Abstract
Background & Aims
Voxilaprevir/velpatasvir/sofosbuvir (VOX/VEL/SOF) is highly effective for re-treatment of direct-acting antiviral (DAA)-experienced patients with chronic HCV infection. In the present study, predictors of virologic treatment response were analyzed in an integrative analysis of three large real-world cohorts.
Methods
Consecutive patients re-treated with VOX/VEL/SOF after DAA failure were enrolled between 2016 and 2021 in Austria, Belgium, Germany, Italy, Spain and Switzerland.
Results
A total of 746 patients were included: median age was 56 (16-88) years and 77% were male. Most patients were infected with HCV genotype 1 (56%) and 3 (32%). 86% of patients carried resistance-associated substitutions in the NS3, NS5A or NS5B regions. Overall, 95.4% (683/716) of patients achieved a sustained virologic response. Treatment effectiveness was significantly affected by advanced liver disease (p <0.001), hepatocellular carcinoma (p <0.001), higher baseline ALT levels (p = 0.02), HCV genotype 3 (p <0.001), and prior VEL/SOF treatment (p = 0.01). In a multivariate analysis, only HCV genotype 3, hepatocellular carcinoma and cirrhosis turned out to be independent predictors of treatment failure. Resistance-associated substitutions, as well as the presence of rare genotypes, did not impact treatment outcome. The effectiveness of rescue therapy with glecaprevir/pibrentasvir and SOF, with or without ribavirin, for 12 to 24 weeks was found to be high (100%).
Conclusions
Infection with HCV genotype 3, the presence of liver cancer and cirrhosis are independently associated with failure of VOX/VEL/SOF re-treatment. It is unclear whether the addition of ribavirin and/or extension of treatment duration may be effective to avoid virologic relapse on VOX/VEL/SOF. However, rescue treatment with glecaprevir/pibrentasvir+SOF seems to be effective.
Impact and implications
Representative data on the effectiveness of voxilaprevir/velpatasvir/sofosbuvir (VOX/VEL/SOF) in clinical practice are still scarce and the collection of a larger number of patients with difficult-to-treat cofactors including the assessment of resistance-associated substitution profiles is required before more specific recommendations for optimal re-treatment in these patients can be given. Thus, we aimed to analyze treatment effectiveness and predictors of virologic response to VOX/VEL/SOF in an integrative analysis of three large real-word cohorts. The study results, derived from a multicenter cohort consisting of 746 patients, demonstrated that re-treatment with VOX/VEL/SOF is an effective salvage therapy associated with an overall per protocol sustained virologic response rate of 95%. Hepatocellular carcinoma onset, cirrhosis and HCV genotype 3 were identified as independent negative predictors of treatment response, whereas resistance-associated substitutions, as well as rare genotypes and chimera, did not impact sustained virologic response rates following re-treatment with VOX/VEL/SOF.
Keywords: Re-treatment, Hepatitis C virus, DAA treatment failure, Voxilaprevir/velpatasvir/sofosbuvir
Graphical abstract
Highlights
-
•
Overall high effectiveness of VOX/VEL/SOF was demonstrated in an integrative analysis of large real-world studies.
-
•
Significantly lower SVR rates were observed in patients with GT3, advanced liver disease, HCC onset and VEL/SOF pretreatment.
-
•
RASs as well as rare genotypes and chimera did not impact SVR rates following VOX/VEL/SOF re-treatment.
-
•
Cirrhosis, GT3 and HCC onset were identified as independent negative predictors of treatment response to VOX/VEL/SOF.
Introduction
With the introduction of direct-acting antivirals (DAAs) for the treatment of HCV in recent years, sustained virological response (SVR) rates to antiviral therapy have dramatically increased, exceeding 95%, regardless of HCV genotype (GT), disease stage, or treatment history.1 However, despite high SVR rates following DAA treatment in both clinical trials and real-world studies, even in optimal settings about 2% of patients fail to achieve an SVR and need alternative treatment options.2 These patients are of particular concern because of the possible presence of negative factors influencing response to DAA therapies.
