Abstract
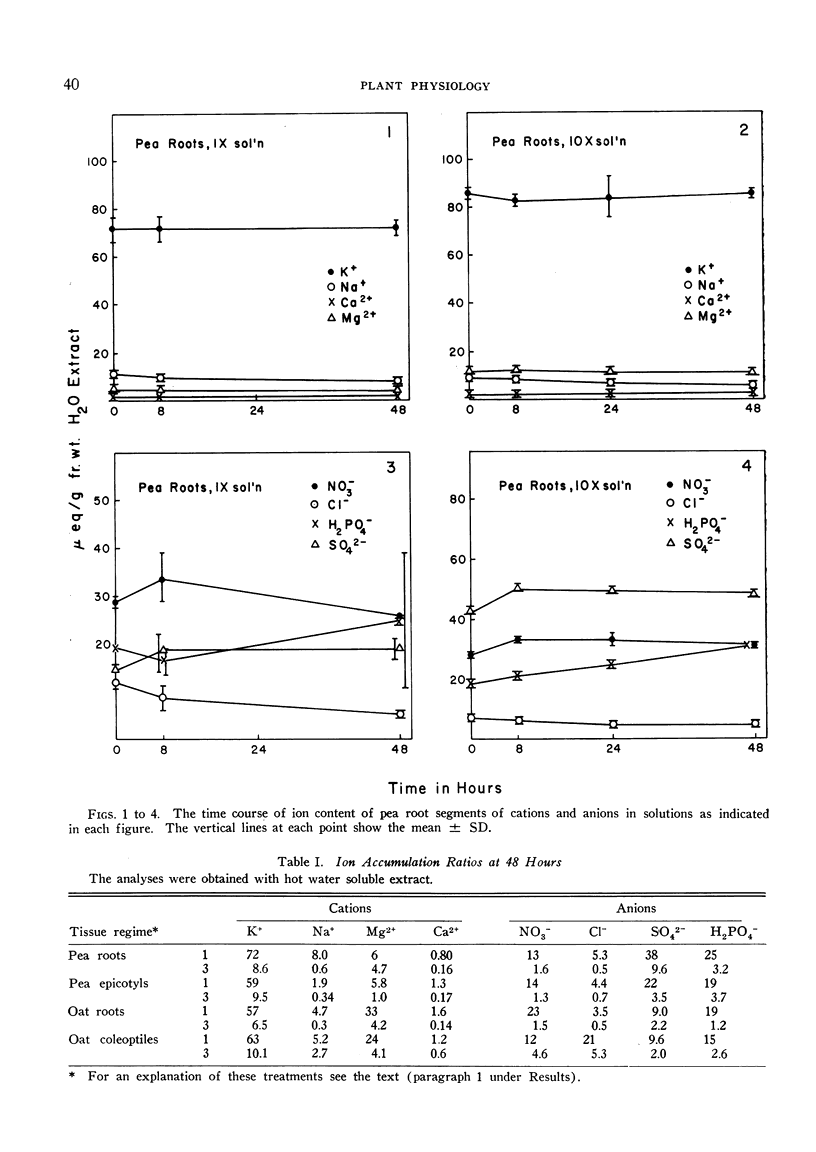
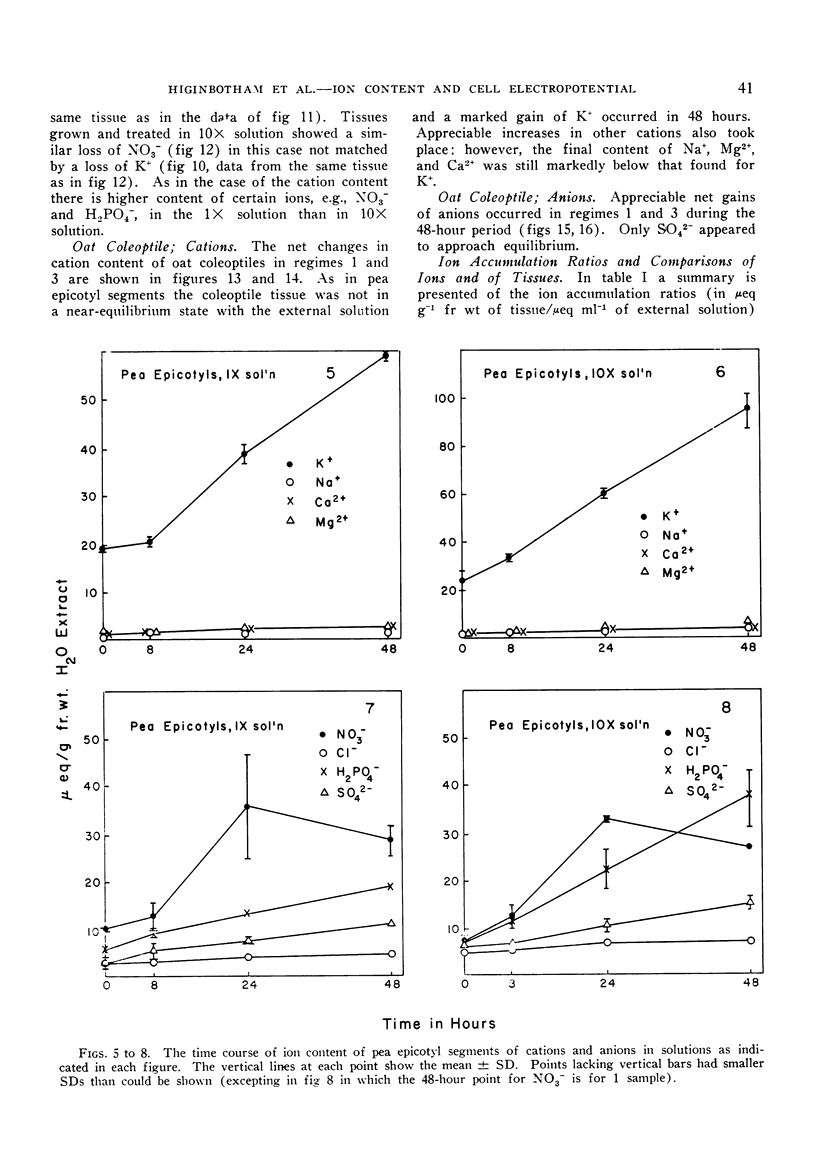
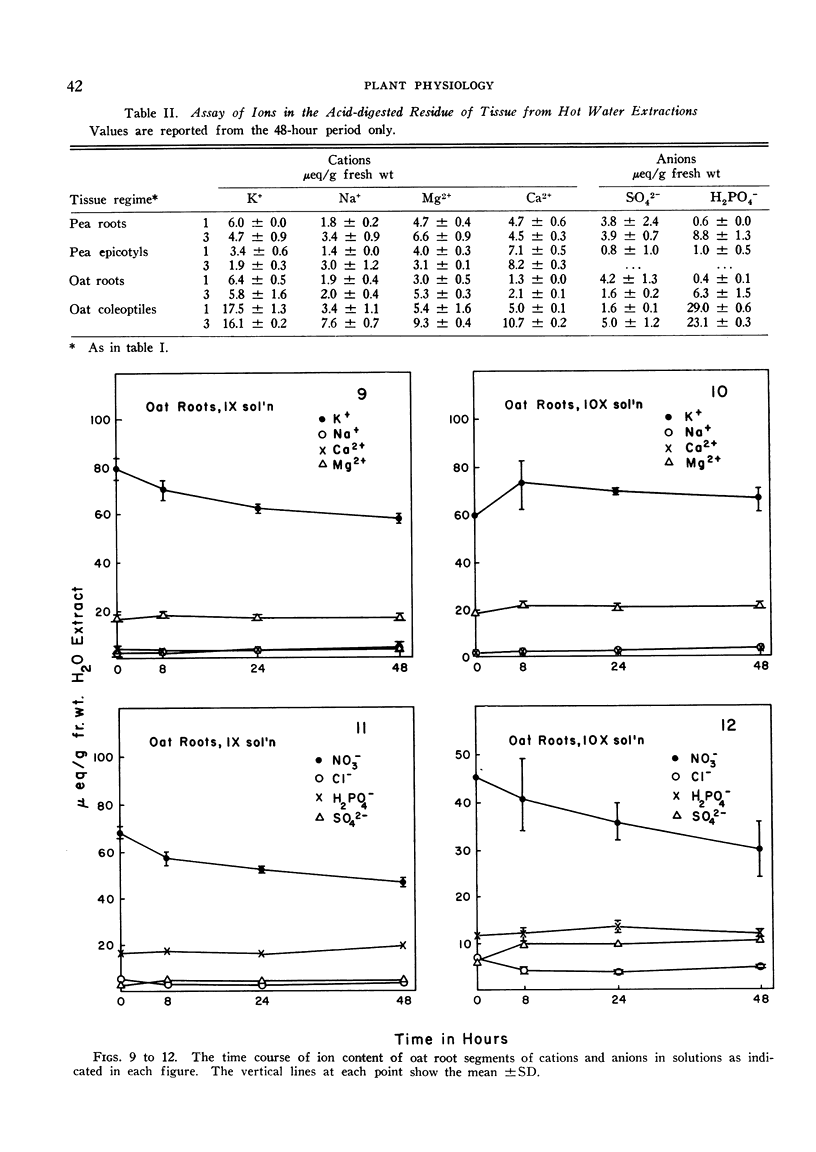
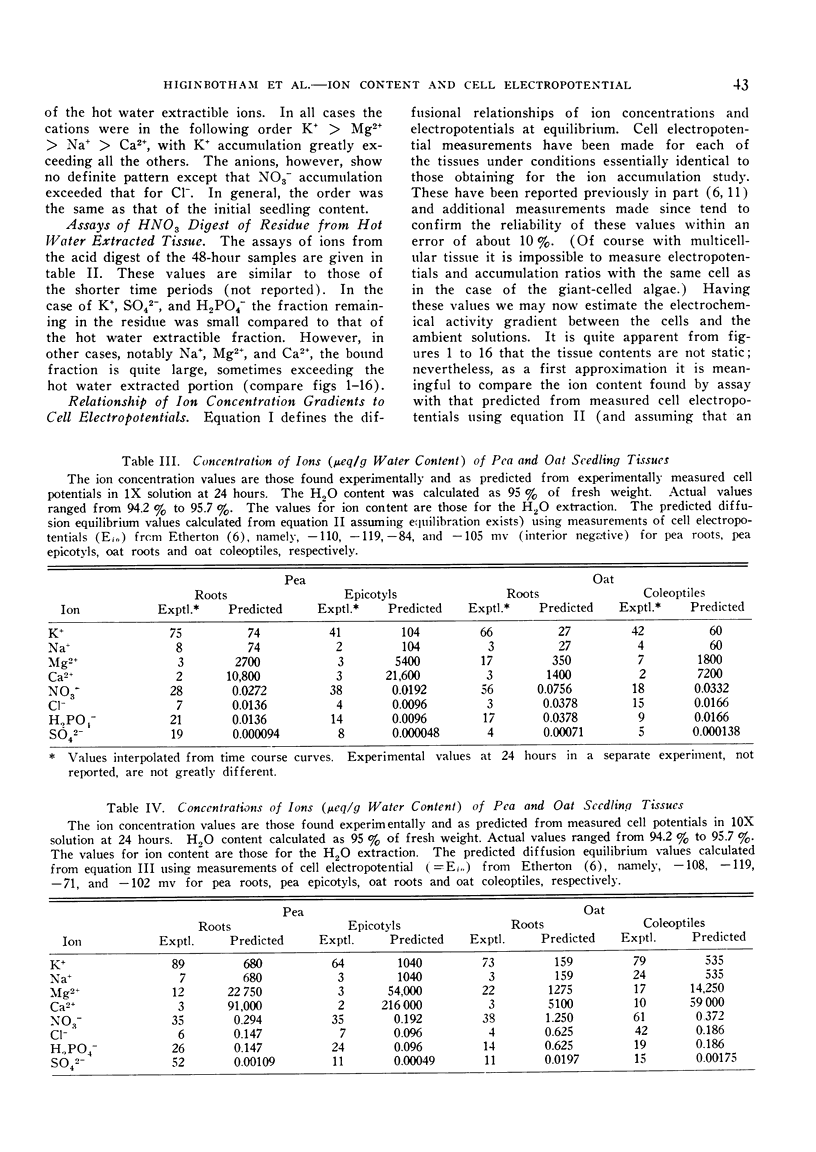
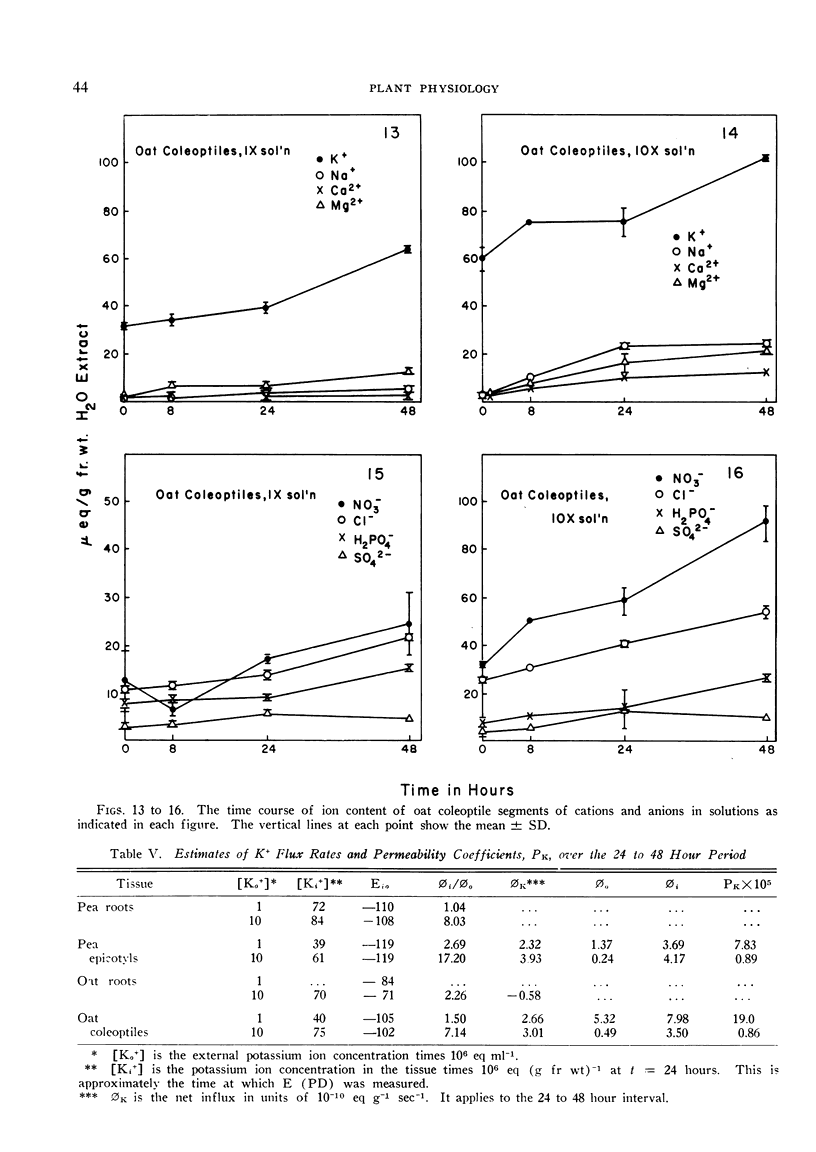
The relationships of concentration gradients to electropotential gradients resulting from passive diffusion processes, after equilibration, are described by the Nernst equation. The primary criterion for the hypothesis that any given ion is actively transported is to establish that it is not diffusing passively. A test was made of how closely the Nernst equation describes the electrochemical equilibrium in seedling tissues. Segments of roots and epicotyl internodes of pea (Pisum sativum var. Alaska) and of roots and coleoptiles of oat (Avena sativa var. Victory) seedlings were immersed and shaken in defined nutrient solutions containing eight major nutrients (K+, Na+, Ca2+, Mg2+, Cl−, NO3−, H2PO4− and