Abstract
Objective:
Reduction of cerebral ischemia-reperfusion injury (IRI)/re-oxygenation injury, is defined as the paradoxical exacerbation of the cellular dysfunction and death, following restoration of the blood flow to previously ischemic tissues. The re-establishment of blood flow is essential to salvage the ischemic tissues. As a result, the treatment of IRI with novel therapies, which have fewer side effects, are of great importance. Therefore, this study aimed to investigate the effects of curcumin nanoparticle (CN) pre-treatment on the cerebral I/R rat model.
Materials and Methods:
In this experimental study, CN was administered to rats orally five days before the bilateral common carotid artery occlusion (BCCAO) and continued for three days. The intensity of oxidative stress, the activities of antioxidant enzymes, glutathione (GSH) content, the activity of mitochondrial enzymes, including succinate dehydrogenase (SDH), malate dehydrogenase (MDH) and lactate dehydrogenase (LDH), curcumin bioavailability, pERK/ERK expression ratio and TFEB protein were studied. Data analysis was performed using Graphpad Prism V.8 software, one-way analysis of variance (ANOVA) with the statistical package for the social sciences (SPSS V.26 software).
Results:
Cerebral IRI-damage significantly increased the oxidative stress (P=0.0008) and decreased the activity of the antioxidant enzymes including catalase (CAT) (P<0.001), super oxide dismutase (SOD) (P<0.001), reduced GSH (P<0.001), mitochondrial enzymes, pERK/ERK expression ratio (P=0.002) and TEFB protein (P=0.005) in rats’ brains. In addition, the pre-treatment of the rats with CN resulted in a decrease in the reactive oxygen species (ROS), and an increase in the activities of antioxidants and mitochondrial enzymes. This in turn up-regulated the pERK/ERK expression ratio and TEFB expression.
Conclusion:
CN has neuroprotective effects on the cerebral IRI condition due to its antioxidant properties and is able to overexpress the pERK and TFEB proteins; thus, it can be considered as a suitable treatment option during and after the incidence of stroke.
Keywords: Curcumin, Ischemia, Lactate Dehydrogenase, Malate Dehydrogenase, Succinate Dehydrogenase
Introduction
Ischemia-reperfusion injury (IRI)/re-oxygenation injury damage following brain ischemic stroke leads to worsening of the condition and reduces the survival rate (1). During the IRI damage, large amounts of free radicals are generated and the brain is exposed to oxidative stress (2). Unfortunately, despite many advances in reducing the IRI damage, no effective drug has yet been found to treat this disease. Recently, however, the use of antioxidants has been suggested as a suitable new strategy because of their neuroprotective effects (3).
Curcumin, with the chemical formula of C21H20O6, is the active ingredient in the rhizome of turmeric plant Curcuma longa, and it is a dietary compound with potent antiinflammatory, antioxidant, and anti-apoptotic properties. Nonetheless, due to its low aqueous solubility, low bioavailability, and rapid first-pass hepatic metabolism, curcumin is limited in therapeutic applications (4). It has been stated that this compound plays an important role in protecting against oxidative damage by scavenging the activity of reactive oxygen species (ROS) (5). Its antioxidant effect is attributed to the phenolic groups or the CH2 group of the β-diketone moiety. In addition, its neuroprotective effects against ischemic brain injury have been reported. However, the blood brain barrier (BBB) appears to prevent free curcumin (FC) from reaching the brain to exert its effects, which in turn reduces the bioavailability of this molecule (6). One possible way to bypass the BBB is the formulation of curcumin nanoparticles, which are proposed to have the capability of crossing the BBB and therefore increasing the concentration of curcumin in the brain.
The mitogen activated protein kinases (MAPK) signaling pathway has been extensively studied in recent years. This pathway plays a critical role in signal transduction in enzymes ranging from protein kinases to protein phosphatases. The most well-known kinase in this pathway is the protein kinase RNA-like ER kinase (ERK). The ERK signaling pathway contains important modulators that are involved in the growth, proliferation, differentiation, aging, and apoptosis of the cells (7). This pathway is activated by growth factors, oxidative stress, an increase in the intracellular calcium level and the stimulation of glutamate receptors. The absence of oxygen leads to ERK up-regulation (8). This kinase is phosphorylated by a three-part MAPK activity, which is required for the inhibition of skeletal muscle differentiation by insulin-like growth factor 1 or fibroblast growth factor 2. In addition, ERK usually increases after the cerebral IRI injury and this may be either protective or destructive (9). Inhibition of ERK phosphorylation stops ischemic injury (10). It has been shown that ERK activation protects neurons under pathological conditions, such as seizures, Alzheimer’s disease, cerebral ischemia, or deprivation of neurotrophic factors (11).
The role of transcription factor EB protein (TFEB) as a critical regulator in the autophagy process is well documented (12). Likewise, TFEB is a branch of the unfolded protein response that confers neuroprotection in ischemic stroke by suppressing protein synthesis. Studies also show that TFEB has beneficial effects on degenerative disorders such as Huntington’s and Parkinson’s diseases (13). Furthermore, the anti-apoptotic role of TFEB in the heart, as well as inhibition of atherosclerosis are also reported (14). TFEB is shown to affect endothelial cell function and regulate angiogenesis after ischemia (15).
This study aimed to investigate the antioxidant and neuroprotection effects of the curcumin nanoparticles (CN) and their impacts on mitochondrial enzymes and ERK and TFEB signaling pathways in the ischemic brains of rats. The reason for investigating the expression levels of TFEB and ERK proteins was their involvement in the autophagy pathway.
