Abstract
Infection of susceptible mouse strains with Borrelia burgdorferi, the agent of Lyme disease, results in the development of arthritis. Components of the innate immune system may be important mediators of this pathology. To investigate the potential role of NK cells in development of experimental Lyme arthritis, we examined their activation in vivo in both resistant and susceptible mouse strains. Following inoculation of B. burgdorferi into the footpad, lymph node NK cells from susceptible C3H/HeJ (C3H) mice produced more gamma interferon than NK cells from resistant DBA/2J mice. Lymph node cells from susceptible C3H and AKR mice also had increased ability to lyse YAC-1 target cells 2 days following infection. Antibody depletion of NK cells from susceptible mice, however, did not alter the development of arthritis following B. burgdorferi challenge. In addition, NK cell depletion had little effect on spirochete burden. Thus, there is a marked activation of NK cells in susceptible mouse strains following infection. Although NK cells are not absolutely required for arthritis, events occurring prior to NK cell activation might be important in mediating pathology in experimental Lyme disease.
Lyme disease is caused by infection with the spirochete Borrelia burgdorferi. In the murine model of Lyme disease, host genetics play an important role in determining the extent of arthritic pathology which develops following infection (10, 56). Challenge of susceptible inbred mouse strains leads to a transient arthritis which peaks at 2 to 3 weeks and then resolves over the next few months (11). Resistant mouse strains develop little pathology and harbor significantly fewer spirochetes in their tissues following intradermal infection (61). This may be due to a more effective early immune response in resistant strains which is capable of limiting bacterial growth and/or persistence (55). Even following footpad injection, when resistant animals have high levels of spirochetes in their ankles, they remain resistant to pathology (16, 32). This observation suggests that pathogenesis is linked to an inappropriate or overexuberant immune response in addition to bacterial load. Mice with the scid mutation are susceptible to development of arthritis, implying that cells of the innate immune system play a role in tissue damage (12, 45, 46). Resistance to arthritis and its resolution, however, appear to require components of the adaptive immune response, i.e., B (14, 48) and/or T (26, 34) cells. In experimentally infected hamsters, macrophages appear to play a direct effector role in the development of B. burgdorferi-induced arthritis (20). The mechanism(s) of immunopathology in humans and mice, however, is still incompletely understood.
NK cells are critical components of the innate immune system (8). In conjunction with macrophages and neutrophils, NK cells make up the first line of cellular defense against viruses, fungi, bacteria, and parasites (57). NK cells play a dual role in early immune responses. They provide direct effector cell function by killing pathogen-infected cells (8) and also indirectly influence the development of the inflammatory and adaptive immune response through the production of gamma interferon (IFN-γ) (51). Both of these roles are important in protection against a wide variety of infectious agents. NK cells can directly lyse Cryptococcus neoformans (29, 39) and human monocytes infected with Mycobacterium tuberculosis (18). Production of IFN-γ by NK cells may also influence the adaptive immune response because it occurs at a critical time during development of T helper type 1 (Th1) and Th2 cells (1, 24, 37). High levels of IFN-γ are capable of suppressing production of interleukin-4 (IL-4) and promoting a Th1-mediated response (33, 53).
Little is known about the role of NK cells during B. burgdorferi infection. Patients with active Lyme disease have suppression of NK cell cytotoxic activity, whereas patients with chronic but nonactive disease show no evidence of NK cell suppression (18). Bone marrow macrophages from both resistant BALB/c and susceptible C3H mice produce nitric oxide (NO) in response to borrelial antigens, and this response can be augmented by the addition of IFN-γ (30). Bone marrow macrophages and IL-2-elicited NK cells from either normal or SCID spleens are able to produce low levels of NO and IFN-γ, respectively, in response to B. burgdorferi antigens when cultured individually but much higher levels when cultured together (31). These results suggest that innate immunity may play an early, critical role after infection with B. burgdorferi. A recent report demonstrated that genetically resistant C57BL/6 (B6) mice depleted of NK cells did not develop arthritis following B. burgdorferi infection, suggesting that NK cells were not required for disease resistance (13). The role of NK cells in mediating Lyme arthritis development or pathology in susceptible animals, however, has not been clearly defined.
To further investigate the role of NK cells during infection with B. burgdorferi, we studied the responses of resistant and susceptible mouse strains in vitro and in vivo. Following challenge, susceptible mouse strains had early activation of NK cells, as assessed by both cytolytic activity and IFN-γ production, whereas resistant mouse strains showed little NK cell activation. Antibody depletion studies, however, indicated that activation of NK cells and early production of IFN-γ were not absolutely required for development of Lyme arthritis. In addition, depletion of NK cells had no effect on dissemination patterns or spirochetal loads in tissues. Therefore, the differential activation of NK cells does not cause but may be a response to the initial events underlying the genetic control of arthritis development.
MATERIALS AND METHODS
Mice.
All mice were females between 4 and 6 weeks of age. C3H/HeJ (C3H), DBA/2J (DBA), B6, BALB/c, AKR, and B6C3F1 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice congenic for the NK1.1 locus (C3H.NK mice) were bred in our facility by crossing B6C3F1 to C3H mice and screening progeny for the expression of NK1.1. NK1.1+ mice were further bred to C3H for four generations and then intercrossed to produce the homozygous C3H.NK mice used in these experiments.