Recently, the fixed dose combination of the HCV NS3/4A protease inhibitor voxilaprevir (VOX), the NS5A inhibitor velpatasvir (VEL) and the NS5B inhibitor sofosbuvir (SOF) has received regulatory approval as a potent salvage regimen for DAA-experienced patients in many countries and is currently recommended as the first-line re-treatment option for compensated patients with HCV by international liver associations like EASL and AASLD.[3], [4], [5] In the approval studies, the combination of VOX/VEL/SOF has been evaluated in patients with and without previous exposure to NS5A inhibitors.6,7 The results demonstrated excellent efficacy and safety, with VOX/VEL/SOF yielding an overall SVR rate of 96% in NS5A inhibitor-experienced patients.6 No epidemiological, clinical, biochemical or virologic parameters were associated with treatment response. In particular, HCV resistance patterns assessed by deep sequencing were also identical before and after VOX/VEL/SOF rescue treatment in the majority of patients and had no influence on treatment outcome.8 However, the number of NS5A-experienced patients with virologic failure was very low (n = 7) and the potential importance of resistance-associated substitutions (RASs) remained unclear. RASs, which are selected by NS5A inhibitors, maintain viral fitness and persist long-term, being detectable even many years after the end of failed treatment.[9], [10], [11]
Consecutive real-world studies from different countries confirmed the observation of an overall high effectiveness of VOX/VEL/SOF as rescue treatment with SVR rates ranging between 91% and 96%.[12], [13], [14], [15], [16], [17], [18], [19] However, all those studies enrolled a limited number of patients and were highly heterogeneous not only with respect to distribution of HCV GT, including HCV GT1a and 3a, but also concerning further difficult-to-treat cofactors such as the presence of cirrhosis, pre-treatment with VEL/SOF as well as the rates of active or previous liver cancer.[12], [13], [14], [15],17 Accordingly, some studies reported significantly lower SVR rates in patients previously treated with VEL/SOF, while this was not observed in others.13,15,17 Μoreover, SVR rates in patients with HCV GT3a and cirrhosis were highly variable between the different studies ranging between 81-92% and 81-90%, respectively.20 Active and previous hepatocellular carcinoma (HCC) also seemed to decrease the rate of cure in some studies.12,13,16 However, the presence of HCC as well as its impact on VOX/VEL/SOF treatment outcome also differed significantly between the studies.
Taken together, treatment with VOX/VEL/SOF over 12 weeks seems to be an effective and safe option for re-treating patients with HCV. However, representative data in clinical practice are still scarce and the collection of a larger number of patients with difficult-to-treat cofactors including the assessment of RAS profiles is required before more specific recommendations for optimal re-treatment in these patients can be given, as no third-line therapies are available in many countries. Thus, the aim of the following study was to observe potential predictors of negative treatment outcomes on VOX/VEL/SOF by performing an integrative analysis of three large multicenter real-life cohorts consisting of representative subgroups with difficult-to-treat patients and a sufficient number of virologic failure patients.
Patients and methods
Patients (n = 746) with chronic hepatitis C, who had previously failed combined treatment with an interferon (IFN)-free regimen were included in the study. Data on the Italian and Spanish cohorts have already been published and details on the study design, assessment and measurement of data from these cohorts are described elsewhere.12,14 Regarding data collection for the Frankfurt resistance database, patients with HCV, consecutively starting antiviral re-treatment with VOX/VEL/SOF after DAA failure were enrolled between March 2016 and October 2021 in a retrospective longitudinal real-life study in 173 centers in Germany, Austria, Belgium and Switzerland. All patients received VOX/VEL/SOF; ribavirin (RBV) was added at the physician’s discretion. In order to conduct routine diagnostic HCV resistance testing, serum samples of each patient were collected prior to re-treatment. Clinical and virological characteristics were recorded at baseline, during and after the end of treatment (EOT). Diagnosis of liver fibrosis and cirrhosis was at the physician’s discretion and was established by ultrasound, laboratory parameters and non-invasive liver fibrosis assessments according to EASL guidelines.21 SVR was defined as undetectable HCV RNA 12 weeks after the EOT (SVR 12) and was calculated by per protocol (PP) analysis.