Materials and Methods
Animals
For this experimental study, specific pathogen-free (SPF) male Wistar rats, aged 30-45 days and weighed approximately 250 to 300 g, were obtained from the Pasteur Institute of Iran. The animals were kept at 25 ± 1.5°C and 65% relative humidity with a 12-hour dark/ light cycle condition and free access to water and food. Before starting the experiments, the animals were allowed to adapt to the laboratory environment. All experimental protocols were approved by the Ethics Committee of the Tehran Medical Sciences Department of the Islamic Azad University (IR.IAU.TNB.REC.1400.022).
Materials
CN were purchased from the Exir Nano Sina Company, Iran. Other materials were of analytical grade and purchased from the Merck Company, Germany.
Induction of the cerebral ischemia/reperfusion
The induction of the cerebral stroke was conducted by the bilateral common carotid artery occlusion (BCCAO) method (16). Firstly, the rats were anesthetized by ketamine (50 mg/kg, Cat# 1867-66-9) and xylazine (2-8 mg/kg, Cat# 23076-35-9), a sagittal incision was made through the neck midline (1 cm in length), and then both carotid arteries were carefully separated from the respective vagal nerves. A 5-0 silk suture loop was made around each CCA, and then all CCAs were occluded by clamps for 10 minutes followed by a 72-hour reperfusion. After the reperfusion step, the wounds were sutured. The sham group underwent surgery without BCCAO. During this period, the animals were examined for rectal temperature of 37°C, a cornea reflex on exposure to light and maintenance of dilated pupils. Animals that did not meet these criteria or developed seizures were excluded from the study. After the period of reperfusion, the animals were sacrificed to remove their brains.
Animals grouping
A total of 48 rats were divided into 5 groups: Group I: Healthy control rats (n=9), Group II: Sham rats (the animals underwent surgery without BCCAO) (n=9), Group III: Cerebral IRI rats (n=10), Groups IV: Cerebral IRI rats receiving FC orally through gavage everyday (25 mg/kg, n=10), and Group V: Cerebral IRI rats receiving 25 mg/kg CN orally through gavage everyday (n=10). FC and CN administrations were started 5 days before the induction of the cerebral IRI and continued until three days after the BCCAO.
Separation of the mitochondria from the brain
Brain homogenization was performed according to the method of Navarro and Boveris (17), using a buffer containing 10 mM KCl, 1.5 mM MgCl2, 0.1 mM Phenylmethylsulfonyl fluoride (PMSF), 20 mM Hepes- KOH and protease inhibitor. The prepared homogenate was centrifuged at 1000 g for 5 minutes at 4°C and then the supernatant was centrifuged at 35000 g for 60 minutes. The resulting pellet containing mitochondria was collected and re-suspended in the same buffer.
The bioavailability of free curcumin and curcumin nanoparticles
After the administration of the last doses of FC or CN, the brain homogenates were prepared at 1, 2, 4, 8, 16, 24, 48, and 72 hour(s) post-treatment to measure the brain concentrations of the FC and CN in the rats. According to the method of Shinde and Devarajan (18), curcumin was extracted from the brain homogenates using the coupled high-performance liquid chromatography (HPLC) with 2487 Dual Absorbance Detector and Waters 515 Pump Reversed Phase C18 Column. The peaks were read at 425 nm.
Interestingly, HPLC has become a routine tool for the separation of complex mixtures. However, the ability to obtain structural information on substances separated using HPLC is limited by the online detector systems and, in most applications, full structural elucidation is performed off-line following separation.
Lipid peroxidation, reactive oxygen species level, succinate dehydrogenase, lactate dehydrogenase and malate dehydrogenase assays
Malondialdehyde (MDA) content, as an indicator of lipid peroxidation, was determined using the method explained by Dhindsa et al. (19). Conversion of the cellpermeant 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) (also known as dichlorofluores cin diacetate) to DCFDA, is used to detect the generation of reactive oxygen intermediates in cells. In the current study, the conversion of H2DCFDA to DCFDA, which detected by spectrometers at 499 nm and 520 nm, respectively, was used as an indicator to measure the ROS levels in brain mitochondria after normalization. The ROS content was expressed as percentages (20).
The measurements of succinate dehydrogenase (SDH) (EC 1.3.5.1, Succinate Dehydrogenase Assay Kit, Cat#MAK197), MDA (EC 1.1.1.37, Malate Dehydrogenase Activity Assay Kit, Cat# MAK196) and lactate dehydrogenase (LDH) (EC 1.1.1.27, LDH Assay kit, Cat# MAK066) were performed using the Sigma- Aldrich kits (St. Louis, Missouri, United States) according to the manufacturer’s instructions.
The measurement of catalase, reduced glutathione and superoxide dismutase activities
The measurement of glutathione (GSH) content was done by the method of Jollow et al. (21); the SOD activity was evaluated by the Kono’s method (22) and the catalase (CAT) activity was estimated by the Greenwald’s method (23).
