Abstract
Owing to the broad applications of quaternary ammonium salts (QAS), we present the synthesis of benzimidazolium-based analogues with variation in the alkyl and alkoxy group at N-1 and N-3 positions. All the compounds were characterized by spectroscopic techniques and found stable to air and moisture both in the solid and solution state. Moreover, molecular structures were established through single-crystal X-ray diffraction studies. The crystal packing of the compounds was stabilized by numerous intermolecular interactions explored by Hirshfeld surface analysis. The enrichment ratio was calculated for the pairs of chemical species to acquire the highest propensity to form contacts. Void analysis was carried out to check the mechanical response of the compounds. Furthermore, theoretical investigations were also performed to explore the optoelectronic properties of compounds. Natural population analysis (NPA) has been conducted to evaluate the distribution of charges on the synthesized compounds, whereas high band gaps of the synthesized compounds by frontier molecular orbital (FMO) analysis indicated their stability. Nonlinear optical (NLO) analysis revealed that the synthesized QAS demonstrates significantly improved NLO behaviour than the standard urea.
Keywords: benzimidazolium, quaternary ammonium salts, synthesis, single crystal and Hirshfeld surface, theoretical analysis
1. Introduction
Quaternary ammonium salts (QAS) comprise a vital class of synthetic products having great significance due to their unique properties like non-flammability, non-volatility, high stability and high polarity [1,2]. They exhibit a wide range of applications in material, medicinal and synthetic chemistry [3]. Benzimidazolium-based QAS receive considerable attention with wide applications [4,5]. These salts having acidic hydrogen at carbon-2 have extensively been used as novel precursors of N-heterocyclic carbenes [6] and ionic liquids [7]. In addition, structural similarities with nucleotide make benzimidazolium salts very interesting pharmaceutical scaffold [8]. Based on their substantial applications, many research groups have focused on synthesizing various benzimidazolium salts by different methods such as coupling, cyclization and condensation reactions [9–12]. For instance, Rivas et al. synthesized different benzimidazolium salts by palladium [13] and copper-catalysed ring closure [14], whereas Türker et al. reported cyanobenzyl substituted benzimidazolium salts by the reaction of N-(alkyl)benzimidazole with 3-bromomethylbenzonitrile as effective inhibitors of α-glycosidase (AG), butyrylcholinesterase (BChE) and acetylcholinesterase [14,15]. Benzimidazole ring can also directly convert to benzimidazolium salt by N-functionalization [16]. These quaternary ammonium salts demonstrate a range of applications such as ionic liquids [17], fluorescence sensors [18], batteries [19] and solar cells [20]. In addition, these salts have been investigated as enzyme inhibitors by several research groups, and studies explore that these salts acquired inhibitory potential against metabolic enzymes [21].
In the recent time, computational investigation has attracted the scientific community because of their useful applications in order to predict and calculate the optoelectronic properties of the newly prepared organic compound such as peptoids [22], chalcones and β-hydroxy carbonyl compounds [23,24], hydrazones [25,26], piperidone derivatives [27], functionalized esters, [28] phosphonates [29], functionalized indoles [30], monocarbonyl curcuminoids [31,32], unsymmetrical acyl thioureas [33], functionalized pyrimidines [34,35], imine-based Zwitterions [36] as well as organic salt systems [37,38]. In continuation of our recent reports on the organic salts [39–43] and benzozole derivatives [44–46], the current study is focused on the design, synthesis and characterization of benzimidazolium salts and the computational exploration of their prospective optoelectronic properties.
2. Experimental part
2.1. Chemicals and instrumentation
Benzimidazole and alkyl halides were commercially purchased from Sigma-Aldrich. Thin layer chromatography (TLC) was carried out using Merck silica-gel 60F-254 aluminium sheet and visualized under a UV lamp. All compounds were purified by column chromatography using silica gel (100–200 mesh). The 1H nuclear magnetic resonance (NMR) spectra were recorded in deuterated methanol (MeOD) using Bruker Advance 400 MHz spectrometer, and 13C NMR spectra were recorded at 125 MHz NMR spectrometer in methanol (MeOD). Chemical shift (δ) values were determined in parts per million (ppm) while coupling constant (J) in Hertz (Hz). The infrared spectra were recorded on the Perkin Elmer Spectrum RX Fourier Transform–IR System and specific peaks were identified. Bruker Kappa Apex-II CCD diffractometer with APEX-II software was used for data collection. SHELXT-2014 [47] and SHELXL 2019/2 [48] software were used for structure solution and refinement, respectively. H-atoms were placed by using riding model except H-atoms of water solvent in DBIC, BMBB and IMBC. H-atoms of water were refined freely for getting the correct orientation of them. PLATON [49] software was used for graphical aims.
2.2. Chemistry and chemical methods
The N-alkyl benzimidazole (where alkyl = methyl, benzyl [50] was synthesized according to the reported procedures.
2.3. General procedure for synthesis of 1-alkylbenzimidazole
The benzimidazole (4 mmol) and 50% aqueous solution of NaOH were added in a round bottom flask. Alkyl halide (5 mmol) was added dropwise and the reaction mixture was stirred for 8 h at room temperature. The reaction was monitored on TLC; after completing, the reaction mixture was extracted with chloroform (30 ml). The solvent was removed under reduced pressure, and the product was purified by column chromatography using ethyl acetate and n-hexane as solvent.
2.4. General procedure for synthesis of 1,3-dialkylbenzimidazolium salt (2a-2b)
Alkyl halide (2 mmol) was added into a round bottom flask containing N-alkylated benzimidazole (2 mmol) and toluene (25 ml). The reaction mixture was heated under reflux for 18–24 h and monitored by TLC. After the completion of the reaction, the product was filtered and washed with ethyl acetate (15 ml) and the collected precipitates were recrystallized in ethanol to obtained pure crystals.
2.5. Synthesis of 3-menthyloxymethyl-1-methyl benzimidazolium chlorides (2c) [51]
Chloromenthyl methyl ether (2 mmol) was added dropwise to 1-methyl benzimidazole (2 mmol) in a round bottom flask under an inert atmosphere. The reaction mixture was allowed to stir for 30 min and diluted with dry diethyl ether. The precipitates were washed with diethyl ether and recrystallized in ethanol to obtain the needle-like crystals of 2c.
2.5.1. 1-benzyl-1-H-benzo[d] imidazole
Solid, yield: 95% IR: 1198.0 (C-N); 1620 (C═N);1646 (C═C aromatic) 2845.7, 2928.6 and 3075.5 (C-H);1H-NMR (400 MHz, CDCl3): δ ppm 8.6 (1H, s). 7.7(2H, d) 7.64 (2H, d), 7.58 (1H, t), 7.34 (3H, t), 7.27 (2H, t), 5.82 (2H, s), 13C-NMR, 144.3, 142.8, 138.2, 134.5, 129.6, 127.8, 125.7, 114.3, 51.9.
2.5.2. 1-methyl-1-H-benzo[d]imidazole
Solid, yield: 94% IR: 1220.0 (C-N); 1628 (C═N);1653 (C═C aromatic) 2901.7, 2947.6 and 3003.5 (C-H);1H-NMR (400 MHz, CDCl3): δ ppm 8.9 (1H, s), 7.68 (1H, d), 7.54 (1H, d), 7.44 (1H, t), 7.37 (1H, t), 4.2 (3H, s), 13C-NMR, 144.3 137.2, 133.5, 133.5, 126.8, 112.3, 122.8, 36.9.
2.5.3. 1-methyl-3-bytyl-1-H-benzo[d]imidazol-3-ium bromide (2a = BMBB)
Reaction time 24 h (toluene). Yield 91%. Crystalline solid, mp 115–120°C. UV. λmax = 276 nm, A = 1.21A IR: 1179.9 (C-N); 1556 (C═N);1962 (C═C aromatic) 2878.5, 2959.2 and 3109.4 (C-H); 1H NMR spectrum,(400 MHz, MeOD): δ ppm: 8.71 (s,1H), 7.8 (d, 1H), 7.57 (d, 1H), 7.28–7.38 (t, 2H), 5.58 (s, CH2), 4.00 (s, CH3), 1.77–1.80 (m, CH2),1.24–1.28 (m, CH2), 0.82–0.84 (t, CH3), 13C NMR spectrum, ppm: 143.76, 133.33, 131.62, 126.89, 126.84, 129.1, 50.52, 42.47, 14.47, 31.29, 20.71, 13.89.
2.5.4. 1,3-dibenzyl-1-H-benzo[d]imidazol-3-ium chloride (2b = DBIC)
Reaction time 21 h (toluene). Yield 89%. Crystalline solid, mp 204–210°C. UV. λmax = 226 nm, A = 1.06A IR: 1189.0 (C-N); 1566 (C═N);1964 (C═C aromatic) 2859.7, 2950.6 and 3070.5 (C-H); 1H NMR spectrum,(400 MHz, MeOD): δ ppm: 12.1 (2H, s), 7.52 (1H, m), 7.45 (2H, m), 7.4 (4H, m), 7.32 (6H, m), 7.29 (1H, m), 5.96–5.97 (CH2, s),5.81–5.82 (CH2, s), 13C NMR spectrum in ppm: 142.96, 131.12, 131.12, 126.84, 126.84, 114.12, 114.12, 129.1, 128.8, 128.4, 50.06 [52].
2.5.5. 1-((1S,2R,4S)-(+)-menthyloxymethyl)-3-methyl Benzimidazolium chloride (2c = IMBC)
White solid, yield 97%. (c = 20 mg/5 ml MeOH). λ max = 273 nm A = 1.41A IR: υ (cm-1) 3389, 2917,(C-H) 1563 (C=N), 1452, 1224(C-N). 1H-NMR (400 MHz, chloroform-d): δ 12.12 (1H, s), 7.92–7.86 (1H, m), 7.71–7.65 (3H, m), 6.23 (1H, d, J = 12.0 Hz), 5.95 (1H, d, J = 12.0 Hz), 4.28 (3H, s), 3.41 (1H, td, J = 10.6, 4.3 Hz), 2.29–2.22 (1H, m), 1.81–1.71 (1H, m), 1.67–1.58 (1H, m), 1.59–1.43 (2H, m), 1.26–1.17 (1H, m), 0.96–0.77 (3H, m), 0.92 (3H, d), 0.73 (3H, d), 0.14 (3H, d). 13C-NMR (100 MHz, chloroform-d): δ 144.4, 132.4, 131.1, 127.6, 127.6, 114.5, 112.6, 79.4, 75.4, 48.0, 40.2, 34.3, 33.8, 31.3, 25.4, 22.8, 22.3, 21.0, 15.25 [51].
