Abstract
The major outer membrane protein (MOMP) of Chlamydia species shares several biochemical properties with classical porin proteins. Secondary structure analysis by circular dichroism now reveals that MOMP purified from Chlamydia psittaci has a predominantly β-sheet content (62%), which is also typical of bacterial porins. Can MOMP form functional ion channels? To directly test the “porin channel” hypothesis at the molecular level, the MOMP was reconstituted into planar lipid bilayers, where it gave rise to multibarreled channels, probably trimers, which were modified by an anti-MOMP monoclonal antibody. These observations are consistent with the well-characterized homo-oligomeric nature of MOMP previously revealed by biochemical analysis and with the triple-barreled behavior of other porins. MOMP channels were weakly anion selective (PCl/PK ∼ 2) and permeable to ATP. They may therefore be a route by which Chlamydia can take advantage of host nucleoside triphosphates and explain why some anti-MOMP antibodies neutralize infection. These findings have broad implications on the search for an effective chlamydial vaccine to control the significant human and animal diseases caused by these organisms.
Members of the order Chlamydiales are distinguishable from other bacteria by their obligate intracellular mode of growth and their distinctive biphasic life cycle in which the small spore-like extracellular and infectious form, the elementary body (EB), alternates with the intracellular vegetative form, the reticulate body (RB). The four main species currently recognized, Chlamydia trachomatis, C. psittaci, C. pneumoniae, and C. pecorum, are diverse pathogens that cause a range of disease in both humans and animals. A common component of all these species is the 40-kDa major outer membrane protein (MOMP), present in both the EB and RB forms. The MOMP is a multifunctional protein which is thought to have a role both in the infectious process (3, 34–36) and in the maintenance of structural rigidity via disulfide bond cross-linking within the EB outer membrane (13, 15, 26).
The antigenic properties of MOMP have been studied in detail since the landmark discovery that MOMP purified from sodium dodecyl sulfate (SDS)-gels was capable of raising antibodies which could neutralize the infectivity of C. trachomatis in vitro (6). Protein sequence comparisons of MOMPs both within (33) and between (18) species, combined with epitope mapping studies (8, 43), have shown that the epitopes responsible for neutralization lie within four variable segments. Vaccine preparations based on chlamydial outer membrane complexes, which are highly enriched for the MOMP in its native form, have been shown to be protective against chlamydial disease in sheep (37), guinea pigs (2), and mice (10, 28). However, experimental vaccines based on denatured or nonnative recombinant MOMP preparations have yielded, at best, only partial protection (28). Most recently, protection was demonstrated in mice administered a DNA vaccine comprising only the MOMP gene (42).
These results clearly make MOMP the primary candidate for a subunit vaccine against chlamydial infection, but despite many years of intensive study, the paucity of structural information leaves unanswered many questions as to how MOMP fulfills its diverse functions. Structural studies are hampered first by the difficulty of growing chlamydiae in bulk and subsequently by problems with purifying and solubilizing a protein which both is highly cross-linked and normally resides in a hydrophobic environment. These factors have precluded attempts to crystallize the protein and have made it necessary to rely on analysis techniques that require relatively small quantities of protein.
A recent report showed that MOMP solubilized with octyl glucoside (OG) in the presence of dithiothreitol (DTT) was oligomeric, with electrophoretic and sedimentation properties consistent with a trimeric structure (21). These oligomers resisted denaturation with SDS in a way similar to that for classical gram-negative porin molecules, which are also trimers (27). The result was consistent with an early observation by Bavoil et al. (3), who used liposome swelling to demonstrate that the chlamydial outer membrane contained pores and, due to its predominance in the outer membrane, that MOMP was the likely pore-forming protein. In this paper, we report direct evidence for porin function obtained by using native, oligomeric MOMP incorporated into planar lipid bilayers. Moreover, due to the traditional view that chlamydiae are required to scavenge ATP from the host cell, we have investigated the transport of ATP through the MOMP channel.
MATERIALS AND METHODS
Chlamydial culture.
The ovine abortion isolate of C. psittaci, S26/3, was grown in McCoy cells as previously described (22). Briefly, infected cells were grown in RPMI 1640 (Gibco) supplemented with 5% (vol/vol) newborn calf serum, 0.2% (wt/vol) sodium hydrogen carbonate, 1% (wt/vol) HEPES, streptomycin (0.1 mg/ml), nystatin (25 U/ml), gentamicin (5 μg/ml), and cycloheximide (1 μg/ml).
Purification of MOMP.
