Abstract
The 37-kDa recombinant protein Asp f 2, encoding an allergen of Aspergillus fumigatus, was expressed in a prokaryotic expression system and immunologically evaluated for its functional and structural properties. The open reading frame for a 310-amino-acid-long protein was shown to encode a signal peptide of 31 amino acids. A native 37-kDa culture filtrate protein and a 55-kDa mycelial glycoprotein (gp55) exhibited complete N-terminal sequence homology to Asp f 2. A GenBank search for homologous proteins revealed 60 and 44% sequence homologies to the cytosolic protein ASPND1 from Aspergillus nidulans and fibrinogen binding protein from Candida albicans, respectively. The glycosylation sites and cysteine molecules are conserved in all the three proteins. The extracellular matrix protein laminin showed a dose-dependent interaction with Asp f 2. This protein, expressed as a major cell-associated protein within 24 h of in vitro fungal culture, comprises 20 to 40% of total fungal protein. Furthermore, both native and recombinant Asp f 2 exhibited specific immunoglobulin (IgE) binding with allergic bronchopulmonary aspergillosis (ABPA) and cystic fibrosis-ABPA patients, whereas A. fumigatus-sensitized allergic asthma and normal control subjects failed to show IgE binding with Asp f 2. These results indicate that Asp f 2 is a major allergen of A. fumigatus exhibiting IgE antibody binding with sera from patients with ABPA. The antigen should be explored further for its potential role in the differential diagnosis of A. fumigatus-associated allergic diseases.
One of the major Aspergillus species responsible worldwide for fungal respiratory disorders is the opportunistic pathogen Aspergillus fumigatus. It is associated with a wide spectrum of diseases in humans and animals such as hypersensitivity pneumonitis, allergic asthma (AA), allergic bronchopulmonary aspergillosis (ABPA), and aspergilloma as well as invasive aspergillosis in immunocompromised and immunodeficient patients (5, 6, 29, 51). ABPA is the most severe A. fumigatus-induced respiratory disease and is usually found in atopic individuals (19–21). Although previously considered a rare disease, ABPA is currently reported to occur in about 1% of A. fumigatus-sensitized asthmatics, while in patients with cystic fibrosis (CF) the incidence varies from 10 to 35% (7, 21, 32, 40). The major diagnostic criteria for ABPA are a history of asthma, immediate wheal and flare, immunoglobulin E (IgE) mast cell-mediated cutaneous reactivity to A. fumigatus, elevated total serum IgE (usually >1,000 ng/ml), elevated serum A. fumigatus-specific IgE and IgG, pulmonary infiltrates, and central bronchiectasis (CB) (19–21, 48). However, these diagnostic criteria are not always present at the same time even in patients with classic ABPA. In addition, CB and pulmonary infiltrates, the hallmarks of ABPA, also occur in CF patients without ABPA. Therefore, the serological detection of A. fumigatus-specific antibodies in patients is considered to be of diagnostic importance in the differential diagnosis of Aspergillus-induced asthma from ABPA and indistinguishable ABPA in CF patients.
The antigens and allergens of A. fumigatus comprise a heterogeneous mixture of proteins, carbohydrates, and glycoproteins (8, 22). Although several A. fumigatus antigenic components have been purified and characterized by various methods, there is still a paucity of purified and characterized antigens for reliable immunodiagnosis of A. fumigatus-induced diseases (2, 29–31, 33, 44, 45, 47). In a recent study, cytoplasmic A. fumigatus allergens were reported to be recognized exclusively by serum IgE of ABPA patients, whereas secretory proteins were recognized by IgE antibodies in A. fumigatus-sensitized asthmatics as well as ABPA patients (13). Hence, purification and characterization of various cell-associated and metabolic A. fumigatus proteins may aid in the differential diagnosis of A. fumigatus-induced diseases. With the advent of DNA technologies and biotechnological procedures, cloning and production of large amounts of pure A. fumigatus allergens are now possible (1, 4, 26, 37). Recently, phage surface display technologies for cloning cDNA have been used for isolation and characterization of a number of major and minor A. fumigatus allergens (13–16, 23).
Recently we have reported the partial nucleotide sequence of a cDNA clone representing the C-terminal region of a major A. fumigatus allergen, Asp f 2 (4). Here we present the complete nucleotide sequence of Asp f 2 and expression of the mature recombinant protein. The expression kinetics and immunochemical properties of both the native and recombinant allergen were studied. The results indicate that Asp f 2 is a major allergen of A. fumigatus, recognizing specific IgE antibodies in ABPA patients.
MATERIALS AND METHODS
The construction of a cDNA library in the λ ZAPII vector was carried out as described previously (26). As the cDNA clone was found to be incomplete at the N-terminal region, a genomic library of A. fumigatus carrying inserts of 9 to 22 kb in λ Fix II vector (Stratagene, La Jolla, Calif.) was screened by using a [32P]dATP-labeled cDNA clone, and a positive plaque was identified. The Asp f 2 gene was amplified by PCR using λ left-arm sequences as the sense primer 5′ATTTGATTACAATTTTGTCCCACTC 3′; the antisense primer 5′CTAAGTGCAATGAAGCTGTCCACC 3′ was designed from the C-terminal-end sequences of Asp f 2 and using λ DNA isolated from the amplified plaque as the template. Long-range PCR was carried out with an XL PCR kit as specified by the manufacturer (Perkin-Elmer, Foster City, Calif.). The resulting 3,000-bp product carrying the complete Asp f 2 gene was then cloned in a TA vector by using a PCR 2.1 cloning kit (Invitrogen, San Diego, Calif.) and sequenced by the chain termination method (49).
Megaprimer PCR amplification of the Asp f 2 gene.
The Asp f 2 gene for overexpression was obtained by megaprimer PCR amplification. A 618-bp PCR product was amplified from the partial cDNA clone of Asp f 2 by using sense primer 5′GTCGGTGCCTACGATGTCATC 3′ and the C-terminal 5′AGTGCAATGAAGCTGTCCACCTTC3′ sequence of Asp f 2 as the antisense primer. This PCR product was used further as the antisense primer along with the Asp f 2 N-terminal sequence 5′GACGCTGGCGCGGTGACCTCGT 3′ as the sense primer for PCR amplification of the complete Asp f 2 gene. The PCR reaction was performed with 300 ng of megaprimer and 80 ng of sense primer; the template was 20 ng of TA cloned Asp f 2 DNA obtained from genomic library of A. fumigatus. The amplification conditions were 30 cycles of 30 s at 94°C and 11 min at 65°C. An amplified PCR product of 804 bp was purified from the gel, subcloned into PCR 2.1 vector (Invitrogen), and sequenced by the dideoxy-chain termination method (49).
Expression of Asp f 2 protein.
To express mature Asp f 2 in Escherichia coli, the amplified PCR product with a BamHI site at the N-terminal end and XhoI site at the C-terminal end was cloned and expressed in pET 23b(+) vector as previously described (4). Recombinant Asp f 1 (rAsp f 1) and rAsp f 12 were expressed in the same way in the pET expression system.
