Abstract
The cerebellum is involved in many motor, autonomic and cognitive functions, and new tasks that have a cerebellar contribution are discovered on a regular basis. Simultaneously, our insight into the functional compartmentalization of the cerebellum has markedly improved. Additionally, studies on cerebellar output pathways have seen a renaissance due to the development of viral tracing techniques. To create an overview of the current state of our understanding of cerebellar efferents, we undertook a systematic review of all studies on monosynaptic projections from the cerebellum to the brainstem and the diencephalon in mammals. This revealed that important projections from the cerebellum, to the motor nuclei, cerebral cortex, and basal ganglia, are predominantly di- or polysynaptic, rather than monosynaptic. Strikingly, most target areas receive cerebellar input from all three cerebellar nuclei, showing a convergence of cerebellar information at the output level. Overall, there appeared to be a large level of agreement between studies on different species as well as on the use of different types of neural tracers, making the emerging picture of the cerebellar output areas a solid one. Finally, we discuss how this cerebellar output network is affected by a range of diseases and syndromes, with also non-cerebellar diseases having impact on cerebellar output areas.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12311-022-01499-w.
Keywords: Cerebellum, Brainstem, Diencephalon, Tracers, Rodents, Non-human primates
Introduction
Paradoxically, recent advances emphasize both heterogeneity and similarity in cerebellar function across different species of mammals. On the one hand, we now understand much better how specific tasks can be localized to certain areas of the cerebellum, in particular in relation to designated zebrin bands [1–7]. On the other hand, it is becoming clear that some cerebellar functions are more widely distributed than previously thought [8–10]. The latter may be related to a more holistic understanding of behavior. The eyeblink reflex, for instance, is not just an isolated contraction of the eyelid muscles, but part of a defensive reflex involving the whole face, or maybe even the entire body [11]. These findings are in agreement with the notion of the cerebellum as a coordinating area for most, if not all, complex motor as well as non-motor functions [5, 8, 12, 13].
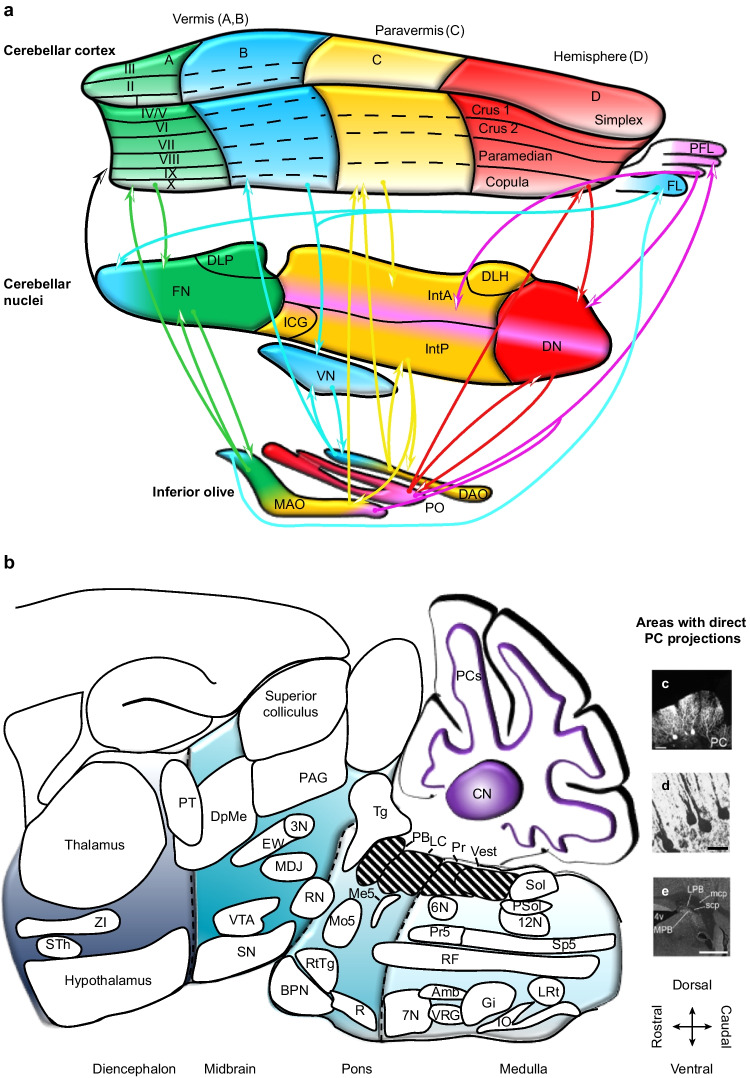
The cerebellum is widely connected with other regions of the central nervous system [14, 15]. Ascending as well as descending input reaches the cerebellum predominantly via two glutamatergic pathways: the climbing fibers [16–18] and the mossy fibers [15, 19]. Both pathways converge in the cerebellar cortex, while also forming collaterals to the cerebellar nuclei [20]. Purkinje cells are the sole output neurons of the cerebellar cortex. They project predominantly to the cerebellar nuclei that form the main output stage of the mammalian cerebellum [20], and that form a feedback projection to the cerebellar cortex [21, 22] (Fig. 1A).
Fig. 1.
Olivocerebellar loops and Purkinje cell projections. a The cerebellar cortex is subdivided from medial to lateral in the vermis, paravermis, and hemispheres and from anterior to posterior in lobules I–X. In rodents, the hemispheric lobules have their own names. Lobule XI, also known as the uvula, and lobule X, sometimes called the nodulus, form together with the flocculus (FL) and the paraflocculus (PFL) the vestibulocerebellum. Purkinje cells in the cerebellar cortex project to the cerebellar nuclei, with the target area depending on the location of the Purkinje cells within the cerebellar cortex. Projections from the cerebellar nuclei to the inferior olive, and from the inferior olive to the cerebellum complement the olivocerebellar loops, organized in parasagittal modules (named A–D), as indicated by the use of different colors. Cerebellar nuclei neurons project also back to the cerebellar cortex. b Cerebellar output originates mostly from the cerebellar nuclei, although the striped areas receive direct input from Purkinje cells. The non-striped areas receive cerebellar input only from the cerebellar nuclei and are their locations are schematically indicated on a sagittal projection inspired by the Paxinos mouse brain atlas [39]. c Retrogradely labeled Purkinje cells after injection of viral tracer in the locus coeruleus (scale bar: 50 µm), reproduced with permission from [36]. The copyrights of this panel are from Nature Publishing Group. d Purkinje cells labeled in the ipsilateral nodulus after HRP injection into lateral vestibular nucleus (scale bar: 50 µm), reproduced from [40]. e Neuronal labeling into parabrachial nucleus is shown after injection of AAV-CMV-hrGFP into cerebellar lobule IX (scale bar: 1 mm), reproduced from [41]. DAO, dorsal accessory olive; DLH, dorsolateral hump; DLP, dorsolateral protuberance; DN, dentate nucleus; FN, fastigial nucleus; ICG, interstitial cell group; IntA, anterior interposed nucleus; IntP, posterior interposed nucleus; LVN, lateral vestibular nucleus; MAO, medial accessory olive; PO, principal olive; Pr, prepositus nucleus
Of the cerebellar nuclei, the fastigial (or medial) nucleus is located nearest to the midline and includes also the dorsolateral protuberance and interstitial cell groups [23–27]. The fastigial nucleus receives input from Purkinje cells in the vermis [7, 20]. Lateral to the fastigial nucleus is the interposed nucleus that consists of an anterior and a posterior part [28]. Only in rodents, the anterior interposed nucleus includes also a separate region termed the dorsolateral hump [24]. The interposed nucleus receives input from Purkinje cells in the paravermis [7, 20]. The dentate (or lateral) nucleus is the most lateral cerebellar nucleus, receiving input from Purkinje cells in the hemispheres, the most lateral part of the cerebellar cortex [7, 20]. The dentate nucleus showed extreme expansion during hominid evolution, possibly related to its contribution to cognitive functions [29–31] (Fig. 1a).
In this systematic review, we summarize the available literature on cerebellar projections to the brainstem and diencephalon in mammals. We questioned whether the advent of viral tracing techniques [32–38] changed our perception of cerebellar output and whether consistent differences between species could be found. In addition, we describe whether specific diseases, whether considered to be cerebellar or non-cerebellar, are associated with modification of certain cerebellar output pathways.
Methods
In May 2021, we searched all available entries on EMBASE, MEDLINE, Web of Science, and Cochrane published after 1975, using a query based on the terms “cerebellar efferents,” “brainstem,” and “monosynaptic” (Table S1). This resulted in 7406 references.
First, we screened the titles and abstracts of these 7406 references manually to exclude papers completely out of topic. During this round, also duplicates, studies that did not include data on mammals, and papers that were not written in English were removed. This resulted in the exclusion of 6544 references.
Of the remaining 862 references, the full text of 52 could not be retrieved through the library of the Erasmus MC, leaving a final list of 810 publications of which we screened the whole text and figures to verify the use of monosynaptic neural tracers and the documentation of the results with at least one microscopic image. The latter condition was included as we argued that the inclusion of original data is essential for the evaluation of the quality of the study. During this second round, 401 papers were selected for further study.
Finally, we read these 401 papers in detail and excluded those that did not report the use of tracers for monosynaptic cerebellar efferents. This resulted in a list of 243 papers as the basis of our systematic review. During this process, 13 new relevant studies were published and added to the final list, consisting now of 256 papers.
We scrutinized the 256 papers for reports on specific projections from the cerebellum to the brainstem and/or the diencephalon. The results are summarized in Table S2. This procedure resulted in first instance in a binary representation of brain areas: reported to receive cerebellar input or not. Table S2 is therefore primarily a resource for finding literature on putative connections. As this list potentially contains false positives, we constructed a confidence score for each monosynaptic projection. We summarized the diversity of studies that reported the existence of each pathway, using a maximum score of 10 points. To calculate the confidence score, we considered the use of both anterograde and retrograde tracers (2 points); the use of both classical and viral tracers (2 points; 1 point if multiple classical or multiple viral tracers were used, but not both); the use of multiple species (2 points for three or more species, 1 point for two species); the use of relatively large animals (2 points if cats, dogs, monkeys, or similarly large species were included as the risk for non-specific staining is less in bigger brains); and the number of replications (2 points for at least ten studies, 1 point for at least five studies) (Fig. 4).
Fig. 4.
Confidence of monosynaptic projections from the cerebellum. Based on the number and types of replications, we assigned a score of 1 to 10 on the reliability of specific projections from the cerebellum (see “Methods” section). In particular, direct projections to cranial and vagal motor nuclei have been described only in single or few studies. Note that the confidence score does not make any implications on the strength of a connection (see Table 1)
In our summary of the literature, we also encountered studies that mentioned cerebellar projections to larger areas than the subdivisions used in our review. Whenever applicable, we mention these results at the start of the relevant sections.
Using the format of a narrative review, we included also data on the fractional anisotropy (FA) of the superior cerebellar peduncle in ataxia, schizophrenia, and autism spectrum disorder. Only papers that compared FA values of patient and control groups were included.
Results
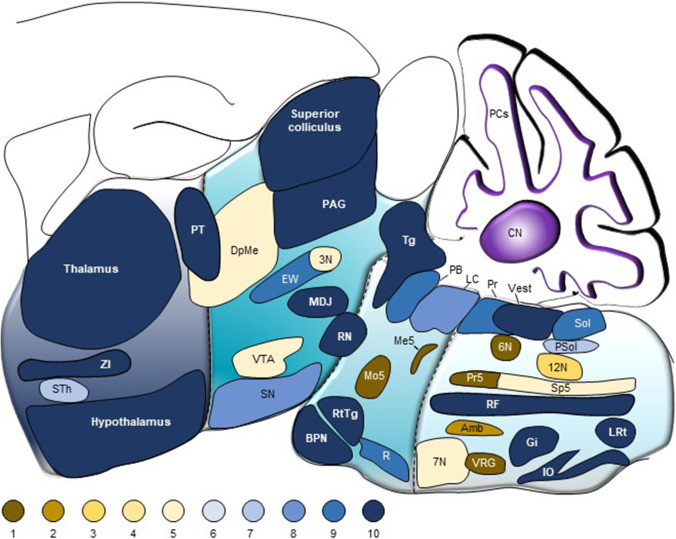
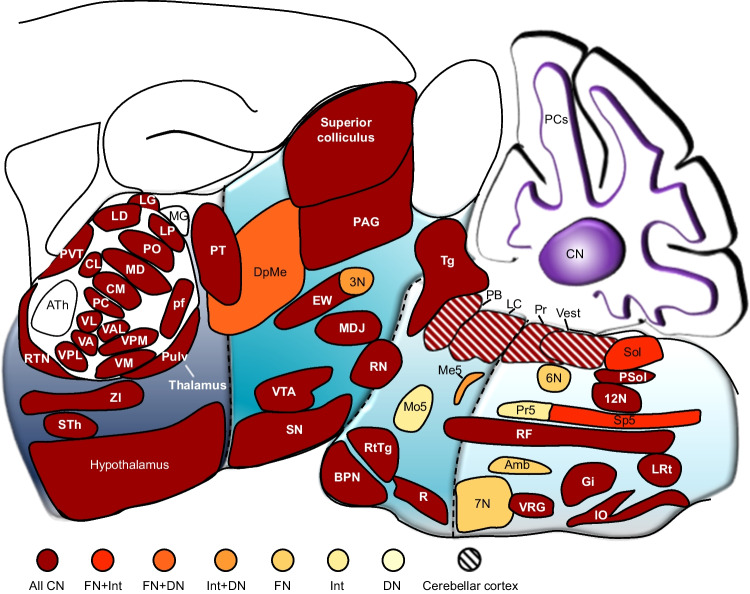
Cerebellar outputs target many structures of the brainstem and diencephalon. To increase readability, we describe only the main structures in the text and in Figs. 1b and 2. Further details on subnuclei can be found in Table S2.
Fig. 2.
Most target areas receive input from all three cerebellar nuclei. All areas in the brainstem and diencephalon that receive monosynaptic input from the cerebellum plotted at their approximate location on a sagittal projection based on the Paxinos mouse atlas [39]. Most of these areas receive input from all three cerebellar nuclei. Abbreviations: 3 N, oculomotor nucleus; 6 N, nucleus abducens; 7 N, facial nucleus; 12 N, hypoglossal nucleus; Amb, nucleus ambiguus; CN, cerebellar nuclei; DN, dentate nucleus; DpMe, deep mesencephalic nucleus; EW, Edinger-Westphal nucleus; FN, fastigial nucleus; Gi, nucleus gigantocellularis; Int, interposed nucleus; IO, inferior olive; LC, locus coeruleus; LRt, lateral reticular nucleus; MDJ, mesodiencephalic junction; Me5, mesencephalic trigeminal nucleus; Mo5, trigeminal nucleus, motor part; PAG, periaqueductal gray; PB, parabrachial complex; Pr, prepositus nucleus; Pr5, principal trigeminal nucleus; PSol, parasolitary nucleus; PT, pretectal complex; R, raphe nuclei; RF, reticular formation; RN, red nucleus; RtTg, nucleus reticularis tegmenti pontis; SN, substantia nigra; Sol, nucleus of the solitary tract; Sp5, spinal trigeminal nucleus; STh, subthalamic nucleus; Tg, tegmentum; Vest, vestibular nuclei; VRG, ventral respiratory group; VTA, ventral tegmental area; ZI, zona incerta. Abbreviations of thalamic subnuclei: AD, antero-dorsal thalamic nucleus; AM, antero-medial thalamic nucleus; AV, antero-ventral thalamic nucleus; CL, centrolateral nucleus; CM, centromedial nucleus; IL, intralaminar nucleus; LD, laterodorsal nucleus; LG, lateral geniculate nucleus; LP, latero-posterior nucleus; MD, mediodorsal thalamic nucleus; MG, medial geniculate nucleus; PC, paracentral nucleus; pf, parafascicular complex; PO, posterior thalamic nuclei; Pulv, pulvinar nucleus; PVT, paraventricular nucleus; RTN, reticular thalamic nuclei; VA, ventro-anterior thalamic nucleus; VAL, ventro-antero thalamic complex; VL, ventrolateral thalamic nucleus; VM, ventromedial nucleus; VPL, ventro-posterior lateral thalamic nucleus; VPM, ventro-posterior medial thalamic nucleus
Projections to the Medulla Oblongata
The most caudal part of the brainstem, the medulla oblongata, is located between the spinal cord and the pons [43]. Three studies describe cerebellar pathways to the medulla oblongata without discriminating between its subdivisions [44–46].
Medullary Reticular Formation (RF)
The medullary reticular formation consists of the gigantocellular and paragigantocellular nuclei, paramedian tract cell groups, medial reticular formation, medullary reticular nucleus, intermediate reticular nucleus, and raphe nuclei. Cerebellar pathways to the medullary reticular formation are described in eleven studies that did not discriminate between its subnuclei [32–34, 44, 47–53]. Twelve other papers describe projections to the gigantocellular nucleus [32–34, 47, 49, 51, 53–58], and four papers state the existence of cerebellar nuclear projections to the paragigantocellular nucleus [32–34, 53]. None of the studies included in this systematic review mentions projections to the paramedian tract cell groups.
The medullary raphe nuclei form a distinct division of the medullary reticular formation and consist of the obscurus, magnus, and pallidus nuclei [59, 60]. These areas contain a large proportion of serotonergic neurons and are involved in many functions such as emotion, response to stress, reward, regulation of appetite, movement, sexual behavior, sleep, respiration, and pain perception [59, 61–63]. The obscurus and magnus nuclei, but not the pallidus nucleus, receive input from the cerebellum, with all three cerebellar nuclei contributing to this output [32, 34, 55, 64–68].
Lateral Reticular Nucleus (LRt)
The lateral reticular nucleus consists of parvocellular, magnocellular, and subtrigeminal divisions [69–72]. All output of the lateral reticular nucleus is directed to the cerebellum [73]. In return, the lateral reticular nucleus receives projections from all cerebellar nuclei. Thirteen studies describe these cerebellar efferents without taking the subdivisions of the lateral reticular nucleus into account [32, 34, 46, 47, 51, 54, 55, 67, 74–77]. Five studies describe the projections to the parvocellular part [32–34, 47] and five to the magnocellular part of the lateral reticular nucleus [32, 34, 49, 51, 54]. While the parvo- and magnocellular parts receive input from all three cerebellar nuclei, the subtrigeminal component receives only input from the fastigial nucleus [49].
Tegmental Areas (Tg)
The tegmentum is an inner lamina of the brainstem, between the tectum and basis. The tegmentum extends along the entire length of the brainstem and is divided in two layers that both contain somatomotor and supplementary motor nuclei [78].
All cerebellar nuclei project to the pontine tegmental nuclei, ventral tegmental nucleus of Gudden, and mesopontine rostromedial tegmental nucleus [79–81]. In particular, the fastigial nucleus projects to the dorsal and medial tegmental area, to the medial pontine tegmentum, paralemniscal tegmental area, and laterodorsal tegmental and peduncular tegmental nuclei [33, 48, 79, 82]. Only the interpositus nucleus projects to the tegmental reticular nucleus [32], and only the dentate nucleus projects to the dorsomedial part of the tegmentum [83], whereas the interpositus and dentate nuclei together project to the ventral mesencephalic tegmentum and ventral midbrain tegmentum [84, 85].
Inferior Olive (IO)
The inferior olive is located near the border of the medulla oblongata and the pons, and it consists of three subnuclei: the principal olive and the medial and dorsal accessory olives [86–88]. The caudal part of medial accessory olive further consists of subnuclei A, B, C, beta, cap of Kooy, ventrolateral protrusion, and dorsomedial cell group [89]. The inferior olive projects exclusively to the cerebellum and is the sole source of climbing fibers innervating cerebellar Purkinje cells [17, 18].
The projections from the cerebellar nuclei to the inferior olive are anatomically well-defined and largely adhere to the sagittal modules of the cerebellar cortex and the corresponding cerebellar nuclei [7]. The fastigial nucleus projects to the medial and dorsal accessory olive, the interposed nucleus to the dorsal accessory olive, and the dentate nucleus to the principal olive [32–34, 44, 54–56, 65, 67, 77, 90–121].
Nucleus of the Solitary Tract (Sol) and Parasolitary Nucleus (PSol)
The nucleus of the solitary tract is located lateral to the motor nucleus of the vagus nerve [122, 123] and is a sensory nucleus of which the rostral part receives gustatory inputs and the caudal part cardiorespiratory input. The nucleus of the solitary tract is the main central recipient of vagal sensory input and receives signals from among others peripheral chemo-, baro-, and stretch receptors [122, 124, 125]. Immediately dorsal and lateral to the nucleus of the solitary tract is the parasolitary nucleus that is involved in the processing of vestibular and autonomic information in relation to postural control [126, 127]. It receives input from all cerebellar nuclei [34, 49, 51, 54, 56]. There are also descriptions of direct input from the fastigial and interposed nuclei to the nucleus of the solitary tract, but possibly a significant part of this input targets the parasolitary nucleus rather than the nucleus of the solitary tract itself [32, 47, 49, 55, 128–130].
Trigeminal Nuclei (5 N)
The trigeminal nerve relates to one motor and three sensory nuclei, whereby the latter consist of a principal and a spinal nucleus in the hindbrain, as well as the mesencephalic trigeminal nucleus [131–133]. All cerebellar nuclei project to the trigeminal nuclei, but not all cerebellar nuclei target all parts of the trigeminal nuclei. The fastigial nucleus projects to the spinal trigeminal nucleus [34, 54], while the interposed nucleus projects to both motor and sensory trigeminal nuclei [32, 34]. The dentate nucleus projects to the mesencephalic trigeminal nucleus and intertrigeminal area, located between the trigeminal motor nucleus and the principal sensory nucleus [34].
