Abstract
Lipoprotein (LP) is a major component of the outer membrane of bacteria in the family Enterobacteriaceae. LP induces proinflammatory cytokine production in macrophages and lethal shock in LPS-responsive and -nonresponsive mice. In this study, the release of LP from growing bacteria was investigated by immuno-dot blot analysis. An immuno-dot blot assay that could detect LP at levels as low as 100 ng/ml was developed. By using this assay, significant levels of LP were detected in culture supernatants of growing Escherichia coli cells. During mid-logarithmic growth, approximately 1 to 1.5 μg of LP per ml was detected in culture supernatants from E. coli. In contrast, these culture supernatants contained 5 to 6 μg/ml of lipopolysaccharide (LPS). LP release was not unique to E. coli. Salmonella typhimurium, Yersinia enterocolitica, and two pathogenic E. coli strains also released LP during in vitro growth. Treatment of bacteria with the antibiotic ceftazidime significantly enhanced LP release. Culture supernatants from 5-h cultures of E. coli were shown to induce in vitro production of interleukin-6 (IL-6) by macrophages obtained from LPS-nonresponsive C3H/HeJ mice. In contrast, culture supernatants from an E. coli LP-deletion mutant were significantly less efficient at inducing IL-6 production in C3H/HeJ macrophages. These results suggest, for the first time, that LP is released from growing bacteria and that this released LP may play an important role in the induction of cytokine production and pathologic changes associated with gram-negative bacterial infections.
Lipopolysaccharide (LPS), or endotoxin, is an integral component of the outer membrane of all gram-negative bacteria. The biologically active lipid A moiety of LPS is believed to play a central pathogenic role in sepsis and septic shock due to gram-negative bacteria (34). LPS is released by different bacteria during both in vitro and in vivo growth, and this release is significantly enhanced when the bacteria are lysed following exposure to antibiotics or human serum (2, 15, 16, 29, 32, 42, 47, 48). LPS released from the microbial surface is also believed to be more biologically active than microbe-associated LPS (28). The in vivo release of LPS has been proposed to be an important mechanism for inducing septic shock (1, 3, 25, 43). Circulating LPS has been detected and implicated in a variety of septic states and, when injected into animals, can evoke pathophysiologic responses that resembles gram-negative-bacterium-induced septic shock (18, 30). LPS does not injure host tissues directly but, rather, through the actions of a variety of inflammatory mediators induced by LPS exposure (13).
Although LPS has been clearly documented to play a potentially important role in septic shock induced by gram-negative bacteria, very little is currently known about the function of other bacterially derived components in septic shock and/or in the induction of cytokine production. In fact, there is significant evidence that other components of gram-negative bacteria also play an important role in the pathology associated with infections mediated by these organisms (11, 17, 26, 44). Although many studies have addressed different aspects of LPS release from bacteria, little is currently known about the fate of other outer membrane components during bacterial growth. We have recently shown that bacterial lipoprotein (LP) is important in the induction and pathogenesis of septic shock. LP was shown to induce in vitro production of tumor necrosis factor alpha and interleukin-6 (IL-6) by mouse and human macrophages (51, 52) and to induce lethal shock and in vivo production of TNF-α and IL-6 in LPS-responsive and nonresponsive mice (53). More importantly, LP was shown to act synergistically with LPS to induce lethal shock and proinflammatory cytokine production, which suggests that LP and LPS activate cells via different mechanisms (53). LP is one of the most abundant proteins in the outer membranes of gram-negative bacteria of the family Enterobacteriaceae (10, 45). The possibility that LP, like LPS, is released by growing bacteria and/or lysed bacteria has never been investigated. Since LP can induce proinflammatory cytokine production, induce lethal shock, and act synergistically with LPS, the possibility that LP is released by growing and/or lysed bacteria becomes an important question for furthering our understanding of its role in the pathogenesis of gram-negative bacterial infections. In this report, we show that LP is released by growing bacteria and that this release is significantly enhanced when bacteria are exposed to the antibiotic ceftazidime. Additionally, we show that bacterial culture supernatants containing LP can induce IL-6 production in macrophages obtained from LPS-nonresponsive mice. These results suggest, for the first time, that LP, like LPS, can be released by growing or lysed bacteria and that this released LP may play an important role in the pathogenesis associated with gram-negative bacterial infections.
MATERIALS AND METHODS
Mice.
C3H/HeJ mice were purchased from Harlan Sprague Dawley (Indianapolis, Ind.). The mice were housed under specific-pathogen-free conditions. Female mice at 8 weeks of age were used in all experiments.
