Abstract
It is well known that as part of their response to infectious agents such as viruses, microglia transition from a quiescent state to an activated state that includes proinflammatory and anti-inflammatory phases; this behavior has been described through in vitro studies. However, recent in vivo studies on the function of microglia have questioned the two-phase paradigm; therefore, a change in the frequency of in vitro studies is expected. A systematic review was carried out to identify the microglial cytokine profile against viral infection that has been further evaluated through in vitro studies (pro-inflammatory or anti-inflammatory), along with analysis of its publication frequency over the years. For this review, 531 articles published in the English language were collected from PubMed, Web of Science, EBSCO and ResearchGate. Only 27 papers met the inclusion criteria for this systematic review. In total, 19 cytokines were evaluated in these studies, most of which are proinflammatory; the most common are IL-6, followed by TNF-α and IL-1β. It should be pointed out that half of the studies were published between 2015 and 2022 (raw data available in https://github.com/dadriba05/SystematicReview.git). In this review, we identified that evaluation of pro-inflammatory cytokines released by microglia against viral infections has been performed more frequently than that of anti-inflammatory cytokines; additionally, a higher frequency of evaluation of the response of microglia cells to viral infection through in vitro studies from 2015 and beyond was noted.
Graphical Abstract
In vitro assessment of microglia-released cytokines upon viral infection has been more frequent since 2015 and has focused more on pro-inflammatory cytokines.
Keywords: Cytokines, Microglia, In Vitro, Viral infection
Introduction
Microglia are cells of the central nervous system with immune function and are found in all regions of the brain. The response of these cells to invading agents such as viruses consists of transition from a quiescent state to an activated state in which they acquire amoeboid morphology and release cytokines to participate in the immune response. This cytokine release process is formed, according to in vitro studies, by a “classical” phase with release of proinflammatory cytokines and an “alternative” phase with release of anti-inflammatory cytokines (Michelucci et al. 2009; Sargsyan et al. 2011; Xiao et al. 2007). However, in vivo studies describe findings that do not coincide with this two-phase paradigm (Chiu et al. 2013; Wes et al. 2016), which could result in a change in the frequency of in vitro studies on the microglial response to infectious agents, a phenomenon thus far not reported in the literature and identification of which may be useful to improve and guide preclinical research in this domain.
On the other hand, cytokines released to generate an inflammatory response against the invader also have deleterious effects on the central nervous system (CNS), damaging the blood‒brain barrier by cytotoxic effects, particularly through proinflammatory cytokines. However, as the anti-inflammatory cytokines also released by microglia partially inhibit the pro-inflammatory state, it is relevant to study this alternating profile (Colonna and Butovsky 2017).
The systematic review in this report aims to identify studies that have evaluated the cytokines expressed by microglia in culture against a viral presence to analyze which cytokine profile is the most assessed (proinflammatory or anti-inflammatory) and how often this type of in vitro study has been reported.
Methods
Search Strategy and Selection Criteria
A systematic review was conducted to identify the frequency of in vitro studies evaluating cytokines expressed by cultured microglia against stimulation with viruses, as well as cytokines that are commonly assessed by such studies. The articles included are reports that assessed the response of microglial cultures to viral infection. This systematic review was conducted under the guidelines of “Cochrane Handbook for Systematic Review of Interventions” and those of “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA).
We performed a sensitive search based on the use of keywords and groups of synonyms, which included articles in the English language and was carried out in the databases PubMed, Web of Science, EBSCO and ResearchGate by using the keywords “Microglia”, “Cytokines”, “Virus Infection/Disease”, “In Vitro” and “Cultures” to identify manuscripts that met the eligibility criteria. In the PubMed database, the resource “Mesh” was used to favor the search with the established keywords.