Vestibular Nuclei (Vest)
The vestibular nuclei consist of four main nuclei: the superior, medial, lateral and spinal vestibular nuclei, and some smaller structures. The spinal vestibular nucleus is also termed descending or inferior vestibular nucleus. The vestibular nuclei are located in the medulla and pons. The vestibular nuclei are involved in the control of posture, head position, equilibrium, and stabilizing vision during movement [134–136].
In contrast to most other brain areas, the vestibular nuclei receive direct input from Purkinje cells (Fig. 1d). From the flocculus, there are Purkinje cell projections to the lateral, medial, superior vestibular nuclei and to cell group Y; from the lingula to the lateral vestibular nucleus and cell group Y; from the nodulus to all vestibular nuclei; and from the uvula to the medial, spinal, and superior vestibular nuclei [40, 41, 137–152]. Furthermore, Purkinje cells in lobule III project to the lateral and inferior vestibular nuclei, and Purkinje cells of lobule IX to the superior and lateral vestibular nuclei [153]. In addition, also the three cerebellar nuclei project to all compartments of the vestibular nuclei [32–34, 40, 47, 48, 51, 52, 54–56, 64, 65, 67, 74, 93, 103, 138, 143, 149, 154, 155].
Hypoglossal Nucleus (12 N)
The hypoglossal nucleus is located at the dorsomedial level of the medulla [156]. It houses the motor neurons of the tongue muscles and thus plays an important role in controlling swallowing, vocalization, respiration, mastication, and suckling [157–161]. Hypoglossal projections from the cerebellum were described in two papers [32, 156].
Perihypoglossal Nucleus (Pr)
The perihypoglossal nucleus, located adjacent to the hypoglossal nucleus, consists of three subnuclei: the nucleus of Staderini, nucleus prepositus hypoglossi, and nucleus of Roller. These areas are involved in the control of eye movements [162, 163]. Cerebellar input to the perihypoglossal nucleus comes from the fastigial nucleus and targets in particular the prepositus nucleus [33, 34, 47, 54, 56, 65, 164, 165]. In addition, the prepositus nucleus receives also input from the interposed nucleus [32, 55, 67] and from Purkinje cells in the flocculus, paraflocculus, and crus I [164, 165].
Nucleus Ambiguus (Amb)
The nucleus ambiguus is located within the medullary reticular formation and is home to vagal motor neurons projecting to upper airway muscles that play a role in respiration, swallowing and speaking, and motor neurons projecting to the cardiovascular system [166]. Although there is an early description of a fastigial projection to the nucleus ambiguus [47], this could not be reproduced later [49, 51], and we did not find other studies confirming the existence of such a projection.
Ventral Respiratory Group (VRG)
The ventral respiratory group is located in the ventrolateral part of the medulla oblongata, and it includes, from rostral to caudal, the Bötzinger and pre-Bötzinger complexes and the rostral and caudal ventral respiratory groups [167]. The ventral respiratory group contains the central pattern generator for inspiration as well as both inspiratory and expiratory pre-motor neurons [167–169]. Only one paper describes a cerebellar nuclear projection bilaterally to the rostral ventral respiratory group [167], while we could not find evidence for cerebellar projections to other compartments of the ventral respiratory group.
Projections to the Metencephalon
Pontine Reticular Formation
The pontine reticular formation is a part of the reticular formation involved in eye movement and sleep-waking cycle [170, 171]. Seven studies describe input from the cerebellum to the pontine reticular formation [33, 55, 65, 172–177], whereas ten studies state, specifically, that the paramedian reticular formation, a subdivision of the pontine reticular formation, receives projections from all cerebellar nuclei [33, 47, 49, 51, 54, 57, 178–180]. This region is represented in the figures within the medullary reticular formation (RF).
Nucleus Reticularis Tegmenti Pontis (RtTg)
The nucleus reticularis tegmenti pontis is a specialized region within the pontine reticular formation that is best known for its involvement in oculomotor control [181–185]. Nineteen studies state that all cerebellar nuclei project to the nucleus reticularis tegmenti pontis [32, 33, 51, 55–57, 64, 65, 79, 105, 110, 117, 186–191].
Basilar Pontine Nuclei (BPN)
The basilar pontine nuclei receive signals from among others the cerebral cortex and provide mossy fibers afferents to the cerebellum. While the basilar pontine nuclei are the main intermediate between the neocortex and the cerebellum, their functions exceed those of a mere relay station [192, 193]. During development, the size of the pontine nuclei increases together with the sizes of the cerebral and cerebellar hemispheres [192]. The basilar pontine nuclei receive projections from all three cerebellar nuclei [33, 47, 54, 65, 92, 105, 110, 117, 172, 186–188, 194–196].
Locus Coeruleus (LC)
The locus coeruleus is located in the pontine region next to the floor of the fourth ventricle [197]. As one of the main sources of noradrenaline, it has widespread innervations serving level setting functions in modulating attention, emotion, stress, and autonomic, respiratory, sensory, as well as motor control [198, 199]. Purkinje cells, mainly from the ipsilateral medial zones, and all cerebellar nuclei, but particularly the fastigial nucleus, project to the locus coeruleus [33, 34, 36, 47, 55, 56, 176, 200, 201] (Fig. 1C).
Parabrachial Complex (PB)
The parabrachial complex surrounds the superior cerebellar peduncle at the level of the dorsal pons. It consists of three main nuclei: the lateral and medial parabrachial nuclei that serve as alarm center, notifying the forebrain of aversive events like pain or bad taste, and the Kölliker-Fuse nucleus that is an autonomic center involved in thermoregulation and respiration [202, 203].
The parabrachial complex is rather unique in that it receives direct input from cerebellar Purkinje cells [64, 153, 204] (Fig. 1e). The medial parabrachial nucleus is targeted by Purkinje cells from lobules VIII–X [41, 144], while the lateral parabrachial nucleus receives input only from lobules IX and X [41, 144]. In addition, also all three cerebellar nuclei project to the lateral and medial parabrachial nuclei, while the rostral fastigial nucleus targets the Kölliker-Fuse nucleus as well [32–34, 49, 201, 204].
Abducens Nucleus (6 N)
The abducens nucleus, located at the level of the pontine tegmentum and ventral to the floor of the fourth ventricle, is responsible of the movement of the eyeball in lateral direction by supplying the lateral rectus muscle [78]. Only the caudal fastigial nucleus projects to the abducens nucleus [51].
Facial Nucleus (7 N)
The facial nucleus is located within the reticular formation at the ventrolateral part of the tegmentum of the pons and consists of motor, sensory, and parasympathetic components [78]. The motor part controls voluntary facial movement, the sensory component receives taste information, and the parasympathetic nuclei are the superior salivatory and lacrimal nuclei [78, 205]. The facial nucleus receives projections from the caudal part of fastigial nucleus and the rostral part of dorsolateral protuberance [33, 51].
Projections to the Mesencephalon
Mesencephalic Reticular Formation
The mesencephalic reticular formation contains the nucleus of the posterior commissure, the central mesencephalic reticular formation, the M-group, and the rostral interstitial nucleus of the medial longitudinal fasciculus. The interstitial nucleus of Cajal belongs both to the mesencephalic reticular formation and the group of nuclei located at the mesodiencephalic junction that is discussed separately. Cerebellar projections to the mesencephalic reticular formation are described in five studies [32, 33, 44, 65, 206] and to the posterior commissure in seven studies [48, 49, 54, 67, 207–209]. This region is represented in the figures within the medullary reticular formation (RF).
Red Nucleus (RN)
The red nucleus in the ventral midbrain consists of a caudal magnocellular and a rostral parvocellular portion and is involved in motor control, sensory processing, and higher-order cognitive functions [210, 211]. The magnocellular portion participates in organizing the execution of learned movements, coordinating the movements of extremities among quadrupedal animals, and grasping [210, 212–214], while the parvocellular portion is required for motor learning and complex cognitive-motor functions affecting the olivocerebellar system [210, 215, 216]. The parvocellular part is also considered to be involved in the group of nuclei that project to the inferior olive and that are located at the mesodiencephalic junction (see below). Both parts receive input from all cerebellar nuclei, although the magnocellular portion receives mainly input from the interposed nucleus [11, 65, 67, 77, 95, 110, 111, 217–224], and the parvocellular part predominantly from the dentate nucleus [65, 79, 110, 111, 117, 218, 220, 221, 225]. Other studies confirm these cerebello-rubral projections, although they do not take the subdivisions into account [32, 34, 48, 54, 55, 64, 83, 85, 93, 105, 121, 217, 226–245].
Ventral Tegmental Area (VTA)
The ventral tegmental area houses dopaminergic neurons projecting to the prefrontal cortex [246–250]. It plays an important role in reward, stress, motivation, social behavior, aversion, and cognition [251–256]. The ventral tegmental area receives projections from all cerebellar nuclei [32, 37, 85, 228, 257–260].
Mesodiencephalic Junction (MDJ)
At the mesodiencephalic junction, a group of nuclei are located that are mainly involved in oculomotor control, such as the nucleus of Darkschewitsch, medial accessory nucleus of Bechterew, parvocellular part of the red nucleus, prerubral field, rostral interstitial nucleus of the medial longitudinal fasciculus, medial accessory oculomotor nucleus, suprarubal reticular formation, nucleus of the fields of Forel, and interstitial nucleus of Cajal. The mesodiencephalic junction forms strong projections to the inferior olive and receives projections from the cerebral cortex and the cerebellum, forming an important hub in cerebro-cerebellar communication [261–272].
All three cerebellar nuclei project to the interstitial nucleus of Cajal, nucleus of Darkschewitsch, medial accessory nucleus of Bechterew, prerubral field, medial oculomotor accessory nucleus, and nucleus of fields of Forel [33, 34, 48, 54, 64, 65, 79, 83, 110, 139, 173, 180, 207, 209, 218, 230, 234, 240, 261, 273–281]. As mentioned above, cerebellar input to the parvocellular part of the red nucleus originates predominantly from the dentate nucleus [65, 79, 110, 111, 117, 218, 220, 221, 225].
Edinger-Westphal (EW) Nucleus
The Edinger-Westphal nucleus lies dorsal to the oculomotor nucleus and controls pupillary constriction [282–285]. It receives projections from all cerebellar nuclei [34, 64, 277, 279].
Oculomotor Nucleus (3 N)
The oculomotor nucleus is a pure motor nucleus. Together with the Edinger-Westphal nucleus, it forms the oculomotor complex involved in both somatic and autonomic functions, by innervating the muscles of the upper eyelid, eye muscles, and pupil [78, 286]. The oculomotor nucleus receives projections from the interposed and dentate nuclei, but not from the fastigial nucleus [32, 34, 230, 277].
Pretectal Complex (PT)
Lateral to the third ventricle and caudal to the posterior thalamus, extending until the superior colliculus, one finds the pretectal complex [287]. It consists of various subnuclei, such as the pretectal olivary nucleus, posterior pretectal nucleus, medial pretectal nucleus, and anterior pretectal nucleus [288]. The pretectal complex is reciprocally connected with the Edinger-Westphal nucleus and regulates the pupillary light reflex and light-evoked blink responses and also plays a role in rapid eye movement sleep and in processing noxious stimuli [208, 287–290]. All cerebellar nuclei project to the pretectal complex [32, 48, 64, 83, 207, 208, 237, 243, 274, 291–296].
Superior Colliculus (SC)
The superior colliculus is composed of superficial, intermediate, and deep cell layers [297]. It integrates visual, auditory, and sensorimotor information in order to orient [298–300]. The cerebellar nuclei project strongly to all layers of the superior colliculus [11, 32, 33, 44, 48, 49, 54, 65, 67, 77, 79, 83, 92, 173, 207, 208, 233, 237, 243, 275, 294, 301–311]
Periaqueductal Gray (PAG)
The periaqueductal gray is a longitudinal gray matter structure surrounding the aqueduct of Sylvius [312–314]. It is involved in pain perception, risk assessment, responses to threats, defensive behaviors, and depression [312, 314–316]. The most rostral region of the periaqueductal gray is in proximity to the posterior commissure and to the rostral level of the third nucleus, whereas the most caudal part of the periaqueductal gray is close to the dorsal tegmental nucleus [317]. The periaqueductal gray is subdivided into four columns: the ventrolateral, lateral, dorsolateral, and dorsomedial columns [318–321]. Furthermore, the periaqueductal gray has a portion that is oculomotor-associated, termed supraoculomotor periaqueductal gray [322].
Five studies demonstrated projections from all cerebellar nuclei to the periaqueductal gray, without taking its subdivisions into account [32, 64, 65, 301, 323]. Other studies show that, in particular, the ventrolateral periaqueductal gray receives projection from all three cerebellar nuclei [32, 33, 35, 38, 48, 83, 208], the dorsolateral column from the interpositus nucleus [67, 83], the dorsomedial column from the dentate nucleus [83], and the supraoculomotor and lateral regions of periaqueductal gray from the fastigial nucleus [275].
Deep Mesencephalic Nucleus (DpMe)
The deep mesencephalic nucleus is located between the superior colliculus, substantia nigra, red nucleus, and periaqueductal gray [324–327]. The deep mesencephalic nucleus is also termed central tegmental field, nucleus mesencephalicus profundus, midbrain reticular formation, and cuneiformis/subcuneiformis complex [328–330]. It has been suggested that the deep mesencephalic nucleus might be involved in nociception, heart rate control, arterial pressure regulation, and locomotion [331, 332]. All cerebellar nuclei project to the deep mesencephalic nucleus [33, 325].
Substantia Nigra (SN)
The substantia nigra consists of GABA and dopaminergic neurons [333, 334] and plays a role in organizing movement, reward processing, cognitive planning, learning, and emotions [333]. All cerebellar nuclei project to the substantia nigra [33, 55, 64, 85, 260].
Projections to the Diencephalon
Hypothalamus
The hypothalamus consists of several regions that manage various vital functions, such as sleep, cardiovascular regulation, stress, metabolism, thermoregulation, electrolyte and water balance, sexual behavior, feeding, and immune and endocrine responses. All these processes are linked to emotional and affective behaviors [335].
All three cerebellar nuclei project to the dorsal, posterior, lateral, ventromedial, and dorsomedial nuclei of the hypothalamus [336–343].
Thalamus
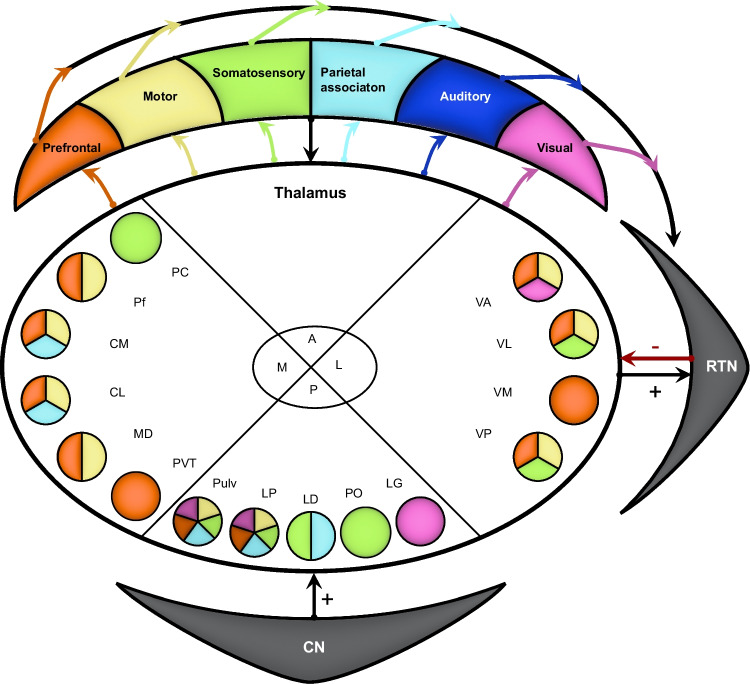
The thalamus is the main gateway for ascending input to the cerebral cortex, while also projecting to the striatum [344, 345]. The thalamus consists of a large number of nuclei that can be organized into groups based on their location: anterior, posterior, medial, and lateral [346–348]. In this classification, the reticular nucleus has a special status as it does not project to the cerebral cortex or the striatum, but instead provides inhibition to the other thalamic nuclei [344]. In turn, the cerebral cortex projects back to the thalamus, both directly as well as indirectly via the reticular nucleus, creating cortico-thalamo-cortical connections that facilitate information exchange between different cortical areas [344, 349–352].
The thalamus is the main intermediate between the cerebellum and the cerebral cortex [294]. The anterior thalamus does not receive direct input from the cerebellum, but most other thalamic nuclei do. Remarkably, if a thalamic nucleus receives input from the cerebellum, it does receive input from all three cerebellar nuclei [11, 32–35, 45, 48, 52, 54, 64, 65, 67, 77, 92, 93, 104, 105, 117, 148, 175, 208, 218, 227, 230, 231, 234, 237, 240, 243, 281, 294–296, 341, 353–381] (Table S2). As different thalamic nuclei innervate different regions of the cerebral cortex [346–348], this implies that cerebellar activity has broad impact on cerebral cortical function [382] (Fig. 3).
Fig. 3.
Cerebello-thalamic projections. The thalamus is an important target area of cerebellar projections and the main gateway to the cerebral cortex. The thalamic nuclei are ordered by their main target area(s) in the cerebral cortex. Most thalamic nuclei receive input from all three cerebellar nuclei. The colors of the thalamic nuclei correspond to the areas of the cerebral cortex to which they project. Abbreviations: Ath, anterior thalamic nuclei; CL, centrolateral nucleus; CM, centromedial nucleus; CN, cerebellar nuclei; IL, intralaminar nucleus; LD, laterodorsal nucleus; LG, lateral geniculate nucleus; LP, latero-posterior nucleus; MD, mediodorsal thalamic nucleus; MG, medial geniculate nucleus; PC, paracentral nucleus; Pf, parafascicular complex; PO, posterior thalamic nuclei; Pulv, pulvinar; PVT, paraventricular nucleus; RTN, reticular thalamic nuclei; VA, ventro-anterior thalamic nucleus; VL, ventrolateral thalamic nucleus; VM, ventromedial nucleus; VP, ventro-posterior thalamic nucleus
Subthalamic Nucleus (Sth)
The subthalamic nucleus is part of the basal ganglia and mainly involved in movement control [383]. Of note, the subthalamic nucleus is also one of the sites used for deep brain stimulation to relieve symptoms of Parkinson’s disease [384–386]. All cerebellar nuclei project to the subthalamic nucleus [64, 104, 227, 387].
Zona Incerta (ZI)
The zona incerta is a subthalamic region with extensive projections to many areas of the brain, such as diencephalon, basal ganglia, brainstem, and spinal cord [388]. It is involved in a wide range of functions, such as visceral and arousal activities, drinking and feeding, nociception, and locomotion [389–393]. The zona incerta receives projections from all three cerebellar nuclei [32–34, 48, 64, 65, 67, 104, 218, 227, 234, 237, 240, 243, 281, 293–296, 366, 377, 381, 394–396].
Discussion
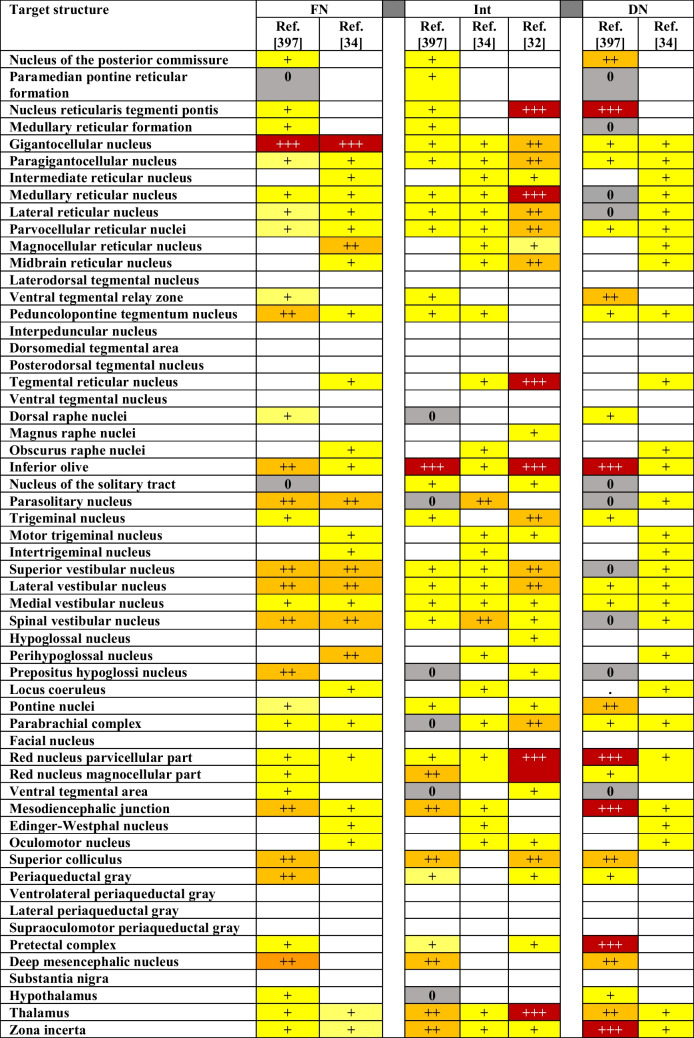
Cerebellar projections are widespread, and there are few regions in the brainstem and diencephalon that do not receive any direct input from the cerebellum. Strikingly, most cerebellar target regions receive input from all three cerebellar nuclei. Thus, despite our increasing understanding of functional compartmentalization in the cerebellum, output streams seem to converge. This does not imply, however, that there is no heterogeneity in projections patterns within target areas or that all projections are equally strong. Based on four studies with a systematic description of multiple projections, we created a table to facilitate the comparison of projection strengths (Table 1). From Table 1, it is immediately clear that interpreting projection strength is not straightforward. It crucially depends on experimental variations and scoring techniques. A functional interpretation is even more difficult, as a weak projection to a specific nucleus can either imply a diffuse projection spreading all over that nucleus, or a targeted projection to a specific subset of neurons. For these reasons, we focus in our systematic review on the existence of evidence in favor of specific connections, rather than on the connection strengths.
