Abstract
Understanding mechanisms that promote the maintenance of biodiversity (genetic and species diversity) has been a central topic in evolution and ecology. Previous studies have revealed that diapause can contribute to coexistence of competing genotypes or species in fluctuating environments via the storage effect. However, they tended to focus on differences in reproductive success (e.g. seed yield) and diapause termination (e.g. germination) timing. Here we tested whether different photoperiodic responses in diapause induction can promote coexistence of two parthenogenetic (asexual) genotypes of Daphnia pulex in Lake Fukami-ike, Japan. Through laboratory experiments, we confirmed that short day length and low food availability induced the production of diapausing eggs. Furthermore, we found that one genotype tended to produce diapausing eggs in broader environmental conditions than the other. Terminating parthenogenetic reproduction earlier decreases total clonal production, but the early diapausing genotype becomes advantageous by assuring reproduction in ‘short’ years where winter arrival is earlier than usual. Empirically parameterized theoretical analyses suggested that different photoperiodic responses can promote coexistence via the storage effect with fluctuations of the growing season length. Therefore, timing of diapause induction may be as important as diapause termination timing for promoting the maintenance of genetic diversity in fluctuating environments.
Keywords: coexistence, Daphnia, diapause induction, diapausing egg, photoperiodic response, storage effect
1. Introduction
How is biodiversity (including genetic and species diversity) maintained despite ecological niche overlap and competition for limited resources? Researchers have investigated this question in evolutionary biology and community ecology for decades [1–3]. One important factor that can promote the maintenance of genetic variation and species coexistence is the production of diapausing stages in fluctuating environments. Many organisms suspend development temporarily in seasonally varying habitats (reviewed in [4,5]). Previous theoretical and empirical studies demonstrated that diapausing stages result in overlapping generations and can promote coexistence of competing genotypes, lineages, or species via the storage effect [6–13]. When reproductive success varies temporally, diapausing stages enable competing genotypes to store products of favourable periods for the purpose of avoiding extinction during unfavourable periods. Without diapausing stages, genotypes with the highest geometric mean growth rate will dominate, and other genotypes will go extinct [6,14,15]. As the ongoing climate change increases extreme weather events, the storage effect with dormancy may become more important in the future [16].
In addition to temporal variation in reproductive success (e.g. seed yield in annual plants), previous studies have shown that variation in the timing of diapause termination, which causes intra-year [17–20] or year-to-year [18,20–22] asynchronous recruitment, can buffer temporal variation in reproductive success and contribute to coexistence of competing genotypes or species via the so-called storage effect, as shown both theoretically [17–19] and empirically [20–22]. Compared to timing of diapause termination, previous studies on the temporal storage effect have not examined the importance of different timing of diapause induction for promoting stable coexistence in fluctuating environments. Researchers have intensively studied adaptive evolution in the optimal timing of diapause induction [23–26], but its potential impact on the maintenance of genetic variation via the storage effect has not attracted so much attention, although timing of diapause induction can be as important as diapause termination.
Here we propose that differences in timing of diapause induction can also contribute to coexistence via the storage effect. Consider a situation where two competing genotypes have different timings of diapause induction and actively reproducing individuals cannot survive after a certain catastrophic event (e.g. the arrival of winter). Earlier production of diapausing offspring enables a genotype to ensure contributions to egg/seed banks even when a growth period is short, whereas later production of diapausing offspring results in longer growth periods for clonal reproduction and larger contributions to egg/seed banks when a growth period is long. Thus, for example, when winter comes earlier than usual in temperate lakes, an active water flea (Daphnia) population of a late-diapausing genotype may disappear before they produce diapausing eggs due to low temperatures, while an early-diapausing genotype produces a small number of diapausing eggs. On the other hand, when winter comes later than usual, the late-diapausing genotype produces more clonal offspring and thus will produce more diapausing eggs than the early-diapausing genotype. These kinds of inter-annual fluctuations in the length of the growing season may produce ‘good’ and ‘bad’ years for competing genotypes, enabling stable coexistence via the temporal storage effect.
In this study, we explored whether differences in timing of diapause induction can contribute to the maintenance of intraspecific genetic variation, and whether photoperiodic environments cause differences in timing of diapause induction between genotypes, by using two parthenogenetic (asexual) genotypes of Daphnia pulex found in Lake Fukami-ike (Nagano Prefecture, Japan). In lake Fukami-ike, water temperature in winter is usually below 6°C through the water column ([27], A Yagi 1978–2016, unpublished data). Such a low temperature decreases phytoplankton production. Because of the low temperature and resulting food shortage for Daphnia, it is likely that D. pulex needs to switch to a diapausing state for overwintering. To explore whether different photoperiodic responses in diapause induction contribute to coexistence of two genotypes, we constructed and analysed a mathematical model. Model parameterization took into consideration the results of two experiments using D. pulex. First, we examined the effects of photoperiod on the speed of clonal reproduction (via parthenogenetic reproduction) between the two genotypes during the active growing phase. Second, we measured the tendency to produce diapausing eggs under different conditions of food abundance and photoperiod.
2. Material and methods
(a) . Experimental materials
Here, we focused on Daphnia pulex, one of the keystone species in lake ecosystems [28]. Genus Daphnia has often been studied to understand the maintenance of genetic variation [29–32] and species coexistence [33]. Some prior studies demonstrated differences in life-history traits of diapause (timing of diapause induction and termination) between genotypes or species [10,33–38]. Genus Daphnia usually propagates by parthenogenetic reproduction but produces diapausing eggs by sexual reproduction in harsh environments, induced by several cues including food abundance, temperature, chemicals released from fish (kairomones) and photoperiod, in addition to overabundance (reviewed in [37]). Photoperiod is an accurate predictor of seasonal changes and thus may be responsible for different timing of diapause induction [37–40]. However, the role of photoperiodic responses in promoting coexistence of competing genotypes, lineages or species has seldom been discussed.
Daphnia pulex in Japan is composed of four lineages (JPN1, JPN2, JPN3 and JPN4), and all of them are obligate parthenogens [41]. Furthermore, they produce diapausing eggs by clonal reproduction [41], and thus we can evaluate the effect of the variation in timing of diapause induction on coexistence via the storage effect without considering sexual interactions. Each lineage has various genotypes that are identified with genetic markers in the control region of mitochondrial DNA and NADH dehydrogenase 5 (ND5), and we use ‘clone’ here to refer to individuals of a culture line that are established from a single female in the laboratory. Several prior studies revealed that multiple lineages in Japanese D. pulex coexist in a few lakes despite their ecological similarities [41] and they have both intra- and inter-lineage variation in life history and morphological traits [42]. However, to our knowledge, no study has examined variation in their diapausing traits. We studied Lake Fukami-ike (35°19N, 137°49E), one of the lakes in which two lineages of D. pulex coexist. The lake is naturally formed and is currently eutrophic, with a maximum depth of 7.8 m and a surface area of 2.2 ha [43]. We collected and analysed the varved sediment core samples from Lake Fukami-ike in a previous study [44]. The previous study found that one genotype of the JPN2 lineage, Jpn2C, established ca 2002–2003 and dominated throughout the research period, and then genotypes belonging to the JPN1 lineage, Jpn1A-C2T2 (more abundant) and Jpn1A-C1T2 (scarce), migrated to Lake Fukami-ike around 2013. Since then, these genotypes, belonging to different lineages, coexisted for about three years in the lake. However, it seems that they went extinct around 2016 probably due to an increase in planktivorous invasive fish [45]. Here we focused on Jpn1A-C2T2 and Jpn2C, which are the dominant genotypes in the JPN1 and JPN2 lineages, respectively, and asked whether these genotypes stably coexisted and if so, what mechanisms would promote their longer coexistence.