Bacteria.
Escherichia coli K-12 and an E. coli K-12 strain that is an LP deletion mutant were obtained from Barbara Bachmann, E. coli Genetic Stock Center (New Haven, Conn.). The E. coli LP deletion mutant (JE 5505) was previously characterized (23, 51). Yersinia enterocolitica WA (O:8), E. coli 51, E. coli 331, Salmonella typhimurium TML, Shigella flexneri SA100, Vibrio cholerae, and Pseudomonas aeruginosa were provided by Robert Brubaker, Department of Microbiology, Michigan State University, East Lansing, Mich., or obtained from sources reported previously (22, 38).
Bacterial cultures.
Bacteria were inoculated into brain heart infusion (BHI) broth (2 ml) and incubated for 16 to 18 h at 37°C with aeration. The overnight culture was used to inoculate 100 ml of fresh BHI broth, which was incubated at 37°C with vigorous shaking. At various time points postinoculation, cultures were either assessed for bacteria (CFU) or sampled for the amount of LP versus LPS present in the culture supernatants. To assess LP versus LPS release from bacteria, 5 ml of bacterial culture was removed and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was collected and assayed for LP and/or LPS content as described below. The bacterial pellet was reconstituted in 1 ml of 6 M guanidine HCl, sonicated for 5 min, and boiled for 10 min. The total volume was brought to 5 ml with phosphate-buffered saline (PBS) and then assessed for LP versus LPS content. To determine bacterial growth at various time points, 1 ml of bacterial culture was removed and 10-fold dilutions were made with PBS. From each dilution, 100 μl was plated onto L-agar plates, and colony counts were determined after overnight incubation at 37°C.
LP and LPS purification.
LP and LPS were purified from E. coli K-12 as previously described (51, 53). LPS was purified from bacteria by hot-phenol extraction. LPS contained no detectable protein, either by silver staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis slab gels or by the Coomassie blue binding assay. LP contained <25 pg of LPS/mg of protein.
Immuno-dot blot analysis.
Various dilutions of purified E. coli LP or bacterial culture supernatants diluted in PBS were loaded (200 μl per well) onto a nitrocellulose membrane under vacuum. The nitrocellulose membranes were blocked at 4°C overnight with 3% gelatin in 20 mM Tris buffer (pH 7.5) containing 0.5 M NaCl and 0.05% Tween 20 (TBST). The membranes were then incubated with a mouse monoclonal antibody (4C4) specific for LP (19) at 1 μg/ml in TBST for 2 h at room temperature. After the membranes were washed (with TBST) six times, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Bio-Rad) was added at a dilution of 1/3,000 in TBST and the mixture was incubated for 1 h at room temperature. Finally, the membranes were washed as described above and developed in an enhanced chemiluminescent substrate (Pierce, Rockford, Ill.) for 5 to 10 min at room temperature before being exposed to medical X-ray film (Fuji Medical Systems U.S.A., Inc., Stamford, Conn.). The LP content of bacterial preparations and supernatants was estimated by comparing the dot blot results with results obtained from serial dilutions of purified LP that was used as a standard in the dot blot assay for comparison.
LPS determination.
LPS was measured by the QCL-1000 Limulus amebocyte lysate (LAL) assay (BioWhittaker, Inc., Walkersville, Md.) as specified by the manufacturer. Briefly, 50 μl of the test sample was added in duplicate to 50 μl of LAL in a pyrogen-free microtiter plate. The mixture was incubated at 37°C for 10 min, and 100 μl of chromogenic substrate solution was added. After incubation at 37°C for 4 min, color development was terminated by addition of 100 μl of 20% acetic acid. The optical density was measured at 405 nm, and the LPS content was determined from a standard curve obtained with E. coli LPS. The sensitivity of the assay was 0.1 EU/ml.
Antibiotic treatment of bacteria.
E. coli cultures were treated with ceftazidime as described by Leeson et al. (29), with minor modifications. Briefly, 2 ml of Trypticase soy broth (BBL Microbiology Systems, Cockeysville, Md.) was inoculated with E. coli K-12 from an agar plate and incubated for 16 to 18 h at 37°C with orbital shaking at 175 rpm. A 0.2-ml sample of this overnight culture was inoculated into 50 ml of fresh Trypticase soy broth and incubated for 1.5 to 2 h at 37°C with shaking at 175 rpm until bacterial counts reached 3 × 108 to 4 × 108 CFU/ml. Ceftazidime in Trypticase soy broth was added at a final concentration of 20 μg/ml for the first 1.5 h of incubation and then at 600 μg/ml for the last 2.5 h of incubation. The culture was then centrifuged at 10,000 × g for 10 min to remove bacterial debris, and the resulting culture supernatant was assessed for LP content.
