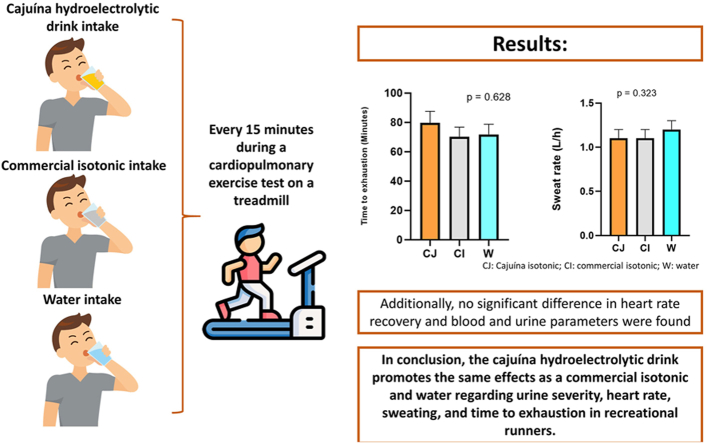
Abstract
Cajuína is a processed drink derived from cashew and is widely consumed in the northeast region of Brazil. This study evaluated the effect of a cajuína-based hydroelectrolytic drink on the aerobic performance and hydration status of recreational runners. Seventeen males (31.9 ± 1.6 years, 51.0 ± 1.4 ml/kg/min) performed three time-to-exhaustion running sessions on a treadmill at 70% VO2max, ingesting cajuína hydroelectrolytic drink (CJ), high carbohydrate commercial hydroelectrolytic drink (CH) and mineral water (W) every 15 min during the running test. The participants ran 80.3 ± 8.4 min in CJ, 70.3 ± 6.8 min in CH and 71.8 ± 6.9 min in W, with no statistical difference between procedures. Nevertheless, an effect size of η2 = 0.10 (moderate) was observed. No statistical difference was observed in the concentrations of sodium, potassium, and osmolality in both serum and urine between the three conditions. However, the effect size was moderate (urine sodium) and high (serum sodium, potassium, and osmolality). Urine specific gravity, sweating rate and heart rate were not significantly different between drinks. The cajuína-based hydroelectrolytic drink promotes similar effects compared to commercial hydroelectrolytic drink and water, considering specific urine gravity, heart rate, sweating, and time to exhaustion in recreational runners.
Keywords: Sports drink, Hydroelectrolytic beverage, Cashew, Physical performance, Hydration status
Graphical abstract
Highlights
-
•
Cajuína hydroelectrolytic drink, commercial isotonic, and mineral water had similar effect on performance.
-
•
No differences in time to exhaustion, heart rate, and sweating rate between drinks.
-
•
A cajuína hydroelectrolytic drink promotes the same effect as a commercial isotonic drink in recreational runners.
1. Introduction
Exercise-induced sweating can cause losses of 2%–6% of body mass, which characterizes a condition of dehydration, a phenomenon regarded to decrease physical performance (Pereira et al., 2017) and important health complications (Liska et al., 2019). Hypohydration before and during exercise potentiates these harmful effects, causing damage to athletes' performance (Batista and dos Santos, 2020). Therefore, adequate water intake before and during sports practice should be encouraged, as it helps in the delay of muscular fatigue, generates less effort perception, reduces the sensation of thirst, and decreases cardiovascular and thermal stress (Cheuvront and Kenefick, 2014).
High electrolytes water not only hydrates but also maintains electrolyte balance, promoting maintenance of extra and intracellular fluid volume and decreasing the incidence of cramps (Rowlands et al., 2021). The inclusion of carbohydrates in isotonic drinks enhances their ergogenic effect (Rowlands et al., 2021; Belval et al., 2019), as they contribute to the maintenance of blood glucose levels, leading to an improvement in physical performance, mainly in prolonged exercises (Vandenbogaerde and Hopkins, 2011). These commercial electrolyte replenishers have an osmolality between 200 and 320 mOsm/kg, around 90 mg of sodium, 24 mg of potassium and 84 mg of chloride in 200 ml of the solution, in addition to being consisted of 6%–9% of carbohydrates (Belval et al., 2019). The main disadvantage of most sports drinks is their high caloric content, mostly derived from sugars, which usually make up 6%–9% of the beverage (Belval et al., 2019).
Cajuína, a traditional drink in the northeast region of Brazil, is a processed juice made of blended cashew apples. It differs from the regular juice since it goes through stages of clarification and heat treatment, with no use of chemical substances during its production (Abreu and Neto, 2007). In addition, cajuína has good antioxidant capacity, with elevated levels of vitamin C (340.3 mg/100 ml), total phenolics (187.4 mg/100 ml), and flavonoids (13, 8 mg/100 ml) (Porto-Luz et al., 2019). This can bring additional benefit as it improves physical performance, considering that the consumption of antioxidant micronutrients helps combat exercise-induced oxidative stress, more specifically aerobic exercises (Pinho and da Silva, 2013). Cajuína is also investigated for antigenotoxic, anticlastogenic properties and antimutagenic properties (Melo-Cavalcante et al., 2011). Due to its nutritional and antioxidant nature, a cajuína-based hydroelectrolytic drink was developed by Universidade Federal do Piauí, in accordance with the Brazilian National Sanitary Vigilance Agency (ANVISA) and made available to the public in 2019 (Galvão, 2020).
A compromise in hydration status (i.e., body water loss) through increased sweating during exercise is well known for developing fatigue (Pereira et al., 2017). Drinking enough water before, during and after physical activity, adjusting fluid intake based on individual sweat rate and environmental conditions, consuming electrolyte-rich fluids to replace lost salts are recommended strategies to avoid dehydration (Deshayes et al., 2020). Consequently, relying solely on thirst to guide fluid consumption and ignoring individual hydration needs such as sweat rate, age, and body size, as well as environmental factors should be avoided (Périard et al., 2021).
The chemical properties cajuína support the hypothesis that it could provide ergogenic action similar or even superior to those of high carbohydrate commercial isotonic drinks, due to its antioxidant compounds, known to delay fatigue (Toscano et al., 2019). Thus, cajuína hydroelectrolytic drink may be a viable alternative strategy for hydration and improvement in the physical performance in athletes.
Therefore, this study aimed to evaluate the effect of cajuína hydroelectrolytic drink on electrolyte replenishment, hydration and aerobic performance in recreational runners compared to high carbohydrate commercial isotonic drinks and mineral water.