Western blot analysis
The brain tissue was isolated from the rats, and subsequently rinsed with cold phosphate-buffered saline (PBS, pH=7.4), and then crushed and homogenized with 1 ml radio immunoprecipitation assay (RIPA) buffer (Cat#ab206996, Abcam, Cambridge, UK) supplemented with 1% protease inhibitor (Cat#P8340, Sigma, St. Louis, Missouri, USA). After centrifugation at 13000g for 15 minutes, the supernatant was collected and the protein concentration was determined using the BCA Kit (Cat#23227, Thermo, Waltham, Massachusetts, USA). 60 μg of the total protein was electrophoresed on 10% polyacrylamide gel at a constant voltage of 80 V for 35 minutes and then 120 V for 45 minutes. The electrophoresed proteins were then transferred to the Polyvinylidene Flouride (PVDF) paper and incubated with ERK (Cat#9102, Cell Signaling, Danvers, Massachusetts, USA), TFEB (Cat# 4240, Cell Signaling, Danvers, Massachusetts, USA) and beta-actin (β-actin) monoclonal antibodies at 4°C overnight. PBS tween-20 (PBST) solution containing 0.1% Tween-20 was used to wash the membrane and then horseradish peroxidase rabbit (HRP-rabbit) antirat secondary antibody (1:10000) against the primary antibodies was incubated on shaker for 190 minutes and washed again. Finally, by adding the Electrochemical Luminescence (ECL) (Cat# WBULS0100, Sigma, St. Louis, Missouri) to PVDF paper, the presence of ERK and TFEB proteins in the samples were investigated. In addition to the samples, the negative control sample (without the primary antibodies) was also electrophoresed with the same protein concentrations. Western blot results were analyzed using the Image-Pro Plus
Statistical analysis
Data analyzing and figure plotting were carried out using the Graphpad Prism V.8 software (Dotmatics, USA). One way analysis of variance (ANOVA) unpaired t test was used to analyze the data (n=6) and post hoc test was done based on the Tukey’s and Duncan’s multiple range tests at a significant level of P<0.05. Data was presented as mean ± SEM.
Results
Bioavailability of curcumin in the brain
The amounts of FC and CN were measured in the rat brain homogenates over time and the outcome showed an increase in the concentration of curcumin in the brain homogenates up to 8 hours after administration. No curcumin was found in the brain homogenates past 48 or 72 hours of curcumin administration. However, the concentration of CN was higher in the brain homogenates compared to the FC and showed an increasing trend for up to 24 hours. After 24 hours, a decreasing trend of CN concentration was observed. However, the presence of CN was observed in the brain homogenates 72 hours after administration (Table 1).
Table 1.
The concentration-time bioavailability of FC and CN in the rat brain homogenate (25 mg/kg, n=3)
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 1 hour | 2 hours | 4 hours | 8 hours | 16 hours | 24 hours | 48 hours | 72 hours |
|
| ||||||||
| FC | 0.12 ± 0.01 | 0.16 ± 0.03 | 0.29 ± 0.04 | 0.68 ± 0.06 | 0.26 ± 0.03 | 0.09 ± 0.05 | Undetectable | Undetectable |
| NC | 1.22 ± 0.11# | 1.89 ± 0.19# | 3.11 ± 0.53# | 4.25 ± 0.62# | 10.88 ± 0.86# | 14.75 ± 0.93# | 5.85 ± 0.37# | 1.78 ± 0.33# |
|
| ||||||||
Data are presented as mean ± SD. FC; Free curcumin, CN; Curcumin nanoparticles, and #; Shows significant differences, based on the unpaired t test at the probability level of P<0.0001.
Mitochondria function
The ROS were overproduced in the cerebral IRI rats. However, a sharp decrease in the ROS production was observed in the cerebral IRI rats receiving CN compared to rats receiving FC. Therefore, it seems that CN is very effective in reducing oxidative stress due to IRI damage. High lipid peroxidation was observed in the cerebral IRI group compared to the control group, indicating damage to the mitochondrial membrane due to the cerebral IRI. However, administration of CN significantly reduced the lipid peroxidation of mitochondrial membranes, and therefore, CN appeared to have stronger protective effects against the cerebral IRI damage compared to FC. Induction of the cerebral IRI in the rats lowered the activities of SDH, MDH and LDH enzymes, which indicates decreased respiratory activity in these rats. However, pre-treatment of the rats with CN increased the activities of these enzymes (Table 2).
Table 2.
The effects of FC and CN on the mitochondria ROS generation, activities of SDH, MDH and LDH in the rat brain homogenate (25 mg/kg, n=5)
|
| |||||
|---|---|---|---|---|---|
| Group | ROS | Lipid peroxidation (µM) | SDH activity (U/mg protein/minutes) | MDH activity (U/mg protein/minutes) | LDH activity (U/mg protein/minutes) |
|
| |||||
| Control | 106 ± 5c | 3.23 ± 0.56b | 5.48 ± 0.53a | 5.98 ± 0.37a | 5.07 ± 0.54a |
| Sham | 112 ± 6c | 3.44 ± 0.63b | 5.42 ±0.58a | 5.84 ±0.32a | 4.85 ± 0.51a |
| Cerebral IRI | 274 ± 18a | 6.23 ±0.78a | 2.88 ± 0.28b | 3.42 ± 0.21c | 3.27 ± 0.25c |
| FC | 243 ± 16b | 5.78 ± 0.73a | 3.42 ± 0.31b | 3.88 ± 0.34b | 3.58 ± 0.39b |
| NC | 131 ± 14c | 3.65 ± 0.53b | 5.22 ± 0.43a | 5.42 ± 0.44ab | 4.86 ± 0.42a |
|
| |||||
Data are presented as mean ± SD. Different letters show significant differences, based on the Duncan’s multiple range test, Sham group underwent surgery without BCCAO. FC; Free curcumin at the concentration of 25 mg/kg, CN; Curcumin nanoparticles at the concentration of 25 mg/kg), ROS; Reactive oxygen species, SDH; Succinate dehydrogenase, MDH; Malate dehydrogenase, LDH; Lactate dehydrogenase, BCCAO; Bilateral common carotid artery occlusion, and IRI; Ischemia-reperfusion injury.
