Abstract
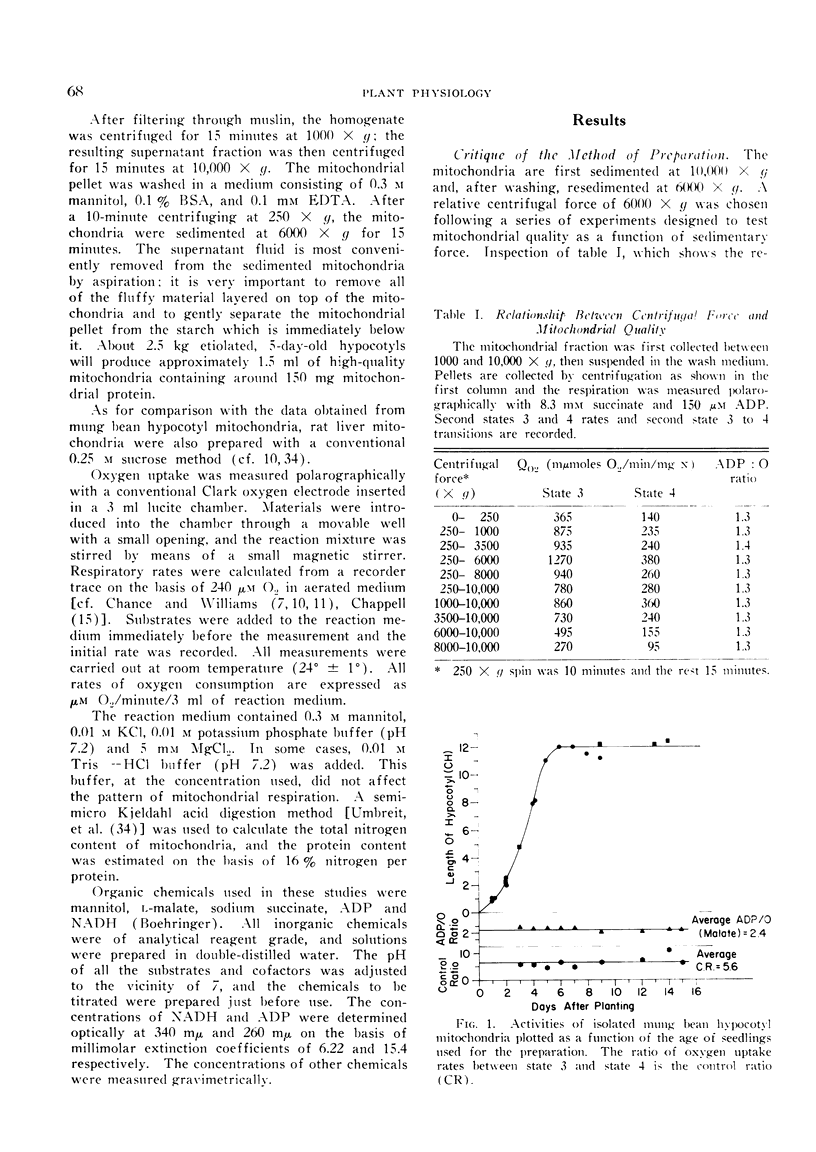
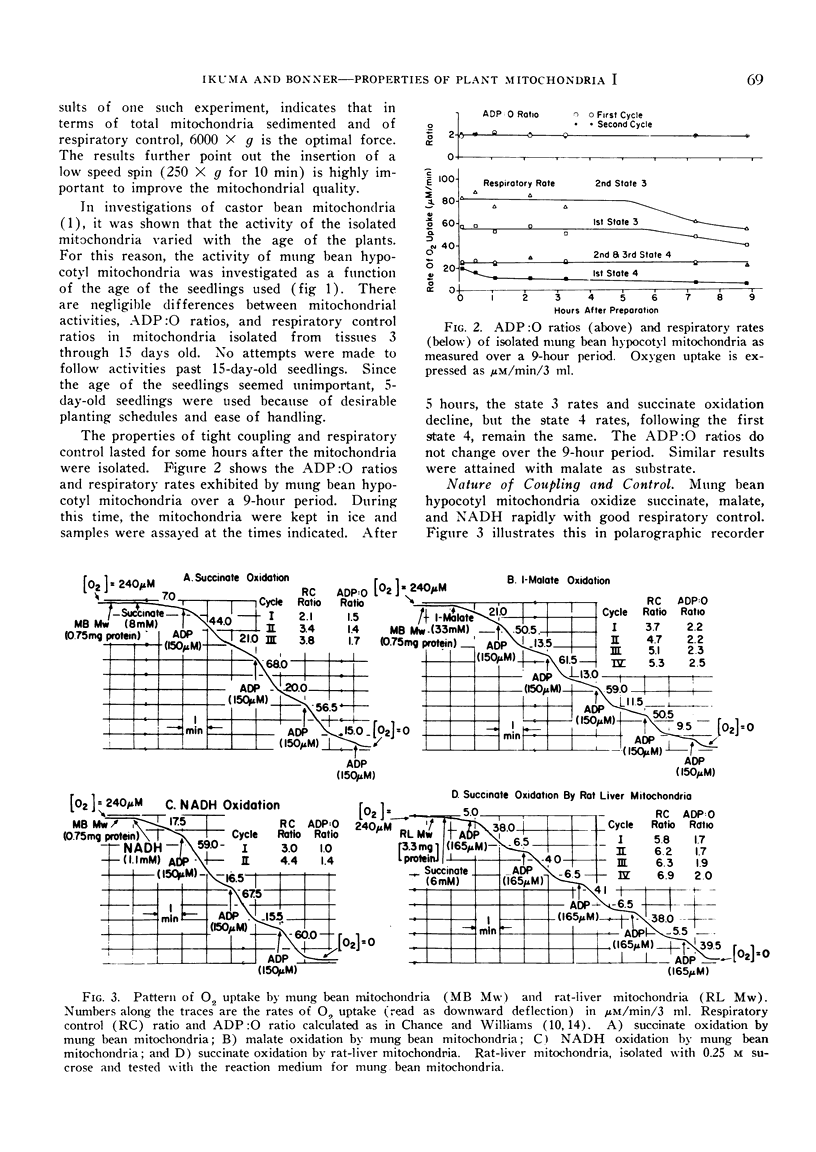
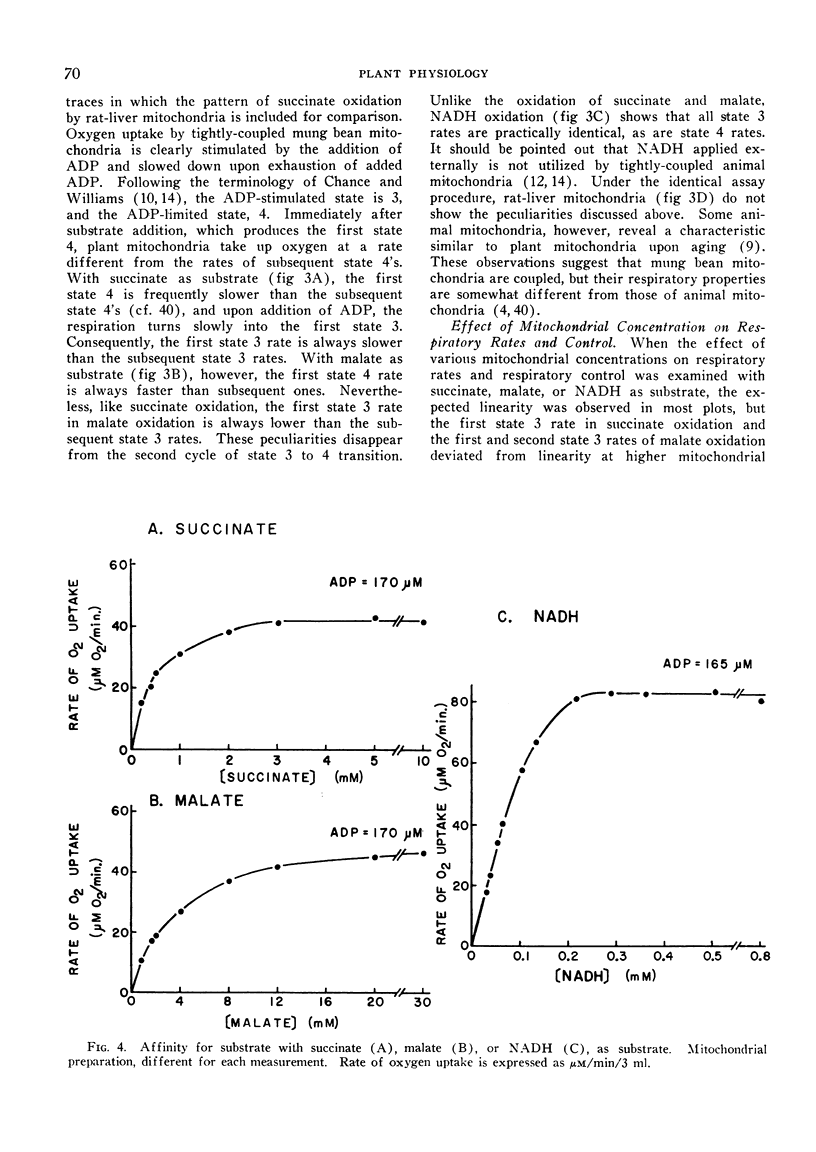
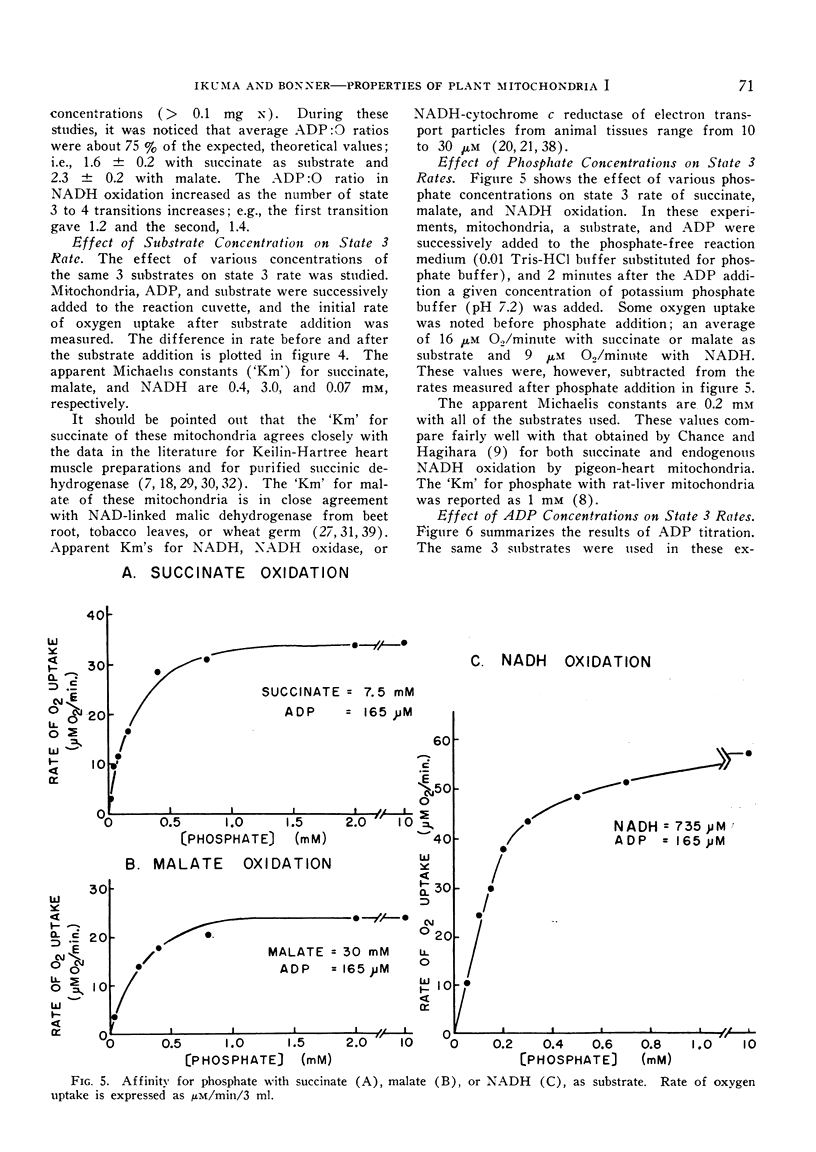
The mitochondria isolated from dark-grown mung bean hypocotyls oxidize succinate, l-malate, and externally added reduced nicotine adenine dinucleotide (NADH) with good respiratory control. While the pattern of respiration resembles that of animal mitochondria, there are 4 basic differences between the respiratory properties of mung bean and animal mitochondria: A) the ability to oxidize NADH, B) the pattern of succinate and malate oxidation, C) the rate of oxygen uptake, and D) the adenosine-5′-diphosphate to oxygen ratios.