In vitro macrophage activation.
Peritoneal exudate macrophages were obtained from C3H/HeJ mice as previously described (53). Adherent macrophages were exposed to medium (RPMI 1640 supplemented with 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5% heat-inactivated fetal calf serum) containing various concentrations of bacterial cultural supernatant obtained from E. coli K-12 or from the E. coli LP deletion mutant (strain 5505). Macrophage culture supernatants were then obtained 8 h after exposure to these E. coli culture supernatants and immediately assessed for IL-6 production by enzyme-linked immunosorbent assay, as previously described (53).
RESULTS AND DISCUSSION
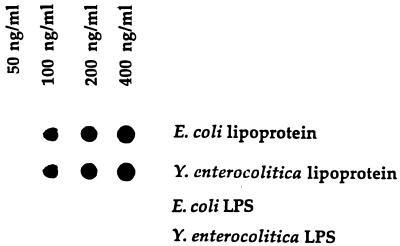
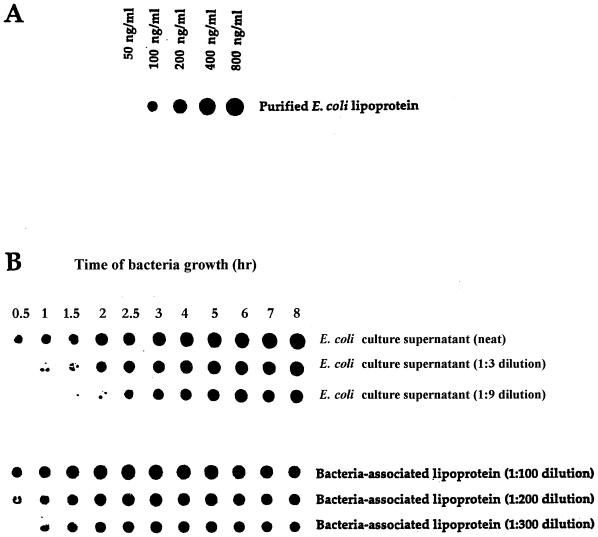
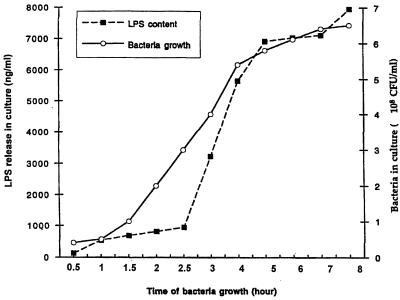
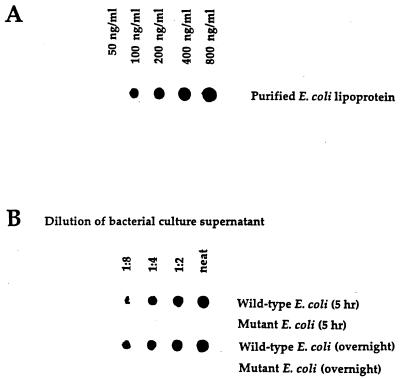
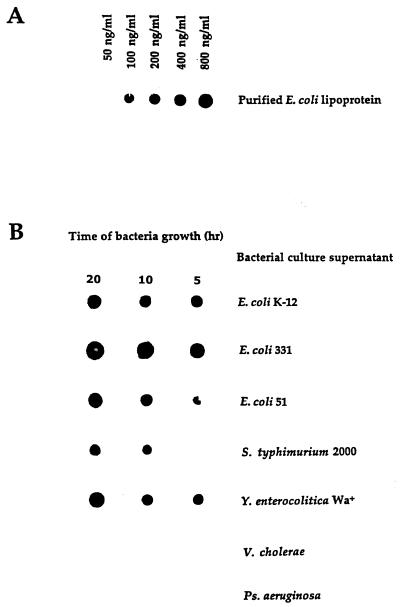
In initial experiments, we screened a number of monoclonal antibodies, specific for LP, for their capacity to function in a dot blot assay that could quantitatively detect purified LP present in BHI broth. Using monoclonal antibody 4C4, we developed a dot blot assay that could detect LP at levels as low as 100 ng/ml (Fig. 1). As seen in Fig. 1, LPS was not detected by this assay. Using this dot blot assay, we then assessed E. coli culture supernatants obtained at different times after initiation of bacterial growth for the presence of LP. As seen in Fig. 2, LP was present in bacterial culture supernatants and its concentration increased in a time-dependent fashion. LP release was detectable within 30 min of bacterial growth, and substantial levels of LP were released after 5 to 8 h of bacterial growth. This period corresponded to the exponential growth phase of these bacteria, which also was the time when the bacteria released LPS (Fig. 3). Using this dot blot assay, we estimated that 1 μg of LP per ml was released from E. coli K-12 after 4 h of culture. While we consider this estimate to be a valid approximation of the amount of LP released by bacteria under these conditions, a more precise determination will be made when a more quantitative assay is developed. This supernatant contained 5.7 μg of LPS per ml as determined by the LAL assay. Thus, LP is present at lower levels (1 to 1.5 μg/ml) than is LPS (5 to 6 μg/ml) in bacterial culture supernatants. However, this concentration of LP is biologically significant when one considers macrophage activation, where 1 ng of LP per ml can activate human or mouse macrophages (51–53). After 4 h of culture, E. coli (5.4 × 108) contained 28.6 μg of LPS (53 fg/bacterium) versus 75 μg of LP (148 fg/bacterium). Previous studies have estimated the amount of LPS associated with a single bacterium to be 30 to 40 fg (27, 36, 49). Our dot blot assay was specific for LP, since culture supernatant from an E. coli LP deletion mutant had no detectable LP (Fig. 4). However, this LP deletion mutant released LPS into the culture medium at levels