Antioxidant enzyme activities and reduced glutathione content
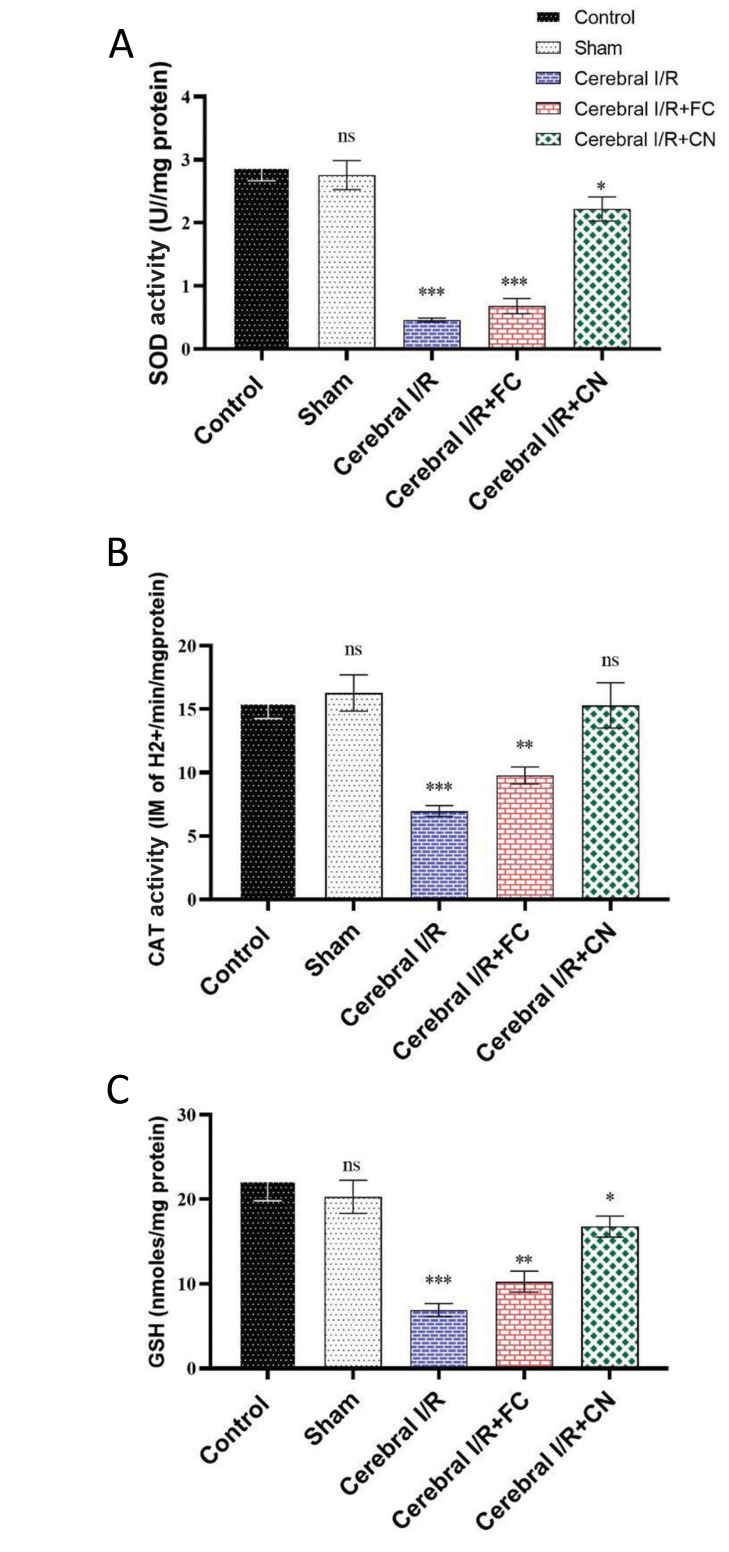
A sharp reduction in the activity of antioxidant enzymes CAT and superoxide dismutase, as well as a decrease in the reduced GSH content due to the cerebral IRI damage, were observed in the rats (P<0.001). However, pre-treatment of CN in the rats with the cerebral IRI recovered the activities of these enzymes to their levels in the healthy control rats, indicating a significant amplification in the antioxidant capacity and an increase in the GSH content, due to the CN administration (Fig .1).
Fig.1.
The changes in anti-oxidant enzymes activities in the rats’ brain after pretreatment with 25/mg/kg FC and CN (n=6). A. SOD, B. CAT, and C. Reduced GSH. FC; Free curcumin, CN; Curcumin nanoparticles, SOD; Superoxide dismutase, CAT; Catalase, GSH; Glutathione, BCCAO; Bilateral common carotid artery occlusion, IRI; Ischemia/reperfusion injury, ns; Not significant, *, **, and ***; Significant differences at the probability levels of P<0.05, P<0.01 and P<0.001, respectively. Sham group underwent surgery without BCCAO.