Purification of Asp f 2.
rAsp f 2 was expressed in the pET system along with a six-histidine tag at its C-terminal end; a Ni2+-nitrilotriacetic acid agarose column (Qiagen, Santa Clarita, Calif.) was used to purify the proteins. Other A. fumigatus antigens such as native Asp f 2 (nAsp f 2) and culture filtrate antigens (AF102 and AF104) were obtained as described earlier (29). The cytosolic fraction complex (CFC) from A. fumigatus and ASPND1 protein from a mycelial extract of Aspergillus nidulans were gifts from F. Leal (University of Salamanca, Salamanca, Spain).
Production of polyclonal antibodies.
Three purified A. fumigatus proteins, rAsp f 2 and two nAsp f 2 proteins isolated from separate strains of A. fumigatus, were used to immunize BALB/c mice. In brief, 50 μg of purified protein was emulsified in an equal volume of complete Freund’s adjuvant and injected subcutaneously. Three consecutive injections were given at weekly intervals with antigen mixed in incomplete Freund’s adjuvant. The animals were bled, and anti-Asp f 2 antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Animals were bled by cardiac puncture, and the serum was stored at −70°C. The rabbit polyclonal serum against the p40 component of A. fumigatus was obtained from F. Leal. All animal studies were approved by the institutional animal studies committee.
Human serum samples.
Serum samples from three groups of patients were used: 10 patients with CF who also had the diagnostic criteria of ABPA (CF/ABPA group), 10 patients with asthma and with ABPA (ABPA group) and 10 patients with asthma with immediate wheal and flare skin reactivity to A. fumigatus and without sufficient features of ABPA (AA group). Sera from the CF/ABPA patients satisfying the criteria for ABPA as reported previously were obtained from the regional CF center at the Medical College of Wisconsin (39). ABPA and AA patients were seen at the Division of Allergy-Immunology of the Northwestern University Medical School or the Medical College of Wisconsin. Serum samples from 10 control (healthy) subjects also were selected. The human study committees of the Medical College of Wisconsin and Northwestern University Medical School approved this investigation.
Aspergillus mycelial extract and culture filtrate antigen preparation.
Aspergillus conidia (107/ml) were grown in Czapek-Dox–AOAC (1:1) liquid medium at 37°C under continuous shaking to isolate mycelial antigens. The cultures were harvested at 24 h, 48 h, 72 h, 96 h, 5 days, 7 days, 14 days, and 21 days, and the mycelia and culture filtrates were collected separately. The mycelia were washed and disrupted in a French press at a pressure of 16,000 lb/in2 and centrifuged. The supernatants were dialyzed against distilled water, and the retentate was freeze-dried (12). The culture filtrates were dialyzed and lyophilized in the same way as mycelial extracts. The protein concentrations of the extracts were determined by BCA (bicinchoninic acid) assay (BCA kit; Pierce Chemicals, Rockford, Ill.).
Kinetics of Asp f 2 production.
An ELISA was carried out to measure Asp f 2 harvested at different time intervals from culture filtrates and mycelial extracts. The amount of Asp f 2 in the preparations was detected by treating antigen (1 μg/ml)-coated microtiter plates with mouse anti-rAsp f 2 antibodies (1:500) for 3 h (27). The subsequent steps, including the addition of biotinylated anti-mouse IgG, enzyme, and substrate, were as described previously (27). Asp f 2 concentrations at different stages of A. fumigatus growth were calculated from a standard curve plotted for concentration of Asp f 2 ranging from 10 to 500 ng/ml.
Antigen-specific ELISA.
The purified rAsp f 2 was evaluated for IgE antibody binding with sera from the various subject groups by ELISA as previously described (10).
Inhibition ELISA.
Monospecific mouse sera raised against recombinant and native Asp f 2 were diluted 1:500 and incubated with various concentrations of rAsp f 2 (1 ng to 10 μg) overnight at 4°C. The serum samples were centrifuged, and the supernatants were transferred to the washed and blocked nAsp f 2-coated plates. Further steps in the assay were carried out with reagents as described before (3, 4). The dose-dependent inhibition of antibody titers was determined from the ELISA (absorbance optical density [OD] values) compared with uninhibited serum.
Asp f 2 production in A. fumigatus cultures.
Antigenic fractions from mycelia and culture filtrates collected at various stages of growth (5 μg per sample) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel and transferred onto a nitrocellulose membrane by using a trans-blot apparatus as described previously (4). After blocking and washing, the membranes were treated with anti-rAsp f 2 antibodies (1:250) and reactivity was analyzed by Western blotting (4).
Western blotting of various Aspergillus antigens with anti-Asp f 2 and anti-p40 antibodies.
The antigenic similarities between Asp f 2, ASPND1 from A. nidulans, and other A. fumigatus antigenic components were analyzed by Western blotting using mouse antisera against rAsp f 2 and rabbit antisera against p40 protein from the cytosolic fraction of A. fumigatus culture. The culture filtrate proteins from strain AF102, the CFC of A. fumigatus (consisting mainly of proteins p90, p60, p40, and p37), rAsp f 2, and protein ASPND1 purified from mycelial extracts of A. nidulans were separated by SDS-PAGE (12% gel) at a concentration of 5 μg sample per lane and transferred onto a nitrocellulose membrane, and reactivities of the resolved proteins were determined by Western blot assay as described previously (28, 29).
IgE binding of Asp f 2 and ASPND1.
An antigen-specific ELISA was carried out to compare the IgE binding of ASPND1 to that of Asp f 2, using sera from three groups of subjects. Ten individual serum samples from the ABPA, AA, and control groups were used, and the ELISA was carried out as described above (3, 27).
Laminin-Asp f 2 interaction.
Wells of microtiter plates were coated overnight with rAsp f 1, rAsp f 2, rAsp f 12, nAsp f 2, and A. fumigatus culture filtrate antigens at 100 μg/ml and incubated first with 10 μg of laminin (Boehringer Mannheim) per ml for 3 h at room temperature and then with mouse antilaminin antibody (Sigma, St. Louis, Mo.) at a 1:1,000 dilution for 1 h. The subsequent incubations were with biotinylated goat anti-mouse IgG (1:1,000) and streptavidin peroxidase (1:10,000) for 1 h each. The color reaction was developed, and the intensity of the reaction was measured as OD as described previously (3, 4).
The binding of Asp f 2 to laminin was evaluated by ELISA where the wells of the microtiter plate were coated with Asp f 2 (4 μg/ml) followed by addition of laminin (1 ng to 10 μg). In another set of experiments, laminin (1 ng to 10 μg) was added directly to the wells. Further steps include addition of mouse antilaminin antibody, biotinylated goat anti-mouse IgG, streptavidin peroxidase, and chromogen substrate as described earlier (3, 4). The reactivities in the two plates were compared.
Statistical analysis.
