Abstract
Background
Among the various pain-related diseases that can be encountered at the clinic, there is a neuropathic pain that is difficult to treat. Numerous methods have been proposed to treat neuropathic pain, such as taking medication, nerve block with lidocaine, or neurolysis with alcohol or phenol. Recently, a method of perineural injection using dextrose instead of lidocaine was proposed. This study was designed to compare the effects of perineural injection therapy (PIT) with buffered 5% dextrose or 0.5% lidocaine on neuropathic pain.
Methods
The data were collected from the database of pain clinic from August 1st, 2019 to December 31st, 2022 without any personal information. The inclusion criteria were patients diagnosed with postherpetic neuralgia (PHN), trigeminal neuralgia (TN), complex regional pain syndrome (CRPS), or peripheral neuropathy (PN), and patients who had undergone PIT with buffered 5% dextrose (Dextrose group) or 0.5% lidocaine (Lidocaine group) for pain control. The data of patients, namely sex, age, and pain score (numerical rating scale, NRS) were collected before PIT. The data of NRS, side effects, and satisfaction grade (excellent, good, fair, or poor) were collected one week after each of the four PIT, and two weeks after the last PIT.
Results
Overall, 112 subjects were enrolled. The Dextrose group included 89 and Lidocaine group included 23 patients. Because the number of patients in the Lidocaine group was too small to allow statistical analysis, the trend in Lidocaine group was just observed in each disease. There were no significant side effects except for a few bruise cases on the site of injection in all groups. The NRS in most Dextrose groups except CRPS were reduced significantly; however, the Lidocaine group showed a trend of pain reduction only in PHN. The Dextrose group except CRPS showed increased satisfaction two weeks after the final PIT.
Conclusion
From the results, it is suggested that PIT with buffered 5% dextrose may have a good effect for neuropathic pain without any side effect except for patients with CRPS. This may offer a window into a new tool that practitioners can employ in their quest to help patients with neuropathic pain.
Keywords: Dextrose, Injections, Lidocaine, Neuropathic Pain
INTRODUCTION
Among the pain that occurs in the facial area managed in the dental field, neuropathic pain, which is classified as incurable, is included. Neuropathic pain refers to chronic pain that persists due to nerve damage or abnormal nerve function, and representative examples include post-herpetic neuralgia (PHN), trigeminal neuralgia (TN), complex regional pain syndrome (CRPS), peripheral neuropathy (PN), failed spine surgery syndrome, and pain due to cancer [1]. Unlike tissue damage, such as muscle or ligament injury, which is commonly experienced when we twist our back, neuropathic pain, in which the nerve itself is damaged, becomes chronic and is prone to hypersensitivity, making mild pain feel more severe or even normal stimulation painful. There are cases where even everyday activities, such as eating, drinking, or brushing teeth, can cause extreme pain, making it impossible to lead a normal life.
Drugs, such as antiepileptics, tricyclic antidepressants, selective serotonin reuptake inhibitors, and norepinephrine reuptake inhibitors are used for intractable chronic pain diseases. These drugs are known to have slightly greater side effects than acetaminophen or non-steroidal anti-inflammatory drugs, which are known as common painkillers [2,3]. Such side effects should be monitored when taken for a long time. If these drug treatments are not satisfied, nerve blocks and even narcotic painkillers may be used as a second line. Several clinicians may observe some side effects with these second line treatments too.
Perineural injection therapy (PIT) is one of the latest advances in regenerative medicine. PIT targets neurogenic inflammation of the subcutaneous nerves, which potentially causes pain [4,5]. It was further improved by Lyftogt [6] using dextrose injections, which provided significant pain control in a series of 300 cases of Achilles tendinopathy.
Since the introduction of PIT, there have been no reports comparing its effectiveness in patients with neuropathic pain. Therefore, it is sought to evaluate the effectiveness of PIT compared to that of the commonly used lidocaine injection method for representative neuropathic pain diseases, such as TN, PHN, CRPS, and PN.
METHODS
The subject of the study was a retrospective data review of patients who were diagnosed with neuropathic pain in the outpatient pain clinic. This study was conducted with approval from the Pusan National University Hospital Research Ethics Committee (IRB No. 2212-006-122) and the informed consent was waived. The inclusion criteria for the study subjects were patients who were older than 18 years of age, patients who visited the outpatient pain clinic at Pusan National University Hospital from August 1, 2019 to December 31, 2022, and had pain due to PHN, TN, CRPS, and PN, and who received a PIT with buffered 5% dextrose or 0.5% lidocaine for pain control. Their medical data, excluding sensitive personal information, were analyzed.
