Abstract
Alzheimer's disease (AD) is acknowledged as the main causative factor of dementia that affects millions of people around the world and is increasing at increasing pace. Okadaic acid (OA) is a toxic compound with ability to inhibit protein phosphatases and to induce tau protein hyperphosphorylation and Alzheimer's-like phenotype. Kolaviron (KV) is a bioflavonoid derived from Garcinia kola seeds with anti-antioxidative and anti-inflammation properties. The main goal of this study was to assess whether kolaviron can exert neuroprotective effect against okadaic acid-induced cognitive deficit. Rats had an intracerebroventricular (ICV) injection of OA and pretreated with KV at 50 or 100 mg/kg and examined for cognition besides histological and biochemical factors. OA group treated with KV at 100 mg/kg had less memory deficit in passive avoidance and novel object discrimination (NOD) tasks besides lower hippocampal levels of caspases 1 and 3, tumor necrosis factor α (TNFα) and interleukin 6 (IL-6) as inflammatory factors, reactive oxygen species (ROS), protein carbonyl, malondialdehyde (MDA), and phosphorylated tau (p-tau) and higher level of acetylcholinesterase (AChE) activity, mitochondrial integrity index, superoxide dismutase (SOD), and glutathione (GSH). Moreover, KV pretreatment at 100 mg/kg attenuated hippocampal CA1 neuronal loss and glial fibrillary acidic protein (GFAP) reactivity as a factor of astrogliosis. In summary, KV was able to attenuate cognitive fall subsequent to ICV OA which is partly mediated through its neuroprotective potential linked to mitigation of tau hyperphosphorylation, apoptosis, pyroptosis, neuroinflammation, and oxidative stress and also improvement of mitochondrial health.
Keywords: Kolaviron, Alzheimer's disease, Cognition, Apoptosis, Inflammation, Pyroptosis, Oxidative stress
List of abbreviations
- AChE
Acetylcholinesterase
- aCSF
Artificial cerebrospinal fluid
- AD
Alzheimer's disease
- Aβ
Amyloid β
- BACE1
beta amyloid precursor protein cleaving enzyme 1
- Cat
Catalase
- DPPH
1,1-Diphenyl-2-picrylhydrazyl
- GFAP
Glial fibrillary acidic protein
- GP
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione
- ICV
Intracerebroventricular
- IL-6
Interleukin 6
- KV
Kolaviron
- MDA
Malondialdehyde
- MMP
Mitochondrial membrane potential
- MPTP
1-methyl-4-phenylpyridinium
- NOD
Novel object discrimination
- OA
Okadaic acid
- PC
Protein carbonyl
- P-tau
Phosphorylated tau
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- STZ
Streptozotocin
- TAA
Total antioxidant activity
- TFC
Total flavonoid content
- TNF-α
Tumor necrosis factor-α
1. Introduction
Alzheimer disease (AD) is acknowledged as the most common causative factor of dementia in the elderly [1]. Brain samples of Alzheimer's patients show higher levels of extracellular amyloid beta (Aβ) deposition as well as heightened intracellular tau neurofibrillary tangles (NFTs) construction [2]. Number of patients with AD and related cognitive problems will be notably higher in the next decades [3]. Moreover, AD patients suffer from memory impairment, non-memory cognitive deficits such as aphasia, executive dysfunction, and apathy, language disturbance, depressive symptoms, and so on [4]. No novel drugs with neuroprotective potential besides its symptomatic relief have been approved for AD [5]. Existing drugs confer little benefit in symptom management and are not practically capable to prevent neuronal death, brain atrophy, and gradual decline of cognition [6]. Although Aβ fragments and streptozotocin (STZ) have long been used to induce murine model of AD, neurotoxicants including okadaic acid (OA) are also used to induce a consistent model of Alzheimer's-like phenotype [7]. The mechanisms for OA neurotoxicity are partly through its inhibition of serine/threonine phosphatases and concomitant tau hyperphosphorylation [8]. NFTs, lower activity of protein phosphatases, and enhanced hyperphosphorylated tau are reported as the chief pathological markers in AD brain which is also observed following OA challenge [[9], [10], [11]]. In addition, intracerebroventricular (ICV) microinjection of OA triggers oxidative stress, neuroinflammation, and apoptosis [12], which is likewise seen in AD [11].
Bioactive compounds in medicinal plants especially their polyphenols have shown potential and promising effects to combat against AD pathophysiological processes [[13], [14], [15]]. Garcinia kola (Garcinia genus, Clusiaceae family, Malpighiales order), commonly known as the bitter kola, is a dioecious, multifunctional, and agroforestry tree which is found in some tropical African and even in Asian countries [16,17]. This amazing and medicinal plant has a multitude of therapeutic applications. The therapeutic power of this plant is mainly attributed to its seed bioflavonoid kolaviron (KV) with neuroprotective, cardioprotective and hepatoprotective activity [18]. KV with anti-oxidant property, anti-inflammation effects, and anti-apoptotic potential has shown beneficial effects in neurodegenerative conditions [19,20]. KV can destabilize Aβ fibrils and act as a potential anti-amyloidogenic factor [21], it has neuroprotective effect against sodium azide or aluminium chloride neurotoxicity [22], and can also exert nootropic property due to its targeting of antioxidant and cholinergic systems in scopolamine-induced model of memory disturbance [23]. Moreover, this plant is able to protect against cognitive decline induced by lipopolysaccharide in rats by mitigation of oxidative stress and neuroinflammation [24]. Nevertheless, there is still no established evidence on the positive effect of KV in some models of AD. Accordingly, this research was designed and performed to evaluate the potential effect of KV on cognitive deficits in OA-provoked AD like phenotype.
2. Materials and methods
2.1. Plant material
Garcinia kola seeds were obtained from local market of Nsukka, Enugu State, Nigeria and was systematically authenticated by Centre for Ethnomedicine and Drug Development (voucher specimen # 17–022010).
2.1.1. Kolaviron preparation
Garcinia kola seeds were peeled, cut, and dried under shade at 25 °C. Kolaviron was separated as mentioned in a former study [25]. Its seeds (100 g) were ground and defatted with 150 ml of petroleum ether and 24 h-incubation in a soxhlet device. It was dried at 25 °C, extracted in acetone (120 ml, 40 °C), concentrated, and re-extracted with ethyl acetate. Major components in the obtained kolaviron include Garcinia Biflavonoid 1, kolaflavanone, and Garcinia Biflavonoid 2 [26].
2.1.2. Kolaviron analytical tests
2.1.2.1. Total antioxidant activity (TAA) measurement
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was measured [27]. For calibration standard curve, different 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) concentrations were used. For this assay (in triplicate), 2 μL of sample were reacted with 250 μl of DPPH reagent. After shaking and incubation in dark for 0.50 h, reading of absorption at 515 nm and data reporting as Trolox equivalent/g of KV (Table 1) were done.
Table 1.
Total phenols, flavonoids, and flavonols in kolaviron.
| Total antioxidant activity (equivalent to Trolox/g) | 2.17 ± 1.5 |
| Total phenol (mg equivalent of gallic acid/gram) | 19.2 ± 1.7 |
| Total flavonoid (mg equivalent of quercetin/gram) | 18.7 ± 1.8 |
| Total flavonol (mg equivalent of rutin/gram) | 16.3 ± 1.4 |
2.1.2.2. Assessment of total flavonoid content (TFC)
Protocol of an earlier study was applied for this test [28]. For this assay (in triplicate), an aliquot of 0.1 g of KV was reacted with 1.5 ml of 95 % alcohol, 0.1 ml of 10 % aluminum chloride hexahydrate, and 0.1 ml of potassium acetate (1 M). After incubation for 40 min, the absorbance was measured at 415 nm. Quercetin was used as the standard and data were shown as mg of quercetin equivalents/g of KV (Table 1).
2.1.2.3. Assessment of total phenolic content
For this test, Folin-Ciocalteu phenol reagent was used [28]. In this test, 0.05 g of KV was reacted with 1.4 ml of deionized water, 1 ml of sodium carbonate (2 %), and 0.05 ml of Folin-Ciocalteau reagent (50 %) in triplicate. After 30 min, the absorbance was taken at 750 nm and with gallic acid as the standard and data were shown as milligram of gallic acid equivalent/g of KV (Table 1).
