disease severity [54]. Such correlations were not homogeneously investigated across the spectrum of oculomotor paradigms, but were skewed towards a few paradigms and hereditary ataxia disorders. Specifically, significant correlations were most consistently and frequently observed in studies with SCA2, SCA3, and FRDA and were rated as moderate or strong in the majority of cases (73%).
The range of parameters that has been shown to correlate with eye movement assessments in the ataxias reported here was broad, including clinical scores rating disease severity, aspects of disease onset/duration, triplet size in repeat expansion disorders, and MRI-based biomarkers quantifying disease pathophysiology. More than half of all significant correlations were linked to saccadic eye movements and were identified in FRDA, NPC, SCA2, SCA3, and SCA17. Significant correlations using other oculomotor parameters such as PEM (noted in A-T and SCA3), qHIT (SCA3 and SCA6), GEN (SCA3), or saccadic intrusions (SCA3) were also reported, but were only investigated in a few studies and diseases. This emphasizes the gaps present in the current literature.
Existing data demonstrates significant correlations of quantitative oculomotor parameters with clinical scores such as SARA, ICARS, and FARS [9, 33, 36, 37, 41, 42]. Currently used clinical scores in hereditary ataxia do not take oculomotor abnormalities into account at all (as SARA [55] or FARS [56]) or represent only one out of several subscales, thus having limited impact on the overall score (as in ICARS [57]). Therefore, the correlations identified by our systematic review indicate that quantitative oculomotor parameters not only reflect damage to specific oculomotor systems, but also capture the severity of the underlying disease across multiple motor domains (as mainly reflected by SARA, FARS, and other clinical scores).
Likewise, changes in oculomotor parameters were also shown to relate to disease duration as a measure of disease severity in FRDA (SEM [33]), SCA2 (SEM [23]), SCA3 (PEM, SEM, and GEN [32]), and SCA17 (PEM and SEM [42]). Such correlations indicate potential responsiveness of the respective quantitative oculomotor parameter over time, and longitudinal studies are recommended to further validate sensitivity to change in trial-relevant time intervals. Importantly, various oculomotor parameters were altered in pre-symptomatic carriers in SCA2 (SEM [39, 40]) and SCA3 (SEM, GEN, and qHIT [9]), and even correlated significantly with the estimated or observed time to manifestation. This highlights the potential utility of using these parameters as outcome measures in early disease stages where disease-modifying therapies are probably most effective.
Correlations between MR imaging and oculomotor paradigms were investigated in SCA2, FRDA, and NPC only, while data is lacking for other hereditary ataxias. Noteworthy, significant correlations were identified only for SEM in SCA2 and NPC, but not in FRDA [49]. Specifically, various SEM parameters correlated with brainstem alterations (i.e., pontine volume and the anterior–posterior pontine diameter) in pre-symptomatic and symptomatic SCA 2 [20] and with both cerebellar (total cerebellar volume/gray matter [21]) and various brainstem measurements (midbrain midsagittal area, pontine-midbrain ratio) [22] in NPC. Furthermore, changes in thickness of different retinal layers on optical coherence tomography (OCT) correlated with SEM in NPC [43]. Thus, in selected hereditary ataxias, a link between structural changes on brainstem and cerebellar MR imaging and eye movement properties could be achieved and thus may be used as a parameter to reflect more global disease damage in future studies.
Quantitative Oculomotor Parameters Suitable to Monitor Disease Progression and Treatment Response
Parameters suitable for monitoring disease progression or improvement (i.e., biomarkers) must be robust and sensitive to change. Noteworthy, the SARA has been shown to reflect progression in hereditary ataxias such as SCA1 [58–60], SCA2 [58, 60], SCA3 [58, 60], SCA6 [60], FRDA [61], and even in pre-symptomatic SCA1 carriers [62]. While validation of quantitative oculomotor parameters was often limited to cross-sectional analysis, only a small number of studies have directly assessed these properties by investigating oculomotor parameters in longitudinal studies [8, 23].
We identified two studies reporting on the suitability of oculomotor parameters for monitoring disease progression in SCA2. Specifically, significant decreases in saccade peak velocity and saccade accuracy and increase in saccade latency were detected over a period of 5 years [8] but was not significant over a shorter—more trial-relevant—follow-up period of 1 year [23] (despite an average decrease in peak velocity of 8°/s per year, n = 30 SCA2 patients). In SCA3, two studies compared oculomotor parameters in patients at different disease stages (e.g., pre-symptomatic far from onset, pre-symptomatic close to onset and symptomatic) cross-sectionally. Specifically, in (pre)symptomatic SCA3 carriers, vertical PEM gains, vertical SEM slopes, horizontal SN and GEN slow-phase velocity, and horizontal video-HIT gains showed stronger alterations in those patients who were symptomatic or became symptomatic soon after oculomotor testing [9, 32].
