Abstract
Objective
This review discusses recent experimental and clinical findings related to ferroptosis, with a focus on the role of MSCs. Therapeutic efficacy and current applications of MSC-based ferroptosis therapies are also discussed.
Background
Ferroptosis is a type of programmed cell death that differs from apoptosis, necrosis, and autophagy; it involves iron metabolism and is related to the pathogenesis of many diseases, such as Parkinson’s disease, cancers, and liver diseases. In recent years, the use of mesenchymal stem cells (MSCs) and MSC-derived exosomes has become a trend in cell-free therapies. MSCs are a heterogeneous cell population isolated from a diverse range of human tissues that exhibit immunomodulatory functions, regulate cell growth, and repair damaged tissues. In addition, accumulating evidence indicates that MSC-derived exosomes play an important role, mainly by carrying a variety of bioactive substances that affect recipient cells. The potential mechanism by which MSC-derived exosomes mediate the effects of MSCs on ferroptosis has been previously demonstrated. This review provides the first overview of the current knowledge on ferroptosis, MSCs, and MSC-derived exosomes and highlights the potential application of MSCs exosomes in the treatment of ferroptotic conditions. It summarizes their mechanisms of action and techniques for enhancing MSC functionality. Results obtained from a large number of experimental studies revealed that both local and systemic administration of MSCs effectively suppressed ferroptosis in injured hepatocytes, neurons, cardiomyocytes, and nucleus pulposus cells and promoted the survival and regeneration of injured organs.
Methods
We reviewed the role of ferroptosis in related tissues and organs, focusing on its characteristics in different diseases. Additionally, the effects of MSCs and MSC-derived exosomes on ferroptosis-related pathways in various organs were reviewed, and the mechanism of action was elucidated. MSCs were shown to improve the disease course by regulating ferroptosis.
Keywords: Mesenchymal stem cells, Ferroptosis, Exosomes, Lipid peroxidation, Lipid reactive oxygen species, Glutathione peroxidase 4
1. Introduction
1.1. Characteristics of mesenchymal stem cells (MSCs) and their therapeutic potential
MSCs have emerged as promising candidates for cell-based therapy for various diseases owing to their availability, ease of harvesting, and safety without a risk of malignant transformation after the infusion of allogeneic cells, and the mechanisms by which MSCs exert their therapeutic effects are multifaceted. Interestingly, MSCs secrete biomolecules such as growth factors, cytokines, and chemokines that assist in their biological activities in an autocrine or paracrine manner, in sync with their microenvironment. Additionally, a basic question is whether biomolecules formed from various types of stem cells have varied protective and therapeutic effects in disease conditions [1]. As the field continues to expand, the escape of MSCs from the immune system has been shown to be mediated by cell-cell interactions via the production of immune regulatory molecules such as IFN-γ, COX-2, PGE2, and IDO [2,3]. Moreover, low expression of major histocompatibility complex (MHC) class Ⅰ molecules, a significant absence of MHC Ⅱ molecules, and the absence of costimulatory ligands, such as CD40,CD80, and CD86, further reduce the immunogenicity of MSCs [4]. Notably, the retention of MSCs within the tissue vasculature and subsequent migration between endothelial cells [5], called homing, plays a prominent role in inflammatory injuries. Several studies have validated their direct role in binding to adhesion molecules and chemokines secreted from injured tissues via self-expressed adhesion molecules and chemokine receptors following the transplantation of MSCs into blood vessels, thereby homing to the site of injury [6]. Furthermore, MSCs are almost tumorigenic owing to their unique gene expression profiles, and have the potential for multidirectional differentiation and self-renewal; consequently, their clinical application prospects are self-evident. For example, MSCs alleviate inflammatory bowel disease (IBD) by regulating inflammatory cytokines in the inflamed gut [7], ameliorate Parkinson’s disease (PD) by enhancing the expression of brain-derived neurotrophic factor and vascular endothelial growth factor to stimulate the potential of damaged dopamine neurons to regenerate in rats [8], protect renal function by reducing serum creatinine and BUN levels, and alleviate acute renal injury (AKI) after ischemic and toxic injury [9]. A better understanding of the mechanisms by which MSCs promote tissue repair and protect organ function in different disease states may provide substantial advances in the therapeutic potential of MSCs. An underexplored mechanism in this respect is the role of MSCs in ferroptosis, and understanding this may establish a novel therapeutic use for MSCs and broaden the spectrum of their clinical applications.
1.2. Characteristics of MSC-derived exosomes and their therapeutic potential
It is no exaggeration to state that we are at the dawn of the exosome era. Exosomes are crucial messengers in biological fluids that have been implicated in multiple physiological and pathological processes [10]. In general, exosomes are extracellular vesicles with a diameter of 20–150 nm and carry a variety of bioactive substances such as proteins, DNA, mRNA, microRNA, lncRNA, and circRNA, which affect recipient cell activity by inducing signaling pathways. Over the past few years, studies have demonstrated that the paracrine effect mediated by stem cell secretion factors is the main mode of action for the therapeutic effects of stem cells [11,12]. It is clinically well-established that exosomes play a major role in the paracrine effect of stem cells [[13], [14], [15]], owing to their capacity to mediate cell microcommunication by transferring mRNA, non-coding RNA, and proteins between cells [16,17], and exhibit lower immunogenicity, facilitating transplantation and storage. An increasing number of studies have identified the anti-inflammatory, anti-aging, and wound-healing effects of MSC-derived exosomes in various in vitro and in vivo models [18]. First, MSCs and MSC-derived exosomes can transform proinflammatory M1-type macrophages into anti-inflammatory M2-type macrophages [19], thus exerting their anti-inflammatory effects. Second, human ASC-exosomes reduce high glucose-induced premature senescence of endothelial progenitor cells (EPCs) and promote wound healing in rats with diabetes [20]. Third, exosomes from pro-inflammatory bone marrow mesenchymal stem cells (BMMSCs) alleviate inflammation and myocardial injury by mediating macrophage polarization [21]. Notably, exosomes appear to be capable of homing to the site of injury. In a mouse model of AKI, DiD-labelled EVs specifically accumulated in the kidneys of mice with AKI compared with healthy controls [22]. As an alternative to MSCs, exosomes have been used in various disease models, including neurological, cardiovascular, immune, renal, musculoskeletal, liver, respiratory, and cancer models [[23], [24], [25], [26], [27]]. Exosomes have advantages over their corresponding MSCs; they are smaller and simpler than cells, and therefore easier to produce and store, which may avoid some of the regulatory issues associated with MSCs. Therefore, MSC-derived exosomes may serve as ideal therapeutic tools for various diseases. Nonetheless, comparative studies of MSC-exosomes based on their tissue origin are still limited, and the exact mechanism of in vivo action of exogenously administered exosomes, their biodistribution, pharmacokinetics, and potential for targeted delivery have not been fully elucidated.
1.3. Ferroptosis
1.3.1. Definition, physiological and pathological role of ferroptosis
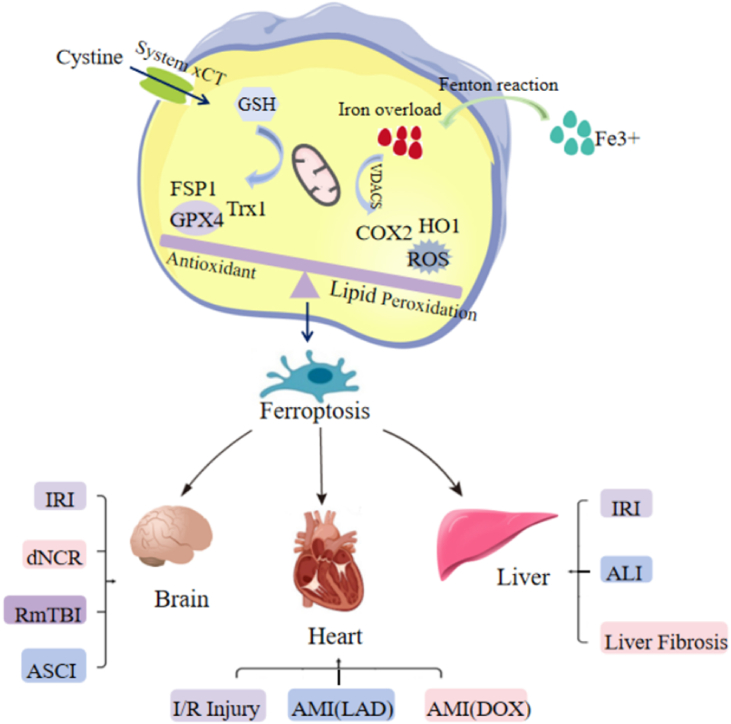
Ferroptosis is a newly identified iron-dependent cell death pathway that is distinct from other forms of cell death, including apoptosis and necrosis, and is characterized by intracellular iron overload and iron-dependent lipid peroxide accumulation. As an essential trace element found in almost all life forms, iron is involved in numerous biological processes, including energy metabolism and synthesis and repair of nucleotides [28]. Iron overload occurs when the body stores excess iron. Accumulated cellular iron, especially labile ferrous iron, can react directly with cellular oxidants to generate cytotoxic hydroxyl radicals via the Fenton reaction, thereby initiating oxidative stress. During oxidative stress, excess lipid reactive oxygen species (ROS) lead to cellular dysfunction and cell death through lipid peroxidation and the oxidation of proteins, DNA, and RNA. Ferroptosis is a form of cell death triggered by the toxic accumulation of lipid peroxides on cellular membranes. It is generally thought that lipid peroxides (PLOOH), particularly lipid hydroperoxides, are key mediators of ferroptosis because of their capacity to damage the lipid bilayer of the membrane and impair cell integrity and function [29]. In the currently proposed model, after the loss of glutathione peroxidase-4 (GPX4) activity, lipid hydroperoxide is generated in the membrane and attacked by ferrous iron, leading to lipid radical formation and increased lipid peroxidation, resulting in lethal membrane damage. Mitochondrial atrophy, accumulation of iron/lipid ROS, and GPX4 inactivation [30,31] are the major markers of ferroptosis. At the molecular level, ferroptosis is characterized by glutathione (GSH) depletion, lipid peroxidation, loss of plasma membrane integrity, cytoplasmic swelling, mitochondrial atrophy, rupture of the mitochondrial outer membrane, and it is biochemically characterized by GPX4 inactivation (seeFig. 1 ).
Fig. 1.
The role of ferroptosis in diseases.
1.3.2. Mechanisms of ferroptosis
The mechanisms underlying the regulation of ferroptosis can be broadly divided into three categories: regulation by iron metabolism, regulation by GPX4, and regulation by lipid metabolism. Most studies investigating the mechanisms of ferroptosis have focused on lipid peroxidation and iron loading.
GPX4 plays a pivotal role in ferroptosis primarily by inhibiting lipid peroxide formation. GPX4 converts GSH to oxidized glutathione (GSSG) and reduces the peroxides of cytotoxic lipids (L-OOH) to their corresponding alcohols (L-OH), which in turn inhibits lipid peroxide formation. The mevalonate (MVA) pathway may affect GPX4 synthesis by regulating selenocysteine tRNA maturation and ferroptosis. More recently, system xc-, an important antioxidant system in cells containing a heterodimer composed of two subunits, SLC7A11 and SLC3A2, was shown to play a key role in the negative regulation of ferroptosis. Cystine and glutamate are exchanged through the plasma membrane by system xc- at a 1:1 rate [32]. Interestingly, a decrease in GSH levels decreased GPX4 activity, which led to a decrease in antioxidant capacity, lipid ROS accumulation, oxidative damage, and ferroptosis. On the one hand, P53, a tumor suppressor gene, inhibits system xc- cystine uptake via negative regulation of SLC7A11 expression, which affect GPX4 activity, resulting in reduced cellular antioxidant capacity, lipid ROS accumulation, and ferroptosis [33]. On the other hand, P53 mediates cell cycle inhibition, senescence, and apoptosis. Moreover, several studies have reported that 90 % of the cells die from lipid ROS after P53 activation, indicating that the activation of P53 suppresses the antioxidant capacity of cells. Furthermore, the P53-SAT1-ALOX15 pathway is involved in the regulation of ferroptosis [34]. The apoptosis-inducing factor mitochondrial 2 (AIFM2) is a previously unrecognized antiferroptotic gene, namely, FSP1, which plays an important role in ferroptosis, catalyzes CoQ10 regeneration through NAD(P)H to inhibit ferroptosis, and inhibits lipid oxidation by reducing CoQ10 to suppress ferroptosis.
Polyunsaturated fatty acids (PUFAs) are a main targets of lipid peroxidation, which increases membrane fluidity, and their high content in cells and organelle membranes facilitates lipid peroxidation, leading to impaired cell integrity and function [35]. Studies have shown that Phosphatidylethanolamine (PE), which contains arachidonic acid (AA) or its derivative adrenaline, is a key phospholipid that induces cellular ferroptosis. Acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are involved in PE biosynthesis and remodeling, activate PUFA, and affect their transmembrane features. PUFA-PE may play additional oxidative roles in lipoxygenase (LOX) catalysis, ultimately inducing ferroptosis in cells [36]. The voltage-dependent anion channels (VDAC) are transmembrane channels that transport ions and metabolites to the outer membrane of mitochondria [37]. The effects of erastin on VDAC cause mitochondrial dysfunction, leading to the release of several oxides, ultimately causing iron-mediated cell death [38].
The uptake of extracellular Fe3+ is mainly mediated by transferrin (TF) and transferrin receptor (TFR). Fe3+-bearing TF binds to TFR on target cells that transport Fe3+ into the cell, where it is reduced to Fe2+ by the six-transmembrane epithelial antigen of prostate 3 (STEAP3), and Fe2+ is subsequently stored in the labile iron pool (LIP) and ferritin, which is mediated by divalent metal transporter 1 (DMT1) or zinc-iron regulatory protein family 8/14 (ZIP8/14) through the Fenton reaction, inducing ferroptosis. Heat shock protein beta-1 (HSPB1) can further reduce intracellular iron levels by inhibiting TRF1 expression; thus, overexpression of HSPB1 can significantly inhibit ferroptosis [39]. In addition, the p62-Keap1-NRF2 and ATG5-ATG7-NCOA4 complexes can efficiently regulate the formation of intracellular iron ions and ROS, and play a regulatory role in ferroptosis (Fig. 2).
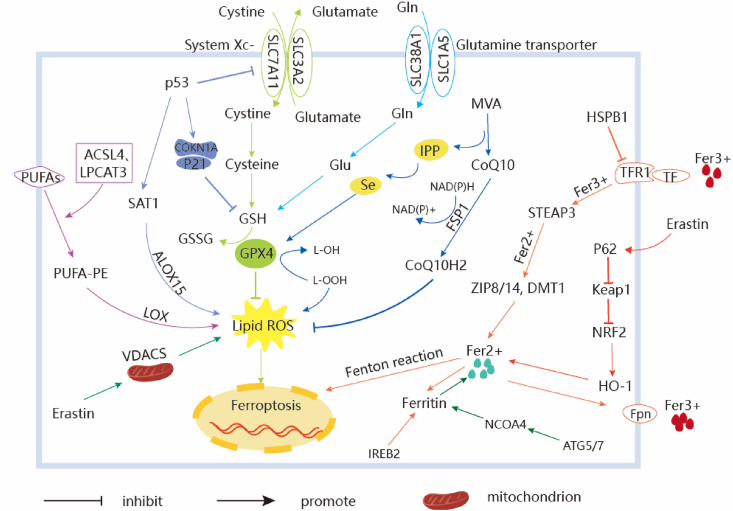
Fig. 2.