Virological failure was defined as an HCV RNA level ≥15 IU/ml. For the various epidemiological, clinical, virological and biochemical parameters, data were not always available from all patients. Thus, medians, ranges, and percentages were always calculated based on the corresponding available data which, at baseline, were as follows: sex, n = 745/746 (99%); age, n = 695/746 (93%); IFN experience, n = 569/746 (76%); stage of liver fibrosis, n = 483/746 (65%); cirrhosis status, n = 736/746 (99%); Child-Turcotte-Pugh (CTP) score, n = 245/286 patients with known cirrhosis (86%); HCC occurrence, n = 696/746 (93%); HCV GT, n = 746/746 (100%); baseline HCV RNA, n = 516/746 (69.2%); alanine aminotransferase (ALT), n = 302/746 (40.5%); gamma-glutamyltransferase, n = 273/746 (36.6%); bilirubin, n = 291/746 (39.0%); albumin, n = 132/746 (17.7%); platelets, n = 302/746 (40.5%); international normalized ratio, n = 250/746 (33.5%); creatinine, n = 299/746 (40.1); previous DAA course, n = 738/746 (98.9%); RBV use in previous DAA course, n = 730/746 (97.9%); RBV use in VOX/VEL/SOF treatment, n = 745/746 (99.9%); liver transplant, n = 603/746 (80.8%); RAS analysis, n = 547/746 (73%); NS3 RASs, n = 420/547 (77%); NS5A RASs, n = 526/547 (96.2%); NS5B RASs, n = 526/547 (96.2%). Information on combined baseline RASs (NS3/NS5A/NS5B) was available in n = 392/547 patients (71.7%).
After exclusion of patients lost to follow-up, those with HCV re-infection, and those with pending results at SVR12, 716/746 cases remained for PP analysis. Sub-analyses of PP data were calculated based on the referring available baseline data, which were as follows: sex, n = 715/716 (99%); age, n = 669/716 (93.4%); IFN experience, n = 544/716 (76.0%); stage of liver fibrosis, n = 471/716 (65.8%); cirrhosis status, n = 706/716 (98.6%); CTP score, n = 238/706 (33.7%); HCC occurrence, n = 672/716 (93.9%); HCV GT, n = 716/716 (100%); baseline HCV RNA, n = 496/716 (69.3%); ALT, n = 301/716 (42.0%); gamma-glutamyltransferase, n = 272/716 (38.0%); bilirubin, n = 290/716 (40.5%); albumin, n = 132/716 (18.4%); platelets, n = 301/716 (42.0%); international normalized ratio, n = 249/716 (34.8%); creatinine, n = 298/716 (41.6%); previous DAA course, n = 708/716 (98.9%); RBV use in previous DAA course, n = 700/716 (97.8%); RBV use in VOX/VEL/SOF, n = 715/716 (99.9%); liver transplant, n = 573/716 (80.0%); RAS analysis, n = 514/716 (71.8%); NS3 RASs, n = 399/514 (77.6%); NS5A RASs, n = 498/514 (96.9%); NS5B RASs, n = 498/514 (96.9%). Information on combined baseline RASs (NS3/NS5A/NS5B) was available in n = 372/514 patients (72.4%).
Recommendations for rescue treatment were given in accordance with EASL/AASLD guidelines, but individual therapy decisions were at the discretion of the treating physician. This study was approved by the ethics committees of the leading centers of this study (University Hospital Frankfurt, Germany; University Hospital Barcelona, Spain and University Hospital Milano, Italy) and conducted in accordance with the Declaration of Helsinki.