The information for analysis, such as age, sex, disease, pain score (NRS), side effects, and satisfaction, were measured in the group using buffered 5% dextrose (Dextrose group) or the group using 0.5% lidocaine (Lidocaine group).
The PIT involved injecting 0.5 to 3 mL of buffered 5% dextrose (by mixing 0.25 cc of sodium bicarbonate with 50 cc of 5% dextrose) or 0.5% lidocaine (by diluting 2% lidocaine with saline) into the subcutaneous tissue with a 27G needle into the distal nerve branch or the relevant pain area. The goal was to inject four times at intervals of one week depending on the intensity of pain. The pain score was expressed using the Numerical Rating Score (NRS), with 0 indicating no pain at all and 10 indicating the most severe pain. The satisfaction was graded from 1 to 4. Grade 1 was poor, grade 2 was fair, grade 3 was good, and grade 4 was excellent. The changes in each set of data were retrospectively assessed by examining the recorded information from several time points: before the first PIT, one week after the first, second, third, and fourth PIT sessions, and two weeks following the fourth PIT session. The data of sex, age, and pain score (numerical rating scale, NRS) were collected before PIT. The data of NRS, side effects, and satisfaction grade (excellent, good, fair, poor) were collected one week after each of the four PIT procedures, and two weeks after the last PIT by reviewing the recorded data.
All values were described as mean ± standard error. For analysis and comparison of the results in each group the StatView® program (version 5.0, SAS Institute INC, Cary, NC, USA) was used to perform the students t-test, and a P value of less than 0.05 was considered as statistically significant.
RESULTS
The data of 112 subjects who met the inclusion criteria were obtained from the medical records room and were analyzed. Notably, PHN (n = 26), TN (n = 18), CRPS (n = 28), and PN (n = 40) were included. The average age was 72 ± 2 years old in PHN, 64 ± 3 years old in TN, 43 ± 2 years old in CRPS, 50 ± 2 years old in PN. Overall, 13 males and 13 females in the PHN group, five males and 13 females in the TN group, 14 males and 14 females in the CRPS group, and 22 males and 18 females in the PN group were included. The sample size of the Lidocaine group, consisting of only 23 participants, was insufficient to permit statistical analysis. The trend in the Lidocaine group was just in each disease. There was no significant side effect in most cases except a few bruises.
1. PHN
The number of patients in each group was 20 in the Dextrose group and 6 in the Lidocaine group. The NRS before treatment was 5.3 ± 0.6 in the Dextrose group and 7.2 ± 0.6 in the Lidocaine group. The NRS at the final follow-up was 3.5 ± 0.7 in the Dextrose group and 5.0 ± 0.6 in the Lidocaine group (Table 1).
Table 1. NRS and satisfaction grade before and after PIT in each group.
| Dextrose group | Lidocaine group | |||||||
|---|---|---|---|---|---|---|---|---|
| NRS | Satisfaction | NRS | Satisfaction | |||||
| P0 | Follow-up | P1 | Follow-up | P0 | Follow-up | P1 | Follow-up | |
| PHN | 5.3 ± 0.6 | 3.5 ± 0.7† | 2.1 ± 0.3 | 2.9 ± 0.3* | 7.2 ± 0.6 | 5.0 ± 0.6 | 1.8 ± 0.7 | 2.3 ± 0.3 |
| TN | 5.9 ± 0.6 | 3.7 ± 0.7* | 2.1 ± 0.3 | 2.9 ± 0.3* | 7.5 ± 2.5 | 2.0 | N/A | N/A |
| CRPS | 7.3 ± 0.4 | 6.9 ± 0.7 | 0.8 ± 0.2 | 0.9 ± 0.3 | 6.8 ± 0.8 | 6.8 ± 0.7 | 0.9 ± 0.3 | 0.3 ± 0.3 |
| PN | 5.7 ± 0.4 | 3.8 ± 0.3† | 1.5 ± 0.2 | 2.7 ± 0.2† | 6.8 ± 0.5 | 7.3 ± 0.5 | 1.1 ± 0.3 | 1.7 ± 0.6 |
CRPS, complex regional pain syndrome; N/A, not available; NRS, numerical rating scale; PHN, post-herpetic neuralgia; PIT, perineural injection therapy; PN, peripheral neuropathy; TN, trigeminal neuralgia.
P0, before PIT; P1, one week after the first PIT; Follow-up, two weeks after the fourth PIT.
