Abstract
An acetaldehyde dehydrogenase from germinating peanut cotyledons has been purified and its properties have been studied. At the highest purification achieved the preparation is free of alcohol dehydrogenase activity.
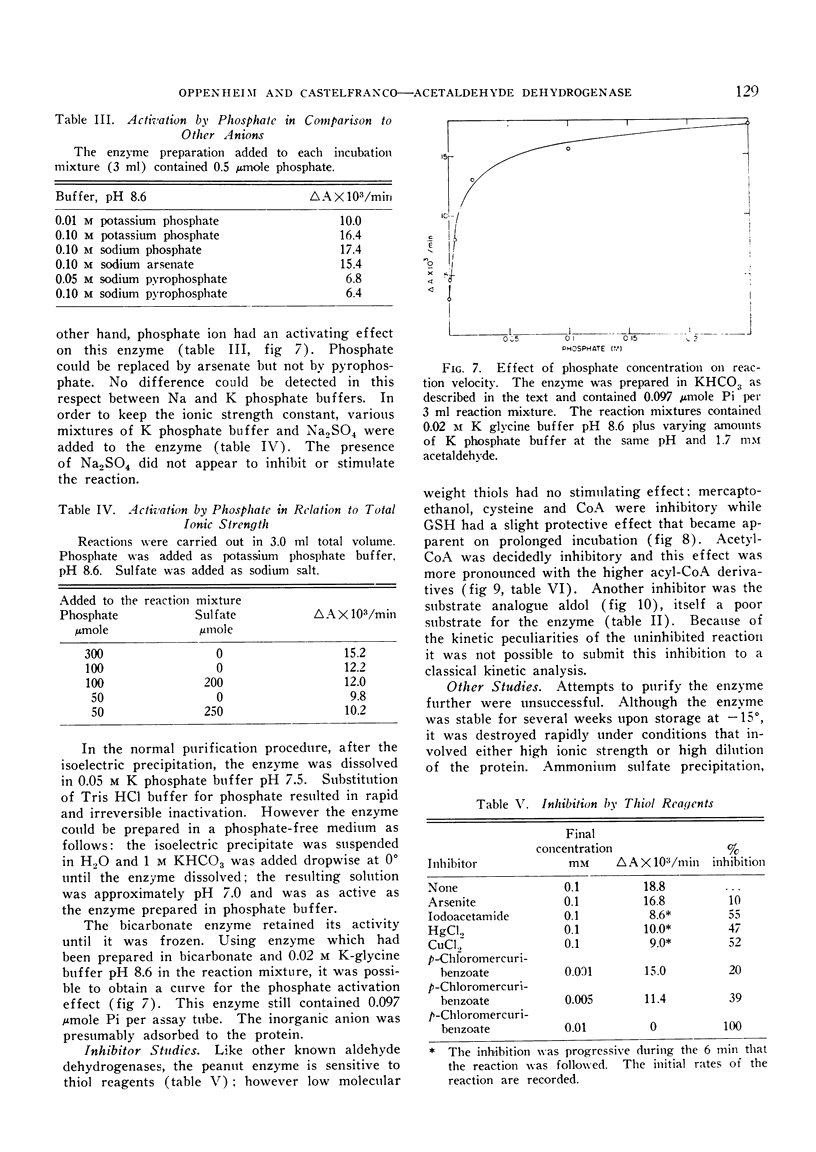
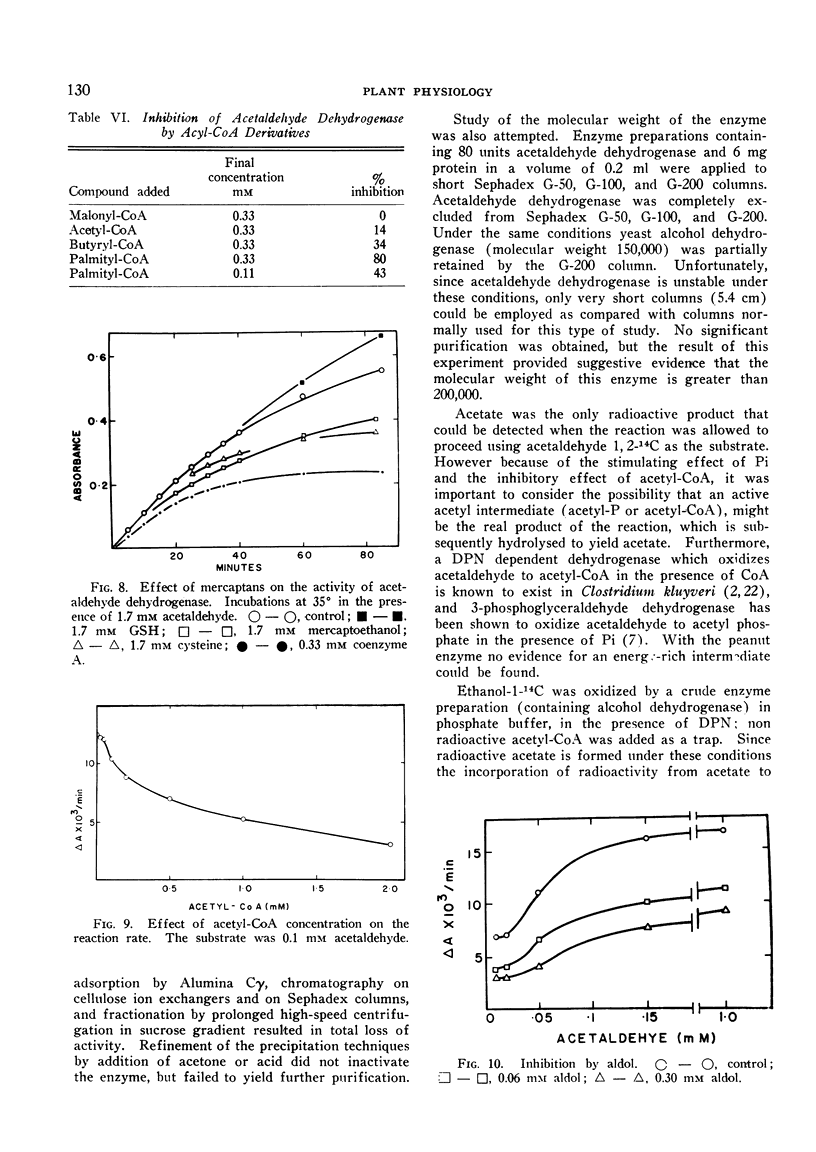
The enzyme is specific toward diphosphopyridine nucleotide, and can oxidize a variety of aldehydes. The highest reaction rate is obtained with acetaldehyde, which is oxidized to acetate. All the attempts to demonstrate the formation of an energy-rich acetyl derivative