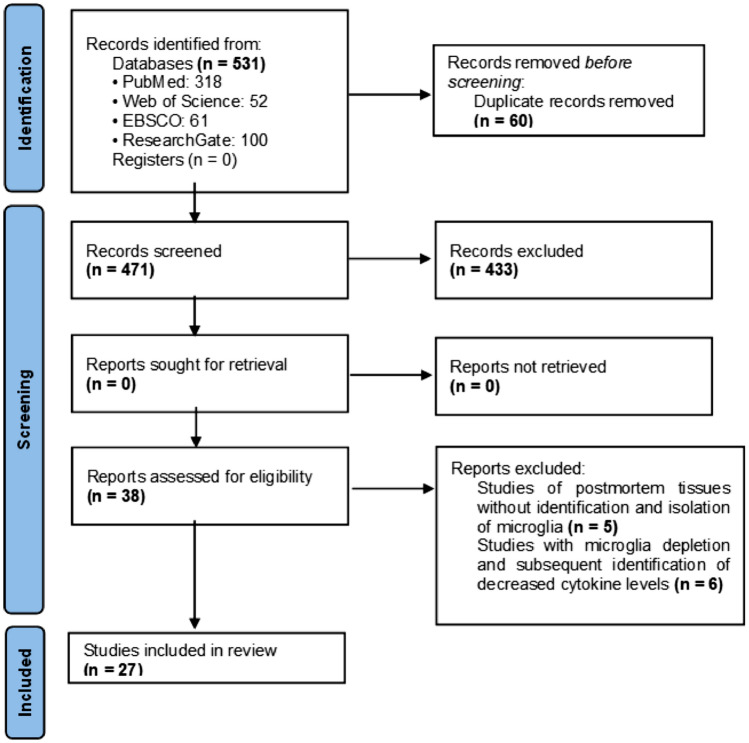
The included reports describe in vitro studies of animal or human microglia directly stimulated with viruses or infected macrophages and the cytokines expressed in response, which were identified either by genetic testing of mRNA or by immunohistochemical methods. Studies of postmortem samples without microglia isolation were not included, nor were studies that evaluated the effects of microglia suppression and consequent changes in cytokine concentration (Fig. 1).
Fig. 1.
The PRISMA flowchart
To identify expression of cytokines in response to viral stimulus, an increase in cytokine concentration with respect to baseline was considered a positive cell response. Cohort points with statistically significant values were established according to the method used to identify cytokines and were individualized for each study.
Risk of Bias Assessment
The risk of bias of the studies included in the review was not evaluated due to the lack of a standardized tool with criteria for in vitro studies. We did not register the protocol in International Prospective Register of Systematic Reviews, but it is available upon request.
Statistical Analysis and Data Availability
A descriptive analysis of the data was performed, for which a database was established in the statistical program IBM SPSS (version 26) ®. The cytokines expressed by microglia in each study were considered nominal variables, and the year of publication of each included report was considered a quantitative variable. Once statistical analysis was performed, the data are provided in tables and plots created with IBM SPSS (version 26) ® and JASP ®. Raw data are available at a public repository in GitHub®: https://github.com/dadriba05/SystematicReview.git.
Results
Literature Search
A total of 531 reports were found, of which 60 were removed prior to screening for duplication. We excluded 433 studies because their focus was to evaluate a change in the morphology of microglia rather than expression of cytokines by stimulation with viruses. In total, 38 studies were eligible, of which 11 were further excluded: 5 due to cytokine assessment in postmortem samples without isolating microglia and 6 for analyzing studies already included in the review (Fig. 1). The characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of the studies included in the systematic review. RT‒PCR: Real Time-PCR; IHC: Immunohistochemistry
| Year of publication | Sample type | Cytokine identification method | Virus | References |
|---|---|---|---|---|