Regulatory pathways of ferroptosis. The regulatory mechanisms of ferroptosis are roughly devided into three categories. The first one is regulated by GSH/GPX4 pathway, such as inhibition of system Xc-, sulfur transfer pathway, MVA pathway, glutamine pathway, and p53 regulatory axis. What’s more, the regulation mechanism of iron metabolism, including iron metabolism pathway, the regulation of ATG5-ATG7-NCOA4 pathway and IREB2 related to ferritin metabolism, and the regulatory pathways of p62-Keap1-NRF2 and HSPB1 all have effects on iron. The third category is related pathways around lipid metabolism, such as P53-SAT1-ALOX15, ACSL4, LPCAT3, etc., which have effects on lipid regulation and ferroptosis. In addition, Erastin acts on mitochondria to induce ferroptosis. Recent studies have also shown that the FSP1-CoQ10- NAD(P)H pathway exists as an independent parallel system that works cooperatively with GPX4 and glutathione to inhibit the peroxidation of phospholipids as well as ferroptosis.
2. Application of MSCs
As a newly identified type of programmed cell death, ferroptosis is associated with the occurrence and development of many diseases, including PD [40], cancer [41], liver injury [42], traumatic brain injury (TBI) [43], stroke [44], myocardial infarction [45]and ischemia-reperfusion injury (IRI) [46]. The intracellular accumulation of lipid peroxides directly affects ferroptosis. Emerging evidence suggests that ferroptosis inhibits GPX4 and induces an increase in lipid ROS by downregulating xc- activity. MSCs can migrate to damaged tissues and organs and rebuild them through direct differentiation and secretion of exosomes, growth factors, and cytokines [47,48]. Studies have shown that MSCs are anchored in the heart, cortex, and hippocampus via vascular networks [49]. The regulatory role of MSCs in ameliorating ferroptosis is well documented. For example, MSCs can inhibit lipid peroxidation and reduce ferroptosis in vivo and in vitro [50], human umbilical cord mesenchymal stem cells (HucMSCs) improve erectile dysfunction in diabetic rats by attenuating ferroptosis [51], MSCs maintain intracellular glutathione levels by directly or indirectly up-regulating the expression of GPX4 to inhibit ferroptosis in myocardial IRI [52], and MSCs alleviate post-resuscitation heart and brain damage by inhibiting ferroptosis in a pig model of cardiac arrest [49].
2.1. MSCs regulate ferroptosis in the brain
Increasing evidence supports the involvement of ferroptosis in various brain diseases. Despite its initial identification in cancer cells [53], ferroptosis plays a significant role in the progression and toxicity of many neurological diseases, including stroke, PD, and Alzheimer’s disease (AD). The involvement of ferroptosis has been suggested by several mechanisms that occur in neurodegenerative diseases such as iron accumulation within the brain, GSH depletion, lipid peroxidation [54], inactivation of GPX4 [55], and abnormal xCT expression [56,57]. In 2017, a study found that inhibition of ferroptosis protected mice from IRI in a model of middle cerebral artery occlusion (MCAO), indicating that ferroptosis contributes to neuronal death following ischemic stroke [58]. Specifically, ischemic stroke occurs when the blood supply to certain parts of the brain is limited, secondary to occlusion of the internal carotid, middle cerebral, or vertebral arteries [59], and ischemia occurs in the brain. The resultant depletion of oxygen and nutrients can cause cells to activate the ischemic cascade, resulting in oxidative stress, mitochondrial damage, and ultimately cell death [60]. Oxidative stress is an important step in ferroptosis and refers to the relative excess of ROS caused by the excessive production and/or impaired degradation of ROS. In contrast, ferroptosis is considered one of the causes of reperfusion injury, which is a key factor in IRI and organ failure [61]. First, the inhibition of tau protein (a major microtubule-associated protein) can result in the accumulation of iron following ischemia, leading to an imbalance in iron metabolism, promoting ROS production, nucleic acid, proteome, and membrane damage, and ultimately cell death [62]. Moreover, several studies have demonstrated the role of ferroptosis in the development of cognitive impairments caused by excessive ROS production. Excessive ROS levels induce lipid peroxidation as well as protein, DNA, and RNA oxidation, leading to neuronal dysfunction and cell death. Depletion of GSH, a significant feature of ferroptosis, has been shown to cause cognitive impairment through the excessive production of ROS [63]. Furthermore, iron overload has been strongly implicated in the pathophysiological process of secondary brain injury reported in recent years [64], particularly TBI, which includes both primary and secondary impairments. Studies have reported that excess intracellular iron affects neurological functions, particularly in compromised brains in TBI. Some types of brain disease-induced dysfunctions can be alleviated through the mechanisms of ferroptosis regulated by MSCs, as shown in Table 1.
Table 1.
Favoring effect of MSCs on brian.
| MSC Origin | Model | Mechanisms | Outcomes | References |
|---|---|---|---|---|
| Mouse bone marrow-derived MSCs | dNCR aged mice | Increase the expression of SIRT1 | Ameliorated cognitive impairment | [65,66] |
| Human umbilical cord blood-derived MSCs | RmTBI mice (CCI4) | Restore GPX activity and GPX4 quantity | Rescue rmTBI-induced neurodegeneration and neuronal loss | [67,68] |
| MSCs-Exo under the hypoxic condition | ASCI mice (NYU impactor M-Ⅲ) | lncGm36569 as a competitive of miR-5627-5p to induce FSP1 upregulation | Promote functional recovery in the ASCI | [69,70] |
| Mouse bone marrow-derived MSCs-EVs | Hippocampal neurons (MCAO, OGD/R) | Upregulate GPX4 expression and inhibit COX-2 expression | Reverse loss of activity in tert-butyl peroxide exposed neurons | [71,72,73] |
Silence-regulating factor 1 (SIRT1) is a nicotinamide adenosine dinucleotide (NAD)-dependent class Ⅲ histone deacetylase [74]. SIRT1 is highly expressed in the hippocampus and hypothalamus [75]. In the nervous system, SIRT1 plays an important role in regulating normal brain functions such as plasticity and memory [76]. A model of delayed neurocognitive recovery (dNCR) in aged mouse hippocampal tissues was used to explore ROS, GSH, malondialdehyde (MDA), and Fer2+ levels and showed that they were increased. SIRT1 is considered as a protective agent against oxidative stress and lipid peroxidation by mediating the levels of nuclear factor-erythroid 2-associated factor 2 (NRF2) and heme oxygenase-1 (HO-1) [65]. The NRF2/HO-1 pathway plays a key role in inhibiting ferroptosis in the nervous system [77,78]. In addition, extensive studies have revealed that NRF2 and HO-1 participate in GPX4 synthesis [65]. SIRT1 expression was found to be downregulated in dNCR mice, and exosomes derived from exogenous MSCs restored the downregulation of SIRT1, which induced increased expression of NRF2 and HO-1 and inhibited hippocampal ferroptosis (Fig. 3) in elderly dNCR mice to improve cognitive impairment. This provides a potential pathway for the treatment of dNCR [66].
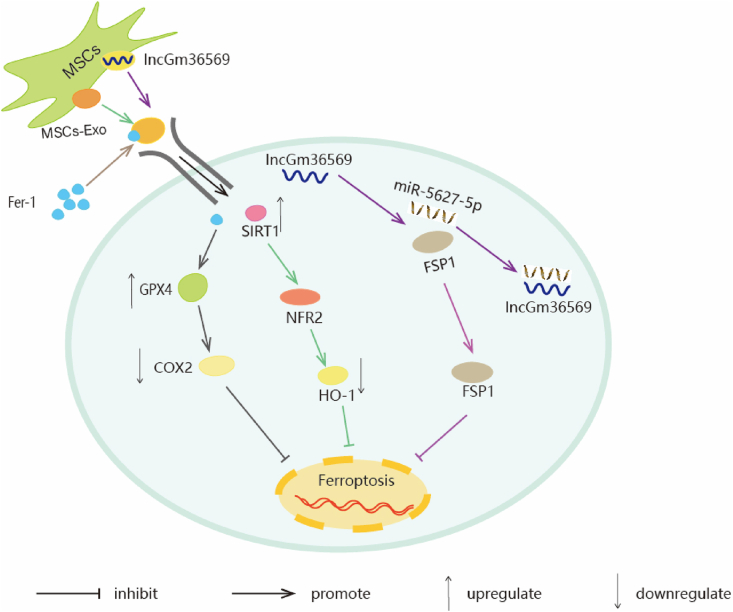
Fig. 3.
The schematic representation of MSC treatment inhibits I/R injury, dNCR, TBI-induced ferroptosis. Fer-1/GPX4/COX2 Signal Pathway: In brain I/R injury, MSCs-EVs/Fer-1 regulates the GPX4/COX-2 axis. MSCs-EVs/Fer-1 upregulates of GPX4 expression and inhibits COX-2 expression, which in turn inhibits ferroptosis, neuronal apoatosis and inflammation. SIRT1/NRF2/HO-1 Signal Pathway: MSCs-Exo upregulates expression level of SIRT1, SIRT1 restoration leads to an increase in NRF2 and HO-1. Taken together, this mechanism inhibits ferroptosis induced by surgery so as to attenuate cognitive impairment. lncGm36569/miR-5627-5p/FSP1 Axis: lncGm36569 was enriched in MSCs-Exo, lncGm36569 acted as a ceRNA of miR-5627-5p to enhance FSP1 expression, consequently suppressing ferroptosis in injured neuronal cells.
Excessive Fer2+ production after TBI disrupts cellular homeostasis and accelerates ROS production via the Fenton response, thereby inducing cellular damage. An imbalance in iron metabolism leads to the abnormal accumulation of iron, which is toxic to brain-specific cells (including microglia, astrocytes, and neurons) [67]. Oxidative damage in the brain mainly manifests as lipid peroxidation, which is caused by an increased content of PUFAs in the membrane [79]. GPX4 inhibits lipid peroxidation by directly reducing hydroperoxides in membrane lipids [80], exhibiting direct detoxification. Wang et al. [68]found that in mice with repetitive mild traumatic brain injury (RmTBI), GPX inactivation, decrease in GPX4 levels, increase in lipid peroxidation, abnormal iron metabolism, and mitochondrial ultrastructural changes, are key risk factors for neurodegeneration, characterized by increased deposition of Aβ and tau proteins and cognitive impairment [81]. By reducing pathological protein deposition after RmTBI and promoting glucose metabolism, MSCs can alleviate neuronal degeneration, effectively regulate protein levels related to iron metabolism, inhibit iron accumulation, reduce RmTBI-induced Fer2+ accumulation, regulate iron metabolism, prevent lipid peroxidation by restoring GPX activity and GPX4 content, and inhibit ferroptosis (Fig. 3). They can exert a protective effect on neurons to reduce the persistent cognitive impairment caused by RmTBI [68].
FSP1 is an important factor in the antioxidant system of non-mitochondrial coenzyme Q, which catalyzes CoQ10 regeneration and inhibits lipid peroxidation through NAD(P)H, thereby inhibiting ferroptosis [69]. lncRNAs regulate ferroptosis in various diseases by acting as miRNAs through competitive endogenous RNA (ceRNA) [82,83]. After acute spinal cord injury, higher levels of ROS and intracellular ferrous iron accumulation were observed in injured mice. Several studies have shown that MSCs inhibit the production of ROS and ferrous iron in mice, and the upregulated expression of ferroptosis inhibitor FSP1 is mainly attributed to the in vivo competitive mechanism of action of lncRNAs in exosomes derived from them. MSC-derived exosomes are rich in lncGm36569, and lncGm36569 coated particles are enriched in miR-5627-5p, showing a strong binding potential. Bioinformatics analysis showed that FSP13 '-UTR contained a binding site of miR-5627-5p, and overexpression of miR-5627-5p enhanced the effects of hypoxia on cell viability, iron, and ROS production, and inhibited the expression of FSP1. lncGm36569, as a ceRNA of miR-5627-5p, can improve the level of FSP1, which is upregulated after transfection with lncGm36569, inhibits ferroptosis (Fig. 3) in damaged neurons, and thus plays a role in the repair of neural function [70].
Brain IRI can lead to irreversible damage, particularly to the hippocampus. Transient cerebral ischemia triggers neuronal death in the hippocampus [84,85], with very high disability and mortality rates. COX-2 is a rate-limiting enzyme that mediates the expression of inflammatory cytokines, growth factors, and tumor promoters [86,87]. In an oxygen-glucose deprivation/reperfusion (OGD/R)-induced brain IRI model, ROS and MDA production increased, GPX4 expression decreased, and COX-2 expression increased. Ferrostatin-1(Fer-1), a powerful ferroptosis inhibitor, is a small antioxidant compound containing aromatic alkyl amines [71]. Bioinformatics studies have verified that Fer-1 has a regulatory effect on the GPX 4/COX 2 axis, and extracellular vesicles (MSC-EVs) derived from BMSCs deliver Fer-1 to hippocampal neurons. MSC-EVs loaded with Fer-1 (MSC-EV/Fer-1) inhibited ferroptosis in hippocampal neurons by upregulating the expression of GPX4 and downregulating the expression of COX-2 (Fig. 3) [72], whereas inactivated COX-2 reduced neuronal damage and prevented cognitive deficits [88]. In addition, Fer-1 treatment reversed SSAT1 upregulation-induced ferroptosis and loss of activity in tert-butyl peroxide-exposed neurons, thereby reducing brain I/R damage [73].
2.2. MSCs regulate ferroptosis in the liver
The liver stores the largest amount of iron in the body, and iron overload and oxidative stress are the two most important causes of liver injury and disease development in most hepatopathies [89,90]. Ferroptosis is emerging as a novel form of regulated necrosis that is implicated in the progression of numerous liver diseases, including hepatocellular carcinoma (HCC), liver fibrosis, viral hepatitis, and IRI. Globally, HCC is the second leading cause of cancer-related death [91]. Recently, several studies have elucidated the essential role of ferroptosis in HCC [92]. Sorafenib, an oral tyrosine kinase inhibitor, was the first drug approved for the treatment of advanced HCC, and has improved the survival rate of patients to a certain degree. Interestingly, many researchers have demonstrated that sorafenib induces ferroptosis by inhibiting SLC7A11 and blocking oxidized cysteine from entering cells [93]. Liver fibrosis is a progressive chronic liver disease that lacks effective therapies worldwide. Iron is abundant in hepatic stellate cells (HSCs), which is a prerequisite for ferroptotic cell death [94]. Recently, direct evidence has emerged regarding the pathogenic role of ferroptosis in iron overload-induced liver damage and fibrosis [95]. In addition, liver IRI can be induced by shock or surgery. Iron overload, lipid peroxidation, and upregulation of the ferroptosis indicator, PTGS2, are typical characteristics of hepatic IRI. During reperfusion, iron-mediated death is thought to be related to oxidative stress caused by ROS [96,97]. More recently, Yamane et al. [98]reported that ferroptosis plays an important role in hepatitis C virus (HCV) replication and that chronic HCV infection promotes iron accumulation, upregulates duodenal ferroportin-1, and downregulates hepatic hepcidin, the master regulator of systemic iron homeostasis [99,100]. Acetaminophen (APAP)-induced liver injury (AILI) is a common liver disease in clinical practice. Recent studies have shown that ferroptosis is responsible for AILI [101,102]. AILI is often accompanied by oxidative stress and dysfunction and continuous oxidative stress can cause hepatocyte death [103]. Lin et al. [104] found that lipid-ROS levels, ROS accumulation, and MDA content ere significantly elevated in the livers of mice with CCl4-induced acute liver injury (ALI). The release of oxidized lipid mediators is a characteristic of ferroptosis [105]. An iron-centered hypothesis has also been proposed for the pathogenesis of nonalcoholic liver disease (NAFLD) [106]. The role of ferroptosis in NAFLD and NASH has not yet been clarified,; however, Loguercio et al. observed that more than 90 % of patients with NAFLD enrolled in their study exhibited elevated levels of lipid peroxidation markers (MDA and 4-hydroxynonenal [4-HNE]) [107]. Although some disease mechanisms remain unknown, the discovery of ferroptosis may provide new treatment strategies for liver disease Table 2.