2.1.2.4. Determination of total flavonols
In this test [28], 1 mL of KV was mixed with 1 ml of aluminium trichloride solution (1 g/50 ml) in 95 % ethanol and 3 ml of sodium acetate solution (2.50 g/50 ml) and after 2.5 h, the absorbance was read at 440 nm and with rutin as the standard and data were shown as milligram of rutin equivalent/g of KV (Table 1). This test was done in triplicate.
2.2. Animals
In this project, forty rats (Rattus norvegicus var. albinos, male gender, 12–13 weeks old, Wistar strain, 220–250 g) were used. Working with animals was in accordance to the ethical guidelines and approval of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.538). Animal house conditions included 12-h lighting photoperiod, a humidity of 45–50 %, and free access to water and food pellet.
2.3. Stereotaxic surgery
Rats were intensely anesthetized with an i.p. injection of an admixture of ketamine and xylazine (80 and 10 mg/kg), positioned in the stereotaxic frame, the scalp was disinfected, incised, and a burr hole was made and OA (Santa Cruz Biotechnology Inc., USA) dissolved in aCSF at a volume of 5 μl was delivered (into the right ventricle) at coordinates 0.2 mm behind Bregma, 1.2 mm in relation to midline, and 3.2 mm below the dura and in conformity with the stereotaxic atlas [29].
2.4. Experimental design
Animals were studied in 5 groups including sham, sham treated with KV 100 mg/kg, OA, and OA groups receiving KV 50 or 100 mg/kg. KV was daily administered intraperitoneally started one week before till one week after the stereotaxic surgery. Doses of kolaviron in this study were obtained from previous studies on its anti-inflammatory and redox regulatory effects in carbon nanotubes-induced model of neurobehavioral deficits and neurotoxicity (Adedara et al., 2020) and its protective effect against ischemia/reperfusion injury [30]. In a previous study by Kalu et al., in 2016, LD50 for kolaviron was determined as 3050 mg/kg [31]. On this basis, selected doses for kolaviron in this study were far lower than its LD50 dose and seem safe for therapeutic purposes. Dose of OA (200 ng/rat) was in accordance to an earlier report on its neurotoxicity in the rat [32]. At 4th week post-surgery, animals were evaluated in behavioral tasks. Experimental scheme is shown in Fig. 1.
Fig. 1.
Experimental design of the study. KV (50 and 100 mg/kg) was injected for 2 weeks. To induce memory impairment, OA was I.C.V. microinjected at 200 ng/kg. Behavioral assessment was done at 4th week and finally biochemical and histochemical assessments were conducted.
2.5. Behavioral assessments
2.5.1. Y maze
Y maze was conducted as mentioned beforehand [33]. This test was based on the curious instinct of the animal and does not use stimuli. At the beginning of the experiment, each rat was placed in the device and observed for 8 min. To evaluate the animal's behavior, the arms to which the animal was inserted were categorized into triple sequences and the categories containing the duplicate arms were ignored. In addition, total number of explored arms was determined and alternation was calculated from the following formula: (number of arms with actual alternation/(total number of arms-2)) × 100.
2.5.2. Novel object recognition
The device to perform this test was an open field chamber in a quiet and noise-free environment with a dim light [33]. Each animal had two 5-min search sessions with a 4-h interval. During the familiarization, each rat was exposed to two identical objects, and in the second experiment, one of the objects was swapped with a new (novel) object. Exploration of objects such as smelling, licking, chewing, or nasal contact was assessed and ratio of discrimination was obtained from the next formula: (t [new] - t [familiar])/(t [new] + t [familiar]) * 100.
2.5.3. Passive avoidance test (shuttle box)
The device had light and dark chambers, an interconnecting door, and a stimulator for shock application [34]. The test was done in three phases including acclimation, acquisition, and retention and recall. For acquisition, each rat was positioned in the light arena and after 5 min, lamp was switched on and door was raised and the animal was allowed to enter the dark chamber. After closing the door, an electric chock (intensity = 1 mA, duration = 1 s) was given. During retention and recall, the same protocol was repeated with no electric chock and time to enter dark chamber was taken as step through latency (STL; cut-off = 300 s).
2.6. Measurement of oxidative stress factors
2.6.1. Homogenate and supernatant preparation
At the end of study, rats were intensely anesthetized with ketamine (130 mg/kg), perfused with normal saline, brains were immediately removed, and they were divided into two halves, right side in 10 % formalin solution for histochemical experiments (n = 6/group) and left side under frozen condition at −70 °C for biochemical assessments (n = 7/group). Specimens were homogenized in Tris buffer (150 mM, pH 7.4, 5 % w/v) and after centrifuging at 7000 rpm for 10 min (4 °C), supernatants were separated and kept at −70 °C.
2.6.2. Protein measurement
Protein measurement was done by the Bradford method in the presence of Coomassie Brilliant Blue G-250 dye [35]. For this measurement, 20 μL of sample were reacted with 250 μl of reagent, incubated for 5 min, its absorbance was taken at 595 nm, and bovine serum albumin was used as the standard.
2.6.3. Reactive oxygen species (ROS) assay
ROS quantification in the hippocampal lysate was done using 2, 7- dichlorofluorescein diacetate (DCF-DA), which is converted to fluorescent DCF (dichlorofluorescein) by cellular peroxides. In this measurement, one hundred microliters of supernatant was reacted with 10 μL of DCF-DA at 37 °C for 30 min. Fluorescent signals were detected using a fluorometer at an excitation of 488 nm and at an emission of 525 nm [36].
2.6.4. Malondialdehyde (MDA) assay
Quantity of MDA as a factor of lipid peroxidation was measured in the presence of 0.5 % thiobarbiturate and 20 % trichloroacetic acid in a thermoblock (95ᵒC) for 30 min. After cooling and centrifuging (1500×g) at 25ᵒC for 5 min, absorption was obtained at 535 nm and with tetraethoxypropane as the standard [37].
2.6.5. Nitrite assay
Lysate nitrite content was measured using Griess reagent containing phosphoric acid (2.5 %), sulfanilamide (1 %) and naphthylethylenediamine dihydrochloride (0.1 %) and after incubation for 10 min, absorption was obtained at 540 nm [38].
2.6.6. Protein carbonyl estimation
Protein carbonyl assay was based on the reactivity of carbonyl compounds and 2, 4 dinitrophenyl hydrazine. One hundred microliters of supernatant were added to 200 μL of 2,4-dinitrophenylhydrazine (10 mM) in HCl (2.5 N) for 60 min. Samples were then precipitated with 300 μl of trichloroacetic acid (20 %), centrifuged for 5 min at 9000 g, the pellet was rinsed with 0.5 ml of ethanol and ethyl acetate, dissolved in 225 μl of guanidine (6 M, 37 °C) for 5 min and its absorbance was obtained at 365 nm [39].
2.6.7. GSH measurement
For GSH assessment, lysate was incubated with 20 % trichloroacetic acid (TCA) and 1 mM EDTA for 5 min and after centrifuging at 10000×g for 30 min (4 °C), obtained product was reacted with 0.1 mM 5.5′-dithiobis 2-nitro-benzoic acid and its absorbance was obtained at 412 nm [40].
2.6.8. Determination of superoxide dismutase (SOD) activity
Activity of this enzyme was analyzed in the presence of nitroblue tetrazolium (NBT) and xanthine with absorbance taken at λ = 560 nm [41]. Tissue lysate (100 μl) was reacted with 60 μl chloroform and 100 μl ethanol. The mixture was centrifuged (15000×g, 30 min), 50 μL of supernatant was separated and reacted with 0.9 ml of the reagent (50 mg of bovine serum albumin, 0.1 mmol/l xanthine, 0.1 mmol/L EDTA, 25 mmol/l nitroblue tetrazolium and 40 mmol/l sodium carbonate; pH 10.2). Xanthine oxidase was then added and kept for 20 min at ambient temperature. The reaction was finished by cupric chloride (0.8 mmol/l) and its absorbance was determined at 560 nm.