2. Methods
2.1. Subjects
Initially, 73 volunteers were recruited to undergo screening, of these 39 were elected to the study because they fully met the inclusion criteria. Of these, 19 agreed to participate in the study, but two volunteers were excluded from the study because they started to ingest food supplements and due to injury. Therefore, this study was conducted with 17 healthy adult male recreational runners (between 18 and 40 years old).
All participants were recreational runners, trained for at least one year, with a weekly frequency of at least five workouts. They had been training for at least three months without interruption. They had no chronic degenerative disease, endocrine and/or thermoregulatory disorders. They were not smokers, alcoholics, did not make continuous use of medications or vitamin-mineral supplements that altered the hydroelectrolyte balance and did not present any dysfunctions in their health history.
This is a clinical trial, randomized, controlled, crossover design. The sample size was calculated according to the protocol by Eng (2003) and considered a previous investigation by Pereira et al. (2017), which evaluated the hydration status of street runners who drank water in seven moments during a half-marathon event. The effect size was 0.87 and an α error of 0.05 and statistical power (β error) of 0.90 were adopted, resulting in a minimum sample size of 13 subjects.
As inclusion criteria, all participants should have at least one year of training in the modality, with a weekly frequency of five training sessions, of which at least three should be running, having been trained for at least three months uninterruptedly. They could not present any chronic degenerative disease, endocrine and/or thermoregulatory disorders, be smokers, be alcoholics, make continuous use of medication or vitamin-mineral supplements that alter the hydroelectrolytic balance, have dysfunctions in the health history or other problem that could compromise the physical integrity and the execution of the research. Participants who changed their usual eating and training habits, who did not ingest the exact amount of drink or controls provided, who had gastrointestinal intolerance and who did not participate in all experimental procedures were excluded.
This study was approved by the Research Ethics Committee of the Federal University of Piaui under number 2.883.417. All procedures were performed after the participants signed an informed consent form, in accordance with the legislation on ethics in research for human beings, of the National Health Council, Resolution 466/2012.
46.4 kcal of energetic value, 10.4 g of carbohydrate, 162 mg of sodium, and 89.4 mg of potassium per 200 ml.
2.2. Procedures
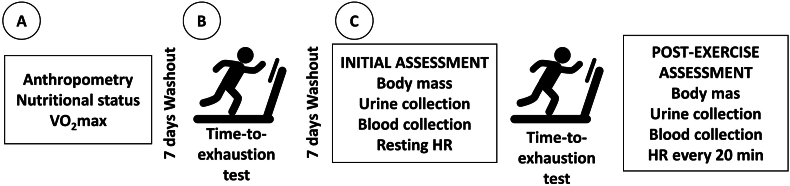
A schematic representation of the study design can be found in Fig. 1. Initially, the volunteers underwent anthropometrical and nutritional assessments, as well as a cardiopulmonary exercise test (CPET) to determine the maximum oxygen consumption (VO2max), to establish the speed at which they would perform the time-to-exhaustion tests. After seven days, they performed the time-to-exhaustion test, without hydration, to determine the volume of fluid lost by sweat, so that the same volume could be used for hydration in the experimental protocols.
Fig. 1.
Graphical scheme of the study design. HR, heart rate; VO2max, maximal oxygen uptake. Panel A: Anthropometric, nutritional, and aerobic evaluations. Panel B: Time-to-exhaustion test without hydration in order to assess the loss of fluid by sweat. Panel C: Experimental approach of the study.
This protocol consisted of determining the body weight loss during training (pre- and post-exercise weight difference) and thus being able to estimate the amount of liquid needed to hydrate the volunteers. It was assumed that 1 g of weight loss is equivalent to 1 ml of moisturizing solution, as proposed by Von Duvillard et al. (Von Duvillard et al., 2004). The final amount of fluid that was administered during the experimental protocol was equivalent to 100% of the weight lost during the protocol.
Seven days later, they were randomized (www.randomizer.org) and performed the time-to-exhaustion ingesting the following drinks: 1 – Cajuína hydroelectrolytic drink (CJ); 2 – high carbohydrate commercial isotonic (CI); and 3 – mineral water (W). All drinks had their temperature monitored and controlled. Time to exhaustion was the primary outcome. Urine volume and specific gravity, blood and urinary osmolality, blood and urinary sodium and potassium, in addition to body mass were also evaluated pre- and post-exercise. HR was monitored at three different periods: at rest, during the physical test, and during recovery, using the Team Pod@ Heart Monitor system (Firstbeat, Finland), according to Vanderlei et al. (2015). Resting HR was evaluated with the volunteers in seated position for 15 min before the exercise. The recovery HR was measured after the exhaustion test, following the same methodology as the resting HR, during 60 min. Data analysis was performed in three cuts of 20 min (20, 40, and 60 min), in order to assess the speed of HR recovery. The data used for the analysis of the recovery HR were the records of the last 5 min of each interval.
2.3. Pre-experimental procedures
The volunteers were instructed to refrain from any physical exercise 48 h prior to the CPET used for determining the aerobic capacity, as well as before the experimental sessions. Additionally, they were requested to refrain from the intake of alcoholic beverages and nutritional supplements during this period, as well as to maintain their usual eating habits and physical training protocols during the study period.
2.4. Cardiopulmonary exercise test and test to exhaustion protocol
Ergospirometric test was performed following the ramp protocol (Bruce et al., 2004) with incremental loads every 3 min to measure VO2max. The time-to-exhaustion tests were carried out in a controlled room in the morning, with a temperature of 23 ± 1.1 °C and a relative humidity of 52 ± 7% (Incoterm®, 7666.02.0.00), measured every 10 min during all experimental protocols. Participants were asked to wear light clothing during the tests and dried their sweat completely before post-test weighing.
The experimental tests followed a protocol designed to assess the running time to exhaustion previously adopted by Toscano et al. (2015), which consisted of starting a treadmill running session at a speed equivalent to 70% of VO2max, maintaining this speed for as long as possible. During the test, the Subjective Perception of Effort Scale with a score ranging from 6 to 20 proposed by Borg (1982) was applied. For this, test interruption criteria were considered: reaching the predicted maximum heart rate (HR), no increase in heart rate, or inability to continue the test (at this time, the athletes were stimulated to produce a maximum final effort).
Peak VO2 was considered the maximum consumption achieved in the last 30 s of the exercise. The lactate threshold was determined by the agreement of the V-Slope and Ventilatory Equivalent methods. The respiratory compensation point was determined from the moment of sustained fall in end-expiratory pressure of CO2 and increase in expiratory pressure of O2 (Herdy et al., 2016).