equivalent to those observed for wild-type E. coli (Fig. 5). As seen in Fig. 6, LP release was not unique to E. coli K-12. S. typhimurium TML, Y. enterocolitica WA, and two pathogenic E. coli strains (331, an enteropathogenic E. coli strain, and 51, an enteroinvasive E. coli strain) also released LP into the culture medium during growth. Interestingly, these bacteria varied significantly in the amounts of LP present in their culture supernatants. E. coli 331 consistently released the highest LP levels. The significance of these findings is unclear and is currently being investigated. In contrast, no LP could be detected in culture supernatants obtained from bacteria (V. cholerae and P. aeruginosa) not in the family Enterobacteriaceae (Fig. 6).
FIG. 1.
Detection of LP by immuno-dot blot analysis. Purified E. coli LP and LPS in BHI broth were loaded onto nitrocellulose membranes and processed as described in Materials and Methods. Data are from one experiment that was representative of three separate experiments.
FIG. 2.
LP can be detected in culture supernatants obtained from growing E. coli. E. coli K-12 was grown in BHI medium. At various times, 5-ml samples were collected and assessed for one of the following: (i) number of bacteria (see Fig. 3), (ii) amount of LPS by the LAL assay (see Fig. 3), or (iii) amount of LP by the dot blot assay (Fig. 1). (A) Dot blot assay involving purified LP in BHI medium, was performed at the same time as the dot blot assay in panel B. (B) Dot blot assay of the amount of LP present in culture supernatants obtained at the times indicated. Culture supernatants were assessed undiluted or after being diluted 1:3 or 1:9 in PBS. Data are from one experiment that was representative of five separate experiments.
FIG. 3.
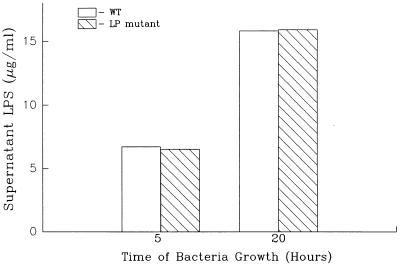
Bacterial growth and LPS release by E. coli K-12. Bacterial growth and LPS contents of bacterial culture supernatants at different times after culture initiation were determined as described in the legend to Fig. 2 and in Materials and Methods. Data are from one experiment that was representative of two separate experiments.
FIG. 4.
LP is not detectable in culture supernatants obtained from an E. coli LP deletion mutant. E. coli K-12 and an E. coli mutant (K-12 strain JE5055) that had a deletion of the lpp gene were grown, and the culture supernatant was assessed as described in the legend to Fig. 2. Data are from one experiment that was representative of two separate experiments.
FIG. 5.
An E. coli LP deletion mutant releases normal levels of LPS during growth. The LPS contents of culture supernatants obtained from wild-type E. coli and an E. coli LP deletion mutant were assessed as described in the legend to Fig. 3. There was no statistical difference (P > 0.05) between the amounts of LPS contained in culture supernatant obtained from wild-type E. coli and the LP deletion mutant. Data are from one experiment that was representative of three separate experiments.