The mean (± standard deviation [SD]) OD values of antigen-antibody reactions by ELISA using serum samples from the four groups of subjects were compared with those of the control group by using Student’s t test (two-tailed independent samples) with the Statworks program (Cricket Software, Inc., Philadelphia, Pa.). Values for ABPA, CF/ABPA, AA, and control groups were compared, and P < 0.05 was taken as significant.
Nucleotide sequence accession number.
The GenBank accession no. for Asp f 2 is U56938.
RESULTS
Nucleotide and deduced amino acid sequences of Asp f 2.
We recently reported the expression of a truncated protein from a cDNA clone of A. fumigatus having strong allergenic activity (4). In the present study, in order to isolate the complete Asp f 2 gene, this cDNA clone was labeled with [32P]dATP and used as a probe to screen the A. fumigatus genomic library. Eleven positive plaques were identified by screening 50,000 plaques. A PCR-amplified product of 939 bp was detected in all plaque lysates, indicating the presence of the correct inserts. One of the 11 plaques was selected for further cloning and sequencing. With isolated λ DNA as the template, the λ left-arm primer as the sense primer, and the C-terminal-end nucleotide sequences from the cDNA clone as the antisense primer, a 3,000-bp PCR product was obtained. The PCR product was cloned into PCR 2.1 vector, and the plasmid DNA was sequenced. The complete nucleotide sequence of the Asp f 2 gene is shown in Fig. 1. Comparison of the cDNA and genomic sequences shows that the Asp f 2 gene consists of three exons encoding a 310-amino-acid-long protein interrupted by two introns of 83 and 52 bp. Both introns have 5′GT and 3′AG dinucleotides at the intron-exon junctions involved in the splicing process, and four possible glycosylation sites are located at Asn 57, 87, 143, and 216 (Fig. 1, boldface). The deduced amino acid sequence of this gene exhibits complete homology with the amino acid sequence of the cDNA clone starting from Glu 65 to the C-terminal-end Thr 310. However, the N-terminal amino acid residues of the nAsp f 2 are located within the open reading frame starting from Asp 43 (Fig. 1, underlined). The N-terminal 20-amino-acid sequence of a previously reported A. fumigatus glycoprotein (gp55) exhibits complete homology to Asp f 2 from Ala 32 to Pro 51 (Fig. 1, italics). This sequence is preceded by a 31-amino-acid-long probable signal peptide with a stretch of hydrophobic amino acids.
FIG. 1.
Complete nucleotide sequence and the deduced amino acid sequence of the Asp f 2 gene of A. fumigatus. The intron sequences are represented in lowercase. The 20 N-terminal amino acids of the purified native mycelial protein gp55 demonstrate sequence homology from amino acid 32 onward, as represented in italics. The A. fumigatus culture filtrate protein with N-terminal sequence homology from Asp 43 onward are underlined; glycosylation sites are in boldface.
Sequence homology of Asp f 2 with cell wall-associated proteins.
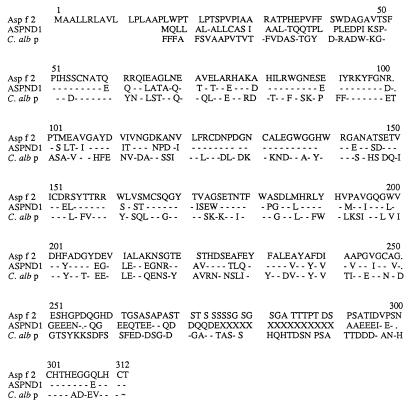
A search of GenBank by using the BLAST program revealed a high degree of sequence homology to ASPND1, a recently described protein from A. nidulans, as well as to cell wall-associated proteins from Candida albicans (12). Figure 2 shows the sequence alignment of Asp f 2, ASPND1, and fibrinogen binding protein from C. albicans. Asp f 2 and ASPND1 exhibited 60% sequence identity and 75% similarity, whereas Asp f 2 had about 44% identity and 65% similarity with fibrinogen binding protein and a similar degree of identity with pH-regulated antigen 1 (PRA1) from C. albicans (12, 50). The four possible glycosylation sites and eight cysteine molecules present in Asp f 2 also are conserved in ASPND1 and C. albican proteins.
FIG. 2.
Conserved sequences of ASPND1 from A. nidulans, a fibrinogen binding protein from C. albicans (C. alb p), and Asp f 2. The cysteine molecules and glycosylation sites are conserved in all the three proteins.
Recombinant and native Asp f 2 are similar in structural conformation.
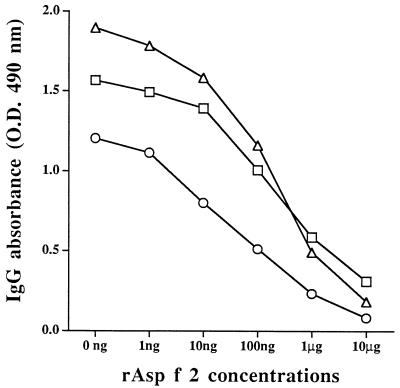
To evaluate the specificity of anti-rAsp f 2 antibodies, an ELISA inhibition was carried out with mouse antibodies raised against rAsp f 2, nAsp f 2 (AF104), and nAsp f 2 (AF102). The antibodies against these three proteins showed binding to solid-phase coated nAsp f 2 (AF104). This binding was inhibited by increasing amounts of recombinant Asp f 2 (Fig. 3). We were able to inhibit more than 80% binding of these antibodies to solid-phase coated nAsp f 2. For anti-nAsp f 2 (AF104), 550 ng of rAsp f 2 was required to obtain 50% inhibition, whereas anti-rAsp f 2 and anti-nAsp f 2 (AF102) required about 75 ng of rAsp f 2 to exhibit 50% inhibition of binding to solid-phase coated native protein.
FIG. 3.
Inhibition of IgG binding to solid-phase coated nAsp f 2. Polyclonal mouse sera raised against nAsp f 2 AF102 (▵), nAsp f 2 AF104 (□), and rAsp f 2 (○) were preincubated overnight with different amounts of rAsp f 2 as indicated on the x axis. Preincubated serum samples were transferred to nAsp f 2 (AF104)-coated wells, and antibody binding was measured.
High expression of Asp f 2 in mycelial extract.