We isolated D. pulex ephippia from the layers of the sediment core samples in which the two distant genotypes, Jpn1A-C2T2 and Jpn2C, coexisted. We then decapsulated the ephippia, isolated diapausing eggs, and exposed the eggs to hatching stimuli, i.e. a long-day photoperiod (16 : 8 h light (L):dark (D)) and 18°C [46]. Hatched individuals were kept in monoclonal cultures in aged tap water under laboratory conditions (23°C, 14 : 10 h L:D, fed 0.63 mg carbon l−1 of chemostat-grown green algae, Scenedesmus: UTEX1359). These clones were identified genetically based on mitochondrial markers in ND5 with primers ND5 f21 and ND5 r1046 [41] and the control region of mitochondrial DNA with primers IAIT fw and IAIT rv1 [41,47]. Full details of these genetic identification processes are described in our previous study [45]. We used seven clones of each genotype, Jpn1-C2T2 and Jpn2C; thus, we used 14 clones in total. The mother individuals hatched from different diapausing eggs, which were picked from different ephippia, respectively.
(b) . Laboratory experiment 1: clonal reproduction
To evaluate the speed of reproducing active populations in the water column, we conducted experiments between two D. pulex genotypes with all possible combinations of seven clones (i.e. a total of 49 combinations) under two photoperiodic conditions: long-day (14 : 10 L : D) and short-day (10 : 14 L : D). The temperature was set to be 18°C according to natural conditions in the spring season in Lake Fukami-ike. We had three replicates for each combination under the two photoperiodic conditions (i.e. 49 × 3 × 2 = 294 in total). Experimental jars were filled with 180 ml aged tap water and four newborn female juveniles of one clone per genotype were added to each jar (i.e. eight individuals were in a jar at the beginning of the experiment). The newborn females were obtained using the following procedure: we transferred clutched females from subculture lines to each experimental photoperiodic condition and the first-generation offspring (generation 1) of each genotype born within a day were transferred to a 200 ml jar that was filled with aged tap water, which was refreshed every 4 days. We fed green algae, Scenedesmus, at high food concentrations (0.63 mg C l−1) every two days from day 0 (i.e. the first day of the experiment) to day 15. After that, we continued the experiment without food until day 30. On days 15 and 30, we sampled individuals after mixing the contents of the jars. On day 15, 40 ml from each jar were poured into a Petri dish and all animals present were fixed with 99% EtOH in a 1.5 ml microtube. On day 30, all living animals that remained in each experimental jar were fixed with 99% EtOH in a 5 ml microtube. Fixed animals were stored at –30°C.
The change in the frequency of each clone was analysed based on the polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method following H Ohtsuki and J Urabe 2015 (personal communication; they developed the method following [48]). Individuals (8–16) were isolated from each sample and fixed with 95% EtOH, and then they were dried for a day. To extract DNA from these individuals, we added 25 µm Quick Extract DNA Extraction Solution (Lucigen, Wisconsin, US) per individual and subjected them to thermal shock of 60°C for 2 h and 95°C for 20 min. PCR amplification of fragments of ND5 of mitochondrial DNA (775 bp) was performed using ExTaq Hot start Version (Takara) and the primer set comprising DpuND5b and DpuND5n. Each 5 µl reaction consisted of 0.5 µl of extracted DNA, 0.02 µl of TaKaRa Ex Taq HS, 0.4 µl of dNTP, 0.5 µl of 10 × ExTaq buffer and 0.2 µmol l−1 of each primer. The thermal cycling conditions were as follows: 94°C for 2 min, followed by 30 cycles of 95°C for 30 s, 48°C for 30 s and 72°C for 1 min, and finished at 72°C for 10 min. We then conducted PCR–RFLP with two restriction enzymes, Hpy 188III and MnII. Individuals' lineages were identified based on the band pattern. Each 10 µl reaction consisted of 5 µl of PCR products, 1.0 µl of CutSmart Buffer (New England Biolabs) and 0.05 µl of each restriction enzyme. The thermal shock consisted of 37°C for 2 h and 65°C for 20 min. Electrophoresis was performed at 50 V for 60 min on a 2% agarose gel. Based on PCR–RFLP, we counted the individual numbers of Jpn1A-C2T2 and Jpn2C.
The results of each combination were evaluated statistically to determine whether Jpn1A-C2T2 or Jpn2C dominated. We used the t-test to check whether the genotype frequency changed significantly at the end of the experiment (day 30) from the initial ratio of Jpn1A-C2T2:Jpn2C = 1:1 on day 0. We then calculated the percentage of Jpn2C as the fraction of the number of clone combinations in which the frequency of Jpn2C significantly exceeded that of Jpn1A-C2T2 at day 30. To evaluate the speed of clonal reproduction of each genotype in each photoperiodic condition, we compared the percentage of Jpn2C between the long-day and short-day conditions with two-sided tests of equality of proportions using the ‘prop.test’ function of R [49].
(c) . Laboratory experiment 2: life history measurement
We measured the prevalence of diapause from 14 clones in each photoperiodic condition (long-day or short-day) as the ratio of ephippial females to the total number of reared individuals. We used third-generation offspring (F3) to reduce the potentially confounding maternal and grandmaternal effects [50]. First, clutched adult females (F0 generation) were isolated from subculture lines and reared individually in 200 ml jars under long-day or short-day experimental photoperiodic conditions. When the second-generation offspring (F2) were born, they were reared individually in 200 ml jars under four experimental conditions: (1) long-day and high-food, (2) long-day and low-food, (3) short-day and high-food, and (4) short-day and low-food. We reared F3 newborn females individually in 50 ml plastic tubes under the above-mentioned four experimental conditions until the release of the third clutch. We changed the water and fed them green algae, Scenedesmus (low-food (0.063 mg C l−1) and high-food (0.63 mg C l−1)) every two days. During this experiment, we observed the reared females daily and recorded whether they had parthenogenetic eggs or ephippia, or they released parthenogenetic offspring or ephippia. When parthenogenetic offspring were born, we determined their sex and counted the number of female and male offspring under a stereo microscope (Nikon smz1500). Genotypes belonging to JPN1 can produce male offspring, but males do not contribute to reproduction [41]. Male offspring were also counted but were not used to calculate the intrinsic population growth rate.
In addition to calculating the prevalence of diapause in each clutch of each clone, to understand the mechanism behind the competition experiment, we calculated the intrinsic population growth rate (r) based on the data until the third clutch of each clone using the Euler–Lotka equation [51]: , where x is the time length of the experiment in days, k is the day of the third clutch for each individual, lx is the survival probability at day x, and mx is the number of offspring released on day x. Because no individuals died during the experiment, we assumed that lx = 1. As values of k and mx were obtained from the experiment, we could calculate r based on the Euler–Lotka equation. To test the effects of genotype (Jpn1A-C2T2 or Jpn2C), photoperiodic conditions, food conditions, and their interactions (explanatory variables) on the prevalence of diapause (a response variable), we analysed the data using a three-way ANOVA. We then compared the prevalence of diapause and intrinsic population growth rate between experimental conditions by multiple comparisons based on Tukey's honestly significant difference (HSD) test followed by Bonferroni correction. In addition, to estimate the effect of the production of diapausing eggs on the competitive ability of each genotype, we tested whether there is a trade-off between the intrinsic population growth rate and prevalence of diapause by the generalized linear model (GLM) with gamma distribution and log-link function. Similarly, a trade-off between the production of parthenogenetic eggs and ephippia was tested by GLM with Poisson distribution and log-link function. In both of these analyses, we tested the effect of prevalence of diapause or the numbers of ephippia from the first to third clutch, genotype, and their interaction (explanatory variables) on intrinsic growth rate or the numbers of parthenogenetic offspring (response variables). All statistical analyses were performed using R version 3.5.2 [49], and p < 0.05 was considered significant.