The MOMP used for reconstitution into planar lipid bilayers was purified from McCoy cells as described previously (3, 21). Secondary structural analysis by circular dichroism (CD) was performed on MOMP purified by nondenaturing hydroxyapatite chromatography as previously described (5). Briefly, 15 to 20 mg of EBs/RBs from tissue culture harvests were suspended in 2% (wt/vol) Sarkosyl and incubated for 1 h at 37°C. After centrifugation for 30 min at 100,000 × g, the pellet was resuspended in 2% (wt/vol) SDS in phosphate-buffered saline (pH 7.4) and incubated for 1 h at 37°C. Seven milligrams of the MOMP-enriched SDS extract was equilibrated with 0.01 M sodium phosphate (pH 6.4) containing 1 mM DTT and 0.1% (wt/vol) SDS (column equilibration buffer). This material was fractionated by hydroxyapatite chromatography in the continued presence of SDS, using the technique of Moss and Rosenblum (24). Briefly, the MOMP-enriched sample was applied to a preequilibrated hydroxyapatite column (Bio-Rad) which was then washed with equilibration buffer, and samples were eluted with a linear gradient of 0.1 to 0.6 M sodium phosphate (pH 6.4) containing 1 mM DTT and 0.1% (wt/vol) SDS. The fractions showing increased absorbance at 280 nm were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and by immunoblotting using the MOMP-specific monoclonal antibody (MAb) 4/11.
The MAbs used in this study, specific to the monomeric (4/11) and oligomeric (4/11 and A11) forms of MOMP, have been previously described (4, 21). SDS-PAGE and Western blotting were carried out as described previously (21). Protein concentrations were determined by gel densitometry using a Bio-Rad GS-670 imaging densitometer and/or bicinchoninic acid protein assay reagent (Pierce).
CD.
CD analysis of hydroxyapatite-purified MOMP was performed on a Jasco J-600 spectrophotometer with a path length of 0.02 cm, averaging 16 scans between wavelengths of 190 nm and 260 nm for each sample. The spectrophotometer was blanked with 0.3 M sodium phosphate (pH 6.4) containing 1 mM DTT and 0.1% (wt/vol) SDS. Secondary structure estimations were obtained by the CONTIN procedure of Provencher and Glockner (30), which essentially compares the spectrum to a database of spectra from known structures.
Planar lipid bilayer reconstitution and channel analysis.
MOMP was incorporated into 0.3-mm-diameter planar lipid bilayers cast at room temperature from a 30-mg/ml decane suspension of diphytanoyl phosphatidylcholine (Avanti Polar Lipids, Alabaster, Ala.), using equipment and techniques similar to those described by Williams (38) as used in previous studies (7, 15). The cis side of the bilayer was voltage clamped relative to the trans side, using a Biologic RK-300 patch-clamp amplifier (Intracel, Royston, United Kingdom). The relative potential applied across the bilayer is called the holding potential or voltage-clamp potential. Transmembrane currents were low-pass filtered (10-kHz cutoff, 8-pole Bessel filter) and digitally recorded. All bilayers used had a conductance of <10 pS and a capacitance of >250 pS. Bilayers of this size have a relatively large capacitance. When large changes are made to the holding potential (e.g., by switching from 0 to ±80 mV), the bilayer momentarily becomes charged, and the charge then dissipates almost immediately to give rise to an exponentially decaying current transient. This is referred to as the bilayer capacitative current transient and is superimposed on channel recordings made immediately after large changes in the holding potential, giving them a characteristic curved appearance.
To incorporate channels, purified detergent-solubilized MOMP was added to the cis side to a final concentration of 1 ng/ml in the presence of a salt gradient, 250 mM KCl cis versus 50 mM KCl trans (buffered with 10 mM Tris-HCl [pH 7.4]). The solutions bathing the bilayer were changed by perfusion (at least 10 volumes) as required. Opening and closing of the ion channels give rise to square-shaped pulses of current which are termed unit currents. Unit currents and holding (voltage clamp) potentials are displayed according to a standard convention, quoting the holding (clamp) potential in the cis chamber, with positive transmembrane (upgoing) currents representing a net flux of cations flowing cis to trans, or a net flux of anions flowing in the opposite direction. Channel recordings were postfiltered (see figure legends) to reduce high-frequency noise and analyzed with the program pClamp 6 (Axon Instruments, Foster City, Calif.).
In the presence of a salt gradient either side of the bilayer, the reversal potential is defined as the holding potential that exactly balances the tendency for ions to diffuse down their chemical concentration gradient. Relative ionic permeabilities were calculated from measured reversal potentials by using appropriate forms of the Goldman-Hodgkin-Katz voltage equation (7, 17). Briefly, with the same monovalent salt in both chambers, Er = −RT/zF.ln{PC[C]t + PA[A]c/PC[C]c + PA[A]t}, where Er is the reversal potential, P is permeability, A and C are the anion and cation, respectively, and c and t represent cis and trans, respectively. R is the gas constant (8.314 JK−1 mol−1), T is room temperature (298 K), z is valency, and F is the Faraday constant (9.6 × 104 C mol−1). Concentrations were corrected for ionic activities by reference to activity coefficients obtained from standard tables.
Addition of components to bilayer solutions. (i) MAb A11.