during the course of the reaction failed. The enzyme is inhibited by aldol; it is sensitive toward sulfhydryl reagents, including arsenite. Reduced glutathione stabilizes the enzyme, while cysteine, mercaptoethanol, and coenzyme A are inhibitory.
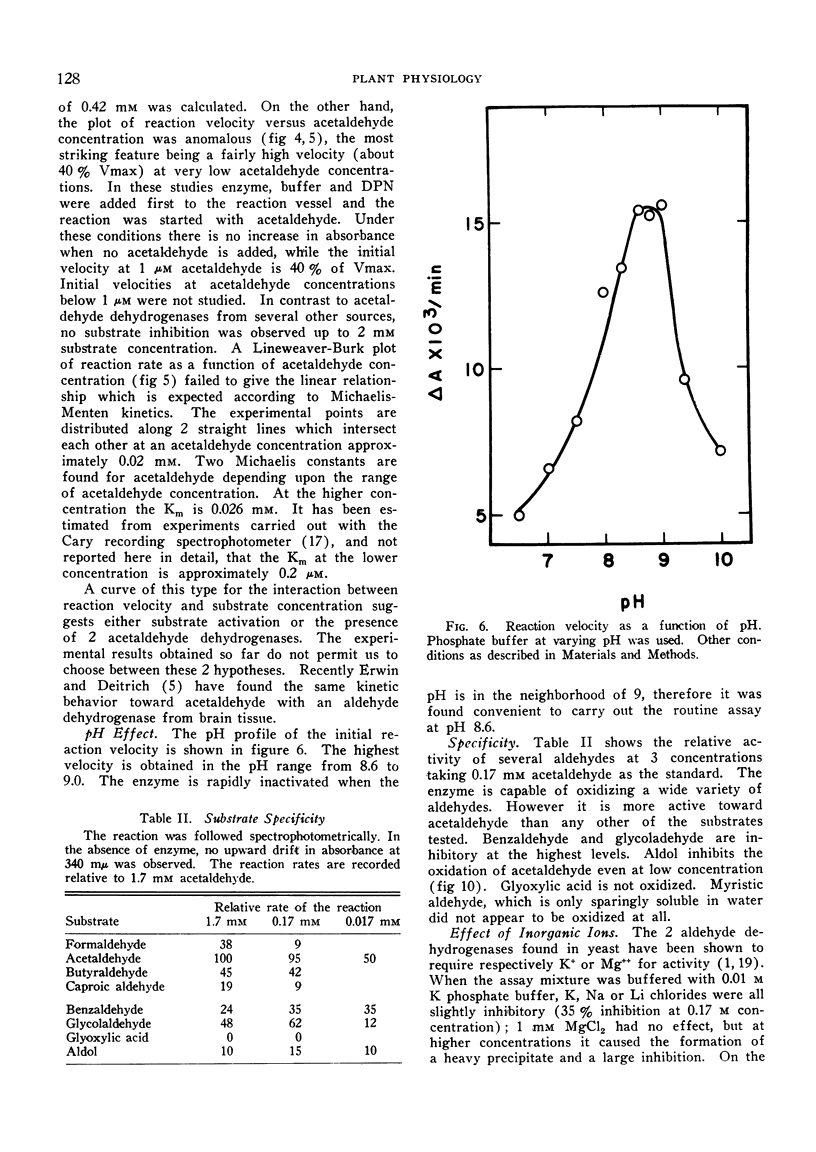
Acetaldehyde dehydrogenase is activated by phosphate and inhibited by fatty acyl-CoA derivatives. It appears to be activated by the substrate, as was deduced from the shape of the plot of reaction velocity against acetaldehyde. These properties suggest that the enzyme is an allosteric protein.