Bacteria and infections.
B. burgdorferi N40 was kindly provided by Steven Barthold (Yale University, New Haven, Conn.). Spirochetes were reisolated from SCID mice, passaged twice in Barbour-Stoenner-Kelly II (BSK) medium (Sigma Chemical Co., St. Louis, Mo.), and frozen in aliquots at −80°C. For infections, an aliquot was thawed, placed in 7 ml of medium, and grown for 5 days at 32°C. Mice were inoculated in both hind footpads with 5 × 105 B. burgdorferi in 50 μl of BSK medium. Tibiotarsal joints were measured weekly, using a metric caliper (Ralmike’s Tool-A-Rama, South Plainfield, N.J.), through the thickest anteroposterior diameter of the ankle. Mice were sacrificed on designated days following infection. In some experiments, ankles, hearts, and skin (ear punches) were frozen for PCR analysis. Blood, heart, spleen, urinary bladder, skin, and ankles were aseptically collected and cultured at 32°C for 14 days in BSK medium. Cultures were read by placing 10 μl of supernatant on a microscope slide under a 22- by 22-mm coverslip and examining 20 high-power fields by dark-field microscopy.
In all experiments, one ankle from each mouse was formalin fixed, embedded in paraffin, stained with hematoxylin and eosin, and blindly evaluated for arthritis severity on a scale of 0 to 3 (12). Grade 0 represents no inflammation, grades 1 and 2 represent mild to moderate inflammation, and grade 3 represents severe inflammation.
Assay for IFN-γ production.
Popliteal lymph node cells were removed from control or infected animals and assayed as pooled groups of two or more animals; 3 × 105 to 5 × 105 cells were cultured in Iscove’s medium containing 10% fetal calf serum and antibiotics, with or without B. burgdorferi sonicate antigen (Bb Ag). All cultures were treated with 10 μg of polymyxin B per ml. Supernatants were harvested 24 h later and assayed for IFN-γ by using monoclonal antibody (MAb) pairs (PharMingen, San Diego, Calif.) in a standard sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
NK cell cytotoxicity assay.
YAC-1 lymphoma target cells were incubated for 1 h with 500 μCi of Na51CrO4 (Amersham, Arlington Heights, Ill.) and then washed three times in Hanks balanced salt solution. Effector cells were obtained from pooled popliteal lymph nodes from naive mice or mice infected 2 days earlier with B. burgdorferi. Spleen cells were removed from mice treated with 100 μg of poly(I-C) (Sigma); 104 target cells were cultured with effectors at effector/target cell (E:T) ratios of 100:1, 50:1, 25:1, and 12.5:1 in a total volume of 200 μl. Plates were incubated for 4 h at 37°C in a CO2 chamber. Chromium release into supernatants was measured in Luma plates (Packard Instrument Co., Meridian, Conn.) with a Packard beta counter. Specific 51Cr release was determined as [(experimental release − spontaneous release)/(maximal release − spontaneous release)] × 100. Maximum release and spontaneous release were determined from wells containing 100 μl of 2% Triton X-100 (Sigma) and medium alone, respectively, in the place of effector cells.
Depletion of NK cells in vivo.
Mice were injected intravenously with 1.85 mg of anti-asialo-GM1 antibody (Waco Chemicals USA, Inc., Richmond, Va.) or 1.85 mg of heat-inactivated normal rabbit serum (Accurate Chemical Co., Westbury, N.Y.) or intraperitoneally with 0.2 mg of anti-NK1.1 (PK136; a generous gift from B. Daniels, VA Medical Center, San Francisco, Calif.) or control rat immunoglobulin G (IgG) (Sigma) on days −7, −3, or 0 of B. burgdorferi infection and weekly thereafter. The success of NK cell depletion in vivo was assessed by subjecting spleen cells to flow cytometry and fluorescence-activated cell sorting (FACS) analysis using phycoerythrin-conjugated anti-NK1.1 and fluorescein isothiocyanate-conjugated anti-CD3 (PharMingen) or for production of IFN-γ in response to Bb Ag. NK cells were routinely depleted to less than 1%.
PCR analysis.
To extract DNA from heart or skin, samples were placed in 0.5 ml of sodium dodecyl sulfate (SDS)-Tris lysis buffer (0.1 mg of proteinase K per ml in 200 mM NaCl–20 mM Tris-HCl [pH 8.0]–50 mM EDTA–0.2% SDS) and incubated overnight at 55°C. Following incubation, debris was pelleted and the supernatants were transferred to tubes containing 0.8 ml of isopropanol. Sample DNA was precipitated on ice for 60 min. DNA was pelleted at 4°C, air dried, and resuspended in 200 μl of Tris-EDTA buffer. To extract DNA from ankles, samples were first incubated in 0.5 ml of 1% collagenase for 6 h at 37°C; 0.25 ml of 3× SDS-Tris lysis buffer was added, and samples were incubated at 55°C overnight. DNA was then precipitated as described above. PCR amplification was performed with 10 μl of DNA diluted 1:100 (hearts and ankles) or 1:10 (ear punches) in Tris-EDTA buffer. DNA from uninfected mice served as negative controls. The presence of B. burgdorferi ospA in sample DNA was detected with 5′ primer TCTTGAAGGAACTTTAACTGCTG and 3′ primer CAAGTTTTGTAATTTCAACTGCTGA. PCRs were performed as follows: denaturation for 60 s at 94°C, followed by 35 cycles of denaturation at 94°C for 60 s, annealing at 60°C for 60 s, and extension at 72°C for 90 s. Amplified products were visualized on a 2.5% agarose gel.