Given this scarcity of longitudinal data, longitudinal validation studies are urgently needed to improve our knowledge of the sensitivity to disease progression of selected oculomotor paradigms and parameters. For a given paradigm and genotype, these studies may have to be tailored to specific disease stages, given that some oculomotor abnormalities are present through almost the entire disease (e.g., saccadic slowing in SCA2), while others rarely occur neither in the pre-symptomatic stage nor in severe disease stages (e.g., saccadic intrusions in SCA2) [63]. Importantly, the large effort of such longitudinal validation studies should be undertaken with the clear aim of regulatory acceptance, focusing on trial-relevant assessment intervals, and complementing quantitative oculomotor assessments by necessary clinician-, patient-, and possibly performance-related outcomes ([64]). In a separate publication (see companion paper Garces et al. 2022a submitted), we provided guidelines for the measurement of those quantitative oculomotor parameters we have reviewed here, including recommendations on acquisition paradigms, measurement conditions, parameter extraction, and biases to consider.
Conclusions
To our knowledge, this is the first systematic literature review on the role of quantitative oculomotor parameters in hereditary ataxias. Where available, we have summarized information on case–control discrimination correlations with measures of disease severity, and evidence of longitudinal and treatment studies separately for each disease and paradigm. Based on this, we provide disease-specific recommendations for selecting the most promising oculomotor paradigms for preparation of future clinical trials, which are firmly placed on the horizon. Additionally, this review highlights current gaps in our knowledge and the critical need for well-designed, genotypically stratified longitudinal validation studies aiming for regulatory approval.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
AAT and AT had the idea for the article. AAT and PG performed the literature search and data analysis. AAT and PG drafted the manuscript. All authors participated in defining the core set of paradigms proposed and the implementation details. All authors critically revised the work and approved it in its final version.
Funding
Open access funding provided by University of Zurich
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethical Approval
Not applicable.
Conflict of Interest
PG is a full-time employee of F. Hoffmann–la Roche Ltd.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park JY, Joo K, Woo SJ. Ophthalmic manifestations and genetics of the polyglutamine autosomal dominant spinocerebellar ataxias: a review. Front Neurosci. 2020;14:892. doi: 10.3389/fnins.2020.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leigh RJ, Zee DS. Appendix C - Tables of ocular motor findings in hereditary ataxia. The neurology of eye movements, 5th edition. New York, USA, Oxford University Press; 2015. pp. 1035–48.
- 3.Zeigelboim BS, Teive HAG, Santos GJB, Severiano MIR, Fonseca VR, Faryniuk JH, Marques JM. Otoneurological findings prevalent in hereditary ataxias. Arq Neuropsiquiatr. 2018;76:131–138. doi: 10.1590/0004-282x20180001. [DOI] [PubMed] [Google Scholar]
- 4.Barsottini OG, Pedroso JL, Jr, Martins CR, Jr, Franca MC, Albernaz PM. Deafness and vestibulopathy in cerebellar diseases: a practical approach. Cerebellum (London, England) 2019;18:1011–6. doi: 10.1007/s12311-019-01042-4. [DOI] [PubMed] [Google Scholar]
- 5.Szmulewicz DJ. Combined central and peripheral degenerative vestibular disorders: CANVAS, idiopathic cerebellar ataxia with bilateral vestibulopathy (CABV) and other differential diagnoses of the CABV phenotype. Curr Otorhinolaryngol Rep. 2017;5:167–174. doi: 10.1007/s40136-017-0161-5. [DOI] [Google Scholar]
- 6.Szmulewicz DJ, Waterston JA, MacDougall HG, Mossman S, Chancellor AM, McLean CA, Merchant S, Patrikios P, Halmagyi GM, Storey E. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis. Ann N Y Acad Sci. 2011;1233:139–147. doi: 10.1111/j.1749-6632.2011.06158.x. [DOI] [PubMed] [Google Scholar]