2.6.9. Determination of catalase (CAT) activity
Enzyme activity was assessed in the presence of hydrogen peroxide, potassium hydroxide, and potassium periodate [42]. For this experiment, 20 μL of supernatant were reacted with 100 μl of buffer and 20 μL of diluted substrate for 20 min. The reaction was terminated by 30 μL of diluted potassium hydroxide. Then, 10 μL of potassium periodate were included and after 5 min, absorbance was taken at 540 nm.
2.6.10. Glutathione peroxidase (GPx) assay
For this measurement [43], changes of absorption in the presence of 50 μl of supernatant and 250 μl of phosphate buffer solution (50 mM, pH 7.4) containing NaN₃ (1.25 mM), ethylenediaminetetraacetic acid (EDTA, 4 mM), H2O2 (0.25 mM), GSH (1 mM), and nicotinamide adenine dinucleotide phosphate cofactor (NADPH, 0.16 mM) were read at 365 nm.
2.6.11. Glutathione reductase assay
For assaying glutathione reductase (GR) activity, 50 μl of supernatant and 450 μl of potassium phosphate buffer solution (0.1 M, pH 7.6), NADPH cofactor (0.1 mM), EDTA (0.5 mM), and glutathione disulfide (1 mM) were mixed and absorption was taken at 340 nm [44].
2.7. Measurement of TNFα, IL-6, and phosphorylated tau
Sandwich ELISA kits (Karmania Pars Gene, Kerman, Iran) were used to quantify TNF-α and IL-6 in samples and reading of absorbance at 450 nm. To quantify phosphorylated tau (p-tau), rabbit anti-phospho-tau (ser 396) antibody and goat anti-rabbit peroxidase IgG were used in accordance to sandwich ELISA protocol from Abcam company, USA.
2.8. Evaluation of apoptotic and pyroptotic factors
The activity of caspase 3 was measured as mentioned before [45]. For caspase 3, 30 μl of lysate was incubated at 37 °C with 120 μL of assaying buffer (50 mM Hepes, 0.2 % 3-([3-cholamidopropyl]-dimethyllammonio)-1-propanesulfonate (CHAPS), 20 % sucrose, EDTA (2 mM), dithiothreitol (10 mM), and 50 μM p-nitroaniline; pH 7.4). After 3 h, absorbance was determined at 405 nm. For caspase 1, Abcam specific kit (USA) was used. In the latter assay, 25 μL of supernatant were reacted with 110 μl of reagent consisting of 10 mM DTT and 0.2 mM YVAD-p-NA substrate for 90 min at 37 °C and reading of absorbance was done at 405 nm.
2.9. Evaluation of acetylcholinesterase (AChE) activity
This assay was conducted as mentioned before [46]. In this test, 20 μl of supernatant was mixed with 130 μL of phosphate buffer solution (0.1 M, pH 8.0) and then 5 μL of DTNB were included. After 2 min, the absorbance was reset to zero. For the substrate, 20 μL of acetylthiocholine-iodide (0.075 M) were included. Absorbance change at 412 nm was taken for 10 min and the change in absorbance per min was obtained.
2.10. Evaluation of BACE-1 activity
This experiment was done as reported before [45,47]. Reaction solution had 50 μL of lysate, 50 μL of sodium acetate buffer solution (50 mM, pH 4.5) and 100 μL of DL-BAPNA (6 mM) at 37 °C. Then, absorption difference was obtained for 65 min at 405 nm and it was reported as the rate of hydrolysis of DL-BAPNA/h (ΔA/h).
2.11. Evaluation of MMP
MMP as a diagnostic factor related to mitochondrial integrity and wellbeing was measured through Rhodamine 123. This substance is a cationic fluorescent dye that accumulates in the mitochondria after entering cells. In this assay, samples were centrifuged at 10000 rpm and then 20 μl of Rhodamine 123 (0.5 mg/5 ml DMSO, SigmaAldrich, USA) and 180 μL of PBS with pH at 7.4 was added to the sediment and after incubation for 30 min (37 °C), emission at 525 nm was determined following excitation at 488 nm [48,49].
2.12. Histological examination
Rotary-microtome cut five-micron hippocampal sections were deparaffinized and stained alternatively for Nissl staining (0.1 % Cresyl violet). Other sections were reacted for glial fibrillary acidic protein (GFAP) immunohistochemistry after incubation in primary mouse antibody against GFAP (monoclonal, 1:75, Santa Cruz Biotechnology, USA) for 12 h at 4 °C, reaction with secondary antibody (HRP-conjugated, Santa Cruz Biotechnology, USA) at room temperature for 4 h and development with 3,3′-diaminobenzidine-tetrahydrochloride (DAB) chromogen in the company of H2O2 and further staining with Hematoxylin for 20 s. Determination of Nissl-stained CA1 pyramidal neuronal number and intensity of GFAP immunoreactivity (IRA) at stereotaxic levels of 3.2–3.6 mm behind the bregma reference point was made in ImageJ (Version 1.53, National Institutes of Health, USA) in a blinded manner on coded slides and findings were reported as number of neurons or as GFAP immunoreactive loci per mm2.
2.13. Statistical judgement
Analyzed data in GraphPad Prism 9.3 (GraphPad Software Inc., USA) were shown as mean ± SEM. Sample size in this study was obtained from pertinent studies in this field [50,51] and using sample and power function in Minitab, version 19.2. After ascertaining normal distribution in Shapiro-Wilk test and testing for outliers in data sets using Grubbs' statistical analysis, we further assessed data using analysis of variance (ANOVA) and Tukey's test and with statistical significance at p < 0.05.
3. Results
Establishment of OA-induced Alzheimer's-like phenotype in this study was verified in our different behavioral tasks as follows.
3.1. The effect of kolaviron on behavioral performance
Fig. 2A shows data of recognitive spatial and short-term memory in the Y maze. Performing one-way ANOVA displayed a significant difference (F [4,35] = 4.95, p < 0.01). Additional assessment with Tukey's multiple range test showed that ICV microinjection of OA significantly reduces alternation score in relation to the sham group (p < 0.01). However, KV administration to OA-injured groups at none of the used doses, i.e., 50 or 100 mg/kg, was successful to significantly improve alternation.
Fig. 2.
Findings for performance in Y-maze (A), novel object recognition (B), and shuttle box (C) tests. * for p < 0.05, ** for p < 0.01 and *** for p < 0.001 compared to the sham group. # for p < 0.05 and ## for p < 0.01 versus OA group.
Performing one-way ANOVA in novel object recognition task (Fig. 2B) showed a significant and marked difference (F [4,35] = 5.91, p < 0.001). Additional Tukey's test showed that discrimination index is notably lower in OA-challenged group in relation to the sham group (p < 0.01) and such significant decrease to a lesser degree was similarly observed for KV-treated OA group at 50 mg/kg (p < 0.05). Contrariwise, OA-injured group receiving KV at 100 mg/kg had a higher discrimination score versus OA group (p < 0.05).
Data analysis with one-way ANOVA for passive avoidance test (Fig. 2C) displayed a significant difference (F [4,35] = 7.54, p < 0.001). Further analysis with Tukey's test indicated that OA-injured group has a significantly lower latency in relation to sham group (p < 0.001) and this reduction was similarly obtained for KV50 + OA group (p < 0.01). In contrast, latency to enter dark chamber was significantly greater in KV100 + OA group versus ICV OA injured group (p < 0.01).