The MOMP oligomer-specific MAb A11 (final dilution of 1/1,000), which has previously been described (4), was added to both the cis and trans chambers in 50 mM KCl–10 mM Tris-HCl (pH 7.4)–1 mg of bovine serum albumin (BSA) per ml.
(ii) Oxidizing agents.
Ten to 500 mM Cu2+-phenanthroline, hydrogen peroxide, or oxidized glutathione in 50 mM KCl–10 mM Tris-HCl (pH 7.4) was added to the cis and trans chambers. Channel activity in bilayers bathed in these buffers was monitored over a period of at least 30 min.
(iii) ATP.
ATP was added by perfusion to the bilayer in the form of 10 mM Na+-ATP–10 mM Tris-HCl (pH 7.4) in both the cis and trans chambers. More highly concentrated solutions were achieved by the addition of Na+-ATP to the chambers from a concentrated stock solution. For the multivalent ATP4− ion in the presence of Na+, the theoretical reverse potential for an ATP4−-selective channel was estimated from the Nernst equation. The activity ratio of 100 mM Na-ATP to 10 mM Na-ATP was measured by using the Na+ ionophore gramicidin (11).
RESULTS
SDS-PAGE and immunoblotting analysis of OG-DTT-solubilized MOMP.
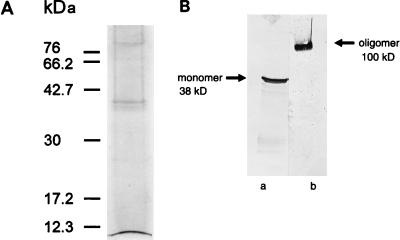
The results of SDS-PAGE and immunoblotting analyses of the MOMP-enriched fraction solubilized with OG-DTT (Fig. 1) illustrate the purity of the MOMP preparation used in planar lipid bilayer reconstitution. Silver staining identified a 38-kDa protein corresponding to the MOMP; contaminant proteins of approximately 90 kDa were also observed (Fig. 1A). These contaminants were identified as the previously described OMP90 (formerly POMP) family (19, 20) by immunoblotting using OMP90-specific MAbs (results not shown). Western blots probed with MAbs specific for the oligomeric (Fig. 1B, lane b) and monomeric (Fig. 1B, lane a) forms of MOMP demonstrated that when MOMP is solubilized in SDS sample buffer at room temperature in the presence of a reducing agent, it migrates with an apparent molecular mass of 100 kDa, which we interpret to represent an oligomer. When fully denatured by boiling, MOMP migrates as a monomer of 38 kDa. These results are typical of those observed with gram-negative porins and are identical to those previously shown by McCafferty et al. (21).
FIG. 1.
SDS-PAGE and Western blot analyses of OG-DTT-solubilized MOMP. (A) SDS-PAGE analysis of 2% (wt/vol) OG–10 mM DTT-solubilized MOMP-enriched preparations by silver staining. (B) The OG-DTT-solubilized preparation was subjected to SDS-PAGE on 12.5% gels, immunoblotted, and probed with MAb 4/11 (sample loaded after boiling; lane a) and MAb A11 (sample loaded without boiling; lane b). Molecular masses are indicated.
Secondary structure determination.
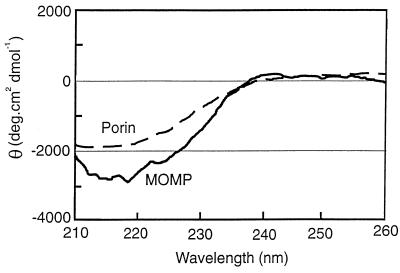
Figure 2 shows silver-stained SDS-PAGE analysis of fractions eluted from a hydroxyapatite column under relatively nondenaturing conditions. Although fractions 20 to 30 contained significant amounts of MOMP oligomer (100 kDa), only fractions 25 to 30 contained very few contaminant proteins while retaining significant amounts of oligomer. MOMP oligomer accounted for >90% of the hydroxyapatite-purified protein (as estimated by densitometry), achieving the criteria for accurate structural analysis by CD. Fractions 25 to 30, which were eluted at a sodium phosphate concentration of approximately 0.3 M, were subjected to CD analysis in the far-UV range; the spectrum obtained for fraction 25 is shown in Fig. 3. The spectrum of purified protein is consistent with the presence of a large percentage of β structure, as indicated by the characteristic absorption minima at approximately 215 nm. Analysis using the CONTIN procedure (30) (see Materials and Methods) estimated 62% β structure, 38% random coil, and 0% α helix. The CD spectrum of bovine heart mitochondrial porin in 0.1% (wt/vol) OG, recorded on the same spectrophotometer and known to contain 60% β structure (29), is shown for comparison.
FIG. 2.
SDS-PAGE analysis of the hydroxyapatite-purified MOMP-enriched preparation. Fractions eluted from the hydroxyapatite column (5 by 1 cm) loaded with the 2% (wt/vol) SDS-solubilized MOMP-enriched preparation (see Materials and Methods) were subjected to SDS-PAGE on a 12.5% gel, under reducing conditions (not boiled), and silver stained. Molecular masses are indicated.