*P < 0.05, †P < 0.01 compared to P0 in NRS
*P < 0.05, †P < 0.01 compared to P1 in Satisfaction
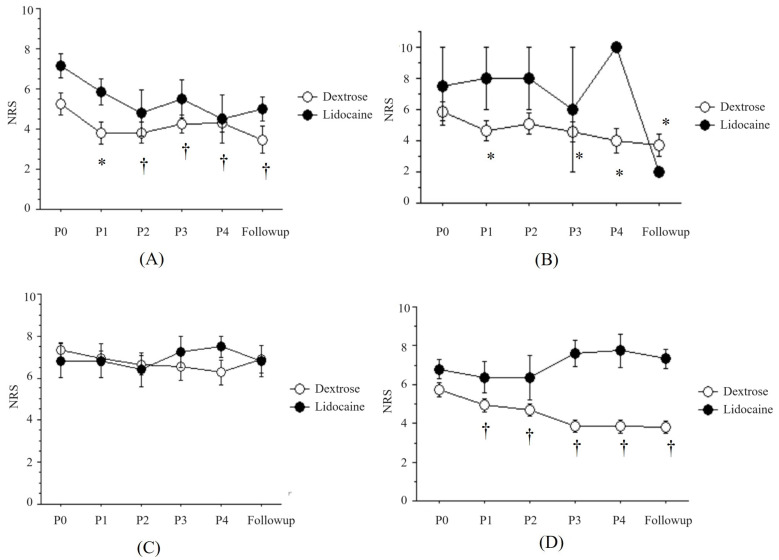
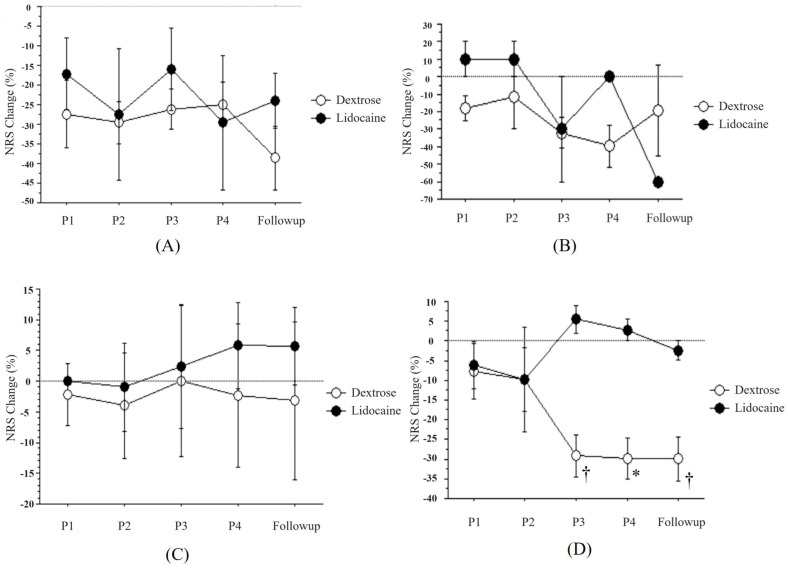
The NRS after each treatment was significantly decreased in the Dextrose group compared to before treatment (P0) (P < 0.01), but not in the Lidocaine group (Fig. 1A). The percent change of NRS after each treatment was from -27.4 ± 8.6% to -38.6 ± 8.2% in the Dextrose group and from -17.2 ± 9.1% to -23.9 ± 7.0% in the Lidocaine group (Fig. 2A).
Fig. 1. The changes of pain intensity (NRS) after PIT with buffered 5% dextrose (Dextrose) or 0.5% lidocaine (Lidocaine). (A) Post-herpetic neuralgia, (B) Trigeminal neuralgia, (C) Complex regional pain syndrome, (D) Peripheral neuropathy. NRS, numerical rating scale; PIT, perineural injection therapy. P0: before PIT, P1: one week after the first PIT, P2: one week after the second PIT, P3: one week after the third PIT, P4: one week after the fourth PIT, Follow-up: two weeks after the fourth PIT. *P < 0.05, †P < 0.01 vs. the change of pain intensity before PIT in the Dextrose group.
Fig. 2. The percent changes of pain intensity (NRS) after PIT with buffered 5% dextrose (Dextrose) or 0.5% lidocaine (Lidocaine). (A) Post-herpetic neuralgia, (B) Trigeminal neuralgia, (C) Complex regional pain syndrome, (D) Peripheral neuropathy. NRS, numerical rating scale; PIT, perineural injection therapy. P1: one week after the first PIT, P2: one week after the second PIT, P3: one week after the third PIT, P4: one week after the fourth PIT, Follow-up: two weeks after the fourth PIT. *P < 0.05, †P < 0.01 vs. the percent change of pain intensity after the first PIT in the Dextr.