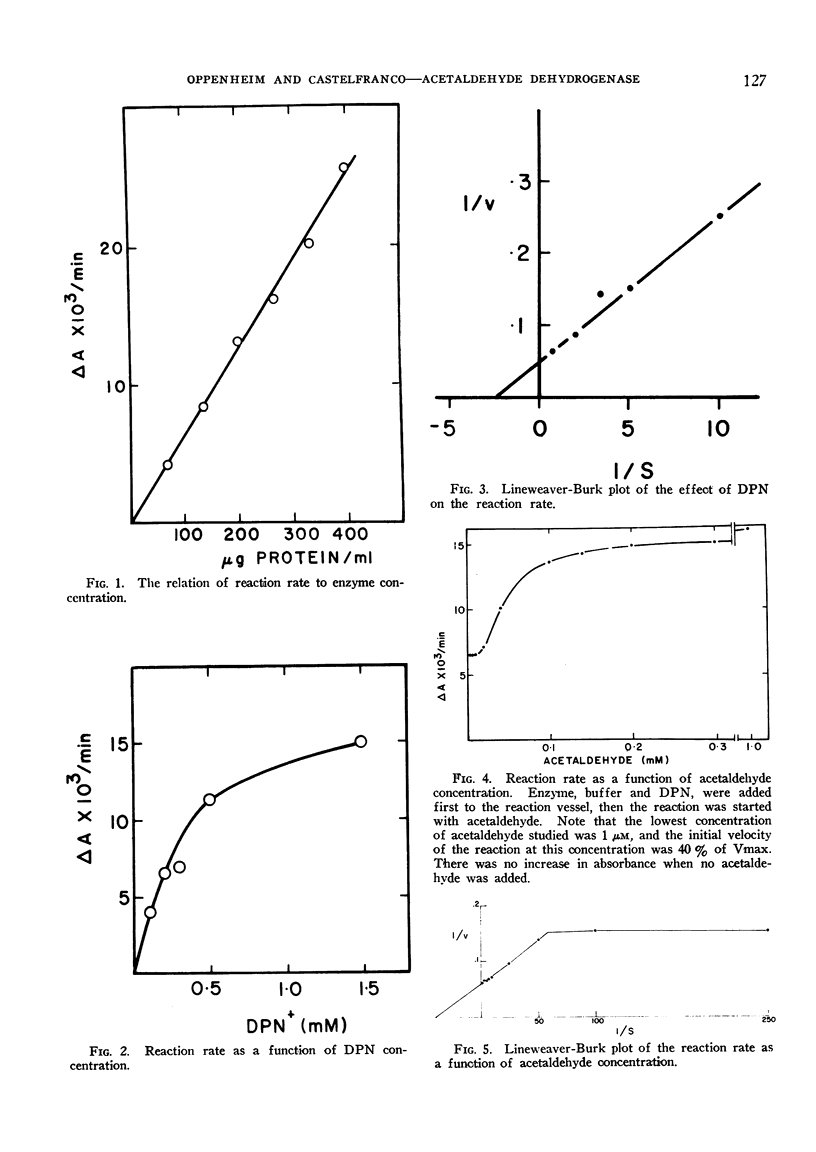
The plot of reaction velocity against substrate concentration is anomalous. The shape of this plot seems to reflect the presence of 2 different enzymatic activities, one with extremely high apparent affinity for acetaldehyde. The 2 activities may reflect 2 conformational states of a single enzyme or 2 separate enzymes.
Experiments with tissue slices indicate that the reaction catalyzed by this enzyme is a step in the oxidation of ethanol to acetyl-CoA. This enzyme may also participate in the oxidation of pyruvate to acetyl-CoA in certain tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. Yeast aldehyde dehydrogenase. Arch Biochem Biophys. 1951 Nov;34(1):86–97. doi: 10.1016/s0003-9861(51)80013-4. [DOI] [PubMed] [Google Scholar]
- BURTON R. M., STADTMAN E. R. The oxidation of acetaldehyde to acetyl coenzyme A. J Biol Chem. 1953 Jun;202(2):873–890. [PubMed] [Google Scholar]
- JAKOBY W. B. Aldehyde oxidation. I. Dehydrogenase from Pseudomonas fluorescens. J Biol Chem. 1958 May;232(1):75–87. [PubMed] [Google Scholar]
- SEEGMILLER J. E. Triphosphopyridine nucleotide-linked aldehyde dehydrogenase from yeast. J Biol Chem. 1953 Apr;201(2):629–637. [PubMed] [Google Scholar]
- Taketa K., Pogell B. M. The effect of palmityl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem. 1966 Feb 10;241(3):720–726. [PubMed] [Google Scholar]