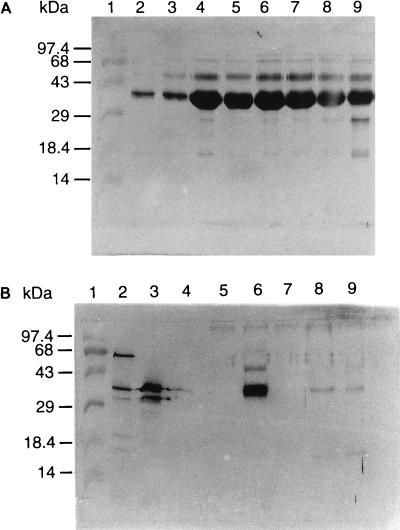
The time kinetics of Asp f 2 production in A. fumigatus culture (AF104) were analyzed by comparing Asp f 2 levels in culture filtrates and mycelial extracts over 21 days of shake culture. Asp f 2 could be detected in the mycelial growth within 24 h of culture. This protein represented about 20% of total mycelial proteins at 96 h and 40% by day 7. This increase was followed by a gradual decrease and stabilization at 30% during weeks 2 and 3 of culture (Table 1). The culture filtrates collected at the same intervals of growth after harvesting of the mycelium demonstrated significantly less secreted Asp f 2. The amount of Asp f 2 varied from 1.6% at 24 h to 0.5% at the 3 weeks of culture. In Western blot analysis using mouse sera against rAsp f 2, both mycelial and culture filtrate preparations at various time intervals exhibited IgG binding in the molecular mass range of 35 to 37 kDa. More than one protein band in this narrow range exhibited distinct antibody binding (Fig. 4). Although the intensity of the antibody binding was different, the mycelial extract showed a very high level of Asp f 2 even at 24 h, whereas the 24-h, 48-h, and 5-day culture filtrates exhibited binding with anti-Asp f 2 in the molecular mass range of 35 to 37 kDa. The reason for complete absence of secretory Asp f 2 at 72 h, 96 h, and 7 days of culture was not clear; it may be due to the involvement of different secretory pathways and regulatory mechanisms at different stages of fungal growth or due to the metabolic degradation of the protein. On the other hand, the stationary culture filtrates collected at various intervals of time exhibited a gradual increase in Asp f 2 production from day 2 onward, reaching the highest concentration at 2 weeks (data not shown). The variation in antigenic profiles of culture filtrates from stationary and shake cultures of A. fumigatus could be due to the complex nature of the fungal morphology and metabolic pathways. For the culture filtrates, a number of proteins appeared to have weak antibody binding with anti-Asp f 2 in the wide range of 18 to 100 kDa (Fig. 4B).
TABLE 1.
Asp f 2 concentration and pH of A. fumigatus culture (AF104) at various time intervals
| Incubation time | Mycelial wt (g) | pH of culture medium | Asp f 2 concn (1,000 ng/ml of crude extract)a
|
|
|---|---|---|---|---|
| Culture filtrate | Mycelia | |||
| 0 h | 7.25 | |||
| 12 h | 0.276 | 7.2 | 10 | |
| 24 h | 0.723 | 6.9 | 16 | 11 |
| 48 h | 3.3 | 6.4 | 8 | 69 |
| 72 h | 3.7 | 5.7 | 12 | 185 |
| 96 h | 5.8 | 5.9 | 9 | 200 |
| 5 days | 6.32 | 5.7 | 7 | 281 |
| 7 days | 8.4 | 5.65 | 9 | 403 |
| 14 days | 4.9 | 4.5 | 9 | 284 |
| 21 days | 3.7 | 4.35 | 5 | 291 |
Calculated from the Asp f 2 standard curve by ELISA, using concentrations of 10 to 500 ng of Asp f 2/ml versus anti-Asp f 2 antibody.
FIG. 4.
Time kinetics of expression of Asp f 2 in A. fumigatus mycelial extracts. Mycelial proteins (5 μg/ml) at various intervals of growth were separated by SDS-PAGE on a 12% gel and transferred onto nitrocellulose membranes. Separated proteins were evaluated for antibody binding by using polyclonal mouse sera against rAsp f 2. Lane 1, molecular weight markers. Different time interval cultures: lane 2, 24 h; lane 3, 48 h; lane 4, 72 h; lane 5, 96 h; lane 6, 5 days; lane 7, 7 days; lane 8, 14 days; lane 9, 21 days. (B) Time kinetics of expression of Asp f 2 in A. fumigatus culture filtrates. The culture filtrate proteins (5 μg/ml) were separated by SDS-PAGE on a 12% gel and transferred onto nitrocellulose membranes. Separated proteins in lanes 2 to 9 are culture filtrates at the same time intervals as for mycelial extracts (A) and were evaluated for antibody binding by using polyclonal mouse sera against rAsp f 2.
ASPND1 and Asp f 2 share common epitopes.
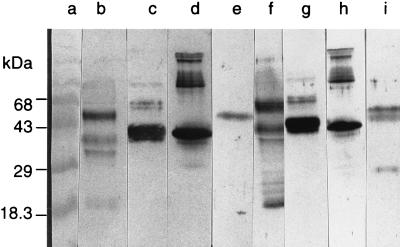
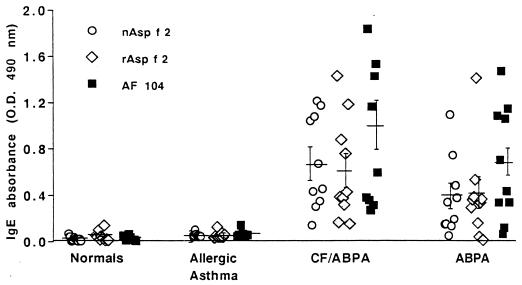
ASPND1, the protein present in the water-soluble extract from A. nidulans, exhibited significant sequence homology with Asp f 2. To ascertain the presence of common epitopes in these proteins, antibody binding of ASPND1 was evaluated by using polyclonal sera against rAsp f 2 and the p40 component of CFC isolated from A. fumigatus culture. In Western blots, rAsp f 2 and ASPND1 exhibited strong IgG binding with sera against rAsp f 2 (Fig. 5, lanes d and e, respectively). Similarly, anti-p40 antibody exhibited strong IgG binding with rAsp f 2 as well as ASPND1 (Fig. 5, lanes h and i, respectively). However, ASPND1 when treated with anti-p40 antibodies demonstrated two additional IgG binding proteins at lower molecular masses. At the same time, the CFC component of A. fumigatus (composed of p90, p60, p40, and p37 antigens) showed similar antibody binding with anti-rAsp f 2 and anti-p40 antibodies, with a doublet type of reaction at 35 to 40 kDa (Fig. 5, lanes c and g). Crude A. fumigatus culture filtrate (AF102) showed multiple anti-Asp f 2 as well as anti-p40 antibody binding proteins in the molecular mass range of 18 to 70 kDa (Fig. 5, lanes b and f). These results strongly suggest the presence of common antigenic epitopes in Asp f 2, ASPND1, and p40 A. fumigatus antigen. Purified ASPND1 and Asp f 2 were evaluated by using an indirect ELISA for specific IgE antibody binding in sera from ABPA (n = 10), AA (n = 10), and control (n = 10) groups (Fig. 6). In ELISAs, the mean IgE absorbance value (OD at 490 nm [OD490]) for ASPND1 with sera from ABPA patients was 0.25, compared to 0.05 for controls (P < 0.001). However, the mean IgE absorbance value for Asp f 2 in sera from ABPA patients was almost threefold higher (OD490 of 0.708) than that with ASPND1 (OD490 of 0.248).
FIG. 5.