During the test, Borg's Rating of Perceived Exertion Scale was applied. The intensity of the exercise sessions was verified through the record of the HR using a Team System Pod @ Heart Monitor (Firstbeat, Finland) connected directly to the Firstbeat SPORTS Individual® software. HR was recorded at rest, during the physical test, and every 20 min of a 60 min post-exercise recovery period.
2.5. Hydration and supplementation protocol
Two hours before each experimental procedure, the participants ingested 500 ml of mineral water to ensure a standardized euhydration status for each experimental procedure. They were instructed to urinate in the period between this intake and the beginning of the test, following the recommendations by the National Athletic Trainer's Association (NATA) (McDermott et al., 2017). The volume of hydration that the CJ, CI, and W groups ingested during the experimental protocols was determined individually considering the amount of sweat after the CPET test performed seven days before the first experimental protocol and under similar climatic conditions. That is, each participant ingested an individualized and controlled amount of liquid every 15 min. In order to calculate the volume of liquid that would be ingested by each participant, the difference in body mass before and after the race was calculated. It was assumed that 1 g of body mass loss is equivalent to 1 ml of hydrating solution, as proposed by Von Duvillard et al. (Von Duvillard et al., 2004). The final amount of liquid that was administered during the three experimental protocols was equivalent to 100% of the lost body mass.
The experimental protocols used a Cajuína hydroelectrolytic drink, a passion-fruit-flavored commercial hydroelectrolytic drink and mineral water. It contains water, cashew juice (cajuína), saccharose, sodium chlorite, sodium citrate, potassium phosphate and citric acid as main ingredients. The cajuína hydroelectrolytic drink has 46.4 kcal of energetic value, 10.4 g of carbohydrate, 162 mg of sodium, and 89.4 mg of potassium per 200 ml servings (Galvão, 2020). Additionally, it contains osmolality of 270.97 ± 0.05 mOs-mol/Kg, sodium concentration of 810.0 ± 0.87 mg/L and potassium around 447.0 ± 0.76 mg/L. The hydroelectrolytic drink was developed at Universidade Federal do Piauí in accordance with the guidelines proposed by the Brazilian National Sanitary Vigilance Agency in 2019. The commercial isotonic drink has 47 kcal of energetic value, 12 g of carbohydrate, 99 mg of sodium, and 28 mg of potassium per 200 ml servings, according to the manufacturer. The mineral bottled water contains 12 mg/L of calcium, 6.1 mg/L of magnesium, 9.6 mg/L of potassium and 3.1 mg/L of sodium, according to the information provided by the manufacturer.
During the exhaustion test, the volunteers were submitted to a programmed hydration at regular intervals every 15 min, starting from the fifteenth minute of exercise until the conclusion at exhaustion. The participants were familiar with the process of drinking water during running. The drink was divided into an individual and personal volume for each runner, so that the total ingestion of the drink was made even before exhaustion. The drinks were labeled in equal portions and stored in squeeze bottles (Plasútil®, Brazil) to prevent any loss of liquid during ingestion, as the drink was ingested during the performance of the physical test. The temperature of the drinks provided was 10 ± 2 °C, as proposed by McDermott et al. (2017).
2.6. Anthropometric assessment
Immediately before the exercise, body mass (kg) and height (cm) were measured, both in triplicate, aiming to minimize intra- and interpersonal errors. Body mass was assessed both before and immediately after the time-to-exhaustion. The clothes and skin of the participants were thoroughly dried before the post-exercise weighing.
2.7. Sweating rate
The sweating rate (SR) was calculated according to the following equation proposed by Murray (1996):
In which: IM: initial body mass in grams; FM: final body mass in grams; FI: volume of fluid ingested in milliliters; U: volume of urine produced in milliliters; T: exercise duration time in minutes. After obtaining the SR in milliliters/minute (mL/min), a conversion to liters/hour (L/h) was performed.
2.8. Blood collection and analysis
Blood collection was performed by a trained and experienced nurse before the time-to-exhaustion test, with the volunteers still at rest, and immediately after the test. Eight ml of venous blood were collected and placed in two vacuum tubes, one containing clot activator and the other lithium heparin vacuette ® (São Paulo, Brazil). The samples were centrifuged at 2500 rpm for 15 min and the supernatant (serum or plasma) transferred to a microtube and refrigerated at −20 °C until analysis.
2.9. Urinary osmolality
Urinary osmolality (mOsm/kg) was measured through the freezing point of the sample using an osmometer, model 5004 Micro-Osmette (Precision Systems®, USA). To determine hydration through plasma osmolality, a cutoff point of <290 mOsm/kg was used for the hydration status (euhydration), as established by Sawka et al. (2007).
2.10. Serum sodium and potassium
Sodium and potassium electrolyte concentrations were analyzed using the ion-selective electrode method with the AU680 device (Beckman Coulter®, United States).
2.11. Urine collection and analysis
Urine was collected at rest before the exhaustion test and immediately after this test. Participants were instructed to empty their bladders at both times. They received plastic bottles with a volume meter and were instructed on the proper procedures for urine collection, minimizing the risk of contamination. After collection, volumes were recorded and samples were placed in standard vials and kept refrigerated at 4 °C until analysis.
Approximately 40 μL of each urine sample were analyzed in triplicate, using a digital refractometer model RTP-20ATC (Instrutherm®, São Paulo, Brazil) to determine the urine specific gravity (USG). Considering the USG in UOsmols, the participants were classified as (McDermott et al., 2017): USG <1010 = well hydrated, 1011 ≤ USG ≤1020 = minimally dehydrated, 1021 ≤ USG ≤1030 = significantly dehydrated, and USG ≥1031 = severely dehydrated.
2.12. Urinary sodium and potassium concentrations
The sodium and potassium electrolyte concentrations were analyzed using the ion-selective electrode method using the AU680 device (Beckman Coulter®, United States).
2.13. Statistical analysis
Data are presented as mean and standard error of the mean. Initially, to verify normality and homogeneity, Shapiro-Wilk and Levene tests were performed, respectively. ANOVA for repeated measures with Tukey post hoc test was conducted to compare the differences between groups. One-way ANOVA was used to compare initial conditions. A 5% confidence level (p < 0.05) was adopted for all tests. Partial eta-squared (η2) was used to examine the effect size. Values of 0.01 to <0.06 was considered small, 0.06 to <0.14 moderate, and 0.14 or higher considered as a large effect size (Cohen, 1988). The procedures were analyzed using the IBM SPSS Statistics 24.0 software.
3. Results
The participants were young adults, eutrophic and had excellent VO2max levels in the health-oriented classifications (Ferretti, 2014), but were below the levels of high-performance runners (González-Mohíno et al., 2020), confirming that they were recreational level athletes (Table 1). At the moments prior to the experimental procedures, the volunteers were statistically equal when the resting HR was evaluated (p > 0.05), as well as all other urinary and blood variables (p > 0.05), as seen in Table 2.