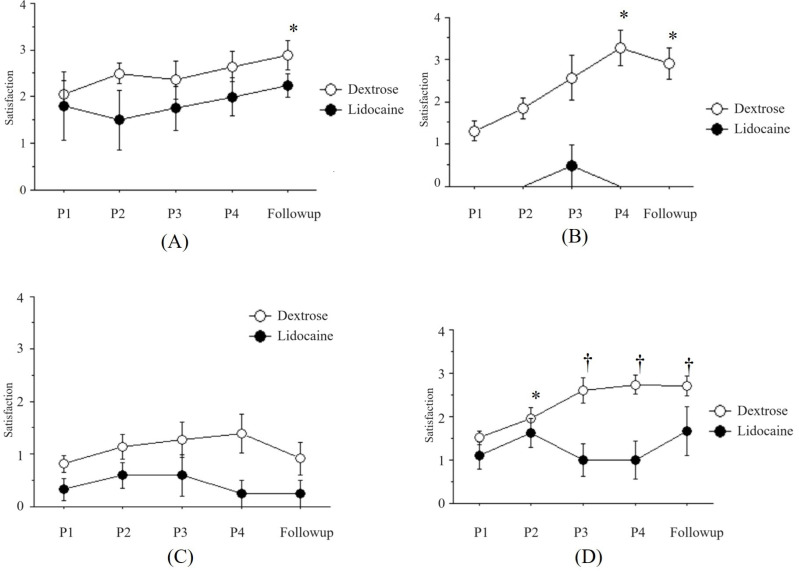
The satisfaction was increased as the number of treatments increased from 2.1 ± 0.3 to 2.9 ± 0.3 in the Dextrose group and from 1.8 ± 0.7 to 2.3 ± 0.3 in the Lidocaine group (Fig. 3A).
Fig. 3. The changes of satisfaction after perineural injection therapy (PIT) with buffered 5% dextrose (Dextrose) or 0.5% lidocaine (Lidocaine). (A) Post-herpetic neuralgia, (B) Trigeminal neuralgia, (C) Complex regional pain syndrome, (D) Peripheral neuropathy. P1: one week after the first PIT, P2: one week after the second PIT, P3: one week after the third PIT, P4: one week after the fourth PIT, Follow-up: two weeks after the fourth PIT. *P < 0.05, †P < 0.01 vs. the satisfaction after the first PIT in Dextrose group.
2. TN
The number of each group was 16 in the Dextrose group and 2 in the Lidocaine group. Therefore, the Lidocaine group could not be analyzed due to the small number. The NRS before treatment was 5.9 ± 0.6 in the Dextrose group and 7.5 ± 2.5 in the Lidocaine group. The NRS at final follow-up was 3.7 ± 0.7 in the Dextrose group and 2.0 in the Lidocaine group (Table 1).
The NRS after each treatment was significantly decreased in the Dextrose group compared to that before treatment (P0) (Figure 1B, P < 0.05). The percent change of NRS after each treatment in the Dextrose group was not significantly different (Fig. 2B).
The satisfaction in the Dextrose group was increased as the number of treatments increased from 2.1 ± 0.3 to 2.9 ± 0.3 (Fig. 3B).
3. CRPS
The number of each group was 22 in the Dextrose group and 6 in the Lidocaine group. The NRS before treatment was 7.3 ± 0.4 in the Dextrose group and 6.8 ± 0.8 in the Lidocaine group. The NRS at the final follow-up was 6.9 ± 0.7 in the Dextrose group and 6.8 ± 0.7 in the Lidocaine group (Table 1). The NRS after each treatment was not significantly changed in both groups (Fig. 1C). The percent change after each treatment was from -2.2 ± 5.1% to -3.2 ± 12.9% in the Dextrose group and from -0.0 ± 0.0% to 5.6 ± 6.3% in the Lidocaine group (Fig. 2C). The variation in each result was so pronounced that it rendered the statistical analysis inconclusive.
The satisfaction was increased as the number of treatments increased from 0.8 ± 0.2 to 0.9 ± 0.3 in the Dextrose group and from 0.9 ± 0.3 to 0.3 ± 0.3 in the Lidocaine group (Fig. 3C).
4. PN
The number in each group was 31 in the Dextrose group and 9 in the Lidocaine group. The NRS before treatment was 5.7 ± 0.4 in the Dextrose group and 6.8 ± 0.5 in the Lidocaine group. The NRS at the final follow-up was 3.8 ± 0.3 in the Dextrose group and 7.3 ± 0.5 in the Lidocaine group (Table 1).