Table 1.
Comparing the strengths of projections. Monosynaptic projections from the fastigial, interposed and dentate nucleus to defined target regions as scored in different studies on mice (but reference [397] concerns rats). 0: reported absence of projection, + sparse projection, + + dense projection, + + + very dense projection. Blank fields concern projections that were not mentioned in a study
While most connections have been found in multiple studies, using both antero- and retrograde tracers, utilizing classical as well as viral tracers, and examining different species, some projections have been described only in one or a few studies. As the interpretation of tracer studies can be obfuscated by non-specific staining, we consider projections described in various ways more reliable than those described only in one or a few studies. We therefore rated all projections, giving a higher confidence score to projections demonstrated under more diverse experimental conditions (Fig. 4). From this confidence rating, we noticed that cerebellar nuclei project with a high degree of confidence to the reticular formation, inferior olive, vestibular nuclei, nucleus of the solitary tract/parasolitary nucleus, locus coeruleus, parabrachial complex, tegmental area, mesodiencephalic junction, Edinger-Westphal nucleus, pontine nuclei, red nucleus, superior colliculus, periaqueductal gray, pretectal complex, thalamus, zona incerta, substantia nigra, and hypothalamus; with moderate degree of confidence to the trigeminal nucleus, facial nucleus, ventral tegmental area, oculomotor nucleus, and deep mesencephalic nucleus; and with low degree of confidence to the hypoglossal nucleus, nucleus ambiguus, nucleus abducens, and motor part of the trigeminal nucleus. It became strikingly apparent that cerebellar projections to cranial and vagal motor nuclei have only been demonstrated in relatively few publications. We consider it therefore likely that direct projections to most motor areas are either relatively sparse, or even non-existent. This argues for the dominance of indirect pathways, in a similar fashion as both the cerebral cortex and the basal ganglia are targeted disynaptically via the thalamus [354, 398]. Also, direct projections to the ventral tegmental area, the ventral respiratory group, and nucleus ambiguus have not been documented extensively, implying that also these connections could very well be either sparse or even absent. Further investigations specifically addressing the presence of these direct connections will be required to settle this issue.
The Impact of Different Tracers
Initially, neural projections were studied by making physical or electrical lesion of the region of interest, causing degeneration of nerve terminals [400]. This method presents various complications, however, such as shrinking of cells and damage to fibers of passage [401]. Hence, we did not include studies using neurodegeneration in this systematic review.
Radioactively labelled amino acids provided more specificity than neurodegeneration, but allowed limited discrimination between labels in terminals or fibers of passage [402]. Later, horseradish peroxidase (HRP) proved to be less toxic, while providing good retrograde transport and moderate anterograde transport [403–405]. Also, HRP conjugated with wheat germ agglutinin (WGA-HRP) has been used as bidirectional tracer [401, 406, 407]. In the 1980s, Phaseolus vulgaris–leucoagglutinin (PHA-L) was demonstrated to be a suitable substitute for HRP as anterograde tracer [401, 408, 409]. Shortly afterwards, several other neural tracers were introduced, of which biotinylated dextran amine (BDA) [410, 411] and cholera toxin subunit B (CTb) [401, 412–415] have been used most often. All these classical tracers lack cell-type selectivity and are ill-suited for transsynaptic tracing [416, 417].
Viruses, such as adeno-associated virus (AAV) [418, 419] and herpes simplex virus [420, 421], can also be used as anterograde tracers, and rabies [422, 423], pseudorabies [424, 425], canine adenovirus [426, 427], and rAAV2-retro [428] as retrograde tracers. The specificity can be optimized by exploiting differences in cell tropism, immunogenicity, and transduction strength of different AAV strains [429, 430]. Furthermore, genetic modification of viruses can introduce conditional expression patterns so that viruses can in principle be optimized for particular experiments, including studying cell-type specific connections. In addition, also the transsynaptic properties of viral transport can be modulated. For instance, deleting external glycoprotein abolishes the ability of rabies virus to be transported transsynaptically [431, 432].
Unfortunately, even the newest conventional and viral tracers have disadvantages that can affect the interpretation of neural tracer experiments. For instance, both conventional [433–435] and viral tracers, such as canine adenovirus, may also be taken up by fibers of passage [36]. Moreover, the spreading of the conventional tracers in the injection site may lead in intense and diffuse labeling reflecting take-up by neurons or glia. This non-specific labeling may lead a unreliable identification of labeled neurons [436, 437]. In the same way, the viral tracers, due to the genome replication, may produce a strong signal, which could lead to false positive findings [438, 439]. Furthermore, different viral tracers may give different labeling results and have different neurotropism [440], creating more heterogeneity and reduced comparability between different experiments. In addition, viral tracers may cause cytotoxicity of infected neurons [416], although viral strains with lower toxicity will probably be increasingly available in the near future [440]. As each specific tracer has advantages and disadvantages, we attributed a higher confidence score to projections that have been demonstrated using both conventional and viral tracers, as well as by using anterograde and retrograde tracing (Fig. 4).
Did the introduction of viral tracers change our view of cerebellar projections? We systematically compared cerebellar projections described with classical and with viral tracers in rodents, and found more similarities than differences in the outcomes (Fig. S1). In general, viral tracers identified more targets than conventional tracers. The oculomotor, trigeminal motor, and hypoglossal nucleus did show up in experiments using viral tracers, but not in those with conventional tracers. These connections are likely to be sparse [156], and this could be an argument for the higher sensitivity of viral tracers. On the other hand, cerebellar projections to the subthalamic nucleus and the ventral respiratory group were found using conventional tracers, but not using viral tracers. Thus, both tracers confirmed the major projections of the cerebellum, but do not always agree on the sparser connections. The latter may also be due to differences in the experimental conditions.
Similarities Between Species
Subsequently, we compared studies performed in cats, monkeys, rats or mice (Figs. S2–S5). In general, the projection patterns seemed similar. However, when examining projections from individual cerebellar nuclei, differences between species were found. A careful comparison of the study designs, however, suggests that most differences relate to variations in study design rather than to differences between species.
Nevertheless, a few exceptions remained. In particular, cerebellar projections to the nucleus abducens and nucleus ambiguus were described in cats, but not in rodents and also not in monkeys, despite searching for these areas [33, 47, 51]. Conversely, projections to the rostral ventral respiratory group, the parabrachial nucleus, the Edinger-Westphal nucleus, the ventral tegmental area, and the trigeminal nuclei were described in rodents and monkeys, but not in cats [32–34, 37, 41, 54, 64, 85, 153, 167, 228, 257–260, 277, 279, 397]. Thus, while the majority of cerebellar projections are present in monkeys, cats, and rodents, a few were not found in all species. Especially cats seem to be an outlier, which might be explained by the popularity of cats in earlier, but not in later studies. We conclude, therefore, that we did not find solid evidence for different projection patterns between mammals as divergent as mice and monkeys. We cannot exclude, however, that minor variations exist.
Heterogeneity in Projections
Most target areas receive monosynaptic input from all three cerebellar nuclei (Fig. 2). This seemingly large convergence of cerebellar output does require some nuance, though. As indicated in the text and in Table S2, there is quite some variation of cerebellar input within certain target areas. Most brain regions are heterogeneous in terms of their neuronal cell types and projection patterns. Likewise, also the cerebellar nuclei contain multiple types of neurons [441]. Altogether, heterogeneity at the sending and the receiving side can create a complex pattern, the unraveling of which will likely entertain neuroanatomists for quite some time. As discussed above, viral tracer techniques are very helpful to study cell-type specificity.
Most projection neurons in the cerebellar nuclei are glutamatergic and they target mostly premotor areas while firing at sustained rates up to around 100 Hz [442, 443]. Excitatory neurons project also to granule and Golgi cells in the cerebellar cortex [22, 77]. Small GABAergic projection neurons target specifically the inferior olive and fire slower than glutamatergic projections neurons [442, 444, 445], but recently also other targets for inhibitory neurons were found, including sensory brainstem structures, medullary reticular nuclei, and pontine nuclei [32]. Moreover, two populations of glycinergic neurons have been found in cerebellar nuclei. One is formed by large, spontaneously active glycinergic neurons in the fastigial nucleus that target the vestibular nuclei [52], and the other is an intrinsically silent neuronal population projecting to the granular layer of the cerebellum [446]. Glycinergic neurons project also to Golgi cells in the cerebellar cortex [21]. Finally, a small population of glycinergic neurons in the fastigial nucleus project to the premotor neurons [447]. All of these cell-types of cerebellar nuclei neurons receive GABAergic projections from Purkinje cells [121, 448, 449].
Within these populations of neurons, further subdivisions can be made, as was recently studied in the fastigial nucleus. The caudal part of the fastigial nucleus expresses SNCA (alpha-synuclein), the rostral part SPP1 (osteopontin), the ventrolateral part CALB2 (calretinin), the caudal dorsolateral protuberance SPP1 and SNCA, and the rostral dorsolateral protuberance SPP1, and these subtypes contact different brainstem regions [33].
Clinical Implications
Cerebellar degeneration, such as that occurring, for instance, in patients with types 1, 2, and 3 of spinocerebellar ataxia (SCA1, SCA2, and SCA3), can be correlated with a lower fractional anisotropy (FA) of the cerebellar peduncles [450–453]. Similarly, patients affected by Friedrich’s ataxia also presented with lower FA of dentato-rubral and dentato-thalamic tracts in the superior cerebellar peduncle [454, 455]. As the cerebellar peduncles carry all afferent and efferent fibers to and from the cerebellum [64], a lower FA could indicate reduced connectivity of the cerebellum with the rest of the brain. Indeed, there seems to be a dissociation between parietal-occipital regions and cerebellar regions in patients with SCA3 [456] and between the cerebellum and supplementary motor area, cingulate cortex, and frontal cortices in patients with Friedreich’s ataxia [457].
A reduced connectivity of cerebellum is not confined to neurodegenerative diseases classically associated with the cerebellum. For instance, also in schizophrenia, a neuropsychiatric disease characterized by hallucinations, thought disorders, social withdrawal, and cognitive dysfunction and by disrupted connectivity of large-scale neural networks [458], there is lower FA of the superior [459–461] and medial cerebellar peduncle [462–464]. This is in line with a reduced connectivity between thalamus and left and right cerebellum in patients with schizophrenia [458], as well as with hypoconnectivity of the thalamus between medial prefrontal cortex and cerebellum [465], and reduction of thalamo-prefrontal-striatal-cerebellar coupling [466]. Some connections involving the cerebellum seem, in contrast, to be stronger in patients with schizophrenia. In particular, hyperconnectivity was present between crus I and the ventral attention, motor, and auditory networks; between crus II and the ventral attention network; between lobule IX and the ventral and dorsal attention, motor, auditory, and cingulo-opercular networks; and between lobule X and the ventral attention, motor, and auditory networks [467]. This hyperconnectivity may be due to a potential association between sensorimotor lobules of the cerebellum and the cortical association networks, and use as a compensatory adaptation to maintain intrinsic baseline resting state, for instance, consciousness, processing sensory signals, and monitoring body position [467, 468].
Finally, we examined the cerebellar functional connectivity in autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by repetitive behavior, and impairments in social interaction and communication [469]. Subjects affected by ASD had decreased white matter in the dentato-rubro-thalamic tract [470] and lower FA of the superior [471, 472] and medial cerebellar peduncle [473–475]. Accordingly, patients with ASD had decreased functional connectivity between various parts of the cerebellar cortex and nuclei and different cortical regions [476–480], which may suggest an important cerebellar role in the pathophysiology of ASD. Like in schizophrenia, ASD was not only associated with reduced connectivity of particular projections, but also with increased strength of others [480–483]. In conclusion, cerebellar connectivity is affected in motor and in non-motor diseases (Fig. 5, Table S3).
Fig. 5.
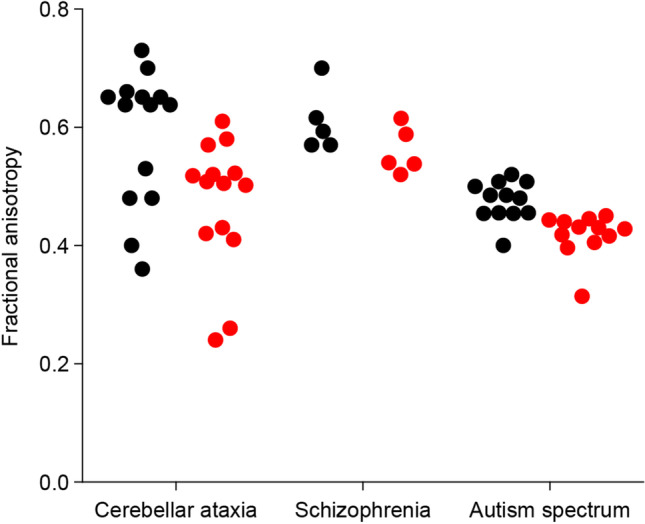
Fractional anisotropy values of superior cerebellar peduncle of patients with cerebellar ataxia, schizophrenia, and autism spectrum disorder. Fractional anisotropy values of superior cerebellar peduncle in diseases (red) and corresponding control (black) groups. Graphs showing that fractional anisotropy values of superior cerebellar peduncle in ataxia, schizophrenia, and autism spectrum disorder were significantly different than in the corresponding control groups. For details and references (see Table S3)
Conclusion
The cerebellum forms widespread projections, innervating most areas in the diencephalon and the brainstem, next to the spinal cord that was not part of this review. Most target areas receive input from all three cerebellar nuclei. The advent of viral tracing techniques will facilitate our understanding of the heterogeneity of these projections. Given the widespread projections, it is quite striking that most brainstem motor nuclei do not receive direct input from the cerebellum, or only sparse projections. This could be in line with modulatory and coordinating roles, rather than with direct motor control [12]. The study of these projections in diseases has just begun, but it is already clear that various diseases implicate variations in connectivity between the cerebellum and the rest of the brain.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Elise Krabbendam from the Erasmus MC Medical Library for creating the search query and performing database searches and advice for the structure of this systematic review, and Dr. Tom Ruigrok for valuable discussions throughout this project.
Author Contribution
Conceptualization: M.N., L.W.J.B., C.I.D.Z. Methodology: M.N., L.W.J.B. Formal analysis and investigation: M.N. Writing-original draft preparation: M.N., L.W.J.B. Writing-review and editing: L.W.J.B., C.I.D.Z. Funding acquisition: L.W.J.B., C.I.D.Z. Supervision: L.W.J.B.
Funding
The work of the authors is supported by the Netherlands Organization for Scientific Research (NWOALW; C.I.D.Z.), the Dutch Organization for Medical Sciences (ZonMW; C.I.D.Z.), BIG (C.I.D.Z.), Medical Neuro-Delta (C.I.D.Z.), INTENSE LSH-NWO (C.I.D.Z.), DBI2 (Zwaartekracht, NWO [C.I.D.Z.]), ERC-adv and ERC-POC (C.I.D.Z.), Van Raamsdonk-fonds (C.I.D.Z.), 3 V-Fonds KNAW (C.I.D.Z.), Albinism Fonds NIN (C.I.D.Z.), and Health Holland (TKI-LSH EMCLSH21017 [L.W.J.B.]).
Data Availability
Not applicable.
Declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/12/2023
A Correction to this paper has been published: 10.1007/s12311-023-01566-w
Contributor Information
Laurens W. J. Bosman, Email: l.bosman@erasmusmc.nl
Chris I. De Zeeuw, Email: c.dezeeuw@erasmusmc.nl
References
- 1.Brochu G, Maler L, Hawkes R. Zebrin II: A polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291:538–552. doi: 10.1002/cne.902910405. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, Schonewille M. Cerebellar modules operate at different frequencies. Elife. 2014;3:e02536. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16:79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Zeeuw CI. Bidirectional learning in upbound and downbound microzones of the cerebellum. Nat Rev Neurosci. 2021;22:92–110. doi: 10.1038/s41583-020-00392-x. [DOI] [PubMed] [Google Scholar]
- 5.King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019;22:1371–1378. doi: 10.1038/s41593-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsutsumi S, Hidaka N, Isomura Y, Matsuzaki M, Sakimura K, Kano M and Kitamura K. Modular organization of cerebellar climbing fiber inputs during goal-directed behavior. eLife 2019: 8. 10.7554/eLife.47021 [DOI] [PMC free article] [PubMed]
- 7.Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, Ebner TJ, Isope P, Jörntell H, Lackey EP, Lawrenson C, Lumb B, Schonewille M, Sillitoe RV, Spaeth L, Sugihara I, Valera A, Voogd J, Wylie DR, Ruigrok TJH. Cerebellar modules and their role as operational cerebellar processing units: a consensus paper [corrected] Cerebellum. 2018;17:654–682. doi: 10.1007/s12311-018-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roostaei T, Nazeri A, Sahraian MA, Minagar A. The human cerebellum: a review of physiologic neuroanatomy. Neurol Clin. 2014;32:859–869. doi: 10.1016/j.ncl.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Yu SY, Ren Z, De Zeeuw CI, Gao Z. A FN-MdV pathway and its role in cerebellar multimodular control of sensorimotor behavior. Nat Commun. 2020;11:6050. doi: 10.1038/s41467-020-19960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju C, Bosman LWJ, Hoogland TM, Velauthapillai A, Murugesan P, Warnaar P, van Genderen RM, Negrello M, De Zeeuw CI. Neurons of the inferior olive respond to broad classes of sensory input while subject to homeostatic control. J Physiol. 2019;597:2483–2514. doi: 10.1113/jp277413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heiney SA, Wojaczynski GJ, Medina JF. Action-based organization of a cerebellar module specialized for predictive control of multiple body parts. Neuron. 2021;109:2981–94.e5. doi: 10.1016/j.neuron.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bina L, Romano V, Hoogland TM, Bosman LWJ, De Zeeuw CI. Purkinje cells translate subjective salience into readiness to act and choice performance. Cell Rep. 2022;38:110362. doi: 10.1016/j.celrep.2022.110362. [DOI] [PubMed] [Google Scholar]
- 13.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosman LW, Houweling AR, Owens CB, Tanke N, Shevchouk OT, Rahmati N, Teunissen WH, Ju C, Gong W, Koekkoek SK, De Zeeuw CI. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Front Integr Neurosci. 2011;5:53. doi: 10.3389/fnint.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruigrok TJH, Sillitoe RV, Voogd J. Chapter 9 - Cerebellum and cerebellar connections. In: Paxinos G, editor. The Rat Nervous System (Fourth Edition) San Diego: Academic Press; 2015. pp. 133–205. [Google Scholar]
- 16.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szentágothai J, Rajkovits K. Über den Ursprung der Kletterfasern des Kleinhirns. Z Anat Entwicklungsgesch. 1959;121:130–141. doi: 10.1007/BF00525203. [DOI] [Google Scholar]
- 18.Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parasuram H, Nair B, Naldi G, D'Angelo E, Diwakar S. Understanding cerebellum granular layer network computations through mathematical reconstructions of evoked local field potentials. Ann Neurosci. 2018;25:11–24. doi: 10.1159/000481905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- 21.Ankri L, Husson Z, Pietrajtis K, Proville R, Léna C, Yarom Y, Dieudonné S and Uusisaari MY. A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. Elife 2015: 4. 10.7554/eLife.06262 [DOI] [PMC free article] [PubMed]
- 22.Gao Z, Proietti-Onori M, Lin Z, Ten Brinke MM, Boele HJ, Potters JW, Ruigrok TJH, Hoebeek FE, De Zeeuw CI. Excitatory cerebellar nucleocortical circuit provides internal amplification during associative conditioning. Neuron. 2016;89:645–657. doi: 10.1016/j.neuron.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buisseret-Delmas C, Angaut P. The cerebellar olivo-corticonuclear connections in the rat. Prog Neurobiol. 1993;40:63–87. doi: 10.1016/0301-0082(93)90048-w. [DOI] [PubMed] [Google Scholar]
- 24.Voogd J, Shinoda Y, Ruigrok TJH, Sugihara I. Cerebellar nuclei and the inferior olivary nuclei: organization and connections. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the Cerebellum and Cerebellar Disorders. Dordrecht, Springer: Netherlands; 2013. pp. 377–436. [Google Scholar]
- 25.Goodman DC, Hallett RE, Welch RB. Patterns of localization in the cerebellar corticonuclear projections of albino rat. J Comp Neurol. 1963;121:51–67. doi: 10.1002/cne.901210106. [DOI] [PubMed] [Google Scholar]
- 26.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Stilling B. Untersuchungen über den Bau des kleinen Gehirns des Menschen. Cassel: T. Kay; 1864.