Table 1.
Characterization of the participants.
| Variables | Mean ± Standard error |
|---|---|
| Age (years) | 31.9 ± 1.6 |
| Body mass (kg) | 72.4 ± 2.0 |
| Height (m) | 1.70 ± 0.01 |
| BMI (kg/m2) | 24.7 ± 0.6 |
| VO2max (ml/kg/min) | 51 ± 1.4 |
Data are presented as mean and standard error of the mean. VO2max: Maximum oxygen consumption.
Table 2.
Conditions of the participants before the experimental protocols.
| CJ | CH | W | p | η2 | |
|---|---|---|---|---|---|
| HR at rest (bpm) | 69.5 ± 2.5 | 68.1 ± 2.0 | 65.8 ± 2.5 | 0.538 | 0.03 |
| Urine volume (ml) | 224.6 ± 49.2 | 208.4 ± 39.3 | 202.9 ± 48.8 | 0.942 | <0.01 |
| USG (g/ml) | 1016 ± 3.1 | 1017 ± 2.7 | 1018 ± 3.2 | 0.912 | <0.01 |
| Urinary sodium (mEq/L) | 71.6 ± 13.6 | 75.1 ± 13.4 | 79.7 ± 11.2 | 0.903 | 0.01 |
| Urinary potassium (mEq/L) | 27.3 ± 5.9 | 24.2 ± 4.7 | 32.8 ± 5.3 | 0.518 | 0.03 |
| Urine osmolality (mOsm/kg) | 432.9 ± 83.1 | 416.9 ± 59.3 | 503.4 ± 81.3 | 0.692 | 0.02 |
| Blood sodium (mEq/L) | 137.6 ± 0.5 | 136.7 ± 0.3 | 136.6 ± 0.6 | 0.331 | 0.06 |
| Blood potassium (mEq/L) | 4.3 ± 0.1 | 4.4 ± 0.1 | 4.5 ± 0.1 | 0.893 | <0.01 |
| Blood osmolality (mOsm/kg) | 279.8 ± 1.3 | 278.2 ± 1.1 | 279.8 ± 1.5 | 0.655 | 0.02 |
Data are presented as mean and standard error of the mean. No statistical differences were observed between the three pre-experimental conditions. One-way ANOVA was used. HR: Heart rate; USG: Urine specific gravity; BMI: Body mass index; USG: Urine specific gravity; CJ: cajuína hydroelectrolytic drink; CH: high carbohydrate commercial hydroelectrolytic drink; W: mineral water; η2: effect size.
The time-to-exhaustion test had an average speed of 10.3 ± 0.2 km/h. Regarding hydration, water intake was 1357.1 ± 134.4 ml in 5.7 ± 0.3 moments in which this volume was provided.
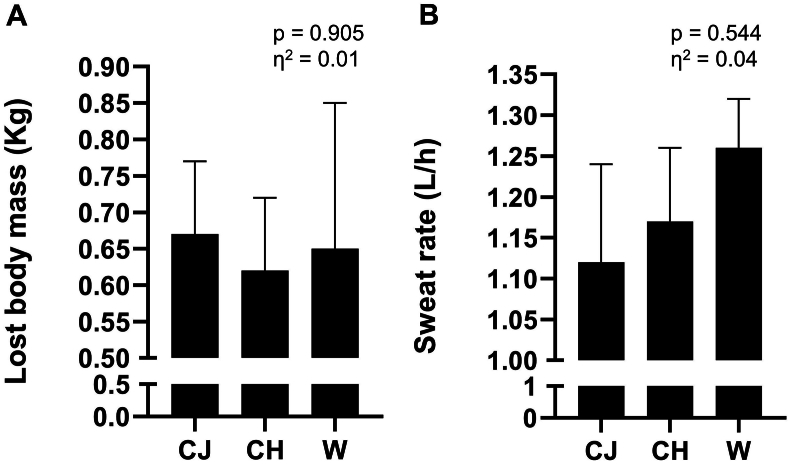
The hydration status of the participants after the time-to-exhaustion tests is represented by the change in body mass and sweating rate. Lost body mass represented a reduction of 0.9 ± 0.1 % after the test in the CJ protocol, which was similar to the 0.85 ± 0.2 % loss in the CI condition and compared to the 0.9 ± 0.2 % loss in the W condition (Fig. 2, Panel A; p > 0.05; η2 = 0.01, classified as small). The reduction in body mass in the three procedures indicated that there was no significant dehydration, according to the classification proposed by McDermott et al. (2017). This also indicates that the volume of hydration in the three conditions was sufficient. There was no significant change in the sweating rate between the three procedures (Fig. 2, Panel B; p > 0.05; η2 = 0.04, classified as small).
Fig. 2.
Hydration status of the participants. Panel A – body mass lost after the exhaustion test; Panel B – sweating rate. Data are presented as mean and standard error of the mean. There were no statistical differences between the procedures for the two variables analyzed (p > 0.05). Repeated measures ANOVA was used. CJ: cajuína hydroelectrolytic drink; CH: high carbohydrate commercial hydroelectrolytic drink; W: mineral water; η2: effect size.
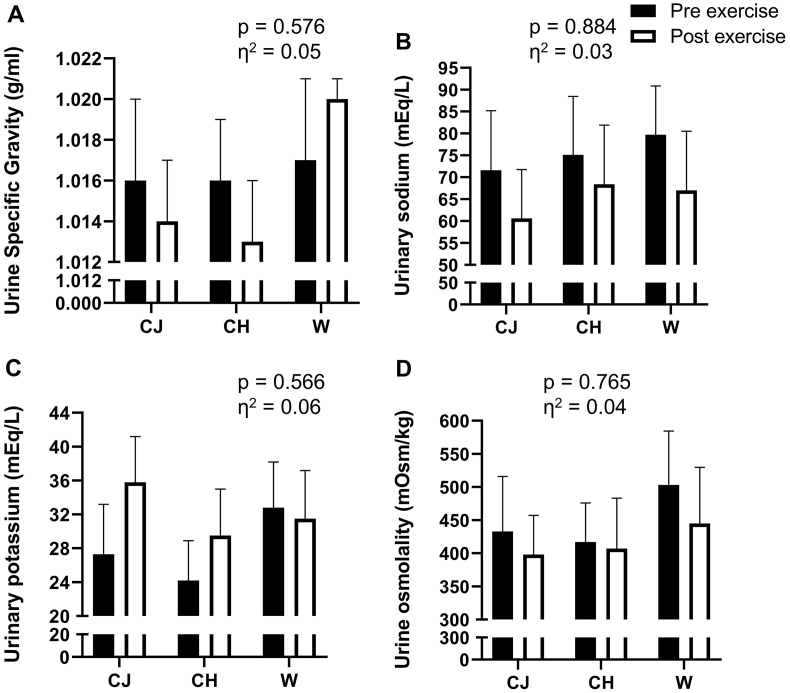
The lack of significant difference in the hydrating capacity of the three drinks, verified by the lost body mass, was confirmed by the analysis of biological material (urine and blood). There was no statistical difference in the USG between pre- and post-exercise moments for the three drinks evaluated (Fig. 3, Panel A; p > 0.05; η2 = 0.05, classified as small). Urinary sodium and potassium also did not differ in the pre- and post-exhaustion moments in the three drinks evaluated (Fig. 3, Panels B and C, respectively; p > 0.05; η2 = 0.03 (small) and 0.06 (moderate), respectively). Urinary osmolality also had similar results, with no significant difference in the pre- and post-exhaustion test moments for the three protocols evaluated (Fig. 3, Panel D; p > 0.05; η2 = 0.04, classified as small).