FIG. 3.
CD spectra of hydroxyapatite-purified MOMP and bovine heart mitochondrial porin in the presence of 0.1% (wt/vol) OG.
Planar lipid bilayer reconstitution.
OG-DTT-solubilized MOMP incorporated into planar lipid bilayers within 5 to 10 min of addition, to give rise to ion-channel-like unit currents (see Materials and Methods). Unit currents were not seen at 0 mV, despite the presence of an ion gradient (250 versus 50 mM KCl [see Materials and Methods]). This finding suggested that channel opening, or insertion, was a voltage-dependent process. Channel incorporation appeared to be autocatalytic, because the rate of incorporation accelerated markedly following the initial appearance of unit currents (40). After the first evidence of channel incorporation, the solution in the cis chamber was changed to limit the channel content of the bilayer. Finally, it was confirmed that the addition of buffer alone had no effect.
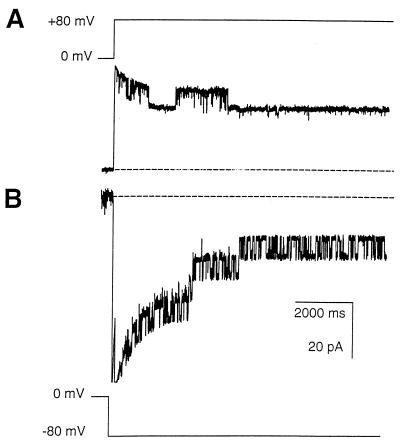
Figure 4 shows typical traces, with the same solutions on both the cis and trans side of a bilayer that had been exposed to MOMP. The data were obtained by switching the holding potential from 0 to either +80 or −80 mV as indicated. Bursts of channel openings are superimposed on bilayer capacitative current transients (see Materials and Methods). The channels exhibit voltage-dependent closure as the high holding potentials are maintained. Normally, the appearances on switching were similar to those shown in Fig. 4, with more open/closed transitions at negative compared to positive holding potentials, but in some experiments all channels became incorporated in the opposite orientation. In addition, the bursts of activity seen after switching to negative holding potentials normally contained more unit currents than those seen at positive potentials. In the presence of diphytanoyl phosphatidylcholine (chosen because it is a very robust bilayer lipid), the membrane potential could be clamped as high as ±200 mV without breakage, but the maximum number of unit currents generally appeared to occur at ±60 mV or above.
FIG. 4.
Ion channel recordings from OG-DTT-solubilized MOMP in a bilayer bathed in 50 mM KCl–10 mM Tris-HCl (pH 7.4). (A) Holding potential switched from 0 to +80 mV. (B) Holding potential switched from 0 to −80 mV. Recordings were low-pass filtered at 100 Hz and show decaying capacitative transients representing the discharge of the typically large bilayer capacitance (∼300 pF).
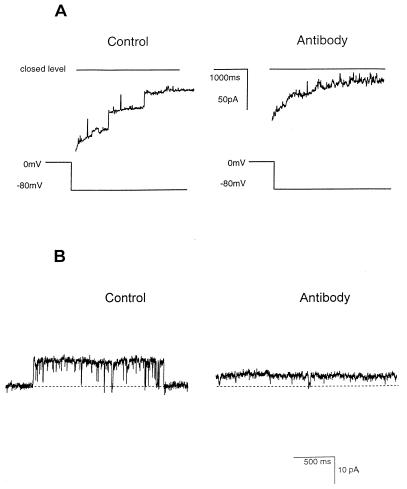
Two types of experiment confirmed that MOMP was the channel-forming protein. First, channel behavior (and rate of channel incorporation) obtained by using similar quantities of MOMP that had been highly purified by hydroxyapatite chromatography was identical to that seen with OG-DTT-solubilized MOMP. This fraction was not reconstituted routinely, because its SDS content destabilized the bilayer. Second, addition of the native MOMP-specific MAb A11 affected both the open/closed transitions of the channel and the amplitude of the unit currents (Fig. 5). These effects could not be reversed by perfusion with antibody-free solutions. Note that the channels did not close completely in the presence of A11 (Fig. 5B). Additions of other immunoglobulins or BSA (1 mg/ml) were without effect (results not shown).
FIG. 5.
Effects of MAb A11 on the MOMP channel. The bilayer was bathed in 50 mM KCl–10 mM Tris-HCl (pH 7.4); MAb A11 was added to both the cis and trans chambers to a final dilution of 1/1,000 in the presence of BSA (1 mg/ml). (A) Traces obtained after switching the holding potential from 0 to −80 mV before (left) and 30 min after (right) addition of MAb A11. In each recording, there is a typical bilayer capacitative current transient superimposed on channel openings, but the openings are of much lower amplitude after addition of MAb A11. (B) Detailed appearance of an individual unit current before (left) and 30 min after (right) addition of MAb A11. Note the difference in amplitude and the reduction in the number of long-lived channel closures. The dotted line represents the baseline level, corresponding to the closure of the unit conductance. Currents were filtered at 100 Hz.