(d) . Theoretical model analysis
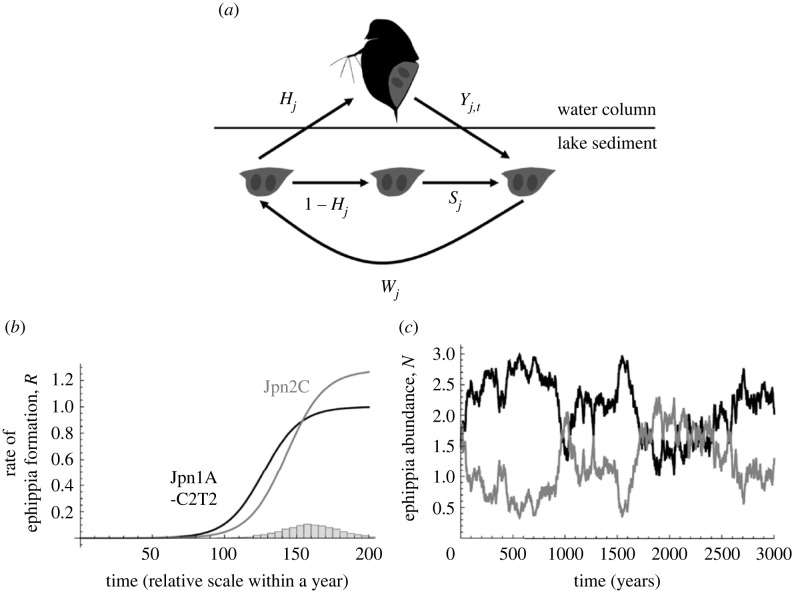
Using a theoretical model (figure 1a), we tested whether the observed difference in timing of diapause induction of the two genotypes, Jpn1A-C2T2 and Jpn2C, can promote coexistence or not. In a difference equation of diapausing egg dynamics of two genotypes [8,33], the number of diapausing eggs of genotype j (j = 1, 2) at year t (Nj,t) changes as follows:
| 2.1 |
where Hj is the hatching rate of diapausing eggs, sj is the probability of survival of diapausing eggs during summer in the egg bank, Yj(et) is the temporally fluctuating per capita production of diapausing eggs (a function of the length of the growing season et) and wj is the probability of survival of diapausing eggs during winter in the egg banks. Assuming the ‘saturating yield’ model [52], Yj(et) can be written as:
| 2.2 |
where K is the total production of diapausing eggs after the growth season and Rj is the temporally fluctuating relative production of diapausing eggs of genotype j. The subscript k in the denominator also represents a genotype. The saturating yield model assumes density-independent production of total diapausing eggs, but there is an equilibrium density and ‘results obtained for the saturating yield model generalize qualitatively to some more realistic models of density-dependent competition’ [8, p. 406]. When the length of the growing season fluctuates, it is likely that both K and R change temporally. For simplicity, however, here we assumed that K is a constant (as like [8]) and R changes depending on the length of the growing season. As the relative amount of diapausing eggs produced in the focal year should be an increasing function of the length of the growing season, we here assume that Rj can be represented as a sigmoidal function for cumulative production of diapausing eggs by the end of the growing season, et:
| 2.3 |
where aj is the maximum production of diapausing eggs by the active individuals of genotype j in the water column, bj is the shape parameter representing the steepness of diapausing egg production, and cj is the threshold to reach half of the maximum production of diapausing eggs. Thus, an early-diapausing genotype may have a smaller c value as it starts producing diapausing eggs earlier than a late-diapausing genotype. However, because of the intrinsic trade-off between production of diapausing eggs and propagation of active individuals (electronic supplementary material, figure S3), the early-diapausing genotype may have a smaller a value than the late-diapausing genotype (figure 1b). It should be noted, however, that the relative production of diapausing eggs of the early-diapausing genotype can be larger than that of the late-diapausing genotype when the length of the growing season is short (e.g. when et < 0.7 in figure 1b). We constructed a model for seasonal dynamics within a year assuming season-dependent growth rate and prevalence of diapause, and used parameters estimated from our life-history experiments to obtain the R functions (electronic supplementary material, appendix S1, figure S1). Although we estimated the parameters based on the laboratory experiments, these can differ from the actual prevalence of diapause in the wild, especially when diapause induction is affected by other additional factors (e.g. the presence of predators). It will be important to develop a method to handle such parameter uncertainty in future studies. We assumed an inter-annual fluctuation in the length of the growing season, et. The timing of winter's onset may vary from year to year in temperate lakes, as seen in the inter-annual variation of lake ice phenology [53]. As D. pulex populations cannot grow in the low temperatures of winter [54], this results in inter-annual fluctuations in the length of the growing season. In equation (2.3), the parameter et represents the length of the growing season, and we assumed that it randomly fluctuates according to a normal distribution with mean μ and variance σ2 (and with the constraint et > 0).
Figure 1.
Assumptions of the theoretical analysis (a,b) and an exemplifying simulation run of stable coexistence of the two genotypes via the temporal storage effect (c). (a) The dynamics of diapausing eggs in equation (2.1). (b) Lines represent cumulative curves of produced diapausing eggs (the black line is Jpn1A-C2T2 and the grey line is Jpn2C). Note that the values on the horizontal axis represent the relative timing of diapause induction, and thus the absolute values themselves are not significant. Parameter values are a1 = 1, a2 = 1.28, b1 = 0.0783, b2 = 0.0764, c1 = 127 and c2 = 143. The histogram in (b) indicates the inter-annual fluctuation in the length of growth season et (in this panel, µ = 160 and σ = 20). Based on the laboratory experiments, we assumed that Jpn1A-C2T2 starts producing diapausing eggs earlier than Jpn2C, and Jpn2C produces more diapausing eggs than Jpn1A-C2T2 when the growth period is long (electronic supplementary material, appendix S1). If the length of the growing season fluctuated among the years as shown by the histogram in (b), the two genotypes can stably coexist (c). Other parameter values are w1 = w2 = 0.9, H1 = H2 = 0.1, s1 = s2 = 0.9 and K = 1.
When only one genotype exists, the equilibrium abundance of diapausing eggs is and the amount of generation overlap is . Coexistence of two genotypes occurs when each genotype can increase when rare, meaning that the geometric mean of the following invasion growth rate is larger than 1 (or the arithmetic mean of log(Ni,t + 1/Ni,t) is larger than 0, where the subscript i indicates ‘invader’):
| 2.4 |
Based on the laboratory experiments, we assumed that Jpn1A-C2T21 starts to produce diapausing eggs earlier than Jpn2C (c1 < c2). Meanwhile, Jpn2C can produce more diapausing eggs at the end of the long growing season than Jpn1A-C2T2 (a1 < a2) because of the higher proportion of active individuals of this genotype at the end of a long growing season. We evaluated the effects of inter-annual growing season fluctuation, diapausing strategies of the two genotypes, and competitive ability in the water column on coexistence of the competing genotypes by numerical simulations of invasion growth rates. We conducted simulations using Wolfram Mathematica 13.3 [55], and evaluated invasibility based on simulations with 10 000 years.
3. Results
(a) . Clonal reproduction of the active population and prevalence of diapause
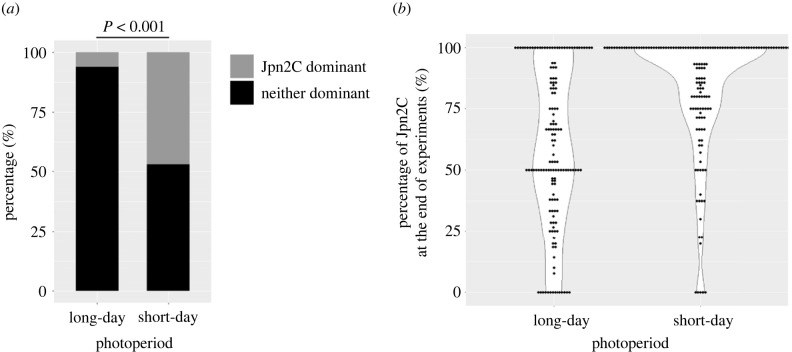
In the clonal reproduction experiments, we found that no match was dominated by Jpn1A-C2T2 and the outcomes were either the dominance of Jpn2C or the domination of neither (figure 2). The fraction of the clone combinations where Jpn2C clones dominated was significantly higher in the short-day condition (46.94%) than in the long-day condition (6.12%, figure 2, p < 0.001). The frequency dynamics of each genotype and the results for all experimental combinations are shown in electronic supplementary material, figure S2.
