Abstract
Background
This study investigated a safe and effective bolus dose and lockout time for patient-controlled sedation (PCS) with dexmedetomidine for dental treatments. The depth of sedation, vital signs, and patient satisfaction were investigated to demonstrate safety.
Methods
Thirty patients requiring dental scaling were enrolled and randomly divided into three groups based on bolus doses and lockout times: group 1 (low dose group, bolus dose 0.05 µg/kg, 1-minute lockout time), group 2 (middle dose group, 0.1 µg/kg, 1-minute), and group 3 (high dose group, 0.2 µg/kg, 3-minute) (n = 10 each). ECG, pulse, oxygen saturation, blood pressure, end-tidal CO2, respiratory rate, and bispectral index scores (BIS) were measured and recorded. The study was conducted in two stages: the first involved sedation without dental treatment and the second included sedation with dental scaling. Patients were instructed to press the drug demand button every 10 s, and the process of falling asleep and waking up was repeated 1-5 times. In the second stage, during dental scaling, patients were instructed to press the drug demand button. Loss of responsiveness (LOR) was defined as failure to respond to auditory stimuli six times, determining sleep onset. Patient and dentist satisfaction were assessed before and after experimentation.
Results
Thirty patients (22 males) participated in the study. Scaling was performed in 29 patients after excluding one who experienced dizziness during the first stage. The average number of drug administrations until first LOR was significantly lower in group 3 (2.8 times) than groups 1 and 2 (8.0 and 6.5 times, respectively). The time taken to reach the LOR showed no difference between groups. During the second stage, the average time required to reach the LOR during scaling was 583.4 seconds. The effect site concentrations (Ce) was significantly lower in group 1 than groups 2 and 3. In the participant survey on PCS, 8/10 in group 3 reported partial memory loss, whereas 17/20 in groups 1 and 2 recalled the procedure fully or partially.
Conclusion
PCS with dexmedetomidine can provide a rapid onset of sedation, safe vital sign management, and minimal side effects, thus facilitating smooth dental sedation.
Keywords: Bispectral Index Monitor, Dental Treatment, Dexmedetomidine, Effect Site Concentration, Patient-Controlled, Sedation
INTRODUCTION
Anxiety and pain during dental procedures are key factors contributing to patients' aversion to dental care [1]. Sedation is used in dental procedures as a method to reduce such anxiety and pain [2,3]. However, the varying sensitivity of patients to sedative drugs makes controlling the depth of sedation difficult, while cardiovascular and respiratory side effects remain an issue [4]. In a deep sedation state, where airway protective reflexes are lost, patients have a high risk of aspiration of cooling water, saliva, disinfectants, and other secretions during dental treatments [5,6]. Furthermore, satisfying both the dentist and the patient is challenging in dental treatments, as patient cooperation is often required, even under sedation [7].
Patient-controlled sedation (PCS), modeled after patient-controlled analgesia (PCA), has been used in clinical practice for over 30 years [8]. However, unlike PCA, PCS can impair cognitive function during the process of losing and regaining consciousness, potentially damaging the feedback system necessary for additional drug administration [9], thus raising questions about the appropriateness of the sedation level [10]. The agents used in PCS should have a rapid onset, easy control over the depth and duration of action, and minimal respiratory or cardiovascular side effects. Propofol is considered beneficial for PCS owing to its rapid sedative action, recovery, and antiemetic effects; however, caution is needed owing to the risk of potential respiratory depression [11]. Midazolam is less likely to cause respiratory depression, and is popular among dentists because of its anxiolytic effects, anterograde amnesia, longer recovery period, and frequent incidence of drowsiness and confusion [12]. The pumps for PCS must be accurate, user-friendly, and safe, allowing bolus doses, infusion rates, and lockout intervals to be set. A minimum infusion rate of 1000 ml/hr is necessary to prevent oversedation due to delayed drug administration in patients requiring higher doses [8,10,11].
Dexmedetomidine, a relatively new, locally introduced intravenous sedative, selectively acts on alpha-2 adrenergic receptors, inhibits sympathetic activity, and ensures sedation, analgesia, and anxiolysis. It is associated with fewer respiratory side effects compared to traditional GABAergic sedatives [13,14]. Typical dosing for conscious sedation involves a loading dose followed by a maintenance dose to adjust the sedation depth. Another advantage of dexmedetomidine is its potential to enhance opioid effects and reduce the required dosage [15]. Its pharmacological effects are dose-dependent, with sedation starting at plasma concentrations of 0.2–0.3 ng/ml, analgesia at 0.7–2.0 ng/ml, and deeper sleep states at levels above 2.7 ng/ml [16,17].
The onset time of a drug is a crucial characteristic in PCS drug selection. Typically, the agents used in PCS have quick onset times, with midazolam taking 2-3 minutes and propofol 15-30 seconds [18]. In contrast, the onset of dexmedetomidine is comparatively slower, usually starting at 5 minutes and peaking at around 15 minutes [19,20].
This study aimed to investigate the efficacy and potential complications of dexmedetomidine treatment in patients with PCS. By adjusting drug doses and lockout times and allowing patients to self-administer at maximum demands, we aimed to establish appropriate conditions for dental treatments and evaluate the depth and safety of sedation, as well as patient satisfaction.
METHODS
This study was approved by the Research Ethics Committee of Seoul National University Dental Hospital (CRI19009) and was registered with the Korea Clinical Research Information Service (KCT0004359). From November 2019 to February 2020, 30 patients who visited our hospital for dental scaling and provided informed consent were recruited. The patients were divided into three groups.
1. Patient selection
The inclusion criteria for the study were: patients classified as ASA (American Society of Anesthesiologists) class 1 or 2, adults aged 20–60 years, and those who volunteered for the study after being informed about the process. The exclusion criteria included patients classified as ASA class 3 or 4; adolescents under 20 years of age or adults over 60 years of age; individuals with a history of cardiovascular or respiratory diseases, cerebrovascular diseases, brain tumors, mental retardation, autism, or other neuropsychiatric disorders; patients who might have difficulty maintaining an airway; or pregnant women.
We calculated the sample size (n = 10) of 3 dose groups based on analysis of variance (ANOVA) with the difference in the maximum infused count of 3, the standard deviation of 1.8, α of 0.05 and β of 0.8, and 10% drop-out rate according to the previous work on PCS based comparison analysis [21].
2. Double-blind, randomized study
After IRB approval, prior to the registration of the first patient, block randomization was conducted using Random Allocation Software version 2.0 to assign 10 patients to each group. The sequences of the groups were sealed and stored. Before sedation, the anesthesiologist was informed of the patient’s group, drug dosage, and lockout time. The anesthesiologist responsible for administering sedation was not involved in random number generation, and was only allowed to open the sealed envelope just before sedation. The random allocation table was managed and kept confidential by an independent third party. Both patients and the dentist performing the scaling were kept blinded to the bolus dose of the sedative and lockout time.
3. Explanation of experimental side effects and process
Before the study, eligibility for sedation was verified through blood tests and vital sign checks. Patients were instructed to fast for 8 h before sedation. During sedation, they were asked to wear a blindfold and close their eyes to minimize noise in the brain's electrical signal conduction from visual stimuli. After the procedure, they were advised to refrain from driving, delicate work, or exercise and were informed of possible symptoms such as dizziness or vomiting.
4. Preparation of dexmedetomidine
Dexmedetomidine HCl: 200 µg/2 ml Vial (brand name: Precedex, manufacturer: Pfizer) was used, with 200 µg of dexmedetomidine diluted to 50 ml with saline in a 50 ml syringe, to achieve a final concentration of 4 µg/ml.
5. Setting up of experimental groups
Thirty patients were randomly divided into three groups with different bolus doses and lockout times (10 patients per group). group 1 (low dose group) was set at a bolus dose of 0.05 µg/kg and a lockout time of 1 min, group 2 (middle dose group) at 0.1 µg/kg, 1 min, and group 3 (high dose group) at 0.2 µg/kg, 3 minutes. Although there are no clear guidelines for the bolus dose and lockout time of the dexmedetomidine PCS, the settings were based on studies by Chi [22], Ahmed [23], and Rodrigo et al. [18,21]. No initial loading dose or basal infusion was set, and the maximum dose was set at 200 µg (50 ml), with an infusion rate of 1500 ml/hr.