Western blot analysis of various Aspergillus proteins, using mouse polyclonal sera against rAsp f 2 and rabbit sera against the p40 cytosolic component of A. fumigatus. Lanes: a, molecular weight markers; b to e, treated with anti-rAsp f 2 sera; f to i, treated with anti-p40 sera; b and f, A. fumigatus culture filtrate antigens (AF102); c and g, CFC fraction from A. fumigatus; d and h, rAsp f 2; e and i, ASPND1 from A. nidulans.
FIG. 6.
IgE binding of purified rAsp f 2 and ASPND1, using 10 serum samples from each of the indicated groups. Solid bars, ELISA absorbance values at 490 nm for rAsp f 2; shaded bars, absorbance values for ASPND1.
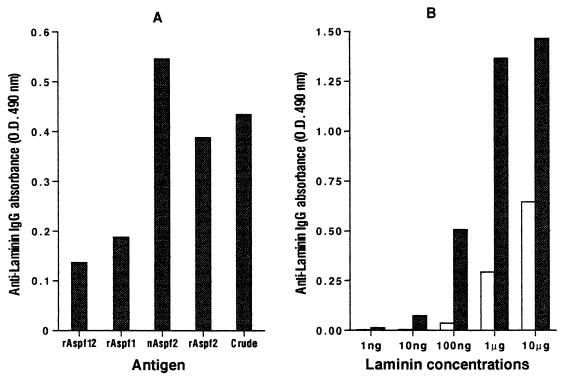
Specific binding of Asp f 2 to extracellular matrix protein laminin.
Both recombinant and native Asp f 2 demonstrated high binding affinity to laminin in a direct solid-phase ELISA. Laminin binding of Asp f 2 was more than twofold higher than that with rAsp f 1 and rAsp f 12. However, native Asp f 2 exhibited higher affinity than rAsp f 2 (Fig. 7A). For dose-dependent binding of laminin to Asp f 2 in wells coated with a constant concentration of Asp f 2, gradual increases in laminin concentration resulted in increased binding with antilaminin antibodies (Fig. 7B), indicating the specific interaction between laminin and Asp f 2. On the other hand, high antilaminin antibody binding was observed in wells directly coated with 100 ng of laminin or more. With 1 μg of laminin, direct laminin-antilaminin interaction demonstrated fourfold-higher ELISA absorbance (OD490 of 1.365) than with Asp f 2-coated plates (OD490 of 0.295).
FIG. 7.
(A) Specific binding of laminin to Asp f 2. rAsp f 1, rAsp f 12, rAsp f 2, nAsp f 2, and crude A. fumigatus culture filtrates (100 μg/well) were incubated with 10 μg of laminin per ml. The subsequent incubation was with a 1:1,000 dilution of mouse antilaminin antibody. The ELISA IgG absorbance was measured at 490 nm (for details, see Materials and Methods). (B) Dose-dependent binding of laminin (1 ng to 10 μg) to solid-phase coated Asp f 2. Open bars, IgG absorbance of laminin-antilaminin interaction on Asp f 2-coated wells; closed bars, absorbance values for direct laminin-antilaminin binding on laminin-coated wells.
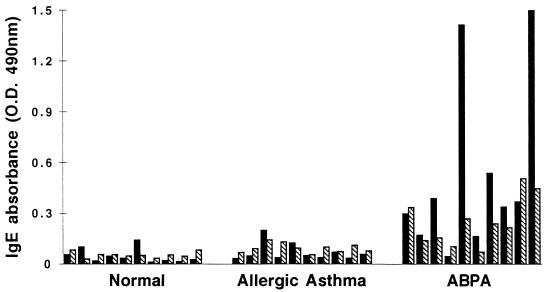
Specific IgE binding of Asp f 2 in ABPA sera.
The biologically active rAsp f 2, nAsp f 2, and crude culture filtrate AF104 were evaluated for IgE antibody with 10 serum samples from each of the CF/ABPA, ABPA, and AA groups of patients along with 10 healthy controls (Fig. 8). Serum IgE antibody against all of the antigens was elevated in CF/ABPA and ABPA patients in comparison with AA patients and controls. The mean IgE absorbance in CF/ABPA samples for both native and recombinant Asp f 2 was higher than in ABPA samples. The difference between rAsp f 2-specific IgE in CF/ABPA (mean 0.606, SD ± 0.438) and control (mean 0.048, SD ± 0.042) groups was statistically significant (P < 0.01). A significant difference was observed also for rAsp f 2-specific IgE antibody in ABPA (mean 0.408, SD ± 0.380) and control groups (P < 0.01).
FIG. 8.
IgE binding of nAsp f 2, rAsp f 2, and A. fumigatus culture filtrate (AF104) with 10 serum samples from each of the indicated groups.
DISCUSSION
Recently we reported the nucleotide sequence of a cDNA clone from A. fumigatus representing the C-terminal region of Asp f 2 (4). The recombinant protein expressed in E. coli without the N-terminal amino acid residues also exhibited distinct IgE binding with sera from ABPA patients and appeared to be a part of a major allergen from A. fumigatus. These results prompted us to isolate and characterize the gene carrying the complete nucleotide sequence for Asp f 2 and also to clone and overproduce the recombinant allergen.
The Asp f 2 sequence encodes a protein with a predicted molecular size of 29 kDa. However, on SDS-PAGE analysis, this protein exhibited a band at 37 kDa. Although the reasons for such differences are not clear, they could be attributed to the amino acids at the C-terminal of the protein. The involvement of C-terminal amino acids in slow migration of Asp f 2 in SDS-PAGE is evident from the differences in the molecular sizes of two recombinant polypeptides from Asp f 2, representing 200 amino acids from either the N- or C-terminal region of Asp f 2 (data not shown). The polypeptide encoding the C-terminal A. fumigatus region migrated during SDS-PAGE to a position about 5 to 6 kDa greater than its predicted size. Similar differences between the predicted molecular size of the native protein and the apparent size on SDS-PAGE have been reported for serine proteases from A. fumigatus, mycelial antigen from A. nidulans, and cell wall antigen from C. albicans (12, 24, 25, 36, 46, 50). For a Ca2+-dependent serine protease from Saccharomyces cerevisiae, the deletion experiments precisely demonstrated that the high negative change of the C-terminal region of the protein is responsible for its slow migration in gel (18).
Comparison of amino acid sequences of the product of the entire Asp f 2 gene with the N-terminal amino acid sequences of other purified proteins exhibited complete homology with a recently purified and characterized 37-kDa concanavalin A-nonbinding protein from an A. fumigatus AF104 culture filtrate and with another previously reported glycoprotein, gp55, characterized after isolation from a water-soluble extract from mycelium of strain NHL5759 (3, 52). Both native proteins demonstrated complete N-terminal sequence homology to the A. fumigatus gene characterized in this study, starting from different N-terminal regions on the same gene. This result indicates that the posttranslational modifications such as phosphorylation, glycosylation, and enzymatic cleavages may be responsible for the differences in the molecular weight of the same core protein under different culture conditions and in different strains of A. fumigatus. The 37-kDa culture filtrate protein as well as deglycosylated gp55 showed high IgE binding, indicating that the core protein without glycosylation in both molecules is involved in IgE binding. Another 58-kDa glycoprotein antigen exhibiting high IgG binding with sera from patients with aspergillosis appeared to be different from Asp f 2 and gp55, as the antibody binding of this protein was totally destroyed by treatment with sodium metaperiodate and partially destroyed by protease treatment (17, 52).