Figure 2.
Percentages of matches where Jpn2C dominated (grey) and neither dominated (black) on day 30 under the long-day condition (14 : 10 h light : dark) and short-day condition (10 : 14 h light : dark). (a) No match was dominated by Jpn1A-C2T2. Results were detected using the t-test. Whether the results were different between long-day and short-day was tested by two-sided tests of equality of proportions. (b) The violin plot representing percentages of Jpn2C on the last day of the experiment. Points represent the percentages of Jpn2C in each match. White areas represent the data distributions. The plot was made using ggplot2 package of R [56].
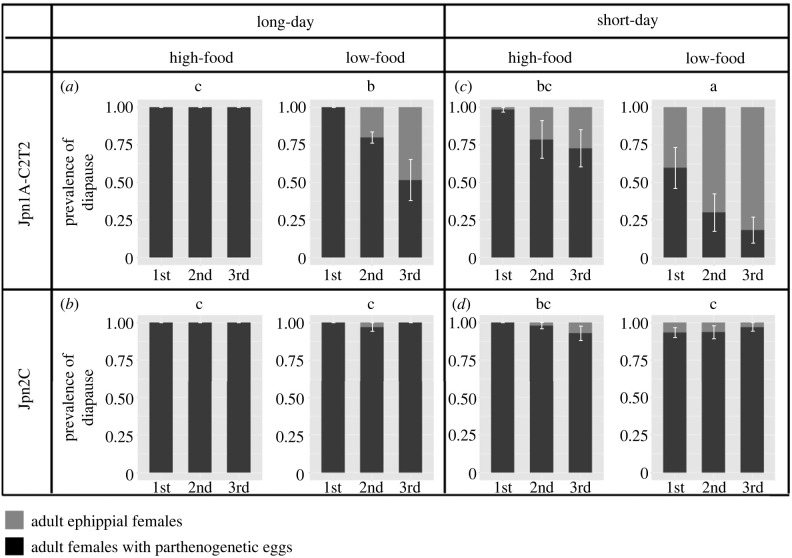
In the life-history experiments, we confirmed that the shorter day length and low food availability induced the production of diapausing eggs (figure 3) as shown by previous studies [37–40]. Interestingly, the two genotypes of D. pulex showed different diapause induction responses to the photoperiodic conditions (figure 3). The Jpn1A-C2T2 genotype showed a higher prevalence of diapause in the short-day condition than the Jpn2C genotype, regardless of food concentration (figure 3c,d). The statistical analysis supported the effect of photoperiodic conditions on the prevalence of diapause (p < 0.001, table 1). The difference in diapausing response showed the effect of interaction between genotype and photoperiodic conditions (p = 0.013, table 1). In addition, the intrinsic growth rate was negatively correlated with the prevalence of diapause (p < 0.001. electronic supplementary material, figure S3a, table S1a). Similarly, the number of parthenogenetic offspring was negatively correlated with the number of ephippia from the first to the third clutch (p < 0.001, see figure S3b and table S1b in the electronic supplementary material). While the intrinsic population growth rate was not significantly associated with genotype and the interaction between prevalence of diapause and genotype, the number of parthenogenetic eggs was significantly associated with both genotype and the interaction between genotype and the number of ephippia (electronic supplementary material, figure S3b, table S1b).
Figure 3.
Prevalence of diapause, the ratio of ephippial females (grey) to the total number of reared individuals, at the first to the third clutches of each genotype under each photoperiodic condition: long-day (14 : 10 h light : dark) (a,b) and short-day (10 : 14 h light : dark) (c,d), and each food condition: high-food (0.63 mg C l−1) and low-food (0.063 mg C l−1). The prevalence of diapause was calculated based on the counts of ephippial females relative to ephippial females plus females with parthenogenetic eggs. The black and grey bars indicate adult females with clonal and diapausing eggs, respectively. The prevalence of diapause of each genotype in each condition was compared using the Tukey HSD test (a-b-c: p < 0.005). The error bars indicate the standard error.
Table 1.
The difference in prevalence of diapause between genotypes, photoperiod and food concentration estimated by ANOVA (significance was considered at p < 0.05).
| d.f. | sum sq | mean sq | F value | Pr (>F) | |
|---|---|---|---|---|---|
| genotype | 1 | 1.553 | 1.553 | 40.065 | <0.001 |
| photoperiod | 1 | 0.767 | 0.767 | 19.797 | <0.001 |
| food | 1 | 1.017 | 1.017 | 26.238 | <0.001 |
| genotype : photoperiod | 1 | 0.256 | 0.256 | 6.611 | 0.013 |
| genotype : food | 1 | 0.757 | 0.757 | 19.544 | <0.001 |
| photoperiod : food | 1 | 0.026 | 0.026 | 0.674 | 0.416 |
| genotype : photoperiod : food | 1 | 0.017 | 0.017 | 0.439 | 0.511 |
| residuals | 48 | 1.860 | 0.039 |
(b) . Mathematical analysis
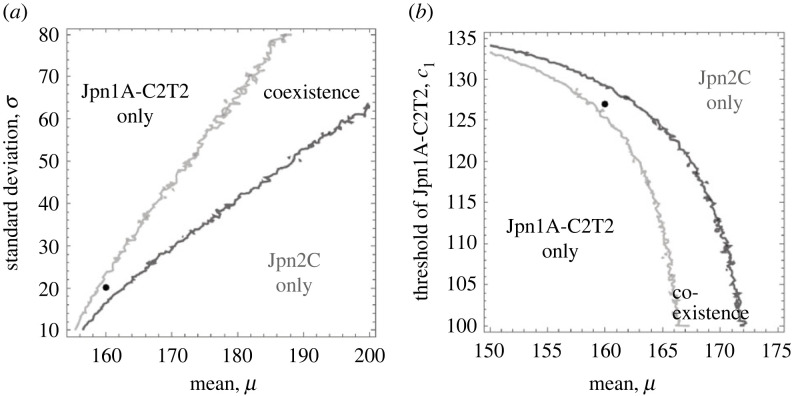
When we assume that the Jpn1A-C2T2 genotype switches to the diapausing phase earlier than the Jpn2C genotype based on the laboratory experiments (figure 3a,b, electronic supplementary material, appendix S1, figure S1), the two genotypes can coexist with the inter-annual fluctuations of the growing season length (figure 1c). Increasing the magnitude of inter-annual fluctuations of the growing season length (σ2) promotes coexistence of the two genotypes (figure 4a). Also, decreasing the parameter c1 expands the parameter space for coexistence because it results in an earlier production of diapausing eggs of the Jpn1A-C2T2 genotype and temporal niche differentiation with the Jpn2C genotype (with c2 = 0.75: figure 4b). On the other hand, increasing b1, the seasonal sensitivity of diapausing egg production of Jpn1A-C2T2 genotype can greatly expand the parameter space for coexistence (electronic supplementary material, figure S5a), while when decreasing a1, the maximum production of diapausing eggs of Jpn1A-C2T2 genotype slightly expands the parameter space for coexistence (electronic supplementary material, figure S5b).
Figure 4.