2.6. Computational methodology
All the theoretical calculations were performed using Gaussian 09 [53] at ωB97xd/6–31+G(d,p) level [54]. The calculations of frontier molecular orbital (FMO), natural population analysis (NPA) and Nonlinear optical (NLO) analyses were also performed at the same level of DFT. The photophysical properties of compounds 2a–2c were explored by utilizing time-dependent DFT (TD-DFT) method involves range separated functionals (ωB97x). The input files were interpreted by Gauss View 5.0 program [55] and the output files were analysed by different software including Avogadro [56], Chem Craft [57], Gauss Sum [58], Multiwfn 3.8 [59] and Origin [60].
2.7. Single crystal X-ray diffraction studies of the synthesized benzimidazolium salts 2a–2c
After synthesis, purification and recrystallization, the good-quality crystals were taken for the measurement of single crystal analysis, the experimental details of all these three crystalline compounds are given in table 1.
Table 1.
Single crystal XRD experimental details of DBIC, BMBB and IMBC.
| compound | DBIC (2b) | BMBB (2a) | IMBC (2c) |
|---|---|---|---|
| CCDC | 2279954 | 2279955 | 2279956 |
| chemical formula | C21H21ClN2O | C12H19BrN2O | C19H31ClN2O2 |
| Mr | 352.85 | 287.19 | 354.91 |
| crystal system, space group | triclinic, P | monoclinic, P21/n | orthorhombic, P212121 |
| temperature (K) | 296 | 296 | 296 |
| a, b, c (Å) | 9.3334 (8), 9.9117 (9), 11.4769 (10) | 5.1650 (9), 19.223 (4), 13.864 (3) | 6.7238 (5), 8.2781 (8), 36.423 (3) |
| α, β, γ (°) | 68.864 (5), 80.344 (5), 70.019 (5) | 94.141 (9) | 90, 90, 90 |
| V (Å3) | 929.49 (15) | 1372.9 (4) | 2027.3 (3) |
| Z | 2 | 4 | 4 |
| radiation type | Mo Kα | Mo Kα | Mo Kα |
| µ (mm−1) | 0.22 | 2.98 | 0.20 |
| crystal size (mm) | 0.40 × 0.28 × 0.26 | 0.40 × 0.34 × 0.16 | 0.38 × 0.32 × 0.24 |
| diffractometer | Bruker Kappa APEXII CCD | Bruker Kappa APEXII CCD | Bruker Kappa APEXII CCD |
| absorption correction | multi-scan | ||
| (SADABS; Bruker, 2007) | multi-scan | ||
| (SADABS; Bruker, 2007) | multi-scan | ||
| (SADABS; Bruker, 2007) | |||
| no. of measured, independent and | |||
| observed [I > 2σ(I)] reflections | 10418, 4144, 2315 | 11319, 3050, 1365 | 13864, 3829, 2738 |
| Rint | 0.032 | 0.076 | 0.048 |
| (sin θ/λ)max (Å−1) | 0.658 | 0.646 | 0.617 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.059, 0.191, 1.05 | 0.060, 0.180, 1.00 | 0.048, 0.112, 1.01 |
| no. of reflections | 4144 | 3050 | 3829 |
| no. of parameters | 232 | 191 | 227 |
| no. of restraints | 3 | 48 | 3 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.42, −0.31 | 0.54, −0.51 | 0.13, −0.17 |
3. Results and discussion
3.1. Chemistry
The target benzimidazolium salts were prepared from the N-functionalization of benzimidazole in two steps (scheme 1). The first alkylation was carried out under aqueous conditions, where the corresponding alkyl halides were reacted with benzimidazole in the presence of 50% NaOH (aq.) at room temperature. The monoalkylated benzimidazoles were further reacted with butyl, benzyl and menthyloxymethyl halides to obtain dialkylated quaternary benzimidazolium salt refluxing in toluene. The corresponding salts (2a-c) were obtained in high yields of 85–95% and further recrystallized in ethanol to recover the pure crystals. Interestingly, all the reactions were carried out under atmospheric conditions where the synthesized quaternary salts were found stable in air and moisture.
Scheme 1.
Synthesis of crystalline benzimidazolium quaternary ammonium salts (2a-c).
3.2. Characterization of the benzimidazolium salts 2a–2c
The complete structural elucidation of all purified benzimidazolium salts has been achieved through the comprehensive application of IR, UV, 1H and 13C NMR spectroscopy and ultimately authenticated by single-crystal X-ray diffraction (SC-XRD) analysis. Furthermore, extensive computational studies were employed to gain a deep understanding of the structural and electronic properties of the synthesized quaternary ammonium salts.
3.2.1. Spectral characterization of the benzimidazolium salts 2a–2c
In the 1H-NMR spectra, a characteristic singlet appeared in the downfield region at 12.1, 9.1 and 12.1 ppm for 2a-c respectively, and verifying the resonance of the NCHN protons of the benzimidazolium ring. Notably, for compound 2b, the H-2 signal was observed at a relatively upfield region (9.1 ppm), which may be attributed to the anisotropic effect of one of the aromatic rings of benzyl groups. The SC-XRD structure of 2a, presented in scheme 1, demonstrates the orientation of the left-side benzyl ring that the C7 proton resides within the shielding area of the aromatic ring. On the other hand, this effect is absent in compounds 2a and 2c, where the H-2 singlet was observed at 12.1 ppm. in the case of compound 2b, 14 aromatic protons were observed in the aromatic region (7.23–7.98 ppm) due to the presence of two benzyls and a benzimidazole ring. In the H-NMR spectra of compounds 2a and 2c, the appearance of singlets for 3Hs at δ 5.97 and 5.58 ppm confirmed the presence of (N-CH3), whereas the signals appearing in the upfield region indicated the presence of different alkyl groups. A characteristic signal for a diastereotopic methylene (N–CH2–O) was appeared at δ 6.23 and 5.95 ppm as doublets were observed due to the presence of a chiral centre in the menthyloxymethyl substitution in compound 2c.
In the 13C-NMR spectra of the synthesized compounds 2a-c, a distinct carbon resonance peak was detected at δ 142.4–143.2 ppm, which is attributed to the resonance of C7 carbon of the benzimidazolium ring. Similarly, benzimidazole and benzyl rings (of 2b) were observed in the aromatic region δ 118.6–162.8 ppm, whereas the aliphatic carbons in compounds 2a and 2c was verified by a set of peaks appearing in the range of δ 53.7–14.2 ppm.
IR spectra of compounds 2a–2c (figure 1) demonstrate the modes of C–N and C–O stretching vibrations at 1100–1250 cm−1, whereas intense peaks detected in the range of 1540 to 1590 cm−1 were observed for the stretching modes of vibrations of the C=C and C=N in the benzimidazolium salts. Moreover, stretching and vibration absorption above 3360–3440 cm−1 were observed for the C-H in both aliphatic and aromatic substitutions.
Figure 1.
IR spectra of benzimidazolium salts 2a-c.
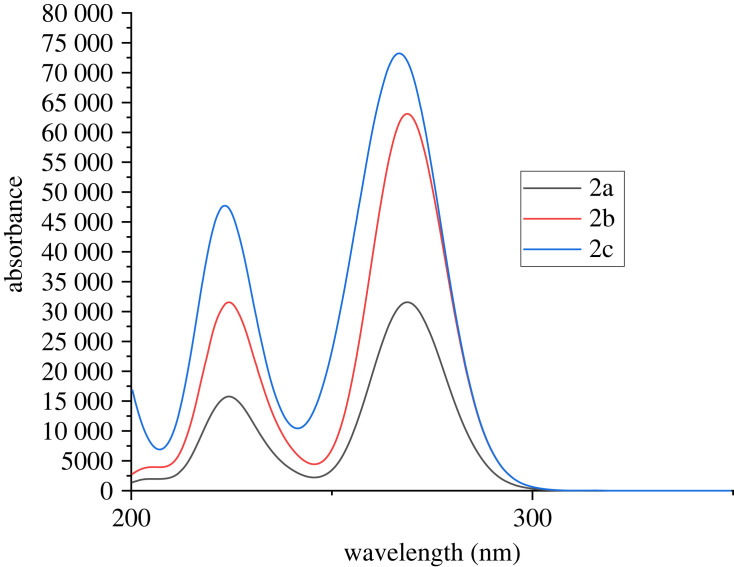
The UV-Vis spectra of benzimidazolium salts 2a–2c in methanol were recorded within 200–400 nm, where the absorption peaks were observed at λmax 226, 276 and 273 nm, respectively (figure 2). Compound 2a, which bears only alkyl substitutions, displayed absorption at a relatively lower wavelength (λmax 226) in comparison with 2b, which features a benzyl group, and 2c, which includes an alkoxy group.
Figure 2.
UV-visible spectra of benzimidazolium salts 2a-c.
3.3. Single crystal X-ray diffraction studies
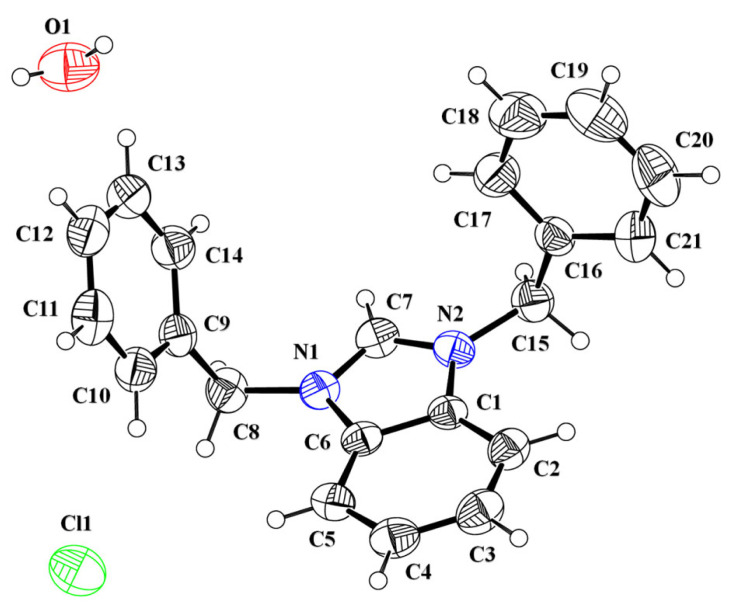
The asymmetric unit of the salt DBIC contained a 1,3-dibenzyl-1H-benzimidazol-3-ium cation (C1-C21/N1/N2), a chloride anion and a water solvent (figure 3, table 1). The central 1H-benzo[d]imidazol-3-ium group A (C1-C7/N1/N2) is planar with root mean square deviation of 0.0052 Å and oriented at the dihedral angle of 73.2 (7)° and 81.8 (7)° with respect to first benzyl group B (C8-C14) and second benzyl group (C15-C21), respectively. The dihedral angles showed that the cation is non-planar.