- 28.Perciavalle V, Apps R, Bracha V, Delgado-García JM, Gibson AR, Leggio M, Carrel AJ, Cerminara N, Coco M, Gruart A, Sánchez-Campusano R. Consensus paper: current views on the role of cerebellar interpositus nucleus in movement control and emotion. Cerebellum. 2013;12:738–757. doi: 10.1007/s12311-013-0464-0. [DOI] [PubMed] [Google Scholar]
- 29.Bond KM, Brinjikji W, Eckel LJ, Kallmes DF, McDonald RJ, Carr CM. Dentate update: imaging features of entities that affect the dentate nucleus. AJNR Am J Neuroradiol. 2017;38:1467–1474. doi: 10.3174/ajnr.A5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matano S. Brief communication: proportions of the ventral half of the cerebellar dentate nucleus in humans and great apes. Am J Phys Anthropol. 2001;114:163–165. doi: 10.1002/1096-8644(200102)114:2<163::AID-AJPA1016>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 31.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Judd EN, Lewis SM and Person AL. Diverse inhibitory projections from the cerebellar interposed nucleus. Elife 2021: 10. 10.7554/eLife.66231 [DOI] [PMC free article] [PubMed]
- 33.Fujita H, Kodama T and Du Lac S. Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. eLife 2020: 9:1–91. 10.7554/eLife.58613 [DOI] [PMC free article] [PubMed]
- 34.Kebschull JM, Richman EB, Ringach N, Friedmann D, Albarran E, Kolluru SS, Jones RC, Allen WE, Wang Y, Cho SW, Zhou H, Ding JB, Chang HY, Deisseroth K, Quake SR and Luo L. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 2020: 370. 10.1126/science.abd5059 [DOI] [PMC free article] [PubMed]
- 35.Frontera JL, Baba Aissa H, Sala RW, Mailhes-Hamon C, Georgescu IA, Léna C and Popa D. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nat Commun 2020: 11. 10.1038/s41467-020-18953-0 [DOI] [PMC free article] [PubMed]
- 36.Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carta I, Chen CH, Schott AL, Dorizan S and Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Sci 2019: 363. 10.1126/science.aav0581 [DOI] [PMC free article] [PubMed]
- 38.Vaaga CE, Brown ST and Raman IM. Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. eLife 2020: 9. 10.7554/eLife.54302 [DOI] [PMC free article] [PubMed]
- 39.Paxinos G, Franklin KBJ. Paxinos and Franklin's the mouse brain in stereotaxic coordinates. Amsterdam: Elsevier Academic Press; 2013. [Google Scholar]
- 40.Ito J, Sasa M, Matsuoka I, Takaori S. Afferent projection from reticular nuclei, inferior olive and cerebellum to lateral vestibular nucleus of the cat as demonstrated by horseradish peroxidase. BRAIN RES. 1982;231:427–432. doi: 10.1016/0006-8993(82)90378-x. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto M, Yamanaka A, Kato S, Tanifuji M, Kobayashi K and Yaginuma H. Anatomical evidence for a direct projection from purkinje cells in the mouse cerebellar vermis to medial parabrachial nucleus. Front Neural Circuits 2018: 12. 10.3389/fncir.2018.00006 [DOI] [PMC free article] [PubMed]
- 42.Dietrichs E, Haines DE. Demonstration of hypothalamo-cerebellar and cerebello-hypothalamic fibres in a prosimian primate (Galago crassicaudatus) Anat Embryol (Berl) 1984;170:313–318. doi: 10.1007/bf00318735. [DOI] [PubMed] [Google Scholar]
- 43.Iordanova R and Reddivari AKR. Neuroanatomy, medulla oblongata. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 44.Bentivoglio M, Kuypers HG. Divergent axon collaterals from rat cerebellar nuclei to diencephalon, mesencephalon, medulla oblongata and cervical cord A fluorescent double retrograde labeling study. Exp Brain Res. 1982;46:339–56. doi: 10.1007/BF00238629. [DOI] [PubMed] [Google Scholar]
- 45.Bentivoglio M, Molinari M. Crossed divergent axon collaterals from cerebellar nuclei to thalamus and lateral medulla oblongata in the rat. BRAIN RES. 1986;362:180–184. doi: 10.1016/0006-8993(86)91414-9. [DOI] [PubMed] [Google Scholar]
- 46.Cobos A, Lima D, Almeida A, Tavares I. Brain afferents to the lateral caudal ventrolateral medulla: A retrograde and anterograde tracing study in the rat. Neuroscience. 2003;120:485–498. doi: 10.1016/s0306-4522(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 47.Moolenaar GW, Rucker HK. Autoradiographic study of brain stem projections from fastigial pressor areas. BRAIN RES. 1976;114:492–496. doi: 10.1016/0006-8993(76)90970-7. [DOI] [PubMed] [Google Scholar]
- 48.Hirai T, Onodera S, Kawamura K. Cerebellotectal projections studied in cats with horseradish peroxidase or tritiated amino acids axonal transport. EXP BRAIN RES. 1982;48:1–12. doi: 10.1007/BF00239567. [DOI] [PubMed] [Google Scholar]
- 49.Andrezik JA, Dormer KJ, Foreman RD, Person RJ. Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neuroscience. 1984;11:497–507. doi: 10.1016/0306-4522(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 50.Schneider JS, Manetto C, Lidsky TI. Substantia nigra projection to medullary reticular formation: Relevance to oculomotor and related motor function in the cat. NEUROSCI LETT. 1985;62:1–6. doi: 10.1016/0304-3940(85)90275-7. [DOI] [PubMed] [Google Scholar]
- 51.Homma Y, Nonaka S, Matsuyama K, Mori S. Fastigiofugal projection to the brainstem nuclei in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1995;23:89–102. doi: 10.1016/0168-0102(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 52.Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, Du Lac S. Glycinergic projection neurons of the cerebellum. J Neurosci. 2009;29:10104–10110. doi: 10.1523/jneurosci.2087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu L, Cao Y, Tokita K, Heck DH, Boughter JD., Jr Medial cerebellar nuclear projections and activity patterns link cerebellar output to orofacial and respiratory behavior. Front Neural Circuits. 2013 doi: 10.3389/fncir.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batton Iii RR, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J COMP NEUROL. 1977;174:281–305. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- 55.Mezey E, Kiss J, Palkovits M. Bidirectional neuronal connections between the cerebellar interpositus nucleus and the brainstem (an autoradiographic study) ACTA MORPHOL HUNG. 1985;33:45–60. [PubMed] [Google Scholar]
- 56.Giuditta M, Ruggiero DA, Del Bo A. Anatomical basis for the fastigial pressor response. Blood Press. 2003;12:175–180. doi: 10.1080/08037050301800. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi M, Sugiuchi Y, Shinoda Y. Convergent synaptic inputs from the caudal fastigial nucleus and the superior colliculus onto pontine and pontomedullary reticulospinal neurons. J Neurophysiol. 2014;111:849–867. doi: 10.1152/jn.00634.2013. [DOI] [PubMed] [Google Scholar]
- 58.Xu F, Zhou T, Gibson T, Frazier DT. Fastigial nucleus-mediated respiratory responses depend on the medullary gigantocellular nucleus. J Appl Physiol. 2001;91:1713–1722. doi: 10.1152/jappl.2001.91.4.1713. [DOI] [PubMed] [Google Scholar]
- 59.Walker EP and Tadi P. Neuroanatomy, nucleus raphe. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 60.Törk I. Anatomy of the serotonergic system. Ann N Y Acad Sci 1990: 600:9–34; discussion -5. 10.1111/j.1749-6632.1990.tb16870.x [DOI] [PubMed]
- 61.Poliacek I, Jakus J, Simera M, Veternik M, Plevkova J. Control of coughing by medullary raphé. Prog Brain Res. 2014;212:277–295. doi: 10.1016/b978-0-444-63488-7.00014-8. [DOI] [PubMed] [Google Scholar]
- 62.Luo M, Zhou J, Liu Z. Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem. 2015;22:452–460. doi: 10.1101/lm.037317.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, Aryal DK, Farrell MS, Lowery-Gionta E, Olsen RH, Wetsel WC, Kash TL, Hurd YL, Tecott LH, Roth BL. Elucidation of The behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology. 2016;41:1404–1415. doi: 10.1038/npp.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Çavdar S, Özgur M, Kuvvet Y, Bay H, Aydogmus E. Cortical, subcortical and brain stem connections of the cerebellum via the superior and middle cerebellar peduncle in the rat. J Integr Neurosci. 2018;17:609–618. doi: 10.3233/JIN-180090. [DOI] [PubMed] [Google Scholar]
- 65.Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. BRAIN RES REV. 1983;5:299–322. doi: 10.1016/0165-0173(83)90017-6. [DOI] [PubMed] [Google Scholar]
- 66.Langer TP, Kaneko CRS. Brainstem afferents to the omnipause region in the cat: a horseradish peroxidase study. J COMP NEUROL. 1984;230:444–458. doi: 10.1002/cne.902300312. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalo-Ruiz A, Leichnetz GR. Connections of the caudal cerebellar interpositus complex in a new world monkey (Cebus apella) BRAIN RES BULL. 1990;25:919–927. doi: 10.1016/0361-9230(90)90189-7. [DOI] [PubMed] [Google Scholar]
- 68.Marcinkiewicz M, Morcos R, Chretien M. CNS connections with the median raphe nucleus: retrograde tracing with WGA-apoHRP-Gold complex in the rat. J COMP NEUROL. 1989;289:11–35. doi: 10.1002/cne.902890103. [DOI] [PubMed] [Google Scholar]
- 69.Ángeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP. Anatomy of the brainstem: a gaze into the stem of life. Seminars in Ultrasound, CT and MRI. 2010;31:196–219. doi: 10.1053/j.sult.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Horn AK. The reticular formation. Prog Brain Res. 2006;151:127–155. doi: 10.1016/s0079-6123(05)51005-7. [DOI] [PubMed] [Google Scholar]
- 71.Mangold SA and Das JM. Neuroanatomy, reticular formation. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 72.Lee HS. Distribution of neurons in the lateral reticular nucleus projecting to cervical, thoracic, and lumbar segments of the spinal cord in the rat. Korean Journal of Biological Sciences. 2000;4:353–359. doi: 10.1080/12265071.2000.9647569. [DOI] [Google Scholar]
- 73.Alstermark B, Ekerot CF. The lateral reticular nucleus: a precerebellar centre providing the cerebellum with overview and integration of motor functions at systems level. A new hypothesis Journal of Physiology-London. 2013;591:5453–5458. doi: 10.1113/jphysiol.2013.256669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hrycyshyn AW, Flumerfelt BA. A light microscopic investigation of the afferent connections of the lateral reticular nucleus in the cat. J COMP NEUROL. 1981;197:477–502. doi: 10.1002/cne.901970309. [DOI] [PubMed] [Google Scholar]
- 75.Qvist H. The cerebellar nuclear afferent and efferent connections with the lateral reticular nucleus in the cat as studied with retrograde transport of WGA-HRP. ANAT EMBRYOL. 1989;179:471–483. doi: 10.1007/bf00319590. [DOI] [PubMed] [Google Scholar]
- 76.Rajakumar N, Hrycyshyn AW, Flumerfelt BA. Afferent organization of the lateral reticular nucleus in the rat: an anterograde tracing study. Anat Embryol (Berl) 1992;185:25–37. doi: 10.1007/BF00213598. [DOI] [PubMed] [Google Scholar]
- 77.Low AYT, Thanawalla AR, Yip AKK, Kim J, Wong KLL, Tantra M, Augustine GJ, Chen AI. Precision of discrete and rhythmic forelimb movements requires a distinct neuronal subpopulation in the interposed anterior nucleus. Cell Rep. 2018;22:2322–2333. doi: 10.1016/j.celrep.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 78.Angeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP. Anatomy of the brainstem: a gaze into the stem of life. Semin Ultrasound CT MR. 2010;31:196–219. doi: 10.1053/j.sult.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalo-Ruiz A, Leichnetz GR, Smith DJ. Origin of cerebellar projections to the region of the oculomotor complex, medial pontine reticular formation, and superior colliculus in new world monkeys: a retrograde horseradish peroxidase study. J COMP NEUROL. 1988;268:508–526. doi: 10.1002/cne.902680404. [DOI] [PubMed] [Google Scholar]
- 80.Leichnetz GR, Carlton SM, Katayama Y, Gonzalo-Ruiz A, Holstege G, DeSalles AA, Hayes RL. Afferent and efferent connections of the cholinoceptive medial pontine reticular formation (region of the ventral tegmental nucleus) in the cat. Brain Res Bull. 1989;22:665–688. doi: 10.1016/0361-9230(89)90087-7. [DOI] [PubMed] [Google Scholar]
- 81.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuller G. Significance of the paralemniscal tegmental area for audio-motor control in the moustached bat, Pteronotus p. Parnellii: The afferent and efferent connections of the paralemniscal area. EUR J NEUROSCI. 1997;9:342–55. doi: 10.1111/j.1460-9568.1997.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 83.Kawamura S, Hattori S, Higo S, Matsuyama T. The cerebellar projections to the superior colliculus and pretectum in the cat: an autoradiographic and horseradish peroxidase study. Neuroscience. 1982;7:1673–1689. doi: 10.1016/0306-4522(82)90026-4. [DOI] [PubMed] [Google Scholar]
- 84.Simon H, Le Moal M, Calas A. Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. BRAIN RES. 1979;178:17–40. doi: 10.1016/0006-8993(79)90085-4. [DOI] [PubMed] [Google Scholar]
- 85.Perciavalle V, Berretta S, Raffaele R. Projections from the intracerebellar nuclei to the ventral midbrain tegmentum in the rat. Neuroscience. 1989;29:109–119. doi: 10.1016/0306-4522(89)90336-9. [DOI] [PubMed] [Google Scholar]
- 86.Paul M, S. and Das J, M. . Neuroanatomy, superior and inferior olnvary Nucleus (superior and inferior olivary complex). StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 87.Ausim AS. … And the olive said to the cerebellum: organization and functional significance of the olivo-cerebellar system. Neuroscientist. 2007;13:616–625. doi: 10.1177/1073858407299286. [DOI] [PubMed] [Google Scholar]
- 88.Ding SL, Royall JJ, Sunkin SM, Ng L, Facer BA, Lesnar P, Guillozet-Bongaarts A, McMurray B, Szafer A, Dolbeare TA, Stevens A, Tirrell L, Benner T, Caldejon S, Dalley RA, Dee N, Lau C, Nyhus J, Reding M, Riley ZL, Sandman D, Shen E, van der Kouwe A, Varjabedian A, Write M, Zollei L, Dang C, Knowles JA, Koch C, Phillips JW, Sestan N, Wohnoutka P, Zielke HR, Hohmann JG, Jones AR, Bernard A, Hawrylycz MJ, Hof PR, Fischl B, LeinReference ES. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 2017;525:407. doi: 10.1002/cne.24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu Y, Fu Y, Watson C. The inferior olive of the C57BL/6 J mouse: a chemoarchitectonic study. Anat Rec (Hoboken) 2014;297:289–300. doi: 10.1002/ar.22866. [DOI] [PubMed] [Google Scholar]
- 90.Dietrichs E, Walberg F. The cerebellar nucleo-olivary projection in the cat. ANAT EMBRYOL. 1981;162:51–67. doi: 10.1007/bf00318094. [DOI] [PubMed] [Google Scholar]
- 91.Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. ANAT EMBRYOL. 1991;184:225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- 92.Lee HS, Kosinski RJ, Mihailoff GA. Collateral branches of cerebellopontine axons reach the thalamus, superior colliculus, or inferior olive: a double-fluorescence and combined fluorescence-horseradish peroxidase study in the rat. Neuroscience. 1989;28:725–734. doi: 10.1016/0306-4522(89)90017-1. [DOI] [PubMed] [Google Scholar]
- 93.Lo LC, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin GF, Henkel CK, King JS. Cerebello olivary fibers: their origin, course and distribution in the North American opossum. EXP BRAIN RES. 1976;24:219–236. doi: 10.1007/BF00235011. [DOI] [PubMed] [Google Scholar]
- 95.Teune TM, Van der Burg J, Ruigrok TJH. Cerebellar projections to the red nucleus and inferior olive originate from separate populations of neurons in the rat: a non-fluorescent double labeling study. BRAIN RES. 1995;673:313–319. doi: 10.1016/0006-8993(94)01431-g. [DOI] [PubMed] [Google Scholar]
- 96.De Zeeuw CI, Holstege JC, Ruigrok TJH, Voogd J. Mesodiencephalic and cerebellar terminals terminate upon the same dendritic spines in the glomeruli of the cat and rat inferior olive: An ultrastructural study using a combination of [3H]leucine and wheat germ agglutinin coupled horseradish peroxidase anterograde tracing. Neuroscience. 1990;34:645–655. doi: 10.1016/0306-4522(90)90171-y. [DOI] [PubMed] [Google Scholar]
- 97.Angaut P, Cicirata F. Cerebello-olivary projections in the rat. An autoradiographic study BRAIN BEHAV EVOL. 1982;21:24–33. doi: 10.1159/000121612. [DOI] [PubMed] [Google Scholar]
- 98.Angaut P, Sotelo C. The dentato-olivary projection in the rat as a presumptive GABAergic link in the olivo-cerebello-olivary loop. An ultrastructural study NEUROSCI LETT. 1987;83:227–231. doi: 10.1016/0304-3940(87)90090-5. [DOI] [PubMed] [Google Scholar]
- 99.Angaut P and Sotelo C. Synaptology of the cerebello-olivary pathway. Double labelling with anterograde axonal tracing and GABA immunocytochemistry in the rat. BRAIN RES 1989: 479:361–5. 10.1016/0006-8993(89)91641-7 [DOI] [PubMed]
- 100.Ikeda Y, Noda H, Sugita S. Olivocerebellar and cerebelloolivary connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J COMP NEUROL. 1989;284:463–488. doi: 10.1002/cne.902840311. [DOI] [PubMed] [Google Scholar]
- 101.Dietrichs E, Walberg F. The cerebellar nucleo-olivary and olivo-cerebellar nuclear projections in the cat as studied with anterograde and retrograde transport in the same animal after implantation of crystalline WGA-HRP. II The fastigial nucleus ANAT EMBRYOL. 1985;173:253–261. doi: 10.1007/bf00316306. [DOI] [PubMed] [Google Scholar]
- 102.Ruigrok THJ, Voogd J. Cerebellar nucleo-olivary projections in the rat: an anterograde tracing study with Phaseoulus vulgaris-leucoagglutinin (PHA-L) J COMP NEUROL. 1990;298:315–333. doi: 10.1002/cne.902980305. [DOI] [PubMed] [Google Scholar]
- 103.Diagne M, Delfini C, Angaut P, Buisseret P, Buisseret-Delmas C. Fastigiovestibular projections in the rat: retrograde tracing coupled with γamino-butyric acid and glutamate immunohistochemistry. Neurosci Lett. 2001;308:49–53. doi: 10.1016/s0304-3940(01)01969-3. [DOI] [PubMed] [Google Scholar]
- 104.Künzle H. Thalamic territories innervated by cerebellar nuclear afferents in the hedgehog tenrec. Echinops telfairi J Comp Neurol. 1998;402:313–326. doi: 10.1002/(sici)1096-9861(19981221)402:3<313::Aid-cne3>3.0.Co;2-e. [DOI] [PubMed] [Google Scholar]
- 105.McCrea RA, Bishop GA, Kitai ST. Morphological and electrophysiological characteristics of projection neurons in the nucleus interpositus of the cat cerebellum. J COMP NEUROL. 1978;181:397–420. doi: 10.1002/cne.901810210. [DOI] [PubMed] [Google Scholar]
- 106.Peltier AC, Bishop GA. The site of origin of calcitonin gene-related peptide-like immunoreactive afferents to the inferior olivary complex of the mouse. Neurosci Res. 1999;34:177–186. doi: 10.1016/s0168-0102(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 107.Tolbert DL, Massopust LC, Murphy MG, Young PA. The anatomical organization of the cerebello olivary projection in the cat. J COMP NEUROL. 1976;170:525–544. doi: 10.1002/cne.901700409. [DOI] [PubMed] [Google Scholar]
- 108.Beitz AJ. The topographical organization of the olivo dentate and dentato olivary pathways in the cat. BRAIN RES. 1976;115:311–317. doi: 10.1016/0006-8993(76)90515-1. [DOI] [PubMed] [Google Scholar]
- 109.De Zeeuw CI, Holstege JC, Calkoen F, Ruigron TJH, Voogd J. A new combination of WGA-HRP anterograde tracing and GABA immunocytochemistry applied to afferents of the cat inferior olive at the ultrastructural level. BRAIN RES. 1988;447:369–375. doi: 10.1016/0006-8993(88)91142-0. [DOI] [PubMed] [Google Scholar]
- 110.Ruigrok TJH and Teune TM. Collateralization of cerebellar output to functionally distinct brainstem areas. A retrograde, non-fluorescent tracing study in the rat. Front Syst Neurosci 2014: 8. 10.3389/fnsys.2014.00023 [DOI] [PMC free article] [PubMed]
- 111.Pong M, Horn KM, Gibson AR. Spinal projections of the cat parvicellular red nucleus. J Neurophysiol. 2002;87:453–468. doi: 10.1152/jn.00950.2000. [DOI] [PubMed] [Google Scholar]
- 112.Dietrichs E, Walberg F. The cerebellar nucleo-olivary and olivocerebellar nuclear projections in the cat as studied with anterograde and retrograde transport in the same animal after implantation of crystalline WGA-HRP. III The interposed nuclei BRAIN RES. 1986;373:373–383. doi: 10.1016/0006-8993(86)90352-5. [DOI] [PubMed] [Google Scholar]