NS3, NS5A and NS5B amplification, sequencing and RAS analysis
This study included data on resistance analysis performed in centers in Germany, Italy and Spain: University Hospital Frankfurt (European HCV resistance database), University Hospital Milan (Lombardia and Veneto Networks) and Vall d’Hebron Barcelona Hospital Campus (Spanish Registry HEPA-C).9,12,14
Population sequencing of NS3, NS5A and NS5B genes were conducted at the leading centers in Frankfurt, Germany; Pavia, Italy; and Barcelona, Spain according to previously published protocols.12,14,[22], [23], [24] After RNA extraction and cDNA synthesis, the NS3, NS5A and NS5B genes were amplified using nested PCRs and sequenced on ABI Prism sequencer at the local study centers. Additionally, deep sequencing was conducted on certain patient samples using MiSeq Illumina platform (Illumina, San Diego, CA), comparing samples collected before starting triple therapy and at treatment failure.25 All sequences were proofread and alignments were created using BioEdit version 7.2.5 (T. Hall, Ibis Therapeutics, Carlsbad). HCV geno- and subtypes were re-evaluated based on nucleotide sequences. RASs were regarded as relevant if they were described in vivo in association with treatment failure and/or they conferred a greater than 2-fold change in drug susceptibility to approved DAAs in comparison to a wild-type reference strain on an in vitro replicon assay.20,26,27
Statistical analysis
Categorial variables were reported as frequencies (percentages) and continuous variables as median (range). Variables with non-normal distribution were analyzed with the Mann-Whitney U test and expressed as median and interquartile range. Categorial variables were compared using the Chi-square or Fisher’s exact test, as appropriate, and expressed as frequencies and percentages. A p value of <0.05 was considered statistically significant. Variables showing p <0.05 in the univariate model were analyzed in a multivariate logistic regression model. Odds ratio (ORs) and 95% CIs were calculated for the independent predictive factors of SVR. Statistical analysis was performed using IBM SPSS 26.0 statistical software package (SPSS/IBM, Munich, Germany).
Results
Patient population
In total, 746 patients consecutively starting VOX/VEL/SOF were enrolled between March 2016 and October 2021. Of those, 430 patients were included in 173 centers of the German Resistance Database in Germany, Austria, Belgium and Switzerland. 179 patients were enrolled in 27 centers of the Lombardia and Veneto Networks (Northern Italy) and 137 patients were included in 27 Spanish centers through a National Registry (HEPA-C) under the auspices of the Spanish Association for the Study of the Liver (AFEH). Concerning data from the Spanish and Italian cohorts, an updated follow-up of already published data was integrated in the current analysis.12,14 Patient characteristics are presented in Table 1. Due to the differing availability of information on baseline characteristics, medians, ranges and percentages of each variable refer to the corresponding available data mentioned in the methods section. Median age was 56 (16-88) years and 573 were males. At baseline, median HCV RNA was 6.03 (1.8-7.9) log IU/ml and median ALT values were 55 (10-538) U/L. Among 483 patients with available information, fibrosis stage was classified as F0-2 in 29% and F3-4 in 72%. At baseline, 286 out of 736 (39%) patients with available information presented with cirrhosis, of whom 220 (77%) had compensated (CTP class A) and 25 (9%) had a history of decompensated (CTP class B/C) cirrhosis. Thirty-seven patients (5%) had a previous history of HCC and 28 patients (4%) presented with HCC during or after re-treatment with VOX/VEL/SOF. Moreover, 19 patients (3%) had received liver transplantation prior to re-treatment. Most patients were infected with HCV GT1 (GT1, 56%; GT1a, 25%; GT1b, 31%) and GT3 (32%), whereas 4% had HCV GT2, 7% GT4, and 0.7% HCV GT6. Data on rare geno/subtypes and chimera were available in the German Resistance Database and were detected in 24 patients (Table S1).
Table 1.
