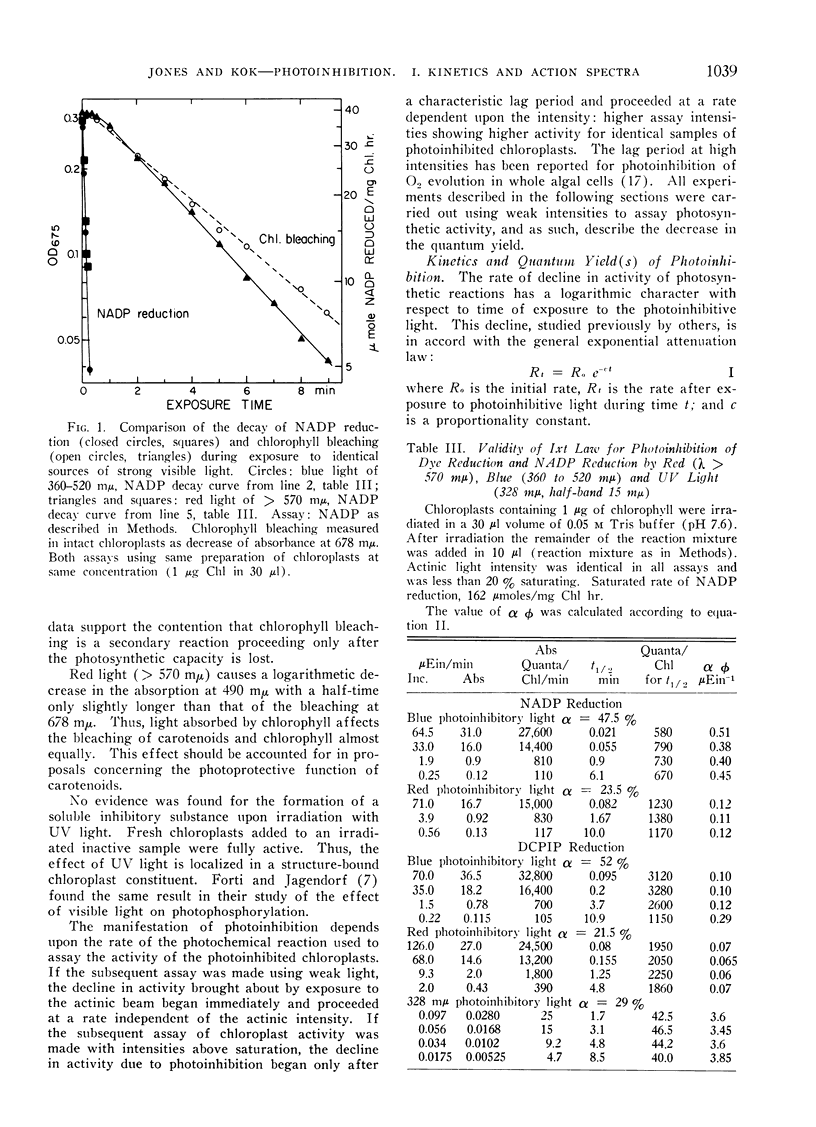
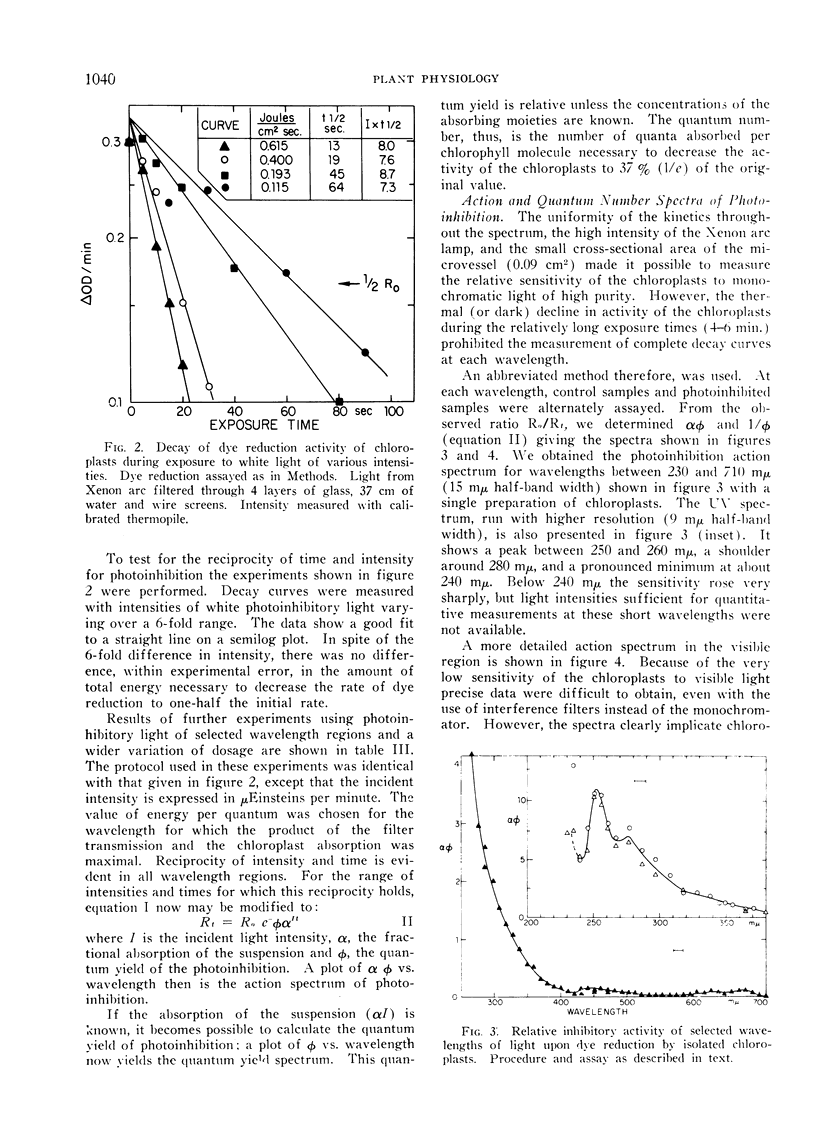
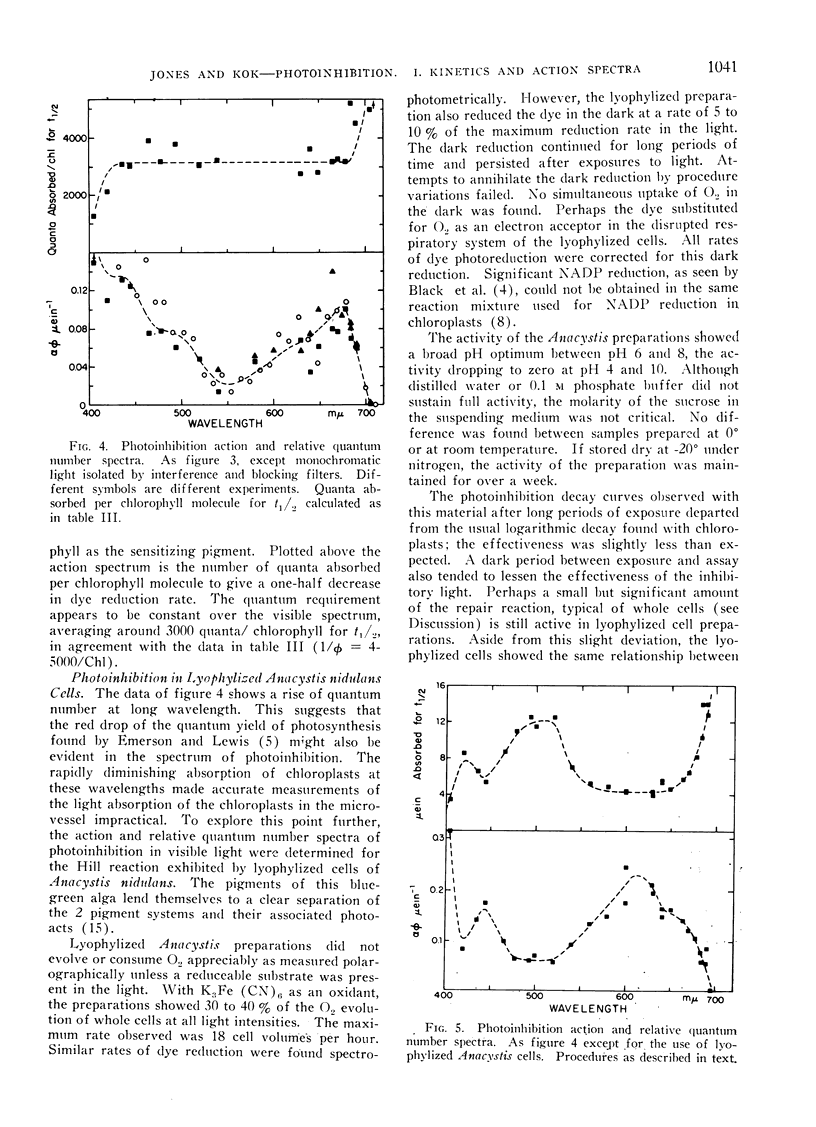
Abstract
A study was made of photoinhibition of spinach chloroplast reactions. The kinetics and spectral characteristics of the photoinhibition over a range between 230 and 700 mμ have been examined. The decline of activity due to preillumination was independent of wavelength, and dependent upon the number of quanta applied, not upon the rate of application. The effectiveness spectra of photoinhibition indicate that active ultraviolet light is absorbed by a pigment which is not a normal light absorber for photosynthesis and acts with a high quantum efficiency (> 0.1) for photoinhibition.