As the correct three-dimensional structure of the allergen is essential for IgE antibody binding, the Asp f 2 allergen overproduced in the prokaryotic expression system is functionally comparable with its native counterpart. In an ELISA inhibition study, mouse antisera against native Asp f 2 preincubated with rAsp f 2 exhibited more than 80% inhibition in binding to solid-phase coated native protein, indicating conformational similarities between these two proteins.
We have evaluated further the IgE antibody binding of recombinant and native Asp f 2 as well as crude culture filtrate in four groups of subjects. None of the AA patients, all of whom showed immediate cutaneous reactivity to A. fumigatus, exhibited IgE binding with Asp f 2 proteins, whereas all patients from the ABPA and CF/ABPA groups showed IgE binding. In a recent study, the polypeptide representing truncated Asp f 2 demonstrated distinct IgE antibody binding with ABPA-CB patients, whereas IgE antibodies in ABPA-S (seropositive for ABPA without CB) patients failed to show significant binding to this protein (4). These observations indicated the possible involvement of Asp f 2 in the acute phase of the disease and bronchial wall injury in patients. The typical immune response of Asp f 2 in ABPA-CB patients emphasized its use in differential diagnosis of ABPA and other A. fumigatus-sensitized patients as well as ABPA-S and ABPA-CB patients. Two recently reported recombinant A. fumigatus allergens, rAsp f 4, and rAsp f 6, also demonstrated distinct IgE binding with ABPA patient sera, and serodiagnosis with these two allergens together showed 100% specificity and greater than 90% sensitivity (13, 15, 23, 34). In the present study, we found 100% specificity in CF/ABPA patients and 90% in ABPA patients, while none of the controls showed significant levels of Asp f 2-specific IgE. The other recombinant A. fumigatus allergens also demonstrated distinct IgE binding in ABPA patients. However, they also reacted with IgE antibodies of A. fumigatus-sensitized non-ABPA patients, and this overlap in IgE binding lowers the specificity when these antigens are used together (13, 37, 38).
The levels of high IgE antibodies against Asp f 2 in CF/ABPA patients indicates the allergen’s usefulness in the diagnosis of patients with CF complicated by ABPA. In a recent study, intracellular rather than secretory A. fumigatus proteins were reported to show distinct IgE antibody binding in CF patients with ABPA but not in CF patients without ABPA (13, 41). Colonization of the fungus in the bronchi and the release of intracellular proteins may be responsible for sensitizing ABPA patients against nonsecretory fungal proteins, whereas the nonavailability of intracellular A. fumigatus protein has been attributed to the lack of specific IgE response against these allergens in A. fumigatus-sensitized allergic asthmatics.
Asp f 2, with its extensive sequence homology to fibrinogen binding protein of C. albicans as well as its specific binding to laminin, appears to be involved in fungal adherence to the extracellular matrix (9, 42, 43). Laminin and fibrinogen are the major structural proteins of the basement membrane and are mainly involved in mediating adherence of conidia to the extracellular matrix. To our knowledge, this is the first A. fumigatus allergen reported to show distinct binding to major extracellular matrix proteins. Thus, one of the initial steps in host colonization may be the recognition in patients of basement membrane laminin by Asp f 2. Recently, a 72-kDa cell wall surface component of A. fumigatus with receptors for laminin was reported (53). The recently reported C. albicans antigen PRA1 demonstrated pH-sensitive expression, with maximum expression at neutral pH and no expression below pH 6.0 (50). Although Asp f 2 exhibited high sequence homology and conserved glycosylation sites with PRA1, the mycelial expression of Asp f 2 appears to be unaffected by the pH of the medium. In shake culture, however, the secretion of Asp f 2 into the medium gradually decreases as the pH changes from neutral to acidic. We cannot explain the high expression of Asp f 2 only at day 5 when the culture is acidic (Fig. 4B), with continuous decreases in concentration in late cultures. The involvement of PRA1 in temperature-dependent hypha formation suggests a possible role of this protein family in fungal morphogenesis.
The extensive sequence homology between Asp f 2 and the mycelial protein ASPND1 from A. nidulans as well as the presence of conserved glycosylation sites and cysteine molecules in these proteins raises the possibilities of common antigenic and allergenic epitopes in the two proteins (11, 12). Indeed, ASPND1 strongly reacted with polyclonal anti-rAsp f 2 antibodies and also exhibited specific IgE binding with sera from patients with ABPA, indicating the presence of common B-cell epitopes in these two proteins. However, the threefold less IgE binding with ASPND1 than with Asp f 2 may be due to the differences in posttranslational modification and three-dimensional structure of these two proteins. The peptide mapping studies of various cytosolic A. fumigatus components demonstrated that p60, p40, and p37 cytosolic proteins are the differentially modified forms of a common peptide core (12). The antisera against the p40 component used in this study reacted strongly with rAsp f 2 as well as ASPND1, indicating the presence of common core protein in all of these antigens.
To obtain well-characterized and reproducible allergen preparations, it is essential to identify the A. fumigatus proteins which are produced by most of the strains and exhibit specific binding with patient sera. Antisera raised against the recombinant allergens expressed both in prokaryotic and eukaryotic systems should be used to screen mycelial and culture filtrate preparations from various strains of A. fumigatus as well as proteins from other Aspergillus species in order to identify the commonly expressed allergens. These allergens can be used for routine diagnosis and analyzed for their involvement in the immunopathogenesis of A. fumigatus-induced respiratory diseases.
In conclusion, the recombinant and native forms of the allergen Asp f 2 evaluated in this study are immunologically comparable, and the distinct IgE binding ability of this major allergen with sera from ABPA patients, particularly those with CB, may be of value for specific diagnosis. Further analysis of this protein in fungal colonization studies may shed new light in the role of A. fumigatus allergens in the immunopathogenesis of ABPA.
ACKNOWLEDGMENTS
This investigation was supported in part by NIH grants AI 42349, the U.S. Veterans Affairs Medical Research Service, and an Ernest S. Bazley grant to Northwestern Memorial Hospital and Northwestern University.
We thank F. Leal for providing us the ASPND1 and CFC proteins and the rabbit anti-p40 serum. We also gratefully acknowledge the technical assistance of Nancy Elms, Laura Castillo, and Kevin Thompson and the editorial assistance of Donna Schrubbe.