Effects of fluctuations in growing season length among years (a) and the diapause timing of the early-diapausing genotype Jpn1A-C2T2 (b) on coexistence of the two genotypes in the theoretical model. (a) Larger variation in the length of the growing season promotes stable coexistence of the two genotypes. (b) Earlier production of diapausing eggs of JPN1 promotes stable coexistence, whereas late production makes coexistence difficult. Horizontal axes are the mean of the normal distribution that the length of the growing season follows. Lines indicate parameter conditions where invasion growth rates (the logarithm of equation (2.4)) are zero. The invasion growth rate of Jpn1A-C2T2 is positive on the left of the black line, and that of Jpn2C is positive on the right of the grey line. Coexistence occurs in the area where the invasion growth rates of both Jpn1A-C2T2 and Jpn2C are positive (i.e. mutually invasible). The black points represent the parameter values in both figure 1c and 1b. The horizontal line in figure 4b indicates the parameter value in figures 1c,b, and 4a. We randomly sampled 10 000 parameter combinations, evaluated invasibility, and drew contour plots for the phase diagrams.
4. Discussion
In the present study, we tested whether diapausing responses to photoperiodic conditions differ between two genotypes of D. pulex and whether these variations in photoperiodic responses can promote coexistence of the genotypes. Our results revealed intraspecific variation in diapausing responses to photoperiodic conditions and showed that this variation can contribute to the maintenance of genetic variation via the storage effect in the presence of inter-annual fluctuations of growth conditions, suggesting that the timing of diapause induction can be as significant as the timing of diapause termination. The inter-annual fluctuations in the length of the growing season can produce ‘good’ and ‘bad’ years for competing genotypes as the early-diapausing genotype is advantageous in ‘short’ years when winter comes earlier by assuring reproduction, whereas the late-diapausing genotype produces more diapausing eggs in ‘long’ years when winter comes later. It should be noted that the effects of ‘bad’ years may not be equivalent for the competing genotypes in the wild if the early-diapausing genotype produces fewer diapausing eggs in ‘long’ years whereas the late-diapausing genotype produces no or much less diapausing eggs in ‘short’ years. The genotype-specific responses to environmental fluctuations, together with overlapping generations due to dormancy, promote stable coexistence of genotypes via the temporal storage effect.
We found a trade-off between entering diapause and the intrinsic growth rate via clonal reproduction. Both intrinsic growth rate and the number of parthenogenetic offspring showed a negative correlation with the prevalence of diapausing and the number of ephippia, respectively (electronic supplementary material figure S3, table S1). This result suggests that the higher prevalence of diapausing of the Jpn1A-C2T2 genotype (figure 3) decreases its asexual population growth rate, thereby making the Jpn2C genotype dominant in the clonal reproduction experiment (figure 2). Since the number of parthenogenetic offspring was significantly associated not only with genotype but also with the interaction between genotype and the number of ephippia (electronic supplementary material figure S3b, table S1b), the trade-off between parthenogenetic reproduction and diapause possibly differs depending on genotypes. The observed negative association between the number of ephippia and parthenogenetic female offspring as well as the induced investment on ephippia by photoperiods as a cue of unfavourable environments are consistent with recent experimental studies [38,57].
The simulation analysis supported the idea that the variation in timing of diapause induction promotes coexistence. It shows that the coexistence region increases because the Jpn1A-C2T2 genotype induces diapause relatively earlier than the Jpn2C genotype (figure 4b). As this early induction of diapause may be advantageous in years with short growth periods (when winter comes earlier than usual years: figure 1), inter-annual fluctuations of the growth period can promote the maintenance of genetic variation through temporal niche partitioning (figure 4) [2,33]. Therefore, our study suggests that photoperiodic conditions may be one of the mechanisms that differentiate phenology [58]. Furthermore, the present study suggests that not only the timing of diapause termination [17,20–22,59] but also the timing of diapause induction is important to maintain coexistence via the storage effect.
Previous studies have investigated adaptive evolution of the optimal timing of diapause induction by using theoretical and empirical approaches (e.g. [23,24]). Particularly, Hairston and Dillon intensively studied timing of diapause induction in copepods (Diaptomus sanguineus). They found that diapause is induced by photoperiod [60] one generation prior to catastrophic events (e.g. the initiation of seasonally intense fish predation) to optimize their fitness [24]. Thus, the observed different timing of diapause induction in our study may also be due to local adaptation to different timing of catastrophic events in different lakes from which each genotype migrated to Lake Fukami-ike [61]. Furthermore, when the catastrophic timing varies from year to year, they found that a bet-hedging strategy may be better than an environmentally cued strategy ([25], see also [26]). Therefore, while our study proposed that genetic variation is stably maintained due to temporal fluctuations in the length of growing season, it may not be evolutionarily stable: after a long period of time, two genotypes may eventually employ the bet-hedging strategy over environmentally-induced diapause and stable coexistence may be lost. It will be interesting to consider how short-term and long-term evolution interact with coexistence of competing genotypes and species in future studies [62,63].
While our study showed that the early-diapausing genotype decreases production of clonal offspring due to the trade-off between diapause and clonal reproduction, the opposite situation (i.e. the growth rate of clonal reproduction of an early-diapausing genotype is higher than that of a later-diapausing genotype) may also promote stable coexistence. This works when the genotype with faster clonal reproduction shows density-dependent diapause induction [64]. As diapausing individuals do not reproduce, the density-dependent production of diapausing stages results in a negative density-dependent population growth (i.e. a population with a high density induces diapause and stops growing). This negative density-dependent population growth of the early-diapausing genotype can promote coexistence, as the late-diapausing genotype can grow without competition after the diapause induction of the early-diapausing genotype (‘protection effect’ [64,65]). Therefore, inter-annual environmental variation and the storage effect are not necessary for the coexistence in this case. It would be interesting to compare the two coexistence mechanisms (i.e. the storage effect and density-dependent diapause) in future studies.
There are other coexistence-promoting factors that we did not evaluate in this study. First, variation in reproductive success and the hatching timing can affect the storage effect [17–19]. Although we implicitly assumed that the hatching from the diapausing egg bank occurs simultaneously in the two genotypes in our model, earlier hatching of the Jpn1A-C2T2 might further promote coexistence by allowing a temporal niche differentiation with Jpn2C. Photoperiodic conditions also affect timing of diapause termination ([66] but see [67–69]). Furthermore, recent studies demonstrated that population growth rates of different Daphnia lineages may vary seasonally in response to temperature and food quality [36], and thus the inter-annual variation of reproductive success may also be an important factor. Second, we did not measure the minimum resource requirement for population growth, R* [70], lineage-specific mortality rates and functional responses [71]. Thus, while we primarily focused on the temporal storage effect in this study, it may be possible for relative nonlinearity to promote coexistence [72–74]. Furthermore, different responses of lineages to predators and primary producers possibly affect coexistence. Two lineages, JPN1 and JPN2, may have different defence traits against predation (e.g. Chaoborus) [75], and different population growth rates when feeding on blue-green algae, cyanobacteria [36] (note that we fed green algae, Scenedesmus, in the laboratory experiments). Although we evaluated the clonal reproduction of two genotypes by short-term experiments in this study, long-term experiments or field observation is necessary to examine the outcome of competition (e.g. [76]). Further long-term (multigenerational) experiments and field observations may enable us to empirically test the relative contributions of different coexistence mechanisms including the resource differentiation, lineage-specific responses to predators, the storage effect and relative nonlinearity [74–79].
In addition, unfortunately, we could not observe the ongoing population dynamics of the two genotypes in Lake Fukami-ike and we cannot determine whether the two genotypes induce diapause with different timing in the wild. The population of D. pulex has reduced drastically (or gone extinct) in Lake Fukami-ike in recent years [44], probably due to the increase of planktivorous invasive fish (Lepomis macrochirus). Moreover, in Lake Fukami-ike, the period when two D. pulex genotypes cooccurred was relatively short, and it is not possible to examine whether the intraspecific difference of diapause induction could promote long-term coexistence. It will be fruitful to observe the dynamics of active populations and egg banks and conduct long-term field studies in other lakes where multiple competitive genotypes coexist in future studies. Researchers should be aware that, while photoperiodic stimuli are in general credible signals of seasonal changes [54], recent climate change may affect the relationship between diapause induction and competitive interactions, as well as the relationship between the hatching of diapausing eggs and competitive interaction [80]. Rudolf [58] suggested that shifts in phenology can affect species interaction, including coexistence, and responses to climate change. Thus, stable coexistence of two genotypes may become difficult in future environments under the ongoing climate change.