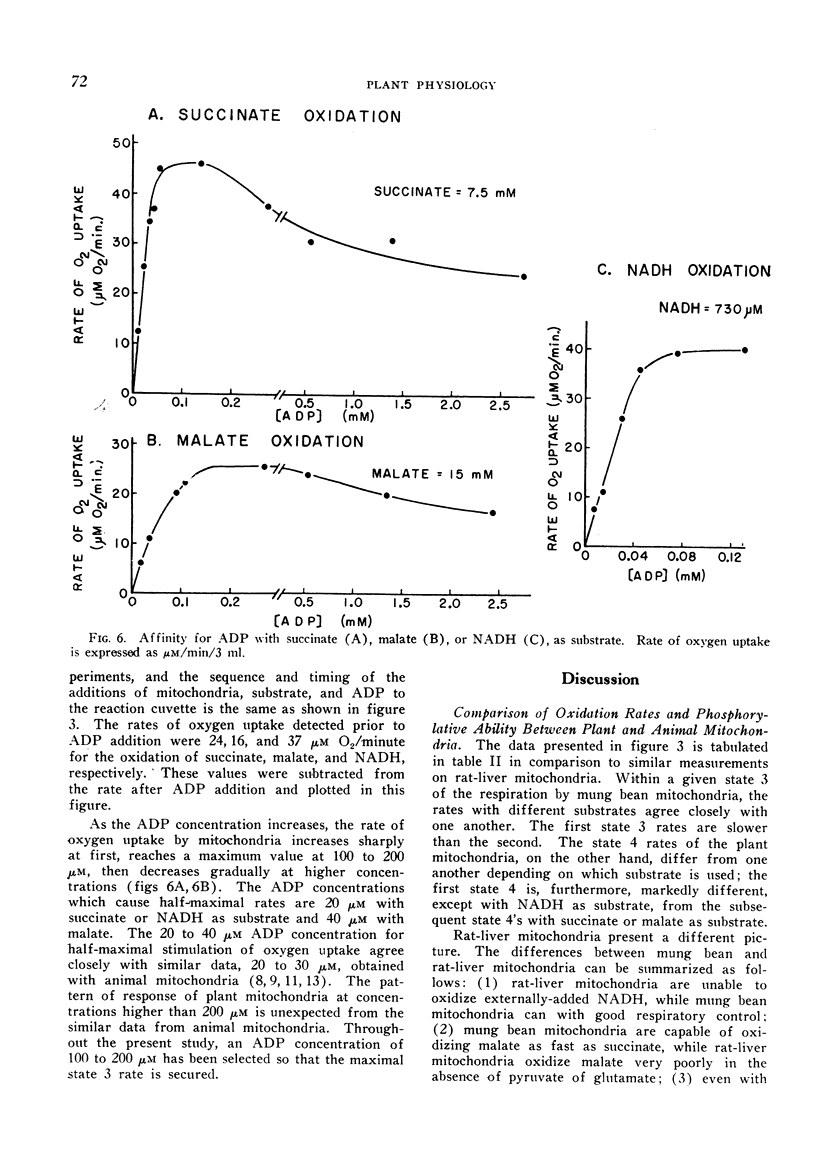
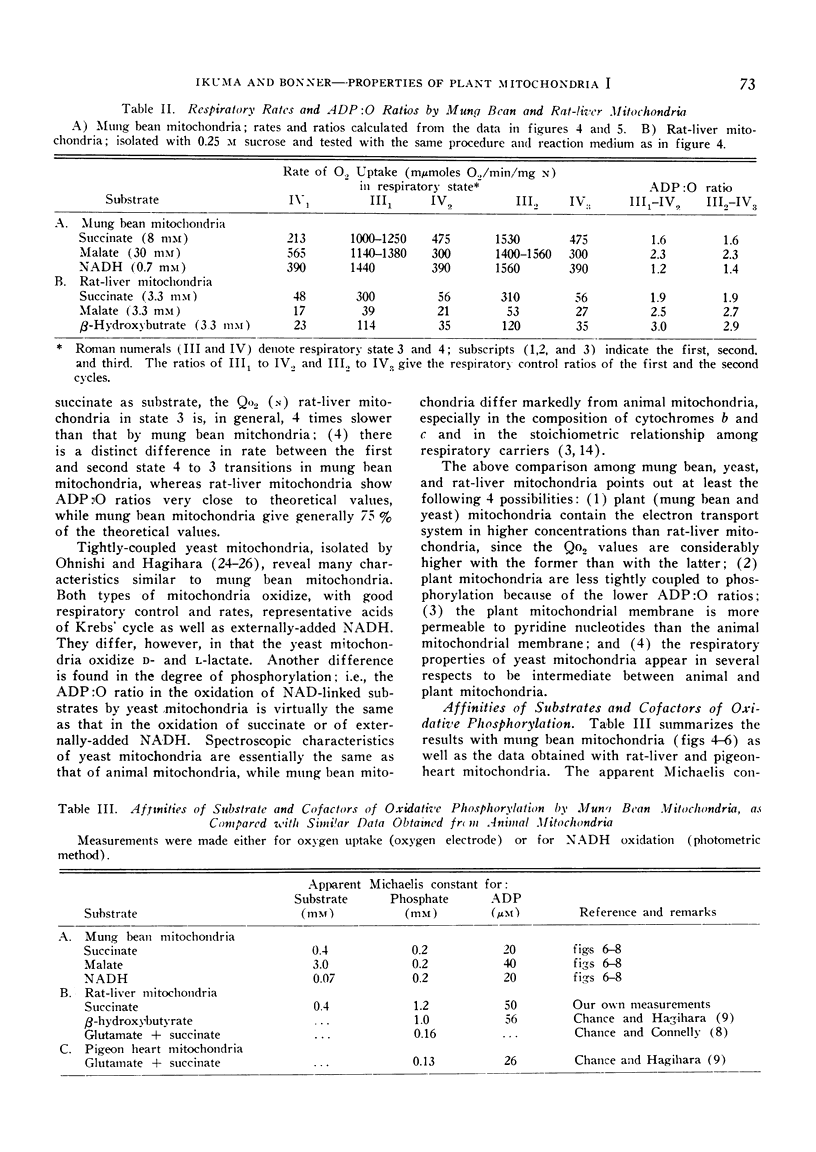
The apparent `Km' for malate of mung bean mitochondria is about one order higher than that expected from malic dehydrogenase in animal mitochondria, whereas the affinity for phosphate is about 5 times higher with plant mitochondria than rat-liver mitochondria. While the half-maximal stimulation of respiration by adenosine-5′-diphosphate is practically identical to that of animal mitochondria, higher concentrations of adenosine-5′-diphosphate cause some decrease in its stimulating action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS H., WALKER D. A. The oxidative activity of particulate fractions from germinating castor beans. Biochem J. 1956 Jan;62(1):114–120. doi: 10.1042/bj0620114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNER J., MILLERD A. Oxidative phosphorylation by plant mitochondria. Arch Biochem Biophys. 1953 Jan;42(1):135–148. doi: 10.1016/0003-9861(53)90247-1. [DOI] [PubMed] [Google Scholar]
- CHANCE B., CONNELLY C. M. A method for the estimation of the increase in concentration of adenosine diphosphate in muscle sarcosomes following a contraction. Nature. 1957 Jun 15;179(4572):1235–1237. doi: 10.1038/1791235a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B. The kinetics and inhibition of cytochrome components of the succinic oxidase system. I. Activity determinations and purity criteria. J Biol Chem. 1952 May;197(2):557–565. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. A simple and rapid assay of oxidative phosphorylation. Nature. 1955 Jun 25;175(4469):1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J Biol Chem. 1955 Nov;217(1):395–407. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., MACKLER B., REPASKE R., MAHLER H. R. DPNH oxidase. Biochim Biophys Acta. 1954 Nov;15(3):435–437. doi: 10.1016/0006-3002(54)90049-9. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., MACKLER B. Studies on the electron transport system. III. The properties of two modified forms of ETP. Biochim Biophys Acta. 1956 Jul;21(1):6–13. doi: 10.1016/0006-3002(56)90087-7. [DOI] [PubMed] [Google Scholar]
- Hanson J. B., Malhotra S. S., Stoner C. D. Action of Calcium on Corn Mitochondria. Plant Physiol. 1965 Nov;40(6):1033–1040. doi: 10.1104/pp.40.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Preparation & some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 1961 Sep;36(5):552–557. doi: 10.1104/pp.36.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNISHI T., HAGIHARA B. PREPARATION OF YEAST MITOCHONDRIA BY AN ENZYMATIC PROCEDURE. J Biochem. 1964 Nov;56:484–486. doi: 10.1093/oxfordjournals.jbchem.a128022. [DOI] [PubMed] [Google Scholar]
- OHNISHI T., HAGIHARA B. PREPARATION OF YEAST MITOCHONDRIA. J Biochem. 1964 May;55:584–585. [PubMed] [Google Scholar]
- Pierpoint W. S. The distribution of succinate dehydrogenase and malate dehydrogenase among components of tobacco-leaf extracts. Biochem J. 1963 Jul;88(1):120–125. doi: 10.1042/bj0880120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. I. Properties of the enzyme purified from extracts of acetone-dried powders. Biochim Biophys Acta. 1961 Nov 25;54:67–76. doi: 10.1016/0006-3002(61)90938-6. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B., MASSEY V. Newer knowledge of succinic dehydrogenase. Adv Enzymol Relat Subj Biochem. 