Statistics.
Data were analyzed by the Student t test for single comparisons or Tukey test for multiple comparisons. Critical values for statistical significance were set at α = 0.05.
RESULTS
Kinetics of NK cell activation in vivo.
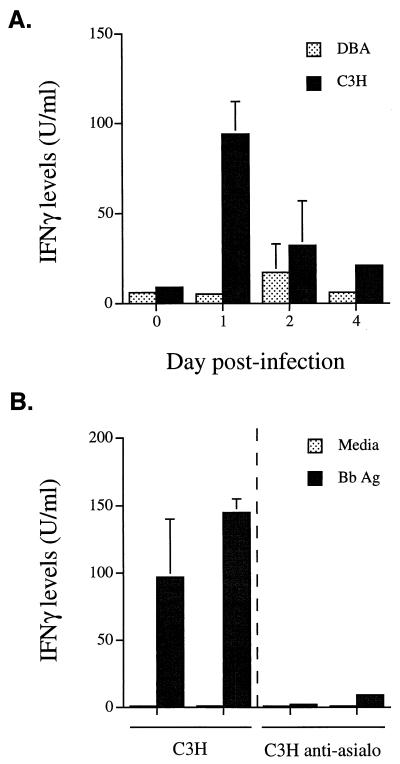
NK cells from arthritis-resistant and -susceptible mouse strains become activated in vitro to produce IFN-γ in response to whole borrelial antigen or purified OspA or OspB (31). To determine if there were differences in activation of NK cells in vivo, we infected resistant and susceptible mice in the hind footpads with B. burgdorferi. Footpad injections were used to provide a site of early, localized focus of the immune response which does not occur following intradermal injection. Injection of media alone into the footpads does not cause histopathological changes in the ankles of mice (15a). Some mouse strains, such as BALB/c, considered resistant by the intradermal route will be intermediate or susceptible when the footpad route of inoculation is used (15a, 25). Consequently, footpad injection was used throughout this study for consistency and to avoid differences in immune responses when various routes of infection were used. FACS analysis revealed similar numbers of NK cells in popliteal lymph nodes of naive arthritis-resistant B6 and -susceptible B6C3F1 mice. By day 1 of infection, the numbers of NK cells were increased 1.9- and 2.3-fold, respectively. To determine if this NK cell expansion also resulted in NK cell activation, we infected DBA and C3H mice and studied their NK cells over time. On days 1, 2, and 4 following infection, draining popliteal lymph nodes were removed and the cells were restimulated in vitro with Bb Ag. Supernatants were collected 24 h later, and production of IFN-γ was measured by ELISA. Lymph node cells from resistant DBA mice produced little IFN-γ during the first 4 days following B. burgdorferi infection. In contrast, lymph node cells from susceptible C3H mice had a rapid response, producing significantly higher levels of IFN-γ on day 1 following infection (Fig. 1A; P < 0.01). Similar levels of IFN-γ were produced by C3H SCID mice (data not shown). The production of IFN-γ by C3H mice declined by day 2 but was still higher than in DBA mice, where it peaked at day 2. By day 4 of infection, the production of IFN-γ by DBA lymph node cells had returned to baseline levels whereas levels in C3H lymph node cells were still slightly elevated. These results demonstrate more rapid and greater production of IFN-γ in the draining lymph nodes of susceptible mice than in those of resistant mice. Although this IFN-γ production was elicited with borrelial extracts, it is unlikely that it was derived from B. burgdorferi-reactive T cells at such an early time point (49). To determine if this increased IFN-γ was produced by NK cells, C3H mice were treated with anti-asialo-GM1 antiserum or control serum and then infected with B. burgdorferi. On day 1 postinfection, draining lymph nodes were tested for the production of IFN-γ. Treatment of mice with anti-asialo-GM1 antiserum completely abolished the production of IFN-γ in response to Bb Ag (Fig. 1B). This result implicates NK cells as the most likely source of IFN-γ in the earliest phase of infection.
FIG. 1.
(A) Kinetics of NK cell activation during early B. burgdorferi infection. Draining popliteal lymph node cells were harvested from C3H and DBA mice infected with B. burgdorferi 1, 2, or 4 days earlier. (B) Depletion of C3H NK cells by anti-asialo-GM1 antibody treatment abolishes production of IFN-γ on day 1 of infection. Results are shown for two control and two anti-asialo-GM1-treated animals. Cultures were assayed in triplicate. Levels of IFN-γ were measured in 24-h supernatants by ELISA as described in Materials and Methods. Sensitivity of the ELISA was 4 U/ml. Results are representative of one of three separate experiments.