As discussed above, there are many traits to be measured in future studies, but our mathematical framework will be helpful to understanding how various trait combinations and the resultant trade-offs affect coexistence. By extending the model to incorporate spatial dynamics, it may be possible to examine whether the intraspecific difference in diapausing strategy can affect migration and range expansion. In Lake Fukami-ike, paleolimnological and population genetic analyses of diapausing eggs revealed that Jpn2C was established earlier than Jpn1A-C2T2 [45]. Further evolutionary and genetic research can contribute to our understanding of evolutionary dynamics with intraspecific interaction and spatial competition–colonization trade-off [81]. Furthermore, comparing the diapause response between a lake with multiple lineages and a lake with a single lineage will be effective to examine the adaptive response to intra-specific competition (i.e. intra-specific ecological character displacement: [19]). In addition, long-term in-depth analysis of field data is necessary to test our hypothesis: whether different timing of diapause induction based on photoperiodic responses can promote stable coexistence of multiple genotypes. Monitoring frequency dynamics of diapausing eggs of each genotype in addition to active populations throughout a year will be one of the best approaches for understanding coexistence mechanisms.
5. Conclusion
This study showed that two genotypes of D. pulex in Japan have different diapausing responses to photoperiodic conditions. Although stopping clonal reproduction earlier by producing diapausing eggs might decrease total clonal production, this variation in diapausing strategy can promote coexistence of the genotypes through the temporal storage effect. Our results suggest that the timing of diapause induction can be as significant as the timing of diapause termination for the storage effect and contribute to deepening our understanding of how genetic and species diversity are maintained in natural environments.
Acknowledgements
We thank Jotaro Urabe and two anonymous reviewers for their suggestions and constructive comments on this study. We also thank Yasushi Matsuzawa and the Fukami-ike-no-shizen-wo-aisuru-kai group for their kind accommodation of our research.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The datasets are available from Dryad Digital Repository: https://doi.org/10.5061/dryad.69p8cz977 [82].
Supplementary material is available online [83].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
Y.O.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing; M.Y.: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing; Y.H.: data curation, investigation, writing—review and editing; H.O.: data curation, investigation, writing—review and editing; T.Y.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was supported by JSPS Research Fellowships for Young Scientists (grant no. 18J22937) to Y.O., KAKENHI (grant no. 19K16223) to M.Y. and KAKENHI (grant nos 17H03730, 21H02560) to T.Y.
References
- 1.Hutchinson GE. 1959. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145-159. ( 10.1086/282070) [DOI] [Google Scholar]
- 2.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343-366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 3.Hedrick PW. 2006. Genetic polymorphism in heterogeneous environments: the age of genomics. Ann. Rev. Ecol. Evol. Syst. 37, 67-93. ( 10.1146/annurev.ecolsys.37.091305.110132) [DOI] [Google Scholar]
- 4.Alekseev VR, Vinogradova EB. 2019. Introduction to dormancy in aquatic invertebrates: mechanism of induction and termination, hormonal and molecular-genetic basis. In Dormancy in Aquatic Organisms Theory, Human Use and Modeling (eds Alekseev V, Pinel-Alloul B), pp. 7-41. ( 10.1007/978-3-030-21213-1_2) [DOI] [Google Scholar]
- 5.Radzikowski J. 2013. Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. J. Plank. Res. 35, 707-723. ( 10.1093/plankt/fbt032) [DOI] [Google Scholar]
- 6.Chesson PL, Warner RR. 1981. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 117, 923-943. ( 10.1086/283778) [DOI] [Google Scholar]
- 7.Ellner S. 1987. Alternate plant life history strategies and coexistence in randomly varying environments. Vegetation 69, 199-208. ( 10.1007/BF00038701) [DOI] [Google Scholar]
- 8.Ellner S, Hairston NG Jr. 1994. Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am. Nat. 143, 403-417. ( 10.1086/285610) [DOI] [Google Scholar]
- 9.Turelli M, Schemske DW, Bierzychudek P. 2001. Stable two-allele polymorphisms maintained by fluctuating fitnesses and seed banks: protecting the blues in Linanthus parryae. Evolution 55, 1283-1298. ( 10.1111/j.0014-3820.2001.tb00651.x) [DOI] [PubMed] [Google Scholar]
- 10.Cáceres CE, Tessier AJ. 2004. To sink or swim: variable diapause strategies among Daphnia species. Limnol. Oceanogr. 49, 1333-1340. ( 10.4319/lo.2004.49.4_part_2.1333) [DOI] [Google Scholar]
- 11.Levine JM, Rees M. 2004. Effects of temporal variability on rare plant persistence in annual systems. Am. Nat. 164, 350-363. ( 10.1086/422859) [DOI] [PubMed] [Google Scholar]
- 12.Angert AL, Huxman TE, Chesson P, Venable DL. 2009. Functional tradeoffs determine species coexistence via the storage effect. Proc. Natl Acad. Sci. USA 106, 11 641-11 645. ( 10.1073/pnas.0904512106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hairston NG Jr, Ellner S, Kearns CM. 1996. Overlapping generations: the storage effect and the maintenance of biotic diversity. In Population dynamics in ecological space and time (eds Rhodes OE Jr, Chesser RK, Smith MH), pp. 109-145. Chicago, IL: University of Chicago Press. [Google Scholar]
- 14.Haldane JBS, Jayakar SD. 1963. Polymorphism due to selection of varying direction. J. Genet. 58, 237-242. ( 10.1007/BF02986143) [DOI] [Google Scholar]
- 15.Dempster ER. 1955. Maintenance of genetic heterogeneity. Cold Spring Harb. Symp. Quant. Biol. 20, 25-32. ( 10.1101/SQB.1955.020.01.005) [DOI] [PubMed] [Google Scholar]
- 16.Coumou D, Rahmstorf S. 2012. A decade of weather extremes. Nat. Clim. Change 2, 491-496. ( 10.1038/nclimate1452) [DOI] [Google Scholar]
- 17.Chesson P, Gebauer RL, Schwinning S, Huntly N, Wiegand K, Ernest MS, Sher A, Novoplansky A, Weltzin JF. 2004. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141, 236-253. ( 10.1007/s00442-004-1551-1) [DOI] [PubMed] [Google Scholar]
- 18.Holt G, Chesson P. 2014. Variation in moisture duration as a driver of coexistence by the storage effect in desert annual plants. Theor. Popul. Biol. 92, 36-50. ( 10.1016/j.tpb.2013.10.007) [DOI] [PubMed] [Google Scholar]
- 19.Kortessis N, Chesson P. 2021. Character displacement in the presence of multiple trait differences: Evolution of the storage effect in germination and growth. Theor. Popul. Biol. 140, 54-66. ( 10.1016/j.tpb.2021.05.003) [DOI] [PubMed] [Google Scholar]
- 20.Li YM, Chesson P. 2018. Seed demographic comparisons reveal spatial and temporal niche differentiation between native and invasive species in a community of desert winter annual plants. Evol. Ecol. Res. 19, 71-84. [Google Scholar]
- 21.Adondakis S, Venable DL. 2004. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology 85, 2582-2590. ( 10.1890/03-0587) [DOI] [Google Scholar]