- 7.Tarnutzer AA, Straumann D, Salman MS. Neuro-ophthalmologic assessment and investigations in children and adults with cerebellar diseases. Handb Clin Neurol. 2018;154:305–327. doi: 10.1016/B978-0-444-63956-1.00019-9. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Labrada R, Velázquez-Pérez L, Auburger G, Ziemann U, Canales-Ochoa N, Medrano-Montero J, Vázquez-Mojena Y, González-Zaldivar Y. Spinocerebellar ataxia type 2: measures of saccade changes improve power for clinical trials. Mov Disord Off J Mov Disord Soc. 2016;31:570–578. doi: 10.1002/mds.26532. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira CM, Leotti VB, Bolzan G, Cappelli AH, Rocha AG, Ecco G, Kersting N, Rieck M, Martins AC, Sena LS, Saraiva-Pereira ML, Jardim LB. Pre-ataxic changes of clinical scales and eye movement in Machado-Joseph disease: BIGPRO study. Mov Disord. 2021;36(4):985–94 . 10.1002/mds.28466. [DOI] [PubMed]
- 10.Luis L, Costa J, Munoz E, de Carvalho M, Carmona S, Schneider E, Gordon CR, Valls-Sole J. Vestibulo-ocular reflex dynamics with head-impulses discriminates spinocerebellar ataxias types 1, 2 and 3 and Friedreich ataxia. J Vestib Res Equilibrium Orientation. 2016;26:327–334. doi: 10.3233/VES-160579. [DOI] [PubMed] [Google Scholar]
- 11.Jensen K, Beylergil SB, Shaikh AG. Slow saccades in cerebellar disease. Cerebellum Ataxias. 2019;6:1. doi: 10.1186/s40673-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costales M, Casanueva R, Suárez V, Asensi JM, Cifuentes GA, Diñeiro M, Cadiñanos J, López F, Álvarez-Marcos C, Otero A, Gómez J, Llorente JL, Cabanillas R. CANVAS: a new genetic entity in the otorhinolaryngologist's differential diagnosis. Otolaryngol Head Neck Surg. 2022;166(1):74–9. 10.1177/01945998211008398. [DOI] [PubMed]
- 13.Lee SU, Kim JS, Kim HJ, Choi JY, Park JY, Kim JM, Yang X. Evolution of the vestibular function during head impulses in spinocerebellar ataxia type 6. J Neurol. 2020;267:1672–1678. doi: 10.1007/s00415-020-09756-w. [DOI] [PubMed] [Google Scholar]
- 14.Tang SY, Shaikh AG. Past and present of eye movement abnormalities in ataxia-telangiectasia. Cerebellum (London, England) 2019;18:556–564. doi: 10.1007/s12311-018-0990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson TJ, MacAskill MR. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. 2013;9:74–85. doi: 10.1038/nrneurol.2012.273. [DOI] [PubMed] [Google Scholar]
- 16.Mariani LL, Rivaud-Pechoux S, Charles P, Ewenczyk C, Meneret A, Monga BB, Fleury MC, Hainque E, Maisonobe T, Degos B, Echaniz-Laguna A, Renaud M, Wirth T, Grabli D, Brice A, Vidailhet M, Stoppa-Lyonnet D, Dubois-d'Enghien C, Le Ber I, Koenig M, Roze E, Tranchant C, Durr A, Gaymard B, Anheim M. Comparing ataxias with oculomotor apraxia: a multimodal study of AOA1, AOA2 and AT focusing on video-oculography and alpha-fetoprotein. Sci Rep. 2017;7:15284. doi: 10.1038/s41598-017-15127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 19.Alexandre MF, Rivaud-Péchoux S, Challe G, Durr A, Gaymard B. Functional consequences of oculomotor disorders in hereditary cerebellar ataxias. Cerebellum (London, England) 2013;12:396–405. doi: 10.1007/s12311-012-0433-z. [DOI] [PubMed] [Google Scholar]
- 20.Reetz K, Rodríguez-Labrada R, Dogan I, Mirzazade S, Romanzetti S, Schulz JB, Cruz-Rivas EM, Alvarez-Cuesta JA, Aguilera Rodríguez R, Gonzalez Zaldivar Y, Auburger G, Velázquez-Pérez L. Brain atrophy measures in preclinical and manifest spinocerebellar ataxia type 2. Ann Clin Transl Neurol. 2018;5:128–137. doi: 10.1002/acn3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walterfang M, Abel LA, Desmond P, Fahey MC, Bowman EA, Velakoulis D. Cerebellar volume correlates with saccadic gain and ataxia in adult Niemann-Pick type C. Mol Genet Metab. 2013;108:85–89. doi: 10.1016/j.ymgme.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Walterfang M, Macfarlane MD, Looi JC, Abel L, Bowman E, Fahey MC, Desmond P, Velakoulis D. Pontine-to-midbrain ratio indexes ocular-motor function and illness stage in adult Niemann-Pick disease type C. Eur J Neurol. 2012;19:462–467. doi: 10.1111/j.1468-1331.2011.03545.x. [DOI] [PubMed] [Google Scholar]