The NRS after each treatment was significantly decreased in the Dextrose group compared to before treatment (P0) (Fig. 1D, P < 0.01), but not in the Lidocaine group. The percent change after each treatment was from -7.8 ± 7.0% to -30.0 ± 5.5% in the Dextrose group and from -6.2 ± 6.0% to -2.4 ± 2.4% in the Lidocaine group (Fig. 2D).
The satisfaction was increased as the number of treatments increased from 1.5 ± 0.2 to 2.7 ± 0.2 in the Dextrose group and from 1.1 ± 0.3 to 1.7 ± 0.6 in the Lidocaine group (Fig. 3D).
DISCUSSION
Chronic pain is a complex and multifaceted problem that is difficult to treat. This frustration is often compounded by patients who are suffering, but cannot receive any relief, and despite their best efforts, the treatment does not seem to help in the long term.
The perineural injection of glucose is a new treatment for peripheral entrapment neuropathy. Additionally, 5% glucose (D5W) has been commonly used in these cases because D5W has an osmolarity like that of normal saline and no harmful effects have been reported in animals and humans [7,8,9,10]. At 10%, dextrose induces thickening of the transverse carpal ligament in rabbits, and there is a cumulative effect of successive injections because hypertonic dextrose can stimulate inflammation [11,12]. In contrast, D5W can reduce neurogenic inflammation. Nonetheless, there are a few case studies and small clinical trials investigating perineural injections of D5W for pain relief [13,14,15].
Despite the increasing popularity of D5W injections, no evidence-based studies had been reported until the trial reported in 2017 by Wu et al. [16]. Although the exact mechanism for the effects of D5W is not clear, it is hypothesized that glucose may reduce neurogenic inflammation by inhibiting transient receptor potential vanilloid receptor-1 (TRPV1). The inhibition of TRPV1 can limit neurogenic inflammation by blocking neurotransmitters, including calcitonin gene-related peptide and substance P [17,18,19,20,21,22].
Soft tissues are innervated by peptidergic sensory nerves with TRPV1. TRPV1 is a receptor found on sensory nerve endings, particularly in pain-sensing neurons called nociceptors. It plays a crucial role in the perception of pain and the body's response to various painful stimuli. The mechanism of TRPV1 on pain involves its sensitivity to multiple stimuli, particularly heat, capsaicin, acidic pH, and certain endogenous substances, such as anandamide. When activated by these stimuli, the TRPV1 channels open and allow the influx of calcium and sodium ions into the nerve cell. This influx of ions triggers a series of events that lead to the generation and propagation of nerve impulses, resulting in the sensation of pain. The activation of TRPV1 receptors leads to the release of neurotransmitters like substance P and CGRP (calcitonin gene-related peptide) from the nerve endings [23,24,25]. These neurotransmitters contribute to the transmission of pain signals to the central nervous system, where they are perceived as pain. The upregulation of TRPV1 receptors in response to the pro-inflammatory signals from damaged tissues leads to the production of substance P and calcitonin gene-related peptide (CGRP) by peptidergic neurons [4]. Substance P causes pain, while CGRP causes the destruction of soft tissue structures, neurogenic inflammation, and inflammation of the surrounding tissues. The nervi nervorum, which are the nerves that supply other nerves, can release neurodegenerative peptides into C-fibers under pathological conditions [26].
Nerve irritation can occur through repetitive muscle contractions and rapid changes in the direction of sensory nerves traveling between the muscles and fascia. TRPV-1 makes nerves vulnerable to irritation or trauma. Irritation of the superficial nerves, which supply cutaneous sensation to the joint, can cause the transmission of ectopic impulses in the anterior and retrograde directions. Bulbar transmission leads to pain perception through the somatosensory cortex and generation of reflex muscle twitches through the spinal ventral horn cells. Reverse propagation towards blood vessels releases substance P [27]. Damage to the superficial nerves can affect deeper structures according to Hilton's law, which states that the nerves supplying a joint supply both the muscles that move the joint and the skin that covers the joint insertions of these muscles because of embryonic development [28]. Cutaneous nerve trauma can cause nerve swelling both proximal and distal to the injury. Swelling along a traumatized cutaneous nerve can reach the fascial penetration point of the nerve and suffocate the affected nerve as it passes through the fascial transition zone, creating a CCI point. This constriction inhibits the flow of nerve growth factors, which are essential for nerve health and repair [29].