REFERENCES
- 1.Arruda L K, Mann B J, Chapman M D. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992;149:3354–3359. [PubMed] [Google Scholar]
- 2.Arruda L K, Platts-Mills T A, Fox J W, Chapman M D. Aspergillus fumigatus allergen I, a major IgE binding protein, is a member of the mitogillin family of cytotoxins. J Exp Med. 1990;172:1529–1532. doi: 10.1084/jem.172.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee B, Kurup V P, Greenberger P A, Hoffman D R, Nair D S, Fink J N. Purification of a major allergen, Asp f 2 binding to IgE in allergic bronchopulmonary aspergillosis, from culture filtrate of Aspergillus fumigatus. J Allergy Clin Immunol. 1997;99:821–827. doi: 10.1016/s0091-6749(97)80017-6. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee B, Kurup V P, Phadnis S, Greenberger P A, Fink J N. Molecular cloning and expression of a recombinant Aspergillus fumigatus protein AspfII with significant immunoglobulin E reactivity in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1996;127:253–262. doi: 10.1016/s0022-2143(96)90093-1. [DOI] [PubMed] [Google Scholar]
- 5.Bardana E J., Jr The clinical spectrum of aspergillosis. Part 1. Epidemiology, pathogenicity, infection in animals and immunology of aspergillosis. Crit Rev Clin Lab Sci. 1981;13:21–83. doi: 10.3109/10408368009106444. [DOI] [PubMed] [Google Scholar]
- 6.Bardana E J., Jr The clinical spectrum of aspergillosis. Part 2. Classification and description of saphorophytic, allergic and invasive variants of human disease. Crit Rev Clin Lab Sci. 1981;13:85–159. doi: 10.3109/10408368009106445. [DOI] [PubMed] [Google Scholar]
- 7.Basich J E, Graves T S, Baz M N, Scanlon G, Hoffman R G, Patterson R, Fink J N. Allergic bronchopulmonary aspergillosis in corticosteroid dependent asthmatics. J Allergy Clin Immunol. 1981;68:98–102. doi: 10.1016/0091-6749(81)90165-2. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein J A, Zeiss C R, Greenberger P A, Patterson R, Marhoul J F, Smith L L. Immunoblot analysis of sera from patients with allergic bronchopulmonary aspergillosis: correlation with disease activity. J Allergy Clin Immunol. 1990;86:532–539. doi: 10.1016/s0091-6749(05)80209-x. [DOI] [PubMed] [Google Scholar]
- 9.Bouchara J P, Tronchin G, Larcher G, Chabasse D. The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol. 1995;8:327–330. doi: 10.1016/s0966-842x(00)88965-9. [DOI] [PubMed] [Google Scholar]
- 10.Brummund W, Resnick A, Fink J N, Kurup V P. Aspergillus fumigatus-specific antibodies in allergic bronchopulmonary aspergillosis and aspergilloma: evidence for a polyclonal antibody response. J Clin Microbiol. 1987;25:5–9. doi: 10.1128/jcm.25.1.5-9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calera J A, Lopez-Medrano R, Ovejero M C, Puente P, Leal F. Variability of Aspergillus nidulans antigens with media and time and temperature of growth. Infect Immun. 1994;62:2322–2333. doi: 10.1128/iai.62.6.2322-2333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calera J A, Ovejero M C, Lopez-Medrano R, Segurado M, Puente P, Leal F. Characterization of the Aspergillus nidulans aspnd1 gene demonstrates that the ASPND1 antigen, which it encodes, and several Aspergillus fumigatus immunodominant antigens belongs to the same family. Infect Immun. 1997;65:1335–1344. doi: 10.1128/iai.65.4.1335-1344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crameri R. Recombinant Aspergillus fumigatus allergens: from the nucleotide sequences to clinical applications. Int Arch Allergy Immunol. 1998;115:99–114. doi: 10.1159/000023889. [DOI] [PubMed] [Google Scholar]
- 14.Crameri R, Blaser K. Cloning Aspergillus fumigatus allergens by the pJuFo filamentous phage display system. Int Arch Allergy Immunol. 1996;110:41–45. doi: 10.1159/000237308. [DOI] [PubMed] [Google Scholar]
- 15.Crameri R, Faith A, Hemmann S, Jaussi R, Ismail C, Menz G, Blaser K. Humoral and cell-mediated autoimmunity in allergy to Aspergillus fumigatus. J Exp Med. 1996;184:265–270. doi: 10.1084/jem.184.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crameri R, Jaussi R, Menz G, Blaser K. Display of expression products of cDNA libraries on phage surfaces. A versatile screening system for selective isolation of genes by specific gene-product/ligand interaction. Eur J Biochem. 1994;226:53–58. doi: 10.1111/j.1432-1033.1994.tb20025.x. [DOI] [PubMed] [Google Scholar]
- 17.Fratamico P M, Buckley H R. Identification and characterization of an immunodominant 58-kilodalton antigen of Aspergillus fumigatus recognized by sera of patients with invasive aspergillosis. Infect Immun. 1991;59:309–315. doi: 10.1128/iai.59.1.309-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller R S, Brake A, Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+ dependent serine protease. Proc Natl Acad Sci USA. 1989;86:1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberger P A. Allergic bronchopulmonary aspergillosis and fungoses. Clin Chest Med. 1988;9:599–608. [PubMed] [Google Scholar]
- 20.Greenberger P A, Patterson R. Diagnosis and management of allergic bronchopulmonary aspergillosis. Ann Allergy. 1986;56:444–448. [PubMed] [Google Scholar]
- 21.Greenberger P A, Patterson R. Allergic bronchopulmonary aspergillosis and the evaluation of the patient with asthma. J Allergy Clin Immunol. 1988;81:646–650. doi: 10.1016/0091-6749(88)91034-2. [DOI] [PubMed] [Google Scholar]
- 22.Hearn V M, Sietsma J H. Chemical and immunological analysis of the Aspergillus fumigatus cell wall. Microbiology. 1994;140:789–795. doi: 10.1099/00221287-140-4-789. [DOI] [PubMed] [Google Scholar]
- 23.Hemmann S, Blaser K, Crameri R. Allergens of Aspergillus fumigatus and Candida boidinii share IgE-binding epitopes. Am J Respir Crit Care Med. 1997;156:1956–1962. doi: 10.1164/ajrccm.156.6.9702087. [DOI] [PubMed] [Google Scholar]
- 24.Jaton-Ogay K, Suter M, Crameri R, Falchetto R, Faith A, Monod M. Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol Lett. 1992;71:163–168. doi: 10.1016/0378-1097(92)90506-j. [DOI] [PubMed] [Google Scholar]
- 25.Kolattukudy P E, Lee J D, Rogers L M, Zimmerman P, Ceselski S, Fox B, Stein B, Copelan E A. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect Immun. 1993;61:2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Reddy L V, Sochanik A, Kurup V P. Isolation and characterization of a recombinant heat shock protein of A. fumigatus. J Allergy Clin Immunol. 1993;91:1024–1030. doi: 10.1016/0091-6749(93)90215-2. [DOI] [PubMed] [Google Scholar]