Properties of unit MOMP channels.
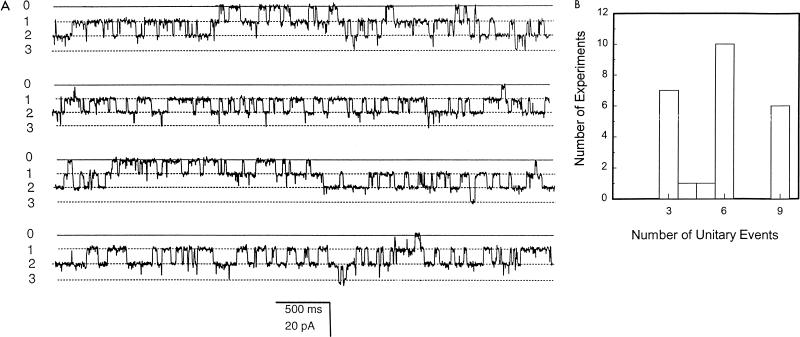
Given that native MOMP tends to migrate as an oligomer, presumably a trimer, under nondenaturing conditions on SDS-PAGE, and knowing that many classical porins are trimers (9, 23, 25), we measured the number of functional unit currents that could be obtained following bilayer reconstitution of MOMP. The number of individual unit currents (e.g., levels 1, 2, and 3 in Fig. 6A) were counted in 25 independent experiments in which incorporated channels were maximally activated by switching between +120 and −120 mV. The numbers of discrete conducting units are summarized in Fig. 6B.
FIG. 6.
Analysis of the minimal conducting unit of the MOMP channel. (A) Contiguous 40-s recording of MOMP activity in a bilayer bathed in 50 mM KCl–10 mM Tris-HCl (pH 7.4) (holding potential −70 mV, filtered at 200 Hz). The closed level of the channel (0) and three unit open levels (1, 2, and 3) are indicated. (B) Maximum number of unit open levels counted in 25 individual reconstitution experiments.
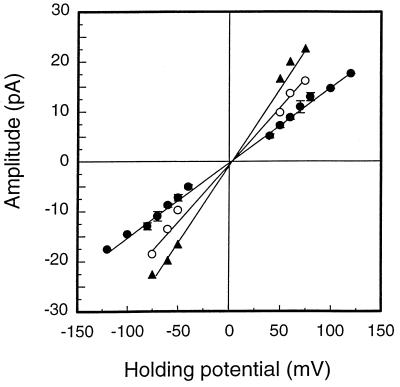
Unit currents were measured (pClamp 6) to construct open-channel current-voltage relationships in symmetrical KCl (the same concentration of KCl in both the cis and trans chambers) (Fig. 7). These were all linear; the slope conductances were 120 ± 18, 210 ± 25, and 310 ± 32 pS in 50, 150, and 300 mM KCl, respectively (mean ± standard deviation [SD], n = 4). Careful inspection of the data revealed that channel closure was incomplete, with a residual conductance equivalent to approximately 5% of the fully open state. In some recordings we also observed subconductance states, but these substates, and the residual conductances of the closed states, were not examined in detail.
FIG. 7.
Current-voltage relationship of MOMP channels in symmetrical concentrations of KCl (each buffered with 10 mM Tris-HCl [pH 7.4]). KCl concentrations are 300 mM (▴), 150 mM (○), and 50 mM (•). Error bars represent ± standard error of the mean for four independent experiments.
In the light of results obtained by liposome swelling indicating that reduction of disulfide bonds was necessary to fully open pores within the chlamydial outer membrane, we attempted to oxidize our fully open MOMP channels and observe any effects on channel behavior. However, in vitro oxidation of bilayer-incorporated MOMP with hydrogen peroxide, oxidized glutathione, or Cu2+-phenanthroline had no observable effect on any channel properties.
Ion selectivity and permeability of MOMP channels.
The relative anion versus cation permeability of reconstituted MOMP channels was calculated from reversal potentials (see Materials and Methods) measured in 250 mM KCl cis versus 50 mM KCl trans. A reversal potential of +10.0 ± 4 mV (mean ± SD, n = 4) indicated that the channel was weakly anion selective: PCl/PK = 2.0 ± 0.84 (mean ± SD, n = 4). The channels were also permeable to ATP. In the presence of 100 mM Na-ATP cis versus 10 mM Na-ATP trans, a reversal potential of −11 ± 1.9 mV (mean ± SD, n = 10) was obtained, with a unit conductance of approximately 80 pS. The ionic activity ratio of 100 mM Na-ATP to 10 mM Na-ATP, measured by using the Na+ ionophore gramicidin (11), was 4.5. This corresponds to a theoretical (Nernstian) reversal potential of −39 mV for a solely Na+ selecting channel and +10 mV for a solely ATP4− selecting channel; hence, a reversal potential of −11 mV suggests that there is substantial ATP transport.