NK cell cytotoxicity in resistant and susceptible mouse strains.
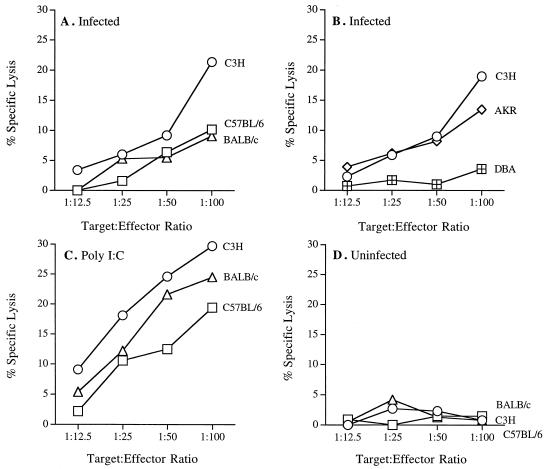
To further assess the activation of NK cells during B. burgdorferi infection, we tested the ability of draining lymph node cells to lyse the YAC-1 tumor cell line. Popliteal lymph nodes were harvested from mice 2 days after infection and tested for lytic capability in a 4-h chromium release assay. Of the three strains tested (Fig. 2A), only cells from infected C3H mice were able to significantly lyse the YAC-1 target cells at an E:T ratio of 100:1 (P < 0.05). Cells from infected resistant BALB/c and B6 mice had minimal lytic capability. To further investigate the correlation between NK cell activation and arthritis resistance or susceptibility, we tested NK cells from resistant DBA and susceptible AKR (47) animals for the ability to lyse YAC-1 cells. Susceptible C3H and AKR mice had significant NK cell cytotoxic activity following infection, while resistant DBA mice did not (Fig. 2B; E:T ratio of 100:1, P < 0.05). The levels of cytotoxicity of NK cells cells from susceptible animals, while low, were reproducible in three separate experiments and most likely reflect the low numbers of NK cells present in the lymph nodes. Others have reported similar low but significant levels of killing by NK cells in other systems (5, 38, 40, 41, 49, 50, 59). Poly(I-C) treatment led to low but significant YAC-1 lysis (49) from all strains tested (Fig. 2C; P < 0.05) and indicated that the failure of resistant strains to lyse YAC-1 cells was not due to a global defect in their ability to activate NK cells. NK cells from susceptible mouse strains were not preactivated for lysis since cells from uninfected mice had no lytic activity in these assays (Fig. 2D).
FIG. 2.
NK cell cytotoxicity in resistant and susceptible mouse strains. Popliteal lymph nodes were pooled from six animals per group from 2-day-infected (A and B) and uninfected (D) mouse strains. Splenocytes from poly(I-C) treated mice were used as positive controls (C). Effector cells were assayed for cytolytic activity against YAC-1 target cells in a 4-h Cr release assay. Similar results were obtained in three other experiments. Values are means of triplicate wells, and standard deviation were less than 10% in all samples.
NK cell depletion studies.
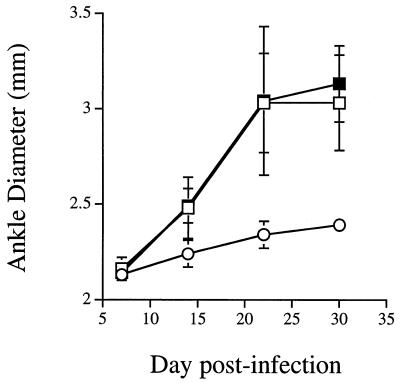
To determine if NK cells were absolutely required for arthritis development in susceptible mice, we depleted infected animals of NK cells. We bred C3H.NK mice or tested arthritis-susceptible B6C3F1 mice in order to use an anti-NK1.1 MAb (PK136) for NK cell depletion (21). NK1.1 is expressed on all NK cells in the mouse (62). MAb antibody staining and flow cytometry were performed weekly to ensure that NK1.1-bearing cells were depleted for the entire experimental period. FACS analysis of splenocytes showed 2 to 3% NK cells in control mice but a reduction of NK cells in antibody-treated mice to less than 1% for the duration of infection. We also stimulated splenocytes with IL-12 and Bb Ag and measured production of IFN-γ after 24 h. IFN-γ production by spleen cells from anti-NK1.1-treated mice was below the limit of detection, while those from IgG-treated control mice produced 19.2 U of IFN-γ per ml, further confirming the efficiency of antibody depletion. This observation is consistent with published studies examining lytic function after a similar protocol of antibody depletion (52). Figure 3 shows that infection of C3H.NK mice resulted in arthritis development even though the NK1.1 locus originated from a genetically resistant B6 mouse. Depletion of NK cells in C3H.NK mice by using anti-NK1.1 antibody had little effect on arthritis development. Beginning 1 week postinfection, ankles of control and anti-NK1.1-treated mice were significantly larger than control DBA ankles (P < 0.001). Similar results were obtained when anti-asialo-GM1 antibody was used to deplete NK cells in C3H mice (data not shown). Thus, using two independent systems of NK cell depletion, we detected no difference in the development of arthritis between control and NK cell-depleted mice. We also found no difference in arthritis development between infected C3H and C3H beige mice (data not shown), confirming the previous work of Barthold and de Souza (13). Spirochete DNA was detected in all tissues examined at day 28 of infection between DBA, C3H.NK, and NK cell-depleted C3H.NK mice as determined by PCR (Table 1). Analysis of histological sections of ankles stained with hematoxylin and eosin revealed no differences between NK cell-depleted and control animals (data not shown). Arthritis severity scores were significantly higher in the susceptible C3H.NK mice than in the resistant DBA mice (Table 1), but there was no difference between the anti-NK1.1-treated and untreated C3H.NK congenic mice. To determine if NK cells might play a role in limiting spirochetal load or dissemination in tissues, B6C3F1 mice were sacrificed on days 5, 10, 15, 20, and 25 postinfection, a time period that spans the peak of arthritis development (14 to 21 days) and spirochete dissemination. The time course of arthritis development after NK cell depletion was unaffected, with little difference in arthritis severity scores at each time point tested (Table 2). Blood, heart, spleen, bladder, skin (ear punches), and ankles were aseptically removed and cultured for 14 days. Spirochete dissemination patterns, as measured by positive cultures, were similar between NK cell-depleted and control mice.