| 1994 | Human microglia | RT‒PCR | MV | (Yamabe et al. 1994) |
| 2000 | Sheep microglia | RT‒PCR | MVV | (Ebrahimi et al. 2000) |
| 2001 | Human microglia | Direct IHC | CMV | (M. C.-J. Cheeran et al. 2001) |
| 2001 | Human microglia | Direct IHC | (HSV) 1 | (Lokensgard et al. 2001) |
| 2001 | Mouse microglia | RT‒PCR | TMEV | (Olson et al. 2001) |
| 2004 | Mouse microglia | RT‒PCR | MHV-A59 | (Li et al. 2004) |
| 2005 | Human microglia | Direct IHC | WNV | (M. C. J. Cheeran et al. 2005) |
| 2010 | Mouse microglia | Direct IHC | JEV | (C. J. Chen et al. 2010) |
| 2011 | Poultry microglia | RT‒PCR | GaHV-2 | (Yang et al. 2011) |
| 2012 | Mouse microglia | Direct IHC | SINV | (Esen et al. 2012) |
| 2012 | Macaque microglia | Direct IHC | VIS | (Renner et al. 2012) |
| 2013 | Mouse microglia | RT‒PCR | RABV | (Zhao et al. 2013) |
| 2015 | Mouse microglia | RT‒PCR | DENV | (Bhatt et al. 2015) |
| 2015 | Mouse microglia | Direct IHC | EV71 | (Chang et al. 2015) |
| 2017 | Mouse microglia | RT‒PCR | JEV | (Deng et al. 2017) |
| 2017 | Human microglia | Direct IHC | JEV | (Lannes et al. 2017) |
| 2017 | Human microglia | Direct IHC | ZIKV | (Lum et al. 2017) |
| 2018 | Human microglia | RT‒PCR | ZIKV | (Diop et al. 2018) |
| 2018 | Human microglia | Direct IHC | VEEV | (Keck et al. 2018) |
| 2018 | Mouse microglia | Direct IHC | ZIKV | (Wang et al. 2018) |
| 2019 | Human microglia | Direct IHC | HHV-6A | (Bortolotti et al. 2019) |
| 2020 | Mouse microglia | RT‒PCR | JEV | (Kumar et al. 2020) |
| 2020 | Human microglia | RT‒PCR | MHV-A59 | (Lavi & Cong 2020) |
| 2020 | Human microglia | Direct IHC | RSV | (Zhang et al. 2021) |
| 2021 | Human microglia | RT‒PCR | SARS-CoV-2 | (Mishra & Banerjea 2021) |
| 2022 | Human microglia | Direct IHC | VI A(H1N1) | (Ding et al. 2022) |
| 2022 | Human microglia | RT‒PCR | SARS-CoV-2 | (Jeong et al. 2022) |
Viruses Used for in vitro Stimulation
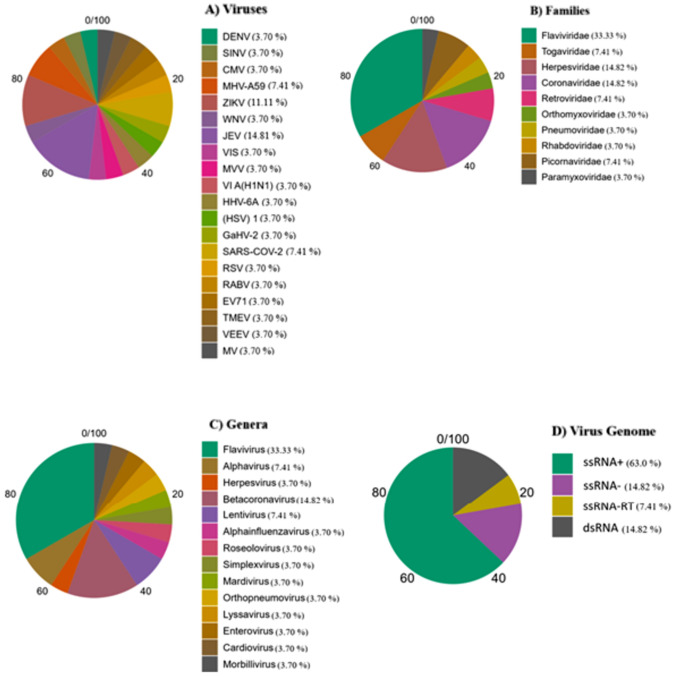
Viral stimulation of microglia in vitro was performed with Japanese encephalitis virus (JEV) in 4 studies (14.8%), Zika virus in 3 studies (11.1%), SARS-CoV-2 in 2 studies (7.4%) and another mouse hepatitis virus strain A59 in 2 studies (MHV-A69, 7.4%). The remaining studies (3.7% for each) used a different virus. By viral family, the most used for cell stimulation was Flaviviridae in 9 (33.3%), followed by Coronaviridae in 4 (14.8%) and Herpesviridae in 4 (14.8%). By genus, the most frequently assessed was Flavivirus in 9 (33.3%) and Betacoronavirus in 4 (14.8%). By viral genome, the viruses assessed were predominantly ssRNA + (Fig. 2).
Fig. 2.