Table 2.
Favoring effect of MSCs on liver.
| MSC/exosomes Origin | Model | Mechanisms | Outcomes | References |
|---|---|---|---|---|
| Exosomes from HO-1 modified BMMSCs | IRI of steatotic liver rats (SHPs-HR) | Knock down IREB2 reduced the level of Fer2+ | Reduce the expression of IREB2 and inhibit ferroptosis and cell damage | [108,109,110] |
| MSCs-Exo derived BECN1 | Mouses of liver fibrosis (10 % CCL4) | BECN1 decreases xCT/GPX4 expression and inhibits HSCs activation | Promote ferroptosis of HSCs | [111,112,113] |
| Exosomes derived from Baicalin-Pretreated MSCs | ALI mouse (LPS/D-GalN) | Activate the Keap1-NRF2 pathway | Inhibit ROS production and lipid peroxide-induced ferroptosis | [114,115,116] |
| Human umbilical cord blood-derived MSCs | (I/R) injury rats | Inhibit the expression of MDA, reduce the release of ROS, and increase the production of GSH | Reduce the accumulation of tissue Fe2+ and alleviate the ferroptosis-related oxidative stress injury | [[117], [118], [119],120,121] |
| Exosomes from HO-1 modified BMMSCs | Rat DCD Liver (IRI) | miR-124-3p inhibit the elevated level of STEAP3 | Inhibit hepatocytes ferroptosis and reduce the IRI of the grafts | [110,122,123] |
Ferroptosis is considered a cause of reperfusion injury and a key factor in triggering IRI and organ failure. Therefore, the role of ferroptosis in IRI-related diseases has attracted increasing attention [124]. Regulation of iron reactive element binding protein 2 (IREB2) expression is one of the mechanisms by which HO-1-modified BMMSCs inhibit IRI ferroptosis in rats fed a high-fat diet. The Fer2+ level and MDA content increased in steatotic liver IRI. IREB2 is a marker gene for ferroptosis [108], and a major regulator of iron cell homeostasis. It binds to iron-responsive elements (IRE) in the 5′-UTR region of FTH1 mRNA (encoding ferritin H, mediating intracellular iron storage) to inhibit its protein translation, and to TFR mRNA (encoding transferrin receptor 1). IRE binding in the 3 '-UTR region mediates cellular iron transport stabilizes its mRNA transcription and prevents its rapid degradation, leading to an increase in intracellular Fe2+ [125]. An increase in intracellular Fe2+ levels is a key factor that induces large lipid accumulation and promotes ferroptosis [109], and knockdown of IREB2 can reduce Fe2+ levels. miR-29a-3p targeting IREB2 is abundant in MSC-derived exosomes, and the paracylsecretory function of BMMSCs is optimized after modification with HO-1 [126]. HO-1/BMMSC exosomes are the mechanistic effectors of HO-1/BMMSCs that inhibit ferroptosis. miR-29a-3p in the exosomes of BMMSCs pretreated with HO-1 reduced IREB2 levels via a post-transcriptional regulatory mechanism (Fig. 4). Overexpression of miR-29a-3p downregulates the expression of IREB2 and inhibits ferroptosis [110]. This provides a potential therapeutic target for IRI in fatty liver disease in rats.
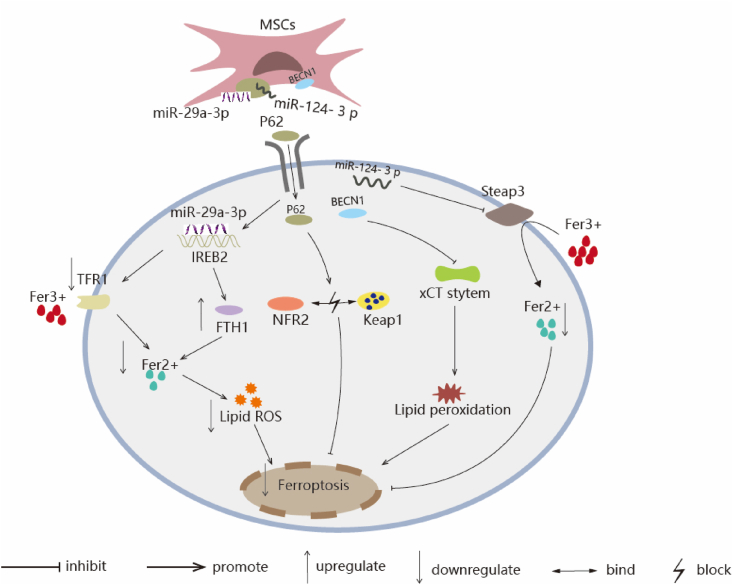
Fig. 4.
The schematic representation of MSCs-Exo inhibit ferroptosis via regulating lipid content in liver. IREB2/FTH1/TFR1 Signal Pathway: miR-29a-3p, which targets IREB2, is abundant in HO-1/BMMSCs-Exo and could decrease the IREB2 protein level. The reduced IREB2 level led to an increase in the level of FTH1 and decrease the level of TFR1 through posttranscriptional regulation, which ultimately reduced the level of Fer2+ and the production of lipid ROS and inhibits the occurrence of ferroptosis. P62-Keap1-NRF2 Pathway: Ba-Exo significantly upregulated P62 protein content in target cells, inhibits NRF2 protein degradation, and reduces Keap1 protein expression via P62, thereby exerting antioxidative stress effect and inhibiting ferroptosis to exerts a protective effect on liver function. xCT/GPX4 Axis: MSCs-Exo can deliver BECN1 protein into HSCs and inhibits SLC7A11/xCT transcription in the nucleus. Decrease SLC7A11/xCT results in cysteine deficiency and GSH reduction in HSCs, leading to GPX4 reduction and ferroptosis. miR-124-3p/Steap3 Signal Pathway: miR-124-3p is enriched in HO-1 modified BMMSCs, STEAP3, acted as an iron reductase to increase the level of Fer2+, the highly expressed miR-124-3p significantly reduces the expression of Steap 3, inhibits ferroptosis in hepatocytes.
GPX4 is a key inhibitor of ferroptosis via the removal of lipid peroxidase [127]. GSH is an important cofactor for GPX4 activity. When GPX4 function is limited, GSH is depleted, and ROS levels are significantly increased, ultimately inducing ferroptosis. GPX4 inhibits cell death by converting GSH to oxidized glutathione (GSSG) and reducing the cytotoxic lipid peroxide (L-OOH) to its corresponding alcohol (L-OH) [111]. xCT is an amino acid reverse transporter that is an important component of the antioxidant system. Cystine and glutamate are transported through the cell membrane via the xc- system, and extracellular cystine is absorbed into cells and rapidly reduced to cysteine, which is involved in GSH synthesis [128]. In mice with LX-2- and CCL4-induced liver fibrosis, MSC-exosomes triggered HSC ferroptosis by promoting ROS formation, mitochondrial dysfunction, Fer2+ release, and lipid peroxidation. BECN1 is a key regulator of ferroptosis. BECN1 induces lipid peroxidation by inhibiting xc- activity and binding to xCT [112]. Increased HSC activation is an important factor leading to the progression of liver fibrosis, and targeted induction of HSC inactivation will help establish more effective anti-fibrosis therapies. MSC-exosome-derived BECN1 promotes xCT/GPX4-mediated HSC ferroptosis by downregulating xc-/GPX4 expression (Fig. 4), thereby delaying the progression of hepatic fibrosis [113]. The use of MSC-exosomes against CCl4-induced liver fibrosis and ferroptosis of HSCs may be a novel therapeutic approach for the treatment of liver fibrosis.
Ferroptosis is cell death caused by the iron-dependent accumulation of lipid hydroperoxides and ROS in cells. In mice with LPS induced ALI, ROS content and lipid peroxidation increased. NRF2 is an important nuclear transcription factor for intracellular antioxidants that negatively regulates ferroptosis in a linear manner, referred to as the p62-keap1-NRF2 pathway. The P62-Keap1-NRF2 pathway is an important signaling pathway for maintaining the balance between oxidative stress and REDOX in vivo [129], and it regulates intracellular iron homeostasis through the transcriptional activation of genes involved in ROS and iron metabolism. The expression of P62 prevents NRF2 degradation and enhances its subsequent nuclear accumulation by inactivating Kelch-like ECH-associated protein 1 (Keap1) [114]. Previous studies have demonstrated that P62 regulates ferroptosis in liver cells by activating the Keap1-NRF2 pathway and has pharmacological effects such as anti-inflammatory, antibacterial, anti-fibrosis, anti-oxidation, and anti-cancer effects [130,131], P62 is highly expressed in exosomes derived from MSCs pretreated with GBP, and P62 inhibits the production of ROS and lipid peroxidation by activating the Keap1-NRF2 pathway (Fig. 4) to suppress ferroptosis [115]. In addition, after APAP-induced ALI, glycosides induced cytoplasmic accumulation of NRF2 in mice, leading to activation of the receptor family pyrin domain containing 3 (NLRP3) inflammasome, followed by increased expression of IL-8, induction of hepatocyte proliferation, and promotion of hepatocyte regeneration [116], thus playing a protective role in liver function. Therefore, optimizing the efficacy of MSC-derived exosomes for the treatment of ALI is an effective and promising strategy.
Donation after circulatory death (DCD) is a major source of organ transplantation. However, DCD donor livers undergo long-term warm ischemic injury, IRI, and intracellular ROS-induced injury, resulting in damage to the structure and function of donor livers, which may lead to primary dysfunction and other complications after transplantation [117]. Normothermic machine perfusion (NMP) is a novel method of organ preservation that mimics the normal metabolic state in vivo to more effectively protect the structure and function of the donor liver, with promising results, potentially broadening the donor pool [118,119]. MSCs are non-hematopoietic stem cells that originate from the stroma and can attenuate hepatocyte injury, accelerate liver regeneration, participate in anti-inflammatory activities, and regulate immunity [[132], [133], [134], [135], [136], [137]]. Donor livers are more susceptible to injury during I/R because of their poor antioxidant defense capacity and high ROS production by the mitochondrial xanthine/xanthine oxidase system [120,121]. In a liver reperfusion model, BMMSCs combined with NMP protected the morphology of mitochondria, reduced the level of intracellular ROS, and reduced ferroptosis in hepatocytes in the DCD donor liver model. In addition, BMSCS combined with the NMP system can stabilize the levels of intracellular free Fe2+ and ROS by regulating the concentration of intracellular free Fe2+, significantly inhibiting the expression of MDA, reducing the release of ROS, and increasing the production of GSH to inhibit hepatocyte ferroptosis. Generally, IRI of the donor liver can be alleviated after circulatory death [117].
IRI inevitably results from a transplanted liver and severe hepatic steatosis worsens hepatic IRI, which can result in early graft and primary dysfunction [138,139]. Steatotic liver is the most common chronic liver disease worldwide and a common type of expanded criteria donor liver (ECD). Severe hepatic steatosis is extremely sensitive to IRI. Li et al. [110] found that Fe2+ and MDA levels are significantly higher in steatotic liver IRI. Excessive free Fe2+ accumulation in the cytoplasm produces a large number of hydroxyl radicals and ROS through the Fenton reaction, which destroys the cell membrane, normal DNA, and cellular proteins, leading to ferroptosis. STEAP3, an iron reductase, can reduce Fe3+ in endosomes to Fe2+ and participates in mediating cellular iron homeostasis [140,141]. The upregulation of STEAP3 leads to a significant increase in Fe2+ generated by reduction, and excess Fe2+ leads to the expansion of LIP, which further exacerbates the Fenton reaction and ultimately leads to cellular ferroptosis [122]. Consistent with predictions from a published database, we found that miR-124-3p, which is differentially expressed, may be a potential miRNA candidate that targets STEAP3. miRNA activity leads to degradation of the mRNA and/or inhibition of translation by targeting the 3' UTR of the mRNA. Studies have shown that miR-124-3p is enriched in HO-1-modified BMMSCs, and highly expressed miR-124-3p significantly reduces the expression of STEAP3 and inhibits ferroptosis (Fig. 4) in hepatocytes by reducing the accumulation of Fe2+ in the endosome and inhibition of IRI [123].
2.3. MSCs regulate ferroptosis in the heart
Ferroptosis is an important driver of hypoxic cardiac injury. It has been shown that excess iron in cardiomyocytes can directly induce ferroptosis through the accumulation of hydroperoxides of phospholipids in the cell membrane [142]. By contrast, Ang II-augmented iron overload induces cardiac fibrosis and promotes neointima formation [143]. Recently, ferroptosis was reported to be a relevant cell death subroutine in cardiomyocytes. Myocardial ferroptosis promotes heart failure (HF), myocardial infarction (MI), heart transplantation, and doxorubicin (DOX)-induced cardiotoxicity [144]. Downregulation of GPX4 induces ferroptosis during acute myocardial injury (AMI) resulting in cardiac cell death and MI [145]. DOX is a second-generation chemotherapeutic drug in the class of anthracyclines, a commonly used antitumor drug with lethal cardiotoxicity that negatively regulates GPX4 and overproduces lipid peroxides in the mitochondria via the DOX-Fe2+ complex, resulting in mitochondria-dependent ferroptosis [146]. The mode of cell death in the process of myocardial IRI has received considerable attention. Zhao et al. [147]identified ferroptosis as a form of cell death in the pathogenesis of IRI in the myocardium, which is closely linked to oxidative stress. Furthermore, ferroptosis has recently been reported to promote the formation of atherosclerotic lesions [148]. Specifically, lipid peroxidation may increase endothelial ROS, decrease nitric oxide, initiate inflammation via macrophage polarization, and accelerate foam cell and atherosclerotic lesion formation [149]. Hypertension is a common cardiovascular disease. One study reported that a reduction in GPX4 and GSH in the brains of hypertensive rats resulted in lipid peroxidation and iron overload, which induced hypertensive brain damage [150]. Recent studies have also indicated that iron overload causes arrhythmia via the promotion of mitochondrial ROS generation and depolarization of the membrane potential, and mitochondrial dysfunction is a key feature of ferroptosis [151]. In addition, cardiac hypertrophy is one of the pathological mechanisms of HF, and Wang et al. [152]found that both proptosis and ferroptosis worsen myocardial hypertrophy and fibrosis. Preliminary studies on the role of MSCs in hypoxic cardiac injury may open a new window of opportunity for novel therapeutic strategies and targets, as shown in Table 3.
Table 3.