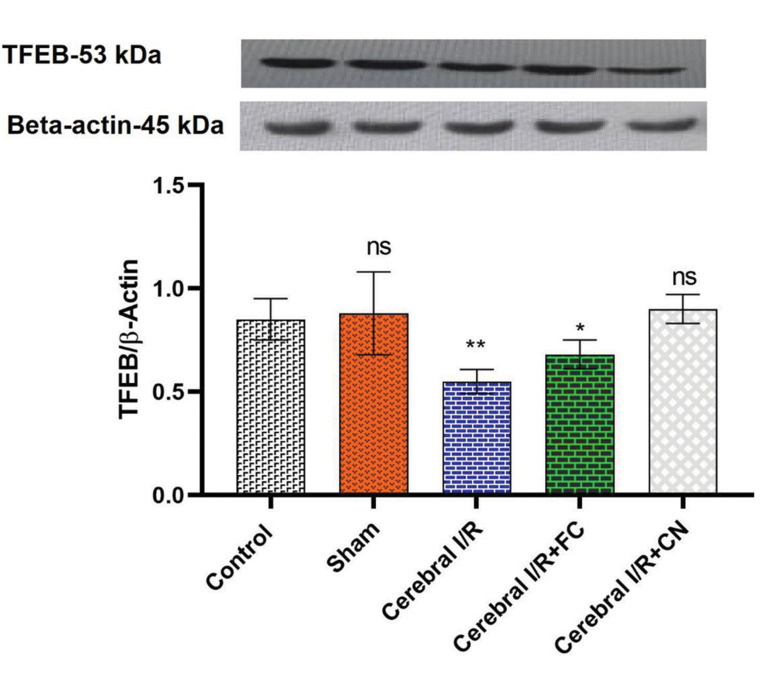
TFEB and pERK/ERK protein expression levels
Induction of the IRI brain injury significantly downregulated the TFEB protein expression in the rat brain compared with the control group (P=0.005). FC and CN both showed positive effects on the TFEB over expression, so that the expression of TFEB protein in the brains of rats receiving CN did not show a significant difference, compared with the control group. Therefore, our data confirm that CN has the ability to up-regulate the TFEB protein expression insignificantly (Fig .2).
Fig.2.
The expression levels of the TFEB/β-Actin proteins in the rat brain homogenate pre-treated with FC (25 mg/kg) and CN (25 mg/kg). FC; Free curcumin, CN; Curcumin nanoparticles, BCCAO; Bilateral common carotid artery occlusion, IRI; Ischemia/reperfusion injury, ns; Not significant, *, and **; Significant differences at the probability levels of P<0.05 and P<0.01, respectively. Sham group underwent surgery without BCCAO.
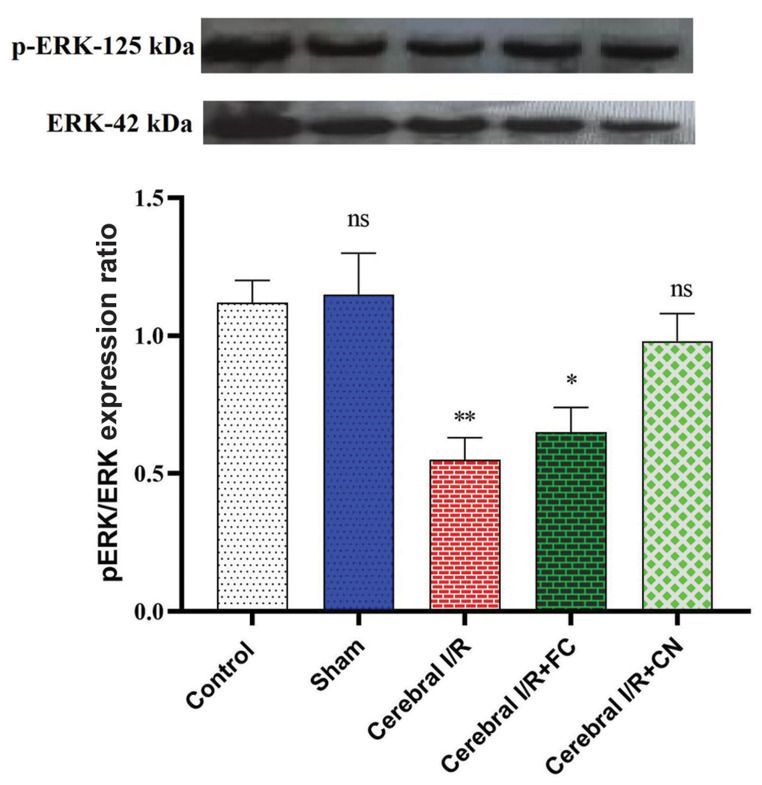
This study also showed that the induction of the cerebral IRI in rats leads to the dephosphorylation of the ERK protein. However, FC and CN administration increased the levels of pERK and subsequently lead to an increase in the pERK/ERK ratio (Fig .3). However, insignificant difference was observed in the brain tissues of the rats receiving only CN concerning the pERK/ERK expression ratio compared to the control group (P=0.243).
Fig.3.
The pERK/ERK expression ratio in the rat brain homogenate pretreated with FC (25 mg/kg) and CN (25 mg/kg), (n=3). FC; Free curcumin, CN; Curcumin nanoparticles, BCCAO; Bilateral common carotid artery occlusion, IRI; Ischemia/reperfusion injury, ns; Not significant, *, and **; Significant differences at the probability levels of P<0.05 and P<0.01, respectively. The sham group underwent surgery without BCCAO.