Figure 3.
ORTEP diagram of DBIC drawn at probability level of 40%. H-atoms are shown by small circles of arbitrary radii.
The cations are not directly interlinked with each other by any H-bonding. Cations are connected with anions by C-H⋯Cl bonding, where CH is from the non-ring CH of benzyl group B and C (figure 4, table 2). No atom of cation acts as H-bond acceptor. The cations are also connected with the water solvent through C-H⋯O bonding, where CH is from the group A. Both H-atoms of water act as H-bond donor for chlorine ions. Each H-atom is involved with H-bonding with a unique symmetry-related chloride anion. So, water solvent plays a significant role in the stabilization of the supramolecular assembly or crystal packing as it forms H-bonding interactions with cation as well as with anion. The water solvent and chloride anions act as bridge to connect cations with each other. The supramolecular assembly is further stabilized by offset π⋯π stacking interactions between rings of the symmetry-related cations with inter-centroid separation range from 3.5231 (14) to 4.2306 (15) Å. Ring offset range is 0.557 to 2.391 Å. Literature crystal structure with CSD [61] reference code JUQWOJ [62] has same cation and a water solvent but has bromide ion instead of chloride ion. EJEMEM [63] has benzyl group attached to one N-atom and methyl group at the other N-atom with bromide ion and water. Bond lengths and bond angles of DBIC are consistent with the corresponding ones in related structures. The cation in JUQWOJ and EJEMEM is also not planar as in DBIC. The intermolecular interactions in JUQWOJ and EJEMEM are not reported in literature.
Figure 4.
Packing diagram of DBIC. Selected H-atoms are shown for clarity.
Table 2.
Hydrogen-bond geometry (Å, °) of DBIC, BMBB and IMBC. Symmetry codes: (i) −x + 1, −y + 1, −z + 1; (ii) x, y−1, z + 1; (iii) −x + 1, −y, −z + 1; (iv) −x + 1, −y + 1, −z; (v) x, y−1, z; (vi) x + 1, y, z; (vii) −x + 3/2, y + 1/2, −z + 1/2; (viii) x−1/2, −y + 1/2, z−1/2; (ix) x + 1/2, −y + 3/2, −z; (x) x + 1, y−1, z; (xi) x−1/2, −y + 3/2, −z.
| D—H···A | D—H | H···A | D···A | <(D—H···A)° | |
|---|---|---|---|---|---|
| DBIC | O1—H1A···Cl1i | 0.82 (1) | 2.45 (2) | 3.224 (3) | 159 (4) |
| O1—H1B···Cl1ii | 0.83 (1) | 2.40 (2) | 3.204 (3) | 164 (4) | |
| C7—H7···O1iii | 0.93 | 2.43 | 3.244 (4) | 146 | |
| C8—H8A···Cl1 | 0.97 | 2.78 | 3.668 (3) | 153 | |
| C8—H8B···Cl1iv | 0.97 | 2.72 | 3.658 (3) | 162 | |
| C15—H15A···Cl1v | 0.97 | 2.66 | 3.603 (3) | 164 | |
| BMBB | O1—H1D···Br1 | 0.82 (1) | 2.56 (1) | 3.374 (5) | 174 (7) |
| O1—H1E···Br1vi | 0.82 (1) | 2.53 (2) | 3.339 (5) | 170 (7) | |
| C5—H5···O1vii | 0.93 | 2.57 | 3.452 (7) | 159 | |
| C7—H7···Br1viii | 0.93 | 2.64 | 3.569 (6) | 173 | |
| IMBC | O2—H2A···Cl1ix | 0.83 (2) | 2.40 (2) | 3.236 (4) | 177 (5) |
| O2—H2B···Cl1 | 0.84 (2) | 2.35 (3) | 3.151 (4) | 161 (5) | |
| C4—H4···Cl1x | 0.93 | 2.76 | 3.519 (4) | 139.81 | |
| C8—H8C···O2xi | 0.96 | 2.37 | 3.317 (6) | 171 | |
| C9—H9A···Cl1vi | 0.97 | 2.81 | 3.691 (4) | 152 |
The asymmetric unit of the salt BMBB contained a 3-butyl-1-methyl-1H-benzimidazol-3-ium cation (C1-C8/C9A-C12A/N1/N2), a bromide anion and a water solvent (figure 5, table 1). The central 1H-benzo[d]imidazol-3-ium group A (C1-C7/N1/N2) is bonded with a methyl group and a butyl group B (C9A-C12A). The butyl group is disordered over two sets of sites with occupancy ratio 0.717(13): 0.283(13). The disorder is treated by using various suitable restraints. Group A is planar with root mean square deviation of 0.0138 Å and oriented at the dihedral angle of 15.4 (1)° and 56.5 (1)° with respect to major and minor part of butyl group, respectively. The dihedral angles inferred that the cation is non-planar.
Figure 5.
ORTEP diagram of BMBB that is drawn at probability level of 40%. H-atoms are shown by small circles of arbitrary radii. Major part of disordered group is shown for clarity.
The cations are not directly interlinked with each other by any H-bonding. Cations are connected with anions by C-H⋯Br bonding, where CH is from group A (figure 6, table 2). No atom of cation acts as H-bond acceptor. The cations are also connected with the water solvent through C-H⋯O bonding, where CH is from the group A. Both H-atoms of water act as H-bond donor for bromide ions. Each H-atom is involved with H-bonding with a unique symmetry-related bromide anion. The water solvent and bromide anions act as bridge to connect cations with each other. The supramolecular assembly is further stabilized by offset π⋯π stacking interactions between rings of the symmetry-related cations with inter-centroid separation of 3.836 (2) Å, and ring offset range is 1.7 to 1.812 Å. Literature crystal structure with CSD reference code KINJUP [64] has isopropyl group bonded with each N-atom of cation and a water solvent but has chloride ion instead of bromide ion, reported as CSD Communication. ACINUY [65] has dinonyl group attached to both N-atoms with bromide ion and water. Bond lengths and bond angles of BMBB are consistent with the corresponding ones in related structures. The crystal packing of ACINUY was stabilized by O-H⋯Br bonding, whereas the crystal packing of BMBB is stabilized by O-H⋯Br, C-H⋯Br and C-H⋯O bonding.
Figure 6.
Packing diagram of BMBB. Selected H-atoms are shown for clarity.
The asymmetric unit of the salt IMBC contained a 3-butyl-1-methyl-1H-benzimidazol-3-ium cation (C1-C19/N1/N2/O1), a chloride anion and a water solvent (figure 7, table 1). The central 1H-benzo[d]imidazol-3-ium group A (C1-C7/N1/N2) is bonded with a methyl group and a 1-isopropyl-2-methoxy-4-methylcyclohexane group C (C9A-C12A). The cyclohexane ring C (C10-C15) is puckered and adopts chair conformation [66]. Group A is planar with root mean square deviation of 0.0105 Å and makes dihedral angle of 88.5 (9)° with group C. The dihedral angle showed that group A is almost perpendicular to group C.
Figure 7.
ORTEP diagram of IMBC that is drawn at probability level of 40%. H-atoms are shown by small circles of arbitrary radii. Major part of disordered group is shown for clarity.
The cations are not directly interlinked with each other by any H-bonding. Cations are connected with anions by C-H⋯Cl bonding, where CH is from group A and B (figure 8, table 2). No atom of cation acts as H-bond acceptor. The cations are also connected with the water solvent through C-H⋯O bonding, where CH is from the methyl group attached to group A. Both H-atoms of water act as H-bond donor for chloride ions. Each H-atom is involved with H-bonding with a unique symmetry-related chloride anion. The water solvent and chloride anions act as bridge to connect cations with each other. No offset π⋯π stacking interaction of significant strength is found in the crystal packing. Literature crystal structure with CSD reference code ENALEL [67] has cyanobenzyl group bonded with one N-atom and methyl group at the other N-atom of cation, water solvent and a bromide ion. Bond lengths and bond angles of IMBC are consistent with the corresponding ones in related structure. The crystal system and space group of IMBC is different from the crystal system and space group of ENALEL. ENALEL was crystallized in monoclinic crystal system with space group P21/n (table 3).
Figure 8.
Packing diagram of IMBC. Selected H-atoms are shown for clarity.
Table 3.
Important bond lengths (Å) and bond angles (°) in DBIC, BMBB and IMBC.
| DBIC | BMBB | IMBC | |
|---|---|---|---|
| N1—C7 | 1.334 (3) | 1.324 (7) | 1.318 (4) |
| N1—C6 | 1.394 (3) | 1.392 (6) | 1.384 (4) |
| N1—C8 | 1.474 (3) | 1.466 (7) | 1.465 (5) |
| N2—C7 | 1.322 (3) | 1.348 (8) | 1.333 (4) |
| N2—C1 | 1.383 (3) | 1.390 (7) | 1.393 (4) |
| C7—N1—C6 | 107.8 (2) | 108.4 (4) | 108.9 (3) |
| C7—N1—C8 | 124.9 (2) | 125.3 (5) | 125.7 (3) |
| C6—N1—C8 | 127.1 (2) | 126.3 (5) | 125.4 (3) |
| C7—N2—C1 | 108.54 (19) | 106.9 (5) | 108.1 (3) |
| N2—C1—C2 | 131.7 (2) | 129.6 (6) | 131.9 (3) |
| N2—C1—C6 | 106.65 (19) | 107.8 (5) | 106.3 (3) |
3.4. Hirshfeld surface analysis
The interest of researchers in designing new crystals with properties better than the already-known crystals is increasing day by day, and for this, a significant knowledge of the intermolecular interactions is required. In this prospective, we are going to explore the intermolecular interactions in DBIC, BMBB and IMBC by Hirshfeld surface analysis by using Crystal Explorer version 21.5 [68–70]. Hirshfeld surface plotted over normalized distances (dnorm) use colour coding to separate short contacts from the longer ones. Red and blue regions on surface showed short and long contacts, respectively, while the white spots showed contacts with distance equal to sum of VdW radii [71–73]. Figure 9a–c are the surfaces for cation of DBIC, BMBB and IMBC, respectively, with red spots showing short contacts. No short contact of significant strength is formed by the cation with the neighbouring cation, but it formed short contacts with anion and water in DBIC, BMBB and IMBC. Short contacts are shown by green dashed lines. Figure 9d–f are the surfaces for anion of DBIC, BMBB and IMBC, respectively, with more than one red spot, showing that anion forms more than one short contact. Anion formed short contacts with cation and water. Figure 9g–i are the surfaces for water of DBIC, BMBB and IMBC, respectively, with both H-atoms of water and O-atom form short contact. Water does not form any short contact with the neighbouring water molecules.
Figure 9.