Fig. 3.
Analysis of urinary variables before and after the exercise protocol for the three conditions evaluated. Panel A – USG (urine specific gravity); Panel B – sodium; Panel C – potassium; Panel D – osmolality. Data are presented as mean and standard error of the mean. There were no statistical differences between the procedures for the four variables analyzed (p > 0.05). Repeated measures ANOVA was used. CJ: cajuína hydroelectrolytic drink; CH: high carbohydrate commercial hydroelectrolytic drink; W: mineral water; η2: effect size.
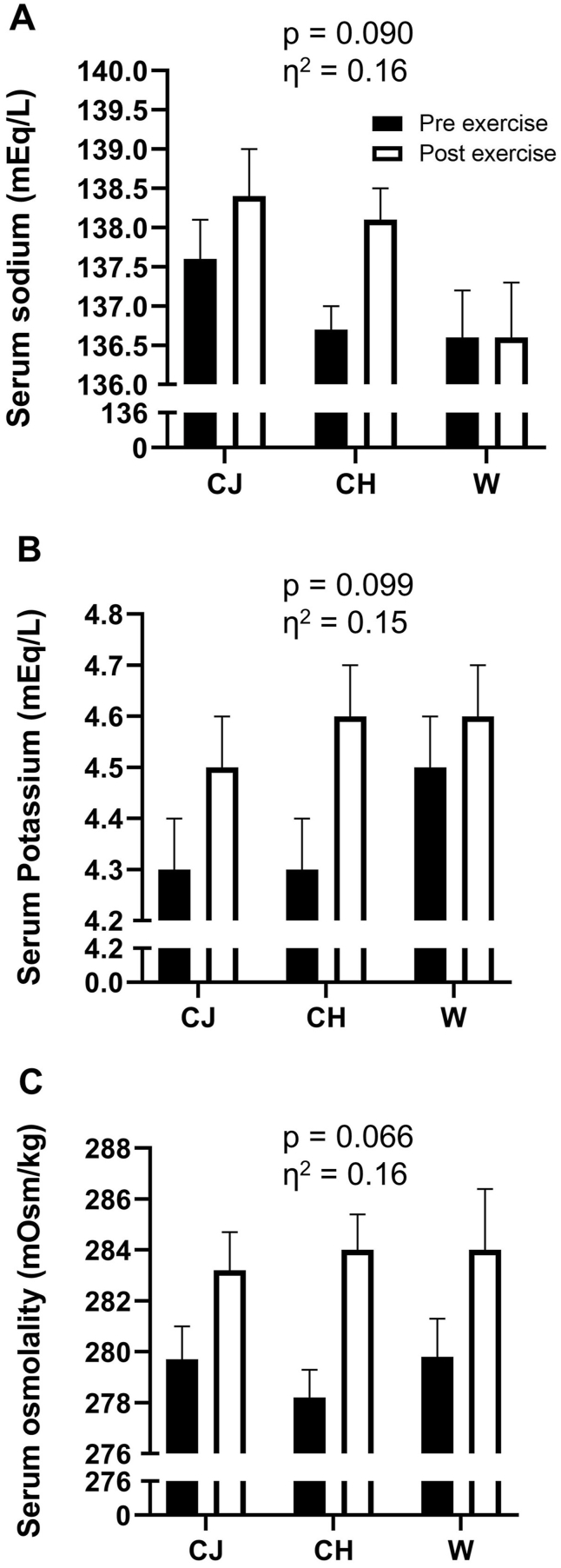
Regarding urine, the serum analysis indicated that blood sodium and potassium did not differ in the pre- and post-exhaustion test moments for the three drinks evaluated (Fig. 4, Panels A and B, respectively; p > 0.05; η2 = 0.16 (large) and 0.15 (large), respectively). Blood osmolality also followed these results, showing no difference in the pre- and post-exhaustion test moments for the three drinks evaluated (Fig. 4, Panel C; p > 0.05; η2 = 0.16, classified as large).
Fig. 4.
Analysis of serum variables before and after the exercise protocol for the three conditions evaluated. Panel A – sodium; Panel B – potassium; Panel C – osmolality. Data are presented as mean and standard error of the mean. There were no statistical differences between the procedures for the three variables analyzed (p > 0.05). Repeated measures ANOVA was used. CJ: cajuína hydroelectrolytic drink; CH: high carbohydrate commercial hydroelectrolytic drink; W: mineral water; η2: effect size.