- 113.Kalil K. Projections of the cerebellar and dorsal column nuclei upon the inferior olive in the rhesus monkey: An autoradiographic study. J COMP NEUROL. 1979;188:43–62. doi: 10.1002/cne.901880105. [DOI] [PubMed] [Google Scholar]
- 114.Buisseret-Delmas C, Batini C. Topology of the pathways to the inferior olive: an HRP study in the cat. NEUROSCI LETT. 1978;10:207–214. doi: 10.1016/0304-3940(78)90227-6. [DOI] [PubMed] [Google Scholar]
- 115.De Zeeuw CI, Lang EJ, Sugihara I, Ruigrok TJH, Eisenman LM, Mugnaini E, Llinás R. Morphological correlates of bilateral synchrony in the rat cerebellar cortex. J NEUROSCI. 1996;16:3412–3426. doi: 10.1523/JNEUROSCI.16-10-03412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carlton SM, Leichnetz GR, Young EG, Mayer DJ. A transcannula method for subcortical HRP gel implants: Inferior olive afferents in the rat. BRAIN RES BULL. 1982;8:581–585. doi: 10.1016/0361-9230(82)90084-3. [DOI] [PubMed] [Google Scholar]
- 117.Schwarz C, Schmitz Y. Projection from the cerebellar lateral nucleus to precerebellar nuclei in the mossy fiber pathway is glutamatergic: a study combining anterograde tracing with immunogold labeling in the rat. J COMP NEUROL. 1997;381:320–334. doi: 10.1002/(sici)1096-9861(19970512)381:3<320::Aid-cne5>3.0.Co;2-4. [DOI] [PubMed] [Google Scholar]
- 118.Wentzel PR, Wylie DR, Ruigrok TJ, De Zeeuw CI. Olivary projecting neurons in the nucleus prepositus hypoglossi, group y and ventral dentate nucleus do not project to the oculomotor complex in the rabbit and the rat. Neurosci Lett. 1995;190:45–48. doi: 10.1016/0304-3940(95)11496-J. [DOI] [PubMed] [Google Scholar]
- 119.Dietrichs E, Walberg F, Nordby T. The cerebellar nucleo-olivary and olivo-cerebellar nuclear projections in the cat as studied with anterograde and retrograde transport in the same animal after implantation of crystalline WGA-HRP. I The dentate nucleus NEUROSCI RES. 1985;3:52–70. doi: 10.1016/0168-0102(85)90038-0. [DOI] [PubMed] [Google Scholar]
- 120.De Zeeuw CI, Gerrits NM, Voogd J, Leonard CS, Simpson JI. The rostral dorsal cap and ventrolateral outgrowth of the rabbit inferior olive receive a GABAergic input from dorsal group Y and the ventral dentate nucleus. J COMP NEUROL. 1994;341:420–432. doi: 10.1002/cne.903410311. [DOI] [PubMed] [Google Scholar]
- 121.Teune TM, Van Der Burg J, De Zeeuw CI, Voogd J, Ruigrok TJH. Single purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: A light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::Aid-cne2>3.0.Co;2-0. [DOI] [PubMed] [Google Scholar]
- 122.AbuAlrob MA and Tadi P. Neuroanatomy, nucleus solitarius. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 123.Cutsforth-Gregory JK, Benarroch EE. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology. 2017;88:1187–1196. doi: 10.1212/WNL.0000000000003751. [DOI] [PubMed] [Google Scholar]
- 124.Zoccal DB, Furuya WI, Bassi M, Colombari DSA and Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Frontiers in Physiology 2014: 5. 10.3389/fphys.2014.00238 [DOI] [PMC free article] [PubMed]
- 125.Escanilla OD, Victor JD, Di Lorenzo PM. Odor-taste convergence in the nucleus of the solitary tract of the awake freely licking rat. J Neurosci. 2015;35:6284–6297. doi: 10.1523/jneurosci.3526-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barmack NH, Yakhnitsa V. Vestibular signals in the parasolitary nucleus. J Neurophysiol. 2000;83:3559–3569. doi: 10.1152/jn.2000.83.6.3559. [DOI] [PubMed] [Google Scholar]
- 127.Barmack NH, Fredette BJ, Mugnaini E. Parasolitary nucleus: a source of GABAergic vestibular information to the inferior olive of rat and rabbit. Journal of Comparative Neurology. 1998;392:352–372. doi: 10.1002/(SICI)1096-9861(19980316)392:3<352::AID-CNE6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 128.Onai T, Takayama K, Miura M. Projections to areas of the nucleus tractus solitarii related to circulatory and respiratory responses in cats. J AUTON NERV SYST. 1987;18:163–175. doi: 10.1016/0165-1838(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 129.Ross CA, Ruggiero DA, Reis DJ. Afferent projections to cardiovascular portions of the nucleus of the tractus solitarius in the rat. BRAIN RES. 1981;223:402–408. doi: 10.1016/0006-8993(81)91155-0. [DOI] [PubMed] [Google Scholar]
- 130.Otake K, Reis DJ, Ruggiero DA. Afferents to the midline thalamus issue collaterals to the nucleus tractus solitarii: an anatomical basis for thalamic and visceral reflex integration. J NEUROSCI. 1994;14:5694–5707. doi: 10.1523/jneurosci.14-09-05694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Viana F. Chemosensory properties of the trigeminal system. ACS Chem Neurosci. 2011;2:38–50. doi: 10.1021/cn100102c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Price S and Daly DT. Neuroanatomy, trigeminal nucleus. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 133.Walker HK. Cranial Nerve V: The Trigeminal Nerve. In: H. K. Walker, W. D. Hall and J. W. Hurst, editors. Clinical methods: the history, physical, and laboratory examinations. Boston, Butterworths Copyright © 1990, Butterworth Publishers, a division of Reed Publishing.; 1990. [PubMed]
- 134.Brodal A, Pompeiano O. The vestibular nuclei in cat. J Anat. 1957;91:438–454. [PMC free article] [PubMed] [Google Scholar]
- 135.Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Research Bulletin 2003. [DOI] [PubMed]
- 136.Hernandez E and Das MJ. Neuroanatomy, nucleus vestibular. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 137.Sato Y, Kawasaki T, Ikarashi K. Zonal organization of the floccular Purkinje cells projecting to the vestibular nucleus in cats. BRAIN RES. 1982;232:1–15. doi: 10.1016/0006-8993(82)90606-0. [DOI] [PubMed] [Google Scholar]
- 138.Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. BRAIN RES. 1983;278:29–51. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- 139.Carpenter MB, Cowie RJ. Connections and oculomotor projections of the superior vestibular nucleus and cell group 'y'. BRAIN RES. 1985;336:265–287. doi: 10.1016/0006-8993(85)90653-5. [DOI] [PubMed] [Google Scholar]
- 140.Langer T, Fuchs AF, Chubb MC. Floccular efferents in the rhesus macaque as revealed by autoradiography and horseradish peroxidase. J COMP NEUROL. 1985;235:26–37. doi: 10.1002/cne.902350103. [DOI] [PubMed] [Google Scholar]
- 141.Shojaku H, Sato Y, Ikarashi K, Kawasaki T. Topographical distribution of Purkinje cells in the uvula and the nodulus projecting to the vestibular nuclei in cats. Brain Res. 1987;416:100–112. doi: 10.1016/0006-8993(87)91501-0. [DOI] [PubMed] [Google Scholar]
- 142.Eisenman LM, Schalekamp MPA, Voogd J. Development of the cerebellar cortical efferent projection: an in-vitro anterograde tracing study in rat brain slices. DEV BRAIN RES. 1991;60:261–266. doi: 10.1016/0165-3806(91)90055-n. [DOI] [PubMed] [Google Scholar]
- 143.Wylie DR, De Zeeuw CI, DiGiorgi PL, Simpson JI. Projections of individual Purkinje cells of identified zones in the ventral nodulus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol. 1994;349:448–463. doi: 10.1002/cne.903490309. [DOI] [PubMed] [Google Scholar]
- 144.Sadakane K, Kondo M, Nisimaru N. Direct projection from the cardiovascular control region of the cerebellar cortex, the lateral nodulus-uvula, to the brainstem in rabbits. Neurosci Res. 2000;36:15–26. doi: 10.1016/s0168-0102(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 145.Ohashi Y, Tsubota T, Sato A, Koyano KW, Tamura K, Miyashita Y. A bicistronic lentiviral vector-based method for differential transsynaptic tracing of neural circuits. Mol Cell Neurosci. 2011;46:136–147. doi: 10.1016/j.mcn.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 146.Shin M, Moghadam SH, Sekirnjak C, Bagnall MW, Kolkman KE, Jacobs R, Faulstich M, du Lac S. Multiple types of cerebellar target neurons and their circuitry in the vestibule-ocular reflex. J Neurosci. 2011;31:10776–10786. doi: 10.1523/jneurosci.0768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pisano TJ, Dhanerawala ZM, Kislin M, Bakshinskaya D, Engel EA, Hansen EJ, Hoag AT, Lee J, de Oude NL, Venkataraju KU, Verpeut JL, Hoebeek FE, Richardson BD, Boele HJ, Wang SS. Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep. 2021;36:109721. doi: 10.1016/j.celrep.2021.109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shi X, Wei H, Chen Z, Wang J, Qu W, Huang Z and Dai C. Whole-brain monosynaptic inputs and outputs of glutamatergic neurons of the vestibular nuclei complex in mice. Hear Res 2021: 401. 10.1016/j.heares.2020.108159 [DOI] [PubMed]
- 150.Bernard JF. Topographical organization of olivocerebellar and corticonuclear connections in the rat - An WGA-HRP study: I Lobules IX, X, and the flocculus. J COMP NEUROL. 1987;263:241–58. doi: 10.1002/cne.902630207. [DOI] [PubMed] [Google Scholar]
- 151.De Zeeuw CI, Wylie DR, DiGiorgi PL, Simpson JI. Projections of individual Purkinje cells of identified zones in the flocculus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol. 1994;349:428–447. doi: 10.1002/cne.903490308. [DOI] [PubMed] [Google Scholar]
- 152.Matsuno H, Kudoh M, Watakabe A, Yamamori T, Shigemoto R, Nagao S. Distribution and structure of synapses on medial vestibular nuclear neurons targeted by cerebellar flocculus Purkinje cells and vestibular nerve in mice: light and electron microscopy studies. PLoS ONE. 2016;11:e0164037. doi: 10.1371/journal.pone.0164037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sugihara I, Fujita H, Na J, Quy PN, Li BY, Ikeda D. Projection of reconstructed single Purkinje cell axons in relation to the cortical and nuclear aldolase C compartments of the rat cerebellum. J Comp Neurol. 2009;512:282–304. doi: 10.1002/cne.21889. [DOI] [PubMed] [Google Scholar]
- 154.Barmack NH, Henkel CK, Pettorossi VE. A subparafascicular projection to the medial vestibular nucleus of the rabbit. Brain Res. 1979;172:339–343. doi: 10.1016/0006-8993(79)90543-2. [DOI] [PubMed] [Google Scholar]
- 155.Paton JFR, La Noce A, Sykes RM, Sebastiani L, Bagnoli P, Ghelarducci B, Bradley DJ. Efferent connections of lobule IX of the posterior cerebellar cortex in the rabbit - Some functional considerations. J AUTON NERV SYST. 1991;36:209–224. doi: 10.1016/0165-1838(91)90045-5. [DOI] [PubMed] [Google Scholar]
- 156.Guo H, Yuan XS, Zhou JC, Chen H, Li SQ, Qu WM, Huang ZL. Whole-brain monosynaptic inputs to hypoglossal motor neurons in mice. Neurosci Bull. 2020;36:585–597. doi: 10.1007/s12264-020-00468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- 158.Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342:538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- 159.Berger AJ, Bayliss DA, Bellingham MC, Umemiya M, Viana F. Postnatal development of hypoglossal motoneuron intrinsic properties. Adv Exp Med Biol. 1995;381:63–71. doi: 10.1007/978-1-4615-1895-2_7. [DOI] [PubMed] [Google Scholar]
- 160.Fregosi RF. Respiratory related control of hypoglossal motoneurons–knowing what we do not know. Respir Physiol Neurobiol. 2011;179:43–47. doi: 10.1016/j.resp.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sokoloff AJ. Topographic segregation of genioglossus motoneurons in the neonatal rat. Neurosci Lett. 1993;155:102–106. doi: 10.1016/0304-3940(93)90683-c. [DOI] [PubMed] [Google Scholar]
- 162.Brodal A. The perihypoglossal nuclei in the macaque monkey and the chimpanzee. J COMP NEUROL. 1983;218:257–269. doi: 10.1002/cne.902180303. [DOI] [PubMed] [Google Scholar]
- 163.McCrea RA, Horn AK. Nucleus prepositus. Prog Brain Res. 2006;151:205–230. doi: 10.1016/S0079-6123(05)51007-0. [DOI] [PubMed] [Google Scholar]
- 164.McCrea RA, Baker R. Anatomical connections of the nucleus prepositus of the cat. J COMP NEUROL. 1985;237:377–407. doi: 10.1002/cne.902370308. [DOI] [PubMed] [Google Scholar]
- 165.Belknap DB, McCrea RA. Anatomical connections of the prepositus and abducens nuclei in the squirrel monkey. J COMP NEUROL. 1988;268:13–28. doi: 10.1002/cne.902680103. [DOI] [PubMed] [Google Scholar]
- 166.Petko B and Tadi P. Neuroanatomy, nucleus ambiguus. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 167.Gaytán SP, Pásaro R. Connections of the rostral ventral respiratory neuronal cell group: an anterograde and retrograde tracing study in the rat. Brain Res Bull. 1998;47:625–642. doi: 10.1016/s0361-9230(98)00125-7. [DOI] [PubMed] [Google Scholar]
- 168.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iwasaki H, Kani K, Maeda T. Neural connections of the pontine reticular formation, which connects reciprocally with the nucleus prepositus hypoglossi in the rat. Neuroscience. 1999;93:195–208. doi: 10.1016/s0306-4522(99)00151-7. [DOI] [PubMed] [Google Scholar]
- 171.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 172.Watt CB, Mihailoff GA. The cerebellopontine system in the rat. I Autoradiographic studies J COMP NEUROL. 1983;215:312–330. doi: 10.1002/cne.902150307. [DOI] [PubMed] [Google Scholar]
- 173.Gonzalo-Ruiz A, Leichnetz GR. Collateralization of cerebellar efferent projections to the paraoculomotor region, superior colliculus, and medial pontine reticular formation in the rat: a fluorescent double-labeling study. EXP BRAIN RES. 1987;68:365–378. doi: 10.1007/BF00248802. [DOI] [PubMed] [Google Scholar]
- 174.Leichnetz GR, Gonzalo-Ruiz A, DeSalles AAF, Hayes RL. The origin of brainstem afferents on the paramedian pontine reticular formation in the cat. BRAIN RES. 1987;422:389–397. doi: 10.1016/0006-8993(87)90951-6. [DOI] [PubMed] [Google Scholar]
- 175.Hazrati LN, Parent A. Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. BRAIN RES. 1992;585:267–271. doi: 10.1016/0006-8993(92)91216-2. [DOI] [PubMed] [Google Scholar]
- 176.Clavier RM. Afferent projections to the self-stimulation regions of the dorsal pons, including the locus coeruleus, in the rat as demonstrated by the horseradish peroxidase technique. BRAIN RES BULL. 1979;4:497–504. doi: 10.1016/0361-9230(79)90034-0. [DOI] [PubMed] [Google Scholar]
- 177.Shammah-Lagnado SJ, Costa MSMO, Ricardo JA. Afferent connections of the parvocellular reticular formation: a horseradish peroxidase study in the rat. Neuroscience. 1992;50:403–425. doi: 10.1016/0306-4522(92)90433-3. [DOI] [PubMed] [Google Scholar]
- 178.Elisevich KV, Hrycyshyn AW, Flumerfelt BA. Cerebellar, medullary and spinal afferent connections of the paramedian reticular nucleus in the cat. BRAIN RES. 1985;332:267–282. doi: 10.1016/0006-8993(85)90596-7. [DOI] [PubMed] [Google Scholar]
- 179.Schnyder H, Reisine H, Hepp K, Henn V. Frontal eye field projection to the paramedian pontine reticular formation traced with wheat germ agglutinin in the monkey. BRAIN RES. 1985;329:151–160. doi: 10.1016/0006-8993(85)90520-7. [DOI] [PubMed] [Google Scholar]
- 180.Sato H, Noda H. Divergent axon collaterals from fastigial oculomotor region to mesodiencephalic junction and paramedian pontine reticular formation in macaques. NEUROSCI RES. 1991;11:41–54. doi: 10.1016/0168-0102(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 181.Crandall WF, Keller EL. Visual and oculomotor signals in nucleus reticularis tegmenti pontis in alert monkey. J Neurophysiol. 1985;54:1326–1345. doi: 10.1152/jn.1985.54.5.1326. [DOI] [PubMed] [Google Scholar]
- 182.Precht W, Strata P. On the pathway mediating optokinetic responses in vestibular nuclear neurons. Neuroscience. 1980;5:777–787. doi: 10.1016/0306-4522(80)90170-0. [DOI] [PubMed] [Google Scholar]
- 183.Maekawa K, Takeda T, Kimura M. Neural activity of nucleus reticularis tegmenti pontis–the origin of visual mossy fiber afferents to the cerebellar flocculus of rabbits. Brain Res. 1981;210:17–30. doi: 10.1016/0006-8993(81)90881-7. [DOI] [PubMed] [Google Scholar]
- 184.Cazin L, Lannou J, Precht W. An electrophysiological study of pathways mediating optokinetic responses to the vestibular nucleus in the rat. Exp Brain Res. 1984;54:337–348. doi: 10.1007/bf00236235. [DOI] [PubMed] [Google Scholar]
- 185.Suzuki DA, Yamada T, Yee RD. Smooth-pursuit eye-movement-related neuronal activity in macaque nucleus reticularis tegmenti pontis. J Neurophysiol. 2003;89:2146–2158. doi: 10.1152/jn.00117.2002. [DOI] [PubMed] [Google Scholar]
- 186.Cicirata F, Panto MR, Angaut P. An autoradiographic study of the cerebellopontine projections in the rat I Projections from the medial cerebellar nucleus. BRAIN RES. 1982;253:303–8. doi: 10.1016/0006-8993(82)90697-7. [DOI] [PubMed] [Google Scholar]
- 187.Gerrits NM, WillemseGeest VDL, Kornet M. Some observations on the cerebellopontine projections in the cat - with a hypothesis to explain species differences. NEUROSCI LETT. 1984;44:65–70. doi: 10.1016/0304-3940(84)90222-2. [DOI] [PubMed] [Google Scholar]
- 188.Angaut P, Cicirata F, Panto MR. An autoradiographic study of the cerebellopontine projections from the interposed and lateral cerebellar nuclei in the rat. J HIRNFORSCH. 1985;26:463–470. [PubMed] [Google Scholar]
- 189.Shammah-Lagnado SJ, Negrao N, Silva BA, Ricardo JA. Afferent connections of the nuclei reticularis pontis oralis and caudalis: a horseradish peroxidase study in the rat. Neuroscience. 1987;20:961–990. doi: 10.1016/0306-4522(87)90256-9. [DOI] [PubMed] [Google Scholar]
- 190.Verveer C, Hawkins RK, Ruigrok TJH, De Zeeuw CI. Ultrastructural study of the GABAergic and cerebellar input to the nucleus reticularis tegmenti pontis. BRAIN RES. 1997;766:289–296. doi: 10.1016/s0006-8993(97)00774-9. [DOI] [PubMed] [Google Scholar]
- 191.Stanton GB. Organization of cerebellar and area "y" projections to the nucleus reticularis tegmenti pontis in macaque monkeys. J Comp Neurol. 2001;432:169–183. doi: 10.1002/cne.1095. [DOI] [PubMed] [Google Scholar]
- 192.Brodal P, Bjaalie JG. Organization of the pontine nuclei. Neurosci Res. 1992;13:83–118. doi: 10.1016/0168-0102(92)90092-Q. [DOI] [PubMed] [Google Scholar]
- 193.Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife. 2013;2:e00400. doi: 10.7554/eLife.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aas JE, Brodal P. GABA and glycine as putative transmitters in subcortical pathways to the pontine nuclei A combined immunocytochemical and retrograde tracing study in the cat with some observations in the rat. NEUROSCIENCE. 1990;34:149–62. doi: 10.1016/0306-4522(90)90309-r. [DOI] [PubMed] [Google Scholar]
- 195.Desmond JE, Rosenfield ME, Moore JW. An HRP study of the brainstem afferents to the accessory abducens region and dorsolateral pons in rabbit: Implications for the conditioned nictitating membrane response. BRAIN RES BULL. 1983;10:747–763. doi: 10.1016/0361-9230(83)90208-3. [DOI] [PubMed] [Google Scholar]
- 196.Mihailoff GA, Watt CB, Burne RA. Evidence suggesting that both the corticopontine and cerebellopontine systems are each composed of two separate neuronal populations: An electron microscopic and horseradish peroxidase study in the rat. J COMP NEUROL. 1981;195:221–242. doi: 10.1002/cne.901950204. [DOI] [PubMed] [Google Scholar]
- 197.de Carvalho D, Patrone LG, Taxini CL, Biancardi V, Vicente MC, Gargaglioni LH. Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2drive to breathe. Front Physiol. 2014;5:288. doi: 10.3389/fphys.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Benarroch EE. Locus coeruleus. Cell Tissue Res. 2018;373:221–232. doi: 10.1007/s00441-017-2649-1. [DOI] [PubMed] [Google Scholar]
- 199.Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morgane PJ, Jacobs MS. Raphe projections to the locus coeruleus in the rat. BRAIN RES BULL. 1979;4:519–534. doi: 10.1016/0361-9230(79)90037-6. [DOI] [PubMed] [Google Scholar]
- 201.Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J COMP NEUROL. 1978;178:1–15. doi: 10.1002/cne.901780102. [DOI] [PubMed] [Google Scholar]