Hirshfeld surface plotted over dnorm for cation of (a) DBIC, (b) BMBB, (c) IMBC, anion of (d) DBIC, (e) BMBB, (f) IMBC, water solvent of (g) DBIC, (h) BMBB, (i) IMBC.
The information that is hidden in the traditional way of crystal packing description can be acquired by two-dimensional finger print plots [74–76]. The plots divide the overall interactions into smaller fragments. Hirshfeld surface containing cation, anion and water is used as input for two-dimensional fingerprint plots analysis. H⋯H contact is the most significant contributor in stabilization of supramolecular assembly in DBIC, BMBB and IMBC with percentage contribution of 50.5% (figure 10a), 59.4% (figure 10e) and 74.5% (figure 10i), respectively. The next larger contributor is H⋯C in DBIC whereas H⋯Br and H⋯Cl in BMBB and IMBC, respectively. Although one water molecule is present in DBIC, BMBB and IMBC, the contribution of H⋯O contact in IMBC is larger than the contribution of the corresponding contact in DBIC and BMBB.
Figure 10.
Two-dimensional fingerprint plots for important contacts in (a-d) DBIC, (e-h) BMBB, (i-k) IMBC.
Enrichment ratio provides the probability of the pair of chemical species forming crystal packing interactions with value greater than one for the higher probable contacts [77,78]. Table 4 lists the results of enrichment ratio calculations. H⋯O and H⋯Cl have same enrichment ratio for DBIC but these pairs have different enrichment ratio for BMBB and IMBC. H⋯Cl contact is the most favourable to form crystal packing interactions in DBIC and IMBC whereas the H⋯O contact is most favourable in BMBB. H⋯H contact is slightly favourable in BMBB but not favourable in DBIC and IMBC.
Table 4.
Enrichment ratio for the pairs of chemical species in DBIC, BMBB, IMBC. Enrichment ratio is not calculated for the pairs with random contact less than 0.99.
| contact % | atom | H | C | N | O | Cl/Br |
|---|---|---|---|---|---|---|
| H | 50.5/59.4/74.5 | 26.8/11.7/6.4 | 0.5/0.8/1.3/1.3 | 3.9/5.3/6.1 | 13.8/16.6/10.7 | |
| C | 26.8/11.7/6.4 | 2.7/1.4/0 | 1.8/1.6/0 | 0/0/0.5 | 0/1.5/0.2 | |
| N | 0.5/0.8/1.3 | 1.8/1.6/0 | 0/0/0 | 0/0/0 | 0/1.7/0.2 | |
| O | 3.9/5.3/6.1 | 0/0/0.5 | 0/0/0 | 0/0/0 | 0/0/0.1 | |
| Cl/Br | 13.8/16.6/10.7 | 0/1.5/0.2 | 0/1.7/0.2 | 0/0/0.1 | 0/0/0 | |
| surface% | 73/76.6/86.75 | 17/8.8/3.55 | 1.15/2.05/0.75 | 1.95/2.65/3.55 | 6.9/9.9/5.6 | |
| random contacts % | ||||||
| H | 53.29/58.68/75.26 | |||||
| C | 24.82/13.48/6.16 | 2.89/0.77/0.13 | ||||
| N | 1.68/3.14/1.30 | 0.39/0.36/0.05 | 0.01/0.04/0.01 | |||
| O | 2.85/4.06/5.81 | 0.66/0.47/0.24 | 0.04/0.11/0.05 | 0.04/0.07/0.11 | ||
| Cl/Br | 10.07/15.17/9.72 | 2.35/1.74/0.40 | 0.16/0.41/0.08 | 0.27/0.52/0.38 | 0.48/0.98/0.31 | |
| Enrichment ratio | ||||||
| H | 0.95/1.01/0.99 | |||||
| C | 1.08/0.87/1.04 | 0.93 | ||||
| N | 0.30/0.2/1.00 | |||||
| O | 1.37/1.31/1.05 | |||||
| Cl/Br | 1.37/1.09/1.10 | 0.00/0.86 | ||||
The key influencer on the crystal packing and mechanical response of the crystal is the voids and cavities. Poor mechanical response is provided by a crystal with large cavities. Voids are calculated by using an idea of the procrystal electron density. For calculating voids, the isosurface is used by the sum of the atomic electron densities located at appropriate nuclear sites [35,79,80]. Figure 11 is the graphical view of voids in DBIC, BMBB and IMBC mapped on 0.002 au isosurface, where 1 au of electron density = 6.748 e Å−3.
Figure 11.
Graphical representation of voids in (a) DBIC, (b) BMBB and (c) IMBC.
The volume of voids in DBIC, BMBB and IMBC is 104.25, 158 and 290.55 Å3, respectively. The space consumed by voids in DBIC, BMBB and IMBC is 11.2%, 11.5% and 14.3%, respectively. The voids occupied a small amount of space, which inferred that there is no large cavity, and compounds are expected to have good mechanical response.
3.5. Structural properties
The optimized geometries of the synthesized compounds 2a, 2b and 2c are displayed in figure 12. The optimized geometries of the compounds were calculated at ωB97xd/6–31+G(d,p) level of DFT. In compound 2a, the theoretically determined values of bond length N1-C7, N1-C6, N1-C8, N2-C7 and N2-C1 were observed as 1.348, 1.392, 1.450, 1.346 and 1.392 Å, respectively, that were in agreement with the experimentally determined bond lengths (1.324 Å for N1-C7, 1.392 Å for N1-C6, 1.466 Å for N1-C8, 1.348 Å for N2-C7 and 1.390 Å for N2-C1). The values bond angles 107.59°, 123.28°, 128.80°, 107.72°, 131.67° and 106.79° for C7-N1-C6, C7-N1-C8, C6-N1-C8, C7-N2-C1, N2-C1-C2 and N2-C1-C6 respectively were also in accordance with the experimentally determined values of bond angle. Similarly for compound 2b and 2c, the values of bond lengths N1-C7 (1.336 Å for 2b and 1.340 Å for 2c), N1-C6 (1.393 Å for 2b and 1.395 Å for 2c), N1-C8 (1.457 Å for 2b and 1.450 Å for 2c), N2-C7 (1.335 Å for 2b and 1.344 Å for 2c) and N2-C1 (1.397 Å for 2b and 1.395 Å for 2c) were in close approximation with the experimentally determined bond lengths N1-C7 (1.334 Å for 2b and 1.318 Å for 2c), N1-C6 (1.394 Å for 2b and 1.384 Å for 2c), N1-C8 (1.474 Å for 2b and 1.465 Å for 2c), N2-C7 (1.322 Å for 2b and 1.333 Å for 2c) and N2-C1 (1.383 Å for 2b and 1.393 Å for 2c). The values for bond angles for 2b were 107.95°, 123.85°, 125.85°, 108.04°, 132.21° and 106.42° for C7-N1-C6, C7-N1-C8, C6-N1-C8, C7-N2-C1, N2-C1-C2 and N2-C1-C6, respectively. The bond angles in the case of 2c were 107.42°, 123.55°, 125.20°, 107.90°, 131.71° and 106.55° for C7-N1-C6, C7-N1-C8, C6-N1-C8, C7-N2-C1, N2-C1-C2 and N2-C1-C6 respectively. The distance of Br from the carbon of imidazole in 2a was 2.59 Å whereas the distance of Cl with the carbon of imidazole in 2b and 2c was 2.68 and 2.54 Å respectively. Moreover, the coordinates of optimized geometry have been placed in the electronic supplementary material. Furthermore, the structural parameters of the compounds 2a–2c after optimization in gaseous and solvent phase have been placed in electronic supplementary material, table S1 (electronic supplementary material, information).
Figure 12.
Optimized geometries of compound.
3.6. Computational findings
3.6.1. Frontier molecular orbital analysis
Frontier molecular orbitals consisting of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) play a crucial role in determining the electric, electronic and optical properties of the compounds [51]. In FMO analysis, HOMO–LUMO energy gap (ELUMO - EHOMO) value is used to explore the stability and reactivity of the compounds. The small value of energy gap depicts high reactivity and low stability while the large value of energy gap depicts low reactivity and high stability of the compound [81]. The energies of HOMO & LUMO and HOMO–LUMO energy gaps of the compounds are given in table 5. In gas phase, the calculated values of energy gap of 2a, 2b and 2c are 7.757, 7.803 and 8.015 eV, respectively, as presented in table 5. The large values of energy gap indicated the low susceptibility of intramolecular electron transfer which leads to low reactivity of the compounds. The energies of HOMO, LUMO and energy gap for 2a–2c after optimization in solvents of their respective solubilities have also been worked out, which showed a little variation and the data has been provided in electronic supplementary material, table S2 (electronic supplementary material, information).
Table 5.
Energies of HOMO & LUMO and HOMO–LUMO energy gap.
| gas phase | |||
|---|---|---|---|
| compounds | EHOMO | ELUMO | Eg |
| 2a | −7.381 | 0.376 | 7.757 |
| 2b | −7.528 | 0.275 | 7.803 |
| 2c | −7.607 | 0.408 | 8.015 |
In compounds 2a, 2b and 2c, the orbital density of HOMO as well as LUMO is scattered over benzimidazole-halogen moiety (figure 13).
Figure 13.
The three-dimensional orbital pictures of HOMO and LUMO of 2a, 2b and 2c.
3.6.2. Global reactivity parameters
Global reactivity parameter (GRP) descriptors of the compounds can also be calculated by the energies of HOMO and LUMO of the compounds. The GRP descriptors of the compounds including ionization energy (I), electron affinity (A), electronegativity (X), global hardness (η), chemical potential (µ), electrophilicity (ω) and global softness (σ) can be calculated by the given equations [82],
| 3.1 |
| 3.2 |
| 3.3 |
| 3.4 |
| 3.5 |
| 3.6 |
| 3.7 |
The stability and reactivity of the entitled compounds is mainly determined by the values of global hardness (η) and global softness (σ). The value of global hardness is maximum in the case of 2c (4.007) that illustrated the high stability and low reactivity of 2c, whereas the value of global hardness is smallest for 2a (3.878) that illustrated the low stability and high reactivity of 2a. Similarly, the greater magnitude of global softness of 2a (0.128) depicts the low stability and high reactivity of 2a whereas small value of global softness of 2c (0.124) depicted its greater stability and lowest reactivity. The values of GRP descriptors of the compounds are given in table 6.
Table 6.