6. Preparation for the study
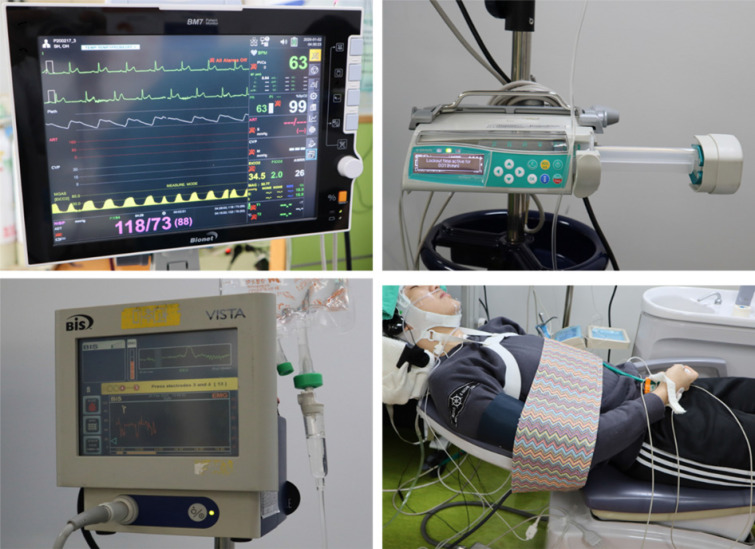
The patients were instructed to fast for 8 h before the procedure. Upon arrival at the clinic, monitoring devices (BM7, Bionet, Korea) were used to monitor the electrocardiogram, pulse, oxygen saturation, blood pressure, end-tidal CO2, respiratory rate, and bispectral index score (BIS) was monitored and recorded. All patient-monitoring data were transmitted and stored on a computer. A 22-gauge intravenous catheter was placed in the patient's left hand or forearm vein and connected to a PCS syringe pump (Perfusor Space, B. Braun Mesungen AG, Germany) and a 500 mL saline bag. The drug demand button was placed on the patient's right hand and fixed to the thumb with tape such that it could not be dropped in cases of loss of consciousness. A nasal cannula was prepared to measure the capnogram and to deliver oxygen in anticipation of hypoxemia. A safety belt was used to secure the patient and prevent unconscious movements and falls during sedation (Fig. 1).
Fig. 1. The equipment used in this study. A vital monitor (upper left, SM7, Bionet, Korea), a syringe pump (upper right, Perfusor Space, B.Braun, Germany) for patient-controlled drug administration, a Bispectral index monitor (lower left, BIS VISTA Monitoring system, Aspect Medical system, USA), a nasal cannula, the safety strap, the drug demand button (lower right) was attached to patient.
7. Conduction of the study
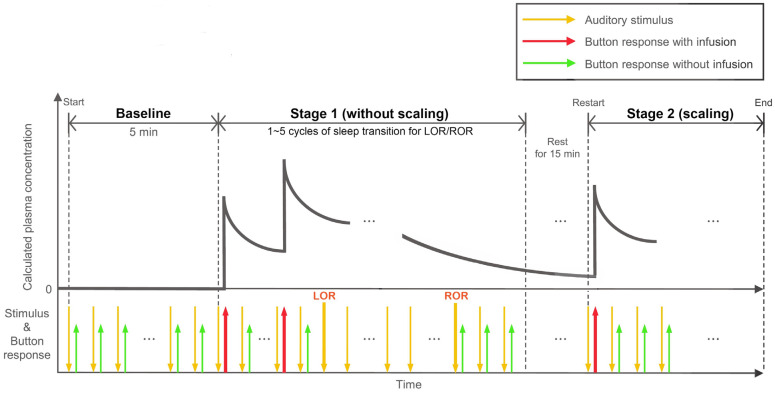
The clinical trial was conducted in two stages: the first stage used only sedation, while in the second stage, dental scaling was performed with PCS. Patients wore headphones that emitted a voice stimulus every 9-11 seconds to press the demand button (“Press the button. Beep.”). The patients responded to the voice stimulus and pressed a button several times prior to the PCS experiment. After turning on the syringe pump, the drug was administered according to the predetermined bolus dose and lockout time when the patient pressed the button following prompting by the voice stimulus. The first bolus dose of the drug was administered with the first button press, and no drug was administered during the lockout time; the drug was administered only after the lockout time elapsed upon pressing the button. The point at which the patient failed to respond to the voice stimulus more than six times was defined as the Loss of Responsiveness point (LOR), and the point at which the patient regained consciousness and responded to the voice stimulus six times in a row was defined as the Return of Responsiveness point (ROR) [24]. Before stopping the operation of the syringe pump, if the patient momentarily regained consciousness in the LOR, they will respond to vocal stimuli by pressing a button. Since the lockout time had already passed, medication would be administered. After repeating LOR and ROR 1-5 times according to the experimental duration and fully regaining consciousness (after more than 15 min rest time from the last ROR), the dental scaling stage under PCS was started. Full-mouth dental scaling was performed according to standard procedures with continuous repetition of the voice stimulus (Fig. 2).
Fig. 2. Experimental designs. Protocols for experimental timeline with the calculated plasma concentration, stimulus onset, and button-response sites during patient-controlled sedation (PCS). LOR, loss of responsiveness point; ROR, return of responsiveness point.
After scaling, drug administration was stopped, and the patient was observed until sufficient recovery of respiration, motor ability, circulation, and consciousness was achieved before moving to the recovery room. Patient recovery and vital signs were monitored in the recovery room until discharge, and intravenous catheters and fluids were maintained until departure in case of emergency. Discharge criteria included good memory, place, time, ability to follow instructions, heart rate and blood pressure within 20% of pre-trial levels, adequate respiratory rate, deep breathing, and oxygen saturation, and assessment by an anesthesiologist that the patient's condition was the same as before sedation.
8. Analysis of data
1) Data recording
The number and duration of effective and ineffective button presses when the drug was and was not administered were recorded using a syringe pump for later analyses. Blood pressure, pulse, oxygen saturation, end-tidal CO2, and respiratory rate were monitored and recorded during sedation to compare the depth of anesthesia and the point of loss of responsiveness.
Calculation of plasma concentration and effect site concentration of dexmedetomidine at loss and recovery of responsiveness
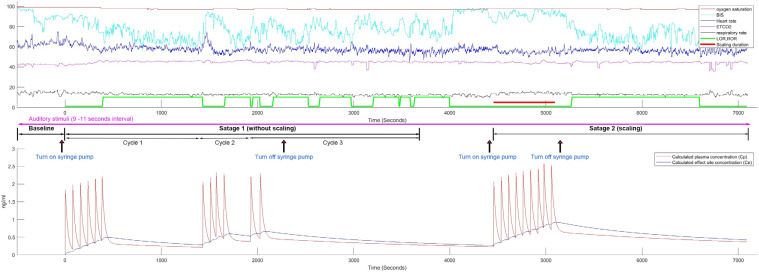
In this study, the plasma concentration (Cp) was calculated using a three-compartment mammillary model, and the indices were obtained from Hannivoort et al. [25]. The effect site concentration (Ce) was calculated from a BIS study by Colin et al. [26] (Fig. 3).
Fig. 3. This figure shows the entire progress of patient-controlled sedation (PCS) and the overall vital signs for one patient in group 1 (low dose group), as well as presenting the calculated plasma concentration (Cp) and effect site concentration (Ce) of dexmedetomidine. BIS, bispectral index; ETCO2, end-tidal CO2; LOR, loss of responsiveness point; ROR, return of responsiveness point.