Characteristics of viruses assessed in the studies included. Percentages by A viruses, B families, C genera and D viral genome
Cytokines Assessed in the in vitro Studies
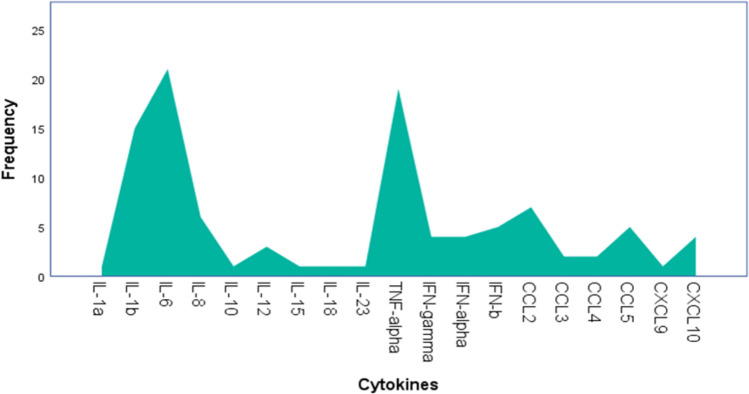
Among the 27 studies included, 19 types of cytokines were assessed and released by microglia. IL-6 was the most frequent (20.38%), followed by TNF-α (18.44%) and IL-1β (14.56%), with these three cytokines far exceeding the others evaluated. Only one cytokine assessed (IL-10) corresponded to the anti-inflammatory profile (Fig. 3).
Fig. 3.
Count of the cytokines evaluated in the studies and expressed by in vitro microglia in response to viral stimulation. In total, 19 types of cytokines were evaluated and expressed by microglia. Of these, IL-10 is the only anti-inflammatory cytokine. IL-6 was the most frequently reported cytokine (20.38%), followed by TNF-α (18.44%), both with a much higher frequency than the other reported cytokines
Publication Frequency of in vitro Reports
We identified the publication frequency over the years. In this distribution, the smallest number corresponds to 1994, the date of the oldest article, and the largest number corresponds to 2022, the date of publication of the most recent report. Thus, 50% of the studies were published between 1994 (minimum) and 2015 (median), and the remaining 50% were published between 2015 and 2022 (maximum) (Fig. 4).
Fig. 4.
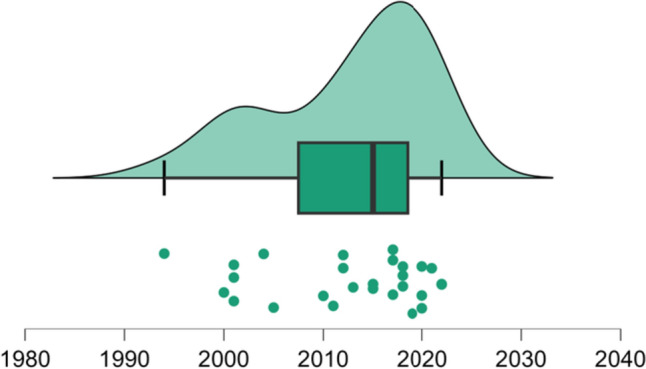
Descriptive analysis of studies’ publication year. Raincloud plot: dots represent the year in which each paper was published; the box plot shows the minimum of the data series that corresponds to year 1994, the maximum to 2022 and median (black line) to 2015 (Color figure online)
Discussion
This systematic review identified that in vitro evaluation of proinflammatory cytokines released by microglia against viral infections, mainly the cytokines IL-6, TNF-α and IL-1β, was frequently compared to the evaluation of anti-inflammatory cytokines. The reason for this might be due to the conceptualization of microglial function in vitro, with an activated state categorized as a first stage called “classical” or “M1”, in which microglia release proinflammatory cytokines with neurotoxic effects, followed by a second stage called “alternative” or “”M2”, with release of anti-inflammatory cytokines and healing activity (Michelucci et al. 2009; Sargsyan et al. 2011; Xiao et al. 2007). However, it is increasingly accepted that microglial function rarely has a tendency toward either of these two stages described by in vitro studies, as based on in vivo models of neurodegeneration that have identified concurrent expression of both neurotoxic and neuroprotective factors by microglia (Chiu et al. 2013; Wes et al. 2016).
Given this fact, it would be expected that studies in vitro have been replaced by those in vivo to better characterize the function of microglia. However, the observed asymmetry in the frequency distribution of the studies’ publication years shows the opposite. This distribution reveals that 50% of the studies were published between 1994 and 2015 and that the remaining percentage was published between 2015 and 2022 (Fig. 4). Therefore, starting in 2015, half of the studies were published within a shorter period, indicating the permanence of a significant frequency of research that evaluates the response of microglia to viral infection through in vitro studies.