GRP descriptors of compounds 2a–2c.
| GRP descriptors | ionization energy (I) | electron affinity (A) | electronegativity (Χ) | global hardness (η) | chemical potential (µ) | electrophilicity (ω) | global softness (σ) |
|---|---|---|---|---|---|---|---|
| 2a | 7.381 | −0.376 | 3.502 | 3.878 | −3.502 | 1.581 | 0.128 |
| 2b | 7.528 | −0.275 | 3.626 | 3.901 | −3.626 | 1.685 | 0.129 |
| 2c | 7.607 | −0.408 | 3.599 | 4.007 | −3.599 | 1.616 | 0.124 |
3.6.3. Density of states analysis:
Density of states (DOS) analysis describes the frequencies of electronic excitation that occur per unit of energy and volume. The understanding of likelihood of possible states per unit volume and energy is necessary to assess a wide range of electronic activities such as total electron scattering, excitation and λmax of the novel materials. The dispersion of electrical characteristics between HOMOs and LUMOs alters as a result of the presence of various electron-activating and deactivating groups in the compounds. The positive values along the x-axis reflected the electrical configuration of LUMOs, while the negative values represented the conductive channel at HOMO (figure 14). The difference that prevailed between these values represents respective energy deficit. In compound 2a, 30.69% of HOMO composition was contributed by imidazole, and 69.31% of HOMO composition was contributed by alkyl halide, whereas 90.02% of LUMO was contributed by imidazole, and 9.8% of LUMO was contributed by alkyl halide. Similarly, in the case of compound 2b, 14.12% and 85.88% HOMO was contributed by imidazole and alkyl halide, while 97.26% and 2.74% of LUMO was contributed by imidazole and alkyl halide, respectively. The 28.52% and 71.48% of HOMO of compound 2c was contributed by imidazole and alkyl halide moiety, respectively, whereas 94.15% and 5.85% of LUMO was contributed by imidazole and alkyl halide, respectively.
Figure 14.
DOS spectra and percentage contribution of fragments of 2a–2c.
3.6.4. Nonlinear optical analysis
The study of optical properties of compounds contributes a lot in the domain of optoelectronics [83]. NLO effect is the result of the interaction of electromagnetic field from several media with the prepared compounds to produce a new field with distinct propagation properties including amplitude, phase and frequency [84]. The materials showing notable NLO effect have significant applications in the field of optical switching, digital signal processing and communication technology [51]. The NLO properties of the respective compounds can be illustrated by comparing their effects with those of urea showing the hyperpolarizability of 0.23 × 10−23 e.s.u and dipole moment of 1.3732 Debye (D) [85]. The NLO properties such as dipole moment (μ), polarizability (αtotal) and hyperpolarizability (βtotal) of the compounds can be determined by the equations (3.8), (3.9) and (3.10), respectively.
| 3.8 |
| 3.9 |
| 3.10 |
The calculated values of μ for 2a, 2b and 2c were found to be 7.940, 9.041 and 7.069 D, respectively (table 7). The values of μ for all the prepared compounds were greater than that of urea (which shows μ value of 1.3732 D), contributing towards relatively better hyperpolarizability of the compounds. Furthermore, the magnitudes of linear polarizability (αtotal) of compounds 2a, 2b and 2c were equal to 185.801, 269.453 and 255.931 arb. units respectively (mentioned in table 8) and the values of hyperpolarizability (βtotal) for compounds 2a, 2b and 2c were 587.001, 918.212 and 661.606 arb. units, respectively (given in table 9), greater than that of urea (reported hyperpolarizability of 0.23 × 10−23 e.s.u and 44.1 arb. units calculated from DFT) reflecting that expected NLO response of prepared compounds might be better than urea. The values of μ, αtotal and βtotal of compounds 2a–2c, achieved after their optimization in solvent phase have also been displayed in tables 7–9.
Table 7.
Dipole moment of compounds 2a–2c.
| dipole moment (Debye) | gas phase |
solvent |
||||
|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2a | 2b | 2c | |
| μx | −2.9677 | −2.7180 | 1.4587 | −6.8625 | 4.3436 | 4.5624 |
| μy | 6.0136 | −7.7228 | 4.2761 | 8.7198 | −14.8221 | −14.8880 |
| μz | 4.2520 | 3.8374 | −5.4375 | 5.3111 | 0.6374 | −3.4333 |
| μtotal | 7.9404 | 9.0418 | 7.0696 | 12.3019 | 15.4586 | 15.9454 |
Table 8.
Linear polarizability of compounds 2a–2c.
| linear polarizability | gas phase |
solvent |
||||
|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2a | 2b | 2c | |
| αxx | 194.928 | 331.844 | 283.921 | 194.781 | 324.362 | 262.213 |
| αyy | 218.464 | 254.292 | 290.049 | 210.888 | 258.174 | 272.722 |
| αzz | 144.013 | 222.224 | 193.823 | 153.199 | 220.831 | 210.933 |
| αtotal(arb. units) | 185.801 | 269.453 | 255.931 | 186.289 | 267.789 | 248.622 |
Table 9.
Hyperpolarizability of compounds 2a–2c.
| hyperpolarizability | gas phase |
solvent |
||||
|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2a | 2b | 2c | |
| βxxx | 110.816 | −11.507 | 94.250 | −147.406 | 165.077 | −20.201 |
| βxxy | −74.366 | −1.008 | −52.247 | 321.993 | −222.403 | −176.370 |
| βxyy | −129.742 | 281.509 | −28.824 | −652.244 | 107.961 | 189.388 |
| βyyy | 596.766 | 743.956 | 346.793 | 1106.463 | −835.660 | −820.836 |
| βxxz | −31.950 | 76.946 | 92.781 | 133.297 | 8.777 | −84.598 |
| βyyz | 211.284 | 229.209 | −275.472 | 399.549 | 35.452 | −157.584 |
| βxzz | 6.023 | 4.145 | −1.342 | −47.715 | 24.587 | 21.370 |
| βyzz | 54.194 | 61.323 | 267.646 | 110.917 | −128.818 | −191.525 |
| βzzz | −70.049 | 41.848 | −160.174 | 65.331 | −11.051 | −143.134 |
| βtotal(arb. units) | 587.001 | 918.212 | 661.606 | 1856.209 | 1224.078 | 1264.065 |
3.6.5. UV-visible spectroscopic analysis
The theoretical calculations of UV-Vis spectral data of compounds 2a–2c were carried out by applying TD-DFT which allows the evaluation of medium sized molecules [86]. The UV-vis spectroscopic analysis was also performed at ωB97xd/6–31+G(d,p) functional level in the presence of methanol as solvent. Theoretically obtained values of energy, wavelength, oscillator strength and major contribution of molecular orbitals are mentioned in electronic supplementary material, table S3 (electronic supplementary material, data). The calculated values of λmax for compound 2a, 2b and 2c in methanol were found to be 268.457, 268.457 and 256.308 nm, respectively. The UV-Vis spectrum of the compounds is shown in figure 15.
Figure 15.
Graphical representation of UV-Vis analysis of compounds 2a–2c.
3.6.6. Natural population analysis
NPA was carried out to determine the charge on each atom of the entitled compounds. The calculations of NPA were performed using Gaussian 09 software by applying ωB97xd/6–31+G(d,p) functional level. In the case of 2a, the largest value of negative charge −0.727 e− was present over bromine (Br12). In 2c, the greater value of negative charge −0.779 e− was observed over chlorine (Cl52). Similarly, the greater negative charge −0.838 e− in 2b was also present over chlorine (Cl43). However, in all the three compounds the maximum positive charge was exhibited by carbon number 8 (C8) due to its direct bond with two highly electronegative nitrogen atoms. The magnitude of positive charge on C8 of 2a, 2b and 2c was 0.321 e−, 0.359 e− and 0.349 e−respectively. The graphical representation of NPA of the compounds is shown in figure 16.
Figure 16.
NPA of compounds 2a–2c.
3.6.7. Molecular electrostatic potential analysis
Molecular electrostatic potential (MEP) analysis is frequently employed for the visual examination of charge distribution in the molecules of interest. MEP plots are used to locate the regions of nucleophilic and electrophilic attack on the molecule [87]. The MEP (V(r)) map around a respective molecule at a given point r along axis is interpreted by the interaction energy between proton present at point r and electrical charge generated by the electrons and nuclei of the molecule. MEP maps can also be defined by the following equation [88]:
| 3.11 |
The MEP maps of the prepared compounds 2a–2c (given in figure 17) consist of various coloured tones (ranging from green to red) revealing different electrostatic potential of the prepared compounds. The red colour area stipulates electrophilic reactive site whereas blue colour stipulates nucleophilic reactive site of the molecules. In all the three compounds 2a–2c, red colour area around halogens (Br and Cl) encompasses their negative electrostatic potential, whereas the blue colour area over the C-H of imidazole encompasses their positive electrostatic potential.
Figure 17.
MEP plots of compounds 2a–2c.
3.6.8. Electron localization function and localized orbital locator analysis
Electron localization function (ELF) and localized orbital locator (LOL) analysis are useful techniques to determine the electron density concentration in the entitled compounds. Through covalent bond analysis, ELF and LOL identify primary active sites in the molecules [89]. The coloured projection charts and contour maps of ELF and LOL (displayed in figure 18) for the prepared compounds were derived using Multiwfn program. The ELF maps were designed in the range of 0 to 1.0 denoting their analogous chemical content. The value of ELF in the range of 0.5 to 1.0 represents bonding and non-bonding localized electrons while ELF magnitude less than 0.5 represent delocalized electrons. LOL expresses more conclusive and flawless representation of electron density concentration in the entitled compounds by the study of topological parameters such as kinetic energy density. Furthermore, the red and blue colours in the scale represent high and low ELF and LOL values.
Figure 18.
ELF and LOL analysis of compounds 2a–2c.
3.6.9. Fourier transform–infrared analysis
DFT calculations were executed to determine the vibrational frequencies of compound 2a–2c. The FT-IR results obtained by DFT analysis were compared with experimental data as given in electronic supplementary material, tables S4–S6. Moreover, the simulated IR spectra for compounds 2a–2c have been placed in electronic supplementary material, figures S1–S3, respectively.
3.6.9.1. C=C vibration
The IR bands for C=C stretching was observed at 1549.38 cm−1 for compound 2a and at 1550.80 cm−1 for compound 2c. The C=C stretching frequencies for three benzene rings of compound 2b were found to be 1543.83 cm−1 for Ben1, 1507.79 cm−1 for Ben2 and 1511.57 cm−1 for Ben3. The experimentally determined values for C=C vibrations in compound 2a, 2b and 2c were 1962, 1964 and 2218 cm−1, respectively.
3.6.9.2. C=N vibration
The C=N stretching frequencies in the imidazole rings of compound 2a, compound 2b and compound 2c were found to be 1605.60, 1625.48 and 1621.20 cm−1, respectively. The experimental values for C=N vibration 1556 cm−1 (2a), 1566 cm−1 (2b) and 1566 cm−1 (2c) were in close proximity with the theoretical values of C=N stretching frequencies.