- 23.Seifried C, Velázquez-Pérez L, Santos-Falcón N, Abele M, Ziemann U, Almaguer LE, Martínez-Góngora E, Sánchez-Cruz G, Canales N, Pérez-González R, Velázquez-Manresa M, Viebahn B, Stuckrad-Barre S, Klockgether T, Fetter M, Auburger G. Saccade velocity as a surrogate disease marker in spinocerebellar ataxia type 2. Ann N Y Acad Sci. 2005;1039:524–527. doi: 10.1196/annals.1325.059. [DOI] [PubMed] [Google Scholar]
- 24.Bremova T, Malinova V, Amraoui Y, Mengel E, Reinke J, Kolnikova M, Strupp M. Acetyl-dl-leucine in Niemann-Pick type C: a case series. Neurology. 2015;85:1368–1375. doi: 10.1212/WNL.0000000000002041. [DOI] [PubMed] [Google Scholar]
- 25.Coin JT, Vance JM. Gabapentin relieves vertigo of periodic vestibulocerebellar ataxia: 3 cases and possible mechanism. Mov Disord. 2021;36(5):1264–67. 10.1002/mds.28491. [DOI] [PubMed]
- 26.Ribaï P, Pousset F, Tanguy ML, Rivaud-Pechoux S, Le Ber I, Gasparini F, Charles P, Béraud AS, Schmitt M, Koenig M, Mallet A, Brice A, Dürr A. Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch Neurol. 2007;64:558–564. doi: 10.1001/archneur.64.4.558. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Díaz JC, Velázquez-Pérez L, Rodríguez Labrada R, Aguilera Rodríguez R, Laffita Pérez D, Canales Ochoa N, Medrano Montero J, Estupiñán Rodríguez A, Osorio Borjas M, Góngora Marrero M, Reynaldo Cejas L, González Zaldivar Y, Almaguer GD. Neurorehabilitation therapy in spinocerebellar ataxia type 2: a 24-week, rater-blinded, randomized, controlled trial. Mov Disord Off J Mov Disord Soc. 2018;33:1481–1487. doi: 10.1002/mds.27437. [DOI] [PubMed] [Google Scholar]
- 28.Rosini F, Federighi P, Pretegiani E, Piu P, Leigh RJ, Serra A, Federico A, Rufa A. Ocular-motor profile and effects of memantine in a familial form of adult cerebellar ataxia with slow saccades and square wave saccadic intrusions. PloS one. 2013;8:e69522. doi: 10.1371/journal.pone.0069522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Zee DS, Straumann D. Effects of 4-aminopyridine on nystagmus and vestibulo-ocular reflex in ataxia-telangiectasia. J Neurol. 2013;260:2728–2735. doi: 10.1007/s00415-013-7046-4. [DOI] [PubMed] [Google Scholar]
- 30.Velázquez-Pérez L, Rodríguez-Chanfrau J, García-Rodríguez JC, Sánchez-Cruz G, Aguilera-Rodríguez R, Rodríguez-Labrada R, Rodríguez-Díaz JC, Canales-Ochoa N, Gotay DA, Almaguer Mederos LE, Laffita Mesa JM, Porto-Verdecia M, Triana CG, Pupo NR, Batista IH, López-Hernandez OD, Polanco ID, Novas AJ. Oral zinc sulphate supplementation for six months in SCA2 patients: a randomized, double-blind, placebo-controlled trial. Neurochem Res. 2011;36:1793–1800. doi: 10.1007/s11064-011-0496-0. [DOI] [PubMed] [Google Scholar]
- 31.Velázquez-Pérez L, Rodríguez-Labrada R, Álvarez-González L, Aguilera-Rodríguez R, Álvarez Sánchez M, Canales-Ochoa N, Galicia Polo L, Haro-Valencia R, Medrano-Montero J, Vázquez-Mojena Y, Peña-Acosta A, Estupiñán-Rodríguez A, Rodríguez PN. Lisuride reduces involuntary periodic leg movements in spinocerebellar ataxia type 2 patients. Cerebellum (London, England) 2012;11:1051–1056. doi: 10.1007/s12311-012-0382-6. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Chen DB, Feng L, Zhou XX, Zhang JW, You HJ, Liang XL, Pei Z, Li XH. Oculomotor deficits in spinocerebellar ataxia type 3: potential biomarkers of preclinical detection and disease progression. CNS Neurosci Ther. 2017;23:321–328. doi: 10.1111/cns.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fielding J, Corben L, Cremer P, Millist L, White O, Delatycki M. Disruption to higher order processes in Friedreich ataxia. Neuropsychologia. 2010;48:235–242. doi: 10.1016/j.neuropsychologia.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Fahey MC, Cremer PD, Aw ST, Millist L, Todd MJ, White OB, Halmagyi M, Corben LA, Collins V, Churchyard AJ, Tan K, Kowal L, Delatycki MB. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain J Neurol. 2008;131:1035–1045. doi: 10.1093/brain/awm323. [DOI] [PubMed] [Google Scholar]