FIG. 3.
Depletion of NK cells in C3H.NK congenic mice does not attenuate development of Lyme arthritis. Groups of C3H.NK (squares) and DBA (circles) mice were treated with phosphate-buffered saline (open symbols) or anti-NK1.1 MAb (closed symbols) and infected with B. burgdorferi as described in Materials and Methods. Ankle diameters were recorded weekly, with measurements pooled from three mice per group. Results are representative of three separate experiments.
TABLE 1.
PCR detection of the presence of B. burgdorferi DNA in selected tissues and arthritis development in ankles of control (IgG-treated) and anti-NK1.1 MAb-treated micea
| Strain (no. tested) | Treatment | PCR result
|
Arthritis severity (mean ± SD) | ||
|---|---|---|---|---|---|
| Heart | Ear | Ankle | |||
| DBA (3) | None | 3 | 3 | 3 | 0.7 ± 0.6 |
| C3H.NK (5) | Rat IgG | 5 | 4 | 5 | 2.0 ± 1.0b |
| Anti-NK1.1 MAb | 5 | 4 | 5 | 2.0 ± 1.0b | |
Mice were infected with 106 B. burgdorferi spirochetes and sacrificed at 28 days. PCR results are presented as number of specimens positive for B. burgdorferi. Tibiotarsal arthritis development was scored on a scale from 0 to 3.
Arthritis severity scores were significantly higher in C3H.NK mice than in DBA mice (P < 0.05).
TABLE 2.
Isolation of B. burgdorferi from selected tissues and arthritis development in ankles of control and anti-NK1.1 MAb-treated B6C3F1 micea
| Treatment | Day | Culture result (n = 3)
|
Arthritis severity (mean ± SD) | |||||
|---|---|---|---|---|---|---|---|---|
| Blood | Heart | Spleen | Bladder | Ear | Ankle | |||
| Control | 5 | 0 | 0 | 0 | 0 | 0 | 3 | 0.3 ± 0.6 |
| Anti-NK1.1 | 5 | 0 | 0 | 0 | 0 | 0 | 3 | 0.3 ± 0.6 |
| Control | 10 | 1 | 0 | 1 | 3 | 0 | 3 | 2.0 ± 0.8 |
| Anti-NK1.1 | 10 | 0 | 1 | 0 | 3 | 0 | 3 | 1.0 ± 0.0 |
| Control | 15 | 0 | 1 | 1 | 3 | 0 | 3 | 2.0 ± 1.0 |
| Anti-NK1.1 | 15 | 0 | 1 | 1 | 3 | 0 | 3 | 2.0 ± 0.0 |
| Control | 20 | 0 | 2 | 1 | 3 | 3 | 3 | 2.5 ± 0.6 |
| Anti-NK1.1 | 20 | 1 | 1 | 0 | 3 | 3 | 3 | 2.2 ± 0.5 |
| Control | 25 | 0 | 3 | 1 | 3 | 3 | 3 | 3.0 ± 0.0 |
| Anti-NK1.1 | 25 | 0 | 1 | 0 | 3 | 3 | 3 | 2.5 ± 0.6 |
Mice were infected with 106 B. burgdorferi spirochetes and sacrificed at 5, 10, 15, 20, and 25 days. Culture results are presented as the number of specimens positive for B. burgdorferi among three tested. Tibiotarsal arthritis development was assessed as for Table 1.
DISCUSSION
We have demonstrated that infection of mice with B. burgdorferi leads to greater activation of NK cells in susceptible strains than in resistant strains. This activation is characterized by both enhanced cytotoxicity against tumor cell targets and increased production of IFN-γ. Increased NK cell activity, however, is not absolutely required for the development of Lyme arthritis since depletion of NK cells in susceptible C3H.NK mice did not alter development of pathology. NK cell activation is also not required for limiting spirochete persistence or dissemination in susceptible animals. Arthritis severity scores and levels of spirochete loads in various tissues, as assessed by PCR or culture, were similar in NK cell-depleted and control mice.