3.6.9.3. C-N vibration
The C-N stretching vibrations of compound 2a, 2b and 2c were observed at the frequencies of 1207.32, 1180.35 and 1205.98 cm−1, respectively. The theoretically calculated values of C-N stretching of compounds 2a, 2b and 2c exhibit excellent concurrence with the experimental values 1179.9 cm−1 (2a), 1189.0 cm−1 (2b) and 1217.1 cm−1 (2c).
3.6.9.4. C-H vibration
The stretching frequency of C-H of diazole ring was observed at 3342.85, 3345.37 and 3353.77 cm−1 for compound 1, 2 and 3, respectively. The symmetric and asymmetric stretching of benzene C-H appeared at 3250.02 and 3241.86 cm−1 for compound 1, 3250.84 and 3241.37 cm−1 for compound 2, and 3255.97 and 3243.68 cm−1 for compound 3. The symmetric C-H stretching of CH3 was observed in compound 1 at 3080.79 and 3148.93 cm−1, and in compound 2 at 3081.97, 3054.58, 3064.12 and 3051.75 cm−1, whereas the asymmetric C-H stretching of CH3 was observed in compound 1 at 3202.61 and 3138.69 cm−1, and in compound 2 at 3192.80, 3128.99, 3146.36 and 3139.23 cm−1. The CH2 symmetric C-H stretching was observed at 3088.41 and 3063.20 cm−1 (compound 1), 3045.97 cm−1 (compound 2) and 3103.97 and 3083.84 cm−1 (compound 3), while CH2 asymmetric C-H stretching was observed at 3155.84 and 3112.04 cm−1 (compound 1), 3110.90 cm−1 (compound 2) and 3154.06 and 3144.76 cm−1 (compound 3). The symmetric and asymmetric C-H stretching frequency of CH2 of cyclohexane in compound 2 were observed at 3077.70 and 3104.61 cm−1, respectively. Moreover, the frequencies of C-H bending, scissoring, rocking, wagging and twisting are given in electronic supplementary material, data (electronic supplementary material, tables S4–S6).
4. Conclusion and prospective
The synthesis of benzimidazolium-based analogues with variation in the alkyl group at N-1 and N-3 positions have been successfully achieved in good yields. All the compounds were characterized by the spectroscopic techniques and found stable to air and moisture both in the solid as well as in solution state. Single crystal XRD of these prepared compounds showed that cations were connected with anions and waters by H-bonding, whereas the components of same nature were not interlinked by H-bonding in compounds. Water and anion act as bridge to connect cations with each other in compounds. Crystal packing of DBIC and BMBB was further stabilized by offset π⋯π stacking interactions, whereas no π⋯π stacking interactions of significant strength was found in IMBC. Hirshfeld surface analysis inferred that H⋯H contact was the most significant contributor of the crystal packing in compounds but the contribution of H⋯C contact was larger in DBIC as compared with in BMBB and IMBC. Void analysis predicted that the compounds will have good mechanical response. The computational findings supported the experimental spectral data, especially those of UV-Vis and IR spectra. Moreover, the results revealed that the the compound 2c was more stable than other compounds. Moreover, NLO response of compound 2b was better than compounds 2a and 2c.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding the work through group Research Project under grant number RGP1/170/44.
Contributor Information
Humaira Yasmeen Gondal, Email: humaira.yasmeen@uos.edu.pk.
Akbar Ali, Email: akbarali@gcuf.edu.pk.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Supplementary material is available online [90].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
S.J.: data curation, methodology; H.Y.G.: project administration, supervision, validation; A.A.: conceptualization, investigation, methodology, writing—original draft; A.H.: resources, software, validation; N.A.: visualization, writing—review and editing; M.N.T.: data curation, software, visualization, writing—original draft; M.A.: formal analysis, software, validation; A.R.R.: conceptualization, supervision; M.N.: data curation, visualization; S.M.: methodology, software, validation, visualization; Z.M.C.: formal analysis, investigation, resources; A.M.: data curation, formal analysis, investigation, software; M.Y.S.: investigation, resources, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received funding from Deanship of Scientific Research at King Khalid University of Saudi Arabia.
References
- 1.Ghandi K. 2014. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 4, 44-53. ( 10.4236/gsc.2014.41008) [DOI] [Google Scholar]
- 2.Giernoth R. 2010. Task-specific ionic liquids. Angew. Chem. Int. Ed. 49, 2834-2839. ( 10.1002/anie.200905981) [DOI] [PubMed] [Google Scholar]
- 3.Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM. 2015. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 86, 19-65. ( 10.1111/cbdd.12462) [DOI] [PubMed] [Google Scholar]
- 4.Yan YL, Cohen SM. 2007. Efficient synthesis of 5-amido-3-hydroxy-4-pyrones as inhibitors of matrix metalloproteinases. Org. Lett. 9, 2517-2520. ( 10.1021/ol0707665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JP, Yoo B, Suresh T, Kang MS, Vital R, Kim KJ. 2009. Novel silane-substituted benzimidazolium iodide as gel electrolyte for dye-sensitized solar cells. Electrochim. Acta 54, 4365-4370. ( 10.1016/j.electacta.2009.03.006) [DOI] [Google Scholar]
- 6.Sandeli AEK, Boulebd H, Khiri-Meribout N, Benzerka S, Bensouici C, Özdemir N, Gürbüz N, Özdemir İ. 2022. New benzimidazolium N-heterocyclic carbene precursors and their related Pd-NHC complex PEPPSI-type: synthesis, structures, DFT calculations, biological activity, docking study, and catalytic application in the direct arylation. J. Mol. Struct. 1248, 131504. ( 10.1016/j.molstruc.2021.131504) [DOI] [Google Scholar]
- 7.Katritzky AR, Jain R, Lomaka A, Petrukhin R, Karelson M, Visser AE, Rogers RD. 2002. Correlation of the melting points of potential ionic liquids (imidazolium bromides and benzimidazolium bromides) using the CODESSA program. J. Chem. Inf. Comput. Sci. 42, 225-231. ( 10.1021/ci0100494) [DOI] [PubMed] [Google Scholar]
- 8.Narasimhan B, Sharma D, Kumar P. 2012. Benzimidazole: a medicinally important heterocyclic moiety. Med. Chem. Res. 21, 269-283. ( 10.1007/s00044-010-9533-9) [DOI] [Google Scholar]
- 9.Hahn FE, Jahnke MC. 2008. Heterocyclic carbenes: synthesis and coordination chemistry. Angew. Chem. Int. Ed. 47, 3122-3172. ( 10.1002/anie.200703883) [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Gu S, Xia Q, Chen W. 2009. New structural motifs of silver and gold complexes of pyridine-functionalized benzimidazolylidene ligands. J. Organomet. Chem. 694, 2359-2367. ( 10.1016/j.jorganchem.2009.03.031) [DOI] [Google Scholar]
- 11.Lee HM, Chiu PL, Hu CH, Lai CL, Chou YC. 2005. Synthesis and structural characterization of metal complexes based on pyrazole/imidazolium chlorides. J. Organomet. Chem. 690, 403-414. ( 10.1016/j.jorganchem.2004.09.053) [DOI] [Google Scholar]
- 12.Yığıt B, Gök Y, Özdemır İ, Günal S. 2012. Synthesis and antimicrobial studies of 1-methyl-2-dimethylaminoethyl-substituted benzimidazolium salts and N-heterocyclic carbene–silver complexes. J. Coord. Chem. 65, 371-379. ( 10.1080/00958972.2012.654469) [DOI] [Google Scholar]
- 13.Rivas FM, Riaz U, Giessert A, Smulik JA, Diver ST. 2001. A versatile synthesis of substituted benzimidazolium salts by an amination/ring closure sequence. Org. Lett. 3, 2673-2676. ( 10.1021/ol016254m) [DOI] [PubMed] [Google Scholar]
- 14.Atlı DD. 2018. Synthesis and characterization of benzimidazolium salts bearing triazole groups. Celal Bayar Univ. J. Sci. 14, 57-60. [Google Scholar]
- 15.Türker F, Barut Celepci D, Aktaş A, Taslimi P, Gök Y, Aygün M, Gülçin İ. 2018. Meta-cyanobenzyl substituted benzimidazolium salts: synthesis, characterization, crystal structure and carbonic anhydrase, α-glycosidase, butyrylcholinesterase, and acetylcholinesterase inhibitory properties. Arch. Pharm. 351, 1800029. ( 10.1002/ardp.201800029) [DOI] [PubMed] [Google Scholar]
- 16.Yiğit M, Yiğit B, Taslimi P, Özdemir İ, Karaman M, Gulcin I. 2020. Novel amine-functionalized benzimidazolium salts: synthesis, characterization, bioactivity, and molecular docking studies. J. Mol. Struct. 1207, 127802. ( 10.1016/j.molstruc.2020.127802) [DOI] [Google Scholar]
- 17.Chaturvedi D. 2011. Recent developments on task specific ionic liquids. Curr. Org. Chem. 15, 1236-1248. ( 10.2174/138527211795202997) [DOI] [Google Scholar]
- 18.Lal AK, Milton MD. 2014. Designed benzimidazolium salts: modulation of fluorescence response towards metal cations in pure aqueous media. Sens. Actuators B 202, 257-262. ( 10.1016/j.snb.2014.05.037) [DOI] [Google Scholar]
- 19.Wang FM, et al. 2017. Robust benzimidazole-based electrolyte overcomes high-voltage and high-temperature applications in 5v-class lithium ion batteries. Chem. Mater. 29, 5537-5549. ( 10.1021/acs.chemmater.7b00824) [DOI] [Google Scholar]
- 20.Zhao Y, Bostrom T. 2015. Application of ionic liquids in solar cells and batteries: a review. Curr. Org. Chem. 19, 556-566. ( 10.2174/1385272819666150127002529) [DOI] [Google Scholar]
- 21.Hamide M, Gök Y, Demir Y, Yakalı G, Tok TT, Aktaş A, Sevinçek R, Güzel B, Gülçin İ. 2022. Pentafluorobenzyl-substituted benzimidazolium salts: synthesis, characterization, crystal structures, computational studies and inhibitory properties of some metabolic enzymes. J. Mol. Struct. 1265, 133266. ( 10.1016/j.molstruc.2022.133266) [DOI] [Google Scholar]