PIT may affect mainly to a TRPV1. In 1997, Caterina et al. [30] announced that the capsaicin receptor is an ion channel activated by heat in the pain pathway. This capsaicin receptor can be represented by transient receptor potential vanilloid 1 (TRPV1), which is known to be an important element of peripheral nociception. In addition, Palazzo et al. [31] reported in TRPV1 and pain development that they are activated by intrinsic inducers in inflammatory pain conditions and will control neuropathic pain conditions.
In a study by Bove and Light [32], peripherin-like immunostaining was observed in the nervi nervorum and vasa nervorum of the rat sciatic nerve, which was associated with blood vessels. It was found that it reached the epineurium and was also associated with the adipose tissue.
TRPV1 receptor is in the nervi-nervorum of the peripheral nervous system, and it was found that it induces the Na+ & Ca++ influx in the nerve cells. When Na+ influx occurs, spike formation and action potential increase electrically, causing neuropathic pain. Ca++ influx releases neuropeptides, such as substance P and calcitonin gene related peptide, resulting in neurogenic inflammation. Using this mechanism, it will be possible to control the pain of many current patients with neuropathic pain [23,24,25].
A nerve cell, like other cells in the body, requires nutrients to keep it healthy, including glucose. When a nerve is injured, inflamed, and lacks glucose, it sends continuous signals that the body interprets as pain. PIT with buffered 5% dextrose is injected near the superficial nerves. Dextrose, when used in PIT, is believed to work by feeding and hydrating the injured nerve and reducing the inflammation around the nerves, thereby alleviating pain in PHN, TN, and NP. The effectiveness of buffered 5% dextrose for pain control can vary depending on the individual and the specific condition being treated. Research and clinical studies have shown promising results for dextrose injections in managing certain types of pain, such as neuropathic pain, chronic joint pain, or musculoskeletal issues. In 2018, it was reported that significant pain relief was achieved by the intrathecal administration of buffered dextrose [33]. After evaluating the effect for six months in patients with carpal tunnel syndrome, it was said that a meaningful effect was achieved [16].
In this study, the pain scores of PHN, TN, and PN were reduced after PIT with dextrose; however, the pain of CRPS did not show any pain reducing effect after PIT with dextrose or lidocaine. Neurogenic inflammation plays an important role in the development of CRPS and is the basis for managing CRPS with PIT [34]. As a study targeting patients with neuropathic pain, there was a report targeting patients with CRPS, showing good effects [35]. Neuropathic pain, which manifests abnormal sensations and hypersensitivity to non-painful stimuli, such as CRPS, goes through a nerve modulation process called peripheral sensitization and central sensitization. Among them, central sensitization is mainly achieved through various mechanisms in the dorsal horn of the spinal cord. In most cases, it occurs through increased excitatory synaptic activity due to the excessive excitation of primary afferent nerves or increased nerve conduction connectivity, such as sprout growth. Those multimodal mechanism of CRPS may affect why PIT did not work in CRPS in this study.
Repetitive muscle dysfunction also changes the myofascial tension and creates chronic constriction injury [36]. Glucose reduces the neurogenic inflammation by binding to the presynaptic calcium channels and inhibiting the release of neurodegenerative peptides. The results are pain reduction, regression of soft tissue edema, relief of chronic constriction injury, restoration of normal nerve growth factor flow, acceleration of nerve repair, and today provide almost immediate analgesic effects that last for several hours [37,38]. In this study, repeated PIT resulted in a stepwise decrease in pain, except CRPS like Figs. 1 and 2.
This study has a limitation. Notably, there were not enough number of patients in the Lidocaine group for statistical analysis. Carrying out of a statistical analysis to discern the differences between the Dextrose group and the Lidocaine group was also not feasible. However, some patients in the Lidocaine group experienced a dramatic reduction in the pain. Thus, its effect could not be completely ruled out. Therefore, further investigation with enough number of patients in each group is required. The other was lack of a long-term follow-up to see the effectiveness of PIT. Due to a limited study time, there was only two weeks after the final PIT to evaluate in this study.
From these results, it is suggested that PIT with buffered 5% dextrose may be a good method to relieve a pain without any side effects for PHN, TN, and NP, and not for CRPS. No side effects occurred, and client satisfaction of the procedure was good. Further clinical acceptance and research into this treatment modality will help advance the understanding of the role of neurogenic inflammation in neuropathic pain conditions. This treatment modality may hold promise for more effective pain management.
ACKNOWLEDGEMENT
The study was funded by the Basic Research Support Project of Pusan National University.
Footnotes
- Haekyu Kim: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing.
- Hyae Jin Kim: Methodology, Resources, Software.