FIG. 6.
LP is present in culture supernatants obtained from different bacteria from the family Enterobacteriaceae. The LP content in culture supernatants obtained from different bacteria was assessed as described in the legend to Fig. 2. Data are from one experiment that was representative of three separate experiments.
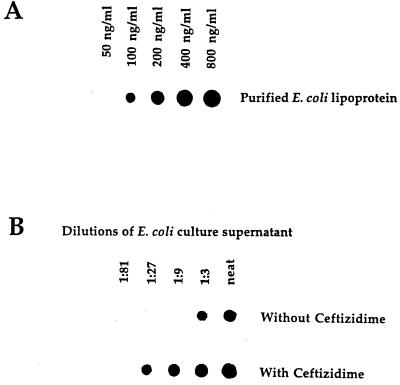
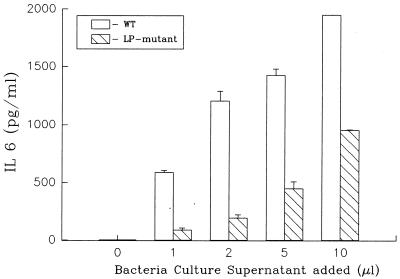
A number of studies have shown that antibiotic treatment of gram-negative bacteria results in an enhanced release of LPS (2, 15, 16, 29, 32, 42, 47, 48). To investigate whether LP release would also be enhanced following bacterial lysis, we treated E. coli with ceftazidime and assessed the culture supernatants for LP content. As seen in Fig. 7, treatment of E. coli with this antibiotic resulted in a significant increase in the amount of LP present in bacterial culture supernatants. Compared to control bacterial cultures (no antibiotic treatment), ceftazidime treatment resulted in an approximately ninefold increase in the amount of LP present in the culture supernatant. Thus, the above results indicate that LP is released by growing or lysed bacteria and is present at levels that we have previously shown would activate human and mouse macrophages (51–53). To further explore this issue, we performed experiments that assessed bacteria culture supernatants for their ability to induce cytokine production in mouse macrophages. To minimize the effects of LPS in these experiments, we used C3H/HeJ mice (LPS nonresponsive). Culture supernatants from E. coli K-12 and the LP deletion mutant E. coli were compared for their ability to induce IL-6 production in peritoneal exudate macrophages. As seen in Fig. 8, E. coli K-12 supernatant obtained at 4 h induced significant levels of IL-6 production in macrophages. In contrast, much lower levels of IL-6 were induced in macrophages exposed to equivalent volumes of culture supernatant obtained from the E. coli LP deletion mutant (undetectable levels of LP). These results suggest that LP, released by growing or from lysed bacteria, could play an important role in the induction of cytokine production and/or pathology associated with gram-negative bacterial infections. However, the biological significance of LP or LPS release in the complex pathology of gram-negative bacterial infections is currently unclear. This study is the first to show that LP is released by growing or lysed bacteria. This observation, coupled with the fact that LP can activate macrophages, induce lethal shock in mice, and act synergistically with LPS to induce these responses, suggests that LP may play a more important role in bacterial pathogenesis than was previously appreciated. In this regard, we have previously shown that heat-killed preparations of LP-deficient E. coli are much less efficient at inducing lethal shock in C3H/HeJ mice than are to heat-killed preparations of wild-type E. coli (53).
FIG. 7.
E. coli treated with ceftazidime shows an enhanced release of LP into culture supernatants. Culture supernatants obtained from untreated E. coli and E. coli treated with ceftazidime were assessed for levels of LP. The culture conditions and ceftazidime treatment are described in Materials and Methods. Data are from one experiment that was representative of three separate experiments.
FIG. 8.
Supernatants obtained from an E. coli LP deletion mutant are less efficient at inducing IL-6 production in macrophages than are supernatants obtained from wild-type E. coli. Peritoneal exudate macrophages were obtained from C3H/HeJ mice and exposed to different concentrations of culture supernatant obtained from wild-type E. coli and an E. coli LP deletion mutant. The supernatants obtained from wild-type and mutant E. coli had equivalent levels of LPS (wild-type, 6.4 μg/ml; mutant, 6.1 μg/ml). Macrophage culture supernatants were collected after 8 h of culture and assessed for IL-6 content by enzyme-linked immunosorbent assay as described in Materials and Methods. Levels of IL-6 in supernatant obtained from macrophages stimulated with any of the different concentrations of culture supernatant obtained from the E. coli LP deletion mutant were statistically different (P < 0.01) from levels of IL-6 in supernatant obtained from macrophages stimulated with culture supernatant obtained from wild-type E. coli. Data are from one experiment that was representative of three separate experiments.