- 22.Concepcion O, et al. 2021. Facile synthesis of diversely functionalized peptoids, spectroscopic characterization, and DFT-based nonlinear optical exploration. ACS Omega 6, 26 016-26 025. ( 10.1021/acsomega.1c02962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali M, et al. 2021. Exploration of structural, electronic and third order nonlinear optical properties of crystalline chalcone systems: monoarylidene and unsymmetrical diarylidene cycloalkanones. J. Mol. Struct. 1241, 130685. ( 10.1016/j.molstruc.2021.130685) [DOI] [Google Scholar]
- 24.Khalid M, Ali A, Din ZU, Tahir MN, de Alcantara Morais SF, Braga AA, Akhtar MN, Imran M, Rodrigues-Filho E. 2021. β-hydroxy carbonyl compounds via aldol reaction: single crystal investigation and quantum chemical exploration for the unveiling of supramolecular behavior . Mol. Struct. 1241, 130650. ( 10.1016/j.molstruc.2021.130650) [DOI] [Google Scholar]
- 25.Khalid M, Ali A, Abid S, Tahir MN, Khan MU, Ashfaq M, Imran M, Ahmad A. 2020. Facile ultrasound-based synthesis, SC-XRD, DFT exploration of the substituted acyl-hydrazones: an experimental and theoretical slant towards supramolecular chemistry. ChemistrySelect 5, 14 844-14 856. ( 10.1002/slct.202003589) [DOI] [Google Scholar]
- 26.Khalid M, Ali A, Asim S, Tahir MN, Khan MU, Vieira LCC, Alexander F, Usman M. 2021. Persistent prevalence of supramolecular architectures of novel ultrasonically synthesized hydrazones due to hydrogen bonding [X–H⋯ O; X=N]: experimental and density functional theory analyses. J. Phys. Chem. Solids 148, 109679. ( 10.1016/j.jpcs.2020.109679) [DOI] [Google Scholar]
- 27.Khalid M, Ali A, Rehman MFU, Mustaqeem M, Ali S, Khan MU, Asim S, Ahmad N, Saleem M. 2020. Exploration of noncovalent interactions, chemical reactivity, and nonlinear optical properties of piperidone derivatives: a concise theoretical approach. ACS Omega 5, 13 236-13 249. ( 10.1021/acsomega.0c01273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan B, Khalid M, Shah MR, Tahir MN, Khan MU, Ali A, Muhammad S. 2019. Efficient synthesis by mono-carboxy methylation of 4,4′-biphenol, X-ray diffraction, spectroscopic characterization and computational study of the crystal packing of ethyl 2-((4′-hydroxy-[1,1′-biphenyl]-4-yl)oxy)acetate. ChemistrySelect 4, 9274-9284. ( 10.1002/slct.201901422) [DOI] [Google Scholar]
- 29.Khalid M, Ali A, De la Torre AF, Marrugo KP, Concepcion O, Kamal GM, Muhammad S, Al-Sehemi AG. 2020. Facile synthesis, spectral (IR, mass, UV–Vis, NMR), linear and nonlinear investigation of the novel phosphonate compounds: a combined experimental and simulation study. ChemistrySelect 5, 2994-3006. ( 10.1002/slct.201904224) [DOI] [Google Scholar]
- 30.Tariq S, Raza AR, Khalid M, Rubab SL, Khan MU, Ali A, Tahir MN, Braga AAC. 2020. Synthesis and structural analysis of novel indole derivatives by XRD, spectroscopic and DFT studies. J. Mol. Struct. 1203, 127438. ( 10.1016/j.molstruc.2019.127438) [DOI] [Google Scholar]
- 31.Khalid M, Ali A, Adeel M, Din ZU, Tahir MN, Rodrigues-Filho E, Iqbal J, Khan MU. 2020. Facile preparation, characterization, SC-XRD and DFT/DTDFT study of diversely functionalized unsymmetrical bis-ayl-α, β-unsaturated ketone derivatives. J. Mol. Struct. 1206, 127755. ( 10.1016/j.molstruc.2020.127755) [DOI] [Google Scholar]
- 32.Ali A, Din ZU, Khalid M, Tahir MN, Rodrigues-Filho E, Ali B, Asim S, Muhammad S. 2020. Crystal and quantum chemical exploration of the potent monocarbonyl curcuminoids to unveil their structural and intriguing electronic properties. ChemistrySelect 5, 3735-3745. ( 10.1002/slct.201904757) [DOI] [Google Scholar]
- 33.Ashfaq M, Tahir MN, Muhammad S, Munawar KS, Ali A, Bogdanov G, Alarfaji SS. 2021. Single-crystal investigation, Hirshfeld surface analysis, and DFT study of third-order NLO properties of unsymmetrical acyl thiourea derivatives. ACS Omega 6, 31 211-31 225. ( 10.1021/acsomega.1c04884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan I, Khalid M, Adeel M, Khan MU, Khan MS, Ahmad N, Ali A, Akram M. 2020. Palladium-catalyzed synthesis of pyrimidine substituted diaryl ethers through Suzuki Miyaura coupling reactions: experimental and DFT studies. Optik 219, 165285. ( 10.1016/j.ijleo.2020.165285) [DOI] [Google Scholar]
- 35.Ali A, Khalid M, Rehman MF, Haq S, Ali A, Tahir MN, Ashfaq M, Rasool F, Braga AA. 2020. Efficient synthesis, SC-XRD, and theoretical studies of O-benzenesulfonylated pyrimidines: role of noncovalent interaction influence in their supramolecular network. ACS Omega 5, 15 115-15 128. ( 10.1021/acsomega.0c00975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik AN, Kuznetsov A, Ali A, Ashfaq M, Tahir MN, Siddique A. 2022. Imine-based zwitterion: synthesis, single-crystal characterization, and computational investigation. J. Mol. Struct. 1253, 132237. ( 10.1016/j.molstruc.2021.132237) [DOI] [Google Scholar]
- 37.Tahir MN, Mirza SH, Khalid M, Ali A, Khan MU, Braga AAC. 2019. Synthesis, single crystal analysis and DFT based computational studies of 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidin-1-ium 3,4,5-trihydroxybenzoate-methanol (DETM). J. Mol. Struct. 1180, 119-126. ( 10.1016/j.molstruc.2018.11.089) [DOI] [Google Scholar]
- 38.Tahir MN, Ashfaq M, Alexander F, Caballero J, Hernández-Rodríguez EW, Ali A. 2019. Rationalizing the stability and interactions of 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidin-1-ium 2-hydroxy-3, 5-dinitrobenzoate salt. J. Mol. Struct. 1193, 185-194. ( 10.1016/j.molstruc.2019.05.003) [DOI] [Google Scholar]
- 39.Mumtaz S, Cano I, Mumtaz N, Abbas A, Dupont J, Gondal HY. 2018. Supramolecular interaction of non-racemic benzimidazolium based ion pairs with chiral substrates. Phys. Chem. Chem. Phys. 20, 20 821-20 826. ( 10.1039/C8CP03881C) [DOI] [PubMed] [Google Scholar]
- 40.Nisar M, Gondal HY, Munir S, Cheema ZM, Alhussain SA, Zaki ME. 2023. New task-specific ionic liquids (TSILs) as efficient bifunctional organocatalysts; synthesis, characterization and catalytic potential. J. Saudi Chem. Soc. 27, 101687. ( 10.1016/j.jscs.2023.101687) [DOI] [Google Scholar]
- 41.Gondal HY, Mumtaz S, Abbaskhan A, Mumtaz N, Cano I. 2020. New alkoxymethyl-functionalized pyridinium-based chiral ionic liquids: synthesis, characterization and properties. Chem. Pap. 74, 2951-2963. ( 10.1007/s11696-020-01135-z) [DOI] [Google Scholar]
- 42.Cheema ZM, Gondal HY, Raza AR, Abbaskhan A. 2020. Nucleophilic phenylation: a remarkable application of alkoxymethyltriphenylphosphonium salts. Mol. Divers. 24, 455-462. ( 10.1007/s11030-019-09966-z) [DOI] [PubMed] [Google Scholar]
- 43.Cheema ZM, Gondal HY, Siddiqui H, Choudhary MI. 2020. Solvent free synthesis of 1-alkoxyphosphonium chlorides for stereoselective multipurpose vinyl ethers. Phosphorus, Sulfur, Silicon Relat. Elem. 195, 37-42. ( 10.1080/10426507.2019.1633533) [DOI] [Google Scholar]
- 44.Nisar M, Gondal HY, Cheema ZM, Abbasskhan A. 2022. Lewis acid catalysed synthesis of alkoxymethyl halides for multipurpose mixed acetal. Lett. Org. Chem. 19, 750-756. ( 10.2174/1570178619666220112105145) [DOI] [Google Scholar]
- 45.Nisar M, Gondal HY, Cheema ZM, Yousaf S, Nadeem H. 2023. New azole-derived hemiaminal ethers as promising acetylcholinesterase inhibitors: synthesis, X-ray structures, in vitro and in silico studies. J. Biomol. Struct. Dyn. 41, 15 535-15 548. ( 10.1080/07391102.2023.2190805) [DOI] [PubMed] [Google Scholar]
- 46.Cheema ZM, Nisar M, Gondal HY, Alhussain SA, Zaki ME, Coldham I. 2023. New benzotriazole-derived α-substituted hemiaminal ethers with enhanced cholinesterase inhibition activity: synthesis, structural, and biological evaluations. J. Saudi Chem. Soc. 27, 101746. ( 10.1016/j.jscs.2023.101746) [DOI] [Google Scholar]
- 47.Sheldrick GM. 2015. SHELXT–integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 71, 3-8. ( 10.1107/S2053273314026370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheldrick GM. 2015. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 71, 3-8. ( 10.1107/S2053229614024218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spek AL. 2009. Structure validation in chemical crystallography. Acta Crystallogr. D Biol. Crystallogr. 65, 148-155. ( 10.1107/S090744490804362X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starikova OV, Dolgushin GV, Larina LI, Ushakov PE, Komarova TN, Lopyrev VA. 2003. Synthesis of 1,3-dialkylimidazolium and 1,3-dialkylbenzimidazolium salts. Russ. J. Org. Chem. 39, 1467-1470. ( 10.1023/B:RUJO.0000010563.79902.47) [DOI] [Google Scholar]
- 51.Eşme ASLI, Sağdınç SG. 2017. Spectroscopic (FT–IR, FT–Raman, UV–Vis) analysis, conformational, HOMO-LUMO, NBO and NLO calculations on monomeric and dimeric structures of 4–pyridazinecarboxylic acid by HF and DFT methods. J. Mol. Struct. 1147, 322-334. ( 10.1016/j.molstruc.2017.06.110) [DOI] [Google Scholar]
- 52.Muskawar PN, Kumar SS, Bhagat PR. 2013. Carboxyl-functionalized ionic liquids based on Benzimidazolium cation: study of Hammett values and catalytic activity towards one-pot synthesis of 1-amidoalkyl naphthols. J. Mol. Catal. A: Chem. 380, 112-117. ( 10.1016/j.molcata.2013.09.026) [DOI] [Google Scholar]
- 53.Frisch A, et al. 2009. Gaussian 09W. Wallingford, CT: Gaussian, Inc. [Google Scholar]
- 54.Lee C, Yang W, Parr RG. 1988. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785. ( 10.1103/PhysRevB.37.785) [DOI] [PubMed] [Google Scholar]
- 55.Nielsen AB, Holder A. 2009. Gauss view 5.0, user's reference. Pittsburgh: GAUSSIAN Inc. [Google Scholar]
- 56.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. 2012. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 17. ( 10.1186/1758-2946-4-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chemcraft. 2019 Chemcraft – graphical software for visualization of quantum chemistry computations. Version 1.8, build 682. See https://www.chemcraftprog.com (accessed 2019).