1957;18:65–111. doi: 10.1002/9780470122631.ch2. [DOI] [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A. Dehydrogenase Activity of Hydroxymalonate and Related Acids in Higher Plants. Plant Physiol. 1956 Mar;31(2):135–141. doi: 10.1104/pp.31.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. J. Properties of mitochondrial malate dehydrogenases. Biochim Biophys Acta. 1962 Jun 4;59:624–633. doi: 10.1016/0006-3002(62)90642-x. [DOI] [PubMed] [Google Scholar]
- THORN M. B. Inhibition by malonate of succinic dehydrogenase in heart-muscle preparations. Biochem J. 1953 Jul;54(4):540–547. doi: 10.1042/bj0540540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNON L. P., MAHLER H. R., SARKAR N. K. Studies on diphosphopyridine nucleotide-cytochrome c reductase. III. Kinetic studies. J Biol Chem. 1952 Dec;199(2):599–606. [PubMed] [Google Scholar]
- Verleur J. D. Studies on the Isolation of Mitochondria from Potato Tuber Tissue. Plant Physiol. 1965 Nov;40(6):1003–1007. doi: 10.1104/pp.40.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleur J. D., Uritani I. Respiratory Activity of the Mitochondrial Fractions Isolated from Healthy Potato Tubers and from Tuber Tissue Incubated after Cutting or Infection with Ceratocystis fimbriata. Plant Physiol. 1965 Nov;40(6):1008–1012. doi: 10.1104/pp.40.6.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K. Kinetics of Malic Dehydrogenase Inhibition by 2,4-Dichlorophenoxyacetic Acid. Plant Physiol. 1963 Mar;38(2):157–164. doi: 10.1104/pp.38.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Young R. E., Biale J. B. Metabolic Processes in Cytoplasmic Particles of the Avocado Fruit. VI. Controlled Oxidations and Coupled Phosphorylations. Plant Physiol. 1964 May;39(3):312–322. doi: 10.1104/pp.39.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]