- 22.Facelli JM, Chesson P, Barnes N. 2005. Differences in seed biology of annual plants in arid lands: a key ingredient of the storage effect. Ecology 86, 2998-3006. ( 10.1890/05-0304) [DOI] [Google Scholar]
- 23.Taylor F. 1980. Optimal switching to diapause in relation to the onset of winter. Theor. Popul. Biol. 18, 125-133. ( 10.1016/0040-5809(80)90044-1) [DOI] [PubMed] [Google Scholar]
- 24.Hairston NG Jr, Munns WR Jr. 1984. The timing of copepod diapause as an evolutionarily stable strategy. Am. Nat. 123, 733-751. ( 10.1086/284236) [DOI] [Google Scholar]
- 25.Hairston NG Jr, Olds EJ, Munns WR Jr. 1985. Bet-hedging and environmentally cued diapause strategies of diaptomid copepods. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 22, 3170-3177. ( 10.1080/03680770.1983.11897852) [DOI] [Google Scholar]
- 26.Taylor F, Spalding JB. 1989. Timing of diapause in relation to temporally variable catastrophes. J. Evol. Biol. 2, 285-297. ( 10.1046/j.1420-9101.1989.2040285.x) [DOI] [Google Scholar]
- 27.Yagi A, Shimodaira I, Terai H, Saijo Y. 1983. Seasonal change of chlorophyll-a and bacteriochlorophyll in Lake Fukami-ike. Jap. J. Limnol. 44, 283–292. ( 10.3739/RIKUSUI.44.283) [DOI] [Google Scholar]
- 28.Miner BE, De Meester L, Pfrender ME, Lampert W, Hairston NG Jr. 2012. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. R. Soc. B 279, 1873-1882. ( 10.1098/rspb.2011.2404). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weider LJ, Makino W, Acharya K, Glenn KL, Kyle M, Urabe J, Elser JJ. 2005. Genotype x environment interactions, stoichiometric food quality effects, and clonal coexistence in Daphnia pulex. Oecologia 143, 537-547. ( 10.1007/s00442-005-0003-x) [DOI] [PubMed] [Google Scholar]
- 30.Weider LJ, Jeyasingh PD, Looper KG. 2008. Stoichiometric differences in food quality: impacts on genetic diversity and the coexistence of aquatic herbivores in a Daphnia hybrid complex. Oecologia 158, 47-55. ( 10.1007/s00442-008-1126-7) [DOI] [PubMed] [Google Scholar]
- 31.Brzezinski T, Dawidowicz P, von Elert E. 2010. The role of food quality in clonal succession in Daphnia: an experimental test. Oecologia 164, 379-388. ( 10.1007/s00442-010-1662-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawford JW, Schrader M, Hall SR, Caceres CE. 2020. Intraspecific variation in resource use is not explained by population persistence or seasonality. Oecologia 193, 135-142. ( 10.1007/s00442-020-04651-7) [DOI] [PubMed] [Google Scholar]
- 33.Cáceres CE. 1997. Temporal variation, dormancy, and coexistence a field test of the storage effect. Proc. Natl Acad. Sci. USA 94, 9171-9175. ( 10.1073/pnas.94.17.9171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hairston NG, Hansen AM, Schaffner WR. 2000. The effect of diapause emergence on the seasonal dynamics of a zooplankton assemblage. Freshw. Biol. 45, 133-145. ( 10.1046/j.1365-2427.2000.00386.x) [DOI] [Google Scholar]
- 35.Rother A, Pitsch M, HÜLsmann S. 2010. The importance of hatching from resting eggs for population dynamics and genetic composition of Daphnia in a deep reservoir. Freshw. Biol. 55, 2319-2331. ( 10.1111/j.1365-2427.2010.02441.x) [DOI] [Google Scholar]
- 36.Schaffner LR, Govaert L, De Meester L, Ellner SP, Fairchild E, Miner BE, Rudstam LG, Spaak P, Hairston NG Jr. 2019. Consumer-resource dynamics is an eco-evolutionary process in a natural plankton community. Nat. Ecol. Evol. 3, 1351-1358. ( 10.1038/s41559-019-0960-9) [DOI] [PubMed] [Google Scholar]
- 37.Gyllström M, Hansson LA. 2004. Dormancy in freshwater zooplankton: Induction, termination and the importance of benthic-pelagic coupling. Aquat. Sci. 66, 274-295. ( 10.1007/s00027-004-0712-y) [DOI] [Google Scholar]
- 38.Lever J, Drapes S, Hall MD, Booksmythe I, Snell-Rood E. 2021. Condition-dependent sexual reproduction is driven by benefits, not costs of sex. Behav. Ecol. 32, 1372-1379. ( 10.1093/beheco/arab103) [DOI] [Google Scholar]
- 39.Stross RG, Hill JC. 1965. Diapause induction in Daphnia requires two stimuli. Science 150, 1462-1464. ( 10.1126/science.150.3702.1462) [DOI] [PubMed] [Google Scholar]
- 40.Kleiven OT, Larsson P, Hobæk A. 1992. Sexual reproduction in Daphnia magna requires three stimuli. Oikos 65, 197-206. ( 10.2307/3545010) [DOI] [Google Scholar]
- 41.So M, Ohtsuki H, Makino W, Ishida S, Kumagai H, Yamaki KG, Urabe J. 2015. Invasion and molecular evolution of Daphnia pulex in Japan. Limnol. Oceanogr. 60, 1129-1138. ( 10.1002/lno.10087) [DOI] [Google Scholar]
- 42.Tian X, Ohtsuki H, Urabe J. 2019. Evolution of asexual Daphnia pulex in Japan: variations and covariations of the digestive, morphological and life history traits. BMC Evol. Biol. 19, 122. ( 10.1186/s12862-019-1453-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagi A, Kai N, Umemura M, Nagano M, Tanaka M, Shimodaira I. 2009. Fukami-ike. In Shimoinashi, Nagano: Nagano prefecture (ed. Shimoina Board of Education), pp. 327-345. [Google Scholar]
- 44.Otake Y, Ohtsuki H, Urabe J, Kimura S, Yamada K, Yoshida T. 2020. Long-term dynamics of a cladoceran community from an early stage of lake formation in Lake Fukami-ike, Japan. Ecol. Evol. 11, 1240-1253. ( 10.1002/ece3.7112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otake Y, Innan H, Ohtsuki H, Urabe J, Yamada K, Yoshida T. 2022. Population genetic dynamics during colonisation and establishment of an obligate parthenogenetic Daphnia pulex population in a small lake of a continental archipelago. Freshwater Biology 67, 1428-1438. ( 10.1111/fwb.13951) [DOI] [Google Scholar]
- 46.Cuenca Cambronero M, Orsini L. 2018. Resurrection of dormant Daphnia magna: protocol and applications. J. Vis. Exp. e56637. ( 10.3791/56637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paland S, Colbourne JK, Lynch M. 2005. Evolutionary history of contagious asexuality in Daphnia pulex. Evolution 59, 800-813. ( 10.1554/04-421) [DOI] [PubMed] [Google Scholar]
- 48.Ishida S, Ohtsuki H, Awano T, Tsugeki NK, Makino W, Suyama Y, Urabe J. 2012. DNA extraction and amplification methods for ephippial cases of Daphnia resting eggs in lake sediments: a novel approach for reconstructing zooplankton population structure from the past. Limnology 13, 261-267. ( 10.1007/s10201-012-0380-x) [DOI] [Google Scholar]
- 49.Team R.C. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- 50.Alekseev V, Lampert W. 2001. Maternal control of resting-egg production in Daphnia. Nature 414, 899-901. ( 10.1038/414899a) [DOI] [PubMed] [Google Scholar]
- 51.Roff DA. 2001. Life history evolution, pp. 1-11. Boston, MA: Sinauer Associates, Inc. [Google Scholar]
- 52.Levin SA, Cohen D, Hastings A. 1984. Dispersal strategies in patchy environments. Theor. Popul. Biol. 26, 165-191. ( 10.1016/0040-5809(84)90028-5) [DOI] [Google Scholar]
- 53.Beyene MT, Jain S, Gupta RC. 2018. Linear-circular statistical modeling of lake ice-out dates. Water Resour. Res. 54, 7841-7858. ( 10.1029/2017wr021731) [DOI] [Google Scholar]
- 54.Alekseev VR. 2007. Diapause in crustaceans: peculiarities of induction. In Diapause in aquatic invertebrates theory and human use, vol. 84, Monographiae Biologicae (eds Alekseev VR, de Stasio BT, Gilbert JJ), pp. 29-63. Dordrecht, The Netherlands: Springer. ( 10.1007/978-1-4020-5680-2_3) [DOI] [Google Scholar]
- 55.Wolfram Research Inc. 2023. Mathematica, v.13.3. Champaign, IL, USA.