This trend has implications for adequate characterization of the pathogenesis of viral encephalitis, as some of its complications are due to the neurotoxic effects of the immune response and the lack of an adequate compensatory response to inhibit it (Z. Chen et al. 2019). This is evident in the case of cognitive dysfunction and memory impairment, which are seen in long-term clinical studies in patients with viral encephalitis (Sadek et al. 2010; Sejvar et al. 2003), both associated with presynaptic membrane damage in the hippocampus, as mediated by microglia through complement activation (Vasek et al. 2016). Consequently, immunosuppressive therapy has emerged as a potential therapeutic alternative to antiviral treatment (Aksamit 2021), and its effectiveness is currently being evaluated in multicenter trials (Stahl 2018). Therefore, the results of in vivo studies do provide valuable information and likely more accurate information than isolated in vitro evaluations.
Conclusion
The findings obtained in this systematic review allowed us to identify a high frequency of in vitro studies evaluating expression of microglial cytokines in response to viral stimulation, particularly since 2015. Furthermore, the majority of these studies have focused on examining pro-inflammatory cytokines, with only one report evaluating their contrasting anti-inflammatory cytokines. The context is noteworthy considering the concurrent presence of both profiles by recent in vivo studies.
This systematic review focuses on recent in vitro evaluations of cultured microglial cell responses to viral infection. By providing this characterization, the review facilitates the potential clinical application of these results in immunotherapeutic trials, contributing to the improvement of the safety and efficacy of therapeutic alternatives for disorders due to viral infection of the brain. Additionally, we show the lack of a standardized tool for assessing the risk of bias in in vitro studies. This gap in the evaluation of preclinical evidence can lead to inappropriate applications of results to clinical practice.
Based on the aforementioned, it is crucial to promote in vivo studies for a more comprehensive characterization of the microglial response to viral infection. This is necessary to address existing gaps regarding the theory of distinct phases (classical and alternative) of the microglial response to viral infection and to better differentiate the anti-inflammatory response in viral encephalitis. The development of a standardized tool for assessing in vitro studies is essential. Such a tool would enhance critical methodological assessment of these studies and have a significant impact on the clinical application of experimental studies.
Author Contributions
MA and DA: conceptualization, methodology, formal analysis, writing—original draft, visualization MA supervision. S: conceptualization, methodology, writing—original draft, visualization. F: conceptualization, writing—original draft. V: Writing—review & editing. IE: writing—review & editing. J: Writing—review & editing. All authors reviewed the manuscript.
Funding
The authors declare that they have not received any financial support for this article’s research, authorship, and/or publication.
Data Availability
Raw data are available on a public repository in GitHub®: https://github.com/dadriba05/SystematicReview.git.
Declarations
Conflict of interest
The authors declare that there are no potential conflicts of interest related to the research, authorship, and/or publication of this article.
Ethical Approval
This is a literature review study. The National Institute of Neurology and Neurosurgery Research Ethics Committee has confirmed that no ethical approval is needed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aksamit AJ (2021) Treatment of viral encephalitis. Neurol Clin 39(1):197–207. 10.1016/J.NCL.2020.09.011 [DOI] [PubMed] [Google Scholar]
- Bhatt RS, Kothari ST, Gohil DJ, D’Souza M, Chowdhary AS (2015) Novel evidence of microglial immune response in impairment of Dengue infection of CNS. Immunobiology 220(10):1170–1176. 10.1016/j.imbio.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Bortolotti D, Gentili V, Rotola A, Caselli E, Rizzo R (2019) HHV-6A infection induces amyloid-beta expression and activation of microglial cells. Alzheimer’s Res Ther. 10.1186/s13195-019-0552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Li JR, Ou YC, Chen WY, Liao SL, Raung SL, Hsiao AL, Chen CJ (2015) Enterovirus 71 infection caused neuronal cell death and cytokine expression in cultured rat neural cells. IUBMB Life 67(10):789–800. 10.1002/iub.1434 [DOI] [PubMed] [Google Scholar]