- 35.Hocking DR, Corben LA, Fielding J, Cremer PD, Millist L, White OB, Delatycki MB. Saccade reprogramming in Friedreich ataxia reveals impairments in the cognitive control of saccadic eye movement. Brain Cogn. 2014;87:161–167. doi: 10.1016/j.bandc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Hocking DR, Fielding J, Corben LA, Cremer PD, Millist L, White OB, Delatycki MB. Ocular motor fixation deficits in Friedreich ataxia. Cerebellum (London, England) 2010;9:411–418. doi: 10.1007/s12311-010-0178-5. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Labrada R, Vázquez-Mojena Y, Canales-Ochoa N, Medrano-Montero J, Velázquez-Pérez L. Heritability of saccadic eye movements in spinocerebellar ataxia type 2: insights into an endophenotype marker. Cerebellum Ataxias. 2017;4:19. doi: 10.1186/s40673-017-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Federighi P, Cevenini G, Dotti MT, Rosini F, Pretegiani E, Federico A, Rufa A. Differences in saccade dynamics between spinocerebellar ataxia 2 and late-onset cerebellar ataxias. Brain J Neurol. 2011;134:879–891. doi: 10.1093/brain/awr009. [DOI] [PubMed] [Google Scholar]
- 39.Velázquez-Pérez L, Seifried C, Abele M, Wirjatijasa F, Rodríguez-Labrada R, Santos-Falcón N, Sánchez-Cruz G, Almaguer-Mederos L, Tejeda R, Canales-Ochoa N, Fetter M, Ziemann U, Klockgether T, Medrano-Montero J, Rodríguez-Díaz J, Laffita-Mesa JM, Auburger G. Saccade velocity is reduced in presymptomatic spinocerebellar ataxia type 2. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2009;120:632–635. doi: 10.1016/j.clinph.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Velázquez-Pérez L, Rodríguez-Labrada R, Cruz-Rivas EM, Fernández-Ruiz J, Vaca-Palomares I, Lilia-Campins J, Cisneros B, Peña-Acosta A, Vázquez-Mojena Y, Diaz R, Magaña-Aguirre JJ, Cruz-Mariño T, Estupiñán-Rodríguez A, Laffita-Mesa JM, González-Piña R, Canales-Ochoa N, González-Zaldivar Y. Comprehensive study of early features in spinocerebellar ataxia 2: delineating the prodromal stage of the disease. Cerebellum (London, England) 2014;13:568–579. doi: 10.1007/s12311-014-0574-3. [DOI] [PubMed] [Google Scholar]
- 41.Huh YE, Kim JS, Kim HJ, Park SH, Jeon BS, Kim JM, Cho JW, Zee DS. Vestibular performance during high-acceleration stimuli correlates with clinical decline in SCA6. Cerebellum (London, England) 2015;14:284–291. doi: 10.1007/s12311-015-0650-3. [DOI] [PubMed] [Google Scholar]
- 42.Hübner J, Sprenger A, Klein C, Hagenah J, Rambold H, Zühlke C, Kömpf D, Rolfs A, Kimmig H, Helmchen C. Eye movement abnormalities in spinocerebellar ataxia type 17 (SCA17) Neurology. 2007;69:1160–1168. doi: 10.1212/01.wnl.0000276958.91986.89. [DOI] [PubMed] [Google Scholar]
- 43.Havla J, Moser M, Sztatecsny C, Lotz-Havla AS, Maier EM, Hizli B, Schinner R, Kümpfel T, Strupp M, Bremova-Ertl T, Schneider SA. Retinal axonal degeneration in Niemann-Pick type C disease. J Neurol. 2020;267:2070–2082. doi: 10.1007/s00415-020-09796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baloh RW, Konrad HR, Honrubia V. Vestibulo-ocular function in patients with cerebellar atrophy. Neurology. 1975;25:160–168. doi: 10.1212/wnl.25.2.160. [DOI] [PubMed] [Google Scholar]
- 45.Dale RT, Kirby AW, Jampel RS. Square wave jerks in Friedreich's ataxia. Am J Ophthalmol. 1978;85:400–406. doi: 10.1016/s0002-9394(14)77738-4. [DOI] [PubMed] [Google Scholar]
- 46.Ell J, Prasher D, Rudge P. Neuro-otological abnormalities in Friedreich's ataxia. J Neurol Neurosurg Psychiatry. 1984;47:26–32. doi: 10.1136/jnnp.47.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furman JM, Perlman S, Baloh RW. Eye movements in Friedreich's ataxia. Arch Neurol. 1983;40:343–346. doi: 10.1001/archneur.1983.04050060043006. [DOI] [PubMed] [Google Scholar]
- 48.Moschner C, Perlman S, Baloh RW. Comparison of oculomotor findings in the progressive ataxia syndromes. Brain J Neurol. 1994;117(Pt 1):15–25. doi: 10.1093/brain/117.1.15. [DOI] [PubMed] [Google Scholar]