Favoring effect of MSCs on heart.
| MSC/exosomes Origin | Model | Mechanisms | Outcomes | References |
|---|---|---|---|---|
| BMSCs derived exosomes | IR mice (ligation of LAD) | LncRNA Mir9-3hg downregulates the expression of Pum2 | Pum2 binds with PRDX6 promoter and restrain PRDX6 expression to alleviate ferroptosis | [153] |
| HuMSCs-Exo under the hypoxic condition | AMI mice (DOX) | Trx1 upregulates phosphorylation of mTORC1 in DOX-treated cardiomyocytes, and promotes GPX4 protein synthesis | Protect cardiomyocyte from DOX-induced ferroptosis | [154,155] |
| Exosomes derived from HuMSCs | AMI mice (permanent ligation of LAD coronary artery) | miR-23a-3p targets DMT1 and suppresses DMT1 expression to prevent the accumulation of Fe2+ in cardiomyocytes | Inhibit ferroptosis and attenuate myocardial injury | [156,157,158] |
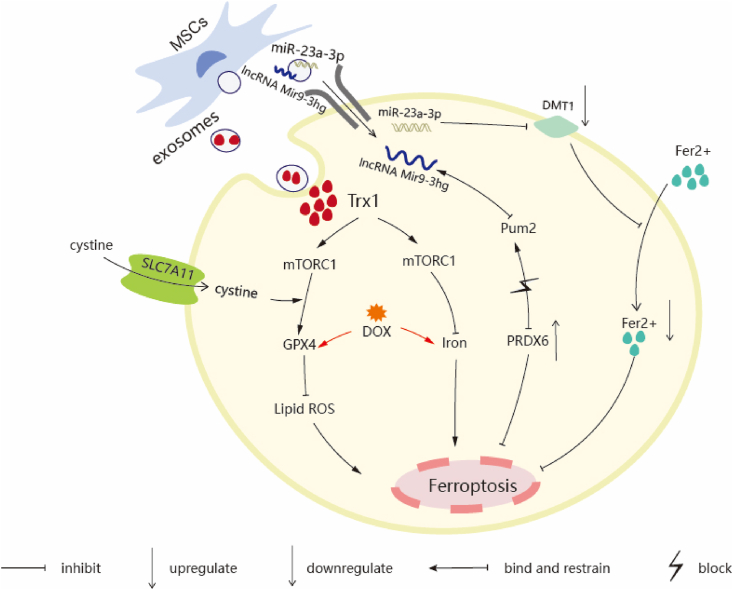
Ferroptosis is an important form of cardiomyocyte death. When the heart tissue is exposed to an ischemia-reperfusion environment, iron overload and associated lipid peroxidation lead to myocardial cell damage, thus affecting heart function. Pumilio (Pum) is a ribonucleic acid-binding protein (RBP) that selectively inhibits gene expression by binding to the 30-untranslated region (30-UTR) of the target mRNA [159]. Peroxido-reducing protein 6 (PRDX6) is a basic negative regulator of ferroptosis sensitivity, and its upregulation significantly inhibits lipid peroxidation and intracellular iron degradation triggered by ferroptosis inducers in various cancer cell lines [160]. Chromatin immunoprecipitation assays showed that Pumilio Na-binding family member 2 (Pum2) bound to the PRDX6 promoter and inhibited PRDX6 expression. Inhibited expression of PRDX6 significantly decreased cell proliferation, GSH content, and GPX4 protein levels in H/R-treated cells, and significantly increased iron ion concentration, ROS content, and ACSL protein levels. However, lncRNA Mir9-3hg, which is highly expressed in BMSCS-derived exosomes, can alleviate ferroptosis by binding to the Pum2 protein, downregulating Pum2 expression, and finally upregulating PRDX6 expression (Fig. 5) [153], thus improving heart injury triggered by ischemia/reperfusion and playing a protective role in heart function.
Fig. 5.
The schematic representation of MSCs-Exo regulate cardiomyocytes ferroptosis by acting on ferroptotic related receptors. Trx1/SLC7A11/mTORC1 Signal Pathway: Schematic illustrate that Trx1 in Hypo-Exo inhibit DOX-induced ferroptosis and promote GPX4 protein synthesis in NRCMs by activating mTORC1. lncRNA Mir9-3hg/Pum2/PRDX6 Axis: lncRNA Mir9-3hg is highly expressed in BMMSCs-derived exosomes. Pum2 bound with PRDX6 promoter and restrained PRDX6 expression. Mir9-3hg bound with Pum2 protein and downregulated Pum2 expression to alleviates cardiomyocute ferroptosis. miR-23a-3p/DMT1 Pathway: The overexpression of DMT1 promotes the accumulation of Fe2+. DMT1 is the target gene of miR-23a-3p, miR-124-3 p was enriched in MSCs-Exo, inhibits DMT1 expression and prevents the accumulation of Fe2+ in cardiomyocytes to inhibit ferroptosis and prevent cardiomyocytes.
SLC7A11 is a component of the cystine glutamate transporter (System xc-), which plays an important role in providing cysteine for GSH biosynthesis. mTORC1 is an evolutionarily conserved serine/threonine kinase that regulates various processes, including cell survival, autophagy, and protein synthesis [161]. Western blotting confirmed that DOX significantly decreased the GPX4 protein levels in mice. Thioredoxin 1 (Trx1) interacts with a variety of signaling molecules and transcription factors, and exhibits anti-ferroptotic effects by activating mTORC1 in neonatal rat cardiomyocytes, which promotes GPX4 protein synthesis through mTORC1 signaling. Trx1-mediated mTORC1 activation is key to ferroptosis resistance in hypoxia-preconditioned MSC-derived exosomes. Trx1 is highly expressed in hypoxia-preconditioned MSC-derived exosomes, and mTORC1, activated by Trx1, promotes GPX4 protein synthesis by utilizing cysteine transported by SLC7A11 to reduce ROS and lipid peroxidation and inhibit ferroptosis (Fig. 5) in mice with AMI [154]. In addition, Trx1 alleviates ferroptosis by reducing H2O2-mediated oxidative stress through mTORC1 [155].
Cardiomyopathy is related to iron overload, and ischemia leads to the accumulation of iron in the myocardium, resulting in myocardial oxidative stress and the production of a large amount of lipid ROS to induce ferroptosis in cardiomyocytes, exacerbating heart damage. DMT1 (SLC11A2) is an Fe2+ transporter that regulates iron content and is a key regulator of iron homeostasis in the body [156]. Relevant studies have shown that the downregulation of DMT1 expression can prevent iron intake [162], and miR-23a-3p is an miRNA enriched in MSC-derived exosomes [157]. Studies have shown that DMT1 is the target gene of miR-23a-3p and that it is significantly upregulated 24 h after the establishment of a mouse model of AMI established by permanent ligation of the LAD coronary artery. Overexpression of DMT1 promoted the accumulation of Fe2+ in H/R-induced cardiomyocytes, leading to ferroptosis. Targeting miR-23a-3p, which is highly expressed in exosomes derived from human cord blood, inhibits the expression of DMT1, prevents the accumulation of Fe2+ in cardiomyocytes, inhibits ferroptosis (Fig. 5), and attenuates MI [158].
2.4. MSCs regulate ferroptosis in other organs
In Table 4, we summarized the currently used BMSCs-secreted EVs and Bone marrow -MSCs that interfere with ferroptosis, the postulated mechanism and the corresponding cellular/animal experimental model.
Table 4.
Favoring effect of MSCs on other organs.
| MSC Origin | Model | Mechanisms | Outcomes | References |
|---|---|---|---|---|
| Mouse BMSCs-secreted EVs | IDD mices (NPCs) | Circ-0072464 competitively binds to miR-431, and targets and inhibits NRF2 expression | Inhibit NPCs ferroptosis and relieve intervertebral disc degeneration | [163] |
| Bone marrow -MSCs | Rats expose to Cr(VI) | upregulate AKT and mTOR phosphorylation increase levels of protein SLC 7A11 | downregulate autophagy-associated proteins and repair Cr(VI)-damaged testes | [164,165] |
As a competitive endogenous RNA (ceRNA), circRNA competes with microRNA (miRNA) to regulate the expression of miRNA target messenger RNA (mRNA), which is a potential biomarker for the treatment of intervertebral disc degeneration (IDD) [166]. NRF2 is a key regulator of cellular antioxidant response and regulates ferroptosis by controlling the expression of genes that counteract oxidative and electrophilic stress. Studies have shown that miR-431 is overexpressed in patients with IDD, and miR-431 targets and inhibits NRF2 expression, thereby reducing the antioxidant capacity of cells and inducing ferroptosis in cells with IDD. Notably, the bioinformatics analysis predicted that circ-0072464 was downregulated in nucleus pulposus of IDD mice. Mechanistically, circ-0072464, which travels through extracellular vesicles of BMMSCs-exosomes, competitively binds to miR-431 to up-regulate NRF2 expression and inhibit ferroptosis by enhancing the antioxidant capacity of the NPC to alleviate IDD. In addition, Circ-0072464 facilitated stromal synthesis and proliferation of NPC by transcellular vesicles in BMMSCs [163], and these mechanisms provide new therapeutic targets for intervertebral disc lesions in mice with IDD.
An estimated 186 million people worldwide suffer from infertility [167], and approximately 40–50 % of these are affected by male infertility [168]. There are various reasons for the decline in sperm quality, among which heavy metal toxicity is a major factors [169]. The testes, which are one of the target organs of heavy metal poisoning, are highly sensitive to heavy metal damage. It has been shown that Cr(VI) can cause reproductive toxicity and further affect the male reproductive ability in mammals [170]. The well-known cellular toxicity of Cr (VI) can be attributed to the generation of ROS, which trigger oxidative stress, DNA damage, and genomic instability [164]. In recent years, autophagy and ferroptosis have emerged as the major mechanisms underlying the toxic effects of heavy metals [[171], [172], [173], [174]]. As a marker of lipid peroxidation and oxidative stress, 4-HNE is also considered a “second toxic messenger of free radicals” and a “toxic product of lipid peroxidation” [175,176]. Cells exposed to Cr (VI) showed significantly increased expression of lipid peroxides (4-HNE), increased lipid ROS levels, and significantly decreased levels of SLC7A11. The AKT/mammalian target of rapamycin (mTOR) pathway is a key autophagy pathway [[177], [178], [179]]. In cancer cells, ferroptosis is suppressed through activation of the PI3K/AKT/mTOR pathway [180]. Taken together, BMSCs repair Cr(VI)-damaged testes by attenuating ferroptosis and downregulating proteins associated with autophagy through the positive regulation of AKT and mTOR phosphorylation [165], this provides a potential therapeutic target for infertility caused by damage by Cr (VI) in the testis.
3. Current challenges of MSC therapy
MSCs have a unique immunophenotypic capacity, tissue repair capacity, and immune dampening activities [181] and are regarded as a promising source of stem cells in personalized cell-based therapies [182]. However, the therapeutic potential of MSCs remains controversial and may depend on different sources of MSC, culture procedures, routes of administration, and cellular heterogeneity. For example, the size of MSCs in monolayer culture in vitro increases at the same time as the passage number; because of their size, MSCs can trigger severe vascular obstruction after intravascular delivery. Few engrafted BMMSCs have been found in the brain because of the inability of these cells [183] to cross the cerebral blood barrier. MSCs are also components of the tumor microenvironment, which can be dictated by tumor associated MSCs (TA-MSCs) and transmuted into the tumor-supporting phenotype to promote tumor growth [184]. Exosomes are naturally occurring vesicles produced by cells that have lower immunogenicity, long circulating half-lives, superior biocompatibility, and the ability to evade phagocytosis, suggesting that Exo-MSCs have promising potential as carriers for the delivery of therapeutics. However, owing to the different sources of MSCs, tumor types, and tumor progression stages, conflicting results have demonstrated that exo-MSCs can suppress tumors through several mechanisms. Exo-MSCs can contribute to drug resistance [185]. For example, some studies have shown that exo-MSCs carry different types of miRNAs that can cause resistance to proteasome inhibitors when co-cultured with multiple myeloma cells. The study of MSC-exos has been in its infancy for some time, and many problems remain to be resolved. The clinical translation of MSC-exos requires further studies on their mass production, isolation, loading, and modification. Overall, optimized MSC isolation protocols and administration routes for clinical use must be well established and standardized. This comprehensive effort should be considered by the scientific community focused on the practical applications of MSCs in tissue repair to optimally prepare MSC-based products for more effective therapies in patients.
4. Discussion
Ferroptosis is a key process involved in many diseases. Nonetheless, the exploration of ferroptosis has been restricted to a few studies and the underlying processes are yet to be completely clarified. Beyond the outlined routes, research should investigate the existence of additional pathways that potentially lead to ferroptosis to investigate the emergence, growth, and subsequent effects of ferroptosis. The exploration of ferroptosis and its involvement in diverse illnesses has led to the development of specialized treatment approaches with significant theoretical and clinical importance. Advancements in biotechnology and use of cross-disciplinary integration techniques will enhance our understanding of ferroptosis. In addition, this review discussed the progress and potential clinical applications of pluripotent stem cells in diseases associated with ferroptosis in the brain, liver, and heart. Further investigations are needed to elucidate the mechanism of mesenchymal stem cells in ferroptosis-associated diseases in other systems to improve and facilitate the clinical use of MSC therapy.
5. Conclusions
Based on existing results, ferroptosis has opened a new platform in disease research, and its clinical importance in disease occurrence, development, and treatment has gradually emerged. Both opportunities and challenges exist in the treatment of multisystem diseases through the regulation of ferroptosis. The important role of MSCs has also been highlighted recently, which strongly indicates MSC involvement in ferroptosis and that may present a strategy that could play an important role in intracellular ROS, lipid peroxidation, and oxidative stress of cells implicated in the pathology of the disease, ultimately contributing to the therapeutic action exerted by MSCs and providing a broader perspective for the clinical application of MSC-based ferroptosis disease. To the best of our knowledge, MSC-exosomes are versatile and can interact with multiple cell types in the immediate vicinity and at distant locations to elicit appropriate cellular responses. Through secreted exosomes, MSCs target maintenance processes to restore tissue homeostasis and allow the cells within the tissue to recover, repair, and regenerate. New MSC-based therapies designed to modulate ferroptosis signaling pathways may represent innovative and attractive strategies for the treatment of numerous diseases.
Funding
The current study was supported by the National Natural Science Foundation of China (grant no. 81860144). Kunming Medical University PhD Innovation Fund Project for 2023 (2023B022). Kunming Medical University Second Affiliated Hospital 2023 First Class Department of Clinical Medicine (J1301907).
Ethical approval
This study was approved by our institutional review board.
Statement of human and animal rights
This article does not contain any studies with human or animal subjects.