2) Satisfaction evaluation
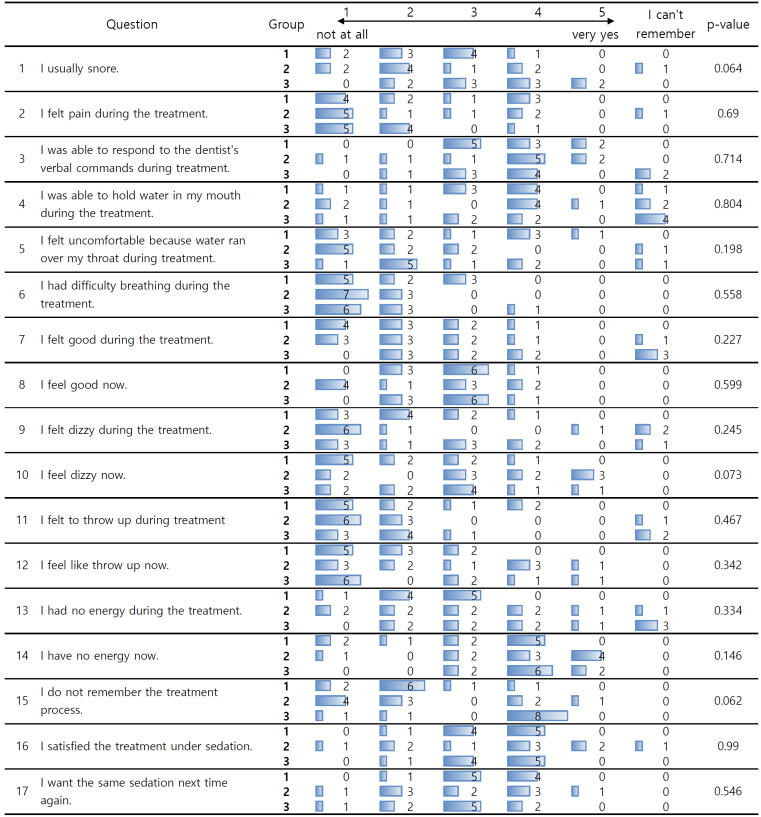
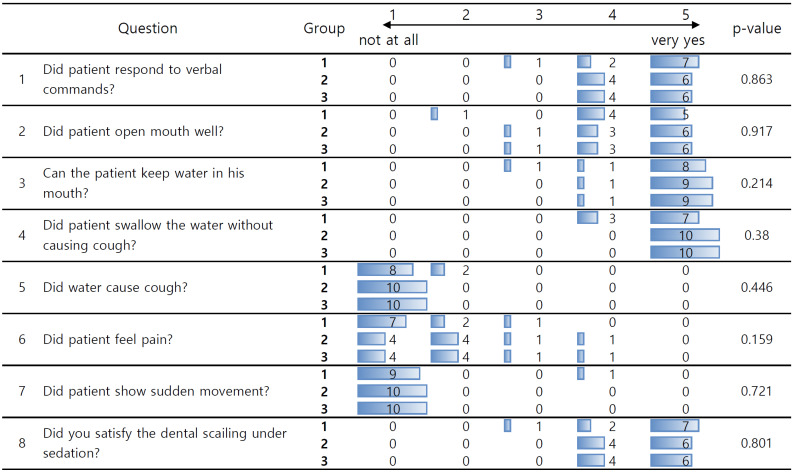
Patient and scaling dentist satisfaction surveys were conducted after PCS. The scaling was performed by a single dentist. Patient satisfaction was assessed on a six-point scale with 17 items (Fig. 4), and dentist satisfaction with the procedure was assessed on a five-point scale with eight items (Fig. 5).
Fig. 4. Survey assessing patient satisfaction with patient-controlled sedation (PCS).
Fig. 5. Survey assessing dentist satisfaction with patient controlled sedation (PCS).
9. Statistical analysis
Comparisons between groups were performed using one-way ANOVA with the Bonferroni Post-hoc test. Non-parametric analyses, including surveys, were conducted using the Kruskal-Wallis rank sum test. The significance level was set at P < 0.05.
RESULTS
1. Participants and PCS characteristics
In this study, 30 patients (22 males and 8 females) were divided into three groups, with 10 participants in each group. All the participants consented to participate in the study and had an average age of 27.1 years. There were no significant differences in demographic data such as age, height, weight, or BMI among the three groups (Table 1).
Table 1. Demographic data and group comparison.
| Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total (n = 30) | P value | |
|---|---|---|---|---|---|
| Gender (M:F) | 7:03 | 7:3 | 8:2 | 22:8 | 0.843 |
| Age (year) | 24.7 (3.3) | 28.2 (9.8) | 28.6 (9.5) | 27.2 (8.0) | 0.505 |
| Height (cm) | 171.7 (9.0) | 171.4 (9.5) | 170.0 (10.4) | 171.0 (9.3) | 0.914 |
| Body weight (kg) | 69.4 (13.4) | 72.6 (13.1) | 72.8 (16.9) | 71.6 (14.2) | 0.840 |
| Body Mass Index (kg/m2) | 23.3 (3.2) | 24.5 (2.9) | 25.1 (5.0) | 24.3 (3.8) | 0.580 |
The values are expressed as the mean (standard deviation). F, female; M, male.
The number of dexmedetomidine administrations, dose per bolus, total dose administered, and total dose administered per body weight of the patients were calculated. The number of doses was significantly lower in group 3, with no significant differences between groups 1 and 2. The total amount of dexmedetomidine administered was clearly lower in group 1, with no significant difference between groups 2 and 3. The total doses per body weight were also lower in group 1, with no significant differences between groups 2 and 3 (Table 2).
Table 2. Comparison of total number of bolus, infusion doses and time between groups during patient-controlled sedation (PCS) using dexmedetomidine.
| PCS parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total (n = 30) | P value |
|---|---|---|---|---|---|
| Total number of doses | 19.2 (2.8) | 16.9 (4.9) | 7.1 (1.4)*12 | 14.4 (6.3) | < 0.001 |
| Dosage per bolus(㎍) | 3.47 (0.67) | 7.26 (1.31)*13 | 14.57 (3.39)*12 | 8.43 (5.12) | < 0.001 |
| Total infusion doses(㎍) | 66.3 (18.3)*23 | 120.1 (34.8) | 101.2 (21.2) | 95.9 (33.3) | < 0.001 |
| Total Infusion doses per body weight (㎍/kg) | 0.96 (0.14)*23 | 1.69 (0.49) | 1.42 (0.27) | 1.36 (0.44) | < 0.001 |
| Total time of stage 1 (minute) | 50.4 (15.0) | 54.1 (11.3) | 41.4 (9.73) | 48.6 (12.9) | 0.073 |
| Total time of stage 2 (minute) | 44.4 (14.7) | 58.9 (19.2) | 50.5 (22.8) | 51.3 (19.5) | 0.255 |
The values are expressed as the mean (standard deviation). An asterisk mark (*) and the subsequent numbers indicate groups with P < 0.05 in the post hoc analysis after performing analysis of variance (ANOVA).
2. Analysis of loss (LOR) and recovery of response (ROR)
1) PCS without dental scaling (stage 1)
PCS was implemented without any stimulation other than voice stimuli prior to the scaling stage to compare the drug responses and dosages. Voice instructions to press the drug demand button came every 9-11 seconds, and the lockout times were 1 min (groups 1 and 2) and 3 min (group 3). Thus, button presses were divided into effective (when the drug was administered) and ineffective (when no drug was administered) (Table 3).
Table 3. Comparison of response to the dexmedetomidine between groups during patient-controlled sedation (PCS) without dental scaling using dexmedetomidine (stage 1).
| Stage 1 Cycle number | PCS parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|
| Cycle 1 | n = 10 | n = 10 | n = 10 | n = 30 | ||
| Number of doses until LOR | 8.0 (2.2) | 6.5 (4.1) | 2.8 (1.1)*12 | 5.8 (3.5) | 0.001 | |
| Number of button press until LOR | 55.4 (16.2) | 44.4 (28.6) | 38.7 (18.9) | 46.2 (22.3) | 0.240 | |
| Infusion doses until LOR (㎍) | 27.2 (7.9) | 45.6 (27.3) | 38.6 (10.9) | 37.1 (18.6) | 0.080 | |
| Infusion doses per body weight until LOR (㎍/kg) | 0.40 (0.11) | 0.65 (0.41) | 0.56 (0.23) | 0.54 (0.29) | 0.149 | |
| Time taken to LOR (seconds) | 568.2 (160.8) | 481.0 (228.8) | 417.7 (232.6) | 489.0 (228.8) | 0.348 | |
| Total sleep time (from LOR to ROR)(seconds) | 446.4 (451.9) | 539.1 (743.5) | 454.7 (389.2) | 480.1 (532.7) | 0.917 | |
| Cylce 2 | n = 10 | n = 9 | n 9 | n = 28 | ||
| Number of doses until LOR | 2.3 (1.4) | 1.6 (0.7) | 1.2 (0.7) | 1.7 (1.1) | 0.079 | |
| Number of button press until LOR | 10.5 (9.2) | 5.4 (3.3) | 6.6 (5.6) | 7.6 (6.8) | 0.234 | |
| Infusion doses until LOR (㎍) | 7.7 (5.2) | 11.4 (5.8) | 17.1 (9.4)*1 | 11.9 (7.8) | 0.024 | |
| Infusion doses per body weight until LOR (㎍/kg) | 0.12 (0.07) | 0.16 (0.07) | 0.24 (0.13)*1 | 0.17 (0.11) | 0.024 | |
| Time taken to LOR (seconds) | 122.5 (118.1) | 71 (51.8) | 68.4 (84.9) | 88.6 (90.8) | 0.350 | |
| Total sleep time (from LOR to ROR)(seconds) | 215.9 (262.6) | 461.7 (525.2) | 612.3 (468.5) | 422.3 (445.1) | 0.145 | |
| Cycle 3 | n = 8 | n = 6 | n = 3 | n = 17 | ||
| Number of doses until LOR | 1 (0) | 2.5 (2.8) | 1.3 (0.6) | 1.6 (1.7) | 0.282 | |
| Number of button press until LOR | 2.9 (1.9) | 11.2 (15.8) | 10.7 (4.7) | 7.2 (10) | 0.259 | |
| Infusion doses until LOR (㎍) | 3.5 (0.8) | 16.0 (14.9) | 19.4 (6.8) | 10.7 (11.2) | 0.027 | |
| Infusion doses per body weight until LOR (㎍/kg) | 0.05 (0) | 0.25 (0.28) | 0.27 (0.12) | 0.16 (0.19) | 0.084 | |
| Time taken to LOR (seconds) | 22.8 (18.6) | 138.7 (216.8) | 149 (37.6) | 85.9 (137.1) | 0.207 | |
| Total sleep time (from LOR to ROR)(seconds) | 118.6 (40.9) | 516.2 (250.6)*1 | 280 (308.5) | 287.4 (257.2) | 0.007 |
The values are expressed as the mean (standard deviation). An asterisk mark (*) and the subsequent numbers indicate groups with P < 0.05 in the post hoc analysis after performing analysis of variance (ANOVA). LOR, loss of responsiveness point; ROR, return of responsiveness point.