Baseline characteristics (n = 746).
| Characteristics | n (%) |
|---|---|
| Age (years), median (range) | 56 (16-88) |
| Males, n (%) | 573 (77) |
| IFN experienced, median (range) | 177 (31) |
| Fibrosis, median (range) | |
| F0–F2 | 137 (29) |
| F3–F4 | 346 (72) |
| n. a. | 263 (35) |
| Cirrhosis, median (range) | 286 (39) |
| CTP score, n (%) | |
| A (5-6) | 220 (77) |
| B (7-9) | 23 (8) |
| C (>10) | 2 (1) |
| n. a. | 41 (14) |
| HCC history, n (%) | 80 (11) |
| Previous HCC | 37 (5) |
| HCC onset during/after treatment | 28 (4) |
| n. a. | 15 (2) |
| HCV genotype, n (%) | |
| 1 | 419 (56) |
| 1a | 183 (25) |
| 1b | 228 (31) |
| Other | 8 (1) |
| 2 | 32 (4) |
| 3 | 236 (32) |
| 4 | 54 (7) |
| 5 | 0 (0) |
| 6 | 5 (0.7) |
| Baseline HCV RNA (log IU/ml), median (range) | 6.03 (1.8-7.9) |
| ALT (U/L), median (range) | 55 (10-538) |
| GGT (U/L), median (range) | 56 (9-708) |
| Bilirubin (IU/L), median (range) | 0.7 (0.2-8.0) |
| Albumin (IU/L), median (range) | 4.2 (2.8-5.1) |
| PLT (103/mm), median (range) | 170 (35-539) |
| INR, median (range) | 1.0 (0.9-3.5) |
| Creatinine (mg/dl), median (range) | 0.8 (0.4-6.8) |
| Previous DAA course, n (%) | |
| Sofosbuvir-based | 465 (62) |
| SIM/SOF | 4 (0.5) |
| DCV/SOF | 109 (15) |
| LDV/SOF | 213 (29) |
| VEL/SOF | 123 (17) |
| OMB/PTV | 15 (2) |
| ELB/GZR | 85 (12) |
| G/P | 58 (8) |
| DSB plus OMB/PTV | 108 (15) |
| Others | 23 (3) |
| RBV use during DAA course, n (%) | 172 (24) |
| RBV use in VOX/VEL/SOF treatment, n (%) | 61 (8) |
| Liver transplant, n (%) | 19 (3) |
ALT, alanine aminotransferase; CTP, Child-Turcotte-Pugh; DCV, daclatasvir; DSB, dasabuvir; ELB, elbasvir; GGT, gamma-glutamyltransferase; G/P, glecaprevir/pibrentasvir; GT, genotype; GZR, grazoprevir; IFN, interferon; INR, international normalized ratio; LDV, ledipasvir; OMB, ombitasvir; PLT, platelet count; PTV, paritaprevir; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
All patients had previously received a DAA-based IFN-free regimen. Features of the prior DAA regimen before re-treatment with VOX/VEL/SOF, which were known in 738 patients, are presented in Table 1 and Table S2. Ledipasvir/sofosbuvir (LDV/SOF) was the most common prior regimen used in 29% of patients, followed by velpatasvir/sofosbuvir (VEL/SOF; 17%), daclatasvir/sofosbuvir (15%), dasabuvir/ombitasvir/paritaprevir (15%) and elbasvir/grazoprevir (12%). Overall, 96% of patients (n = 711) had failed an NS5A- and 37% an NS3/4A-containing regimen (n = 270) and a total of 3 out of 738 patients with available information had twice failed to achieve an SVR after being treated with protease- and/or a NS5A inhibitor-containing regimen.
Concerning re-treatment duration, most patients (99%) received VOX/VEL/SOF for 12 weeks. However, in a few cases a shortened treatment duration was observed: four patients were treated with VOX/VEL/SOF over 4 weeks and one patient over 8 weeks. Add-on administration of RBV was observed in 8% of re-treatment courses. Patients receiving RBV more frequently carried RASs at baseline (p = 0.04) but did not significantly differ from others with respect to further clinical features such as the prevalence of cirrhosis (p = 0.41), HCV GT (HCV GT 3 vs. others: p = 0.20) and the type of previous DAA course (SOF vs. non-SOF: p = 1.00).