- Cheeran MC-J, Hu S, Yager SL, Gekker G, Peterson PK, Lokensgard JR (2001) Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J NeuroVirol 7:135–147 [DOI] [PubMed] [Google Scholar]
- Cheeran MCJ, Hu S, Sheng WS, Rashid A, Peterson PK, Lokensgard JR (2005) Differential responses of human brain cells to West Nile virus infection. J Neurovirol 11(6):512–524. 10.1080/13550280500384982 [DOI] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Lin SY, Raung SL, Liao SL, Lai CY, Chen SY, Chen JH (2010) Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol 91(4):1028–1037. 10.1099/vir.0.013565-0 [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhong D, Li G (2019) The role of microglia in viral encephalitis: a review. J Neuroinflammation. 10.1186/S12974-019-1443-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ETA, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, Myers RM, Maniatis T (2013) A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4(2):385–401. 10.1016/J.CELREP.2013.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468. 10.1146/ANNUREV-IMMUNOL-051116-052358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Du G, Zhao J, Du X (2017) miR-146a negatively regulates the induction of proinflammatory cytokines in response to Japanese encephalitis virus infection in microglial cells. Adv Virol 162(6):1495–1505. 10.1007/s00705-017-3226-3 [DOI] [PubMed] [Google Scholar]
- Ding XM, Wang YF, Lyu Y, Zou Y, Wang X, Ruan SM, Wu WH, Liu H, Sun Y, Zhang RL, Zhao H, Han Y, Zhao BT, Pan J, Han XY, Wang CR, Zhao HL, Yang GL, Liu LZ, Fang SS (2022) The effect of influenza A (H1N1) pdm09 virus infection on cytokine production and gene expression in BV2 microglial cells. Virus Res. 10.1016/j.virusres.2022.198716 [DOI] [PubMed] [Google Scholar]
- Diop F, Vial T, Ferraris P, Wichit S, Bengue M, Hamel R, Talignani L, Liegeois F, Pompon J, Yssel H, Marti G, Missé D (2018) Zika virus infection modulates the metabolomic profile of microglial cells. PLoS one. 10.1371/journal.pone.0206093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi B, Allsopp TE, Fazakerley JK, Harkiss GD (2000) Phenotypic characterisation and infection of ovine microglial cells with Maedi-Visna virus. J Neurovirol 6(4):320–328. 10.3109/13550280009030758 [DOI] [PubMed] [Google Scholar]
- Esen N, Blakely PK, Rainey-Barger EK, Irani DN (2012) Complexity of the microglial activation pathways that drive innate host responses during lethal alphavirus encephalitis in mice. ASN Neuro 4(4):207–221. 10.1042/AN20120016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong GU, Lyu J, Kim K-D, Chung YC, Yoon GY, Lee S, Hwang I, Shin W-H, Ko J, Lee J-Y, Kwon Y-C (2022) SARS-CoV-2 infection of microglia elicits proinflammatory activation and apoptotic cell death. Microbiol Spectr. 10.1128/spectrum.01091-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck F, Kortchak S, Bakovic A, Roberts B, Agrawal N, Narayanan A (2018) Direct and indirect pro-inflammatory cytokine response resulting from TC-83 infection of glial cells. Virulence 9(1):1403–1421. 10.1080/21505594.2018.1509668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kalita J, Sinha RA, Singh G, Anjum B, Shukla M, Tiwari S, Dhole TN, Misra UK (2020) Impaired Autophagy flux is associated with proinflammatory microglia activation following japanese encephalitis virus infection. Neurochem Res 45(9):2184–2195. 10.1007/s11064-020-03080-5 [DOI] [PubMed] [Google Scholar]
- Lannes N, Neuhaus V, Scolari B, Kharoubi-Hess S, Walch M, Summerfield A, Filgueira L (2017) Interactions of human microglia cells with Japanese encephalitis virus. Virol J. 10.1186/s12985-016-0675-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E, Cong L (2020) Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp Mol Pathol. 10.1016/j.yexmp.2020.104474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fu L, Gonzales DM, Lavi E (2004) Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol 78(7):3398–3406. 10.1128/jvi.78.7.3398-3406.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokensgard JR, Hu S, Sheng W, Vanoijen M, Cox D, Cheeran MC-J, Peterson PK (2001) Robust expression of TNF-, IL-1, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J NeuroVirol 7:208–219 [DOI] [PubMed] [Google Scholar]