Discussion
This study aimed to investigate the antioxidant and neuroprotection effects of CN, and its effects on the mitochondrial enzymes, ERK and TFEB signaling pathways in ischemic brains of rats, as IRI models. This study investigated the effects of pretreatment with FC and CN on the cerebral IRI in rats. The results showed promising positive effects of CN in reducing oxidative stress, increasing the activities of antioxidant enzymes and GSH content, recovering SDH, LDH and MDH activities in mitochondria and overexpressing TFEB proteins and increasing pERK/ERK ratio in the brain.
Increased oxidative stress in IRI is commonly reported in different studies and especially during the reperfusion stage, high levels of damage due to oxidative stress is very likely (24). Therefore, pretreatment with antioxidant compounds can be very effective in reducing the fatal effects of the oxidative stress in the reperfusion phase (25). The protective role of curcumin against ischemic injury is reported in previous studies. However, due to the low bioavailability of this compound, its use has been limited (26). Therefore, due to their small sizes, the use of curcumin nanoparticles may be a solution to this problem. Numerous studies have shown that curcumin nanoparticles successfully cross the BBB (27). This study showed that when CN was given to the rats, even 48 hours after administration, significant amounts of curcumin were still found in the rat brain homogenate samples. This confirms both CN passage through the BBB as well as sustained release of curcumin from the CN. Therefore, CN can be considered as a good therapeutic approach in prevention of oxidative stress induced by the cerebral IRI.
Increased oxidative stress in IRI is commonly reported in different studies and especially during the reperfusion stage, high levels of damage due to oxidative stress is very likely (24). Therefore, pretreatment with antioxidant compounds can be very effective in reducing the fatal effects of the oxidative stress in the reperfusion phase (25). The protective role of curcumin against ischemic injury is reported in previous studies. However, due to the low bioavailability of this compound, its use has been limited (26). Therefore, due to their small sizes, the use of curcumin nanoparticles may be a solution to this problem. Numerous studies have shown that curcumin nanoparticles successfully cross the BBB (27). This study showed that when CN was given to the rats, even 48 hours after administration, significant amounts of curcumin were still found in the rat brain homogenate samples. This confirms both CN passage through the BBB as well as sustained release of curcumin from the CN. Therefore, CN can be considered as a good therapeutic approach in prevention of oxidative stress induced by the cerebral IRI.
In this study, decreases in the activities of SDH, MDH and LDH enzymes were observed after the occurrence of cerebral IRI in the rat brain mitochondria, indicating impairment in the electron transfer chain and oxidative phosphorylation. However, in pre-treated rats with CN, slight decreases in the activities of these enzymes were observed, suggesting a protective role for FC from the mitochondrial respiratory function after the I/R injury. The cerebral I/R damage leads to mitochondrial dysfunction, and ROS produced by complexes I and II plays an important role in this damage (32). Our findings also revealed that administration of CN to the rats prevented the reduction of SDH, MDH and LDH enzymes in the cerebral I/R rats. The antioxidant properties of curcumin and its role in increasing the activities of antioxidant enzymes appear to play an important role in prevention of the mitochondrial and respiratory chain damages. Impaired mitochondrial respiration is associated with the oxidative stress, and the results of this study showed that CN has a protective role on the mitochondrial function in cerebral I/R condition.
The Mitogen-activated protein kinases (MAPK) signaling pathway plays an important role in the signal transduction of protein kinases and phosphatases (33). The most important kinase of this pathway is ERK, which contains important modulators for cell growth and proliferation. Induction of the cerebral ischemia is reported to rapidly activate the MAPKs in different areas of the brain, causing phosphorylation and activation of ERKs, thus protecting neurons under the pathological conditions. The neuronal protection mechanism of ERK has been attributed to the activation of tyrosine kinase receptors, induction of B-cell lymphoma 2 (Bcl- 2), BCL2-associated agonist of cell death (Bad) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) expressions, or inhibition of cytochrome C release (34). A reduction in the pERK/ERK ratio was observed after reperfusion in the brain homogenates, indicating a decrease in the neuronal protection. However, CN significantly increased the pERK/ERK expression ratio, compared to the I/R group rats, suggesting a neuroprotective role for CN. Nonetheless, the role of ERK in the ischemia model has been controversial; ERK activity has been shown in both neuronal protection and worsening of the ischemic injury. It has been suggested that its activity may exacerbate inflammation and necrosis by up regulating the expression of interleukin-1beta (IL- 1β) (35).
Nevertheless, another report stated that ERK positively regulates caspase-3 expression in the brain after ischemia, leading to cell death (36). In the present study, we observed that up regulation of pERK and down regulation of ERK in the cerebral I/R rats receiving CN played an important role in protecting against neurodegeneration. However, more research is needed in this area to better understand the mechanism of this phenomenon. Elevated levels of phosphorylated ERK proteins have been reported following curcumin treatment in depressive conditions (37), indicating the anti-inflammatory effects of this secondary metabolite. However, since the neuroprotective effects of curcumin depends on crossing the BBB, size reduction due to the application of nano-formulation intensifies the protective effects of neurons. It is therefore reasonable to conclude that this experiment had a sound internal and external validity.