Active visible light is absorbed by the pigments which sensitize photosynthesis (chlorophyll, carotenoids). A very low quantum efficiency (about 10−4) was observed for the photoinhibition with visible light.
The action spectrum of the photoinhibition of dye reduction by chloroplasts and lyophylized Anacystis cells indicated that the damage caused by visible light is due to quanta absorbed by photosystem II. However, since system I might not be involved in dye reduction, the spectra may reflect only damage to photosystem II.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMESZ J. SPECTROPHOTOMETRIC EVIDENCE FOR THE PARTICIPATION OF A QUINONE IN PHOTOSYNTHESIS OF INTACT BLUE-GREEN ALGAE. Biochim Biophys Acta. 1964 Mar 30;79:257–265. [PubMed] [Google Scholar]
- AVRON M. Light inactivation of photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 Oct 21;44:41–48. doi: 10.1016/0006-3002(60)91520-1. [DOI] [PubMed] [Google Scholar]
- BLACK C. C., FEWSON C. A., GIBBS M. Photochemical reduction of triphosphopyridine nucleotide by cell-free extracts of blue-green algae. Nature. 1963 Apr 6;198:88–88. doi: 10.1038/198088a0. [DOI] [PubMed] [Google Scholar]
- FORTI G., JAGENDORF A. T. Inactivation by light of the phosphorylative activity of chloroplasts. Biochim Biophys Acta. 1960 Oct 21;44:34–40. doi: 10.1016/0006-3002(60)91519-5. [DOI] [PubMed] [Google Scholar]
- FREDRICKS W. W., JAGENDORF A. T. A SOLUBLE COMPONENT OF THE HILL REACTION IN ANACYSTIS NIDULANS. Arch Biochem Biophys. 1964 Jan;104:39–49. doi: 10.1016/s0003-9861(64)80032-1. [DOI] [PubMed] [Google Scholar]
- HAXO F. T., BLINKS L. R. Photosynthetic action spectra of marine algae. J Gen Physiol. 1950 Mar;33(4):389–422. doi: 10.1085/jgp.33.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLT A. S., BROOKS I. A., ARNOLD W. A. Some effects of 2537 A on green algae and chloroplast preparations. J Gen Physiol. 1951 May;34(5):627–645. doi: 10.1085/jgp.34.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R., Walker D. A. Pyocyanine and Phosphorylation with Chloroplasts. Plant Physiol. 1959 May;34(3):240–245. doi: 10.1104/pp.34.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of Chloroplast Reactions. II. Multiple Effects. Plant Physiol. 1966 Jun;41(6):1044–1049. doi: 10.1104/pp.41.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Myers J. Enhancement in the Blue-Green Alga, Anacystis nidulans. Plant Physiol. 1964 Nov;39(6):938–946. doi: 10.1104/pp.39.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDLER O., SIRONVAL C. Photoxidation processes in normal green Chlorella cells. II. Effects on metabolism. Biochim Biophys Acta. 1959 May;33(1):207–215. doi: 10.1016/0006-3002(59)90515-3. [DOI] [PubMed] [Google Scholar]
- KOK B. On the inhibition of photosynthesis by intense light. Biochim Biophys Acta. 1956 Aug;21(2):234–244. doi: 10.1016/0006-3002(56)90003-8. [DOI] [PubMed] [Google Scholar]
- Kok B., Gassner E. B., Rurainski H. J. Photoinhibition of chloroplast reactions. Photochem Photobiol. 1966 Mar;4(2):215–227. doi: 10.1111/j.1751-1097.1965.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- LYMAN H., EPSTEIN H. T., SCHIFF J. A. Studies of chloroplast development in Euglena. I. Inactivation of green colony formation by u.v. light. Biochim Biophys Acta. 1961 Jun 24;50:301–309. doi: 10.1016/0006-3002(61)90328-6. [DOI] [PubMed] [Google Scholar]
- REDFORD E. I., MYERS J. Some effects of ultraviolet radiations on the metabolism of Chlorella. J Cell Physiol. 1951 Oct;38(2):217–243. doi: 10.1002/jcp.1030380208. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M. The quantum efficiency of the photochemical reduction of quinone and ferricyanide by lyophilized and whole Chlorella cells. Arch Biochem Biophys. 1955 Nov;59(1):5–16. doi: 10.1016/0003-9861(55)90457-4. [DOI] [PubMed] [Google Scholar]
- SIRONVAL C., KANDLER O. Photoxidation processes in normal green Chlorella cells. I. The bleaching process. Biochim Biophys Acta. 1958 Aug;29(2):359–368. doi: 10.1016/0006-3002(58)90195-1. [DOI] [PubMed] [Google Scholar]