- 58.O'Boyle NM, Tenderholt AL, Langner KM. 2008. cclib: a library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839-845. ( 10.1002/jcc.20823) [DOI] [PubMed] [Google Scholar]
- 59.Lu T. 2014. Multiwfn. Software manual. Version 3, 6. See http://sobereva.com/multiwfn/old/3.6/Multiwfn_3.6.pdf. [Google Scholar]
- 60.May RA, Stevenson KJ. 2009. Software review of Origin 8. J. Am. Chem. Soc. 131, 872. ( 10.1021/Ja809638X) [DOI] [Google Scholar]
- 61.Groom CR, Bruno IJ, Lightfoot MP, Ward SC. 2016. The Cambridge structural database. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 72, 171-179. ( 10.1107/S2052520616003954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams TJ, et al. 2015. Mechanistic elucidation of the arylation of non-spectator N-heterocyclic carbenes at copper using a combined experimental and computational approach. Organometallics 34, 3497-3507. ( 10.1021/acs.organomet.5b00093) [DOI] [Google Scholar]
- 63.Patil S, Deally A, Gleeson B, Müller-Bunz H, Paradisi F, Tacke M. 2010. Synthesis, cytotoxicity and antibacterial studies of symmetrically and non-symmetrically benzyl- or p-cyanobenzyl-substituted N-heterocyclic carbene–silver complexes. Appl. Organomet. Chem. 24, 781-793. ( 10.1002/aoc.1702) [DOI] [Google Scholar]
- 64.Huynh HV, Lam TT, Luong HTT. 2018. Anion influences on reactivity and NMR spectroscopic features of NHC precursors. RSC Adv. 8, 34 960-34 966. ( 10.1039/C8RA05839C) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fatima T, Haque RA, Razali MR, Ahmad A, Asif M, Khadeer Ahamed MB, Abdul Majid AMS. 2017. Effect of lipophilicity of wingtip groups on the anticancer potential of mono N-heterocyclic carbene silver(I) complexes: synthesis, crystal structures and in vitro anticancer study. Appl. Organomet. Chem. 31, e3735. ( 10.1002/aoc.3735) [DOI] [Google Scholar]
- 66.Cremer D, Pople JA. 1975. General definition of ring puckering coordinates. J. Am. Chem. Soc. 97, 1354-1358. ( 10.1021/ja00839a011) [DOI] [Google Scholar]
- 67.Patil S, Deally A, Gleeson B, Müller-Bunz H, Paradisi F, Tacke M. 2011. Novel benzyl-substituted N-heterocyclic carbene–silver acetate complexes: synthesis, cytotoxicity and antibacterial studies. Metallomics 3, 74-88. ( 10.1039/c0mt00034e) [DOI] [PubMed] [Google Scholar]
- 68.Spackman PR, Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Jayatilaka D, Spackman MA. 2021. Crystal Explorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 54, 1006-1011. ( 10.1107/S1600576721002910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubab SL, et al. 2023. Synthesis, crystal structure, DFT calculations, Hirshfeld surface analysis and in silico drug-target profiling of (R)-2-(2-(1,3-dioxoisoindolin-2-yl)propanamido)benzoic acid methyl ester. Molecules 28, 4375-4394. ( 10.3390/molecules28114375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simsek O, Ashfaq M, Tahir MN, Ozturk S, Agar E. 2023. Synthesis and characterizations of the Schiff base derived from 2-hydroxy-5-nitrobenzaldehyde along with Hirshfeld surface analysis and computational study. J. Struct. Chem. 64, 942-953. ( 10.1134/S0022476623050128) [DOI] [Google Scholar]
- 71.Spackman MA, Jayatilaka D. 2009. Hirshfeld surface analysis. CrystEngComm 11, 19-32. ( 10.1039/B818330A) [DOI] [Google Scholar]
- 72.Ali A, et al. 2022. Synthesis, structural, and intriguing electronic properties of symmetrical bis-aryl-α,β-unsaturated ketone derivatives. ACS Omega 7, 39 294-39 309. ( 10.1021/acsomega.2c05441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ali A, et al. 2023. Acid catalyzed one-pot approach towards the synthesis of curcuminoid systems: unsymmetrical diarylidene cycloalkanones, exploration of their single crystals, optical and nonlinear optical properties. RSC Adv. 13, 4476-4494. ( 10.1039/D2RA07681K) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKinnon JJ, Jayatilaka D, Spackman MA. 2007. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. ChemComm. 37, 3814-3816. ( 10.1039/B704980C) [DOI] [PubMed] [Google Scholar]
- 75.Faihan AS, AlShammari RH, Ashfaq M, Muhammad S, Al-Jibori SA, Tahir MN, Hatshan MR, Al-Janabi AS, Al-Moayid SM. 2023. Synthesis, spectroscopic, crystallographic, quantum and molecular docking investigations of cis-4,5-diphenylimidazolidine-2-thione. J. Mol. Struct. 1286, 135633. ( 10.1016/j.molstruc.2023.135633) [DOI] [Google Scholar]
- 76.Al-Jibori SA, Al-Jibori GHH, Ashfaq M, Khalil T, Laguna M, Wagner C, Tahir MN, Al-Janabi ASM. 2023. Synthesis, characterization, crystal structure, Hirshfeld surface analysis of Cd(II)-1,2-benzisothiazol-3(2H)-one complexes. J. Mol. Struct. 1289, 135803. ( 10.1016/j.molstruc.2023.135803) [DOI] [Google Scholar]
- 77.Jelsch C, Ejsmont K, Huder L. 2014. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 1, 119-128. ( 10.1107/S2052252514003327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurbanova M, Ashfaq M, Tahir MN, Maharramov A, Dege N, Koroglu A. 2023. Synthesis, crystal structure, supramolecular assembly exploration by Hirshfeld surface analysis and computational study of 6-bromo-2-oxo-2H-chromene-3-carbonitrile (BOCC). J. Struct. Chem. 64, 302-313. ( 10.1134/S0022476623020142) [DOI] [Google Scholar]
- 79.Turner MJ, McKinnon JJ, Jayatilaka D, Spackman MA. 2011. Visualisation and characterisation of voids in crystalline materials. CrystEngComm 13, 1804-1813. ( 10.1039/C0CE00683A) [DOI] [Google Scholar]
- 80.Kurbanova M, Ashfaq M, Tahir MN, Maharramov A, Dege N, Ramazanzade N, Cinar EB. 2023. Synthesis, crystal structure, supramolecular assembly inspection by Hirshfeld surface analysis and computational exploration of 4-phenyl-6-(p-tolyl)pyrimidin-2(1H)-one (PPTP). J. Struct. Chem. 64, 437-449. ( 10.1134/S0022476623030095) [DOI] [Google Scholar]
- 81.Mumit MA, Pal TK, Alam MA, Islam MA, Paul S, Sheikh MC. 2020. DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2, 4, 5-trimethoxyphenylmethylene) hydrazinecarbodithioate. J. Mol. Struct. 1220, 128715. ( 10.1016/j.molstruc.2020.128715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elik MU, Serdaroğlu GO. 2017. A computational study of 1-substituted methyl 9-methyl-9H-pyrido[3,4-b]indole-3-carboxylate: quantum chemical descriptors, FMO and NBO analysis. Cumhuriyet Sci. J. 38, 138-155. ( 10.17776/csj.356185) [DOI] [Google Scholar]
- 83.Manjusha P, Prasana JC, Muthu S, Rizwana BF. 2020. Spectroscopic elucidation (FT-IR, FT-Raman and UV-visible) with NBO, NLO, ELF, LOL, drug likeness and molecular docking analysis on 1-(2-ethylsulfonylethyl)-2-methyl-5-nitro-imidazole: an antiprotozoal agent. Comput. Biol. Chem. 88, 107330. ( 10.1016/j.compbiolchem.2020.107330) [DOI] [PubMed] [Google Scholar]
- 84.Chand S, Al-Omary FA, El-Emam AA, Shukla VK, Prasad O, Sinha L. 2015. Study on molecular structure, spectroscopic behavior, NBO, and NLO analysis of 3-methylbezothiazole-2-thione. Spectrochim. Acta, Part A 146, 129-141. ( 10.1016/j.saa.2015.03.068) [DOI] [PubMed] [Google Scholar]
- 85.Cassidy C, Halbout JM, Donaldson W, Tang CL. 1979. Nonlinear optical properties of urea. Opt. Commun. 29, 243-246. ( 10.1016/0030-4018(79)90027-0) [DOI] [Google Scholar]
- 86.Atac A, Karabacak M, Karaca C, Kose E. 2012. NMR, UV, FT-IR, FT-Raman spectra and molecular structure (monomeric and dimeric structures) investigation of nicotinic acid N-oxide: a combined experimental and theoretical study. Spectrochim. Acta, Part A 85, 145-154. ( 10.1016/j.saa.2011.09.048) [DOI] [PubMed] [Google Scholar]
- 87.Politzer P, Truhlar DG. (eds) 2013. Chemical applications of atomic and molecular electrostatic potentials: reactivity, structure, scattering, and energetics of organic, inorganic, and biological systems. New York, NY: Springer Science & Business Media. [Google Scholar]
- 88.Politzer P, Murray JS. 2002. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 108, 134-142. ( 10.1007/s00214-002-0363-9) [DOI] [Google Scholar]
- 89.Arulaabaranam K, Muthu S, Mani G, Geoffrey AB. 2021. Speculative assessment, molecular composition, PDOS, topology exploration (ELF, LOL, RDG), ligand-protein interactions, on 5-bromo-3-nitropyridine-2-carbonitrile. Heliyon 7, e07061. ( 10.1016/j.heliyon.2021.e07061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jamil S, et al. 2024. Benzimidazolium quaternary ammonium salts: synthesis, single crystal and Hirshfeld surface exploration supported by theoretical analysis. Figshare. ( 10.6084/m9.figshare.c.7065749) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jamil S, et al. 2024. Benzimidazolium quaternary ammonium salts: synthesis, single crystal and Hirshfeld surface exploration supported by theoretical analysis. Figshare. ( 10.6084/m9.figshare.c.7065749) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Supplementary material is available online [90].