- Young-Hoon Jung: Visualization, Writing – original draft.
- Wangseok Do: Data curation, Resources, Visualization, Writing – original draft.
- Eun-Jung Kim: Data curation, Methodology, Resources.
DECLARATION OF INTEREST: The authors declare that they have no conflicts of interest.
References
- 1.Smith PA. Neuropathic pain; what we know and what we should do about it. Front Pain Res (Lausanne) 2023;4:1220034. doi: 10.3389/fpain.2023.1220034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park HJ, Moon DE. Pharmacologic management of chronic pain. Korean J Pain. 2010;23:99–108. doi: 10.3344/kjp.2010.23.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riediger C, Schuster T, Barlinn K, Maier S, Weitz J, Siepmann T. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol. 2017;8:307. doi: 10.3389/fneur.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldman SD. Pain Management. 2nd ed. Saunders Elsevier; 2011. [Google Scholar]
- 5.Geppetti P, Holzer P. Neurogenic Inflammation. Taylor & Francis; 1996. [Google Scholar]
- 6.Lyftogt J. Subcutaneous prolotherapy for achilles tendinopathy: the best solution? Australasian Musculoskeletal Medicine. 2007;12:107–109. [Google Scholar]
- 7.Hashimoto K, Sakura S, Bollen AW, Ciriales R, Drasner K. Comparative toxicity of glucose and lidocaine administered intrathecally in the rat. Reg Anesth Pain Med. 1998;23:444–450. doi: 10.1016/s1098-7339(98)90025-6. [DOI] [PubMed] [Google Scholar]
- 8.Sakura S, Chan VW, Ciriales R, Drasner K. The addition of 7.5% glucose does not alter the neurotoxicity of 5% lidocaine administered intrathecally in the rat. Anesthesiology. 1995;82:236–240. doi: 10.1097/00000542-199501000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Tsui BCH, Kropelin B. The electrophysiological effect of dextrose 5% in water on single-shot peripheral nerve stimulation. Anesth Analg. 2005;100:1837–1839. doi: 10.1213/01.ANE.0000153020.84780.A5. [DOI] [PubMed] [Google Scholar]
- 10.Dufour E, Donat N, Jaziri S, Kurdi O, Couturier C, Dreyfus JF, et al. Ultrasound-guided perineural circumferential median nerve block with and without prior dextrose 5% hydrodissection: a prospective randomized double-blinded noninferiority trial. Anesth Analg. 2012;115:728–733. doi: 10.1213/ANE.0b013e31825fa37d. [DOI] [PubMed] [Google Scholar]
- 11.Yoshii Y, Zhao C, Schmelzer JD, Low PA, An KN, Amadio PC. The effects of hypertonic dextrose injection on connective tissue and nerve conduction through the rabbit carpal tunnel. Arch Phys Med Rehabil. 2009;90:333–339. doi: 10.1016/j.apmr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshii Y, Zhao C, Schmelzer JD, Low PA, An KN, Amadio PC. Effects of hypertonic dextrose injections in the rabbit carpal tunnel. J Orthop Res. 2011;29:1022–1027. doi: 10.1002/jor.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MY, Na YM, Moon JH. Comparison on treatment effects of dextrose water, saline, and lidocaine for trigger point injection. Ann Rehabil Med. 1997;21:967–973. [Google Scholar]
- 14.Weglein AD. Neural Prolotherapy. Journal of Prolotherapy. 2011;3:639–643. [Google Scholar]
- 15.Conaway E, Browning B. Neural prolotherapy for neuralgia. Journal of Prolotherapy. 2014;6:e928–e931. [Google Scholar]
- 16.Wu YT, Ho TY, Chou YC, Ke MJ, Li TY, Tsai CK, et al. Six-month efficacy of perineural dextrose for carpal tunnel syndrome: a prospective, randomized, double-blind, controlled trial. Mayo Clin Proc. 2017;92:1179–1189. doi: 10.1016/j.mayocp.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Yelland MJ, Sweeting KR, Lyftogt JA, Ng SK, Scuffham PA, Evans KA. Prolotherapy injections and eccentric loading exercises for painful achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421–428. doi: 10.1136/bjsm.2009.057968. [DOI] [PubMed] [Google Scholar]