- Lum FM, Low DKS, Fan Y, Tan JJL, Lee B, Chan JKY, Rénia L, Ginhoux F, Ng LFP (2017) Zika virus infects human fetal brain microglia and induces inflammation. Clin Infect Dis 64(7):914–920. 10.1093/cid/ciw878 [DOI] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P (2009) Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-β. J Neuroimmunol 210(1–2):3–12. 10.1016/J.JNEUROIM.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Mishra R, Banerjea AC (2021) SARS-CoV-2 spike targets USP33-IRF9 axis via exosomal miR-148a to activate human microglia. Front Immunol. 10.3389/fimmu.2021.656700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Girvin AM, Miller SD (2001) Direct activation of innate and antigen-presenting functions of microglia following infection with theiler’s virus. J Virol 75(20):9780–9789. 10.1128/jvi.75.20.9780-9789.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner NA, Sansing HA, Morici LA, Inglis FM, Lackner AA, MacLean AG (2012) Microglia activation by SIV-infected macrophages: alterations in morphology and cytokine secretion. J Neurovirol 18(3):213–221. 10.1007/s13365-012-0100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek JR, Pergam SA, Harrington JA, Echevarria LA, Davis LE, Goade D, Harnar JA, Nofchissey RA, Sewell CM, Ettestad P, Haaland KY (2010) Persistent neuropsychological impairment associated with West Nile virus infection. J Clin Exp Neuropsychol 32(1):81–87. 10.1080/13803390902881918 [DOI] [PubMed] [Google Scholar]
- Sargsyan SA, Blackburn DJ, Barber SC, Grosskreutz J, De Vos KJ, Monk PN, Shaw PJ (2011) A comparison of in vitro properties of resting SOD1 transgenic microglia reveals evidence of reduced neuroprotective function. BMC Neurosci. 10.1186/1471-2202-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR (2003) Neurologic manifestations and outcome of West Nile virus infection. JAMA 290(4):511–515. 10.1001/JAMA.290.4.511 [DOI] [PubMed] [Google Scholar]
- Stahl, J. P. (2018). Dexamethasone in Herpes Simplex Virus Encephalitis (DexEnceph) - Open label Randomized Controlled Trial. https://beta.clinicaltrials.gov/study/NCT03084783
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, Funk K, DeMasters BK, Jiang X, Bowen JR, Mennerick S, Robinson JK, Garbow JR, Tyler KL, Suthar MS, Klein RS (2016) A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534(7608):538–543. 10.1038/NATURE18283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu J, Zhou R, Ding X, Zhang Q, Zhang C, Li L (2018) Zika virus infected primary microglia impairs NPCs proliferation and differentiation. Biochem Biophys Res Commun 497(2):619–625. 10.1016/j.bbrc.2018.02.118 [DOI] [PubMed] [Google Scholar]
- Wes PD, Holtman IR, Boddeke EWGM, Möller T, Eggen BJL (2016) Next generation transcriptomics and genomics elucidate biological complexity of microglia in health and disease. Glia 64(2):197–213. 10.1002/GLIA.22866 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, Appel SH (2007) Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem 102(6):2008–2019. 10.1111/J.1471-4159.2007.04677.X [DOI] [PubMed] [Google Scholar]
- Yamabe T, Dhir G, Cowan EP, Wolf AL, Bergey GK, Krumholz A, Barry E, Hoffman PM, Dhib-Jalbut S (1994) Cytokine-gene expression in measles-infected adult human glial cells. J Neuroimmunol. 10.1016/0165-5728(94)90193-7 [DOI] [PubMed] [Google Scholar]
- Yang Q, Wei P, Chen H (2011) Cytokine responses and inducible nitrous oxide synthase expression patterns in neonatal chicken brain microglia infected with very virulent Marek’s disease virus strain YL040920. Vet Immunol Immunopathol 142(1–2):14–24. 10.1016/j.vetimm.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhang XC, Yu HY, Wang Y, Chen J, Wang Y, Yu L, Zhu GX, Cao XJ, Huang SH (2021) Respiratory syncytial virus infection of microglia exacerbates SH-SY5Y neuronal cell injury by inducing the secretion of inflammatory cytokines: a transwell in vitro study. Iran J Basic Med Sci 24(2):213–221. 10.22038/ijbms.2020.49193.11263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Yang Y, Feng H, Zhao L, Qin J, Zhang T, Wang H, Yang S, Xia X (2013) Global gene expression changes in BV2 microglial cell line during rabies virus infection. Infect Genet Evol 20:257–269. 10.1016/j.meegid.2013.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available on a public repository in GitHub®: https://github.com/dadriba05/SystematicReview.git.