All 30 participants in the three groups achieved sedation using PCS with dexmedetomidine. The number of drug administrations reaching the first LOR was significantly lower in group 3 at 2.8 times compared to group 1 (8.0 times) and 2 (6.5 times).
The amount of drug administered until the first LOR and the dose per body weight of the participants were showed no significant differences between groups. The average amount of dexmedetomidine administered until the LOR was 37.1 µg, and the average dose per body weight was 0.54 µg/kg. No significant differences were observed in the time required to reach the first LOR among the three groups. The average was 489.0 seconds, with 568.2 seconds in the group 1, 481.0 seconds in the group 2, and 417.7 seconds in the group 3 (Table 3).
After a certain period of time in LOL when consciousness returns, the patient responds to continuous auditory stimuli and pressed the demand button. In this case, the blood concentration of dexmedetomidine had already increased to some extent, resulting in a rapid loss of consciousness. The PCS characteristics of the PCS in the second and third cycles are presented in Table 3.
2) PCS during dental scaling (stage 2)
The second stage compared the responses to dexmedetomidine among the three groups during scaling (Table 4). The average duration of dental scaling performed using PCS was 500.8 seconds, with no significant differences among the groups. The average of 3.1 drug administrations in group 3 was lower than that in the other two groups (6.9 and 7.1 times in groups 1 and 2, respectively). The total amount of dexmedetomidine administered during the entire duration of scaling did not differ significantly between the groups per participant body weight. One participant in groups 1 and 3 and two in group 2 fell asleep (LOL) during dental scaling for an average of 70.3 seconds, while the remaining 25 participants stayed awake throughout. One participant in group 3 requested to stop the experiment because of a non-specific response to dexmedetomidine administered before the start of scaling (continuous complaints of dizziness and feeling buoyant).
Table 4. Comparison of response to the dexmedetomidine between groups during patient-controlled sedation (PCS) with dental scaling using dexmedetomidine (stage 2).
| Stage 2 PCS parameter | Group 1 (Low dose group) n=10 | Group 2 (Middle dose group) n = 10 | Group 3 (High dose group) n = 9 | Total n = 29 | P value |
|---|---|---|---|---|---|
| Duration of dental scaling (second) | 476.9 (79.0) | 500.3 (107.1) | 527.8 (79.6) | 500.8 (89.1) | 0.482 |
| Number of doses during dental scaling | 6.9 (1.2) | 7.1 (1.9) | 3.1 (0.3)*12 | 5.8 (2.2) | < 0.001 |
| 1st sleep time during dental scaling (second) | 57 (n = 1) | 57.5 (74.3) (n = 2) | 109 (n = 1) | 70.3 (50.1) (n = 4) | |
| Number of doses until LOR | 7.3 (1.8) | 6.7 (1.4) | 3.5 (1.3)*12 | 5.8 (2.2) | < 0.001 |
| Number of button press until LOR | 66.2 (16.5) | 51.6 (16.8) | 45.4 (15.3)*1 | 54.5 (18.0) | 0.022 |
| Infusion doses until LOR (㎍) | 24.1 (6.9)*23 | 51.5 (18.4) | 45.9 (13.4) | 40.31 (18.0) | < 0.001 |
| Infusion doses per body weight until LOR (㎍/kg) | 0.34 (0.06)*23 | 0.71 (0.19) | 0.62 (0.07) | 0.56 (0.20) | < 0.001 |
| Time taken to LOR (seconds) | 664.5 (168.1) | 569.6 (158.6) | 508.6 (112.6) | 583.4 (158.1) | 0.091 |
| Total sleep time (from LOR to ROR)(seconds) | 894.9 (823.7) | 1223.7 (1150.5) | 895.7 (530.2) | 1050.9 (882.3) | 0.630 |
The values are expressed as the mean (standard deviation). An asterisk mark (*) and the subsequent numbers indicate groups with P < 0.05 in the post hoc analysis after performing analysis of variance (ANOVA). LOR, loss of responsiveness point; ROR, return of responsiveness point.
3. Comparison of effect site concentrations
The effect site concentrations (Ce) among the three groups were compared at the start (LOR), and end of the asleep state (ROR), and the highest concentrations of the effect site sleep states in both stages 1 and 2. Just as group 1 required a smaller amount of medication to reach the LOR than groups 2 and 3, group 1 experienced LOR at a lower Ce than groups 2 and 3 (Table 5).
Table 5. Comparison of effect-site concentration between groups during patient-controlled sedation (PCS).
| Stage | Cycle | Effect-site concentration | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Stage 1 (without scailing) | Cycle 1 | n | 10 | 10 | 10 | 30 | |
| Ce (ng/ml) when start of infusion | 0 | 0 | 0 | 0 | |||
| Ce (ng/ml) at LOR | 0.59 (0.14) | 1.01 (0.46)*1 | 0.85 (0.28) | 0.82 (0.35) | 0.024 | ||
| Peak Ce (ng/ml) at sleep state | 0.60 (0.14) | 1.03 (0.48)*1 | 0.87 (0.26) | 0.83 (0.36) | 0.019 | ||
| Ce (ng/ml) at ROR | 0.51 (0.21) | 0.76 (0.19) | 0.71 (0.34) | 0.66 (0.27) | 0.076 | ||
| Cycle 2 | n | 10 | 9 | 9 | 28 | ||
| Ce (ng/ml) at LOR | 0.64 (0.19) | 0.95 (0.21) | 1.03 (0.38)*1 | 0.86 (0.31) | 0.011 | ||
| Peak Ce (ng/ml) at sleep state | 0.65 (0.19)*23 | 0.98 (0.21) | 1.10 (0.36) | 0.90 (0.32) | 0.003 | ||
| Ce (ng/ml) at ROR | 0.61 (0.20) | 0.80 (0.21) | 0.85 (0.43) | 0.75 (0.30) | 0.188 | ||
| Cycle 3 | n | 8 | 6 | 3 | 17 | ||
| Ce (ng/ml) at LOR | 0.71 (0.18) | 1.00 (0.43) | 1.47 (0.58)*1 | 0.95 (0.44) | 0.024 | ||
| Peak Ce (ng/ml) at sleep state | 0.72 (0.18) | 1.05 (0.41) | 1.47 (0.58)*1 | 0.97 (0.44) | 0.021 | ||
| Ce (ng/ml) at ROR | 0.69 (0.18) | 0.81 (0.23) | 1.34 (0.67)*1 | 0.85 (0.38) | 0.027 | ||
| Stage 2 (with scailing) | n | 10 | 10 | 9 | 29 | ||
| Ce (ng/ml) when start of infusion | 0.36 (0.13) | 0.52 (0.20) | 0.48 (0.19) | 0.45 (0.18) | 0.135 | ||
| Peak Ce (ng/ml) at awake state | 0.86 (0.10)*23 | 1.55 (0.30) | 1.33 (0.15) | 1.24 (0.36) | < 0.001 | ||
| Ce (ng/ml) at LOR after scaling | 0.79 (0.10)*23 | 1.51 (0.28) | 1.32 (0.15) | 1.20 (0.36) | < 0.001 | ||
| Ce (ng/ml) at ROR after scaling | 0.58 (0.18)*2 | 1.07 (0.47) | 0.94 (0.29) | 0.86 (0.34) | 0.010 | ||
The values are expressed as the mean (standard deviation). An asterisk mark (*) and the subsequent numbers indicate groups with P < 0.05 in the post hoc analysis after performing analysis of variance (ANOVA). Ce, effect-site concentration; LOR, loss of responsiveness point; ROR, return of responsiveness point.