The main regulator of autophagy-lysosomal pathway (ALP) is TFEB, which upon expression leads to increased lysosome biogenesis. The therapeutic role of this protein has been reported in various studies, in all of which it has been stated that this protein has a protective effect on neurons by clearing proteins involved in the neurodegenerative diseases, such as Parkinson’s and Alzheimer’s diseases (38). The results of our study also showed that the expression levels of the TFEB protein were downregulated in the cerebral I/R rats. This is not a desirable sign, because this protein plays a role in lysosomal biogenesis and autophagy in the cerebral IRI conditions. Nevertheless, administration of CN was significantly up-regulated TFEB protein expression. As mentioned earlier, the role of TFEB in prevention of the accumulation of autophagosomes and protein clearance has been reported. Collectively, this protein is located inside the cytosol at the lysosomal surface, where it interacts with the mammalian target of rapamycin (mTOR) (39). Under stressful conditions, this protein is transported into the nucleus, where it activates the genes involved in autophagy, which is important in the survival of nerve cells due to the clearance of defective mitochondria (40). Therefore, up-regulation of the TFEB protein expression in the IRI rats receiving CN can be extremely important as it leads to increased autophagy of defective mitochondria.
The first limitation of this study, was the use of an animal model, which required clinical studies to confirm the protective effects of the CN and FC. The second was a limited sample size. The strength point of this study is the neuroprotective effects of the CN and FC in the cerebral IRI of the rats.
Conclusion
The results of this study suggest that CN has strong neuroprotective effects in the cerebral IRI conditions due to its antioxidant properties and ability to upregulate the pERK/ERK and TFEB protein expressions, and therefore, may be considered as a suitable adjuvant therapy option in stroke conditions.
Acknowledgments
The authors would like to express their sincere gratitude to the members of the Faculty of the Biological Sciences, Tehran Islamic Azad University, for their generous assistance. There is no financial support and conflict of interest in this study.
Author’s Contributions
Y.S.; Writing original draft, Methodology, and Software. M.E.; Writing-Reviewing and Supervision. M.M., M.T.; Software and Validation. All authors read and approved the final draft of the manuscript.
References
- 1.Zhang DM, Zhang T, Wang MM, Wang XX, Qin YY, Wu J, et al. TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic Biol Med. 2019;137:13–23. doi: 10.1016/j.freeradbiomed.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Janyou A, Wicha P, Jittiwat J, Suksamrarn A, Tocharus C, Tocharus J. Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Sci Rep. 2017;7(1):10556–10556. doi: 10.1038/s41598-017-11181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malekiyan R, Abdanipour A, Sohrabi D, Jafari Anarkooli I. Antioxidant and neuroprotective effects of lycopene and insulin in the hippocampus of streptozotocin-induced diabetic rats. Biomed Rep. 2019;10(1):47–54. doi: 10.3892/br.2018.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahams S, Haylett WL, Johnson G, Carr JA, Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: a review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Malik P, Singh M. Study of curcumin antioxidant activities in robust oil-water nanoemulsions. New J Chem. 2017;41(21):12506–12519. [Google Scholar]
- 6.Velásquez-Jiménez D, Corella-Salazar DA, Zuñiga-Martínez BS, Domínguez-Avila JA, Montiel-Herrera M, Salazar-López NJ, et al. Phenolic compounds that cross the blood-brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021;12(21):10356–10369. doi: 10.1039/d1fo02017j. [DOI] [PubMed] [Google Scholar]
- 7.Wang YC, Li X, Shen Y, Lyu J, Sheng H, Paschen W, et al. PERK (Protein Kinase RNA-Like ER Kinase) branch of the unfolded protein response confers neuroprotection in ischemic stroke by suppressing protein synthesis. Stroke. 2020;51(5):1570–1577. doi: 10.1161/STROKEAHA.120.029071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Liu L, Xie ZY, Wang F, Zhu L, Zhang C, et al. Protein kinase RNA-like ER kinase/eukaryotic translation initiation factor 2α pathway attenuates tumor necrosis factor alpha-induced apoptosis in nucleus pulposus cells by activating autophagy. J Cell Physiol. 2019;234(7):11631–11645. doi: 10.1002/jcp.27820. [DOI] [PubMed] [Google Scholar]
- 9.Xu D, Kong T, Zhang S, Cheng B, Chen J, Wang C. Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/ mTOR pathway. Cell Signal. 2021;79:109839–109839. doi: 10.1016/j.cellsig.2020.109839. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed SK, Ahmed AAE, Elmorsy EM, Nofal S. ERK activation by zeranol has neuroprotective effect in cerebral ischemia reperfusion. Life Sci. 2019;227:137–144. doi: 10.1016/j.lfs.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Wiciński M, Socha M, Malinowski B, Wódkiewicz E, Walczak M, Górski K, et al. Liraglutide and its neuroprotective properties-focus on possible biochemical mechanisms in alzheimer’s disease and cerebral ischemic events. Int J Mol Sci. 2019;20(5):1050–1050. doi: 10.3390/ijms20051050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nnah IC, Wang B, Saqcena C, Weber GF, Bonder EM, Bagley D, et al. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy. 2019;15(1):151–164. doi: 10.1080/15548627.2018.1511504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantiya P, Thonusin C, Chattipakorn N, Chattipakorn SC. Mitochondrial abnormalities in neurodegenerative models and possible interventions: focus on Alzheimer’s disease, Parkinson’s disease, Huntington’s disease. Mitochondrion. 