- 18.Rabago D, Patterson JJ, Mundt M, Kijowski R, Grettie J, Segal NA, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229–237. doi: 10.1370/afm.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand H, Kyriazis M, Reeves KD, Lyftogt J, Rabago D. Topical mannitol reduces capsaicin-induced pain: results of a pilot-level, double-blind, randomized controlled trial. PM R. 2015;7:1111–1117. doi: 10.1016/j.pmrj.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Murakawa Y, Zhang W, Pierson CR, Brismar T, Ostenson CG, Efendic S, et al. Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab Res Rev. 2002;18:473–483. doi: 10.1002/dmrr.326. [DOI] [PubMed] [Google Scholar]
- 21.Zamami Y, Takatori S, Yamawaki K, Miyashita S, Mio M, Kitamura Y, et al. Acute hyperglycemia and hyperinsulinemia enhance adrenergic vasoconstriction and decrease calcitonin gene-related peptide-containing nerve-mediated vasodilation in pithed rats. Hypertens Res. 2008;31:1033–1044. doi: 10.1291/hypres.31.1033. [DOI] [PubMed] [Google Scholar]
- 22.Wei Z, Wang L, Han J, Song J, Yao L, Shao L, et al. Decreased expression of transient receptor potential vanilloid 1 impaires the postischemic recovery of diabetic mouse hearts. Circ J. 2009;73:1127–1132. doi: 10.1253/circj.cj-08-0945. [DOI] [PubMed] [Google Scholar]
- 23.Willis WD., Jr The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp Brain Res. 2009;196:5–11. doi: 10.1007/s00221-009-1760-2. [DOI] [PubMed] [Google Scholar]
- 24.Brito R, Sheth S, Mukherjea D, Rybak LP, Ramkumar V. TRPV1: a potential drug target for treating various diseases. Cells. 2014;3:517–545. doi: 10.3390/cells3020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyftogt J. Subcutaneous prolotherapy treatment of refractory knee, shoulder, and lateral elbow pain. Australasian Musculoskeletal Medicine. 2007;12:110–112. [Google Scholar]
- 27.Pybus P Rheumatoid Disease Foundation, (U. S.) Intraneural injections for rheumatoid arthritis and osteoarthritis: and reprint for physicians and layman, the control of pain in arthritis of the knee. Rheumatoid Disease Foundation; 1988. [Google Scholar]
- 28.Hilton J. The classic: on rest and pain: lecture XIV. Clin Orthop Relat Res. 2009;467:2208–2214. doi: 10.1007/s11999-009-0927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 30.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 31.Palazzo E, Luongo L, de Novellis V, Rossi F, Marabese I, Maione S. Transient receptor potential vanilloid type 1 and pain development. Curr Opin Pharmacol. 2012;12:9–17. doi: 10.1016/j.coph.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Bove GM, Light AR. Calcitonin gene-related peptide and peripherin immunoreactivity in nerve sheaths. Somatosens Mot Res. 1995;12:49–57. doi: 10.3109/08990229509063141. [DOI] [PubMed] [Google Scholar]
- 33.Maniquis-Smigel L, Reeves KD, Rosen HJ, Lyftogt J, Graham-Coleman C, Cheng AL, et al. Analgesic effect and potential cumulative benefit from caudal epidural d5w in consecutive participants with chronic low-back and buttock/leg pain. J Altern Complement Med. 2018;24:1189–1196. doi: 10.1089/acm.2018.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci Lett. 2008;437:199–202. doi: 10.1016/j.neulet.2008.03.081. [DOI] [PubMed] [Google Scholar]
- 35.Thor JA, Mohamed Hanapi NH, Halil H, Suhaimi A. Perineural injection therapy in the management of complex regional pain syndrome: a sweet solution to pain. Pain Med. 2017;18:2041–2045. doi: 10.1093/pm/pnx063. [DOI] [PubMed] [Google Scholar]
- 36.Buntragulpoontawee M, Chang KV, Vitoonpong T, Pornjaksawan S, Kitisak K, Saokaew S, et al. The effectiveness and safety of commonly used injectates for ultrasound-guided hydrodissection treatment of peripheral nerve entrapment syndromes: a systematic review. Front Pharmacol. 2021;11:621150. doi: 10.3389/fphar.2020.621150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu YT, Wu CH, Lin JA, Su DC, Hung CY, Lam SKH. Efficacy of 5% dextrose water injection for peripheral entrapment neuropathy: a narrative review. Int J Mol Sci. 2021;22:12358. doi: 10.3390/ijms222212358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen LC, Ho TY, Shen YP, Su YC, Li TY, Tsai CK, et al. Perineural dextrose and corticosteroid injections for ulnar neuropathy at the elbow: a randomized double-blind trial. Arch Phys Med Rehabil. 2020;101:1296–1303. doi: 10.1016/j.apmr.2020.03.016. [DOI] [PubMed] [Google Scholar]