Our results also indicate that other bacterial components that are also capable of activating macrophages, but different from LP or LPS, are released by growing bacteria. Different gram-negative bacteria have been shown to release or produce a number of proteins that could act either alone or in combination with each other or with LP and/or LPS to activate macrophages. In 1945, Binkley et al. (9) reported the isolation of protein from LPS-protein complexes and demonstrated that it was toxic to mice. Since this observation, a number of outer membrane components, different from LPS, have been characterized with regard to their capacity to activate different immune functions. Two such components are endotoxin protein (19, 20, 45) and lipid A-associated protein (7, 35). Endotoxin protein preparations were shown to contain at least 12 proteins, ranging in molecular size from 5 to 80 kDa (19). The predominant proteins present in endotoxin protein preparations were the porins, protein II, and LP (19). It is not known whether all of these proteins are also released by bacteria or synergize with each other to induce cytokine production and/or disease.
In contrast to our results and the above evidence that a number of bacterial proteins can activate monocytes and macrophages, Leeson et al. (29) have published data suggesting that LPS is the predominant proinflammatory mediator in supernatants of antibiotic-treated bacteria. In this study, LPS and proteins contained in bacterial culture supernatants obtained from antibiotic-treated bacteria were separated by isopycnic density gradient ultracentrifugation in cesium chloride or by velocity sedimentation in sucrose gradients. The predominant monocyte-stimulating activity was found in the LPS-containing fractions that were inhibited by polymyxin B. Surprisingly, the protein fractions obtained by these purification techniques had little to no activity with regard to activating monocytes. Since we have previously shown (51–53) that LP is as potent as LPS at activating human and mouse macrophages, LP and possibly other proteins may have been lost or altered as a result of the purification techniques used in this study. Alternatively, LP and/or other proteins may have aggregated and/or formed complexes with LPS.
LP is the most abundant protein in the outer membrane of bacteria in the Enterobacteriaceae (16, 45). Melchers et al. (33) were the first to show that LP was mitogenic for mouse B cells. Much of the current knowledge of the biologic properties of LP has come from work by Bessler and colleagues (4–6, 8, 21, 24). LP and synthetic lipopeptides activate mouse and human macrophages (21, 24). LPs from bacteria not in the Enterobacteriaceae could also play important roles in the pathologic findings in diseases caused by these bacteria. For example, spirochetal LPs are key inflammatory mediators in syphilis and Lyme disease. LPs obtained from Treponema pallidum and Borrelia burgdorferi or lipopeptides corresponding to the N-terminal domains of these LPs activate monocytes/macrophages, B cells, and endothelial cells (12, 31, 41, 46, 50). Interestingly, these LPs were shown to activate cells via signaling pathways and/or receptors that appear to be different from those used by LPS (37, 39, 40). The possibility that these LPs are also released during growth and/or lysis has not been fully explored.
In summary, we have shown that LP is released by growing bacteria and that this release is dramatically enhanced after treatment with the antibiotic ceftazidime. More importantly, LP is released at levels that can induce cytokine production in macrophages. Collectively, these results suggest that LP plays an important role in the pathology associated with infections caused by gram-negative bacteria from the family Enterobacteriaceae.