SO42−) at 1-fold and 10-fold concentrations. The tissue content of each ion was assayed at 0, 8, 24, and 48 hours. A near-equilibrium condition was approached by roots for most ions; however, the segments of shoot tissue generally continued to show a net accumulation of some ions, mainly K+ and NO3−. Only K+ approached a reasonable fit to the Nernst equation and this was true for the 1-fold concentration but not the 10-fold. The data suggest that for Na+, Mg2+, and Ca2+ the electrochemical gradient is from the external solution to the cell interior; thus passive diffusion should be in an inward direction. Consequently, some mechanism must exist in plant tissue either to exclude these cations or to extrude them (e.g., by an active efflux pump). For each of the anions the electrochemical gradient is from the tissue to the solution; thus an active influx pump for anions seems required. Root segments approach ionic equilibrium with the solution concentration in which the seedlings were grown. Segments of shoot tissue, however, are far removed from such equilibration. Thus in the intact seedling the extracellular (wall space) fluid must be very different from that of the nutrient solution bathing the segments; it would appear that the root is the site of regulation of ion uptake in the intact plant although other correlative mechanisms may be involved.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ETHERTON B., HIGINBOTHAM N. Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science. 1960 Feb 12;131(3398):409–410. doi: 10.1126/science.131.3398.409. [DOI] [PubMed] [Google Scholar]
- Etherton B. Relationship of Cell Transmembrane Electropotential to Potassium and Sodium Accumulation Ratios in Oat and Pea Seedlings. Plant Physiol. 1963 Sep;38(5):581–585. doi: 10.1104/pp.38.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Hope A. B., Findlay G. P. Electrical Resistance of Cell Membranes of Avena coleoptiles. Science. 1964 Mar 27;143(3613):1448–1449. doi: 10.1126/science.143.3613.1448. [DOI] [PubMed] [Google Scholar]
- LLOYD A. G. Studies on sulphatases. 24. The use of barium chloranilate in the determination of enzymically liberated sulphate. Biochem J. 1959 May;72(1):133–136. doi: 10.1042/bj0720133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G., Macdonald I. R., Dainty J. Influence of the Counter-ion on the Absorption Isotherm for Chloride at Low Temperature. Plant Physiol. 1964 Mar;39(2):254–262. doi: 10.1104/pp.39.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J. The influence of the intracellular potential on potassium uptake by beetroot tissue. J Gen Physiol. 1966 Jan;49(3):551–563. doi: 10.1085/jgp.49.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]