- 49.Spieker S, Schulz JB, Petersen D, Fetter M, Klockgether T, Dichgans J. Fixation instability and oculomotor abnormalities in Friedreich's ataxia. J Neurol. 1995;242:517–521. doi: 10.1007/bf00867423. [DOI] [PubMed] [Google Scholar]
- 50.Baloh RW, Yee RD, Boder E. Eye movements in ataxia-telangiectasia. Neurology. 1978;28:1099–1104. doi: 10.1212/wnl.28.11.1099. [DOI] [PubMed] [Google Scholar]
- 51.Lewis RF, Crawford TO. Slow target-directed eye movements in ataxia-telangiectasia. Invest Ophthalmol Vis Sci. 2002;43:686–691. [PubMed] [Google Scholar]
- 52.Lewis RF, Lederman HM, Crawford TO. Ocular motor abnormalities in ataxia telangiectasia. Ann Neurol. 1999;46:287–295. doi: 10.1002/1531-8249(199909)46:3<287::aid-ana3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 53.Velazquez-Perez L, Rodriguez-Labrada R, Gonzalez-Garces Y, Vazquez-Mojena Y, Perez-Rodriguez R, Ziemann U. Neurophysiological features in spinocerebellar ataxia type 2: prospects for novel biomarkers. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2021;135:1–12. doi: 10.1016/j.clinph.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull. 1955;52:281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Fancellu R. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 56.Subramony SH, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, Taylor P, Wilson R, Ashizawa T, Cooperative AG. Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology. 2005;64:1261–1262. doi: 10.1212/01.WNL.0000156802.15466.79. [DOI] [PubMed] [Google Scholar]
- 57.Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 58.Jacobi H, du Montcel ST, Bauer P, Giunti P, Cook A, Labrum R, Parkinson MH, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, Panzeri M, Rakowicz M, Sulek A, Sobanska A, Schmitz-Hubsch T, Schols L, Hengel H, Baliko L, Melegh B, Filla A, Antenora A, Infante J, Berciano J, van de Warrenburg BP, Timmann D, Szymanski S, Boesch S, Kang JS, Pandolfo M, Schulz JB, Molho S, Diallo A, Klockgether T. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14:1101–1108. doi: 10.1016/S1474-4422(15)00202-1. [DOI] [PubMed] [Google Scholar]
- 59.Nigri A, Sarro L, Mongelli A, Castaldo A, Porcu L, Pinardi C, Grisoli M, Ferraro S, Canafoglia L, Visani E, Bruzzone MG, Nanetti L, Taroni F, Mariotti C. Spinocerebellar ataxia type 1: one-year longitudinal study to identify clinical and MRI measures of disease progression in patients and presymptomatic carriers. Cerebellum. 2022;21(1):133–44. 10.1007/s12311-021-01285-0. [DOI] [PubMed]
- 60.Diallo A, Jacobi H, Tezenas du Montcel S, Klockgether T. Natural history of most common spinocerebellar ataxia: a systematic review and meta-analysis. J Neurol. 2021;268:2749–56. doi: 10.1007/s00415-020-09815-2. [DOI] [PubMed] [Google Scholar]
- 61.Reetz K, Dogan I, Hilgers RD, Giunti P, Mariotti C, Durr A, Boesch S, Klopstock T, de Rivera FJR, Schols L, Klockgether T, Burk K, Rai M, Pandolfo M, Schulz JB, Group ES Progression characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS): a 2 year cohort study. Lancet Neurol. 2016;15:1346–54. doi: 10.1016/S1474-4422(16)30287-3. [DOI] [PubMed] [Google Scholar]
- 62.Jacobi H, Reetz K, du Montcel ST, Bauer P, Mariotti C, Nanetti L, Rakowicz M, Sulek A, Durr A, Charles P, Filla A, Antenora A, Schols L, Schicks J, Infante J, Kang JS, Timmann D, Di Fabio R, Masciullo M, Baliko L, Melegh B, Boesch S, Burk K, Peltz A, Schulz JB, Dufaure-Gare I, Klockgether T. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. Lancet Neurol. 2013;12:650–658. doi: 10.1016/S1474-4422(13)70104-2. [DOI] [PubMed] [Google Scholar]
- 63.Stephen CD, Schmahmann JD. Eye movement abnormalities are ubiquitous in the spinocerebellar ataxias. Cerebellum (London, England) 2019;18:1130–1136. doi: 10.1007/s12311-019-01044-2. [DOI] [PubMed] [Google Scholar]
- 64.Administration USFD. Patient-focused drug development: selecting, developing, or modifying fit-for-purpose clinical outcome assessments. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-selecting-developing-or-modifying-fit-purpose-clinical-outcome. Accessed 9th Sept 2022.