RASs at baseline in patients re-treated with VOX/VEL/SOF
Resistance testing was available in 547 patients (73%) at baseline (start of VOX/VEL/SOF treatment; Table 2), of whom 471 patients (86%) harbored RASs prior to re-treatment with VOX/VEL/SOF. NS5A RASs were observed to be the most frequent (82%), followed by NS5B (34%) and NS3 RASs (30%; Table 2). Combined RASs were detected in 174 out of 392 patients (44%) with available data (NS5A + NS3 in 17%; NS5A + NS5B in 17%, NS5A + NS3 + NS5B in 10%; Table 2). Regarding patients harboring NS5A RASs at baseline, most of them carried one single NS5A RAS (47%), whereas multiple RASs (≥3 RASs) could be detected in a minority of patients (5%), only. Y93H was found to be the most prevalent NS5A RAS in all DAA treatment-failing patients (56%).
Table 2.
RASs at baseline in all patients re-treated with VOX/VEL/SOF with a complete follow-up.
| Resistance testing | n = 547 |
|---|---|
| No RAS | 76 (14%) |
| Any RAS | 471 (86%) |
| RAS types and variants | |
| Any NS3 | 124 (30%) |
| Any NS5A | 430 (82%) |
| Any Y93H | 295 (56%) |
| Any NS5B | 177 (34%) |
| Combined RAS | |
| NS5A + NS3 | 65 (17%) |
| NS5A + NS5B | 68 (17%) |
| NS5A + NS3 + NS5B | 41 (10%) |
| Frequencies of NS5A RAS | |
| 0 | 96 (18%) |
| 1 | 248 (47%) |
| 2 | 158 (30%) |
| 3 | 19 (4%) |
| 4 | 2 (0.4%) |
| >4 | 3 (0.6%) |
RASs, resistance-associated substitutions; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
Treatment effectiveness of VOX/VEL/SOF
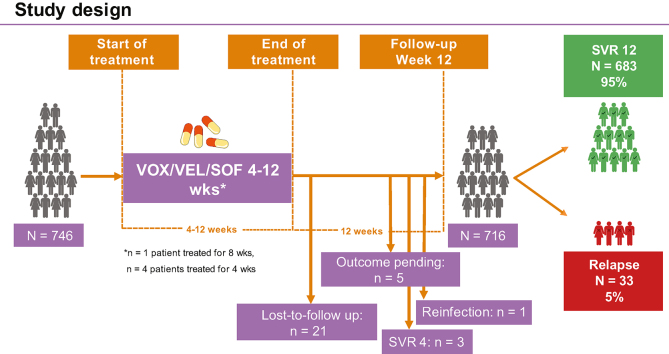
By intention-to-treat analysis, 683 of the 746 patients (92%) who commenced on VOX/VEL/SOF achieved SVR12. Overall, 21 patients (3%) were lost-to-follow-up during or after the EOT and one patient was reinfected. At the end of follow-up, outcomes of re-treatment were pending in five patients (0.6%) and SVR4 was observed in three patients (0.4%). These cases were excluded from PP SVR analysis, since SVR12 results were not available. As such, among 746 patients re-treated with VOX/VEL/SOF, 716 patients had available outcomes 12 weeks after the EOT and the PP SVR12 rate was 95% (Fig. 1).
Fig. 1.
Rates of SVR according to the most important baseline and on-treatment features.
Categorial variables were compared using the χ2 or the Fisher’s exact test; continuous variables were compared using the Mann-Whitney U test or the Kruskall-Wallis test, when appropriate. HCC, hepatocellular carcinoma; RASs, resistance-associated substitutions; RBV, ribavirin; SVR, sustained virologic response.