3.2. Kolaviron effect on oxidative stress factors
Analysis of oxidative stress factors including MDA (F [4,30] = 10.49, p < 0.001), ROS (F [4,30] = 5.38, p < 0.01), nitrite (F [4,30] = 4.72, p < 0.01), protein carbonyl (F [4,30] = 12.87, p < 0.001), GSH (F [4,30] = 4.36, p < 0.01), catalase (F [4,30] = 9.23, p < 0.001), SOD (F [4,30] = 5.69, p < 0.01), glutathione reductase (GR) (F [4,30] = 3.34, p < 0.05) and glutathione peroxidase (GPx) (F [4,30] = 4.02, p < 0.01) (Table 2) showed that intracerebroventricular injection of OA is associated with significant elevation of protein carbonyl (p < 0.001), MDA (p < 0.001), ROS (p < 0.001), and nitrite (p < 0.05) and lower levels or activities of GR (p < 0.05), GPx (p < 0.05), GSH (p < 0.01), catalase (p < 0.001), and SOD (p < 0.01) in relation to the sham. Conversely, treatment of ICV OA group with kolaviron at 100 mg/kg significantly reduced protein carbonyl (p < 0.05), ROS (p < 0.05), and MDA (p < 0.01) and significantly enhanced GSH (p < 0.05) and SOD (p < 0.05) and with no significant change of GR, GPx, nitrite, and catalase. Furthermore, kolaviron treatment at 50 mg/kg did not produce such significant and reversing effects.
Table 2.
Hippocampal levels of apoptosis-, oxidative stress-, and neuroinflammation-associated factors.
| Groups | Sham | Sham+ KV100 mg/kg | OA | OA+ KV50 mg/kg | OA+ KV100 mg/kg |
|---|---|---|---|---|---|
| Oxidative stress and antioxidants (n=7/group) | |||||
| MDA (nmol/mg) | 1.03 ± 0.09 | 1.15 ± 0.14 | 2.18 ± 0.17*** | 1.73 ± 0.18* | 1.29 ± 0.15## |
| Estimated ROS (RFU) | 5.17 ± 0.49 | 4.95 ± 0.53 | 8.91 ± 0.85** | 6.92 ± 0.81 | 5.79 ± 0.75# |
| Nitrite (μg/mg) | 5.91 ± 0.67 | 5.73 ± 0.75 | 9.82 ± 0.91* | 8.32 ± 0.83 | 7.03 ± 0.78 |
| Protein carbonyl (μmol/g) | 7.85 ± 1.12 | 8.27 ± 1.34 | 20.15 ± 1.62*** | 15.91 ± 1.59** | 13.82 ± 1.54*# |
| GSH (nmol/mg) | 6.71 ± 0.49 | 6.03 ± 0.51 | 3.86 ± 0.55** | 5.35 ± 0.52 | 6.07 ± 0.54# |
| Catalase (unit/mg) | 4.91 ± 0.33 | 4.51 ± 0.39 | 2.07 ± 0.45*** | 2.51 ± 0.43** | 3.46 ± 0.41 |
| SOD (unit/mg) | 7.51 ± 0.49 | 7.05 ± 0.53 | 4.17 ± 0.59** | 6.09 ± 0.56 | 6.67 ± 0.52# |
| Glutathione reductase (nmol/min/mg) | 73.52 ± 5.68 | 70.21 ± 4.97 | 47.32 ± 5.93* | 57.80 ± 5.82 | 65.81 ± 6.34 |
| Glutathione peroxidase (nmol/min/mg) | 38.95 ± 2.85 | 41.76 ± 3.25 | 25.17 ± 3.55* | 30.93 ± 3.29 | 33.85 ± 3.37 |
| Inflammation (n=7/group) | |||||
| TNFα (pg/mg) | 27.83 ± 2.57 | 26.58 ± 2.91 | 55.14 ± 3.46*** | 41.09 ± 3.55*# | 38.25 ± 3.18## |
| IL-6 (pg/mg) | 25.13 ± 2.38 | 30.38 ± 2.85 | 48.51 ± 3.57*** | 42.35 ± 3.76** | 35.21 ± 3.17# |
| Apoptosis and pyroptosis (n=7/group) | |||||
| Caspase 1 (OD) | 0.31 ± 0.05 | 0.35 ± 0.06 | 0.79 ± 0.11** | 0.57 ± 0.08 | 0.45 ± 0.07# |
| Caspase 3 (OD) | 0.27 ± 0.04 | 0.25 ± 0.06 | 0.67 ± 0.08** | 0.48 ± 0.07 | 0.36 ± 0.07# |
| Phosphorylated tau (n=7/group) | |||||
| P-tau (pg/mg) | 31.95 ± 2.87 | 33.51 ± 3.09 | 65.82 ± 3.89*** | 50.19 ± 3.75**# | 46.37 ± 3.52*## |
* for p < 0.05, ** for p < 0.01, *** for p < 0.001 versus sham group; # for p < 0.05, ## for p < 0.01 versus OA group.
3.3. The effect of kolaviron on inflammation factors
Analysis of inflammation factors (Table 2) by one-way ANOVA displayed a significant difference for TNFα (F [4,30] = 13.46, p < 0.001) and for IL-6 (F [4,30] = 8.47, p < 0.001). Further analysis by Tukey test displayed significant elevation of both TNFα and IL-6 (p < 0.001) in OA group in relation to the sham. Contrariwise, kolaviron given to OA group at 100 mg/kg attenuated both TNFα (p < 0.01) and IL-6 (p < 0.05) in relation to OA-injured group. Again, treatment of OA group with KV at 50 mg/kg reduced only TNF-α level at a significant level (p < 0.05).
3.4. Kolaviron effect on apoptotic and pyroptotic factors
Analysis of data for apoptotic factor caspase 3 (F [4,30] = 7.25, p < 0.001) and pyroptotic factor caspase 1 (F [4,30] = 6.10, p < 0.01) (Table 2) by one-way ANOVA displayed a significant difference. Further Tukey analysis showed that hippocampal levels of caspase 3 and caspase 1 are significantly higher (p < 0.01 for both of them) in OA group in relation to the sham. Additionally, kolaviron given at 100 mg/kg to OA group was associated with significantly lower level of caspase 1 and caspase 3 (p < 0.05) in relation to OA group (Table 2).
3.5. Kolaviron effect on hippocampal level of phosphorylated tau (p-tau)
One-way ANOVA for hippocampal level of p-tau as a diagnostic indicator of tauopathy and AD development (F [4,30] = 16.10, p < 0.001) indicated significant difference. Further Tukey test showed that phosphorylated tau is markedly higher in OA group (p < 0.001) and OA groups pretreated with kolaviron at 50 mg/kg (p < 0.01) and 100 mg/kg (p < 0.05) in relation to sham. In contrast, kolaviron given to OA group at 50 mg/kg (p < 0.05) and 100 mg/kg (p < 0.01) led to lower hippocampal amount of phosphorylated tau in relation to OA-injured group (Table 2).
3.6. The effect of kolaviron on activity of BACE-1 and AChE
Conductance of one-way ANOVA for hippocampal activity of BACE-1 (F [4,30] = 5.42, p < 0.01) (Fig. 3A) and AChE (F [4,30] = 7.29, p < 0.001) (Fig. 3B) displayed a significant difference. Further Tukey analysis displayed that hippocampal activity of BACE-1 and AChE in the OA group is distinctly more (p < 0.01) and less (p < 0.01), respectively, in relation to sham. Contrariwise, KV given at 100 mg/kg to OA group caused non-significant reduction of BACE-1 activity (p < 0.05) and significant improvement of AChE activity (p < 0.05) in relation to OA group.
Fig. 3.
Hippocampal level of BACE 1 (A) and AChE activity (B). These factors were evaluated three weeks after OA injection. * for p < 0.05 and ** for p < 0.01 (versus the sham); # for p < 0.05 (versus the OA group).