In stage 1, where only PCS was performed without dental scaling, the average Ce at the LOR after starting drug administration was 0.82 ng/ml, and 0.66 ng/ml at the point of ROR. The highest Ce value after the LOR was 0.83 ng/ml. The general trend showed an increase in Ce from the start of drug administration, reaching the highest Ce after the LOR point and then gradually decreasing to the ROR point.
In stage 2, where dental scaling was performed with the PCS, Ce was on average higher than that in stage 1. Owing to the experimental design, stage 2 started after stage 1; therefore, the Ce at the start of stage 2 was not 0, but averaged 0.59 ng/ml, with no differences among the groups. As the average scaling time was only 500.8 seconds, there were only four cases (out of 29) where LOR occurred during scaling. The peak Ce while awake is shown in Table 5. The highest Ce value in group 2 before LOR was 1.55 ng/ml. After scaling was completed, the operation of the syringe pump was stopped. Although Ce decreased, all 29 subjects reached the LOR after the scaling stimulus ceased, and the Ce at that time was calculated. The Ce at the point of LOR after falling asleep during stage 2 was 1.35 ng/ml, and 0.99 ng/ml at the point of return of response.
4. Observation of vital signs
The average BIS score was 83.4 before drug administration, 86.1, 73.7 after falling asleep, with no significant differences among the groups (Table 6). The average oxygen saturation was 97.4% after falling asleep, minimum oxygen saturation was 96.5%, with no significant differences among the groups (Table 7). The average respiratory rate was 16.0 /min after falling asleep, minimum respiratory rate was 13 /min, with no significant differences among the groups (Table 8). end-tidal CO2 (Table 9), heart rate (Table 10), and blood pressure (Table 11) showed no significant differences between groups.
Table 6. Comparison of bispectral index (BIS) between groups during patient-controlled sedation (PCS) using dexmedetomidine.
| Stage | Cycle | BIS parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Before infusion | average BIS | 78.1 (28.1) | 83.6 (15.5) | 88.5 (7.5) | 83.4 (19) | 0.492 | |
| minimum BIS | 73.2 (27.5) | 71.3 (30.9) | 82.2 (12.2) | 75.7 (24.2) | 0.588 | ||
| Stage 1 (without scailing) | Cycle 1 | average BIS before LOR | 76.9 (27.7) | 87 (7.4) | 88.9 (5.1) | 84.2 (17.3) | 0.260 |
| average BIS during sleep | 67.9 (25.2) | 78.6 (7.7) | 75.2 (6.6) | 73.7 (16) | 0.335 | ||
| minimum BIS during sleep | 59.2 (23.5) | 61.7 (24.9) | 64.1 (10.6) | 61.7 (19.9) | 0.867 | ||
| Cycle 2 | average BIS before LOR | 71.1 (26.7) | 78.9 (6.9) | 77.7 (6.3) | 75.6 (16.9) | 0.581 | |
| average BIS during sleep | 66.2 (24.7) | 68.9 (18.4) | 69.5 (8.9) | 68.1 (18.2) | 0.923 | ||
| minimum BIS during sleep | 58.8 (23.2) | 43.9 (34.1) | 56 (10.9) | 53.1 (24.6) | 0.395 | ||
| Cycle 3 | average BIS before LOR | 75.8 (4.1) | 69 (35.1) | 85.2 (4.1) | 75.1 (20.7) | 0.569 | |
| average BIS during sleep | 75.6 (6.5) | 60.4 (31.2) | 78.6 (6.4) | 70.8 (19.8) | 0.289 | ||
| minimum BIS during sleep | 67.5 (9.7) | 48.5 (25.5) | 71.8 (12.2) | 61.5 (19.1) | 0.103 | ||
| Stage 2 (scailing) | average BIS during scaling | 80.3 (28.7) | 73.4 (34) | 88.8 (2.8) | 80.5 (26.1) | 0.452 | |
| average BIS during sleep | 68.7 (24.9) | 58.4 (31.8) | 75.6 (8.5) | 67.3 (24.4) | 0.311 | ||
| minimum BIS during sleep | 51.5 (20.6) | 34.6 (26.5) | 56.6 (13.2) | 47.2 (22.4) | 0.073 |
The values are expressed as the mean (standard deviation). BIS, bispectral index; LOR, loss of responsiveness point.
Table 7. Comparison of oxygen saturation (SPO2) bwtween groups during patient-controlled sedation (PCS) using dexmedetomidine.
| Stage | Cycle | SPO2 parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Before infusion | average SPO2 (%) | 98.4 (0.7) | 98.1 (0.9) | 98.1 (0.7) | 98.2 (0.7) | 0.644 | |
| minimum SPO2 (%) | 98.1 (0.7) | 97.8 (0.8) | 97.7 (1.2) | 97.9 (0.9) | 0.602 | ||
| Stage 1 (without scailing) | Cycle 1 | average SPO2 before LOR (%) | 98.2 (0.6) | 98.1 (0.7) | 98.2 (0.7) | 98.2 (0.6) | 0.862 |
| average SPO2 during sleep (%) | 97.5 (0.5) | 97.6 (0.7) | 97.1 (1) | 97.4 (0.7) | 0.382 | ||
| minimum SPO2 during sleep (%) | 97.2 (0.6) | 96.3 (1.9) | 96 (1.6) | 96.5 (1.5) | 0.191 | ||
| Cycle 2 | average SPO2 before LOR (%) | 97.4 (0.7) | 97.9 (0.6) | 97.6 (1) | 97.6 (0.8) | 0.514 | |
| average SPO2 during sleep (%) | 97.5 (0.5) | 97.7 (0.7) | 97.5 (0.6) | 97.6 (0.6) | 0.830 | ||
| minimum SPO2 during sleep (%) | 97 (0.8) | 96.8 (1.5) | 96.3 (1.3) | 96.7 (1.2) | 0.497 | ||
| Cycle 3 | average SPO2 before LOR (%) | 97.1 (0.8) | 97.9 (0.9) | 97.7 (0.5) | 97.5 (0.9) | 0.228 | |
| average SPO2 during sleep (%) | 97.5 (0.7) | 97.8 (0.6) | 97.7 (0.5) | 97.6 (0.6) | 0.631 | ||
| minimum SPO2 during sleep (%) | 97 (0.9) | 97.3 (0.8) | 97 (1) | 97.1 (0.9) | 0.770 | ||
| Stage 2 (scailing) | average SPO2 during scaling (%) | 96.9 (1.1) | 97.4 (0.7) | 97 (1.1) | 97.1 (1) | 0.499 | |
| average SPO2 during sleep (%) | 97.5 (0.6) | 97.7 (0.6) | 97.5 (0.5) | 97.6 (0.5) | 0.594 | ||
| minimum SPO2 during sleep (%) | 96.3 (1.1) | 96.2 (1.4) | 95.1 (1.8) | 95.9 (1.5) | 0.155 |
The values are expressed as the mean (standard deviation). LOR, loss of responsiveness point; SPO2, oygen saturation of pulse oxymetry.
Table 8. Comparison of respiratory rate between groups during patient-controlled sedation (PCS) using dexmedetomidine.
| Stage | Cycle | RR parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Before infusion | average RR (/min) | 17.3 (3.7) | 15.4 (3.3) | 16.3 (3.3) | 16.3 (3.4) | 0.453 | |
| minimum RR (/min) | 14.8 (4) | 13.5 (3.4) | 13.9 (2.5) | 14.1 (3.3) | 0.679 | ||
| Stage 1 (without scailing) | Cycle 1 | average RR before LOR (/min) | 17.1 (3.3) | 15.8 (1.8) | 16.4 (2.9) | 16.4 (2.7) | 0.585 |
| average RR during sleep (/min) | 16.3 (2.5) | 16 (1.2) | 15.5 (2.9) | 16 (2.3) | 0.726 | ||
| minimum RR during sleep (/min) | 13.9 (2.5) | 13 (1.5) | 12.2 (2.9) | 13 (2.4) | 0.290 | ||
| Cycle 2 | average RR before LOR (/min) | 15.6 (2.6) | 15.7 (1.7) | 15.9 (3.4) | 15.7 (2.6) | 0.973 | |
| average RR during sleep (/min) | 16.2 (2.7) | 16.3 (1.6) | 16.2 (2.9) | 16.2 (2.4) | 0.996 | ||
| minimum RR during sleep (/min) | 14.4 (2.6) | 14.2 (2) | 12.8 (2) | 13.8 (2.3) | 0.253 | ||
| Cycle 3 | average RR before LOR (/min) | 15.6 (2.7) | 15.8 (1.3) | 14 (1.5) | 15.4 (2.1) | 0.477 | |
| average RR during sleep (/min) | 16.5 (2.6) | 16.8 (1.4) | 14.4 (2.1) | 16.2 (2.2) | 0.305 | ||
| minimum RR during sleep (/min) | 14.9 (2.1) | 14.2 (2.3) | 12 (2.7) | 14.1 (2.4) | 0.208 | ||
| Stage 2 (scailing) | average RR during scaling (/min) | 16.7 (2.8) | 16.6 (1.8) | 17.3 (3.2) | 16.8 (2.6) | 0.799 | |
| average RR during sleep (/min) | 15.8 (2.3) | 15.8 (1.8) | 15.8 (2.7) | 15.8 (2.2) | 0.995 | ||
| minimum RR during sleep (/min) | 12.3 (2) | 10.5 (2) | 9.9 (3.4) | 10.9 (2.6) | 0.106 |
The values are expressed as the mean (standard deviation). LOR, loss of responsiveness point; RR, respiratory rate.