Statement of informed consent
There are no human subjects in this article and informed consent is not applicable.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Mengling Cui: Conceptualization. Fukun Chen: Project administration. Lishi Shao: Funding acquisition. Chanyan Wei: Methodology. Weihu Zhang: Software. Wenmei Sun: Methodology. Jiaping Wang: Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jiaping Wang reports financial support was provided by the current study was supported by the National Natural Science Foundation of China (grant no. 81860144). Jiaping Wang reports financial support was provided by Kunming Medical University Second Affiliated Hospital 2023 First Class Department of Clinical Medicine (J1301907). Lishi Shao reports financial support was provided by Kunming Medical University PhD Innovation Fund Project for 2023 (2023B022). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviation
- PD
Parkinson’s disease
- MSCs
mesenchymal stem cells
- NPCs
nucleus pulposus cells
- MHC
major histocompatibility complex
- IBD
inflammatory bowel disease
- AKI
acute renal injury
- EPCs
endothelial progenitor cells
- BMMSCs
bone marrow mesenchymal stem cells
- ROS
Reactive Oxygen Species
- GPX4
glutathione peroxidase-4
- GSH
glutathione
- AIFM2
apoptosis-inducing factor mitochondrial 2
- PUFAs
Polyunsaturated fatty acids
- ACSL4
Acyl-CoA synthetase long chain family member 4
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- VDAC
Voltage-dependent anion channel
- TF
transferrin
- TFR
transferrin receptor
- STEAP3
six-transmembrane epithelial antigen of prostate 3
- DMT1
divalent metal transporter 1
- HSPB1
heat shock protein beta-1
- TBI
traumatic brain injury
- IRI
ischemia-reperfusion injury
- HucMSCs
human umbilical cord mesenchymal stem cells
- AD
Alz-heimer's disease
- MCAO
middle cerebral artery occlusion
- SIRT1
Silence-regulating factor 1
- dNCR
delayed neurocognitive recovery
- MDA
malondialdehyde
- NRF2
nuclear factor-erythroid 2-associated factor 2
- HO-1
heme oxygenase-1
- RmTBI
repetitive mild traumatic brain injury
- Fer-1
Ferrostatin-1
- HCC
hepatocellular carcinoma
- HSCs|
hepatic stellate cells
- HCV
hepatitis C virus
- AILI
Acetaminophen (APAP)-induced liver injury
- ALI
acute liver injury
- NAFLD
non-alcoholic liver disease
- 4-HNE
4-hydroxynonenal
- Keap1
Kelch-like ECH associated protein 1
- NLRP3
receptor family pyrin domain containing 3
- DCD
Donation after circulatory death
- NMP
Normothermic machine perfusion
- ECD
expanded criteria donor liver
- HF
heart failure
- MI
myocardial infarction
- DOX
doxorubicin
- AMI
acute myocardial injury
- PRDX6
Peroxido-reducing protein 6
- Pum2
PumiliorNa-binding family member 2
- Trx1
Thioredoxin 1
- IDD
intervertebral disc degeneration
References
- 1.Sheykhhasan M., Heidari F., Eslami Farsani M., Azimzadeh M., Kalhor N., Ababzadeh S., Seyedebrahimi R. Dual role of Exosome in neurodegenerative diseases: a review study. Curr. Stem Cell Res. Ther. 2023 doi: 10.2174/1574888X18666230726161035. Epub ahead of print. PMID: 37496136. [DOI] [PubMed] [Google Scholar]
- 2.Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F., Romagnani P., Maggi E., Romagnani S., Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cell. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. Epub 2005 Aug 25. PMID: 16123384. [DOI] [PubMed] [Google Scholar]
- 3.DelaRosa O., Lombardo E., Beraza A., Mancheño-Corvo P., Ramirez C., Menta R., Rico L., Camarillo E., García L., Abad J.L., Trigueros C., Delgado M., Büscher D. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng. 2009;15(10):2795–2806. doi: 10.1089/ten.TEA.2008.0630. PMID: 19231921. [DOI] [PubMed] [Google Scholar]
- 4.Cho P.S., Messina D.J., Hirsh E.L., Chi N., Goldman S.N., Lo D.P., Harris I.R., Popma S.H., Sachs D.H., Huang C.A. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008 Jan 1;111(1):430–438. doi: 10.1182/blood-2007-03-078774. Epub 2007 Oct 1. PMID: 17909081. [DOI] [PubMed] [Google Scholar]
- 5.Nitzsche F., Müller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade-Insights into homing and Transendothelial migration. Stem Cell. 2017 Jun;35(6):1446–1460. doi: 10.1002/stem.2614. Epub 2017 Apr 3. PMID: 28316123. [DOI] [PubMed] [Google Scholar]
- 6.Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J. Stem Cell. 2021 Jun 26;13(6):619–631. doi: 10.4252/wjsc.v13.i6.619. PMID: 34249231; PMCID: PMC8246245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Salam A.G., Ata H.M., Salman T.M., Rashed L.A., Sabry D., Schaalan M.F. Potential therapeutic utility of mesenchymal stem cells in inflammatory bowel disease in mice. Int. Immunopharm. 2014 Oct;22(2):515–521. doi: 10.1016/j.intimp.2014.07.030. Epub 2014 Aug 13. PMID: 25133649. [DOI] [PubMed] [Google Scholar]
- 8.Glavaski-Joksimovic A., Bohn M.C. Mesenchymal stem cells and neuroregeneration in Parkinson's disease. Exp. Neurol. 2013 Sep;247:25–38. doi: 10.1016/j.expneurol.2013.03.016. Epub 2013 Mar 28. PMID: 23542820. [DOI] [PubMed] [Google Scholar]
- 9.Sávio-Silva C., Soinski-Sousa P.E., Balby-Rocha M.T.A., Lira Á.O., Rangel É.B. Mesenchymal stem cell therapy in acute kidney injury (AKI): review and perspectives. Rev. Assoc. Med. Bras. 2020 Jan 13;66(Suppl 1):s45–s54. doi: 10.1590/1806-9282.66.S1.45. PMID: 31939535. [DOI] [PubMed] [Google Scholar]
- 10.Yeo R.W., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh Bj, Lim S.K. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013 Mar;65(3):336–341. doi: 10.1016/j.addr.2012.07.001. Epub 2012 Jul 7. PMID: 22780955. [DOI] [PubMed] [Google Scholar]
- 11.Timmers L., Lim S.K., Arslan F., Armstrong J.S., Hoefer I.E., Doevendans P.A., Piek J.J., El Oakley R.M., Choo A., Lee C.N., Pasterkamp G., de Kleijn D.P. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007 Nov;1(2):129–137. doi: 10.1016/j.scr.2008.02.002. Epub 2008 Mar 8. PMID: 19383393. [DOI] [PubMed] [Google Scholar]
- 12.Liang X., Ding Y., Zhang Y., Tse H.F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi: 10.3727/096368913X667709. PMID: 23676629. [DOI] [PubMed] [Google Scholar]
- 13.Liang X., Ding Y., Zhang Y., Tse H.F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi: 10.3727/096368913X667709. PMID: 23676629. [DOI] [PubMed] [Google Scholar]
- 14.Phinney D.G., Pittenger M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem cells. 2017 Apr;35(4):851–858. doi: 10.1002/stem.2575. Epub 2017 Mar 10. Erratum in: Stem Cells. 2017 Sep;35(9):2103. PMID: 28294454. [DOI] [PubMed] [Google Scholar]
- 15.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S., Salto-Tellez M., Timmers L., Lee C.N., El Oakley R.M., Pasterkamp G., de Kleijn D.P., Lim S.K. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010 May;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. Epub 2010 Jan 4. PMID: 20138817. [DOI] [PubMed] [Google Scholar]
- 16.Roefs M.T., Sluijter J.P.G., Vader P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 2020 Dec;30(12):990–1013. doi: 10.1016/j.tcb.2020.09.009. Epub 2020 Oct 14. PMID: 33069512. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020 Feb 7;367(6478) doi: 10.1126/science.aau6977. PMID: 32029601; PMCID: PMC7717626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha D.H., Kim H.K., Lee J., Kwon H.H., Park G.H., Yang S.H., Jung J.Y., Choi H., Lee J.H., Sung S., Yi Y.W., Cho B.S. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020 May 7;9(5):1157. doi: 10.3390/cells9051157. PMID: 32392899; PMCID: PMC7290908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K., Yan G., Huang H., Zheng M., Ma K., Cui X., Lu D., Zheng L., Zhu B., Cheng J., Zhao J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnol. 2022 Jan 20;20(1):38. doi: 10.1186/s12951-021-01236-1. PMID: 35057811; PMCID: PMC8771624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Xie X., Lian W., Shi R., Han S., Zhang H., Lu L., Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018 Apr 13;50(4):1–14. doi: 10.1038/s12276-018-0058-5. PMID: 29651102; PMCID: PMC5938041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R., Zhang F., Chai R., Zhou W., Hu M., Liu B., Chen X., Liu M., Xu Q., Liu N., Liu S. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J. Cell Mol. Med. 2019 Nov;23(11):7617–7631. doi: 10.1111/jcmm.14635. Epub 2019 Sep 26. PMID: 31557396; PMCID: PMC6815833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grange C., Tapparo M., Bruno S., Chatterjee D., Quesenberry P.J., Tetta C., Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014 May;33(5):1055–1063. doi: 10.3892/ijmm.2014.1663. Epub 2014 Feb 20. PMID: 24573178; PMCID: PMC4020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han C., Sun X., Liu L., Jiang H., Shen Y., Xu X., Li J., Zhang G., Huang J., Lin Z., Xiong N., Wang T. Exosomes and their therapeutic potentials of stem cells. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/7653489. Epub 2015 Dec 6. PMID: 26770213; PMCID: PMC4684885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phinney D.G., Pittenger M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cell. 2017 Apr;35(4):851–858. doi: 10.1002/stem.2575. Epub 2017 Mar 10. Erratum in: Stem Cells. 2017 Sep;35(9):2103. PMID: 28294454. [DOI] [PubMed] [Google Scholar]
- 25.Lou G., Chen Z., Zheng M., Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017 Jun 16;49(6):e346. doi: 10.1038/emm.2017.63. PMID: 28620221; PMCID: PMC5519012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nooshabadi V.T., Mardpour S., Yousefi-Ahmadipour A., Allahverdi A., Izadpanah M., Daneshimehr F., Ai J., Banafshe H.R., Ebrahimi-Barough S. The extracellular vesicles-derived from mesenchymal stromal cells: a new therapeutic option in regenerative medicine. J. Cell. Biochem. 2018 Nov;119(10):8048–8073. doi: 10.1002/jcb.26726. Epub 2018 Jun 22. PMID: 29377241. [DOI] [PubMed] [Google Scholar]
- 27.Mendt M., Rezvani K., Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019 Aug;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. PMID: 31431712. [DOI] [PubMed] [Google Scholar]
- 28.Ganz T. Systemic iron homeostasis. Physiol. Rev. 2013;93(4):1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 29.Catalá A., Díaz M. Editorial: impact of lipid peroxidation on the physiology and pathophysiology of cell membranes. Front. Physiol. 2016 Sep 22;7:423. doi: 10.3389/fphys.2016.00423. PMID: 27713704; PMCID: PMC5031777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuno H., Kubota C., Takigawa Y., Shintoku R., Kannari N., Muraoka T., Obinata H., Yoshimoto Y., Kanazawa M., Koshiishi I., Torii S. 2,2,6,6-Tetramethylpiperidine-1-oxyl acts as a volatile inhibitor of ferroptosis and neurological injury. J. Biochem. 2022 Jul 25;172(2):71–78. doi: 10.1093/jb/mvac044. PMID: 35512114. [DOI] [PubMed] [Google Scholar]
- 31.Angeli J.P.F., Shah R., Pratt D.A., Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol. Sci. 2017 May;38(5):489–498. doi: 10.1016/j.tips.2017.02.005. Epub 2017 Mar 28. PMID: 28363764. [DOI] [PubMed] [Google Scholar]
- 32.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012 May 25;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. PMID: 22632970; PMCID: PMC3367386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang L., Hickman J.H., Wang S.J., Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14(18):2881–2885. doi: 10.1080/15384101.2015.1068479. PMID: 26218928; PMCID: PMC4825584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou Y., Wang S.J., Li D., Chu B., Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. U. S. A. 2016 Nov 1;113(44):E6806–E6812. doi: 10.1073/pnas.1607152113. Epub 2016 Oct 3. PMID: 27698118; PMCID: PMC5098629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017 Jan 15;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. Epub 2017 Feb 3. PMID: 28212725; PMCID: PMC5319403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., Yang Q., Proneth B., Klein-Seetharaman J., Watkins S., Bahar I., Greenberger J., Mallampalli R.K., Stockwell B.R., Tyurina Y.Y., Conrad M., Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017 Jan;13(1):81–90. doi: 10.1038/nchembio.2238. Epub 2016 Nov 14. PMID: 27842066; PMCID: PMC5506843.s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skonieczna M., Cieslar-Pobuda A., Saenko Y., Foksinski M., Olinski R., Rzeszowska-Wolny J., Wiechec E. The impact of DIDS-induced inhibition of voltage-dependent anion channels (VDAC) on cellular response of lymphoblastoid cells to ionizing radiation. Med. Chem. 2017;13(5):477–483. doi: 10.2174/1573406413666170421102353. PMID: 28427245. [DOI] [PubMed] [Google Scholar]
- 38.Yagoda N., von Rechenberg M., Zaganjor E., Bauer A.J., Yang W.S., Fridman D.J., Wolpaw A.J., Smukste I., Peltier J.M., Boniface J.J., Smith R., Lessnick S.L., Sahasrabudhe S., Stockwell B.R. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007 Jun 14;447(7146):864–868. doi: 10.1038/nature05859. PMID: 17568748; PMCID: PMC3047570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gammella E., Recalcati S., Rybinska I., Buratti P., Cairo G. Iron-induced damage in cardiomyopathy: oxidative-dependent and independent mechanisms. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/230182. Epub 2015 Mar 24. PMID: 25878762; PMCID: PMC4387903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masaldan S., Belaidi A.A., Ayton S., Bush A.I. Cellular senescence and iron dyshomeostasis in alzheimer's disease. Pharmaceuticals. 2019 Jun 19;12(2):93. doi: 10.3390/ph12020093. PMID: 31248150; PMCID: PMC6630536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyokuni S. The origin and future of oxidative stress pathology: from the recognition of carcinogenesis as an iron addiction with ferroptosis-resistance to non-thermal plasma therapy. Pathol. Int. 2016 May;66(5):245–259. doi: 10.1111/pin.12396. Epub 2016 Mar 2. PMID: 26931176. [DOI] [PubMed] [Google Scholar]
