Abstract
Leaves of dark-grown corn (Zea mays) were illuminated for periods ranging from 3 minutes to 12 hours. The changes in the activities of ribose-5-phosphate isomerase, ribulose-5-phosphate kinase, and ribulose-1,5-diphosphate carboxylase were followed.
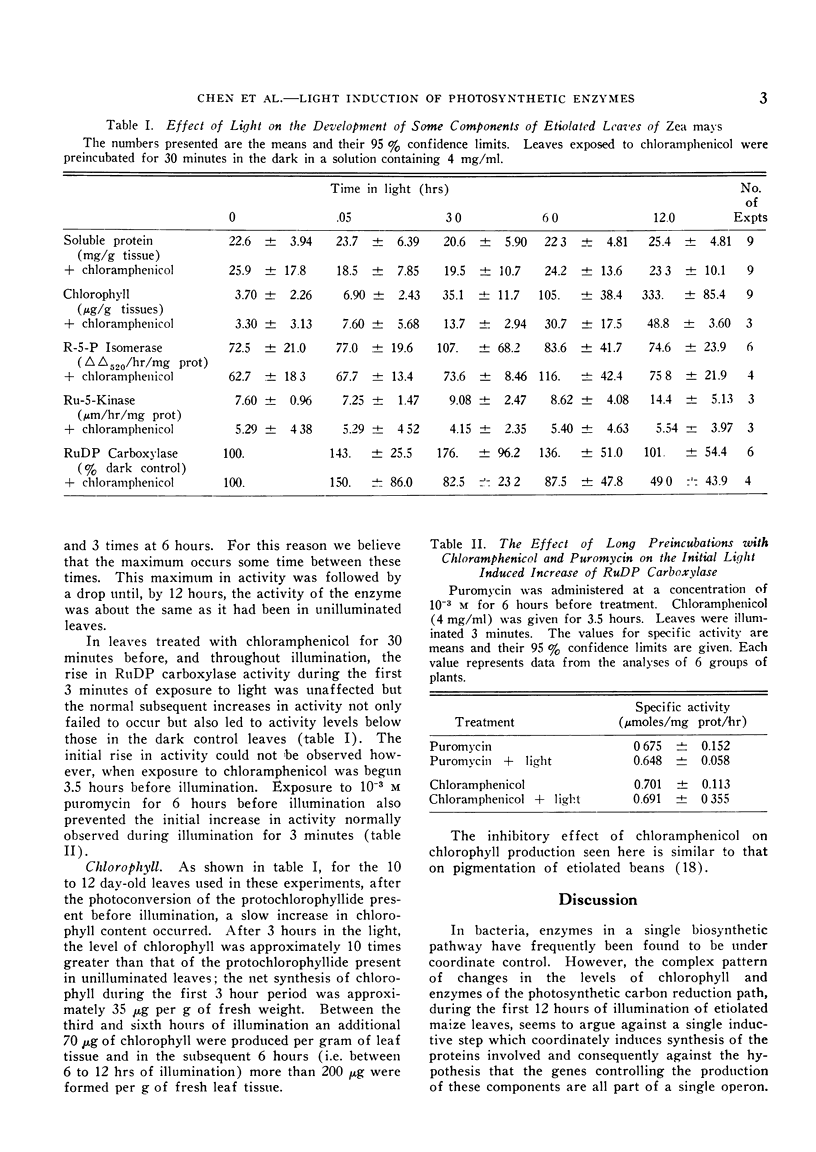
The activity of ribose-5-phosphate isomerase did not change significantly until between 12 and 24 hours of illumination. An increase in ribulose-5-phosphate kinase activity occurred after a lag of about 6 hours. The increase in carboxylase activity began after 3 minutes of illumination and increased until after 3 to 6 hours in the light, after which it began to decline. The increases in these enzymes appear to be the result of protein synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Briggs W. R. The relation between structure and pigments during the first stages of proplastid greening. Biochim Biophys Acta. 1966 Jan 4;112(1):45–53. doi: 10.1016/s0926-6585(96)90006-0. [DOI] [PubMed] [Google Scholar]
- Fuller R. C., Gibbs M. Intracellular and Phylogenetic Distribution of Ribulose 1,5-Diphosphate Carboxylase and D-Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Physiol. 1959 May;34(3):324–329. doi: 10.1104/pp.34.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL D. O., HUFFAKER R. C., SHANNON L. M., WALLACE A. Influence of light on dark carboxylation reactions in etiolated barley leaves. Biochim Biophys Acta. 1959 Oct;35:540–542. doi: 10.1016/0006-3002(59)90406-8. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Fernández-Morán H., Kieras F. J., Van Bruggen E. F. Electron microscopic and biochemical characterization of fraction I protein. Science. 1965 Dec 17;150(3703):1598–1601. doi: 10.1126/science.150.3703.1598. [DOI] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudock G. A., McLeod G. C., Moravkova-Kiely J., Levine R. P. The Relation of Oxygen Evolution to Chlorophyll and Protein Synthesis in a Mutant Strain of Chlamydomonas reinhardi. Plant Physiol. 1964 Nov;39(6):898–903. doi: 10.1104/pp.39.6.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Swift H., Bogorad L. Cytochemical studies concerning the occurrence and distribution of RNA in plastids of Zea mays. J Cell Biol. 1963 Jun;17:557–570. doi: 10.1083/jcb.17.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPKE D. W. Correlation of a soluble leaf protein with chlorophyll accumulation. J Biol Chem. 1962 Oct;237:3287–3291. [PubMed] [Google Scholar]
- LASCELLES J. The formation of ribulose 1:5-diphosphate carboxylase by growing cultures of Athiorhodaceae. J Gen Microbiol. 1960 Dec;23:499–510. doi: 10.1099/00221287-23-3-499. [DOI] [PubMed] [Google Scholar]
- Marcus A. Photocontrol of Formation of Red Kidney Bean Leaf Triphosphopyridine Nucleotide Linked Triosephosphate Dehydrogenase. Plant Physiol. 1960 Jan;35(1):126–128. doi: 10.1104/pp.35.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light Dependent Development of Seedlings of Phaseolus vulgaris var. Black Valentine, With Particular Reference to Development of Photosynthetic Activity. Plant Physiol. 1962 Jul;37(4):473–480. doi: 10.1104/pp.37.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Relationship Between Red Light Mediated Glyceraldehyde-3-Phosphate Dehydrogenase Formation and Light Dependent Development of Photosynthesis. Plant Physiol. 1965 Jan;40(1):57–61. doi: 10.1104/pp.40.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- SARKISSIAN I. V., HUFFAKER R. C. Depression and stimulation by chloramphenicol of development of carboxylating enxyme activity in inbred and hybrid barley. Proc Natl Acad Sci U S A. 1962 May 15;48:735–743. doi: 10.1073/pnas.48.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- TROWN P. W. AN IMPROVED METHOD FOR THE ISOLATION OF CARBOXYDISMUTASE. PROBABLE IDENTITY WITH FRACTION I PROTEIN AND THE PROTEIN MOIETY OF PROTOCHLOROPHYLL HOLOCHROME. Biochemistry. 1965 May;4:908–918. doi: 10.1021/bi00881a018. [DOI] [PubMed] [Google Scholar]