3.7. The effect of kolaviron on hippocampal MMP
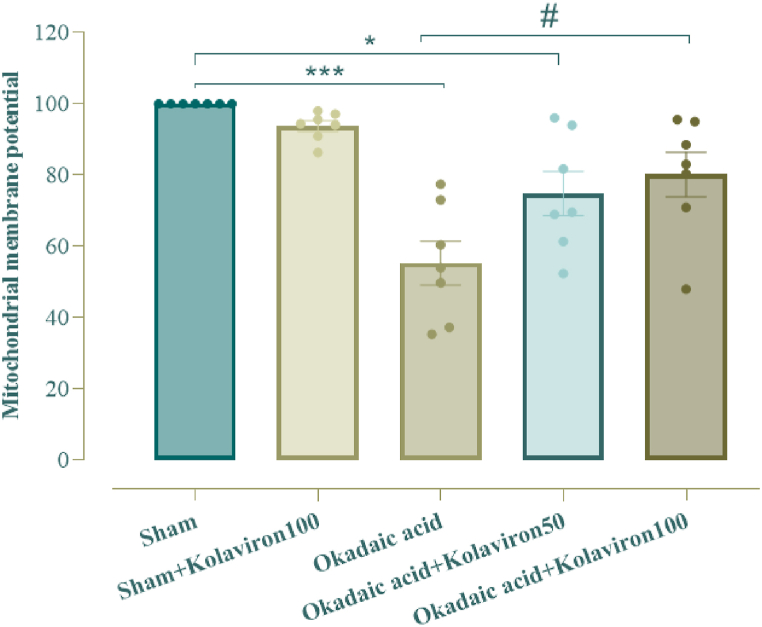
One-way ANOVA test for hippocampal level of MMP as factor of mitochondrial integrity showed a significant inter-group difference (F [4,30] = 12.97, p < 0.001). MMP in OA group was notably lower (p < 0.001) in relation to sham. Conversely, KV administration to OA group at 100 mg/kg significantly prevented such reduction of MMP (p < 0.05). Additionally, KV given at 50 mg/kg to OA group did not produce a significant effect (Fig. 4).
Fig. 4.
Hippocampal level of mitochondrial membrane potential (MMP). * for p < 0.05 and *** for p < 0.001 (relative to the sham), # for p < 0.05 (versus the OA group).
3.8. The effect of kolaviron on hippocampal histochemistry
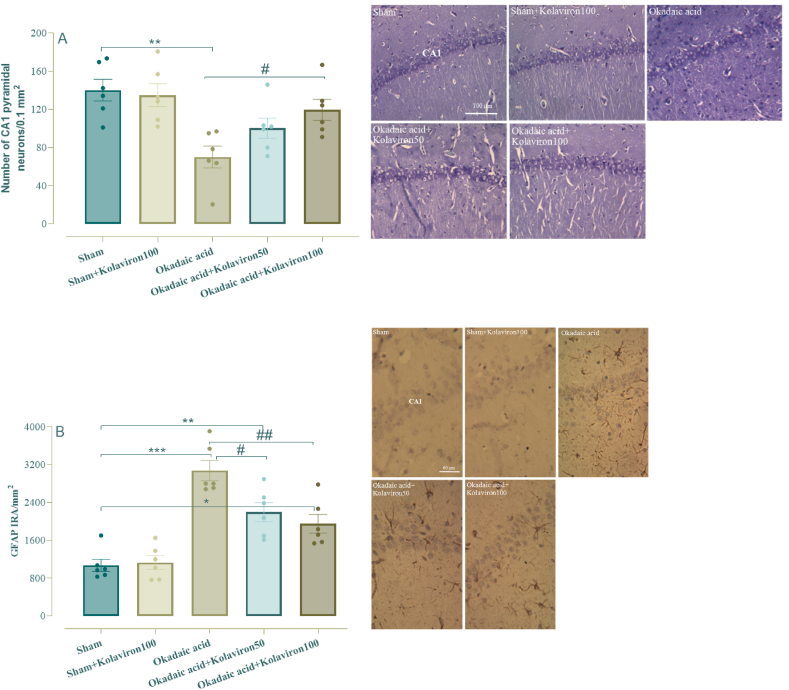
One-way ANOVA analysis showed a significant difference for number of CA1 pyramidal neurons (F [4,25] = 6.34, p < 0.01) and GFAP immunoreactivity (F [4,25] = 21.2, p < 0.001). Further Tukey analysis showed that ICV microinjection of OA is associated with significantly lower density of CA1 neurons in Nissl staining (Fig. 5A) (p < 0.01) and higher GFAP immunoreactivity as a special indicator of astrocytes (Fig. 5B) (p < 0.001). In contrast, OA group under treatment with kolaviron at 100 mg/kg, but not administered at 50 mg/kg, had a significantly higher density of pyramidal neurons (p < 0.05) and lower GFAP reactivity (p < 0.01), clearly suggestive of neuroprotective potential of kolaviron and also its ability to attenuate astrogliosis.
Fig. 5.
Density of CA1 pyramidal neurons (A) and immunoreactivity for glial fibrillar acidic protein (GFAP). * for p < 0.05, ** for p < 0.01 and *** for p < 0.001 versus the sham group; # for p < 0.05 and ## for p < 0.01 versus the OA group.
4. Discussion
The main goal of this research was to show potential protective effect of kolaviron in ICV OA murine model of Alzheimer's like phenotype regarding memory function, apoptosis, oxidative stress, neuroinflammation, and neuronal loss. ICV microinjection of OA was associated with impairment of memory functions, as demonstrated by inferior alternation in Y maze, disturbed discrimination score in novel object discrimination (NOD) test, and recall deficit in passive avoidance task which was in agreement with past reports [12,52]. Contrarywise, kolaviron administration at 100 mg/kg improved discrimination and raised latency in OA-injected rats, clearly indicating its improvement of information consolidation and retrieval besides its restoration of recognition. These results for kolaviron are corroborating past reports [23,53,54].
Loss and down-regulation of brain cholinergic activity as an indicator of degeneration of cholinergic neuronal system has been reported in AD phenotype [50,55,56]. Since we observed lower activity of AChE besides neuronal loss in the hippocampus in the OA group, this clearly justify cholinergic impairment in our study. However, as a limitation, it had been better to determine also AChE activity and expression status of cholinergic receptors within cerebral cortex. This important issue certainly requires further investigation. KV administered at 100 mg/kg to ICV OA-injected group caused significant improvement of hippocampal AChE which may be attributed to its neuroprotective effect and preservation of the cholinergic system. In line with this finding, it has been shown that KV at 100 and 200 mg/kg is able to improve cortical and striatal activity of AChE in a cerebral ischemic model [57].
AD is pathologically typified by the development of two abnormal assemblies, i.e., Aβ plaques and tau-associated neurofibrillary tangles [58]. Although we did not measure amyloid beta plaques in this study which is a limitation, our ICV injection of OA was associated with higher hippocampal level of phosphorylated tau. In agreement with this finding, in a study by Foidl et al., in 2018, it was shown that exposure of brain slices obtained from wild type mice to okadaic acid induces tau hyperphosphorylation [59]. Additionally, Çakır et al., in 2023 showed enhanced level of phosphorylated-tau in OA model of AD in cortical and hippocampal areas [60]. In contrast, kolaviron pretreatment of OA group at 50 and 100 mg/kg was capable to appropriately reverse this change regarding p-tau. There is still no report how the kolaviron can target tauopathy and amyloidogenesis which warrants further investigation.
ICV OA injection in rats is associated with oxidative stress load, apoptosis, cellular inflammation, and neurodegenerative changes in cortical and hippocampal areas [61], which was also observed in this study by elevated hippocampal levels of MDA, protein carbonyl, ROS, nitrite and concomitant depression of antioxidants such as SOD and catalase in addition to higher activity for caspases 1 and 3. In contrast, kolaviron treatment of ICV OA-challenged group was associated with attenuation of these inappropriate changes. In line with our findings, it has been shown that KV can alleviate busulfan-induced brain damage via partial suppression of oxidative stress load, apoptosis, and neuroinflammation [62]. Furthermore, it has been shown that kolaviron can suppress 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced striatal load of oxidative stress, degeneration of dopaminergic neurons besides its down-regulation of caspase 3 in a model of Parkinson's disease [63]. However, there is no report on the effect of kolaviron on caspase 1 and this still warrants further research studies.
Development of astrogliosis has been reported following ICV OA [64,65]. Such findings were also obtained in our study, as demonstrated by higher hippocampal GFAP immunoreactivity as a specific feature of astrogliosis. Conversely, KV treatment of ICV OA group was able to significantly attenuate astrogliosis. Of relevance to this finding, it has been proven that kolaviron can protect prefrontal cortex against sodium azide neurotoxicity through attenuation of astrocyte activation [66].