- 42.Bai T., Lei P., Zhou H., Liang R., Zhu R., Wang W., Zhou L., Sun Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J. Cell Mol. Med. 2019 Nov;23(11):7349–7359. doi: 10.1111/jcmm.14594. Epub 2019 Sep 10. PMID: 31507082; PMCID: PMC6815844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenny E.M., Fidan E., Yang Q., Anthonymuthu T.S., New L.A., Meyer E.A., Wang H., Kochanek P.M., Dixon C.E., Kagan V.E., Bayir H. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit. Care Med. 2019 Mar;47(3):410–418. doi: 10.1097/CCM.0000000000003555. PMID: 30531185; PMCID: PMC6449247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zille M., Karuppagounder S.S., Chen Y., Gough P.J., Bertin J., Finger J., Milner T.A., Jonas E.A., Ratan R.R. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017 Apr;48(4):1033–1043. doi: 10.1161/STROKEAHA.116.015609. Epub 2017 Mar 1. PMID: 28250197; PMCID: PMC5613764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frangogiannis N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2015 Sep 20;5(4):1841–1875. doi: 10.1002/cphy.c150006. PMID: 26426469. [DOI] [PubMed] [Google Scholar]
- 46.Guan X., Li X., Yang X., Yan J., Shi P., Ba L., Cao Y., Wang P. The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 2019 Oct 15;235 doi: 10.1016/j.lfs.2019.116795. Epub 2019 Aug 27. PMID: 31470002. [DOI] [PubMed] [Google Scholar]
- 47.Zhai Q.Y., Ren Y.Q., Ni Q.S., Song Z.H., Ge K.L., Guo Y.L. Transplantation of human umbilical cord mesenchymal stem cells-derived neural stem cells pretreated with Neuregulin1β ameliorate cerebral ischemic reperfusion injury in rats. Biomolecules. 2022 Mar 10;12(3):428. doi: 10.3390/biom12030428. PMID: 35327620; PMCID: PMC8945978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Zhang X., Liu Y., Pan R., Liang X., Huang L., Yang C. Exosomes derived from mesenchyml stem cells ameliorate oxygen-glucose deprivation/reoxygenation-induced neuronal injury via transferring MicroRNA-194 and targeting Bach1. Tissue Cell. 2021 Dec;73:101651. doi: 10.1016/j.tice.2021.101651. Epub 2021 Sep 17. PMID: 34600339. [DOI] [PubMed] [Google Scholar]
- 49.Xu J., Zhang M., Liu F., Shi L., Jiang X., Chen C., Wang J., Diao M., Khan Z.U., Zhang M. Mesenchymal stem cells alleviate post-resuscitation cardiac and cerebral injuries by inhibiting cell pyroptosis and ferroptosis in a swine model of cardiac arrest. Front. Pharmacol. 2021 Dec 9;12 doi: 10.3389/fphar.2021.793829. PMID: 34955860; PMCID: PMC8696260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong Y., Chen L., Lin Z., Hu Y., Panayi A.C., Zhou W., Sun Y., Cao F., Liu G., Dai G., Mi B., Liu G. The regulatory role of ferroptosis in bone homeostasis. Stem Cell. Int. 2022 Jul 13;2022 doi: 10.1155/2022/3568597. PMID: 35873534; PMCID: PMC9300333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng H., Liu Q., Deng Z., Li H., Zhang H., Song J., Liu X., Liu J., Wen B., Wang T. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res. Ther. 2022 Sep 5;13(1):450. doi: 10.1186/s13287-022-03147-w. PMID: 36064453; PMCID: PMC9444126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang J.W., Park J.H., Park B.W., Kim H., Kim J.J., Sim W.S., Mishchenko N.P., Fedoreyev S.A., Vasileva E.A., Ban K., Park H.J., Baek S.H. Histochrome attenuates myocardial ischemia-reperfusion injury by inhibiting ferroptosis-induced cardiomyocyte death. Antioxidants. 2021 Oct 15;10(10):1624. doi: 10.3390/antiox10101624. PMID: 34679760; PMCID: PMC8533175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012 May 25;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. PMID: 22632970; PMCID: PMC3367386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichert C.O., de Freitas F.A., Sampaio-Silva J., Rokita-Rosa L., Barros P.L., Levy D., Bydlowski S.P. Ferroptosis mechanisms involved in neurodegenerative diseases. Int. J. Mol. Sci. 2020 Nov 20;21(22):8765. doi: 10.3390/ijms21228765. PMID: 33233496; PMCID: PMC7699575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hambright W.S., Fonseca R.S., Chen L., Na R., Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017 Aug;12:8–17. doi: 10.1016/j.redox.2017.01.021. Epub 2017 Feb 1. PMID: 28212525; PMCID: PMC5312549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massie A., Schallier A., Kim S.W., Fernando R., Kobayashi S., Beck H., De Bundel D., Vermoesen K., Bannai S., Smolders I., Conrad M., Plesnila N., Sato H., Michotte Y. Dopaminergic neurons of system x(c)⁻-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. Faseb. J. 2011 Apr;25(4):1359–1369. doi: 10.1096/fj.10-177212. Epub 2010 Dec 29. PMID: 21191088. [DOI] [PubMed] [Google Scholar]
- 57.Ashraf A., Jeandriens J., Parkes H.G., So P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: evidence of ferroptosis. Redox Biol. 2020 May;32 doi: 10.1016/j.redox.2020.101494. Epub 2020 Mar 5. PMID: 32199332; PMCID: PMC7083890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuo Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L., Liuyang Z.Y., Roisman L., Zhang S.T., Ayton S., Wang Q., Crouch P.J., Ganio K., Wang X.C., Pei L., Adlard P.A., Lu Y.M., Cappai R., Wang J.Z., Liu R., Bush A.I. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatr. 2017 Nov;22(11):1520–1530. doi: 10.1038/mp.2017.171. Epub 2017 Sep 8. PMID: 28886009. [DOI] [PubMed] [Google Scholar]
- 59.Au A., Griffiths L.R., Irene L., Kooi C.W., Wei L.K. The impact of APOA5, APOB, APOC3 and ABCA1 gene polymorphisms on ischemic stroke: evidence from a meta-analysis. Atherosclerosis. 2017 Oct;265:60–70. doi: 10.1016/j.atherosclerosis.2017.08.003. Epub 2017 Aug 19. PMID: 28865324. [DOI] [PubMed] [Google Scholar]
- 60.Brouns R., De Deyn P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009 Jul;111(6):483–495. doi: 10.1016/j.clineuro.2009.04.001. Epub 2009 May 14. PMID: 19446389. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Ma N., Xu J., Zhang Y., Yang P., Su X., Xing Y., An N., Yang F., Zhang G., Zhang L., Xing Y. Targeting ferroptosis: pathological mechanism and treatment of ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2021 Oct 28;2021 doi: 10.1155/2021/1587922. PMID: 34745412; PMCID: PMC8568519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J., Guo Z.N., Yan X.L., Huang S., Ren J.X., Luo Y., Yang Y. Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Front. Cell. Neurosci. 2020 Oct 2;14 doi: 10.3389/fncel.2020.577403. PMID: 33132849; PMCID: PMC7566169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González-Fraguela M.E., Blanco L., Fernández C.I., Lorigados L., Serrano T., Fernández J.L. Glutathione depletion: starting point of brain metabolic stress, neuroinflammation and cognitive impairment in rats. Brain Res. Bull. 2018 Mar;137:120–131. doi: 10.1016/j.brainresbull.2017.11.015. Epub 2017 Nov 26. PMID: 29183693. [DOI] [PubMed] [Google Scholar]
- 64.Imai T., Iwata S., Hirayama T., Nagasawa H., Nakamura S., Shimazawa M., Hara H. Intracellular Fe2+ accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci. Rep. 2019 Apr 17;9(1):6228. doi: 10.1038/s41598-019-42370-z. PMID: 30996325; PMCID: PMC6470176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y., Xu W., McBurney M.W., Longo V.D. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metabol. 2008 Jul;8(1):38–48. doi: 10.1016/j.cmet.2008.05.004. PMID: 18590691; PMCID: PMC2822839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J., Huang J., Zhang Z., Zhang R., Sun Q., Zhang Z., Liu Y., Ma B. Mesenchymal stem cell-derived exosomes ameliorate delayed neurocognitive recovery in aged mice by inhibiting Hippocampus ferroptosis via activating SIRT1/nrf2/HO-1 signaling pathway. Oxid. Med. Cell. Longev. 2022 Sep 30;2022 doi: 10.1155/2022/3593294. PMID: 36238648; PMCID: PMC9553403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angeli J.P.F., Shah R., Pratt D.A., Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol. Sci. 2017 May;38(5):489–498. doi: 10.1016/j.tips.2017.02.005. Epub 2017 Mar 28. PMID: 28363764. [DOI] [PubMed] [Google Scholar]
- 68.Wang D., Zhang S., Ge X., Yin Z., Li M., Guo M., Hu T., Han Z., Kong X., Li D., Zhao J., Wang L., Liu Q., Chen F., Lei P. Mesenchymal stromal cell treatment attenuates repetitive mild traumatic brain injury-induced persistent cognitive deficits via suppressing ferroptosis. J. Neuroinflammation. 2022 Jul 14;19(1):185. doi: 10.1186/s12974-022-02550-7. PMID: 35836233; PMCID: PMC9281149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., Bassik M.C., Nomura D.K., Dixon S.J., Olzmann J.A. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019 Nov;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. Epub 2019 Oct 21. PMID: 31634900; PMCID: PMC6883167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao C., Chen Y., Yang T., Zhao H., Li D. Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis via lncGm36569/miR-5627-5p/FSP1 Axis in acute spinal cord injury. Stem Cell Rev Rep. 2022 Mar;18(3):1127–1142. doi: 10.1007/s12015-022-10327-x. Epub 2022 Mar 7. PMID: 35257299. [DOI] [PubMed] [Google Scholar]
- 71.Dangol S., Chen Y., Hwang B.K., Jwa N.S. Iron- and reactive oxygen species-dependent ferroptotic cell death in rice-magnaporthe oryzae interactions. Plant Cell. 2019 Jan;31(1):189–209. doi: 10.1105/tpc.18.00535. Epub 2018 Dec 18. PMID: 30563847; PMCID: PMC6391706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J., Zhou Y., Xie C., Li C., Ma L., Zhang Y. Anti-ferroptotic effects of bone marrow mesenchymal stem cell-derived extracellular vesicles loaded with ferrostatin-1 in cerebral ischemia-reperfusion injury associate with the GPX4/COX-2 Axis. Neurochem. Res. 2022 Nov 2 doi: 10.1007/s11064-022-03770-2. Epub ahead of print. PMID: 36322371. [DOI] [PubMed] [Google Scholar]
- 73.Kuang H., Wang T., Liu L., Tang C., Li T., Liu M., Wang T., Zhong W., Wang Y. Treatment of early brain injury after subarachnoid hemorrhage in the rat model by inhibiting p53-induced ferroptosis. Neurosci. Lett. 2021 Sep 25;762 doi: 10.1016/j.neulet.2021.136134. Epub 2021 Jul 24. PMID: 34311053. [DOI] [PubMed] [Google Scholar]
- 74.Ramadori G., Lee C.E., Bookout A.L., Lee S., Williams K.W., Anderson J., Elmquist J.K., Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J. Neurosci. 2008 Oct 1;28(40):9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. PMID: 18829956; PMCID: PMC2578850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao W., Dou Y., Li A. Resveratrol boosts cognitive function by targeting SIRT1. Neurochem. Res. 2018 Sep;43(9):1705–1713. doi: 10.1007/s11064-018-2586-8. Epub 2018 Jun 25. PMID: 29943083. [DOI] [PubMed] [Google Scholar]
- 76.Yu H., Zhang F., Guan X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signaling pathway in olfactory bulbectomized rats. Phytother Res. 2019 May;33(5):1480–1489. doi: 10.1002/ptr.6340. Epub 2019 Mar 8. PMID: 30848526. [DOI] [PubMed] [Google Scholar]
- 77.Song X., Long D. Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front. Neurosci. 2020 Apr 21;14:267. doi: 10.3389/fnins.2020.00267. PMID: 32372896; PMCID: PMC7186402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang T., Cheng H., Su J., Wang X., Wang Q., Chu J., Li Q. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicol. Vitro. 2020 Feb;62 doi: 10.1016/j.tiv.2019.104715. Epub 2019 Nov 5. PMID: 31698019. [DOI] [PubMed] [Google Scholar]
- 79.Cheng Y., Qu W., Li J., Jia B., Song Y., Wang L., Rui T., Li Q., Luo C. Ferristatin II, an iron uptake inhibitor, exerts neuroprotection against traumatic brain injury via suppressing ferroptosis. ACS Chem. Neurosci. 2022 Mar 2;13(5):664–675. doi: 10.1021/acschemneuro.1c00819. Epub 2022 Feb 10. PMID: 35143157. [DOI] [PubMed] [Google Scholar]
- 80.Yang L., Du B., Zhang S., Wang M. RXRγ attenuates cerebral ischemia-reperfusion induced ferroptosis in neurons in mice through transcriptionally promoting the expression of GPX4. Metab. Brain Dis. 2022 Jun;37(5):1351–1363. doi: 10.1007/s11011-022-00988-5. Epub 2022 Apr 29. PMID: 35486208. [DOI] [PubMed] [Google Scholar]
- 81.Xing F., Meng T., Therriault J., Luo J., Zhang H., Alzheimer’s Disease Neuroimaging Initiative Apolipoprotein E ε4 and ε3 alleles associate with cerebrospinal fluid tau and cognition in the presence of amyloid-β in mild cognitive impairment but not in Alzheimer's disease. J. Integr. Neurosci. 2021 Jun 30;20(2):277–286. doi: 10.31083/j.jin2002027. PMID: 34258926. [DOI] [PubMed] [Google Scholar]
- 82.Wang M., Mao C., Ouyang L., Liu Y., Lai W., Liu N., Shi Y., Chen L., Xiao D., Yu F., Wang X., Zhou H., Cao Y., Liu S., Yan Q., Tao Y., Zhang B. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26(11):2329–2343. doi: 10.1038/s41418-019-0304-y. Epub 2019 Feb 20. Erratum in: Cell Death Differ. 2019 Aug 5;: PMID: 30787392; PMCID: PMC6889193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y., Tai W., Lu N., Li T., Liu Y., Wu W., Li Z., Pu L., Zhao X., Zhang T., Dong Z. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging (Albany NY) 2020 May 26;12(10):9085–9102. doi: 10.18632/aging.103176. Epub 2020 May 26. PMID: 32453709; PMCID: PMC7288977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu F., Ma R., Zhang G., Wang S., Yin J., Wang E., Xiong E., Zhang Q., Li Y. Estrogen and propofol combination therapy inhibits endoplasmic reticulum stress and remarkably attenuates cerebral ischemia-reperfusion injury and OGD injury in hippocampus. Biomed. Pharmacother. 2018 Dec;108:1596–1606. doi: 10.1016/j.biopha.2018.09.167. Epub 2018 Oct 9. PMID: 30372862. [DOI] [PubMed] [Google Scholar]
- 85.Tian X., An R., Luo Y., Li M., Xu L., Dong Z. Tamibarotene improves Hippocampus injury induced by focal cerebral ischemia-reperfusion via modulating PI3K/akt pathway in rats. J. Stroke Cerebrovasc. Dis. 2019 Jul;28(7):1832–1840. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.017. Epub 2019 May 9. PMID: 31078389. [DOI] [PubMed] [Google Scholar]
- 86.Eibl G., Bruemmer D., Okada Y., Duffy J.P., Law R.E., Reber H.A., Hines O.J. PGE(2) is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2003 Jul 11;306(4):887–897. doi: 10.1016/s0006-291x(03)01079-9. PMID: 12821125. [DOI] [PubMed] [Google Scholar]
- 87.Kalinski P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012 Jan 1;188(1):21–28. doi: 10.4049/jimmunol.1101029. PMID: 22187483; PMCID: PMC3249979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu X., Yao Y., Yang J., Zhengxie J., Li X., Hu S., Zhang A., Dong J., Zhang C., Gan G. COX-2-PGE2 signaling pathway contributes to hippocampal neuronal injury and cognitive impairment in PTZ-kindled epilepsy mice. Int. Immunopharm. 2020 Oct;87 doi: 10.1016/j.intimp.2020.106801. Epub 2020 Jul 20. PMID: 32702600. [DOI] [PubMed] [Google Scholar]
- 89.Brown K.E., Dennery P.A., Ridnour L.A., Fimmel C.J., Kladney R.D., Brunt E.M., Spitz D.R. Effect of iron overload and dietary fat on indices of oxidative stress and hepatic fibrogenesis in rats. Liver Int. 2003 Aug;23(4):232–242. doi: 10.1034/j.1600-0676.2003.00832.x. PMID: 12895262. [DOI] [PubMed] [Google Scholar]