Table 9. Comparison of end-tidal CO2 between groups during patient-controlled sedation (PCS) using dexmedetomidine.
| Stage | Cycle | ETCO2 parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Before infusion | average ETCO2 (mmHg) | 41.5 (2) | 42.5 (2.1) | 40.1 (5.9) | 41.4 (3.8) | 0.399 | |
| maximum ETCO2 (mmHg) | 42.5 (1.9) | 43.5 (2.2) | 42.1 (5.5) | 42.7 (3.5) | 0.668 | ||
| Stage 1 (without scailing) | Cycle 1 | average ETCO2 before LOR (mmHg) | 42.3 (1.9) | 42.9 (2) | 42.3 (2.7) | 42.5 (2.1) | 0.791 |
| average ETCO2 during sleep (mmHg) | 43.2 (2.1) | 44.2 (2.6) | 42.6 (6.8) | 43.3 (4.3) | 0.719 | ||
| maximum ETCO2 during sleep (mmHg) | 45.3 (2.1) | 46.3 (2.1) | 45.1 (5.9) | 45.5 (3.7) | 0.750 | ||
| Cycle 2 | average ETCO2 before LOR (mmHg) | 42.9 (2.6) | 44.5 (2.6) | 41.3 (7.5) | 42.9 (4.7) | 0.382 | |
| average ETCO2 during sleep (mmHg) | 43.6 (2.3) | 44.9 (2.1) | 41.1 (6.8) | 43.2 (4.4) | 0.185 | ||
| maximum ETCO2 during sleep (mmHg) | 44.9 (2.4) | 46.5 (2) | 44.6 (6.2) | 45.3 (3.9) | 0.563 | ||
| Cycle 3 | average ETCO2 before LOR (mmHg) | 44 (2.2) | 44.1 (2.2) | 42.9 (4.4) | 43.9 (2.5) | 0.796 | |
| average ETCO2 during sleep (mmHg) | 43.6 (1.7) | 44.2 (1.7) | 45.8 (2.4) | 44.2 (1.9) | 0.212 | ||
| maximum ETCO2 during sleep (mmHg) | 45 (1.4) | 45.7 (1.8) | 48 (2) | 45.8 (1.9) | 0.060 | ||
| Stage 2 (scailing) | average ETCO2 during scaling (mmHg) | 41.3 (1.9) | 42.3 (2.3) | 39.6 (4.7) | 41.1 (3.2) | 0.213 | |
| average ETCO2 during sleep (mmHg) | 43.2 (2.2) | 43.9 (2.2) | 41.5 (5.6) | 42.9 (3.5) | 0.368 | ||
| maximum ETCO2 during sleep (mmHg) | 45.4 (2) | 47 (2.5) | 45.5 (4.3) | 46 (3) | 0.448 |
The values are expressed as the mean (standard deviation). ETCO2, end-tidal CO2; LOR, loss of responsiveness point.
Table 10. Comparison of heart rate during patient-controlled sedation (PCS) using dexmedetomidine.
| Stage | Cycle | HR parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Before infusion | average HR (/min) | 67.3 (7.9) | 73.1 (11.8) | 71.1 (10) | 70.5 (10) | 0.433 | |
| minimum HR (/min) | 62 (8.3) | 69.1 (11.9) | 66.9 (8.4) | 66 (9.8) | 0.262 | ||
| Stage 1 (without scailing) | Cycle 1 | average HR before LOR (/min) | 63.6 (9.3) | 67.4 (8.5) | 64.2 (6.7) | 65.2 (8) | 0.591 |
| minimum HR before LOR (/min) | 51.4 (14.5) | 55.2 (7.6) | 53 (5.4) | 53.2 (9.7) | 0.695 | ||
| average HR during sleep (/min) | 58.4 (10.2) | 62.1 (9.8) | 62.6 (5.6) | 61 (8.6) | 0.509 | ||
| minimum HR during sleep (/min) | 52.4 (14.1) | 57.1 (8.6) | 55.5 (6.6) | 55 (10.1) | 0.588 | ||
| Cycle 2 | average HR before LOR (/min) | 60.6 (8.7) | 60.3 (8.7) | 59.9 (5.3) | 60.3 (7.4) | 0.984 | |
| minimum HR before LOR (/min) | 52.5 (16.1) | 56.9 (8.8) | 55.8 (7.6) | 55 (11.4) | 0.697 | ||
| average HR during sleep (/min) | 58.8 (8.9) | 60.1 (8.9) | 59.2 (5.3) | 59.4 (7.6) | 0.937 | ||
| minimum HR during sleep (/min) | 51.5 (14.5) | 54.1 (8.6) | 45.4 (12.2) | 50.4 (12.2) | 0.314 | ||
| Cycle 3 | average HR before LOR (/min) | 60.7 (9.8) | 57.4 (8.8) | 58.6 (4.6) | 59.1 (8.3) | 0.797 | |
| minimum HR before LOR (/min) | 53.5 (16.9) | 54.8 (10.8) | 50 (8.2) | 53.4 (13.1) | 0.887 | ||
| average HR during sleep (/min) | 58.8 (9.1) | 54.8 (8.5) | 60 (3.3) | 57.7 (7.9) | 0.612 | ||
| minimum HR during sleep (/min) | 50.5 (16.3) | 49.3 (8.8) | 56.3 (4.9) | 51.1 (12.2) | 0.733 | ||
| Stage 2 (scailing) | average HR during scaling (/min) | 57.7 (5.6) | 56.3 (9.9) | 57.5 (6.1) | 57.2 (7) | 0.923 | |
| minimum HR during scaling (/min) | 47.5 (12.3) | 42.7 (16.7) | 45.9 (10.2) | 45.3 (13.1) | 0.722 | ||
| average HR during sleep (/min) | 57.9 (7.1) | 60.9 (7.3) | 59.8 (6.1) | 59.4 (6.7) | 0.677 | ||
| minimum HR during sleep (/min) | 49 (13.3) | 47.1 (10.4) | 50.9 (8.1) | 48.9 (10.6) | 0.752 |
The values are expressed as the mean (standard deviation). HR, herat rate; LOR, loss of responsiveness point.
Table 11. Comparison of mean blood pressure between groups during patient-controlled sedation (PCS) using dexmedetomidine.
| Stage | Cycle | ETCO2 parameter | Group 1 (Low dose group) | Group 2 (Middle dose group) | Group 3 (High dose group) | Total | P value |
|---|---|---|---|---|---|---|---|
| Before infusion | average mBP (mmHg) | 91.2 (8.5) | 90.3 (10) | 93.7 (8.5) | 91.7 (8.8) | 0.692 | |
| minimum mBP (mmHg) | 85.8 (16.5) | 71.4 (34.7) | 76.5 (29) | 77.9 (27.5) | 0.510 | ||
| Stage 1 (without scailing) | Cycle 1 | average mBP before LOR (mmHg) | 90.9 (9.5) | 93.1 (9) | 96.3 (9.2) | 93.5 (9.2) | 0.591 |
| average mBP during sleep (mmHg) | 85 (9.1) | 92.7 (11) | 86 (8.7) | 87.7 (9.8) | 0.509 | ||
| Cycle 2 | average mBP before LOR (mmHg) | 84.6 (9.2) | 89.5 (12.8) | 82.2 (9) | 85.3 (10.3) | 0.984 | |
| average mBP during sleep (mmHg) | 82.7 (10.4) | 89 (12.3) | 83.1 (8.2) | 84.7 (10.3) | 0.937 | ||
| Cycle 3 | average mBP before LOR (mmHg) | 83.4 (10.3) | 89 (11.6) | 88.3 (7.2) | 86.2 (10.1) | 0.797 | |
| average mBP during sleep (mmHg) | 83 (12) | 90.2 (10.5) | 93.9 (18.2) | 87.3 (12.7) | 0.612 | ||
| Stage 2 (scailing) | average mBP during scaling (mmHg) | 80.9 (9.9) | 88 (8.9) | 89.1 (8.7) | 85.6 (9.7) | 0.923 | |
| average mBP during sleep (mmHg) | 76.6 (9) | 87.7 (11.3) | 82.1 (9.5) | 81.9 (10.6) | 0.067 |
The values are expressed as the mean (standard deviation). LOR, loss of responsiveness point; mBP, mean blood pressure.