REFERENCES
- 1.Abernathy R S, Spink W W. Studies with Brucella endotoxin in humans: significance of susceptibility to endotoxin in the pathogenesis of brucellosis. J Clin Invest. 1958;37:219–231. doi: 10.1172/JCI103601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen B M, Solberg O. The endotoxin-liberating effect of antibiotics on meningococci in vitro. Acta Pathol Microbiol Scand Sect B. 1980;88:231–236. doi: 10.1111/j.1699-0463.1980.tb02633.x. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz F E, Vallabh P, Altman D I, Diamantes F, VanWyk H J, Stroucken J M. Jarisch-Herxheimer reaction in meningococcal meningitis. Am J Dis Child. 1983;137:599–603. doi: 10.1001/archpedi.1983.02140320075018. [DOI] [PubMed] [Google Scholar]
- 4.Bessler W G, Resch K, Hancock E, Hantke K. Induction of lymphocyte-proliferation and membrane changes by lipoprotein derivatives of the lipoprotein from the outer membrane of E. coli. Z Immunitaetsforsch. 1977;153:11–19. [PubMed] [Google Scholar]
- 5.Bessler W G, Ottenbreit B P. Studies on the mitogenic principal of lipoprotein from the outer membrane of E. coli. Biochem Biophys Res Commun. 1977;76:239–246. doi: 10.1016/0006-291x(77)90717-3. [DOI] [PubMed] [Google Scholar]
- 6.Bessler W G, Cox M, Lex A, Suhs B, Wiesmuller K H, Jung G. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol. 1985;135:1900–1912. [PubMed] [Google Scholar]
- 7.Betz S, Morrison D C. Chemical and biological properties of a protein-rich fraction of bacterial LPS: the in vitro murine lymphocyte response. J Immunol. 1977;119:1475–1480. [PubMed] [Google Scholar]
- 8.Biesert L, Scheuer W, Bessler W G. Interaction of mitogenic bacterial lipoprotein and a synthetic analogue with mouse lymphocytes: isolation and characterization of binding proteins. Eur J Biochem. 1987;162:651–657. doi: 10.1111/j.1432-1033.1987.tb10687.x. [DOI] [PubMed] [Google Scholar]
- 9.Binkley F, Goebel W F, Perlman E. Studies in the Flexner group of dysentery bacilli. II. The chemical degradation of the specific antigen of type Z Shigella paradysenteiae (Flexner) J Exp Med. 1945;81:331–341. doi: 10.1084/jem.81.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun V, Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43:89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter A, Evans T J, Buurman W A, Bremelmans M H, Moyes D, Cohen J. Differences in the shedding of soluble TNF receptors between endotoxin-sensitive and endotoxin-resistant mice in response to LPS or live bacterial challenge. J Immunol. 1995;155:2005–2012. [PubMed] [Google Scholar]
- 12.DeOgny L, Pramanik B C, Arndt L L, Jones J D, Rush J, Slaughter C A, Radolf J D, Norgard M V. Solid-phase synthesis of biologically active lipopeptides as analogs for spirochetal lipoproteins. Peptide Res. 1994;7:91–97. [PubMed] [Google Scholar]
- 13.Dinarello C A. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 14.DiRienzo J M, Nakamura K, Inouye M. The outer membrane proteins of gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–491. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- 15.Dofferhoff A S, Nijland J H, de Vries-Hospers H G, Mulder P O, Weits J, Bom V J. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in vitro and in vivo study. Scand J Infect Dis. 1991;23:745–754. doi: 10.3109/00365549109024303. [DOI] [PubMed] [Google Scholar]
- 16.Evans M E, Pollack M. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–1343. doi: 10.1093/infdis/167.6.1336. [DOI] [PubMed] [Google Scholar]
- 17.Evans T J, Strivens E, Carpenter A, Cohen J. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous Gram-negative infection. J Immunol. 1993;150:5033–5040. [PubMed] [Google Scholar]
- 18.Glauser M P, Zanetti G, Baumgartner J D, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–739. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 19.Goldman R C, White D, Leive L. Identification of outer membrane proteins including known lymphocyte mitogens of the endotoxin protein of E. coli O111. J Immunol. 1981;127:1290–1294. [PubMed] [Google Scholar]
- 20.Goodman G, Sultzer B. Further studies on the activation of lymphocytes by endotoxin protein. J Immunol. 1979;122:1329–1335. [PubMed] [Google Scholar]
- 21.Hauschildt S, Hoffmann P, Beuscher H U, Dufhues G, Henrich P, Wiesmuller K H, Jung G, Bessler W G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990;20:63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- 22.Hess C B, Niesel D W, Cho Y J, Klimpel G R. Bacterial invasion of fibroblasts induces IFN production. J Immunol. 1987;138:3949–3950. [PubMed] [Google Scholar]
- 23.Hirota Y, Suzuki H, Nishimura Y, Yasuda S. On the process of cellular division in E. coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci USA. 1977;74:1417–1421. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann P, Heinle S, Schade U F, Loppnow H, Ulmer A J, Flad H D, Jung G, Bessler W G. Stimulation of human and mouse adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology. 1988;177:158–170. doi: 10.1016/S0171-2985(88)80036-6. [DOI] [PubMed] [Google Scholar]