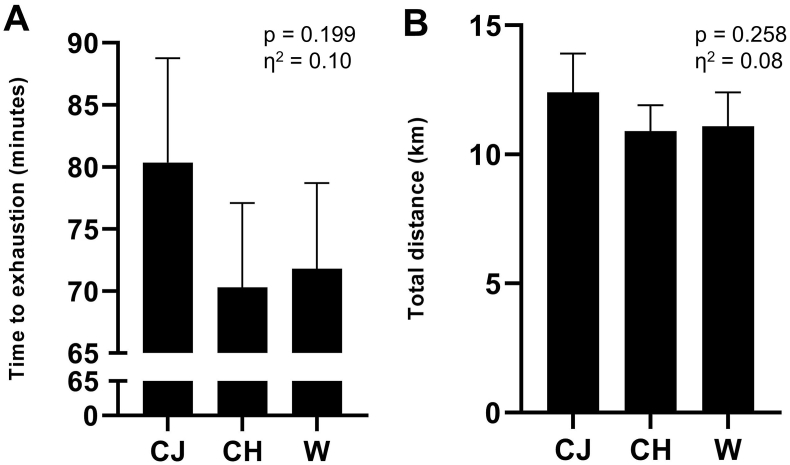
Considering the euhydration between the three procedures, the running time to exhaustion was evaluated to verify possible effects of the beverage composition on the physical performance of the participants. As seen in Fig. 5, the running time to exhaustion was 80.3 ± 8.4 min in the CJ protocol, 70.3 ± 6.8 min in CH and 71.8 ± 6.9 min in W, with 11 out of the 17 participants improving their performance with CJ intake compared to W. Although the running time in the CJ condition was 14.2% longer than that of the CH condition and 11.8% longer than that of the W condition, these differences were not statistically significant (Fig. 5, Panel A; p > 0.05; η2 = 0.10, classified as moderate). The distance run by the volunteers was 12.4 ± 1.5 km for the CJ, 10.9 ± 1.1 km for the CH and 11.1 ± 1.1 km for the W. Likewise, 11 out of the 17 volunteers increased their total distance with CJ intake compared to water, representing 64.7% of the subjects. However, similarly to the time to exhaustion, although the distance covered in the CJ condition was 13.8% greater than that of the CH condition and 11.7% greater than that of the W condition, these differences were not significant (Fig. 5, Panel B; p > 0.05, η2 = 0.08, classified as moderate).
Fig. 5.
Performance of the time-to-exhaustion test. Panel A – time to exhaustion. Panel B – total distance of the test. Data are presented as mean and standard error of the mean. There were no statistical differences between the procedures (p > 0.05). Repeated measures ANOVA was used. CJ: cajuína hydroelectrolytic drink; CH: high carbohydrate commercial hydroelectrolytic drink; W: mineral water; η2: effect size.
The HR behavior is shown in Table 3. HR did not present statistical difference between the procedures in any of the evaluated moments (p > 0.05). Despite this, the effect size analysis showed a classification considered small during the exercise and at 40th minute during recovery and moderate at the 20th and 60th minute during the recovery period.
Table 3.
Heart rate at rest (bpm), during exercise, and during recovery of the participants.
| CJ | CH | W | p | η2 | |
|---|---|---|---|---|---|
| During exercise | 156 ± 3.3 | 153 ± 3.1 | 153 ± 3.0 | 0.795 | 0.04 |
| 20 min recovery | 88 ± 3.7 | 85 ± 3.3 | 83 ± 2.6 | 0.583 | 0.09 |
| 40 min recovery | 84 ± 4.3 | 83 ± 3.7 | 80 ± 2.4 | 0.381 | 0.04 |
| 60 min recovery | 80 ± 4.5 | 79 ± 2.9 | 75 ± 2.3 | 0.191 | 0.06 |
Data are mean and standard error of the mean. Statistical differences were not observed between the procedures for all evaluated moments. Repeated measures ANOVA was used. CJ: cajuína hydroelectrolytic drink; CH: high carbohydrate commercial hydroelectrolytic drink; W: mineral water; η2: effect size.
4. Discussion
The data from this study showed that cajuína hydroelectrolytic drink promotes the same effects on hydroelectrolyte balance and physical performance as a high carbohydrate commercial hydroelectrolytic drink during a time-to-exhaustion test at 70% of VO2max in a recreational runner. Despite the statistical similarity between the three drinks tested, CJ resulted in a time to exhaustion 11.8% longer than CH and 9.9% longer compared to W, with an effect size of moderate level. Likewise, even with no statistical difference, CJ promoted a reduction in urinary sodium and an increase in urinary potassium (with small and moderate effect sizes, respectively) compared to the other drinks. Serum sodium and potassium levels increased (large effect size), although there was no statistical difference.
In summary, the data from this study indicate that, based on the p value of the statistical test, CJ and CH did not improve physical performance or hydroelectrolytic parameters. However, based on the effect size, CJ increased the time to exhaustion and both CJ and CH influenced the balance electrolyte in recreational runners.
The loss of body mass after physical exercise is widely used as a measure of dehydration (Belval et al., 2019). The participants in this study lost less than 1% of body mass, indicating that they finished the time-to-exhaustion test at a satisfactory level of hydration (Sawka et al., 2007). Thus, the data from the present study indicate that the methodological protocol of the ingested water volume was adequate, as it was done individually, which is more suitable to prevent dehydration than ad libitum methods (Melo-Marins et al., 2018). Considering the data regarding the loss in body mass, the sweating rate was between 1 and 1.25 L/h, also without showing statistical difference between the conditions evaluated. This was also expected since, in addition to the fact that the volume of hydration ingested during the exercise was the same, time-to-exhaustion tests were carried out in an environment with controlled temperature (23 ± 1.1 °C) and relative humidity (52 ± 7%).
It was expected that the body osmolality and the serum and urinary concentration of minerals would differ between the procedures since the mineral compounds of CJ and CI are similar. However, we hypothesized that these differences would be evident in comparison with W. In fact, no significant difference was found based on the p value of the statistical test, but the effect size classified as high (sodium) and moderate (potassium) revealed that the minerals of both cajuína hydroelectrolytic drink and the commercial isotonic drinks leads to higher serum concentration after the exercise session. These data are corroborated by the effect size of these same variables in the urinary analysis, classified as moderate both for the reduction of sodium and for the increase of potassium. This confirms the influence of experimental drinks on the electrolyte balance of recreational runners, at least by the effect size, if not by p value. A possible explanation for this lack of statistical difference in blood and urinary parameters assessed by p value may have been the exercise time, which was not enough to promote osmolar change. Literature has shown that exercises lasting around 1 h do not promote a mineral deficit (Dion et al., 2013; Del Coso et al., 2017), but only in longer events, such as marathon, ultramarathon and triathlon electrolyte change occurs (Rogers et al., 2011; Knechtle et al., 2019).
Considering the euhydration between the three procedures, the duration of the time-to-exhaustion test was evaluated to verify possible effects of the drink's composition on the physical performance of the participants. Once again, the statistical test revealed a p value indicating that the experimental beverages do not alter physical performance. Despite this, the effect size was considered moderate (η2 = 0.10), and this prevents us from discarding the possibility that cajuína hydroelectrolytic drink promotes a greater delay in fatigue, as the time to exhaustion of the CJ condition was 11.8% longer than that of the CH condition and 9.9% higher than that of the W condition. This statistical evidence of the effect size corroborates with the practical implication in the sport context. The considerable advantage in practice, which represented an increase of almost 10 min when cajuína hydroelectrolytic drink was ingested, is relevant in the sports context, since it significantly modifies the result in running competitions.