- 65.Chang Z, Chen Z, Stephen CD, Schmahmann JD, Wu HT, Sapiro G, Gupta AS. Accurate detection of cerebellar smooth pursuit eye movement abnormalities via mobile phone video and machine learning. Sci Rep. 2020;10:18641. doi: 10.1038/s41598-020-75661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anastasopoulos D, Haslwanter T, Fetter M, Dichgans J. Smooth pursuit eye movements and otolith-ocular responses are differently impaired in cerebellar ataxia. Brain J Neurol. 1998;121(Pt 8):1497–1505. doi: 10.1093/brain/121.8.1497. [DOI] [PubMed] [Google Scholar]
- 67.Bour LJ, van Rootselaar AF, Koelman JH, Tijssen MA. Oculomotor abnormalities in myoclonic tremor: a comparison with spinocerebellar ataxia type 6. Brain J Neurol. 2008;131:2295–2303. doi: 10.1093/brain/awn177. [DOI] [PubMed] [Google Scholar]
- 68.Bürk K, Fetter M, Skalej M, Laccone F, Stevanin G, Dichgans J, Klockgether T. Saccade velocity in idiopathic and autosomal dominant cerebellar ataxia. J Neurol Neurosurg Psychiatry. 1997;62:662–664. doi: 10.1136/jnnp.62.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crowdy KA, Hollands MA, Ferguson IT, Marple-Horvat DE. Evidence for interactive locomotor and oculomotor deficits in cerebellar patients during visually guided stepping. Exp Brain Res. 2000;135:437–454. doi: 10.1007/s002210000539. [DOI] [PubMed] [Google Scholar]
- 70.Kim JS, Kim JS, Youn J, Seo DW, Jeong Y, Kang JH, Park JH, Cho JW. Ocular motor characteristics of different subtypes of spinocerebellar ataxia: distinguishing features. Mov Disord Off J Mov Disord Soc. 2013;28:1271–1277. doi: 10.1002/mds.25464. [DOI] [PubMed] [Google Scholar]
- 71.Yue Q, Jen JC, Nelson SF, Baloh RW. Progressive ataxia due to a missense mutation in a calcium-channel gene. Am J Hum Genet. 1997;61:1078–1087. doi: 10.1086/301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zee DS, Yee RD, Cogan DG, Robinson DA, Engel WK. Ocular motor abnormalities in hereditary cerebellar ataxia. Brain J Neurol. 1976;99:207–234. doi: 10.1093/brain/99.2.207. [DOI] [PubMed] [Google Scholar]
- 73.Saglam M, Lehnen N. Gaze stabilization in chronic vestibular-loss and in cerebellar ataxia: interactions of feedforward and sensory feedback mechanisms. J Vestib Res Equilibrium Orientation. 2014;24:425–431. doi: 10.3233/VES-140538. [DOI] [PubMed] [Google Scholar]
- 74.Ciuffreda KJ, Kenyon RV, Stark L. Eye movements during reading: further case reports. Am J Optom Physiol Opt. 1985;62:844–852. doi: 10.1097/00006324-198512000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Wessel K, Moschner C, Wandinger KP, Kömpf D, Heide W. Oculomotor testing in the differential diagnosis of degenerative ataxic disorders. Arch Neurol. 1998;55:949–956. doi: 10.1001/archneur.55.7.949. [DOI] [PubMed] [Google Scholar]
- 76.Bürk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, Didierjean O, Brice A, Klockgether T. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain J Neurol. 1996;119(Pt 5):1497–1505. doi: 10.1093/brain/119.5.1497. [DOI] [PubMed] [Google Scholar]
- 77.Buttner N, Geschwind D, Jen JC, Perlman S, Pulst SM, Baloh RW. Oculomotor phenotypes in autosomal dominant ataxias. Arch Neurol. 1998;55:1353–1357. doi: 10.1001/archneur.55.10.1353. [DOI] [PubMed] [Google Scholar]
- 78.Kerber KA, Jen JC, Perlman S, Baloh RW. Late-onset pure cerebellar ataxia: differentiating those with and without identifiable mutations. J Neurol Sci. 2005;238:41–45. doi: 10.1016/j.jns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Rufa A, Federighi P. Fast versus slow: different saccadic behavior in cerebellar ataxias. Ann N Y Acad Sci. 2011;1233:148–154. doi: 10.1111/j.1749-6632.2011.06126.x. [DOI] [PubMed] [Google Scholar]
- 80.Pretegiani E, Piu P, Rosini F, Federighi P, Serchi V, Tumminelli G, Dotti MT, Federico A, Rufa A. Anti-saccades in cerebellar ataxias reveal a contribution of the cerebellum in Executive functions. Front Neurol. 2018;9:274. doi: 10.3389/fneur.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghasia FF, Wilmot G, Ahmed A, Shaikh AG. Strabismus and micro-opsoclonus in Machado-Joseph disease. Cerebellum (London, England) 2016;15:491–497. doi: 10.1007/s12311-015-0718-0. [DOI] [PubMed] [Google Scholar]