Multifunctional phytocompound kolaviron has been presented as an amyloid beta fibrils disruptor and may be considered as a potential anti-amyloidogenic agent after further studies [21]. Although ICV microinjection of OA in this study was associated with significantly higher BACE1 (β-secretase 1) activity as one of the key enzymes in amyloidogenic cascade, however, KV treatment even at 100 mg/kg was not able to significantly lower BACE1 and only a 34.7 % reduction in enzyme activity was obtained in this regard which may have been due to its insufficient administered dose and/or time duration of administration.
OA exposure is associated with lower MMP as a marker for mitochondrial health and integrity [67] which was also detected in this research. On the contrary, KV treatment prevented loss of MMP in OA-instigated hippocampal injury. In conformance to this result, it has been displayed that flavonoid-rich kolaviron can abrogate MMP loss following busulfan-instigated brain injury in rats [62].
In the current research, we preferred to have a pretreatment, preventive, and neuroprotective strategy and not a post-treatment and restorative design and accordingly we administered kolaviron before okadaic acid microinjection. Such design for Alzheimer's-like phenotype and under neurotoxic conditions is according to literature [[68], [69], [70], [71]]. However, as a limitation, it is recommended to evaluate the beneficial and restorative effect of kolaviron post-treatment in OA model of AD like phenotype in future studies. In addition, in this study, we administered our therapeutic kolaviron through intraperitoneal route to avoid potential changes in its pharmacokinetics due to absorption and also having its effectiveness to a greater degree. However, since medicinal plants and their by-products are usually given orally, thus, it is recommended to administer kolaviron through oral route to assess its possible effectiveness in animal models of AD. Lack of immunoblotting and gene expression studies in addition to absence of assessment of amyloid deposition were other limitations of this study.
In conclusion, KV was able to attenuate cognitive fall subsequent to ICV OA which is partly mediated through its neuroprotective potential linked to mitigation of tau hyperphosphorylation, apoptosis, pyroptosis, neuroinflammation, and oxidative stress and also improvement of mitochondrial health.
Funding statement
This research study was the results of PhD student thesis project that was approved and supported by Iran University of Medical Sciences, Tehran, Iran (Grant no. 99-2-4-18769).
Consent for publication
All authors have read the manuscript and approved its submission.
Ethics approval
This study was approved by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.538).
Availability of data
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Morteza Nazari-Serenjeh: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Tourandokht Baluchnejadmojarad: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Masoud Hatami-Morassa: Writing – original draft, Investigation. Javad Fahanik-Babaei: Writing – review & editing, Writing – original draft, Methodology, Investigation. Soraya Mehrabi: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Mahsa Tashakori-Miyanroudi: Writing – review & editing, Writing – original draft, Methodology, Investigation. Samira Ramazi: Writing – review & editing, Writing – original draft, Investigation. Seyed-Mahdi Mohamadi-Zarch: Writing – review & editing, Writing – original draft, Methodology, Investigation. Davood Nourabadi: Writing – review & editing, Writing – original draft, Methodology, Investigation. Mehrdad Roghani: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Takeda S. Progression of Alzheimer's disease, tau propagation, and its modifiable risk factors. Neurosci. Res. 2019;141:36–42. doi: 10.1016/j.neures.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Busche M.A., Hyman B.T. Synergy between amyloid-β and tau in Alzheimer's disease. Nat. Neurosci. 2020;23(10):1183–1193. doi: 10.1038/s41593-020-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali G-C G.M., Wu Y.-T., Prince M., Prina M. The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. 2015:10–29. 2015. [Google Scholar]
- 4.Apostolova L.G. Alzheimer disease. Continuum. 2016;22(2 Dementia):419–434. doi: 10.1212/CON.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atri A. Current and future treatments in Alzheimer's disease. Semin. Neurol. 2019;39(2):227–240. doi: 10.1055/s-0039-1678581. [DOI] [PubMed] [Google Scholar]
- 6.Vaz M., Silvestre S. Alzheimer's disease: Recent treatment strategies. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173554. [DOI] [PubMed] [Google Scholar]
- 7.Çakır M., Tekin S., Doğanyiğit Z., Erden Y., Soytürk M., Çiğremiş Y., Sandal S. Cannabinoid type 2 receptor agonist JWH-133, attenuates Okadaic acid induced spatial memory impairment and neurodegeneration in rats. Life Sci. 2019;217:25–33. doi: 10.1016/j.lfs.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 8.Fu L.-l., Zhao X.-y., Ji L.-d., Xu J. Okadaic acid (OA): toxicity, detection and detoxification. Toxicon. 2019;160:1–7. doi: 10.1016/j.toxicon.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Kamat P.K., Nath C. Okadaic acid: a tool to study regulatory mechanisms for neurodegeneration and regeneration in Alzheimer's disease. Neural Regen Res. 2015;10(3):365–367. doi: 10.4103/1673-5374.153679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z., Simpkins J.W. An okadaic acid-induced model of tauopathy and cognitive deficiency. Brain Res. 2010;1359:233–246. doi: 10.1016/j.brainres.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamat P.K., Rai S., Nath C. Okadaic acid induced neurotoxicity: an emerging tool to study Alzheimer's disease pathology. Neurotoxicology. 2013;37:163–172. doi: 10.1016/j.neuro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Dwivedi S., Nagarajan R., Hanif K., Siddiqui H.H., Nath C., Shukla R. Standardized extract of bacopa monniera attenuates okadaic acid induced memory dysfunction in rats: effect on Nrf 2 pathway. Evid. Based Complement. Alternat. Med. 2013 doi: 10.1155/2013/294501. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Márquez J.M., Forbes-Hernández T.Y., Navarro-Hortal M.D., Quirantes-Piné R., Grosso G., Giampieri F., Lipari V., Sánchez-González C., Battino M., Quiles J.L. Molecular mechanisms of the protective effects of olive leaf polyphenols against Alzheimer's disease. Int. J. Mol. Sci. 2023;24(5) doi: 10.3390/ijms24054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas-García L., Romero-Márquez J.M., Navarro-Hortal M.D., Esteban-Muñoz A., Giampieri F., Sumalla-Cano S., Battino M., Quiles J.L., Llopis J., Sánchez-González C. Unravelling potential biomedical applications of the edible flower Tulbaghia violacea. Food Chem. 2022;381 doi: 10.1016/j.foodchem.2022.132096. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Márquez J.M., Navarro-Hortal M.D., Orantes F.J., Esteban-Muñoz A., Pérez-Oleaga C.M., Battino M., Sánchez-González C., Rivas-García L., Giampieri F., Quiles J.L., Forbes-Hernández T.Y. In vivo anti-alzheimer and antioxidant properties of avocado (persea americana mill.) honey from southern Spain. Antioxidants. 2023;12(2) doi: 10.3390/antiox12020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maňourová A., Chinheya I.P., Kalousová M., Ruiz-Chután J.A., Okafor U.C., Tchoundjeu Z., Tsobeng A., Van Damme P., Lojka B. Domestication potential of Garcinia kola heckel (Clusiaceae): searching for diversity in south Cameroon. Plants. 2023;12(4):742. doi: 10.3390/plants12040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dogara A.M., Hamad S.W., Hama H.A., Bradosty S.W., Kayfi S., Al-Rawi S.S., Lema A.A. Biological evaluation of Garcinia kola heckel. Advances in Pharmacological and Pharmaceutical Sciences. 2022;2022 doi: 10.1155/2022/3837965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erukainure O.L., Salau V.F., Chukwuma C.I., Islam M.S. Kolaviron: a biflavonoid with numerous health benefits. Curr. Pharm. Des. 2021;27(4):490–504. doi: 10.2174/1381612826666201113094303. [DOI] [PubMed] [Google Scholar]