The in vivo contribution of NK cells to the development of experimental Lyme arthritis has been reported in one other study to date. Barthold and de Souza studied the development of arthritis in C3H and B6 mice with the beige mutation (13). beige mice and patients with Chediak-Higashi syndrome have defective vesicular transport to and from the lysosome and late endosome (9). There is dysregulated fusion of intracellular vesicles and compartmental missorting of proteins (17, 23). This results in defective granulocytes, cytotoxic T cells, and NK cells that are not cytolytic but are capable of IFN-γ production (44). B6 mice are normally resistant to Lyme pathology; however, B6 beige mice developed severe arthritis similar to that of C3H control animals (13). These results indicated that the NK cell or granulocytic function perturbed in beige mice contributes to resistance to arthritis development. Few differences in immune responses were detected between control and beige mutant C3H mice, although the authors speculated this was because C3H mice already had severe arthritis which could not be exacerbated. Depletion of NK cells, granulocytes, or macrophages in vivo in this study failed to identify the cell type responsible for resistance to arthritis in B6 animals. Granulocyte depletion, which had the greatest effect, increased the incidence but not the severity of arthritis development in these mice. The authors concluded that NK cells had no role in resistance to Lyme arthritis in resistant mice. The present study lends support to this conclusion by showing very little activation of NK cells in resistant animals.
Production of IFN-γ by NK cells is important in many infectious disease models (7). Much of our understanding of the role of NK cells in innate immunity has come from the study of infections of mice with Listeria monocytogenes (58). Listeria-infected macrophages release tumor necrosis factor alpha (TNF-α) and IL-12, which activate NK cells to secrete IFN-γ in an antigen-independent manner. The IFN-γ activates macrophages to produce NO and become bactericidal (6). This cytokine-inducible pathway of macrophage activation is not unique to Listeria but operates during infections with other viral (15), bacterial (4, 40), and protozoan (22, 49) pathogens and has recently been shown to occur in response to bacterial DNA (5). This same cytokine-inducible pathway is activated following stimulation of C3H or BALB/c spleen cells in vitro with Bb Ag or with OspA or OspB (31). Thus, in the present study, the early activation and production of IFN-γ by NK cells in the susceptible C3H mice could cause the activation of macrophages and release of NO. Production of NO is detrimental in other models of arthritis (36), and its inhibition by NG-monomethyl-l-arginine (NMMA) decreases development of arthritis (35, 60). In contrast, treatment of B. burgdorferi-infected mice with NMMA had no effect on development of arthritis or on bacterial burdens in either resistant or susceptible mouse strains (54).
NK cell-mediated production of IFN-γ could also contribute to pathology by influencing the development of Th cell subsets. Resistance or susceptibility to Lyme arthritis correlates with the development of a Th2 or Th1 phenotype, respectively (27, 34). T cells can be activated in vitro by either whole Bb Ag or OspA (28). Further evidence for the participation of T cells in the pathogenesis of Lyme disease comes from the association of chronic Lyme disease with HLA-DR4 (56) or of chronic inflammation in joints and hearts of certain H-2 haplotypes of inbred mouse strains (47). T cells from vaccinated inbred hamsters were capable of conferring the ability to develop severe destructive arthritis in naive recipients upon challenge with nonpathogenic levels of B. burgdorferi (30). In contrast, a Th2 clone was able to confer protection to naive mice against challenge with B. burgdorferi (43). Cytokine production by Th cells may be of importance in mediating pathology. Treatment of arthritis-resistant mice with anti-IL-4 antibody increased disease severity, while treatment of susceptible mice with anti-IFN-γ antibody attenuated arthritis development (27, 34). Thus, the early activation of NK cells in susceptible mice could favor development of a Th1 phenotype and result in an enhanced inflammatory response and disease. In contrast, studies with immunodeficient mice suggest that T-cell responses do not determine disease outcome (12). In the present study, the in vivo depletion of NK cells resulted in the loss of early IFN-γ production. After footpad inoculation of C3H and BALB/c mice with B. burgdorferi, both strains develop a complex pattern of early cytokine production (25). This finding suggests that the regulation of inflammation is more complex than the simple balance between IL-4 and IFN-γ levels. Indeed, treatment of C3H/HeN mice with antibody to IL-12 also resulted in a decrease in IFN-γ production and Th1 responses (2). Anti-IL-12 treatment, however, in contrast to the results of the present study, produced a reduction in peak arthritis severity and an increase in the number of spirochetes in ear tissue. Thus, the overall levels of IFN-γ may not be the most critical factor in determining resistance or susceptibility to Lyme borreliosis.