- 90.Mancardi D., Mezzanotte M., Arrigo E., Barinotti A., Roetto A. Iron overload, oxidative stress, and ferroptosis in the failing heart and liver. Antioxidants. 2021 Nov 24;10(12):1864. doi: 10.3390/antiox10121864. PMID: 34942967; PMCID: PMC8698778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018 Nov;68(6):394–424. doi: 10.3322/caac.21492. Epub 2018 Sep 12. Erratum in: CA Cancer J Clin. 2020 Jul;70(4):313. PMID: 30207593. [DOI] [PubMed] [Google Scholar]
- 92.You H., Wang L., Bu F., Meng H., Huang C., Fang G., Li J. Ferroptosis: shedding light on mechanisms and therapeutic opportunities in liver diseases. Cells. 2022 Oct 20;11(20):3301. doi: 10.3390/cells11203301. PMID: 36291167; PMCID: PMC9600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Louandre C., Ezzoukhry Z., Godin C., Barbare J.C., Mazière J.C., Chauffert B., Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer. 2013 Oct 1;133(7):1732–1742. doi: 10.1002/ijc.28159. Epub 2013 Apr 8. PMID: 23505071. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Z., Yao Z., Wang L., Ding H., Shao J., Chen A., Zhang F., Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14(12):2083–2103. doi: 10.1080/15548627.2018.1503146. Epub 2018 Aug 21. PMID: 30081711; PMCID: PMC6984765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H., An P., Xie E., Wu Q., Fang X., Gao H., Zhang Z., Li Y., Wang X., Zhang J., Li G., Yang L., Liu W., Min J., Wang F. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017 Aug;66(2):449–465. doi: 10.1002/hep.29117. Epub 2017 May 16. PMID: 28195347; PMCID: PMC5573904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliveira T.H.C., Marques P.E., Proost P., Teixeira M.M.M. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab. Invest. 2018 Jan;98(1):51–62. doi: 10.1038/labinvest.2017.90. Epub 2017 Sep 18. PMID: 28920945. [DOI] [PubMed] [Google Scholar]
- 97.Luo L., Mo G., Huang D. Ferroptosis in hepatic ischemia-reperfusion injury: regulatory mechanisms and new methods for therapy. Mol. Med. Rep. 2021 Mar;23(3):225. doi: 10.3892/mmr.2021.11864. Epub 2021 Jan 26. PMID: 33495834. [DOI] [PubMed] [Google Scholar]
- 98.Yamane D., Hayashi Y., Matsumoto M., Nakanishi H., Imagawa H., Kohara M., Lemon S.M., Ichi I. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem. Biol. 2022 May 19;29(5):799–810.e4. doi: 10.1016/j.chembiol.2021.07.022. Epub 2021 Sep 13. PMID: 34520742; PMCID: PMC8913804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen J., Li X., Ge C., Min J., Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022 Mar;29(3):467–480. doi: 10.1038/s41418-022-00941-0. Epub 2022 Jan 24. PMID: 35075250; PMCID: PMC8901678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanda T., Goto T., Hirotsu Y., Moriyama M., Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int. J. Mol. Sci. 2019 Mar 18;20(6):1358. doi: 10.3390/ijms20061358. PMID: 30889843; PMCID: PMC6470669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niu B., Lei X., Xu Q., Ju Y., Xu D., Mao L., Li J., Zheng Y., Sun N., Zhang X., Mao Y., Li X. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol. Toxicol. 2022 Jun;38(3):505–530. doi: 10.1007/s10565-021-09624-x. Epub 2021 Aug 17. PMID: 34401974. [DOI] [PubMed] [Google Scholar]
- 102.Wang M., Liu C.Y., Wang T., Yu H.M., Ouyang S.H., Wu Y.P., Gong H.B., Ma X.H., Jiao G.L., Fu L.L., Wu Q.S., Kurihara H., Li Y.F., Shen T., He R.R. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. 2020 Sep 19;11(9):781. doi: 10.1038/s41419-020-02961-5. PMID: 32951003; PMCID: PMC7502081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moles A., Torres S., Baulies A., Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial-Lysosomal Axis in acetaminophen hepatotoxicity. Front. Pharmacol. 2018 May 15;9:453. doi: 10.3389/fphar.2018.00453. PMID: 29867464; PMCID: PMC5968389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin F., Chen W., Zhou J., Zhu J., Yao Q., Feng B., Feng X., Shi X., Pan Q., Yu J., Li L., Cao H. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022 Mar 26;13(3):271. doi: 10.1038/s41419-022-04708-w. PMID: 35347117; PMCID: PMC8960810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., Basavarajappa D., Rådmark O., Kobayashi S., Seibt T., Beck H., Neff F., Esposito I., Wanke R., Förster H., Yefremova O., Heinrichmeyer M., Bornkamm G.W., Geissler E.K., Thomas S.B., Stockwell B.R., O'Donnell V.B., Kagan V.E., Schick J.A., Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014 Dec;16(12):1180–1191. doi: 10.1038/ncb3064. Epub 2014 Nov 17. PMID: 25402683; PMCID: PMC4894846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gautheron J., Gores G.J., Rodrigues C.M.P. Lytic cell death in metabolic liver disease. J. Hepatol. 2020 Aug;73(2):394–408. doi: 10.1016/j.jhep.2020.04.001. Epub 2020 Apr 13. PMID: 32298766; PMCID: PMC7371520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loguercio C., De Girolamo V., de Sio I., Tuccillo C., Ascione A., Baldi F., Budillon G., Cimino L., Di Carlo A., Di Marino M.P., Morisco F., Picciotto F., Terracciano L., Vecchione R., Verde V., Del Vecchio Blanco C. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J. Hepatol. 2001 Nov;35(5):568–574. doi: 10.1016/s0168-8278(01)00192-1. Erratum in: J Hepatol 2002 May;36(5):713. PMID: 11690701. [DOI] [PubMed] [Google Scholar]
- 108.Terzi E.M., Sviderskiy V.O., Alvarez S.W., Whiten G.C., Possemato R. Iron-sulfur cluster deficiency can be sensed by IRP2 and regulates iron homeostasis and sensitivity to ferroptosis independent of IRP1 and FBXL5. Sci. Adv. 2021 May 26;7(22) doi: 10.1126/sciadv.abg4302. PMID: 34039609; PMCID: PMC8153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021 Feb;31(2):107–125. doi: 10.1038/s41422-020-00441-1. Epub 2020 Dec 2. PMID: 33268902; PMCID: PMC8026611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X., Wu L., Tian X., Zheng W., Yuan M., Tian X., Zuo H., Song H., Shen Z. miR-29a-3p in exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells alleviates steatotic liver ischemia-reperfusion injury in rats by suppressing ferroptosis via iron responsive element binding protein 2. Oxid. Med. Cell. Longev. 2022 Jun 9;2022 doi: 10.1155/2022/6520789. PMID: 35720183; PMCID: PMC9203237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014 Jan 16;156(1-2):317–331. doi: 10.1016/j.cell.2013.12.010. PMID: 24439385; PMCID: PMC407641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song X., Zhu S., Chen P., Hou W., Wen Q., Liu J., Xie Y., Liu J., Klionsky D.J., Kroemer G., Lotze M.T., Zeh H.J., Kang R., Tang D. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Cc- activity. Curr. Biol. 2018 Aug 6;28(15):2388–2399.e5. doi: 10.1016/j.cub.2018.05.094. Epub 2018 Jul 26. PMID: 30057310; PMCID: PMC6081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan Y., Huang Y., Mei R., Mao F., Yang D., Liu J., Xu W., Qian H., Yan Y. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. 2022 Apr 8;13(4):319. doi: 10.1038/s41419-022-04764-2. PMID: 35395830; PMCID: PMC8993870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016 Jan;63(1):173–184. doi: 10.1002/hep.28251. Epub 2015 Nov 26. PMID: 26403645; PMCID: PMC4688087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao S., Huang M., Yan L., Zhang H., Shi C., Liu J., Zhao S., Liu H., Wang B. Exosomes derived from baicalin-pretreated mesenchymal stem cells alleviate hepatocyte ferroptosis after acute liver injury via the keap1-NRF2 pathway. Oxid. Med. Cell. Longev. 2022 Jul 21;2022 doi: 10.1155/2022/8287227. PMID: 35910831; PMCID: PMC9334037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi L., Zhang S., Huang Z., Hu F., Zhang T., Wei M., Bai Q., Lu B., Ji L. Baicalin promotes liver regeneration after acetaminophen-induced liver injury by inducing NLRP3 inflammasome activation. Free Radic. Biol. Med. 2020 Nov 20;160:163–177. doi: 10.1016/j.freeradbiomed.2020.05.012. Epub 2020 Jul 17. PMID: 32682928. [DOI] [PubMed] [Google Scholar]
- 117.Choi E.K., Jung H., Kim K.J., Kang S.J., Kim H.J., Lim J.A., Kwak K.H., Lim D.G. Sodium nitrite attenuates hepatic ischemia-reperfusion injury in rats. Exp Clin Transplant. 2019 Jun;17(3):348–354. doi: 10.6002/ect.2018.0169. Epub 2018 Dec 31. PMID: 30602366. [DOI] [PubMed] [Google Scholar]
- 118.Sun D., Yang L., Zheng W., Cao H., Wu L., Song H. Protective effects of bone marrow mesenchymal stem cells (BMMSCS) combined with normothermic machine perfusion on liver grafts donated after circulatory death via reducing the ferroptosis of hepatocytes. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2021 Jun 11;27 doi: 10.12659/MSM.930258. PMID: 34112750; PMCID: PMC8204680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ravikumar R., Jassem W., Mergental H., Heaton N., Mirza D., Perera M.T., Quaglia A., Holroyd D., Vogel T., Coussios C.C., Friend P.J. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (First-in-Man) clinical trial. Am. J. Transplant. 2016 Jun;16(6):1779–1787. doi: 10.1111/ajt.13708. Epub 2016 Mar 7. PMID: 26752191. [DOI] [PubMed] [Google Scholar]
- 120.Saidi R.F., Kenari S.K. Liver ischemia/reperfusion injury: an overview. J. Invest. Surg. 2014 Dec;27(6):366–379. doi: 10.3109/08941939.2014.932473. Epub 2014 Jul 24. PMID: 25058854. [DOI] [PubMed] [Google Scholar]
- 121.Fernández L., Carrasco-Chaumel E., Serafín A., Xaus C., Grande L., Rimola A., Roselló-Catafau J., Peralta C. Is ischemic preconditioning a useful strategy in steatotic liver transplantation? Am. J. Transplant. 2004 Jun;4(6):888–899. doi: 10.1111/j.1600-6143.2004.00447.x. PMID: 15147422. [DOI] [PubMed] [Google Scholar]
- 122.Yoshida M., Minagawa S., Araya J., Sakamoto T., Hara H., Tsubouchi K., Hosaka Y., Ichikawa A., Saito N., Kadota T., Sato N., Kurita Y., Kobayashi K., Ito S., Utsumi H., Wakui H., Numata T., Kaneko Y., Mori S., Asano H., Yamashita M., Odaka M., Morikawa T., Nakayama K., Iwamoto T., Imai H., Kuwano K. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019 Jul 17;10(1):3145. doi: 10.1038/s41467-019-10991-7. PMID: 31316058; PMCID: PMC6637122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu L., Tian X., Zuo H., Zheng W., Li X., Yuan M., Tian X., Song H. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J. Nanobiotechnol. 2022 Apr 22;20(1):196. doi: 10.1186/s12951-022-01407-8. PMID: 35459211; PMCID: PMC9026664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li X., Ma N., Xu J., Zhang Y., Yang P., Su X., Xing Y., An N., Yang F., Zhang G., Zhang L., Xing Y. Targeting ferroptosis: pathological mechanism and treatment of ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2021 Oct 28;2021 doi: 10.1155/2021/1587922. PMID: 34745412; PMCID: PMC8568519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Katsarou A., Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol. Aspect. Med. 2020 Oct;75 doi: 10.1016/j.mam.2020.100866. Epub 2020 Jun 18. PMID: 32564977. [DOI] [PubMed] [Google Scholar]
- 126.Sun D., Cao H., Yang L., Lin L., Hou B., Zheng W., Shen Z., Song H. MiR-200b in heme oxygenase-1-modified bone marrow mesenchymal stem cell-derived exosomes alleviates inflammatory injury of intestinal epithelial cells by targeting high mobility group box 3. Cell Death Dis. 2020 Jun 25;11(6):480. doi: 10.1038/s41419-020-2685-8. PMID: 32587254; PMCID: PMC7316799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., Basavarajappa D., Rådmark O., Kobayashi S., Seibt T., Beck H., Neff F., Esposito I., Wanke R., Förster H., Yefremova O., Heinrichmeyer M., Bornkamm G.W., Geissler E.K., Thomas S.B., Stockwell B.R., O'Donnell V.B., Kagan V.E., Schick J.A., Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014 Dec;16(12):1180–1191. doi: 10.1038/ncb3064. Epub 2014 Nov 17. PMID: 25402683; PMCID: PMC4894846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang J.J., Du J., Kong N., Zhang G.Y., Liu M.Z., Liu C. Mechanisms and pharmacological applications of ferroptosis: a narrative review. Ann. Transl. Med. 2021 Oct;9(19):1503. doi: 10.21037/atm-21-1595. PMID: 34805365; PMCID: PMC8573439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mousa A.M., El-Sammad N.M., Hassan S.K., Aena M., Hashim A.N., Moustafa E.S., Bakry S.M., Elsayed E.A. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Compl. Alternative Med. 2019 Dec 2;19(1):345. doi: 10.1186/s12906-019-2760-9. PMID: 31791313; PMCID: PMC6888969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu J., Wang J., Sheng Y., Zou Y., Bo L., Wang F., Lou J., Fan X., Bao R., Wu Y., Chen F., Deng X., Li J. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035523. Epub 2012 May 8. PMID: 22590504; PMCID: PMC3348138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Motoo Y., Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994 Oct 28;86(1):91–95. doi: 10.1016/0304-3835(94)90184-8. PMID: 7954360. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y., Xu Z., Jin T., Xu K., Liu M., Xu H. Ferroptosis in low-grade glioma: a new marker for diagnosis and prognosis. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020 Jun 2;26 doi: 10.12659/MSM.921947. PMID: 32484805; PMCID: PMC7291787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng P., Hu X., Lou Y., Tang K. A rabbit model of osteochondral regeneration using three-dimensional printed polycaprolactone-hydroxyapatite scaffolds coated with umbilical cord blood mesenchymal stem cells and chondrocytes. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019 Oct 1;25:7361–7369. doi: 10.12659/MSM.915441. PMID: 31570688; PMCID: PMC6784681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang L., Cao H., Sun D., Lin L., Zheng W.P., Shen Z.Y., Song H.L. Normothermic machine perfusion combined with bone marrow mesenchymal stem cells improves the oxidative stress response and mitochondrial function in rat donation after circulatory death livers. Stem Cell. Dev. 2020 Jul 1;29(13):835–852. doi: 10.1089/scd.2019.0301. Epub 2020 May 5. PMID: 32253985; PMCID: PMC7336881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang L., Cao H., Sun D., Hou B., Lin L., Shen Z.Y., Song H.L. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020 Aug;381(2):239–254. doi: 10.1007/s00441-020-03202-z. Epub 2020 Apr 29. PMID: 32347385; PMCID: PMC7369267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cao H., Yang L., Hou B., Sun D., Lin L., Song H.L., Shen Z.Y. Heme oxygenase-1-modified bone marrow mesenchymal stem cells combined with normothermic machine perfusion to protect donation after circulatory death liver grafts. Stem Cell Res. Ther. 2020 Jun 5;11(1):218. doi: 10.1186/s13287-020-01736-1. Retraction in: Stem Cell Res Ther. 2022 Nov 1;13(1):510. PMID: 32503631; PMCID: PMC7275432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 137.Salama N.M., Zaghlol S.S., Mohamed H.H., Kamar S.S. Suppression of the inflammation and fibrosis in Asherman syndrome rat model by mesenchymal stem cells: histological and immunohistochemical studies. Folia Histochem. Cytobiol. 2020;58(3):208–218. doi: 10.5603/FHC.a2020.0024. Epub 2020 Sep 30. PMID: 32996119. [DOI] [PubMed] [Google Scholar]