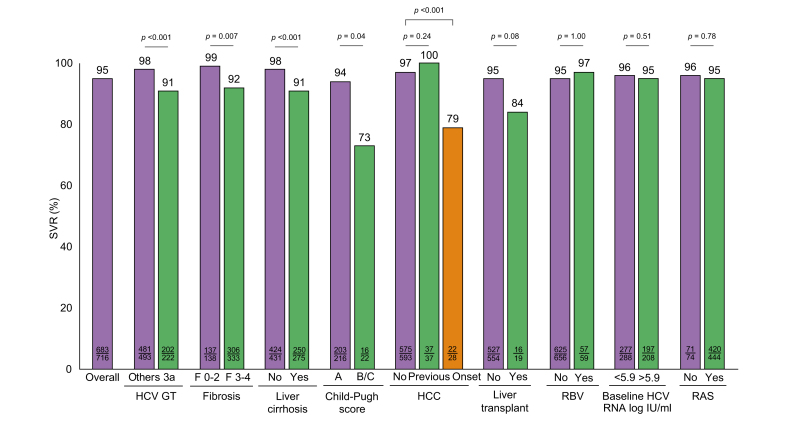
Analyzing PP SVR12 rates according to baseline and on-treatment factors, treatment effectiveness was significantly affected by higher baseline ALT values (p = 0.02) and by HCV GT (p = 0.001; Table 3). Further features that were significantly associated with reduced SVR rates were the presence of liver fibrosis (p = 0.001), cirrhosis (p <0.001) and HCC (p <0.001) (Table 3 and Fig. 1). Among patients with cirrhosis, significantly lower SVR rates were observed in those with higher CTP: 75% and 50% of patients with CTP B and C achieved SVR12, whilst treatment response of patients with compensated cirrhosis (CTP A) was comparably high (SVR12: 94%, p = 0.008). Concerning further baseline and on-treatment factors, no significant differences in SVR rates could be detected according to sex (p = 0.53), baseline HCV RNA (p = 0.59), RBV use (p = 1.00), baseline RASs (p = 0.78) and liver transplant status (p = 0.08; Table 3 and Fig. 1). Analysis of the subgroup of patients with the additional administration of RBV showed no significant differences to the remaining patients (Tables S7 and S8).
Table 3.
Per protocol analysis of clinical factors associated with an SVR (n = 716).
| SVR (n = 683) | Non-SVR (n = 33) | p value | |
|---|---|---|---|
| Males, n (%) | 526 (96) | 24 (5) | 0.53 |
| Age (years), median (range) | 56 (16-88) | 58 (31-71) | 0.52 |
| ALT, median (range) | 54 (10-538) | 75 (43-211) | 0.02 |
| GGT, median (range) | 56 (9-708) | 77 (39-253) | 0.08 |
| PLT (103/mm3), median (range) | 172 (35-539) | 134 (51-233) | 0.41 |
| Bilirubin (mg/dl), median (range) | 0.8 (0.2-8.00) | 0.7 (0.3-3.2) | 0.41 |
| Albumin (mg/dl), median (range) | 4.2 (3-5.1) | 4.2 (2.8-4.4) | 0.42 |
| INR, median (range) | 1.00 (0.9-3.5) | 1.1 (0.9-1.4) | 0.52 |
| Creatinine (mg/dl), median (range) | 0.8 (0.4-6.8) | 0.8 (0.6-1.3) | 0.70 |
| Genotype, n (%) | 0.001 | ||
| 1 | 395 (98) | 10 (2) | 0.002 |
| 1a | 169 (95) | 8 (5) | <0.001 |
| 1b | 218 (99) | 2 (1) | <0.001 |
| 1 (other) | 8 (100) | 0 (0) | 1.00 |
| 2 | 30 (100) | 0 (0) | 0.39 |
| 3 | 202 (91) | 20 (9) | <0.001 |