Our inability to alter the development of arthritis in susceptible mice through the depletion of NK cells has several possible explanations. First, the ligand for the antibody treatments used, anti-NK1.1, is not expressed exclusively on NK cells and thus may have unexpected effects (21). It is also possible that due to the redundancy found in the immune system, the critical role played by early NK cell production of IFN-γ in inducing arthritis development might be circumvented in NK cell-deficient mice. Indeed, Orange and Biron (41) recently demonstrated that in murine cytomegalovirus-infected mice, IFN-αβ, TNF, and IL-12 could all be produced in an NK- and T cell-independent fashion. Also, each of these cytokines had antiviral activities which were independent of NK or T-cell activity. It was recently shown that ablation of IL-12 exacerbated Lyme arthritis development in SCID mice (3), in contrast to a reduction in arthritis severity following IL-12 depletion in normal mice (2). These studies suggest that downregulation of innate immune responses, at least in some cases, can be compensated for through the activity of T and/or B cells. Thus, in the present study, spirochetal products could activate macrophages in NK-deficient mice to produce high levels of IL-12 and drive the development of Th1 cells. Susceptible mouse strains favor a Th1 phenotype (27, 34). Production of IFN-γ from these T cells could replace that lost from NK cell depletion and drive the further activation of macrophages, effectively bypassing the need for NK cell activation. Since T-cell activation precedes spirochetal dissemination and arthritis development, these processes could proceed normally even though an integral component of the inflammatory pathway, NK cells, was missing. This scenario, however, places the critical events responsible for the genetic differences between resistant and susceptible mouse strains earlier in the inflammatory pathway. It is possible that differential activation of macrophages or granulocytes by borrelial antigens or even differences in their recruitment into the sites of infection underlies resistance or susceptibility to pathology. Studies are under way to explore these possibilities.
ACKNOWLEDGMENTS
This work was supported by NIH grant AR 44042 and by the Burroughs Wellcome Fund.
We thank Dan Brown, Kevin Swier, and Helena Shiels for critical reading of the manuscript and Jennifer Bird for excellent technical assistance.
REFERENCES
- 1.Afonso L C C, Scharton T M, Vieira L Q, Wysucka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin-12 treatment on murine Lyme borreliosis. J Clin Investig. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anguita J, Samanta S, Barthold S W, Fikrig E. Ablation of interleukin-12 exacerbates Lyme arthritis in SCID mice. Infect Immun. 1997;65:4334–4336. doi: 10.1128/iai.65.10.4334-4336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelberg R, Castro A G, Pedrosa J, Silva R A, Orme I M, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and T-cell-dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballas Z K, Rasmussen W L, Krieg A M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 6.Bancroft G J, Sheehan K C F, Schreiber R D, Unanue E R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- 7.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 8.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa M D F S, Nguyen Q A, Tchernev V T, Ashley J A, Detter J C, Blaydes S M, Brandt S J, Chotai D, Hodgman C, Solari R C E, Lovett M, Kingsmore S F. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K O. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 11.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 13.Barthold S W, de Souza M. Exacerbation of Lyme arthritis in beige mice. J Infect Dis. 1995;172:778–784. doi: 10.1093/infdis/172.3.778. [DOI] [PubMed] [Google Scholar]
- 14.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in serum of mice actively infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 15.Biron C A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 15a.Brown, C. Unpublished data.
- 16.Brown, C. R., and S. L. Reiner. Clearance of Borrelia burgdorferi may not be required for resistance to experimental Lyme arthritis. Infect. Immun. 66:2065–2071. [DOI] [PMC free article] [PubMed]
- 17.Burkhardt J K, Wiebel F A, Hester S, Argon Y. The giant organelles in beige and Chediak-Higashi fibroblasts are derived from late endosomes and mature lysosomes. J Exp Med. 1993;176:1845–1856. doi: 10.1084/jem.178.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dattwyler R J, Thomas J A, Benach J L, Golightly M G. Cellular immune response in Lyme disease: the response to mitogens, live Borrelia burgdorferi, NK cell function and lymphocyte subsets. Zentralbl Bakteriol Hyg A. 1986;263:151–159. doi: 10.1016/s0176-6724(86)80118-3. [DOI] [PubMed] [Google Scholar]
- 19.Denis M. Interleukin-12 (IL-12) augments cytolytic activity of natural killer cells toward Mycobacterium tuberculosis-infected human monocytes. Cell Immunol. 1994;156:529–536. doi: 10.1006/cimm.1994.1196. [DOI] [PubMed] [Google Scholar]
- 20.Du Chateau B K, England D M, Callister S M, Lim L C, Lovrich S D, Schell R F. Macrophages exposed to Borrelia burgdorferi induce Lyme arthritis in hamsters. Infect Immun. 1996;64:2540–2547. doi: 10.1128/iai.64.7.2540-2547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehl S, Nuesch R, Tanaka T, Myasaka M, Hengartner H, Zinkernagel R. A comparison of efficacy and specificity of three NK depleting antibodies. J Immunol Methods. 1996;199:149–153. doi: 10.1016/s0022-1759(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 22.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcombe R F, Jones K L, Stewart R M. Lysosomal enzyme activities in Chediak-Higashi syndrome: evaluation of lymphoblastoid cell lines and review of the literature. Immunodeficiency. 1994;5:131–140. [PubMed] [Google Scholar]