- 202.Varga AG, Maletz SN, Bateman JT, Reid BT, Levitt ES. Neurochemistry of the Kölliker-Fuse nucleus from a respiratory perspective. J Neurochem. 2021;156:16–37. doi: 10.1111/jnc.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. Parabrachial complex: a hub for pain and aversion. J Neurosci. 2019;39:8225–8230. doi: 10.1523/JNEUROSCI.1162-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Supple WF, Jr, Kapp BS. Anatomical and physiological relationships between the anterior cerebellar vermis and the pontine parabrachial nucleus in the rabbit. BRAIN RES BULL. 1994;33:561–574. doi: 10.1016/0361-9230(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 205.Seneviratne SO and Patel BC. Facial nerve anatomy and clinical applications. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 206.Shammah Lagnado SJ, Ricardo JA, Sakamoto NTMN, Negrao N. Afferent connections of the mesencephalic reticular formation: a horseradish peroxidase study in the rat. Neuroscience. 1983;9:391–409. doi: 10.1016/0306-4522(83)90302-0. [DOI] [PubMed] [Google Scholar]
- 207.Sugimoto T, Mizuno N, Uchida K. Distribution of cerebellar fiber terminals in the midbrain visuomotor areas: an autoradiographic study in the cat. Brain Res. 1982;238:353–370. doi: 10.1016/0006-8993(82)90110-X. [DOI] [PubMed] [Google Scholar]
- 208.Person RJ, Andrezik JA, Dormer KJ, Foreman RD. Fastigial nucleus projections in the midbrain and thalamus in dogs. Neuroscience. 1986;18:105–120. doi: 10.1016/0306-4522(86)90182-x. [DOI] [PubMed] [Google Scholar]
- 209.Berretta S, Bosco G, Giaquinta G, Smecca G, Perciavalle V. Cerebellar influences on accessory oculomotor nuclei of the rat: a neuroanatomical, immunohistochemical, and electrophysiological study. J COMP NEUROL. 1993;338:50–66. doi: 10.1002/cne.903380105. [DOI] [PubMed] [Google Scholar]
- 210.Kennedy PR. Corticospinal, rubrospinal and rubro-olivary projections: a unifying hypothesis. Trends Neurosci. 1990;13:474–479. doi: 10.1016/0166-2236(90)90079-p. [DOI] [PubMed] [Google Scholar]
- 211.Habas C, Guillevin R, Abanou A. In vivo structural and functional imaging of the human rubral and inferior olivary nuclei: a mini-review. Cerebellum. 2010;9:167–173. doi: 10.1007/s12311-009-0145-1. [DOI] [PubMed] [Google Scholar]
- 212.Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- 213.Ulfig N, Chan WY. Differential expression of calcium-binding proteins in the red nucleus of the developing and adult human brain. Anat Embryol (Berl) 2001;203:95–108. doi: 10.1007/s004290000147. [DOI] [PubMed] [Google Scholar]
- 214.Basile GA, Quartu M, Bertino S, Serra MP, Boi M, Bramanti A, Anastasi GP, Milardi D, Cacciola A. Red nucleus structure and function: from anatomy to clinical neurosciences. Brain Struct Funct. 2021;226:69–91. doi: 10.1007/s00429-020-02171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lang EJ, Apps R, Bengtsson F, Cerminara NL, De Zeeuw CI, Ebner TJ, Heck DH, Jaeger D, Jörntell H, Kawato M, Otis TS, Ozyildirim O, Popa LS, Reeves AM, Schweighofer N, Sugihara I, Xiao J. The roles of the olivocerebellar pathway in motor learning and motor control. A Consensus Paper Cerebellum. 2017;16:230–252. doi: 10.1007/s12311-016-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reid EK, Norris SA, Taylor JA, Hathaway EN, Smith AJ, Yttri EA, Thach WT. Is the parvocellular red nucleus involved in cerebellar motor learning? Curr Trends Neurol. 2009;3:15–22. [PMC free article] [PubMed] [Google Scholar]
- 217.Olmstead CE, Villablanca JR, Sonnier BJ, McAllister JP, Gómez F. Reorganization of cerebellorubral terminal fields following hemispherectomy in adult cats. Brain Res. 1983;274:336–340. doi: 10.1016/0006-8993(83)90714-X. [DOI] [PubMed] [Google Scholar]
- 218.Naus CG, Flumerfelt BA, Hrycyshyn AW. Topographic specificity of aberrant cerebellorubral projections following neonatal hemicerebellectomy in the rat. BRAIN RES. 1984;309:1–15. doi: 10.1016/0006-8993(84)91005-9. [DOI] [PubMed] [Google Scholar]
- 219.Rosenfield ME, Dovydaitis A, Moore JW. Brachium conjuntivum and rubrobulbar tract: Brain stem projections of red nucleus essential for the conditioned nictitating membrane response. PHYSIOL BEHAV. 1985;34:751–759. doi: 10.1016/0031-9384(85)90374-9. [DOI] [PubMed] [Google Scholar]
- 220.Angaut P, Batini C, Billard JM, Daniel H. The cerebellorubral projection in the rat: Retrograde anatomical study. NEUROSCI LETT. 1986;68:63–68. doi: 10.1016/0304-3940(86)90230-2. [DOI] [PubMed] [Google Scholar]
- 221.Daniel H, Billard JM, Angaut P and Batini C. The interposito-rubrospinal system. Anatomical tracing of a motor control pathway in the rat. Neurosci Res 1987: 5:87–112. [DOI] [PubMed]
- 222.Robinson FR, Houk JC, Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J COMP NEUROL. 1987;257:553–577. doi: 10.1002/cne.902570406. [DOI] [PubMed] [Google Scholar]
- 223.Song WJ, Murakami F. Ipsilateral interpositorubral projection in the kitten and its relation to post-hemicerebellectomy plasticity. DEV BRAIN RES. 1990;56:75–85. doi: 10.1016/0165-3806(90)90166-v. [DOI] [PubMed] [Google Scholar]
- 224.Beitzel CS, Houck BD, Lewis SM, Person AL. Rubrocerebellar feedback loop isolates the interposed nucleus as an independent processor of corollary discharge information in mice. J Neurosci. 2017;37:10085–10096. doi: 10.1523/jneurosci.1093-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Angaut P, Cicirata F and Serapide MF. The dentatorubral projection. An autoradiographic study in rats. Brain Behav Evol 1987: 30:272–81. [DOI] [PubMed]
- 226.Flumerfelt BA, Caughell KA. A horseradish peroxidase study of the cerebellorubral pathway in the rat. EXP NEUROL. 1978;58:95–101. doi: 10.1016/0014-4886(78)90124-3. [DOI] [PubMed] [Google Scholar]
- 227.Hendry SHC, Jones EG, Graham J. Thalamic relay nuclei for cerebellar and certain related fiber systems in the cat. J COMP NEUROL. 1979;185:679–713. doi: 10.1002/cne.901850406. [DOI] [PubMed] [Google Scholar]
- 228.Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: A horseradish peroxidase study in the rat. J COMP NEUROL. 1979;187:117–144. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 229.Dekker JJ. Anatomical evidence for direct fiber projections from the cerebellar nucleus interpositus to rubrospinal neurons A quantitative EM study in the rat combining anterograde and retrograde intra-axonal tracing methods. BRAIN RES. 1981;205:229–44. doi: 10.1016/0006-8993(81)90335-8. [DOI] [PubMed] [Google Scholar]
- 230.Kalil K. Projections of the cerebellar and dorsal column nuclei upon the thalamus of the rhesus monkey. J Comp Neurol. 1981;195:25–50. doi: 10.1002/cne.901950105. [DOI] [PubMed] [Google Scholar]
- 231.Walberg F, Dietrichs E. Is there a reciprocal connection between the red nucleus and the interposed cerebellar nuclei? Conclusions based on observations of anterograde and retrograde transport of peroxidase-labelled lectin in the same animal. Brain Res. 1986;397:73–85. doi: 10.1016/0006-8993(86)91370-3. [DOI] [PubMed] [Google Scholar]
- 232.Walberg F, Dietrichs E, Nordby T. The origin and termination of the dentatorubral fibres in the cat as studied with retrograde and anterograde transport of peroxidase labelled lectin. EXP BRAIN RES. 1986;63:294–300. doi: 10.1007/BF00236846. [DOI] [PubMed] [Google Scholar]
- 233.May PJ, Hall WC. The cerebellotectal pathway in the grey squirrel. EXP BRAIN RES. 1987;65:200–212. doi: 10.1007/BF00243843. [DOI] [PubMed] [Google Scholar]
- 234.Asanuma C, Ohkawa R, Stanfield BB, Cowan WM. Observations on the development of certain ascending inputs to the thalamus in rats. I Postnatal development DEV BRAIN RES. 1988;41:159–170. doi: 10.1016/0165-3806(88)90179-4. [DOI] [PubMed] [Google Scholar]
- 235.Bernays RL, Heeb L, Cuenod M, Streit P. Afferents to the rat red nucleus studied by means of D-[3H] aspartate, [3H]choline and non-selective tracers. Neuroscience. 1988;26:601–619. doi: 10.1016/0306-4522(88)90168-6. [DOI] [PubMed] [Google Scholar]
- 236.Ostrowska A, Zguczynski L, Zimny R. Spatial arrangement of the interpositorubral projection in the rabbit: a retrograde HRP study. BIOL STRUCT MORPHOG. 1992;4:129–143. [PubMed] [Google Scholar]
- 237.Vaudano E, Legg CR. Cerebellar connections of the ventral lateral geniculate nucleus in the rat. ANAT EMBRYOL. 1992;186:583–588. doi: 10.1007/BF00186981. [DOI] [PubMed] [Google Scholar]
- 238.Giuffrida R, Aicardi G, Canedi A, Rapisarda C. Excitatory amino acids as neurotransmitters of cortical and cerebellar projections to the red nucleus: an immunocytochemical study in the guinea pig. SOMATOSENS MOT RES. 1993;10:365–376. doi: 10.3109/08990229309028844. [DOI] [PubMed] [Google Scholar]
- 239.Ostrowska A, Sikora E, Mierzejewska-Krzyzowska B, Zimny R. The dentatorubral projection in the rabbit with emphasis on distinction from the interpositorubral connectivity: an HRP retrograde tracer study. J HIRNFORSCH. 1993;34:9–23. [PubMed] [Google Scholar]
- 240.Sakai ST, Patton K. Distribution of cerebellothalamic and nigrothalamic projections in the dog: a double anterograde tracing study. J COMP NEUROL. 1993;330:183–194. doi: 10.1002/cne.903300204. [DOI] [PubMed] [Google Scholar]
- 241.Daly RD. Cerebellar terminations in the red nucleus of Macaca fascicularis: an electron-microscopic study utilizing the anterograde transport of WGA:HRP. SOMATOSENS MOT RES. 1994;11:101–107. doi: 10.3109/08990229409028863. [DOI] [PubMed] [Google Scholar]
- 242.Olyntho-Tokunaga HHV, Pinto ML, Souccar C, Schoorlemmer GHM, Lapa RCRS. Projections from the anterior interposed nucleus to the red nucleus diminish with age in the mouse. J Vet Med Ser C Anat Histol Embryol. 2008;37:438–441. doi: 10.1111/j.1439-0264.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 243.Kuramoto E, Fujiyama F, Nakamura KC, Tanaka Y, Hioki H, Kaneko T. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur J Neurosci. 2011;33:95–109. doi: 10.1111/j.1460-9568.2010.07481.x. [DOI] [PubMed] [Google Scholar]
- 244.Del Rio-Bermudez C, Plumeau AM, Sattler NJ, Sokoloff G, Blumberg MS. Spontaneous activity and functional connectivity in the developing cerebellorubral system. J Neurophysiol. 2016;116:1316–1327. doi: 10.1152/jn.00461.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hara S, Kaneyama T, Inamata Y, Onodera R, Shirasaki R. Interstitial branch formation within the red nucleus by deep cerebellar nuclei-derived commissural axons during target recognition. J Comp Neurol. 2016;524:999–1014. doi: 10.1002/cne.23888. [DOI] [PubMed] [Google Scholar]
- 246.Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- 247.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 248.Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fox ME, Lobo MK. The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol Psychiatry. 2019;24:1798–1815. doi: 10.1038/s41380-019-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 255.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 256.Borland JM, Grantham KN, Aiani LM, Frantz KJ, Albers HE. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology. 2018;95:128–137. doi: 10.1016/j.psyneuen.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Baek SJ, Park J, Kim J, Yamamoto Y and Tanaka-Yamamoto K. VTA-projecting cerebellar neurons mediate stress-dependent depression-like behavior. bioRxiv 2021:2021.08.25.457606. 10.1101/2021.08.25.457606 [DOI] [PMC free article] [PubMed]
- 258.Parker KL, Narayanan NS and Andreasen NC. The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci 2014: 8. 10.3389/fnsys.2014.00163 [DOI] [PMC free article] [PubMed]
- 259.Snider RS, Maiti A, Snider SR. Cerebellar pathways to ventral midbrain and nigra. EXP NEUROL. 1976;53:714–728. doi: 10.1016/0014-4886(76)90150-3. [DOI] [PubMed] [Google Scholar]
- 260.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 261.Wang X, Novello M, Gao Z, Ruigrok TJH, De Zeeuw CI. Input and output organization of the mesodiencephalic junction for cerebro-cerebellar communication. J Neurosci Res. 2022;100:620–637. doi: 10.1002/jnr.24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Carlton SM, Leichnetz GR, Mayer DJ. Projections from the nucleus parafascicularis prerubralis to medullary raphe nuclei and inferior olive in the rat: a horseradish peroxidase and autoradiography study. Neurosci Lett. 1982;30:191–197. doi: 10.1016/0304-3940(82)90398-6. [DOI] [PubMed] [Google Scholar]
- 263.Onodera S. Olivary projections from the mesodiencephalic structures in the cat studied by means of axonal transport of horseradish peroxidase and tritiated amino acids. J Comp Neurol. 1984;227:37–49. doi: 10.1002/cne.902270106. [DOI] [PubMed] [Google Scholar]
- 264.Brown JT, Chan-Palay V, Palay SL. A study of afferent input to the inferior olivary complex in the rat by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1977;176:1–22. doi: 10.1002/cne.901760102. [DOI] [PubMed] [Google Scholar]
- 265.Rutherford JG, Anderson WA, Gwyn DG. A reevaluation of midbrain and diencephalic projections to the inferior olive in rat with particular reference to the rubro-olivary pathway. J Comp Neurol. 1984;229:285–300. doi: 10.1002/cne.902290213. [DOI] [PubMed] [Google Scholar]
- 266.Voogd J, Ruigrok TJ. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol. 2004;33:5–21. doi: 10.1023/B:NEUR.0000029645.72074.2b. [DOI] [PubMed] [Google Scholar]
- 267.De Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocytochemistry. J Comp Neurol. 1989;284:12–35. doi: 10.1002/cne.902840103. [DOI] [PubMed] [Google Scholar]
- 268.Kubo R, Aiba A, Hashimoto K. The anatomical pathway from the mesodiencephalic junction to the inferior olive relays perioral sensory signals to the cerebellum in the mouse. J Physiol. 2018;596:3775–3791. doi: 10.1113/jp275836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Leichnetz GR, Spencer RF, Smith DJ. Cortical projections to nuclei adjacent to the oculomotor complex in the medial dien-mesencephalic tegmentum in the monkey. J COMP NEUROL. 1984;228:359–387. doi: 10.1002/cne.902280306. [DOI] [PubMed] [Google Scholar]
- 270.Linauts M, Martin GF. The organization of olivo-cerebellar projections in the opossum, didelphis virginiana, as revealed by the retrograde transport of horseradish peroxidase. J COMP NEUROL. 1978;179:355–381. doi: 10.1002/cne.901790207. [DOI] [PubMed] [Google Scholar]
- 271.Nakamura Y, Kitao Y, Okoyama S. Cortico-Darkschewitsch-olivary projection in the cat: an electron microscope study with the aid of horseradish peroxidase tracing technique. Brain Res. 1983;274:140–143. doi: 10.1016/0006-8993(83)90529-2. [DOI] [PubMed] [Google Scholar]
- 272.Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats An anterograde study using autoradiographic and axonal degeneration techniques. NEUROSCIENCE. 1983;8:259–75. doi: 10.1016/0306-4522(83)90064-7. [DOI] [PubMed] [Google Scholar]
- 273.Ostrowska A, Zimny R, Zguczynski L, Sikora E. Subcortical afferents to the interstitial nucleus of Cajal: an anatomical retrograde tracing study in the rabbit. J HIRNFORSCH. 1990;31:747–759. [PubMed] [Google Scholar]
- 274.Bohlen MO, Gamlin PD, Warren S, May PJ. Cerebellar projections to the macaque midbrain tegmentum: possible near response connections. Vis Neurosci. 2021;38:E007. doi: 10.1017/s0952523821000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gonzalo-Ruiz A, Leichnetz GR, Hardy SGP. Projections of the medial cerebellar nucleus to oculomotor-related midbrain areas in the rat: an anterograde and retrograde HRP study. J COMP NEUROL. 1990;296:427–436. doi: 10.1002/cne.902960308. [DOI] [PubMed] [Google Scholar]
- 276.Fukushima K, Terashima T, Kudo J. Projections of the group y of the vestibular nuclei and the dentate and fastigial nuclei of the cerebellum to the interstitial nucleus of Cajal. NEUROSCI RES. 1986;3:285–299. doi: 10.1016/0168-0102(86)90021-0. [DOI] [PubMed] [Google Scholar]
- 277.da Silva AV, Torres KR, Haemmerle CA, Céspedes IC, Bittencourt JC. The Edinger-Westphal nucleus II: hypothalamic afferents in the rat. J Chem Neuroanat. 2013;54:5–19. doi: 10.1016/j.jchemneu.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 278.De Zeeuw CI, Ruigrok TJH. Olivary projecting neurons in the nucleus of Darkschewitsch in the cat receive excitatory monosynaptic input from the cerebellar nuclei. BRAIN RES. 1994;653:345–350. doi: 10.1016/0006-8993(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 279.May PJ, Porter JD, Gamlin PDR. Interconnections between the primate cerebellum and midbrain near-response regions. J COMP NEUROL. 1992;315:98–116. doi: 10.1002/cne.903150108. [DOI] [PubMed] [Google Scholar]
- 280.Rutherford JG, Zuk-Harper A, Gwyn DG. A comparison of the distribution of the cerebellar and cortical connections of the nucleus of Darkschewitsch (ND) in the cat: a study using anterograde and retrograde HRP tracing techniques. ANAT EMBRYOL. 1989;180:485–496. doi: 10.1007/BF00305124. [DOI] [PubMed] [Google Scholar]
- 281.Sakai ST, Inase M, Tanji J. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. Journal of Comparative Neurology. 1996;368:215–228. doi: 10.1002/(SICI)1096-9861(19960429)368:2<215::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 282.Heiland Hogan MB, Subramanian S and J MD. Neuroanatomy, Edinger–Westphal nucleus (accessory oculomotor nucleus). StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- 283.McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–473. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Szabadi E. Functional organization of the sympathetic pathways controlling the pupil: light-inhibited and light-stimulated pathways. Front Neurol. 2018;9:1069. doi: 10.3389/fneur.2018.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Che Ngwa E, Zeeh C, Messoudi A, Büttner-Ennever JA, Horn AK. Delineation of motoneuron subgroups supplying individual eye muscles in the human oculomotor nucleus. Front Neuroanat. 2014;8:2. doi: 10.3389/fnana.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Joyce C, Le PH and Peterson DC. Neuroanatomy, Cranial Nerve 3 (Oculomotor). StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 287.Hutchins B, Weber JT. The pretectal complex of the monkey: a reinvestigation of the morphology and retinal terminations. J Comp Neurol. 1985;232:425–442. doi: 10.1002/cne.902320402. [DOI] [PubMed] [Google Scholar]
- 288.Gamlin PDR. The pretectum: connections and oculomotor-related roles. In: Büttner-Ennever JA, editor. Progress in Brain Research. Elsevier; 2006. pp. 379–405. [DOI] [PubMed] [Google Scholar]
- 289.Magoun HW, Ranson SW. The central path of the light reflex: a study of the effect of lesions. Arch Ophthalmol. 1935;13:791–811. doi: 10.1001/archopht.1935.00840050069006. [DOI] [Google Scholar]
- 290.Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: discharge patterns of pretectal neurons. J Neurophysiol. 2000;84:964–974. doi: 10.1152/jn.2000.84.2.964. [DOI] [PubMed] [Google Scholar]
- 291.Nakamura H, Wu R, Watanabe K, Onozuka M, Itoh K. Projections of glutamate decarboxylase positive and negative cerebellar neurons to the pretectum in the cat. Neurosci Lett. 2006;403:30–34. doi: 10.1016/j.neulet.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 292.Bull MS, Berkley KJ. Cerebellar projections to the somatic pretectum in the cat. SOMATOSENS MOT RES. 1991;8:117–126. doi: 10.3109/08990229109144736. [DOI] [PubMed] [Google Scholar]
- 293.Pong M, Horn KM, Gibson AR. Pathways for control of face and neck musculature by the basal ganglia and cerebellum. Brain Res Rev. 2008;58:249–264. doi: 10.1016/j.brainresrev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 294.Schäfer CB, Hoebeek FE. Convergence of primary sensory cortex and cerebellar nuclei pathways in the whisker system. Neuroscience. 2018;368:229–239. doi: 10.1016/j.neuroscience.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 295.Aumann TD, Rawson JA, Pichitpornchai C, Horne MK. Projections from the cerebellar interposed and dorsal column nuclei to the thalamus in the rat: a double anterograde labelling study. Journal of Comparative Neurology. 1996;368:608–619. doi: 10.1002/(SICI)1096-9861(19960513)368:4<608::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 296.Aumann TD, Horne MK. Ramification and termination of single axons in the cerebellothalamic pathway of the rat. J Comp Neurol. 1996;376:420–430. doi: 10.1002/(sici)1096-9861(19961216)376:3<420::Aid-cne5>3.0.Co;2-4. [DOI] [PubMed] [Google Scholar]