- 27.Kurup V P. Enzyme-linked immunosorbent assay in the detection of specific antibodies against Aspergillus in patients’ sera. Zentralbl Bakteriol Mikrobiol Hyg Reiche A. 1986;261:509–516. doi: 10.1016/s0176-6724(86)80084-0. [DOI] [PubMed] [Google Scholar]
- 28.Kurup V P, Greenberger P A, Fink J N. Antibody response to low-molecular-weight antigens of Aspergillus fumigatus in allergic bronchopulmonary aspergillosis. J Clin Microbiol. 1989;27:1312–1316. doi: 10.1128/jcm.27.6.1312-1316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurup V P, Kumar A. Immunodiagnosis of aspergillosis. Clin Microbiol Rev. 1991;4:439–456. doi: 10.1128/cmr.4.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurup V P, Resnick A, Scribner G H, Kalbfleisch J H, Fink J N. Comparison of antigens and serological methods in Aspergillus fumigatus antibody detection. Mykosen. 1984;27:43–50. doi: 10.1111/j.1439-0507.1984.tb01981.x. [DOI] [PubMed] [Google Scholar]
- 31.Kurup V P, Ting E Y, Fink J N. Immunochemical characterization of Aspergillus fumigatus antigens. Infect Immun. 1983;41:698–701. doi: 10.1128/iai.41.2.698-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laufer P, Fink J N, Bruns W T, Unger G F, Kalbfleisch J H, Greenberger P A, Patterson R. Allergic bronchopulmonary aspergillosis in cystic fibrosis. J Allergy Clin Immunol. 1984;73:44–48. doi: 10.1016/0091-6749(84)90482-2. [DOI] [PubMed] [Google Scholar]
- 33.Leser C, Kauffman H F, Virchow C, Menz G. Specific serum immunopatterns in clinical phases of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1992;90:589–599. doi: 10.1016/0091-6749(92)90131-k. [DOI] [PubMed] [Google Scholar]
- 34.Mayer C, Hemmann S, Faith A, Blaser K, Crameri R. Cloning, production, characterization and IgE cross-reactivity of different manganese superoxide dismutases in individuals sensitized to Aspergillus fumigatus. Int Arch Allergy Immunol. 1997;113:213–215. doi: 10.1159/000237550. [DOI] [PubMed] [Google Scholar]
- 35.Monod M, Paris S, Sarfati J, Jaton-Ogay K, Ave P, Latge J P. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol Lett. 1993;80:39–46. doi: 10.1111/j.1574-6968.1993.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 36.Moser M, Crameri R, Brust E, Suter M, Menz G. Diagnostic value of recombinant Aspergillus fumigatus allergen I/a for skin testing and serology. J Allergy Clin Immunol. 1994;93:1–11. doi: 10.1016/0091-6749(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 37.Moser M, Crameri R, Menz G, Schneider T, Dudler T, Virchow C, Gmachl M, Blaser K, Suter M. Cloning and expression of recombinant Aspergillus fumigatus allergen I/a (rAsp f I/a) with IgE binding and type I skin test activity. J Immunol. 1992;149:454–460. [PubMed] [Google Scholar]
- 38.Moser M, Menz G, Blaser K, Crameri R. Recombinant expression and antigenic properties of a 32-kilodalton extracellular alkaline protease, representing a possible virulence factor from Aspergillus fumigatus. Infect Immun. 1994;62:936–946. doi: 10.1128/iai.62.3.936-942.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murali P S, Pathial K, Saff R H, Splaingard M L, Atluru D, Kurup V P, Fink J N. Immune responses to Aspergillus fumigatus and Pseudomonas aeruginosa antigens in cystic fibrosis and allergic bronchopulmonary aspergillosis. Chest. 1994;106:513–519. doi: 10.1378/chest.106.2.513. [DOI] [PubMed] [Google Scholar]
- 40.Nikolaizik W H, Moser M, Crameri R, Little S, Warner J O, Blaser K, Schoni M H. Identification of allergic bronchopulmonary aspergillosis in cystic fibrosis patients by recombinant Aspergillus fumigatus I/a-specific serology. Am J Respir Crit Care Med. 1995;152:634–639. doi: 10.1164/ajrccm.152.2.7633719. [DOI] [PubMed] [Google Scholar]
- 41.Nikolaizik W H, Crameri R, Blaser K, Schoni M H. Skin test reactivity to recombinant Aspergillus fumigatus allergen I/a in patients with cystic fibrosis. Int Arch Allergy Immunol. 1996;111:403–408. doi: 10.1159/000237399. [DOI] [PubMed] [Google Scholar]
- 42.Ovejero M C. Aislamiento y caracterización de antigenos importantes en el immunodiagnóstico de las aspergilosis. Ph.D. thesis. Salamanca, Spain: Universidad de Salamanca; 1993. [Google Scholar]
- 43.Penalver M C, O’Connor J E, Martinez J P, Gil M L. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect Immun. 1996;64:1146–1153. doi: 10.1128/iai.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piechura J E, Huang C J, Cohen S H, Kidd J M, Kurup V P, Calvanico N J. Antigens of Aspergillus fumigatus. II. Electrophoretic and clinical studies. Immunology. 1983;49:657–665. [PMC free article] [PubMed] [Google Scholar]
- 45.Piechura J E, Riefel R S, Daft L J. Electrophoretic and serological analyses of cytoplasmic antigens from Aspergillus fumigatus during growth of conidia to mature mycelia. J Med Vet Mycol. 1987;25:243–254. [PubMed] [Google Scholar]
- 46.Ramesh M V, Sirakova T, Kolattukudy P E. Isolation, characterization, and cloning of cDNA and the gene for an elastinolytic serine proteinase from Aspergillus flavus. Infect Immun. 1994;62:79–85. doi: 10.1128/iai.62.1.79-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed C. Variability of antigenicity of Aspergillus fumigatus. J Allergy Clin Immunol. 1978;61:227–229. doi: 10.1016/0091-6749(78)90189-6. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg M, Patterson R, Mintzer R, Cooper B J, Roberts M, Harris K E. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86:405–414. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 49.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sentandreu M, Elorza M V, Sentandreu R, Fonzi W A. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J Bacteriol. 1998;180:282–289. doi: 10.1128/jb.180.2.282-289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheffer A L, Taggart V S. Expert panel report and guidelines for the diagnosis and management of asthma. Med Care. 1993;31:MS20–MS28. [PubMed] [Google Scholar]
- 52.Teshima R, Ikebuchi H, Sawada J, Miyachi S, Kitani S, Iwama M, Irie M, Ichinoe M, Terao T. Isolation and characterization of a major allergenic component (gp55) of Aspergillus fumigatus. J Allergy Clin Immunol. 1993;92:698–706. doi: 10.1016/0091-6749(93)90013-6. [DOI] [PubMed] [Google Scholar]
- 53.Tronchin G, Esnault K, Renier G, Filmon R, Chabasse D, Bouchara J P. Expression and identification of a laminin-binding protein in Aspergillus fumigatus conidia. Infect Immun. 1997;65:9–15. doi: 10.1128/iai.65.1.9-15.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]