- 25.Hopkin D A. Too-rapid destruction of gram-negative organisms. Lancet. 1977;ii:603–604. doi: 10.1016/s0140-6736(77)91445-3. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins W J, Gendron-Fitzpatrick A, McCarthy D O, Haine J E, Uehling D T. LPS-responder and nonresponder C3H mouse strains are equally susceptible to an induced Escherichia coli urinary tract infection. Infect Immun. 1996;64:1369–1374. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurley J. Endotoxemia: methods of detection and clinical correlates. Clin Microbiol Rev. 1995;8:268–283. doi: 10.1128/cmr.8.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leeson M C, Morrison D C. Induction of proinflammatory responses in human monocytes by particulate and soluble forms of LPS. Shock. 1994;2:235–245. doi: 10.1097/00024382-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Leeson M C, Fujihara Y, Morrison D C. Evidence for lipopolysaccharide as the predominant proinflammatory mediator in supernatants of antibiotic-treated bacteria. Infect Immun. 1994;62:4975–4980. doi: 10.1128/iai.62.11.4975-4980.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowry S F. Sepsis and its complications, clinical definitions and therapeutic prospects. Crit Care Med. 1994;22:S1–S2. [PubMed] [Google Scholar]
- 31.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machowiak P A. Relationship between growth temperature and shedding of lipopolysaccharide by gram-negative bacteria. Eur J Clin Microbiol. 1984;3:406–410. doi: 10.1007/BF02017360. [DOI] [PubMed] [Google Scholar]
- 33.Melchers F, Braun V, Galanos C. The lipoprotein of the outer membrane of E. coli: a B lymphocyte mitogen. J Exp Med. 1975;142:473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 35.Morrison D C, Betz S J, Jacobs D M. Isolation of a lipid A bound polypeptide responsible for LPS-initiated mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976;144:840–850. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neidhardt F C. Chemical composition of Escherichia coli. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 3–6. [Google Scholar]
- 37.Norgard M V, Arndt L L, Akins D R, Curetty L L, Harrich D A, Radolf J D. Activation of human monocytic cells by Treponema palladium and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of LPS but involves the transcriptional activator NF-κB. Infect Immun. 1996;64:3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry R D, Brubaker R R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983;40:166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis: analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 40.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 41.Sellati T J, Abrescia L D, Radolf J D, Furie M B. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–3187. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shenep J L, Barton R P, Mogan K A. Role of antibiotic class in the rate of liberation of endotoxin during the therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1985;151:1012–1018. doi: 10.1093/infdis/151.6.1012. [DOI] [PubMed] [Google Scholar]
- 43.Shenep J L, Flynn P M, Barrett F F, Stidham G L, Westenkirchner D F. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 44.Stone R. Search for sepsis drugs goes on despite past failures. Science. 1994;264:365–366. doi: 10.1126/science.8153620. [DOI] [PubMed] [Google Scholar]
- 45.Sultzer B M, Goodman G W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976;144:821–830. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai K F, Ma Y, Weis J J. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tauber M G, Shibl A M, Hackbarth C J, Larrick J W, Sande M A. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J Infect Dis. 1987;156:456–462. doi: 10.1093/infdis/156.3.456. [DOI] [PubMed] [Google Scholar]
- 48.Tesh V L, Morrison D C. The interaction of Escherichia coli and normal human serum: factors affecting the capacity of serum to mediate lipopolysaccharide release. Microb Pathog. 1988;4:175–187. doi: 10.1016/0882-4010(88)90068-x. [DOI] [PubMed] [Google Scholar]
- 49.Watson S W, Novitsky T J, Quinby H L, Valois F W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977;33:940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weis J J, Ma Y, Erdile L F. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect Immun. 1994;62:4632–4636. doi: 10.1128/iai.62.10.4632-4636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Kaur I, Niesel D W, Seetharamaiah G S, Peterson J W, Prabhakar B S, Klimpel G R. Lipoprotein from Yersinia enterocolitica contains epitopes that crossreact with the human thyrotropin receptor. J Immunol. 1997;158:1976–1983. [PubMed] [Google Scholar]
- 52.Zhang H, Kaur I, Niesel D W, Seetharamaiah G S, Peterson J W, Justement L B, Prabhakar B S, Klimpel G R. Yersinia enterocolitica envelope proteins that are crossreactive with the thyrotropin receptor (TSHR) also have B-cell mitogenic activity. J Autoimmun. 1996;9:509–516. doi: 10.1006/jaut.1996.0068. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Peterson J W, Niesel D W, Klimpel G R. Bacterial lipoprotein and LPS act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–4878. [PubMed] [Google Scholar]