- 19.Farombi E.O., Awogbindin I.O., Farombi T.H., Oladele J.O., Izomoh E.R., Aladelokun O.B., Ezekiel I.O., Adebambo O.I., Abah V.O. Neuroprotective role of kolaviron in striatal redo-inflammation associated with rotenone model of Parkinson's disease. Neurotoxicology. 2019;73:132–141. doi: 10.1016/j.neuro.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Farombi E.O., Awogbindin I.O., Olorunkalu P.D., Ogbuewu E., Oyetunde B.F., Agedah A.E., Adeniyi P.A. Kolaviron protects against nigrostriatal degeneration and gut oxidative damage in a stereotaxic rotenone model of Parkinson's disease. Psychopharmacology (Berl.) 2020;237(11):3225–3236. doi: 10.1007/s00213-020-05605-w. [DOI] [PubMed] [Google Scholar]
- 21.Adewole K.E., Gyebi G.A., Ibrahim I.M. Amyloid β fibrils disruption by kolaviron: molecular docking and extended molecular dynamics simulation studies. Comput. Biol. Chem. 2021;94 doi: 10.1016/j.compbiolchem.2021.107557. [DOI] [PubMed] [Google Scholar]
- 22.Olajide O.J., Asogwa N.T., Moses B.O., Oyegbola C.B. Multidirectional inhibition of cortico-hippocampal neurodegeneration by kolaviron treatment in rats. Metab. Brain Dis. 2017;32(4):1147–1161. doi: 10.1007/s11011-017-0012-6. [DOI] [PubMed] [Google Scholar]
- 23.Ishola I.O., Adamson F.M., Adeyemi O.O. Ameliorative effect of kolaviron, a biflavonoid complex from Garcinia kola seeds against scopolamine-induced memory impairment in rats: role of antioxidant defense system. Metab. Brain Dis. 2017;32(1):235–245. doi: 10.1007/s11011-016-9902-2. [DOI] [PubMed] [Google Scholar]
- 24.Onasanwo S.A., Adebimpe-John O.E., Olopade F.E., Olajide O.O. Kolaviron protects rats from cognitive decline induced by Lipopolysaccharide in Wistar rat: the memory-enhancing activity of kolaviron in Wistar rat, Niger. J. Physiol. Sci. 2021;36(1):67–76. [PubMed] [Google Scholar]
- 25.Ayepola O.R., Cerf M.E., Brooks N.L., Oguntibeju O.O. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine. 2014;21(14):1785–1793. doi: 10.1016/j.phymed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Rotimi D., Amanze C.J., Ojo B.A., Iyobhebhe M., Elebiyo C.T., Ojo A.O. Kolaviron, A biflavonoid compound: its pharmacological activity and therapeutic efficacy. Curr. Bioact. Compd. 2022;18(5):21–29. [Google Scholar]
- 27.Zhao L.J., Liu W., Xiong S.H., Tang J., Lou Z.H., Xie M.X., Xia B.H., Lin L.M., Liao D.F. Determination of total flavonoids contents and antioxidant activity of ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018 doi: 10.1155/2018/8195784. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J.-Y., Tang C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. [Google Scholar]
- 29.Paxinos G., Watson C. second ed. Academic Press; New York: 1986. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 30.Akinmoladun A.C., Akinrinola B.L., Olaleye M.T., Farombi E.O. Kolaviron, a Garcinia kola biflavonoid complex, protects against ischemia/reperfusion injury: pertinent mechanistic insights from biochemical and physical evaluations in rat brain. Neurochem. Res. 2015;40(4):777–787. doi: 10.1007/s11064-015-1527-z. [DOI] [PubMed] [Google Scholar]
- 31.Kalu W.O., Okafor P.N., Ijeh, Eleazu C. Effect of kolaviron, a biflavanoid complex from Garcinia kola on some biochemical parameters in experimentally induced benign prostatic hyperplasic rats. Biomed. Pharmacother. 2016;83:1436–1443. doi: 10.1016/j.biopha.2016.08.064. [DOI] [PubMed] [Google Scholar]
- 32.Dashniani M.G., Chighladze M.R., Solomonia R.O., Burjanadze M.A., Kandashvili M., Chkhikvishvili N.C., Beselia G.V., Kruashvili L.B. Memantine treatment prevents okadaic acid induced neurotoxicity at the systemic and molecular levels. Neuroreport. 2020;31(4):281–286. doi: 10.1097/WNR.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoudi N., Kiasalari Z., Rahmani T., Sanaierad A., Afshin-Majd S., Naderi G., Baluchnejadmojarad T., Roghani M. Diosgenin attenuates cognitive impairment in streptozotocin-induced diabetic rats: underlying mechanisms. Neuropsychobiology. 2021;80(1):25–35. doi: 10.1159/000507398. [DOI] [PubMed] [Google Scholar]
- 34.Kiasalari Z., Heydarifard R., Khalili M., Afshin-Majd S., Baluchnejadmojarad T., Zahedi E., Sanaierad A., Roghani M. Ellagic acid ameliorates learning and memory deficits in a rat model of Alzheimer's disease: an exploration of underlying mechanisms. Psychopharmacology (Berl.) 2017;234(12):1841–1852. doi: 10.1007/s00213-017-4589-6. [DOI] [PubMed] [Google Scholar]
- 35.Mohamadi-Zarch S.-M., Baluchnejadmojarad T., Nourabadi D., Ramazi S., Nazari-Serenjeh M., Roghani M. Esculetin alleviates acute liver failure following lipopolysaccharide/D-galactosamine in male C57bl/6 mice. Iran. J. Med. Sci. 2021;46(5):373–382. doi: 10.30476/ijms.2020.84909.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arya A., Sethy N.K., Singh S.K., Das M., Bhargava K. Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int J Nanomedicine. 2013;8:4507–4520. doi: 10.2147/IJN.S53032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feriyani F., Maulanza H., Lubis R.R., Balqis U., Darmawi D. Effects of binahong (anredera cordifolia (tenore) steenis) extracts on the levels of malondialdehyde (MDA) in cataract goat lenses. Sci. World J. 2021;2021 doi: 10.1155/2021/6617292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teixeira F.C., Gutierres J.M., Soares M.S.P., da Siveira de Mattos B., Spohr L., do Couto C.A.T., Bona N.P., Assmann C.E., Morsch V.M., da Cruz I.B.M., Stefanello F.M., Spanevello R.M. Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacology (Berl.) 2020;237(3):811–823. doi: 10.1007/s00213-019-05419-5. [DOI] [PubMed] [Google Scholar]
- 39.Reznick A.Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 40.Hemmati A.A., Alboghobeish S., Ahangarpour A. Effects of cinnamic acid on memory deficits and brain oxidative stress in streptozotocin-induced diabetic mice. KOREAN J. PHYSIOL. PHARMACOL. 2018;22(3):257–267. doi: 10.4196/kjpp.2018.22.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namgyal D., Ali S., Hussain M.D., Kazi M., Ahmad A., Sarwat M. Curcumin ameliorates the Cd-induced anxiety-like behavior in mice by regulating oxidative stress and neuro-inflammatory proteins in the prefrontal cortex region of the brain. Antioxidants. 2021;10(11) doi: 10.3390/antiox10111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alzokaky A.A.-m., Abdelkader E.M., El-Dessouki A.M., Khaleel S.A., Raslan N.A. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: role of HMGB1/NLRP3/NF-κB pathway. Basic Clin. Pharmacol. Toxicol. 2020;127(4):265–277. doi: 10.1111/bcpt.13415. [DOI] [PubMed] [Google Scholar]
- 43.de Souza Gonçalves B., de Moura Valadares J.M., Alves S.L.G., Silva S.C., Rangel L.P., Cortes V.F., Villar J., Barbosa L.A., de Lima Santos H. Evaluation of neuroprotective activity of digoxin and semisynthetic derivatives against partial chemical ischemia. J. Cell. Biochem. 2019;120(10):17108–17122. doi: 10.1002/jcb.28971. [DOI] [PubMed] [Google Scholar]