DISCUSSION
In the search for an effective antichlamydial vaccine, efforts have mainly concentrated on the proteins of the outer membrane. These proteins are likely to be the key virulence determinants of Chlamydia, involved in attachment, internalization, and the prevention of phagolysosomal fusion, and are an obvious starting point for vaccine development. Primarily, research has focused on the MOMP which is the predominant constituent of chlamydial outer membranes, comprising approximately 60% of the protein mass. The MOMP remains the only chlamydial protein shown unequivocally to be surface exposed, and antibodies specific to the protein have been shown to be protective (2, 28, 37). For these reasons, MOMP remains the primary candidate for a chlamydial vaccine.
Problems purifying native MOMP or obtaining recombinant MOMP in a native form have hampered the study of structure-function relationships for this protein. Despite this, MOMP has been implicated in many aspects of chlamydial pathogenesis (3, 12, 34–36). Perhaps one of the most convincing, and widely accepted, proposed functions is that of a “chlamydial porin.” Using the technique of liposome swelling, Bavoil et al. (3) demonstrated that outer membrane complexes of C. trachomatis contained water-filled pores with an exclusion limit of 850 to 2250 Da. Due to its abundance in these outer membrane preparations, it was postulated that MOMP, as a chlamydial porin, was responsible. This hypothesis is amply supported by both the structural and functional results presented in this report.
The protocol used to purify MOMP for structural studies could not be employed during the purification of MOMP for reconstitution experiments due to the destabilizing effects of SDS on the bilayer. Unfortunately, dialysis to remove SDS from hydroxyapatite-purified fractions was not feasible because of the prohibitively small amounts of protein involved. However, both methods, solubilization in OG-DTT and purification by hydroxyapatite chromatography, resulted in the formation of SDS-resistant MOMP oligomers typical of classical gram-negative porin proteins. Structural analysis by CD indicated that the secondary structure of MOMP was mainly β sheet (62%), similar to the high β-sheet content of bacterial porins previously characterized by X-ray crystallography (29). The relatively high proportion of random coil, calculated from the MOMP spectrum, may be attributed to its four surface-exposed variable segments (1, 16, 41).
At a functional level, the insertion of MOMP into planar lipid bilayers seemed to be autocatalytic, resembling that seen with other porins (40). It is possible that the correct insertion of one channel assisted the correct orientation of other channel proteins, thereby accelerating subsequent insertion. The preference shown by MOMP for a particular bilayer orientation was demonstrated by the asymmetric response of the channel to holding potentials of opposite polarity. Presumably this asymmetry of behavior, seen in other porins, has a structural basis (9, 23). The modification of channel amplitude and open/closed transitions by the neutralizing MOMP-specific MAb A11 was of particular significance, for it not only confirmed that MOMP was the channel-forming protein but also reinforced our belief that MOMP is arranged as an oligomer in the bilayer, since A11 recognizes only oligomeric MOMP upon Western blotting (4, 21). This posed the question, What is the minimal conducting unit of the MOMP channel? From the results summarized in Fig. 6, we suggest that each MOMP molecule inserts into the bilayer as a trimer to give rise to three pores through the membrane, and that switching to holding potentials of ±60 mV and above promotes the opening of all three pores together. Thus, two MOMP trimers could give rise to up to six pores with six unit currents. Voltage-dependent closure at maintained high holding potentials meant that within a few seconds, only one or two protochannels in each trimer were seen to be open at any one time. This type of multibarrel behavior is common in porins (25), and a similar phenomenon has been observed, and analyzed in detail, in eukaryotic channel proteins (e.g., references 7 and 15).
What ions and metabolites would be translocated across the chlamydial outer membrane by a MOMP porin? Previously, chlamydiae were thought to require host-derived nucleoside triphosphates as an energy source and precursor of RNA synthesis, prompting us to look at their transfer across the bilayer. The reversal potential of −11 mV observed in asymmetric concentrations of Na-ATP corresponded to the passage of substantial amounts of ATP across the bilayer. If the MOMP channel were Na+ selective, allowing only the passage of Na+, we would theoretically expect to see a reversal potential of −39 mV; alternatively, if the channel allowed the passage of only ATP4− across the bilayer, a reversal potential of +10 mV would be observed. Therefore, a reversal potential of −11 mV demonstrates the passage of both Na+ and ATP4− through the MOMP channel. The identification of a route by which chlamydiae can take advantage of host nucleoside triphosphates and other nutrients may also explain why antibodies specific to MOMP neutralize infection. With the identification of genes encoding ATP biosynthetic pathways by the Chlamydia Genome Sequencing Project (32), it now appears unlikely that chlamydiae derive all of their ATP from the host cell.