- 297.Zubricky RD and Das JM. Neuroanatomy, superior colliculus. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 298.Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci. 2011;34:205–231. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.King AJ. The superior colliculus. Curr Biol. 2004;14:R335–R338. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 300.Sparks DL, Gandhi NJ. Single cell signals: an oculomotor perspective. Prog Brain Res. 2003;142:35–53. doi: 10.1016/s0079-6123(03)42005-0. [DOI] [PubMed] [Google Scholar]
- 301.Beitz AJ. Possible origin of glutamatergic projections to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain Res Bull 1989: 23:25–35. 0361–9230(89)90159–7 [pii] [DOI] [PubMed]
- 302.Cadusseau J, Roger M. Afferent projections to the superior colliculus in the rat, with special attention to the deep layers. J HIRNFORSCH. 1985;26:667–681. [PubMed] [Google Scholar]
- 303.Künzle H. Connections of the superior colliculus with the tegmentum and the cerebellum in the hedgehog tenrec. NEUROSCI RES. 1997;28:127–145. doi: 10.1016/s0168-0102(97)00034-5. [DOI] [PubMed] [Google Scholar]
- 304.Kurimoto Y, Kawaguchi S, Murata M. Cerebellotectal projection in the rat: Anterograde and retrograde WGA-HRP study of individual cerebellar nuclei. NEUROSCI RES. 1995;22:57–71. doi: 10.1016/0168-0102(95)00874-s. [DOI] [PubMed] [Google Scholar]
- 305.Roldan M, Reinoso-Suarez F. Cerebellar projections to the superior colliculus in the cat. J NEUROSCI. 1981;1:827–834. doi: 10.1523/jneurosci.01-08-00827.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J COMP NEUROL. 1979;184:309–330. doi: 10.1002/cne.901840207. [DOI] [PubMed] [Google Scholar]
- 307.Covey E, Hall WC, Kobler JB. Subcortical connections of the superior colliculus in the mustache bat. Pteronotus parnellii J COMP NEUROL. 1987;263:179–197. doi: 10.1002/cne.902630203. [DOI] [PubMed] [Google Scholar]
- 308.Katoh YY, Benedek G. Cerebellar fastigial neurons send bifurcating axons to both the left and right superior colliculus in cats. Brain Res. 2003;970:246–249. doi: 10.1016/S0006-8993(03)02359-X. [DOI] [PubMed] [Google Scholar]
- 309.Katoh YY, Arai R, Benedek G. Bifurcating projections from the cerebellar fastigial neurons to the thalamic suprageniculate nucleus and to the superior colliculus. Brain Res. 2000;864:308–311. doi: 10.1016/s0006-8993(00)02156-9. [DOI] [PubMed] [Google Scholar]
- 310.Uchida K, Mizuno N, Sugimoto T. Direct projections from the cerebellar nuclei to the superior colliculus in the rabbit: An HRP study. J COMP NEUROL. 1983;216:319–326. doi: 10.1002/cne.902160308. [DOI] [PubMed] [Google Scholar]
- 311.Gayer NS, Faull RLM. Connections of the paraflocculus of the cerebellum with the superior colliculus in the rat brain. BRAIN RES. 1988;449:253–270. doi: 10.1016/0006-8993(88)91042-6. [DOI] [PubMed] [Google Scholar]
- 312.Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 313.Menant O, Andersson F, Zelena D, Chaillou E. The benefits of magnetic resonance imaging methods to extend the knowledge of the anatomical organisation of the periaqueductal gray in mammals. J Chem Neuroanat. 2016;77:110–120. doi: 10.1016/j.jchemneu.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 314.Mokhtar M and Singh P. Neuroanatomy, Periaqueductal Gray. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 315.Walker P, Carrive P. Role of ventrolateral periaqueductal gray neurons in the behavioral and cardiovascular responses to contextual conditioned fear and poststress recovery. Neuroscience. 2003;116:897–912. doi: 10.1016/s0306-4522(02)00744-3. [DOI] [PubMed] [Google Scholar]
- 316.Ho YC, Lin TB, Hsieh MC, Lai CY, Chou D, Chau YP, Chen GD, Peng HY. Periaqueductal gray glutamatergic transmission governs chronic stress-induced depression. Neuropsychopharmacology. 2018;43:302–312. doi: 10.1038/npp.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 1995: 46:575–605. 0301–0082(95)00009-K [pii] [DOI] [PubMed]
- 318.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 319.Dampney RA, Furlong TM, Horiuchi J, Iigaya K. Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Auton Neurosci. 2013;175:17–25. doi: 10.1016/j.autneu.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 320.Subramanian HH. Descending control of the respiratory neuronal network by the midbrain periaqueductal grey in the rat in vivo. J Physiol. 2013;591:109–122. doi: 10.1113/jphysiol.2012.245217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Torigoe Y, Blanks RHI, Precht W. Anatomical studies on the nucleus reticularis tegmenti pontis in the pigmented rat II Subcortical afferents demonstrated by the retrograde transport of horseradish peroxidase. Journal of Comparative Neurology. 1986;243:88–105. doi: 10.1002/cne.902430108. [DOI] [PubMed] [Google Scholar]
- 323.Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- 324.Veazey RB, Severin CM. Efferent projections of the deep mesencephalic nucleus (pars lateralis) in the rat. J Comp Neurol. 1980;190:231–244. doi: 10.1002/cne.901900203. [DOI] [PubMed] [Google Scholar]
- 325.Veazey RB, Severin CM. Afferent projections to the deep mesencephalic nucleus in the rat. J COMP NEUROL. 1982;204:134–150. doi: 10.1002/cne.902040204. [DOI] [PubMed] [Google Scholar]
- 326.Hay-Schmidt A, Mikkelsen JD. Demonstration of a neuronal projection from the entopeduncular nucleus to the substantia nigra of the rat. Brain Res. 1992;576:343–347. doi: 10.1016/0006-8993(92)90702-b. [DOI] [PubMed] [Google Scholar]
- 327.Yasui Y, Tsumori T, Ando A, Domoto T, Kayahara T, Nakano K. Descending projections from the superior colliculus to the reticular formation around the motor trigeminal nucleus and the parvicellular reticular formation of the medulla oblongata in the rat. Brain Res. 1994;656:420–426. doi: 10.1016/0006-8993(94)91489-3. [DOI] [PubMed] [Google Scholar]
- 328.Olszewski J and Baxter D. Cytoarchitecture of the human brainstem. By Jerzy Olszewski and Donald Baxter. Published and distributed in North America for S. Karger by J. B. Lippincott Company, Philadelphia and Montreal. 1954. 199 pages. Price $16.00 (Reviewed by Gerhardt von Bonin). Journal of Comparative Neurology 1954: 101:825-. 10.1002/cne.901010308
- 329.Taber E. The cytoarchitecture of the brain stem of the cat I Brain stem nuclei of cat. J Comp Neurol. 1962;116:27–69. doi: 10.1002/cne.901160104. [DOI] [PubMed] [Google Scholar]
- 330.Valverde F. Reticular formation of the albino rat's brain stem cytoarchitecture and corticofugal connections. J Comp Neurol. 1962;119:25–53. doi: 10.1002/cne.901190105. [DOI] [PubMed] [Google Scholar]
- 331.Wang XM, Yuan B, Hou ZL. Role of the deep mesencephalic nucleus in the antinociception induced by stimulation of the anterior pretectal nucleus in rats. Brain Res. 1992;577:321–325. doi: 10.1016/0006-8993(92)90291-g. [DOI] [PubMed] [Google Scholar]
- 332.Rodríguez M, Abdala P, Barroso-Chinea P, González-Hernández T. The deep mesencephalic nucleus as an output center of basal ganglia: morphological and electrophysiological similarities with the substantia nigra. J Comp Neurol. 2001;438:12–31. doi: 10.1002/cne.1299. [DOI] [PubMed] [Google Scholar]
- 333.Sonne J, Reddy V and Beato MR. Neuroanatomy, Substantia Nigra. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 334.Haber SN, Groenewegen HJ. Interrelationship of the distribution of neuropeptides and tyrosine hydroxylase immunoreactivity in the human substantia nigra. Journal of Comparative Neurology. 1989;290:53–68. doi: 10.1002/cne.902900105. [DOI] [PubMed] [Google Scholar]
- 335.Baroncini M, Jissendi P, Balland E, Besson P, Pruvo JP, Francke JP, Dewailly D, Blond S, Prevot V. MRI atlas of the human hypothalamus. Neuroimage. 2012;59:168–180. doi: 10.1016/j.neuroimage.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 336.Dietrichs E, Haines DE. Observations on the cerebello-hypothalamic projection, with comments on non-somatic cerebellar circuits. Arch Ital Biol. 1985;123:133–139. [PubMed] [Google Scholar]
- 337.Haines DE, Dietrichs E. An HRP study of hypothalamo-cerebellar and cerebello-hypothalamic connections in squirrel monkey (Saimiri sciureus) J COMP NEUROL. 1984;229:559–575. doi: 10.1002/cne.902290409. [DOI] [PubMed] [Google Scholar]
- 338.Çavdar S, Tangül ŞAN, Aker R, Şehirli Ü, Onat F. Cerebellar connections to the dorsomedial and posterior nuclei of the hypothalamus in the rat. J Anat. 2001;198:37–45. doi: 10.1017/s0021878200007172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Li B, Zhuang QX, Gao HR, Wang JJ, Zhu JN. Medial cerebellar nucleus projects to feeding-related neurons in the ventromedial hypothalamic nucleus in rats. Brain Struct Funct. 2017;222:957–971. doi: 10.1007/s00429-016-1257-2. [DOI] [PubMed] [Google Scholar]
- 340.Keifer J and Lustig DG. Comparison of cortically and subcortically controlled motor systems. II. Distribution of anterogradely labeled terminal boutons on intracellularly filled rubrospinal neurons in rat and turtle. J Comp Neurol 2000: 416:101–11. 10.1002/(sici)1096-9861(20000103)416:1 < 101::Aid-cne8 > 3.0.Co;2-r [DOI] [PubMed]
- 341.Haines DE, May PJ, Dietrichs E. Neuronal connections between the cerebellar nuclei and hypothalamus in Macaca fascicularis: cerebello-visceral circuits. J Comp Neurol. 1990;299:106–122. doi: 10.1002/cne.902990108. [DOI] [PubMed] [Google Scholar]
- 342.Haines DE, Sowa TE, Dietrichs E. Connections between the cerebellum and hypothalamus in the tree shrew (Tupaia glis) Brain Res. 1985;328:367–373. doi: 10.1016/0006-8993(85)91051-0. [DOI] [PubMed] [Google Scholar]
- 343.Çavdar S, Onat F, Aker R, Sehirli U, Tangul SAN, Yananli HR. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. J Anat. 2001;198:463–472. doi: 10.1017/s0021878201007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;19:533–541. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- 345.Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, Ables JL, Aleyasin H, Khibnik LA, Hodes GE, Ben-Dor GA, Deisseroth K, Shapiro ML, Malenka RC, Ibanez-Tallon I, Han MH, Russo SJ. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18:962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lambert C, Simon H, Colman J, Barrick TR. Defining thalamic nuclei and topographic connectivity gradients in vivo. Neuroimage. 2017;158:466–479. doi: 10.1016/j.neuroimage.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 347.Morel A. Stereotactic Atlas of the Human Thalamus and Basal Ganglia (1st ed.). Informa Healthcare 2007.
- 348.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 349.Briggs F, Usrey WM. Emerging views of corticothalamic function. Curr Opin Neurobiol. 2008;18:403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mitchell AS. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev. 2015;54:76–88. doi: 10.1016/j.neubiorev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 351.Antunes FM, Malmierca MS. Corticothalamic pathways in auditory processing: recent advances and insights from other sensory systems. Front Neural Circuits. 2021;15:721186. doi: 10.3389/fncir.2021.721186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Crabtree JW. Functional diversity of thalamic reticular subnetworks. Front Syst Neurosci. 2018;12:41. doi: 10.3389/fnsys.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Stepniewska I, Kosmal A. Subcortical afferents to the mediodorsal thalamic nucleus of the dog. Acta Neurobiol Exp (Warsz) 1986;46:323–339. [PubMed] [Google Scholar]
- 354.Hoshi E, Tremblay L, Féger J, Carras PL and Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci 2005: 8:1491–3. nn1544 [pii] 10.1038/nn1544 [DOI] [PubMed]
- 355.Craig AD. Retrograde analyses of spinothalamic projections in the macaque monkey: Input to the ventral lateral nucleus. Journal of Comparative Neurology. 2008;508:315–328. doi: 10.1002/cne.21672. [DOI] [PubMed] [Google Scholar]
- 356.Deniau JM, Kitai ST. Patterns of termination of cerebellar and basal ganglia efferents in the rat thalamus Strictly segregated and partly overlapping projections. NEUROSCI LETT. 1992;144:202–6. doi: 10.1016/0304-3940(92)90750-2. [DOI] [PubMed] [Google Scholar]
- 357.Ilinsky IA, Kultas-Ilinsky K. Sagittal cytoarchitectonic maps of the Macaca mulatta thalamus with a revised nomenclature of the motor-related nuclei validated by observations on their connectivity. J Comp Neurol. 1987;262:331–364. doi: 10.1002/cne.902620303. [DOI] [PubMed] [Google Scholar]
- 358.Anderson ME, DeVito JL. An analysis of potentially converging inputs to the rostral ventral thalamic nuclei of the cat. EXP BRAIN RES. 1987;68:260–276. doi: 10.1007/BF00248792. [DOI] [PubMed] [Google Scholar]
- 359.Sato F, Nakamura Y, Shinoda Y. Three-dimensional analysis of cerebellar terminals and their postsynaptic components in the ventral lateral nucleus of the cat thalamus. Journal of Comparative Neurology. 1996;371:537–551. doi: 10.1002/(SICI)1096-9861(19960805)371:4<537::AID-CNE4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 360.Ilinsky IA, Kultas-Ilinsky K, Rosina A, Haddy M. Quantitative evaluation of crossed and uncrossed projections from basal ganglia and cerebellum to the cat thalamus. Neuroscience. 1987;21:207–227. doi: 10.1016/0306-4522(87)90334-4. [DOI] [PubMed] [Google Scholar]
- 361.Okuda B. Cerebello-thalamo-cerebral projection from the dentate nucleus onto the frontal eye field in the cat. Acta Physiol Scand. 1994;151:1–6. doi: 10.1111/j.1748-1716.1994.tb09715.x. [DOI] [PubMed] [Google Scholar]
- 362.Cornwall J, Phillipson OT. Afferent projections to the parafascicular thalamic nucleus of the rat, as shown by the retrograde transport of wheat germ agglutinin. BRAIN RES BULL. 1988;20:139–150. doi: 10.1016/0361-9230(88)90171-2. [DOI] [PubMed] [Google Scholar]
- 363.Royce GJ, Bromley S, Gracco C. Subcortical projections to the centromedian and parafascicular thalamic nuclei in the cat. J COMP NEUROL. 1991;306:129–155. doi: 10.1002/cne.903060110. [DOI] [PubMed] [Google Scholar]
- 364.Tracey DJ, Asanuma C, Jones EG, Porter R. Thalamic relay to motor cortex: afferent pathways from brain stem, cerebellum, and spinal cord in monkeys. J NEUROPHYSIOL. 1980;44:532–554. doi: 10.1152/jn.1980.44.3.532. [DOI] [PubMed] [Google Scholar]
- 365.Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. BRAIN RES REV. 1983;5:237–265. doi: 10.1016/0165-0173(83)90015-2. [DOI] [PubMed] [Google Scholar]
- 366.Sugimoto T, Mizuno N, Itoh K. An autoradiographic study on the terminal distribution of cerebellothalamic fibers in the cat. BRAIN RES. 1981;215:29–47. doi: 10.1016/0006-8993(81)90489-3. [DOI] [PubMed] [Google Scholar]
- 367.Kyuhou S, Kawaguchi S. Cerebellocerebral projection from the fastigial nucleus onto the frontal eye field and anterior ectosylvian visual area in the cat. J Comp Neurol. 1987;259:571–590. doi: 10.1002/cne.902590407. [DOI] [PubMed] [Google Scholar]
- 368.Jimenez-Castellanos J, Jr, Reinoso-Suarez F. Topographical organization of the afferent connections of the principal ventromedial thalamic nucleus in the cat. J COMP NEUROL. 1985;236:297–314. doi: 10.1002/cne.902360303. [DOI] [PubMed] [Google Scholar]
- 369.Nakamura H. Cerebellar projections to the ventral lateral geniculate nucleus and the thalamic reticular nucleus in the cat. J Neurosci Res. 2018;96:63–74. doi: 10.1002/jnr.24105. [DOI] [PubMed] [Google Scholar]
- 370.Halverson HE, Lee I, Freeman JH. Associative plasticity in the medial auditory thalamus and cerebellar interpositus nucleus during eyeblink conditioning. J Neurosci. 2010;30:8787–8796. doi: 10.1523/JNEUROSCI.0208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Stepniewska I, Sakai ST, Qi HX, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: a potential substrate for parkinsonian tremor. Journal of Comparative Neurology. 2003;455:378–395. doi: 10.1002/cne.10499. [DOI] [PubMed] [Google Scholar]
- 372.Çavdar S, lýz Onat FY, Yananli HR, Şehirli ÜS, Tulay C, Saka E and Gürdal E. Cerebellar connections to the rostral reticular nucleus of the thalamus in the rat. J Anat 2002: 201:485–91. 10.1046/j.1469-7580.2002.00119.x [DOI] [PMC free article] [PubMed]
- 373.Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to 'AIP'. Cereb Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 374.Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- 375.Sakayori N, Kato S, Sugawara M, Setogawa S, Fukushima H, Ishikawa R, Kida S and Kobayashi K. Motor skills mediated through cerebellothalamic tracts projecting to the central lateral nucleus. Mol Brain 2019: 12. 10.1186/s13041-019-0431-x [DOI] [PMC free article] [PubMed]
- 376.Mason A, Ilinsky IA, Beck S, KultasIlinsky K. Reevaluation of synaptic relationships of cerebellar terminals in the ventral lateral nucleus of the rhesus monkey thalamus based on serial section analysis and three-dimensional reconstruction. Exp Brain Res. 1996;109:219–239. doi: 10.1007/BF00231783. [DOI] [PubMed] [Google Scholar]
- 377.Aumann TD, Horne MK. A comparison of the ultrastructure of synapses in the cerebello-rubral and cerebello-thalamic pathways in the rat. Neurosci Lett. 1996;211:175–178. doi: 10.1016/0304-3940(96)12757-9. [DOI] [PubMed] [Google Scholar]
- 378.Rodrigo-Angulo ML, Reinoso-Suarez F. Cerebellar projections to the lateral posterior-pulvinar thalamic complex in the cat. Brain Res. 1984;322:172–176. doi: 10.1016/0006-8993(84)91200-9. [DOI] [PubMed] [Google Scholar]
- 379.Çavdar S, Özgür M, Uysal SP, Amuk ÖC. Motor afferents from the cerebellum, zona incerta and substantia nigra to the mediodorsal thalamic nucleus in the rat. J Integr Neurosci. 2014;13:565–578. doi: 10.1142/s0219635214500198. [DOI] [PubMed] [Google Scholar]
- 380.Berkley KJ. Spatial relationships between the terminations of somatic sensory motor pathways in the rostral brainstem of cats and monkeys II Cerebellar projections compared with those of the ascending somatic sensory pathways in lateral diencephalon. J COMP NEUROL. 1983;220:229–51. doi: 10.1002/cne.902200210. [DOI] [PubMed] [Google Scholar]
- 381.Erickson SL, Melchitzky DS, Lewis DA. Subcortical afferents to the lateral mediodorsal thalamus in cynomolgus monkeys. Neuroscience. 2004;129:675–690. doi: 10.1016/j.neuroscience.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 382.Habas C, Manto M, Cabaraux P. The cerebellar thalamus. Cerebellum. 2019;18:635–648. doi: 10.1007/s12311-019-01019-3. [DOI] [PubMed] [Google Scholar]
- 383.Basinger H and Joseph J. Neuroanatomy, subthalamic nucleus. StatPearls. Treasure Island (FL), StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 384.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 385.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/nejm199810153391603. [DOI] [PubMed] [Google Scholar]
- 386.Houeto JL, Damier P, Bejjani PB, Staedler C, Bonnet AM, Arnulf I, Pidoux B, Dormont D, Cornu P, Agid Y. Subthalamic stimulation in Parkinson disease: a multidisciplinary approach. Arch Neurol. 2000;57:461–465. doi: 10.1001/archneur.57.4.461. [DOI] [PubMed] [Google Scholar]
- 387.Çavdar S, Özgür M, Çakmak YÖ, Kuvvet Y, Kunt SK and Sağlam G. Afferent projections of the subthalamic nucleus in the rat: emphasis on bilateral and interhemispheric connections. Acta Neurobiol Exp (Wars) 2018: 78:251–63. 10.21307/ane-2018-023 [PubMed]
- 388.Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 389.Spencer SE, Sawyer WB, Loewy AD. l-Glutamate stimulation of the zona incerta in the rat decreases heart rate and blood pressure. Brain Res. 1988;458:72–81. doi: 10.1016/0006-8993(88)90497-0. [DOI] [PubMed] [Google Scholar]