- 138.Eggenhofer E., Groell A., Junger H., Kasi A., Kroemer A., Geissler E.K., Schlitt H.J., Scherer M.N. Steatotic livers are more susceptible to ischemia reperfusion damage after transplantation and show increased γδ T cell infiltration. Int. J. Mol. Sci. 2021 Feb 18;22(4):2036. doi: 10.3390/ijms22042036. PMID: 33670793; PMCID: PMC7922678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X., Walkey C.J., Maretti-Mira A.C., Wang L., Johnson D.L., DeLeve L.D. Susceptibility of rat steatotic liver to ischemia-reperfusion is treatable with liver-selective matrix metalloproteinase inhibition. Hepatology. 2020 Nov;72(5):1771–1785. doi: 10.1002/hep.31179. Epub 2020 Oct 22. PMID: 32060938; PMCID: PMC7523533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohgami R.S., Campagna D.R., Greer E.L., Antiochos B., McDonald A., Chen J., Sharp J.J., Fujiwara Y., Barker J.E., Fleming M.D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005 Nov;37(11):1264–1269. doi: 10.1038/ng1658. Epub 2005 Oct 16. PMID: 16227996; PMCID: PMC2156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohgami R.S., Campagna D.R., McDonald A., Fleming M.D. The Steap proteins are metalloreductases. Blood. 2006 Aug 15;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. Epub 2006 Apr 11. PMID: 16609065; PMCID: PMC1785011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fang X., Wang H., Han D., Xie E., Yang X., Wei J., Gu S., Gao F., Zhu N., Yin X., Cheng Q., Zhang P., Dai W., Chen J., Yang F., Yang H.T., Linkermann A., Gu W., Min J., Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 2019 Feb 12;116(7):2672–2680. doi: 10.1073/pnas.1821022116. Epub 2019 Jan 28. PMID: 30692261; PMCID: PMC6377499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ishizaka N., Saito K., Mitani H., Yamazaki I., Sata M., Usui S., Mori I., Ohno M., Nagai R. Iron overload augments angiotensin II-induced cardiac fibrosis and promotes neointima formation. Circulation. 2002 Oct 1;106(14):1840–1846. doi: 10.1161/01.cir.0000031161.77536.02. PMID: 12356639. [DOI] [PubMed] [Google Scholar]
- 144.Guo Y., Zhang W., Zhou X., Zhao S., Wang J., Guo Y., Liao Y., Lu H., Liu J., Cai Y., Wu J., Shen M. Roles of ferroptosis in cardiovascular diseases. Front Cardiovasc Med. 2022 May 23;9 doi: 10.3389/fcvm.2022.911564. PMID: 35677693; PMCID: PMC9168067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park T.J., Park J.H., Lee G.S., Lee J.Y., Shin J.H., Kim M.W., Kim Y.S., Kim J.Y., Oh K.J., Han B.S., Kim W.K., Ahn Y., Moon J.H., Song J., Bae K.H., Kim D.H., Lee E.W., Lee S.C. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019 Nov 4;10(11):835. doi: 10.1038/s41419-019-2061-8. PMID: 31685805; PMCID: PMC6828761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tadokoro T., Ikeda M., Ide T., Deguchi H., Ikeda S., Okabe K., Ishikita A., Matsushima S., Koumura T., Yamada K.I., Imai H., Tsutsui H. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020 May 7;5(9) doi: 10.1172/jci.insight.132747. PMID: 32376803; PMCID: PMC7253028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao W.K., Zhou Y., Xu T.T., Wu Q. Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2021 Oct 23;2021 doi: 10.1155/2021/9929687. PMID: 34725566; PMCID: PMC8557044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oudit G.Y., Trivieri M.G., Khaper N., Liu P.P., Backx P.H. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J. Mol. Med. (Berl.) 2006 May;84(5):349–364. doi: 10.1007/s00109-005-0029-x. Epub 2006 Apr 8. PMID: 16604332; PMCID: PMC7095819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020 Feb;20(2):95–112. doi: 10.1038/s41577-019-0215-7. Epub 2019 Sep 26. PMID: 31558839. [DOI] [PubMed] [Google Scholar]
- 150.Yang J., Wang M., Wang S., Li G., Gao Y. Study on ferroptosis pathway that operates in hypertensive brain damage. Clin. Exp. Hypertens. 2020 Nov 16;42(8):748–752. doi: 10.1080/10641963.2020.1783545. Epub 2020 Jun 20. PMID: 32564622. [DOI] [PubMed] [Google Scholar]
- 151.Gordan R., Fefelova N., Gwathmey J.K., Xie L.H. Iron overload, oxidative stress and calcium mishandling in cardiomyocytes: role of the mitochondrial permeability transition pore. Antioxidants. 2020 Aug 16;9(8):758. doi: 10.3390/antiox9080758. PMID: 32824344; PMCID: PMC7465659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang J., Deng B., Liu Q., Huang Y., Chen W., Li J., Zhou Z., Zhang L., Liang B., He J., Chen Z., Yan C., Yang Z., Xian S., Wang L. Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis. 2020 Jul 24;11(7):574. doi: 10.1038/s41419-020-02777-3. PMID: 32710001; PMCID: PMC7382480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang J.K., Zhang Z., Guo Z.A., Fu Y., Chen X.J., Chen W.J., Wu H.F., Cui X.J. The BMSC-derived exosomal lncRNA Mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr. Metabol. Cardiovasc. Dis. 2022 Feb;32(2):515–527. doi: 10.1016/j.numecd.2021.10.017. Epub 2021 Nov 3. PMID: 34953631. [DOI] [PubMed] [Google Scholar]
- 154.Yu Y., Wu T., Lu Y., Zhao W., Zhang J., Chen Q., Ge G., Hua Y., Chen K., Ullah I., Zhang F. Exosomal thioredoxin-1 from hypoxic human umbilical cord mesenchymal stem cells inhibits ferroptosis in doxorubicin-induced cardiotoxicity via mTORC1 signaling. Free Radic. Biol. Med. 2022 Oct 12;193(Pt 1):108–121. doi: 10.1016/j.freeradbiomed.2022.10.268. Epub ahead of print. PMID: 36241072. [DOI] [PubMed] [Google Scholar]
- 155.Oka S.I., Hirata T., Suzuki W., Naito D., Chen Y., Chin A., Yaginuma H., Saito T., Nagarajan N., Zhai P., Bhat S., Schesing K., Shao D., Hirabayashi Y., Yodoi J., Sciarretta S., Sadoshima J. Thioredoxin-1 maintains mechanistic target of rapamycin (mTOR) function during oxidative stress in cardiomyocytes. J. Biol. Chem. 2017 Nov 17;292(46):18988–19000. doi: 10.1074/jbc.M117.807735. Epub 2017 Sep 22. PMID: 28939765; PMCID: PMC5704480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pujol-Giménez J., Hediger M.A., Gyimesi G. A novel proton transfer mechanism in the SLC11 family of divalent metal ion transporters. Sci. Rep. 2017 Jul 28;7(1):6194. doi: 10.1038/s41598-017-06446-y. PMID: 28754960; PMCID: PMC5533754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferguson S.W., Wang J., Lee C.J., Liu M., Neelamegham S., Canty J.M., Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci. Rep. 2018 Jan 23;8(1):1419. doi: 10.1038/s41598-018-19581-x. PMID: 29362496; PMCID: PMC5780426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Song Y., Wang B., Zhu X., Hu J., Sun J., Xuan J., Ge Z. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021 Feb;37(1):51–64. doi: 10.1007/s10565-020-09530-8. Epub 2020 Jun 13. PMID: 32535745. [DOI] [PubMed] [Google Scholar]
- 159.Re A., Joshi T., Kulberkyte E., Morris Q., Workman C.T. RNA-protein interactions: an overview. Methods Mol. Biol. 2014;1097:491–521. doi: 10.1007/978-1-62703-709-9_23. PMID: 24639174. [DOI] [PubMed] [Google Scholar]
- 160.Lu B., Chen X.B., Hong Y.C., Zhu H., He Q.J., Yang B., Ying M.D., Cao J. Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol. Sin. 2019 Oct;40(10):1334–1342. doi: 10.1038/s41401-019-0233-9. Epub 2019 Apr 29. PMID: 31036877; PMCID: PMC6786318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. PMID: 22500797; PMCID: PMC3331679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Du X., Xu H., Shi L., Jiang Z., Song N., Jiang H., Xie J. Activation of ATP-sensitive potassium channels enhances DMT1-mediated iron uptake in SK-N-SH cells in vitro. Sci. Rep. 2016 Sep 20;6 doi: 10.1038/srep33674. PMID: 27646472; PMCID: PMC5028757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu X., Xu H., Liu Q., Wang Y., Wang S., Lu R., Jiang Y., Kang H., Hu W. circ_0072464 shuttled by bone mesenchymal stem cell-secreted extracellular vesicles inhibits nucleus pulposus cell ferroptosis to relieve intervertebral disc degeneration. Oxid. Med. Cell. Longev. 2022 Jun 29;2022 doi: 10.1155/2022/2948090. PMID: 35814268; PMCID: PMC9259290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem. Res. Toxicol. 2005 Jan;18(1):3–11. doi: 10.1021/tx049774+. PMID: 15651842. [DOI] [PubMed] [Google Scholar]
- 165.Zhuge R., Li Z., He C., Ma W., Yan J., Xue Q., Wang R., Liu Y., Lu R., Du H., Yin F., Guo L. Bone marrow mesenchymal stem cells repair hexavalent chromium-induced testicular injury by regulating autophagy and ferroptosis mediated by the AKT/mTOR pathway in rats. Environ. Toxicol. 2022 Nov 23 doi: 10.1002/tox.23713. Epub ahead of print. PMID: 36416502. [DOI] [PubMed] [Google Scholar]
- 166.Huo Z., Li H., Tian L., Li J., Zhang K., Li Z., Li G., Du L., Xu H., Xu B. Construction of a potentially functional circRNA-miRNA-mRNA network in intervertebral disc degeneration by bioinformatics analysis. BioMed Res. Int. 2021 Aug 4;2021 doi: 10.1155/2021/8352683. PMID: 34395625; PMCID: PMC8357516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inhorn M.C., Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update. 2015 Jul-Aug;21(4):411–426. doi: 10.1093/humupd/dmv016. Epub 2015 Mar 22. PMID: 25801630. [DOI] [PubMed] [Google Scholar]
- 168.Boivin J., Bunting L., Collins J.A., Nygren K.G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum. Reprod. 2007 Jun;22(6):1506–1512. doi: 10.1093/humrep/dem046. Epub 2007 Mar 21. Erratum in: Hum Reprod. 2007 Oct;22(10):2800. PMID: 17376819. [DOI] [PubMed] [Google Scholar]
- 169.Pizent A., Tariba B., Živković T. Reproductive toxicity of metals in men. Arh. Hig. Rada. Toksikol. 2012;63(Suppl 1):35–46. doi: 10.2478/10004-1254-63-2012-2151. PMID: 22548851. [DOI] [PubMed] [Google Scholar]
- 170.Pizent A., Tariba B., Živković T. Reproductive toxicity of metals in men. Arh. Hig. Rada. Toksikol. 2012;63(Suppl 1):35–46. doi: 10.2478/10004-1254-63-2012-2151. PMID: 22548851. [DOI] [PubMed] [Google Scholar]
- 171.Lv Y., Li T., Yang M., Su L., Zhu Z., Zhao S., Zeng W., Zheng Y. Melatonin attenuates chromium (VI)-Induced spermatogonial stem cell/progenitor mitophagy by restoration of METTL3-mediated RNA N6-methyladenosine modification. Front. Cell Dev. Biol. 2021 Jun 4;9 doi: 10.3389/fcell.2021.684398. PMID: 34150779; PMCID: PMC8212693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meng P., Zhang S., Jiang X., Cheng S., Zhang J., Cao X., Qin X., Zou Z., Chen C. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf. 2020 May;194 doi: 10.1016/j.ecoenv.2020.110360. Epub 2020 Mar 6. PMID: 32151864. [DOI] [PubMed] [Google Scholar]
- 173.Wei S., Qiu T., Yao X., Wang N., Jiang L., Jia X., Tao Y., Wang Z., Pei P., Zhang J., Zhu Y., Yang G., Liu X., Liu S., Sun X. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J. Hazard Mater. 2020 Feb 15;384 doi: 10.1016/j.jhazmat.2019.121390. Epub 2019 Oct 5. PMID: 31735470. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y., Bian H., Ma Y., Xiao Y., Xiao F. Cr(VI)-induced overactive mitophagy contributes to mitochondrial loss and cytotoxicity in L02 hepatocytes. Biochem. J. 2020 Jul 31;477(14):2607–2619. doi: 10.1042/BCJ20200262. PMID: 32597464. [DOI] [PubMed] [Google Scholar]
- 175.Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Aspect. Med. 2003 Aug-Oct;24(4-5):281–291. doi: 10.1016/s0098-2997(03)00023-2. PMID: 12893006. [DOI] [PubMed] [Google Scholar]
- 176.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. PMID: 1937131. [DOI] [PubMed] [Google Scholar]
- 177.Liang Q., Xiao Y., Liu K., Zhong C., Zeng M., Xiao F. Cr(VI)-Induced autophagy protects L-02 hepatocytes from apoptosis through the ROS-AKT-mTOR pathway. Cell. Physiol. Biochem. 2018;51(4):1863–1878. doi: 10.1159/000495713. Epub 2018 Nov 30. PMID: 30504711. [DOI] [PubMed] [Google Scholar]
- 178.Zeng Z., Liang J., Wu L., Zhang H., Lv J., Chen N. Exercise-induced autophagy suppresses sarcopenia through akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front. Physiol. 2020 Nov 2;11 doi: 10.3389/fphys.2020.583478. PMID: 33224037; PMCID: PMC7667253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun H., Wang Z., Yakisich J.S. Natural products targeting autophagy via the PI3K/Akt/mTOR pathway as anticancer agents. Anti Cancer Agents Med. Chem. 2013 Sep;13(7):1048–1056. doi: 10.2174/18715206113139990130. PMID: 23293890. [DOI] [PubMed] [Google Scholar]
- 180.Yi J., Zhu J., Wu J., Thompson C.B., Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. U. S. A. 2020 Dec 8;117(49):31189–31197. doi: 10.1073/pnas.2017152117. Epub 2020 Nov 23. PMID: 33229547; PMCID: PMC7733797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014 Nov;15(11):1009–1016. doi: 10.1038/ni.3002. PMID: 25329189. [DOI] [PubMed] [Google Scholar]
- 182.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014 Mar;32(3):252–260. doi: 10.1038/nbt.2816. Epub 2014 Feb 23. PMID: 24561556; PMCID: PMC4320647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cerri S, Greco R, Levandis G, Ghezzi C, Mangione AS, Fuzzati-Armentero MT, Bonizzi A, Avanzini MA, Maccario R, Blandini F. Intracarotid Infusion of Mesenchymal Stem Cells in an Animal Model of Parkinson’s Disease, Focusing on Cell Distribution and Neuroprotective and Behavioral Effects. Stem Cells Transl Med. 2015 Sep;4(9):1073–1085. doi: 10.5966/sctm.2015-0023. Epub 2015 Jul 21. PMID: 26198165; PMCID: PMC4542871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dufrane D. Impact of Age on Human Adipose Stem Cells for Bone Tissue Engineering. Cell Transplant. 2017 Sep;26(9):1496–1504. doi: 10.1177/0963689717721203. PMID: 29113460; PMCID: PMC5680951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1):336. doi: 10.1186/s13287-018-1078-8. PMID: 30526687; PMCID: PMC6286545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.