5. Patient and dentist satisfaction with PCS
After PCS, a survey was conducted once the patients had fully recovered to assess the satisfaction of both the patient and the dentists who performed the scaling. The Kruskal-Wallis rank-sum test was used for comparison between groups. There were no items with a P value < 0.05 due to the small number of patients.
1) Patient satisfaction with PCS
The overall data are shown in Fig. 4. In statistical analysis, data stating "I can't remember" was excluded. There was no significant difference in the degree of pain felt by patients during treatment across the three groups, but in group 3, nine out of ten patients either did not feel or barely felt pain compared to approximately six in groups 1 and 2. The level of response to the dentist’s verbal commands during treatment was mostly at a stage where some response was possible (answer 4) in all groups.
One patient in group 3 reported difficulty breathing during treatment, but the rest largely did not complain of discomfort. In group 1, one patient complained of discomfort due to water flowing down the back of the throat during scaling, and in group 2, one patient was unable to hold water in their mouth during treatment. During the treatment, 12 patients reported not feeling dizziness at all, and one felt severe dizziness, whereas after the treatment, four felt severely dizzy and 3 slightly dizzy. Regarding memory of the treatment process, in group 3, 8 out of 10 patients reported slightly not remembering, whereas in groups 1 and 2, 17 out of 20 patients chose answers 1 and 2, indicating that they remembered all or most of them.
There was no difference in overall satisfaction with the PCS between the groups. Of the 29 patients who underwent scaling, 13 reported being slightly satisfied (answer 4), and 12 gave a neutral response (answer 3) to the question of whether they would like to undergo scaling again.
2) Dentist satisfaction with PCS
The overall data are shown in Fig. 5. There were no significant differences in the eight items among the three groups. Regarding responses to verbal commands, 18 participants were highly cooperative. Regarding opening their mouths well, 16 participants were cooperative.
Among the 29 patients who underwent scaling, only one in group 3 was recorded as not holding water well in the mouth and inducing coughing. In cases of sudden movements, only two out of 29 showed slight movement.
In response to the question on overall satisfaction, five out of nine in group 3, six out of ten in group 2, and seven out of ten in group 1 reported being very satisfied.
DISCUSSION
This study involved a total of 30 patients (22 males, 8 females), with 10 in each group, participating in the clinical trial. Scaling was performed in 29 patients, excluding one who complained of dizziness during PCS before the scaling procedure.
Dexmedetomidine is typically administered as a loading dose of 1 µg/kg over approximately 10 minutes, followed by a maintenance dose of 0.5-1 µg/kg/hr, which is the conventional method for procedural sedation [27,28]. Although there are many studies on the continuous administration of dexmedetomidine, few have focused on bolus administration for sedation, and most of these involved children, making it difficult to consider procedural sedation [29,30]. Studies on PCS using drugs such as midazolam, propofol, and ketamine exist; however, studies using dexmedetomidine are rare [11,12,22,31,32]. In this study, PCS was conducted using dexmedetomidine during dental scaling, and the changes in patients and their satisfaction levels were investigated.
The onset time of a drug is an important characteristic when selecting a medication for PCS. Generally, drugs used for this purpose, such as midazolam (2-3 minutes) and propofol (15-30 seconds), are preferred for their quick onset [18,33,34,35,36]. In contrast, the onset of dexmedetomidine typically starts at around 5 minutes and peaks around 15 minutes, which is relatively slow [19,20]. In this study, bolus doses of 0.05, 0.1, 0.2 µg/kg were administered to the patients in each group until the point of LOR. The time to reach this point was on average 417.7 seconds in group 3, which is faster than is generally known. In groups 2 and 1, the averages were 481 seconds and 568.2 seconds, respectively, which were similar to or faster than the usual onset time after the loading dose.
The dose of dexmedetomidine administered to reach the first LOR was on average 0.54 µg/kg for all patients, which is less than the typical loading dose of 1 µg/kg, suggesting a similar or faster sedative effect. Although no significant difference was observed between the groups, in group 3, the average dose was 0.56 µg/kg, almost half the usual loading dose, and a sedative effect was achieved within a shorter time (average 417.7 seconds).
The number of dexmedetomidine administrations until LOR was significantly lower in group 3 (2.8 times) compared to those in groups 2 (6.5 times) and 1 (8 times). Considering patient comfort, speed of sleep onset, and the amount of drug administered, group 3 appeared to be slightly more efficient in terms of overall sedative effectiveness.
Dental scaling was performed in 29 of 30 patients. Considering patient safety, one patient was excluded from scaling after experiencing excessive dizziness during the self-regulation of sedation. The average time required for dental scaling was 500.8 seconds, with no significant differences between the groups. The number of drug administrations during scaling was significantly lower in group 3, at 3.1 times. This could be due to the longer lockout time (3 min) in the high-dose group, which prevented multiple administrations during a similar scaling time (average 500.7 seconds). Of the 29 patients, 4 reached the LOR during scaling, and the time from this point to the end of scaling was on average 70.23 seconds, indicating a short duration of sleep compared to the total scaling time. This suggests that continuous stimulation of dental scaling might delay the LOR compared to the first stage without stimulation.
The Ce during the experiment showed that group 1 also experienced LOR at a lower Ce than groups 2 and 3. In the first stage without stimulation, LOR started at an average concentration of 0.82 ng/ml, whereas in the second stage with scaling stimulation, the effective concentration rose to 1.24 ng/ml for LOR onset.
Dexmedetomidine induces a biphasic hemodynamic response [37]. At lower blood concentrations, sympathetic effects dominate, reducing blood pressure and heart rate, whereas at higher concentrations, peripheral vasoconstriction effects dominate, increasing blood pressure and pulse rate [38,39]. In this study, the heart rate decreased immediately after reaching the LOR, gradually increasing thereafter. In some group 3 patients, the heart rate decreased immediately after drug administration, but quickly recovered.
Satisfaction with the PCS using dexmedetomidine was evaluated through surveys of patients and dentists. While most responses showed no significant differences between the groups, there was a significant difference in the memory of the procedure. Compared to other sedative drugs, dexmedetomidine is not frequently associated with anterograde amnesia compared to other sedative drugs [40,41]. However, in this study, 8 of the 10 patients in group 3 reported slight memory loss. This suggests that anterograde amnesia occurred even if patients did not fall asleep during treatment, leading to a lack of memory of the difficult parts of the procedure. According to the dentist survey, except for one patient in the high-dose group, eight patients followed verbal commands well and were cooperative, suggesting appropriate characteristics for conscious sedation. In the patient survey, positive responses were more common in the areas of pain, breathing, and aspiration; however, there were more negative responses regarding dizziness during the experiment and mood at the time. However, 13 of 29 patients responded positively in terms of overall satisfaction. Practitioners were generally satisfied with the verbal command compliance, pain, breathing, aspiration, and sudden movement of the patients.
The limitations of this study include the short duration of dental scaling, which did not allow for sufficient observation of patients' sleep during dental procedures. More invasive and longer procedures may address this issue in future studies.
In this study, we investigated the effects of dexmedetomidine on PCS. Research on PCS with dexmedetomidine bolus administration is scarce. The results of this study show that it is possible to achieve safe and rapid sedation with a lower dose than the usual loading dose (1 µg, administered over 10 minutes). Overall, our results indicate that PCS with dexmedetomidine can be performed efficiently with relatively quick sedative effects, safe vital sign management, and minimal side effects.
Footnotes
- Seung-Hyun Rhee: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft.
- Young-Seok Kweon: Data curation, Investigation, Software.
- Dong-Ok Won: Conceptualization, Data curation, Investigation, Methodology, Software.
- Seong-Whan Lee: Funding acquisition, Supervision.
- Kwang-Suk Seo: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Visualization, Writing – review & editing.
DECLARATIONS OF INTEREST: The authors declare no conflicts of interest.
FUNDING: This study was supported by grant no 04-2018-0099 from the Seoul National University Dental Hospital Research Fund.