- 24.Hsieh C, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Listeria-induced Th1 development in αβ-TCR transgenic CD4+ T cells occurs through macrophage production of IL-12. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 25.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane-Myers A, Nickell S P. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;154:1770–1776. [PubMed] [Google Scholar]
- 27.Keane-Myers A, Nickell S P. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 28.Lahesmaa R, Shannafelt M-C, Allsup A, Soderberg C, Anzola J, Freitas V, Turek C, Steinman L, Peltz G. Preferential usage of T cell antigen receptor V region gene segment Vβ5.1 by Borrelia burgdorferi antigen-reactive T cell clones isolated from a patient with Lyme disease. J Immunol. 1993;150:4125–4135. [PubMed] [Google Scholar]
- 29.Levitz S M, Dupont M P, Smail E H. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect Immun. 1994;62:194–202. doi: 10.1128/iai.62.1.194-202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim L C L, England D M, Chateau B K, Glowacki N G, Schell R F. Borrelia burgdorferi-specific T lymphocytes induce severe destructive Lyme arthritis. Infect Immun. 1995;63:1400–1408. doi: 10.1128/iai.63.4.1400-1408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Seiler K P, Tai K F, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manetti R, Parronchi P, Giudizi M G, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (NKSF/IL-12) induces Th1-type specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matyniak J, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCartney-Francis N, Allen J B, Mizel D E, Xie Q-W, Nathan C F, Wahl S M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993;178:749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInnes I B, Leung B P, Field M, Wei X Q, Huang F-P, Sturrock R D, Kinninmonth A, Weidner J, Mumford R, Liew F Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKnight A J, Zimmer G J, Fogelman I, Wolf S F, Abbas A K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 38.Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272:405–408. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- 39.Murphy J W, Hidore M R, Wong S C. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Investig. 1993;91:1553–1566. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orange J S, Biron C A. Characterization of early IL-12, IFN-αβ, and TNF effects on antiviral state and NK responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 42.Rao T D, Fischer A, Frey A B. CD4+ Th2 cells elicited by immunization confer protective immunity to experimental Borrelia burgdorferi infection. Ann NY Acad Sci. 1994;730:364–366. doi: 10.1111/j.1749-6632.1994.tb44294.x. [DOI] [PubMed] [Google Scholar]
- 43.Rao T D, Frey A B. Protective resistance to experimental Borrelia burgdorferi infection of mice by adoptive transfer of a CD4+ T cell clone. Cell Immunol. 1995;162:225–234. doi: 10.1006/cimm.1995.1073. [DOI] [PubMed] [Google Scholar]
- 44.Saunders B M, Cheers C. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect Immun. 1996;64:4236–4241. doi: 10.1128/iai.64.10.4236-4241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaible U E, Kramer M D, Museteanu C, Zimmer G, Mossmann H, Simon M M. The severe combined immunodeficiency (scid) mouse: a laboratory model for analysis of Lyme arthritis and carditis. J Exp Med. 1989;170:1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaible U E, Gay S, Museteanu C, Kramer M D, Zimmer G, Eichmann K, Museteanu U, Simon M M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joint, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 47.Schaible U E, Kramer M D, Wallich R, Tran T, Simon M M. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur J Immunol. 1991;21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 48.Schaible U E, Wallich R, Kramer M D, Nerz G, Stehle T, Museteanu C, Simon M M. Protection against Borrelia burgdorferi infection in scid mice is conferred by presensitized spleen cells and partially by B but not T cells alone. Int Immunol. 1994;6:671–681. doi: 10.1093/intimm/6.5.671. [DOI] [PubMed] [Google Scholar]
- 49.Scharton T M, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharton-Kersten T, Afonso L C C, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 51.Scott P, Trinchieri G. The role of natural killer cells in host-parasite interactions. Curr Opin Immunol. 1995;7:34–40. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 52.Seaman W, Sleisenger M, Erikson E, Koo G. Depletion of natural killer cells in mice by monoclonal antibody to NK 1.1. J Immunol. 1987;138:4539–4544. [PubMed] [Google Scholar]
- 53.Seder R A, Gazzinelli R, Sher A, Paul W E. IL-12 acts directly on CD4+ T cells to enhance priming for IFN-γ production and diminishes IL-4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seiler K P, Vavrin Z, Eichwald E, Hibbs J B, Jr, Weis J J. Nitric oxide production during murine Lyme disease: lack of involvement in host resistance or pathology. Infect Immun. 1995;63:3886–3895. doi: 10.1128/iai.63.10.3886-3895.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shih C-M, Telford III S R, Pollack R J, Spielman A. Rapid dissemination by the agent of Lyme disease in hosts that permit fulminating infection. Infect Immun. 1993;61:2396–2399. doi: 10.1128/iai.61.6.2396-2399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang B, Biron C, She J, Higgins K, Sunshine M-J, Lacy E, Lonberg N, Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc Natl Acad Sci USA. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinberg J B, Granger D L, Pisetsky D S, Seldin M F, Misukonis M A, Mason S N, Pippen A M, Ruiz P, Wood E R, Gilkeson G S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRl-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-l-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokahama W M, Seaman W E. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]