- 44.Mohandas J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984;33(11):1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 45.Morroni F., Tarozzi A., Sita G., Bolondi C., Zolezzi Moraga J.M., Cantelli-Forti G., Hrelia P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology. 2013;36:63–71. doi: 10.1016/j.neuro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 47.Ogunsuyi O.B., Oboh G., Oluokun O.O., Ademiluyi A.O., Ogunruku O.O. Gallic acid protects against neurochemical alterations in transgenic Drosophila model of Alzheimer's disease. Advances in Traditional Medicine. 2020;20(1):89–98. [Google Scholar]
- 48.Ding J., Yu H.L., Ma W.W., Xi Y.D., Zhao X., Yuan L.H., Feng J.F., Xiao R. Soy isoflavone attenuates brain mitochondrial oxidative stress induced by β-amyloid peptides 1-42 injection in lateral cerebral ventricle. J. Neurosci. Res. 2013;91(4):562–567. doi: 10.1002/jnr.23163. [DOI] [PubMed] [Google Scholar]
- 49.Pourmohammadi S., Roghani M., Kiasalari Z., Khalili M. Paeonol ameliorates cuprizone-induced hippocampal demyelination and cognitive deficits through inhibition of oxidative and inflammatory events. J. Mol. Neurosci. 2022;72(4):748–758. doi: 10.1007/s12031-021-01951-2. [DOI] [PubMed] [Google Scholar]
- 50.Rajasekar N., Dwivedi S., Tota S.K., Kamat P.K., Hanif K., Nath C., Shukla R. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur. J. Pharmacol. 2013;715(1–3):381–394. doi: 10.1016/j.ejphar.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 51.Sachdeva A.K., Chopra K. Naringin mitigate okadaic acid-induced cognitive impairment in an experimental paradigm of Alzheimer's disease. J. Funct.Foods. 2015;19:110–125. [Google Scholar]
- 52.Hamidi N., Nozad A., Sheikhkanloui Milan H., Amani M. Okadaic acid attenuates short-term and long-term synaptic plasticity of hippocampal dentate gyrus neurons in rats. Neurobiol. Learn. Mem. 2019;158:24–31. doi: 10.1016/j.nlm.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Onasanwo S.A., Adebimpe-John O.E., Olopade F.E., Olajide O.O. Kolaviron protects rats from cognitive decline induced by Lipopolysaccharide in Wistar rat, Niger. J. Physiol. Sci. 2021;36(1):67–76. [PubMed] [Google Scholar]
- 54.Omotoso G.O., Ukwubile, Arietarhire L., Sulaimon F., Gbadamosi I.T. Kolaviron protects the brain in cuprizone-induced model of experimental multiple sclerosis via enhancement of intrinsic antioxidant mechanisms: possible therapeutic applications? Pathophysiology. 2018;25(4):299–306. doi: 10.1016/j.pathophys.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Levin E.D. Nicotinic receptor subtypes and cognitive function. J. Neurobiol. 2002;53(4):633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 56.Kamat P.K., Tota S., Rai S., Shukla R., Ali S., Najmi A.K., Nath C. Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats. Eur. J. Pharmacol. 2012;690(1–3):90–98. doi: 10.1016/j.ejphar.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Akinmoladun A.C., Saliu I.O., Olowookere B.D., Ojo O.B., Olaleye M.T., Farombi E.O., Akindahunsi A.A. Improvement of 2-vessel occlusion cerebral ischaemia/reperfusion-induced corticostriatal electrolyte and redox imbalance, lactic acidosis and modified acetylcholinesterase activity by kolaviron correlates with reduction in neurobehavioural deficits. Ann. Neurosci. 2018;25(1):53–62. doi: 10.1159/000484517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernández F., Llorens-Martín M., Bolós M., Pérez M., Cuadros R., Pallas-Bazarra N., Zabala J.C., Avila J. New beginnings in Alzheimer's disease: the most prevalent tauopathy. J. Alzheimers Dis. 2018;64(s1):S529–s534. doi: 10.3233/JAD-179916. [DOI] [PubMed] [Google Scholar]
- 59.Foidl B.M., Humpel C. Differential hyperphosphorylation of tau-S199, -T231 and -S396 in organotypic brain slices of alzheimer mice. A model to study early tau hyperphosphorylation using okadaic acid. Front. Aging Neurosci. 2018;10:113. doi: 10.3389/fnagi.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Çakır M., Yüksel F., Mustafa Özkut M., Durhan M., Kaymak E., Tekin S., Çiğremiş Y. Neuroprotective effect of transient receptor potential Vanilloid 1 agonist capsaicin in Alzheimer's disease model induced with okadaic acid. Int. Immunopharm. 2023;118 doi: 10.1016/j.intimp.2023.109925. [DOI] [PubMed] [Google Scholar]
- 61.Cakir M., Duzova H., Tekin S., Taslıdere E., Kaya G.B., Cigremis Y., Ozgocer T., Yologlu S. ACA, an inhibitor phospholipases A2 and transient receptor potential melastatin-2 channels, attenuates okadaic acid induced neurodegeneration in rats. Life Sci. 2017;176:10–20. doi: 10.1016/j.lfs.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Tesi E.P., Ben-Azu B., Mega O.O., Mordi J., Knowledge O.O., Awele E.D., Rotu R.A., Emojevwe V., Adebayo O.G., Eneni O.A. Kolaviron, a flavonoid-rich extract ameliorates busulfan-induced chemo-brain and testicular damage in male rats through inhibition of oxidative stress, inflammatory, and apoptotic pathways. J. Food Biochem. 2022;46(4) doi: 10.1111/jfbc.14071. [DOI] [PubMed] [Google Scholar]
- 63.Farombi E.O., Awogbindin I.O., Owoeye O., Abah V.O., Izomoh E.R., Ezekiel I.O. Kolaviron ameliorates behavioural deficit and injury to striatal dopaminergic terminals via modulation of oxidative burden, DJ-1 depletion and CD45R(+) cells infiltration in MPTP-model of Parkinson's disease. Metab. Brain Dis. 2020;35(6):933–946. doi: 10.1007/s11011-020-00578-3. [DOI] [PubMed] [Google Scholar]
- 64.Broetto N., Hansen F., Brolese G., Batassini C., Lirio F., Galland F., Dos Santos J.P., Dutra M.F., Gonçalves C.A. Intracerebroventricular administration of okadaic acid induces hippocampal glucose uptake dysfunction and tau phosphorylation. Brain Res. Bull. 2016;124:136–143. doi: 10.1016/j.brainresbull.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Kamat P.K., Tota S., Rai S., Swarnkar S., Shukla R., Nath C. A study on neuroinflammatory marker in brain areas of okadaic acid (ICV) induced memory impaired rats. Life Sci. 2012;90(19–20):713–720. doi: 10.1016/j.lfs.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Olajide O.J., Enaibe B.U., Bankole O.O., Akinola O.B., Laoye B.J., Ogundele O.M. Kolaviron was protective against sodium azide (NaN3) induced oxidative stress in the prefrontal cortex. Metab. Brain Dis. 2016;31(1):25–35. doi: 10.1007/s11011-015-9674-0. [DOI] [PubMed] [Google Scholar]
- 67.Jiang W., Luo T., Li S., Zhou Y., Shen X.Y., He F., Xu J., Wang H.Q. Quercetin protects against okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nillert N., Pannangrong W., Welbat J.U., Chaijaroonkhanarak W., Sripanidkulchai K., Sripanidkulchai B. Neuroprotective effects of aged garlic extract on cognitive dysfunction and neuroinflammation induced by β-amyloid in rats. Nutrients. 2017;9(1) doi: 10.3390/nu9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mataram M.B.A., Hening P., Harjanti F.N., Karnati S., Wasityastuti W., Nugrahaningsih D.A.A., Kusindarta D.L., Wihadmadyatami H. The neuroprotective effect of ethanolic extract Ocimum sanctum Linn. in the regulation of neuronal density in hippocampus areas as a central autobiography memory on the rat model of Alzheimer's disease. J. Chem. Neuroanat. 2021;111 doi: 10.1016/j.jchemneu.2020.101885. [DOI] [PubMed] [Google Scholar]
- 70.Som S., Antony J., Dhanabal S., Ponnusankar S. Neuroprotective role of Diosgenin, a NGF stimulator, against Aβ (1-42) induced neurotoxicity in animal model of Alzheimer's disease. Metab. Brain Dis. 2022;37(2):359–372. doi: 10.1007/s11011-021-00880-8. [DOI] [PubMed] [Google Scholar]
- 71.Abdelsayed E.M., Medhat D., Mandour Y.M., Hanafi R.S., Motaal A.A. Niazimicin: a thiocarbamate glycoside from Moringa oleifera Lam. seeds with a novel neuroprotective activity. J. Food Biochem. 2021;45(12) doi: 10.1111/jfbc.13992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.