- 56.Wickam H. 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- 57.Hadany L, Otto SP. 2007. The evolution of condition-dependent sex in the face of high costs. Genetics 176, 1713-1727. ( 10.1534/genetics.107.074203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rudolf VHW. 2019. The role of seasonal timing and phenological shifts for species coexistence. Ecol. Lett. 22, 1324-1338. ( 10.1111/ele.13277) [DOI] [PubMed] [Google Scholar]
- 59.Chesson P, Huntly NJ, Roxburgh SH, Pantastico-Caldas M, Facelli JM. 2012. The storage effect: definition and tests in two plant communities. In Temporal dynamics and ecological process (eds Kelly CK, Bowler MG, Fox GA), pp. 11-40. Cambridge University Press. ( 10.1017/CBO9781139048170.003) [DOI] [Google Scholar]
- 60.Hairston NG, Dillon TA. 1990. Fluctuating selection and response in a population of freshwater copepods. Evolution 44, 1796-1805. ( 10.2307/2409508) [DOI] [PubMed] [Google Scholar]
- 61.Hairston NG, Olds EJ. 1984. Population differences in the timing of diapause: adaptation in a spatially heterogeneous environment. Oecologia 61, 42-48. ( 10.1007/BF00379086) [DOI] [PubMed] [Google Scholar]
- 62.Ellner SP, Hairston NG Jr, Kearns CM, Babai D. 1999. The roles of fluctuating selection and long-term diapause in microevolution of diapause timing in a freshwater copepod. Evolution 53, 111-122. ( 10.1111/j.1558-5646.1999.tb05337.x) [DOI] [PubMed] [Google Scholar]
- 63.Yamamichi M, Letten AD, Schreiber SJ. 2023. Eco-evolutionary maintenance of diversity in fluctuating environments. Ecol. Lett. 26, S152-S167. ( 10.1111/ele.14286) [DOI] [PubMed] [Google Scholar]
- 64.Montero-Pau J, Serra M. 2011. Life-cycle switching and coexistence of species with no niche differentiation. PLoS One 6, e20314. ( 10.1371/journal.pone.0020314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aránguiz-Acuña A, Ramos-Jiliberto R. 2014. Diapause may promote coexistence of zooplankton competitors. J. Plank. Res. 36, 978-988. ( 10.1093/plankt/fbu034) [DOI] [Google Scholar]
- 66.Gyllström M. 2004. Induction and termination of diapause in a freshwater zooplankton community. Archiv für Hydrobiologie 161, 81-97. ( 10.1127/0003-9136/2004/0161-0081) [DOI] [Google Scholar]
- 67.Davison J. 1969. Activation of the ephippial egg of Daphnia pulex. J. Gen. Physiol. 53, 562-575. ( 10.1085/jgp.53.5.562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinceel T, Vanschoenwinkel B, Uten J, Brendonck L. 2013. Mechanistic and evolutionary aspects of light-induced dormancy termination in a temporary pond crustacean. Freshwater Science 32, 517-524. ( 10.1899/12.157.1) [DOI] [Google Scholar]
- 69.Ślusarczyk M, Flis S. 2019. Light quantity, not photoperiod terminates diapause in the crustacean Daphnia. Limnology and Oceanography 64, 124-130. ( 10.1002/lno.11023) [DOI] [Google Scholar]
- 70.Tilman D. 1982. Resource competition and community structure. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 71.Holling CS. 1965. The functional response of predators to prey density and its role in mimicry and population regulation. The Memoirs of the Entomological Society of Canada 97, 5-60. ( 10.4039/entm9745fv) [DOI] [Google Scholar]
- 72.Armstrong RA, McGehee R. 1980. Competitive exclusion. Am. Nat. 115, 151-170. ( 10.1086/283553) [DOI] [Google Scholar]
- 73.Chesson P. 2003. Quantifying and testing coexistence mechanisms arising from recruitment fluctuations. Theor. Popul. Biol. 64, 345-357. ( 10.1016/s0040-5809(03)00095-9) [DOI] [PubMed] [Google Scholar]
- 74.Yuan C, Chesson P. 2015. The relative importance of relative nonlinearity and the storage effect in the lottery model. Theor. Popul. Biol. 105, 39-52. ( 10.1016/j.tpb.2015.08.001) [DOI] [PubMed] [Google Scholar]
- 75.Tian X-F, Toyota M, Ohtsuki H, Urabe J. 2024. Lineage-specific trait variations and plasticity of obligate parthenogenetic animals following the expansion of distribution range to a continental archipelago. J. Syst. Evol. ( 10.1111/jse.13015) [DOI] [Google Scholar]
- 76.Letten AD, Dhami MK, Ke PJ, Fukami T. 2018. Species coexistence through simultaneous fluctuation-dependent mechanisms. Proc. Natl Acad. Sci. USA 115, 6745-6750. ( 10.1073/pnas.1801846115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwabuchi T, Urabe J. 2012. Food quality and food threshold: implications of food stoichiometry to competitive ability of herbivore plankton. Ecosphere 3, 6. ( 10.1890/es12-00098.1) [DOI] [Google Scholar]
- 78.Armitage DW, Jones SE. 2019. Negative frequency-dependent growth underlies the stable coexistence of two cosmopolitan aquatic plants. Ecology 100, e02657. ( 10.1002/ecy.2657) [DOI] [PubMed] [Google Scholar]
- 79.Zepeda V, Martorell C. 2019. Fluctuation-independent niche differentiation and relative non-linearity drive coexistence in a species-rich grassland. Ecology 100, e02726. ( 10.1002/ecy.2726) [DOI] [PubMed] [Google Scholar]
- 80.Jones NT, Gilbert B. 2016. Changing climate cues differentially alter zooplankton dormancy dynamics across latitudes. J. Anim. Ecol. 85, 559-569. ( 10.1111/1365-2656.12474) [DOI] [PubMed] [Google Scholar]
- 81.Tilman D. 1994. Competition and biodiversity in spatially structured habitats. Ecology 75, 2-16. ( 10.2307/1939377) [DOI] [Google Scholar]
- 82.Otake Y, Yamamichi M, Hirata Y, Odagiri H, Yoshida T. 2024. Data from: Different photoperiodic responses in diapause induction can promote the maintenance of genetic diversity via the storage effect in Daphnia pulex. Dryad Digital Repository. ( 10.5061/dryad.69p8cz977) [DOI] [PMC free article] [PubMed]
- 83.Otake Y, Yamamichi M, Hirata Y, Odagiri H, Yoshida T. 2024. Different photoperiodic responses in diapause induction can promote the maintenance of genetic diversity via the storage effect in Daphnia pulex. Figshare. ( 10.6084/m9.figshare.c.7041933) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Otake Y, Yamamichi M, Hirata Y, Odagiri H, Yoshida T. 2024. Data from: Different photoperiodic responses in diapause induction can promote the maintenance of genetic diversity via the storage effect in Daphnia pulex. Dryad Digital Repository. ( 10.5061/dryad.69p8cz977) [DOI] [PMC free article] [PubMed]
- Otake Y, Yamamichi M, Hirata Y, Odagiri H, Yoshida T. 2024. Different photoperiodic responses in diapause induction can promote the maintenance of genetic diversity via the storage effect in Daphnia pulex. Figshare. ( 10.6084/m9.figshare.c.7041933) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets are available from Dryad Digital Repository: https://doi.org/10.5061/dryad.69p8cz977 [82].
Supplementary material is available online [83].