References
- 1.Locker D, Shapiro D, Liddell A. Negative dental experiences and their relationship to dental anxiety. Community dental health. 1996;13:86–92. [PubMed] [Google Scholar]
- 2.Chanpong B, Haas DA, Locker D. Need and demand for sedation or general anesthesia in dentistry: a national survey of the canadian population. Anesth Prog. 2005;52:3–11. doi: 10.2344/0003-3006(2005)52[3:NADFSO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milgrom P, Newton JT, Boyle C, Heaton LJ, Donaldson N. The effects of dental anxiety and irregular attendance on referral for dental treatment under sedation within the national health service in london. Community Dent Oral Epidemiol. 2010;38:453–459. doi: 10.1111/j.1600-0528.2010.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanborn PA, Michna E, Zurakowski D, Burrows PE, Fontaine PJ, Connor L, et al. Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology. 2005;237:288–294. doi: 10.1148/radiol.2371041415. [DOI] [PubMed] [Google Scholar]
- 5.Dionne RA, Yagiela JA, Moore PA, Gonty A, Zuniga J, Beirne OR. Comparing efficacy and safety of four intravenous sedation regimens in dental outpatients. J Am Dent Assoc. 2001;132:740–751. doi: 10.14219/jada.archive.2001.0271. [DOI] [PubMed] [Google Scholar]
- 6.Green SM, Mason KP, Krauss BS. Pulmonary aspiration during procedural sedation: a comprehensive systematic review. Br J Anaesth. 2017;118:344–354. doi: 10.1093/bja/aex004. [DOI] [PubMed] [Google Scholar]
- 7.Manley MC, Skelly AM, Hamilton AG. Dental treatment for people with challenging behaviour: General anaesthesia or sedation? Br Dent J. 2000;188:358–360. doi: 10.1038/sj.bdj.4800480. [DOI] [PubMed] [Google Scholar]
- 8.Rudkin GE, Osborne GA, Curtis NJ. Intra-operative patient-controlled sedation. Anaesthesia. 1991;46:90–92. doi: 10.1111/j.1365-2044.1991.tb09345.x. [DOI] [PubMed] [Google Scholar]
- 9.Thorpe SJ, Balakrishnan VR, Cook LB. The safety of patient-controlled sedation. Anaesthesia. 1997;52:1144–1150. doi: 10.1111/j.1365-2044.1997.247-az0395.x. [DOI] [PubMed] [Google Scholar]
- 10.Seo KS. Patient-controlled sedation for dental treatment. J Korean Dent Soc Anesthesiol. 2013;13:81–87. [Google Scholar]
- 11.Rudkin GE, Osborne GA, Finn BP, Jarvis DA, Vickers D. Intra-operative patient-controlled sedation. Comparison of patient-controlled propofol with patient-controlled midazolam. Anaesthesia. 1992;47:376–381. doi: 10.1111/j.1365-2044.1992.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 12.Garip H, Gürkan Y, Toker K, Göker K. A comparison of midazolam and midazolam with remifentanil for patient-controlled sedation during operations on third molars. Br J Oral Maxillofac Surg. 2007;45:212–216. doi: 10.1016/j.bjoms.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268. doi: 10.2165/00003495-200059020-00012. discussion 269-70. [DOI] [PubMed] [Google Scholar]
- 14.Coughlan MG, Lee JG, Bosnjak ZJ, Schmeling WT, Kampine JP, Warltier DC. Direct coronary and cerebral vascular responses to dexmedetomidinesignificance of endogenous nitric oxide synthesis. Anesthesiology. 1992;77:998–1006. doi: 10.1097/00000542-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–128. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–1076. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigo C. Patient-controlled sedation. Anesth Prog. 1998;45:117–126. [PMC free article] [PubMed] [Google Scholar]
- 19.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Comparison of dexmedetomidine and midazolam sedation and antagonism of dexmedetomidine with atipamezole. J Clin Anesth. 1993;5:194–203. doi: 10.1016/0952-8180(93)90014-6. [DOI] [PubMed] [Google Scholar]
- 20.Mason KP, Zgleszewski SE, Dearden JL, Dumont RS, Pirich MA, Stark CD, et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg. 2006;103:57–62. doi: 10.1213/01.ane.0000216293.16613.15. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo MR, Fung SC. Comparison of two techniques of patient-controlled sedation with midazolam. Br J Oral Maxillofac Surg. 1999;37:472–476. doi: 10.1054/bjom.1999.0118. [DOI] [PubMed] [Google Scholar]
- 22.Chi SI, Kim HJ, Seo KS. Dexmedetomidine intravenous sedation using a patient-controlled sedation infusion pump: a case report. J Dent Anesth Pain Med. 2016;16:55–59. doi: 10.17245/jdapm.2016.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed SS, Unland T, Slaven JE, Nitu ME. High dose dexmedetomidine: effective as a sole agent sedation for children undergoing mri. Int J Pediatr. 2015;2015:397372. doi: 10.1155/2015/397372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeom SK, Won DO, Chi SI, Seo KS, Kim HJ, Müller KR, et al. Spatio-temporal dynamics of multimodal eeg-fnirs signals in the loss and recovery of consciousness under sedation using midazolam and propofol. PLoS One. 2017;12:e0187743. doi: 10.1371/journal.pone.0187743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannivoort LN, Eleveld DJ, Proost JH, Reyntjens KM, Absalom AR, Vereecke HE, et al. Development of an optimized pharmacokinetic model of dexmedetomidine using target-controlled infusion in healthy volunteers. Anesthesiology. 2015;123:357–367. doi: 10.1097/ALN.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 26.Colin PJ, Hannivoort LN, Eleveld DJ, Reyntjens KMEM, Absalom AR, Vereecke HEM, et al. Dexmedetomidine pharmacokinetic-pharmacodynamic modelling in healthy volunteers: 1. Influence of arousal on bispectral index and sedation. Br J Anaesth. 2017;119:200–210. doi: 10.1093/bja/aex085. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115:171–182. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 28.Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawes J, Myers D, Görges M, Zhou G, Ansermino JM, Montgomery CJ. Identifying a rapid bolus dose of dexmedetomidine (ED 50) with acceptable hemodynamic outcomes in children. Paediatr Anaesth. 2014;24:1260–1267. doi: 10.1111/pan.12468. [DOI] [PubMed] [Google Scholar]
- 30.Siddappa R, Riggins J, Kariyanna S, Calkins P, Rotta AT. High-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth. 2011;21:153–158. doi: 10.1111/j.1460-9592.2010.03502.x. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson A, Steinvall I, Bak Z, Sjöberg F. Patient controlled sedation using a standard protocol for dressing changes in burns: patients' preference, procedural details and a preliminary safety evaluation. Burns. 2008;34:929–934. doi: 10.1016/j.burns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Tokumine J, Iha H, Okuda Y, Shimabukuro T, Shimabukuro T, Ishigaki K, et al. Appropriate method of administration of propofol, fentanyl, and ketamine for patient-controlled sedation and analgesia during extracorporeal shock-wave lithotripsy. J Anesth. 2000;14:68–72. doi: 10.1007/s005400050069. [DOI] [PubMed] [Google Scholar]
- 33.Barends CR, Absalom A, van Minnen B, Vissink A, Visser A. Dexmedetomidine versus midazolam in procedural sedation. a systematic review of efficacy and safety. PLoS One. 2017;12:e0169525. doi: 10.1371/journal.pone.0169525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarkson K, Power CK, O'Connell F, Pathmakanthan S, Burke CM. A comparative evaluation of propofol and midazolam as sedative agents in fiberoptic bronchoscopy. Chest. 1993;104:1029–1031. doi: 10.1378/chest.104.4.1029. [DOI] [PubMed] [Google Scholar]
- 35.Dundee JW, Halliday NJ, Harper KW, Brogden RN. Midazolam. A review of its pharmacological properties and therapeutic use. Drugs. 1984;28:519–543. doi: 10.2165/00003495-198428060-00002. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava VK, Agrawal S, Kumar S, Mishra A, Sharma S, Kumar R. Comparison of dexmedetomidine, propofol and midazolam for short-term sedation in postoperatively mechanically ventilated neurosurgical patients. J Clin Diagn Res. 2014;8:GC04–GC07. doi: 10.7860/JCDR/2014/8797.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colin PJ, Hannivoort LN, Eleveld DJ, Reyntjens KMEM, Absalom AR, Vereecke HEM, et al. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Haemodynamic profile. Br J Anaesth. 2017;119:211–220. doi: 10.1093/bja/aex086. [DOI] [PubMed] [Google Scholar]
- 38.Figueroa XF, Poblete MI, Boric MP, Mendizábal VE, Adler-Graschinsky E, Huidobro-Toro JP. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial α2-adrenoceptor activation. Br J Pharmacol. 2001;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Cheung CW, Ying CL, Chiu WK, Wong GT, Ng KF, Irwin MG. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia. 2007;62:1132–1138. doi: 10.1111/j.1365-2044.2007.05230.x. [DOI] [PubMed] [Google Scholar]
- 41.Levänen J, Mäkelä ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82:1117–1125. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]