- 390.Tonelli L, Chiaraviglio E. Enhancement of water intake in rats after lidocaine injection in the zona incerta. Brain Res Bull. 1993;31:1–5. doi: 10.1016/0361-9230(93)90002-S. [DOI] [PubMed] [Google Scholar]
- 391.Shammah-Lagnado SJ, Negrao N, Ricardo JA. Afferent connections of the zona incerta: a horseradish peroxidase study in the rat. Neuroscience. 1985;15:109–134. doi: 10.1016/0306-4522(85)90127-7. [DOI] [PubMed] [Google Scholar]
- 392.Tonelli L, Chiaraviglio E. Dopaminergic neurons in the zona incerta modulates ingestive behavior in rats. Physiol Behav. 1995;58:725–729. doi: 10.1016/0031-9384(95)00128-6. [DOI] [PubMed] [Google Scholar]
- 393.Nicolelis MA, Chapin JK, Lin RC. Development of direct GABAergic projections from the zona incerta to the somatosensory cortex of the rat. Neuroscience. 1995;65:609–631. doi: 10.1016/0306-4522(94)00493-o. [DOI] [PubMed] [Google Scholar]
- 394.Aguirre JA, Covenas R, Burgos C, Castro T. Incertal projections from the brainstem and cerebellum: a horseradish peroxidase study in the cat. J HIRNFORSCH. 1989;30:449–455. [PubMed] [Google Scholar]
- 395.Roger M, Cadusseau J. Afferents to the zona incerta in the rat: a combined retrograde and anterograde study. J COMP NEUROL. 1985;241:480–492. doi: 10.1002/cne.902410407. [DOI] [PubMed] [Google Scholar]
- 396.Mitrofanis J, De Fonseka R. Organisation of connections between the zona incerta and the interposed nucleus. Anat Embryol. 2001;204:153–159. doi: 10.1007/s004290100187. [DOI] [PubMed] [Google Scholar]
- 397.Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- 398.Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N. A cortico-cerebellar loop for motor planning. Nature. 2018;563:113–116. doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Fujita H, Kodama T and du Lac S. Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 2020: 9. 10.7554/eLife.58613 [DOI] [PMC free article] [PubMed]
- 400.Fink RP, Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967;4:369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- 401.Lanciego JL, Wouterlood FG. A half century of experimental neuroanatomical tracing. J Chem Neuroanat. 2011;42:157–183. doi: 10.1016/j.jchemneu.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 402.Taylor AC, Weiss P. Demonstration of axonal flow by the movement of tritium-labeled protein in mature optic nerve fibers. Proc Natl Acad Sci U S A. 1965;54:1521–1527. doi: 10.1073/pnas.54.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kristensson K, Olsson Y. Uptake and retrograde axonal transport of peroxidase in hypoglossal neurons Electron microscopical localization in the neuronal perikaryon. Acta Neuropathol. 1971;19:1–9. doi: 10.1007/bf00690948. [DOI] [PubMed] [Google Scholar]
- 404.Kristensson K, Olsson Y. Retrograde axonal transport of protein. Brain Res. 1971;29:363–365. doi: 10.1016/0006-8993(71)90044-8. [DOI] [PubMed] [Google Scholar]
- 405.LaVail JH, LaVail MM. Retrograde axonal transport in the central nervous system. Science. 1972;176:1416–1417. doi: 10.1126/science.176.4042.1416. [DOI] [PubMed] [Google Scholar]
- 406.Gonatas NK, Harper C, Mizutani T, Gonatas JO. Superior sensitivity of conjugates of horseradish peroxidase with wheat germ agglutinin for studies of retrograde axonal transport. J Histochem Cytochem. 1979;27:728–734. doi: 10.1177/27.3.90065. [DOI] [PubMed] [Google Scholar]
- 407.Schwab ME, Agid I. Labelled wheat germ agglutinin and tetanus toxin as highly sensitive retrograde tracers in the CNS: the afferent fiber connections of the rat nucleus caudatus. Int J Neurol. 1979;13:117–126. [PubMed] [Google Scholar]
- 408.Cowan WM. The emergence of modern neuroanatomy and developmental neurobiology. Neuron. 1998;20:413–426. doi: 10.1016/s0896-6273(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 409.Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Veenman CL, Reiner A, Honig MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- 411.Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 412.Ericson H, Blomqvist A. Tracing of neuronal connections with cholera toxin subunit B: light and electron microscopic immunohistochemistry using monoclonal antibodies. J Neurosci Methods. 1988;24:225–235. doi: 10.1016/0165-0270(88)90167-7. [DOI] [PubMed] [Google Scholar]
- 413.Lencer WI, Tsai B. The intracellular voyage of cholera toxin: going retro. Trends Biochem Sci. 2003;28:639–645. doi: 10.1016/j.tibs.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 414.de Sousa TB, de Santana MA, Silva Ade M, Guzen FP, Oliveira FG, Cavalcante JC, Cavalcante Jde S, Costa MS, Nascimento ES., Jr Mediodorsal thalamic nucleus receives a direct retinal input in marmoset monkey (Callithrix jacchus): a subunit B cholera toxin study. Ann Anat. 2013;195:32–38. doi: 10.1016/j.aanat.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 415.Scalia F, Rasweiler JJt and Danias J. Retinal projections in the short-tailed fruit bat, Carollia perspicillata, as studied using the axonal transport of cholera toxin B subunit: comparison with mouse. J Comp Neurol. 2015;523:1756–91. doi: 10.1002/cne.23723. [DOI] [PubMed] [Google Scholar]
- 416.Ugolini G. Advances in viral transneuronal tracing. J Neurosci Methods. 2010;194:2–20. doi: 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 417.Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 418.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, Zhang LI. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron. 2017;93:33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, Beyer J, Forsayeth J, Bankiewicz KS. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20:348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci U S A. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Zeng WB, Jiang HF, Gang YD, Song YG, Shen ZZ, Yang H, Dong X, Tian YL, Ni RJ, Liu Y, Tang N, Li X, Jiang X, Gao D, Androulakis M, He XB, Xia HM, Ming YZ, Lu Y, Zhou JN, Zhang C, Xia XS, Shu Y, Zeng SQ, Xu F, Zhao F, Luo MH. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol Neurodegener. 2017;12:38. doi: 10.1186/s13024-017-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol. 1995;356:457–480. doi: 10.1002/cne.903560312. [DOI] [PubMed] [Google Scholar]
- 423.Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: Retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999;414:167–192. doi: 10.1002/(sici)1096-9861(19991115)414:2<167::Aid-cne3>3.0.Co;2-p. [DOI] [PubMed] [Google Scholar]
- 424.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Card JP, Enquist LW. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- 426.Soudais C, Skander N, Kremer EJ. Long-term in vivo transduction of neurons throughout the rat CNS using novel helper-dependent CAV-2 vectors. Faseb j. 2004;18:391–393. doi: 10.1096/fj.03-0438fje. [DOI] [PubMed] [Google Scholar]
- 427.Peltékian E, Garcia L, Danos O. Neurotropism and retrograde axonal transport of a canine adenoviral vector: a tool for targeting key structures undergoing neurodegenerative processes. Mol Ther. 2002;5:25–32. doi: 10.1006/mthe.2001.0517. [DOI] [PubMed] [Google Scholar]
- 428.Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 431.Etessami R, Conzelmann KK, Fadai-Ghotbi B, Natelson B, Tsiang H, Ceccaldi PE. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J Gen Virol. 2000;81:2147–2153. doi: 10.1099/0022-1317-81-9-2147. [DOI] [PubMed] [Google Scholar]
- 432.Callaway EM, Luo L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci. 2015;35:8979–8985. doi: 10.1523/jneurosci.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Dado RJ, Burstein R, Cliffer KD, Giesler GJ., Jr Evidence that fluoro-gold can be transported avidly through fibers of passage. Brain Res. 1990;533:329–333. doi: 10.1016/0006-8993(90)91358-n. [DOI] [PubMed] [Google Scholar]
- 434.Chen S, Aston-Jones G. Evidence that cholera toxin B subunit (CTb) can be avidly taken up and transported by fibers of passage. BRAIN RES. 1995;674:107–111. doi: 10.1016/0006-8993(95)00020-q. [DOI] [PubMed] [Google Scholar]
- 435.Conte WL, Kamishina H, Reep RL. Multiple neuroanatomical tract-tracing using fluorescent Alexa Fluor conjugates of cholera toxin subunit B in rats. Nat Protoc. 2009;4:1157–1166. doi: 10.1038/nprot.2009.93. [DOI] [PubMed] [Google Scholar]
- 436.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Henriksen S, Pang R, Wronkiewicz M. A simple generative model of the mouse mesoscale connectome. Elife. 2016;5:e12366. doi: 10.7554/eLife.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Osakada F, Callaway EM. Design and generation of recombinant rabies virus vectors. Nat Protoc. 2013;8:1583–1601. doi: 10.1038/nprot.2013.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Sun L, Tang Y, Yan K, Yu J, Zou Y, Xu W, Xiao K, Zhang Z, Li W, Wu B, Hu Z, Chen K, Fu ZF, Dai J, Cao G. Differences in neurotropism and neurotoxicity among retrograde viral tracers. Mol Neurodegener. 2019;14:8. doi: 10.1186/s13024-019-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Chan-Palay V. The cerebellar dentate nucleus. Cerebellar Dentate Nucleus 1977.
- 442.Uusisaari M, Obata K, Knopfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–911. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- 443.Zheng N, Raman IM. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Uusisaari MY, Knöpfel T. Diversity of neuronal elements and circuitry in the cerebellar nuclei. Cerebellum. 2012;11:420–421. doi: 10.1007/s12311-011-0350-6. [DOI] [PubMed] [Google Scholar]
- 445.Lefler Y, Yarom Y, Uusisaari MY. Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations. Neuron. 2014;81:1389–1400. doi: 10.1016/j.neuron.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 446.Uusisaari M, Knöpfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2010;9:42–55. doi: 10.1007/s12311-009-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Houck BD, Person AL. Cerebellar loops: a review of the nucleocortical pathway. Cerebellum. 2014;13:378–385. doi: 10.1007/s12311-013-0543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.De Zeeuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. EUR J NEUROSCI. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 449.Uusisaari M, Knöpfel T. GABAergic synaptic communication in the GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Neuroscience. 2008;156:537–549. doi: 10.1016/j.neuroscience.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 450.Solodkin A, Peri E, Chen EE, Ben-Jacob E, Gomez CM. Loss of intrinsic organization of cerebellar networks in spinocerebellar ataxia type 1: correlates with disease severity and duration. Cerebellum. 2011;10:218–232. doi: 10.1007/s12311-010-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Mandelli ML, De Simone T, Minati L, Bruzzone MG, Mariotti C, Fancellu R, Savoiardo M, Grisoli M. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR Am J Neuroradiol. 2007;28:1996–2000. doi: 10.3174/ajnr.A0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Park YW, Joers JM, Guo B, Hutter D, Bushara K, Adanyeguh IM, Eberly LE, Öz G, Lenglet C. Assessment of Cerebral and cerebellar white matter microstructure in spinocerebellar ataxias 1, 2, 3, and 6 using diffusion MRI. Front Neurol. 2020;11:411. doi: 10.3389/fneur.2020.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wu X, Liao X, Zhan Y, Cheng C, Shen W, Huang M, Zhou Z, Wang Z, Qiu Z, Xing W, Liao W, Tang B, Shen L. Microstructural alterations in asymptomatic and symptomatic patients with spinocerebellar ataxia type 3: a tract-based spatial statistics study. Front Neurol. 2017;8:714. doi: 10.3389/fneur.2017.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Akhlaghi H, Yu J, Corben L, Georgiou-Karistianis N, Bradshaw JL, Storey E, Delatycki MB, Egan GF. Cognitive deficits in Friedreich ataxia correlate with micro-structural changes in dentatorubral tract. Cerebellum. 2014;13:187–198. doi: 10.1007/s12311-013-0525-4. [DOI] [PubMed] [Google Scholar]
- 455.Vieira Karuta SC, Raskin S, de Carvalho NA, Gasparetto EL, Doring T, Teive HA. Diffusion tensor imaging and tract-based spatial statistics analysis in Friedreich's ataxia patients. Parkinsonism Relat Disord. 2015;21:504–508. doi: 10.1016/j.parkreldis.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 456.Huang SR, Wu YT, Jao CW, Soong BW, Lirng JF, Wu HM, Wang PS. CAG repeat length does not associate with the rate of cerebellar degeneration in spinocerebellar ataxia type 3. Neuroimage Clin. 2017;13:97–105. doi: 10.1016/j.nicl.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zalesky A, Akhlaghi H, Corben LA, Bradshaw JL, Delatycki MB, Storey E, Georgiou-Karistianis N, Egan GF. Cerebello-cerebral connectivity deficits in Friedreich ataxia. Brain Struct Funct. 2014;219:969–981. doi: 10.1007/s00429-013-0547-1. [DOI] [PubMed] [Google Scholar]
- 458.Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O'Leary D, Mueller BA, Lim KO, McEwen SC, Calhoun VD, Diaz M, Glover G, Greve D, Wible CG, Vaidya JG, Potkin SG, Mathalon DH. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med. 2018;48:2492–2499. doi: 10.1017/s003329171800003x. [DOI] [PubMed] [Google Scholar]
- 459.Liu H, Fan G, Xu K, Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 2011;34:1430–1438. doi: 10.1002/jmri.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Magnotta VA, Adix ML, Caprahan A, Lim K, Gollub R, Andreasen NC. Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: a pilot study. Psychiatry Res. 2008;163:193–200. doi: 10.1016/j.pscychresns.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Okugawa G, Nobuhara K, Minami T, Takase K, Sugimoto T, Saito Y, Yoshimura M, Kinoshita T. Neural disorganization in the superior cerebellar peduncle and cognitive abnormality in patients with schizophrenia: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1408–1412. doi: 10.1016/j.pnpbp.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 462.Okugawa G, Nobuhara K, Sugimoto T, Kinoshita T. Diffusion tensor imaging study of the middle cerebellar peduncles in patients with schizophrenia. Cerebellum. 2005;4:123–127. doi: 10.1080/14734220510007879. [DOI] [PubMed] [Google Scholar]
- 463.Koch K, Wagner G, Dahnke R, Schachtzabel C, Schultz C, Roebel M, Güllmar D, Reichenbach JR, Sauer H, Schlösser RG. Disrupted white matter integrity of corticopontine-cerebellar circuitry in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:419–426. doi: 10.1007/s00406-009-0087-0. [DOI] [PubMed] [Google Scholar]
- 464.Okugawa G, Nobuhara K, Minami T, Tamagaki C, Takase K, Sugimoto T, Sawada S, Kinoshita T. Subtle disruption of the middle cerebellar peduncles in patients with schizophrenia. Neuropsychobiology. 2004;50:119–123. doi: 10.1159/000079101. [DOI] [PubMed] [Google Scholar]
- 465.Zhang M, Palaniyappan L, Deng M, Zhang W, Pan Y, Fan Z, Tan W, Wu G, Liu Z, Pu W. Abnormal thalamocortical circuit in adolescents with early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2021;60:479–489. doi: 10.1016/j.jaac.2020.07.903. [DOI] [PubMed] [Google Scholar]
- 466.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Kim DJ, Moussa-Tooks AB, Bolbecker AR, Apthorp D, Newman SD, O'Donnell BF, Hetrick WP. Cerebellar-cortical dysconnectivity in resting-state associated with sensorimotor tasks in schizophrenia. Hum Brain Mapp. 2020;41:3119–3132. doi: 10.1002/hbm.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 469.Haghighat H, Mirzarezaee M, Araabi BN, Khadem A. Functional networks abnormalities in autism spectrum disorder: age-related hypo and hyper connectivity. Brain Topogr. 2021;34:306–322. doi: 10.1007/s10548-021-00831-7. [DOI] [PubMed] [Google Scholar]
- 470.Jeong JW, Chugani DC, Behen ME, Tiwari VN, Chugani HT. Altered white matter structure of the dentatorubrothalamic pathway in children with autistic spectrum disorders. Cerebellum. 2012;11:957–971. doi: 10.1007/s12311-012-0369-3. [DOI] [PubMed] [Google Scholar]
- 471.Hanaie R, Mohri I, Kagitani-Shimono K, Tachibana M, Azuma J, Matsuzaki J, Watanabe Y, Fujita N, Taniike M. Altered microstructural connectivity of the superior cerebellar peduncle is related to motor dysfunction in children with autistic spectrum disorders. Cerebellum. 2013;12:645–656. doi: 10.1007/s12311-013-0475-x. [DOI] [PubMed] [Google Scholar]
- 472.Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, Curran S, Robertson D, Murphy DG. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 473.Shukla DK, Keehn B, Lincoln AJ, Müller RA. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(1269–78):78.e1–2. doi: 10.1016/j.jaac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ogur T, Boyunaga OL. Relation of behavior problems with findings of cranial diffusion tensor MRI and MR spectroscopy in autistic children. Int J Clin Exp Med. 2015;8:5621–5630. [PMC free article] [PubMed] [Google Scholar]
- 475.Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 476.Arnold Anteraper S, Guell X, D'Mello A, Joshi N, Whitfield-Gabrieli S, Joshi G. Disrupted cerebrocerebellar intrinsic functional connectivity in young adults with high-functioning autism spectrum disorder: a data-driven, whole-brain, high-temporal resolution functional magnetic resonance imaging study. Brain Connect. 2019;9:48–59. doi: 10.1089/brain.2018.0581. [DOI] [PubMed] [Google Scholar]
- 477.Ramos TC, Balardin JB, Sato JR, Fujita A. Abnormal cortico-cerebellar functional connectivity in autism spectrum disorder. Front Syst Neurosci. 2018;12:74. doi: 10.3389/fnsys.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Olivito G, Clausi S, Laghi F, Tedesco AM, Baiocco R, Mastropasqua C, Molinari M, Cercignani M, Bozzali M, Leggio M. Resting-state functional connectivity changes between dentate nucleus and cortical social brain regions in autism spectrum disorders. Cerebellum. 2017;16:283–292. doi: 10.1007/s12311-016-0795-8. [DOI] [PubMed] [Google Scholar]
- 479.Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, Emsell L, Boets B, Noens I, Steyaert J, Lagae L, De Cock P, Rommel N, Sunaert S. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. Neuroimage Clin. 2014;4:374–382. doi: 10.1016/j.nicl.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hanaie R, Mohri I, Kagitani-Shimono K, Tachibana M, Matsuzaki J, Hirata I, Nagatani F, Watanabe Y, Katayama T, Taniike M. Aberrant cerebellar-cerebral functional connectivity in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2018;12:454. doi: 10.3389/fnhum.2018.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Khan AJ, Nair A, Keown CL, Datko MC, Lincoln AJ, Müller RA. Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry. 2015;78:625–634. doi: 10.1016/j.biopsych.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Anteraper SA, Guell X, Taylor HP, D'Mello A, Whitfield-Gabrieli S, Joshi G. Intrinsic functional connectivity of dentate nuclei in autism spectrum disorder. Brain Connect. 2019;9:692–702. doi: 10.1089/brain.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JD, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Almeida A, Cobos A, Tavares I, Lima D. Brain afferents to the medullary dorsal reticular nucleus: A retrograde and anterograde tracing study in the rat. Eur J Neurosci. 2002;16:81–95. doi: 10.1046/j.1460-9568.2002.02058.x. [DOI] [PubMed] [Google Scholar]
- 485.Bharos TB, Kuypers HGJM, Lemon RN, Muir RB. Divergent collaterals from deep cerebellar neurons to thalamus and tectum, and to medulla oblongata and spinal cord: retrograde fluorescent and electrophysiological studies. EXP BRAIN RES. 1981;42:399–410. doi: 10.1007/BF00237505. [DOI] [PubMed] [Google Scholar]
- 486.Jiang MC, Alheid GF, Nunzi MG, Houk JC. Cerebellar input to magnocellular neurons in the red nucleus of the mouse: synaptic analysis in horizontal brain slices incorporating cerebello-rubral pathways. Neuroscience. 2002;110:105–121. doi: 10.1016/s0306-4522(01)00544-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.