In the EB, outer membrane proteins are disulfide bond cross-linked, rendering the outer membrane largely impermeable. The transition into the intracellular RB is coupled with a reduction of outer membrane disulfide bonds, increasing membrane permeability. It is clear that MOMP’s pore-forming activity would be primarily utilized at the RB stage of the chlamydial life cycle. Bavoil et al. (3) proposed that the reduction of outer membrane disulfide bonds opened chlamydial pores, allowing the uptake of ATP and other nutrients. Evidence supporting this hypothesis included activation of the pores by treatment with DTT and the blocking of reoxidation with iodoacetamide. Chemical modification was not required to maintain functional open channels from our OG-DTT-solubilized MOMP. We also noted that we could not reoxidize the putative free SH groups of bilayer-incorporated MOMP to close or inactivate the channels (mimicking the EB-to-RB transition). However, the reoxidation of disulfide bonds in the correct pairings to close the channel may require the presence of other proteins, such as the cysteine-rich proteins absent from the OG-DTT-solubilized sample.
In this study, we have shown that MOMP functions as a porin and have demonstrated a possible physiological function with the passage of nucleotide triphosphates through the channel. A particularly significant aspect of the work is that it provides a measurable criterion by which to assess recombinant MOMP for native function. Indeed, we have recently reconstituted a recombinant MOMP into the planar lipid bilayer resulting in the formation of ion channels with properties identical to those of the native protein (39). These results have broad implications on the search for an effective chlamydial vaccine.
ACKNOWLEDGMENTS
This work was supported by the Scottish Office Agriculture, Environment and Fisheries Department.
We thank N. C. Price and S. M. Kelly, Scottish CD Facility, Stirling University, Stirling, United Kingdom, for help with CD analysis and G. Lindsay and T. Lever, Glasgow University, Glasgow, United Kingdom, for use of their bovine heart mitochondrial porin CD spectra. MAb A11 was kindly provided by A. A. Andersen, National Animal Disease Center, U.S. Department of Agriculture, Ames, Iowa. In addition, we thank M. Livingstone, Moredun Research Institute, for help with the culture and growth of chlamydiae.
REFERENCES
- 1.Baehr W, Zhang Y X, Joseph T, Su H, Nano F E, Everett K D, Caldwell H D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci USA. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batteiger B E, Rank R G, Bavoil P M, Soderberg L S. Partial protection against genital reinfection by immunization of guinea pigs with isolated outer membrane proteins of the chlamydial agent of guinea pig inclusion conjunctivitis. J Gen Microbiol. 1993;139:2965–2972. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- 3.Bavoil P, Ohlin A, Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984;44:479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzoni-Gatel D, Bernard F, Andersen A, Rodolakis A. Protective effect of polyclonal and monoclonal antibodies against abortion in mice infected by Chlamydia psittaci. Vaccine. 1990;8:342–346. doi: 10.1016/0264-410x(90)90092-z. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell H D, Perry L J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982;38:745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark A G, Murray D, Ashley R H. Single-channel properties of a rat brain endoplasmic reticulum anion channel. Biophys J. 1997;73:168–178. doi: 10.1016/S0006-3495(97)78057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan J W, Clarke I N, Ward M E. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species- and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol Microbiol. 1988;2:673–679. doi: 10.1111/j.1365-2958.1988.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 9.Dahan D, Vachon V, Laprade R, Coulton J W. Voltage gating of porins from Haemophilus influenzae type b. Biochim Biophys Acta. 1994;1189:204–211. doi: 10.1016/0005-2736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 10.De Sa C, Souriau A, Bernard F, Salinas J, Rodolakis A. An oligomer of the major outer membrane protein of Chlamydia psittaci is recognized by monoclonal antibodies which protect mice from abortion. Infect Immun. 1995;63:4912–4916. doi: 10.1128/iai.63.12.4912-4916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gennis R B. Biomembranes. New York, N.Y: Springer; 1989. pp. 288–320. [Google Scholar]
- 12.Gutierrez-Martin C B, Ojcius D M, Hsia R C, Hellio R, Bavoil P M, Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- 13.Hackstadt T, Todd W J, Caldwell H D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae. J Bacteriol. 1985;161:25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatch T P, Allan I, Pearce J H. Structural and polypeptide differences between envelopes of infective and reproductive life-cycle forms of Chlamydia spp. J Bacteriol. 1984;157:13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayman K A, Ashley R H. Structural features of a multisubstate cardiac mitoplast anion channel—inferences from single-channel recording. J Membr Biol. 1993;136:191–197. doi: 10.1007/BF02505763. [DOI] [PubMed] [Google Scholar]