- 82.Caspi A, Zivotofsky AZ, Gordon CR. Multiple saccadic abnormalities in spinocerebellar ataxia type 3 can be linked to a single deficiency in velocity feedback. Invest Ophthalmol Vis Sci. 2013;54:731–738. doi: 10.1167/iovs.12-10689. [DOI] [PubMed] [Google Scholar]
- 83.Lemos J, Novo A, Duque C, Castelhano J, Eggenberger E, Januário C. "Pinball" intrusions in spinocerebellar ataxia type 3. Neurology. 2018;90:36–37. doi: 10.1212/wnl.0000000000004772. [DOI] [PubMed] [Google Scholar]
- 84.Gordon CR, Zivotofsky AZ, Caspi A. Impaired vestibulo-ocular reflex (VOR) in spinocerebellar ataxia type 3 (SCA3): bedside and search coil evaluation. J Vestib Res Equilibrium Orientation. 2014;24:351–355. doi: 10.3233/ves-140527. [DOI] [PubMed] [Google Scholar]
- 85.Geisinger D, Elyoseph Z, Zaltzman R, Mintz M, Gordon CR. Angular vestibulo ocular reflex loss with preserved saccular function in Machado-Joseph disease. J Neurol Sci. 2021;424:117393. doi: 10.1016/j.jns.2021.117393. [DOI] [PubMed] [Google Scholar]
- 86.Ribeiro RS, Pereira MM, Pedroso JL, Braga-Neto P, Barsottini OG, Manzano GM. Cervical and ocular vestibular evoked potentials in Machado-Joseph disease: Functional involvement of otolith pathways. J Neurol Sci. 2015;358:294–298. doi: 10.1016/j.jns.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 87.Takegoshi H, Murofushi T. Vestibular evoked myogenic potentials in patients with spinocerebellar degeneration. Acta Otolaryngol. 2000;120:821–824. doi: 10.1080/000164800750061660. [DOI] [PubMed] [Google Scholar]
- 88.Christova P, Anderson JH, Gomez CM. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch Neurol. 2008;65:530–536. doi: 10.1001/archneur.65.4.530. [DOI] [PubMed] [Google Scholar]
- 89.Takeichi N, Fukushima K, Sasaki H, Yabe I, Tashiro K, Inuyama Y. Dissociation of smooth pursuit and vestibulo-ocular reflex cancellation in SCA-6. Neurology. 2000;54:860–866. doi: 10.1212/wnl.54.4.860. [DOI] [PubMed] [Google Scholar]
- 90.Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, Zee DS, Clark HB, Anderson JH. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42:933–950. doi: 10.1002/ana.410420616. [DOI] [PubMed] [Google Scholar]
- 91.Matsuda S, Matsumoto H, Furubayashi T, Fukuda H, Emoto M, Hanajima R, Tsuji S, Ugawa Y, Terao Y. Top-down but not bottom-up visual scanning is affected in hereditary pure cerebellar ataxia. PloS one. 2014;9:e116181. doi: 10.1371/journal.pone.0116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, Taylor AM, Zee DS. Gaze fixation deficits and their implication in ataxia-telangiectasia. J Neurol Neurosurg Psychiatry. 2009;80:858–864. doi: 10.1136/jnnp.2008.170522. [DOI] [PubMed] [Google Scholar]
- 93.Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, Carey JP, Nguyen KD, Zee DS. Ataxia telangiectasia: a "disease model" to understand the cerebellar control of vestibular reflexes. J Neurophysiol. 2011;105:3034–3041. doi: 10.1152/jn.00721.2010. [DOI] [PubMed] [Google Scholar]
- 94.Bremova T, Krafczyk S, Bardins S, Reinke J, Strupp M. Vestibular function in patients with Niemann-Pick type C disease. J Neurol. 2016;263:2260–2270. doi: 10.1007/s00415-016-8247-4. [DOI] [PubMed] [Google Scholar]
- 95.Solomon D, Winkelman AC, Zee DS, Gray L, Büttner-Ennever J. Niemann-Pick type C disease in two affected sisters: ocular motor recordings and brain-stem neuropathology. Ann N Y Acad Sci. 2005;1039:436–445. doi: 10.1196/annals.1325.041. [DOI] [PubMed] [Google Scholar]
- 96.Moreno-Ajona D, Álvarez-Gómez L, Manrique-Huarte R, Rivas E, Martínez-Vila E, Pérez-Fernández N. VEMPs and dysautonomia assessment in definite cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a case series study. Cerebellum. 2021;20(5):717–23. 10.1007/s12311-019-01061-1. [DOI] [PubMed]
- 97.Yacovino DA, Zanotti E, Hain TC. Is cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS) a vestibular ganglionopathy? J Int Adv Otol. 2019;15:304–308. doi: 10.5152/iao.2019.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rey-Martinez J, Batuecas-Caletrio A, Matino E, Trinidad-Ruiz G, Altuna X, Perez-Fernandez N. Mathematical methods for measuring the visually enhanced vestibulo-ocular reflex and preliminary results from healthy subjects and patient groups. Front Neurol. 2018;9:69. doi: 10.3389/fneur.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.