Taking into consideration the interpretation of the data by the effect size, the explanation for this effect of moderate magnitude can be explained by the antioxidant composition of cajuína (Porto-Luz et al., 2019). In fact, other foods high in antioxidants have been shown to improve physical performance, such as grape juice, which increased the time to exhaustion of runners by 18.7% (p = 0.009) 15; açaí, which increased the time to exhaustion of runners by approximately 14% (p < 0.05) (Carvalho-Peixoto et al., 2015); dark chocolate, which increased the total distance covered by moderately trained men by 17% (p = 0.001) (Patel et al., 2015); and saffron, which increased time to exhaustion in healthy men by 24.9% (p < 0.001) (Meamarbashi and Rajabi, 2015). Several other foods also improved sports performance, as shown in a systematic review and meta-analysis (Costa et al., 2020; Doma et al., 2020). The antioxidant effect of these foods has been reported by researchers as one of the explanations for their ergogenic effects.
The data from this study point to a possibility of an ergogenic effect of cajuína, since the interpretations of p value and effect size are conflicting. The main explanation for this might be the small sample size, which was significantly reduced during the course of the investigation. For an effect size of 0.63, a minimum sample size of 22 participants would be necessary for a statistical power of 80%, a larger number than the one recruited in the present study. Therefore, this study indicates the need to reproduce the protocol adopted here in future investigations with a larger sample size, with the perspective that, in this way, the p value and effect size data point in the same direction and confirm the ergogenic potential of cajuína.
Other limitations can be pointed out for the present study. Even though the participants ran at 70% of VO2max, the time-to-exhaustion test was not able to generate electrolyte imbalance, as the sweating rate was not high enough, with a loss of body mass below 1%. Another limitation was the thermoneutral environment with relative humidity favorable to evaporation of the sweat to which the participants were exposed, reducing thermal stress. Therefore, we also suggest further studies evaluating the supplementation of cajuína at higher temperature and relative humidity far from 60%, which is the value considered ideal (Maughan et al., 2012), as well as studies that can evaluate cajuína in high-level athletes and in different sport disciplines.
5. Conclusion
From our results, the cajuína-based hydroelectrolytic drink has a beneficial effect on the hydroelectrolytic balance and physical performance, promoting a moderate delay in time to exhaustion and maintenance of the hydroelectrolytic state of recreational runners, similarly to the effects promoted by a commercial hydroelectrolytic drink rich in carbohydrate. This suggests future studies with the objective of investigating the possible use of cajuína-based hydroelectrolytic drink to improve the performance of physical exercise practitioners in other sport disciplines. Further studies are needed in this direction.
CRediT authorship contribution statement
Valmir Oliveira Silvino: contributed to the concept of the study, contributed to the data acquisition, contributed to the data analysis and interpretation, drafted the manuscript. Mara Cristina Carvalho Batista: contributed to the concept of the study, contributed to the data acquisition, contributed to the data analysis and interpretation, drafted the manuscript. Marcos Antonio Pereira dos Santos: contributed to the concept of the study, critically revised the manuscript. André Luiz Berzoti Ribeiro: contributed to the data acquisition, Paulo Pedro do Nascimento: contributed to the data acquisition, Esmeralda Maria Lustosa Barros: contributed to the data acquisition, Rayane Carvalho de Moura: contributed to the data acquisition, Karen Christie Gomes Sales: contributed to the data acquisition, Luanne Morais Vieira Galvão: contributed to the data acquisition, Lívio César Cunha Nunes. contributed to the data acquisition, Manoel Miranda Neto: contributed to the data analysis and interpretation. Alessandra Durazzo: contributed to the data analysis and interpretation. Alexandre Sérgio Silva. contributed to the data analysis and interpretation. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Funding
This research received no external funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the volunteer athletes and the staff at Federal University of Piauí for their support, as well as Fundação de Amparo à Pesquisa do Estado do Maranhão (FAPEMA BD-02488/21).
Data availability
Data will be made available on request.
References
- Abreu F., Neto R. Embrapa technological information; 2007. Cajuína. Brasília. [Google Scholar]
- Batista M.C.C.C., dos Santos M.A.P.A.P. Impact of hydration on exercise performance and physiological responses. Curr. Nutr. Food Sci. 2020;16:1–7. [Google Scholar]
- Belval L.N., Hosokawa Y., Casa D.J., et al. Practical hydration solutions for sports. Nutrients. 2019;11 doi: 10.3390/nu11071550. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Bruce R.A., Blackmon J.R., Jones J.W., et al. Exercising testing in adult normal subjects and cardiac patients. Pediatrics. 2004;32:291–303. doi: 10.1111/j.1542-474X.2004.93003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Peixoto J., Moura M.R.L., Cunha F.A., et al. Consumption of açai (Euterpe oleracea Mart.) functional beverage reduces muscle stress and improves effort tolerance in elite athletes: a randomized controlled intervention study. Appl. Physiol. Nutr. Metabol. 2015;40:725–733. doi: 10.1139/apnm-2014-0518. [DOI] [PubMed] [Google Scholar]
- Cheuvront S.N., Kenefick R.W. Dehydration: physiology, assessment, and performance effects. Compr. Physiol. 2014;4:257–285. doi: 10.1002/cphy.c130017. [DOI] [PubMed] [Google Scholar]
- Cohen J. second ed. Lawrence Erlbaum; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Costa M.S., Toscano L.T., Toscano L. de LT., et al. Ergogenic potential of foods for performance and recovery: a new alternative in sports supplementation? A systematic review. Crit. Rev. Food Sci. Nutr. 2020 doi: 10.1080/10408398.2020.1844137. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Del Coso J., Salinero J.J., Lara B., et al. A comparison of the physiological demands imposed by competing in a half-marathon vs. a marathon. J. Sports Med. Phys. Fit. 2017;57:1399–1406. doi: 10.23736/S0022-4707.17.07056-6. [DOI] [PubMed] [Google Scholar]