- 16.Herring A J, Tan T W, Inglis N F, Dunbar S M. Sequence analysis of the major outer membrane protein gene of an ovine abortion strain of Chlamydia psittaci. FEMS Microbiol Lett. 1989;65:153–158. doi: 10.1016/0378-1097(89)90383-2. [DOI] [PubMed] [Google Scholar]
- 17.Hille B. Ionic channels of excitable membranes. Sunderland, Mass: Sinauer; 1992. pp. 341–348. [Google Scholar]
- 18.Kaltenboeck B, Kousoulas K G, Storz J. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol. 1993;175:487–502. doi: 10.1128/jb.175.2.487-502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longbottom D, Russell M, Dunbar S M, Jones G E, Herring A J. Molecular cloning and characterization of the genes coding for the highly immunogenic cluster of 90-kilodalton envelope proteins from the Chlamydia psittaci subtype that causes abortion in sheep. Infect Immun. 1998;66:1317–1324. doi: 10.1128/iai.66.4.1317-1324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longbottom D, Russell M, Jones G E, Lainson F A, Herring A J. Identification of a multigene family coding for the 90 kDa proteins of the ovine abortion subtype of Chlamydia psittaci. FEMS Microbiol Lett. 1996;142:277–281. doi: 10.1111/j.1574-6968.1996.tb08443.x. [DOI] [PubMed] [Google Scholar]
- 21.McCafferty M C, Herring A J, Andersen A A, Jones G E. Electrophoretic analysis of the major outer membrane protein of Chlamydia psittaci reveals multimers which are recognized by protective monoclonal antibodies. Infect Immun. 1995;63:2387–2389. doi: 10.1128/iai.63.6.2387-2389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClenaghan M, Herring A J, Aitken I D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984;45:384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzl K, Maier E, Chakraborty T, Benz R. HlyA hemolysin of Vibrio cholerae O1 biotype el tor—identification of the hemolytic complex and evidence for the formation of anion selective ion permeable channels. Eur J Biochem. 1996;240:646–654. doi: 10.1111/j.1432-1033.1996.0646h.x. [DOI] [PubMed] [Google Scholar]
- 24.Moss B, Rosenblum U M. Hydroxylapatite chromatography of protein sodium dodecyl sulphate complexes. J Biol Chem. 1972;247:5194–5198. [PubMed] [Google Scholar]
- 25.Nakae T, Ischii J, Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the outer membrane of Escherichia coli. J Biol Chem. 1979;254:1457–1461. [PubMed] [Google Scholar]
- 26.Newhall W J, Jones R B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983;154:998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992;6:435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 28.Pal S, Theodor I, Peterson E M, De La Maza L M. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price N C. Circular dichroism in protein analysis. In: Meyers R A, editor. Encyclopedia of molecular biology and molecular medicine. Vol. 1. Weinheim, Germany: VCH; 1996. pp. 384–396. [Google Scholar]
- 30.Provencher S W, Glockner J. Estimation of globular protein secondary structure from circular dichroism. J Biochem. 1981;20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 31.Schulz G E. Porins—general to specific, native to engineered passive pores. Curr Opin Struct Biol. 1996;6:485–490. doi: 10.1016/s0959-440x(96)80113-8. [DOI] [PubMed] [Google Scholar]
- 32.Stephens, R. S., S. Kalman, C. Fenner, and R. Davis. Chlamydia Genome Sequencing Project (http://chlamydia-www.berkeley.edu:4231/).
- 33.Stephens R S, Sanchez-Pescador R, Wagar E A, Inouye C, Urdea M S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su H, Raymond L, Rockey D D, Fischer E, Hackstadt T, Caldwell H D. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc Natl Acad Sci USA. 1996;93:11143–11148. doi: 10.1073/pnas.93.20.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Watkins N G, Zhang Y X, Caldwell H D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su H, Zhang Y X, Barrera O, Watkins N G, Caldwell H D. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect Immun. 1988;56:2094–2100. doi: 10.1128/iai.56.8.2094-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan T W, Herring A J, Anderson I E, Jones G E. Protection of sheep against Chlamydia psittaci infection with a subcellular vaccine containing the major outer membrane protein. Infect Immun. 1990;58:3101–3108. doi: 10.1128/iai.58.9.3101-3108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams A J. The measurement of the function of ion channels reconstituted into artificial membranes. In: Ashley R H, editor. Ion channels—a practical approach. Oxford, United Kingdom: IRL Press; 1995. pp. 43–67. [Google Scholar]
- 39.Wyllie, S., R. H. Ashley, A. J. Herring, and D. Longbottom. Unpublished data.
- 40.Xu X F, Colombini M. Self-catalyzed insertion of proteins into phospholipid membranes. J Biol Chem. 1996;271:23675–23682. doi: 10.1074/jbc.271.39.23675. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Y, Zhang Y X, Watkins N G, Caldwell H D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989;57:1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D J, Yang X, Berry J, Shen C X, McClarty G, Brunham R C. DNA vaccination with the major outer membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 43.Zhong G, Reid R E, Brunham R C. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990;58:1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]