2020;55:14–47. doi: 10.1016/j.mito.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhou P, Xie W, Luo Y, Lu S, Dai Z, Wang R, et al. Inhibitory effects of ginsenoside Rb1 on early atherosclerosis in ApoE-/- mice via inhibition of apoptosis and enhancing autophagy. Molecules. 2018;23(11):2912–2912. doi: 10.3390/molecules23112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Y, Lu H, Liang W, Garcia-Barrio MT, Guo Y, Zhang J, et al. Endothelial TFEB (Transcription Factor EB) positively regulates postischemic angiogenesis. Circ Res. 2018;122(7):945–957. doi: 10.1161/CIRCRESAHA.118.312672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saghari Y, Movahedi M, Tebianian M, Entezari M. The neuroprotective effect of betanin nanoparticles on brain ischemia-reperfusion injury. Animal Gene. 2023;27:200145–200145. [Google Scholar]
- 17.Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- 18.Shinde RL, Devarajan PV. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017;24(1):152–161. doi: 10.1080/10717544.2016.1233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32(1):93–101. [Google Scholar]
- 20.Batandier C, Fontaine E, Kériel C, Leverve XM. Determination of mitochondrial reactive oxygen species: methodological aspects. J Cell Mol Med. 2002;6(2):175–187. doi: 10.1111/j.1582-4934.2002.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzeneinduced liver necrosis.Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 22.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186(1):189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald RA. Handbook methods for oxygen radical research. 1st ed.Boca Raton. CRC Press; 2017. [Google Scholar]
- 24.Zhou H, Toan S. Pathological roles of mitochondrial oxidative stress and mitochondrial dynamics in cardiac microvascular ischemia/reperfusion injury. Biomolecules. 2020;10(1):85–85. doi: 10.3390/biom10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torki A, Khalaji-Pirbalouty V, Lorigooini Z, Rafieian-Kopaei M, Sadeghimanesh A, Rabiei Z. Anchusa italica extract: phytochemical and neuroprotective evaluation on global cerebral ischemia and reperfusion. BJPS. 2018;54(1):1–9. [Google Scholar]
- 26.Rahmati F. Microencapsulation of Lactobacillus acidophilus and Lactobacillus plantarum in Eudragit S100 and alginate chitosan under gastrointestinal and normal conditions. Appl Nanosci. 2020;10(9):391–399. [Google Scholar]
- 27.Barbara R, Belletti D, Pederzoli F, Masoni M, Keller J, Ballestrazzi A, et al. Novel curcumin loaded nanoparticles engineered for Blood- Brain Barrier crossing and able to disrupt Abeta aggregates. Int J Pharm. 2017;526(1-2):413–424. doi: 10.1016/j.ijpharm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Bodega G, Alique M, Puebla L, Carracedo J, Ramírez RM. Microvesicles: ROS scavengers and ROS producers. J Extracell Vesicles. 2019;8(1):1626654–1626654. doi: 10.1080/20013078.2019.1626654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raefsky SM, Furman R, Milne G, Pollock E, Axelsen P, Mattson MP, et al. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2018;66:165–176. doi: 10.1016/j.neurobiolaging.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X, Huang X, Gong Y, Xiao H, McClements DJ, Hu K. Enhancement of curcumin water dispersibility and antioxidant activity using core-shell protein-polysaccharide nanoparticles. Food Res Int. 2016;87:1–9. doi: 10.1016/j.foodres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Rahmati F, Hosseini SS, Mahuti Safai S, Asgari Lajayer B, Hatami M. New insights into the role of nanotechnology in microbial food safety. 3 Biotech. 2020;10(10):425–425. doi: 10.1007/s13205-020-02409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelova PR, Abramov AY. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592(5):692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 33.Klemm C, Bruchhagen C, van Krüchten A, Niemann S, Löffler B, Peters G, et al. Mitogen-activated protein kinases (MAPKs) regulate IL-6 over-production during concomitant influenza virus and Staphylococcus aureus infection. Sci Rep. 2017;7:42473–42473. doi: 10.1038/srep42473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM. Melatonin prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high glucose-treated schwann cells via upregulation of Bcl2, NF-κB, mTOR, Wnt signalling pathways. Antioxidants (Basel) 2019;8(7):198–198. doi: 10.3390/antiox8070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660–110660. doi: 10.1016/j.biopha.2020.110660. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D, Han S, Wang S, Luo Y, Zhao L, Li J. cPKCγ-mediated down-regulation of UCHL1 alleviates ischaemic neuronal injuries by decreasing autophagy via ERK-mTOR pathway. J Cell Mol Med. 2017;21(12):3641–3657. doi: 10.1111/jcmm.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan C, Li Y, Lan T, Wang W, Mao X, Yu SY. Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss. Food Funct. 2021;12(22):11202–11213. doi: 10.1039/d1fo02676c. [DOI] [PubMed] [Google Scholar]
- 38.Lindestam Arlehamn CS, Garretti F, Sulzer D, Sette A. Roles for the adaptive immune system in Parkinson’s and Alzheimer’s diseases. Curr Opin Immunol. 2019;59:115–120. doi: 10.1016/j.coi.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabanal-Ruiz Y, Korolchuk VI. mTORC1 and nutrient homeostasis: the central role of the lysosome. Int J Mol Sci. 2018;19(3):818–818. doi: 10.3390/ijms19030818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damme M, Suntio T, Saftig P, Eskelinen EL. Autophagy in neuronal cells: general principles and physiological and pathological functions. Acta Neuropathol. 2015;129(3):337–362. doi: 10.1007/s00401-014-1361-4. [DOI] [PubMed] [Google Scholar]