- Deshayes T.A., Jeker D., Goulet E.D.B. Impact of pre-exercise hypohydration on aerobic exercise performance, peak oxygen consumption and oxygen consumption at lactate threshold: a systematic review with meta-analysis. Sports Med. 2020;50:581–596. doi: 10.1007/s40279-019-01223-5. [DOI] [PubMed] [Google Scholar]
- Dion T., Savoie F.A., Asselin A., et al. Half-marathon running performance is not improved by a rate of fluid intake above that dictated by thirst sensation in trained distance runners. Eur. J. Appl. Physiol. 2013;113:3011–3020. doi: 10.1007/s00421-013-2730-8. [DOI] [PubMed] [Google Scholar]
- Doma K., Gahreman D., Connor J. Fruit supplementation reduces indices of exercise-induced muscle damage: a systematic review and meta-analysis. Eur. J. Sport Sci. 2020:1–18. doi: 10.1080/17461391.2020.1775895. [DOI] [PubMed] [Google Scholar]
- Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–313. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]
- Ferretti G. Maximal oxygen consumption in healthy humans: theories and facts. Eur. J. Appl. Physiol. 2014;114:2007–2036. doi: 10.1007/s00421-014-2911-0. [DOI] [PubMed] [Google Scholar]
- Galvão L.M.V. Universidade Federal do Piauí; 2020. Desenvolvimento de uma bebida isotônica a base de cajuína. PhD Thesis. [Google Scholar]
- González-Mohíno F., Santos-Concejero J., Yustres I., et al. The effects of interval and continuous training on the oxygen cost of running in recreational runners: a systematic review and meta-analysis. Sports Med. 2020;50:283–294. doi: 10.1007/s40279-019-01201-x. [DOI] [PubMed] [Google Scholar]
- Herdy A.H., Ritt L.E.F., Stein R., et al. Cardiopulmonary exercise test: background, applicability and interpretation. Arq. Bras. Cardiol. 2016;107:467–481. doi: 10.5935/abc.20160171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knechtle B., Chlíbková D., Papadopoulou S., et al. Exercise-associated hyponatremia in endurance and ultra-endurance performance–aspects of sex, race location, ambient temperature, sports discipline, and length of performance: a narrative review. Méd. 2019;55 doi: 10.3390/medicina55090537. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska D., Mah E., Brisbois T., et al. Narrative review of hydration and selected health outcomes in the general population. Nutrients. 2019;11 doi: 10.3390/nu11010070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan R.J., Otani H., Watson P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2012;112:2313–2321. doi: 10.1007/s00421-011-2206-7. [DOI] [PubMed] [Google Scholar]
- McDermott B.P., Anderson S.A., Armstrong L.E., et al. National athletic trainers' association position statement: fluid replacement for the physically active. J. Athl. Train. 2017;52:877–895. doi: 10.4085/1062-6050-52.9.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meamarbashi A., Rajabi A. Preventive effects of 10-day supplementation with saffron and indomethacin on the delayed-onset muscle soreness. Clin. J. Sport Med. 2015;25:105–112. doi: 10.1097/JSM.0000000000000113. [DOI] [PubMed] [Google Scholar]
- Melo-Cavalcante A.A., Dantas S.M.M.D.M., Leite A.D.S., et al. In vivo antigenotoxic and anticlastogenic effects of fresh and processed cashew (Anacardium occidentale) apple juices. J. Med. Food. 2011;14:792–798. doi: 10.1089/jmf.2010.0153. [DOI] [PubMed] [Google Scholar]
- Melo-Marins D., Souza-Silva A.A., da Silva-Santos G.L.L., et al. Personalized hydration strategy attenuates the rise in heart rate and in skin temperature without altering cycling capacity in the heat. Front. Nutr. 2018;5 doi: 10.3389/fnut.2018.00022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. Dehydration, hyperthermia, and athletes: science and practice. J. Athl. Train. 1996;31:248–252. [PMC free article] [PubMed] [Google Scholar]
- Patel R.K., Brouner J., Spendiff O. Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. J Int Soc Sports Nutr. 2015;12 doi: 10.1186/s12970-015-0106-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira E.R., De A'Ndrade M.T., Mendes T.T., et al. Evaluation of hydration status by urine, body mass variation and plasma parameters during an official half-marathon. J. Sports Med. Phys. Fit. 2017;57:1499–1503. doi: 10.23736/S0022-4707.16.06836-5. [DOI] [PubMed] [Google Scholar]
- Périard J.D., Eijsvogels T.M.H., Daanen H.A.M. Exercise under heat stress: thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021;101:1873–1979. doi: 10.1152/physrev.00038.2020. [DOI] [PubMed] [Google Scholar]
- Pinho W., da Silva A. Efeitos do exercício físico sobre a formação de espécies reativas de oxigênio e os compostos antioxidantes da dieta. Rev. Bras Nutr. Esportiva. 2013;7:77–87. [Google Scholar]
- Porto-Luz R.G.L., de Moura A.J.B., da Silva B., et al. Identification and quantification of antioxidant compounds in clarified cashew apple juice ‘cajuína’. Curr. Nutr. Food Sci. 2019;16:585–591. [Google Scholar]
- Rogers I.R., Hook G., Stuempfle K.J., et al. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin. J. Sport Med. 2011;21:200–203. doi: 10.1097/JSM.0b013e31821a6450. [DOI] [PubMed] [Google Scholar]
- Rowlands D.S., Kopetschny B.H., Badenhorst C.E. The hydrating effects of hypertonic, isotonic and hypotonic sports drinks and waters on central hydration during continuous exercise: a systematic meta-analysis and perspective. Sports Med. 2021 doi: 10.1007/s40279-021-01558-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka M.N., Burke L.M., Eichner E.R., et al. American college of sports medicine position stand: exercise and fluid replacement. Med. Sci. Sports Exerc. 2007;39:377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- Toscano L.L.T., Toscano R.L., Silva LT da, et al. Potential ergogenic activity of grape juice in runners. Appl. Physiol. Nutr. Metabol. 2015;40:899–906. doi: 10.1139/apnm-2015-0152. [DOI] [PubMed] [Google Scholar]
- Toscano L.L.T., Silva A.S., França ACL de, et al. A single dose of purple grape juice improves physical performance and antioxidant activity in runners: a randomized, crossover, double-blind, placebo study. Eur. J. Nutr. 2019 doi: 10.1007/s00394-019-02139-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbogaerde T.J., Hopkins W.G. Effects of acute carbohydrate supplementation on endurance performance: a meta-analysis. Sports Med. 2011;41:773–792. doi: 10.2165/11590520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Vanderlei F.M., Moreno I.L., Vanderlei L.C.M., et al. Comparison of the effects of hydration with water or isotonic solution on the recovery of cardiac autonomic modulation. Int. J. Sport Nutr. Exerc. Metabol. 2015;25:145–153. doi: 10.1123/ijsnem.2014-0004. [DOI] [PubMed] [Google Scholar]
- Von Duvillard S.P., Braun W.A., Markofski M., et al. Fluids and hydration in prolonged endurance performance. Nutrition. 2004;20:651–656. doi: 10.1016/j.nut.2004.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.