| 4 | 51 (96) | 2 (4) | 1.00 |
| 6 | 5 (100) | 0 (0) | 1.00 |
| Fibrosis, n (%) | <0.001 | ||
| F0–F2 | 136 (99) | 2 (1) | |
| F3-4 | 306 (92) | 27 (8) | |
| n. a. | 241 (98) | 4 (2) | |
| Liver cirrhosis, n (%) | 250 (91) | 25 (9) | <0.001 |
| CTP score, n (%) | 0.008 | ||
| A | 203 (94) | 13 (6) | |
| B | 15 (75) | 5 (25) | |
| C | 1 (50) | 1 (50) | |
| HCC history, n (%) | 68 (86) | 11 (14) | <0.001 |
| Previous HCC history | 37 (100) | 0 (0) | 0.39 |
| HCC onset during/after treatment | 22 (79) | 6 (21) | <0.001 |
| Liver transplant, n (%) | 16 (84) | 3 (16) | 0.08 |
| IFN experienced, n (%) | 166 (95) | 8 (5) | 1.00 |
| Previous DAA combination, n (%) | |||
| Sofosbuvir-based | 422 (95) | 24 (5) | 0.19 |
| SIM/SOF | 4 (100) | 0 (0) | 1.00 |
| DCV/SOF | 98 (92) | 8 (8) | 0.12 |
| LDV/SOF | 202 (98) | 5 (2) | 0.11 |
| VEL/SOF | 104 (90) | 11 (10) | 0.01 |
| PTV/RTV, OMB ± DSB | 120 (98) | 2 (2) | 0.15 |
| ELB/GZR | 78 (98) | 2 (2) | 0.57 |
| G/P | 49 (92) | 4 (8) | 0.29 |
| Other | 21 (100) | 0 (0) | 0.62 |
| n. a. | 7 (88) | 1 (12) | |
| RBV use in previous DAA treatment, n (%) | 160 (94) | 11 (6) | 0.21 |
| RBV use in VOX/VEL/SOF treatment, n (%) | 57 (97) | 2 (3) | 1.00 |
| Baseline HCV RNA, log IU/ml, n (%) | 6.0 (1.8-7.9) | 5.9 (3.2-7.4) | 0.59 |
| RASs, n (%) | |||
| Any RAS | 420 (95) | 24 (5) | 0.78 |
| NS5A | 386 (95) | 21 (5) | 0.61 |
| Y93H | 265 (95) | 14 (5) | 0.69 |
| Multiple RAS | 275 (95) | 14 (5) | 0.70 |
ALT, alanine aminotransferase; CTP, Child-Turcotte-Pugh; DCV, daclatasvir; DSB, dasabuvir; ELB, elbasvir; GGT, gamma-glutamyltransferase; G/P, glecaprevir/pibrentasvir; GT, genotype; GZR, grazoprevir; IFN, interferon; INR, international normalized ratio; LDV, ledipasvir; OMB, ombitasvir; PLT, platelet count; PTV, paritaprevir; RASs, resistance-associated substitutions; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir. Categorial variables were compared using the χ2 or the Fisher’s exact test; continuous variables were compared using the Mann-Whitney U test or the Kruskall-Wallis test, when appropriate.
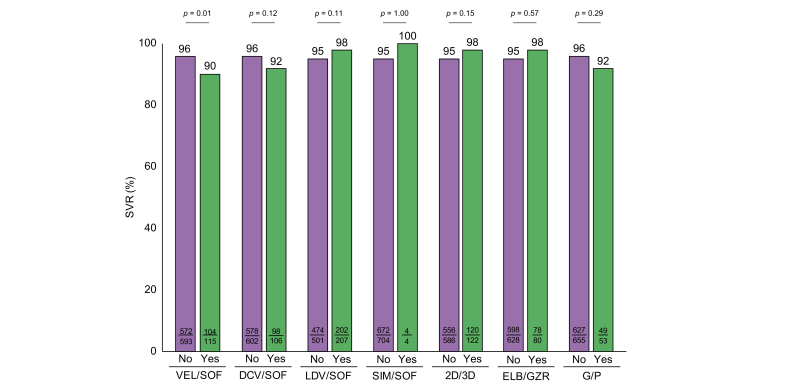
Analyzing PP SVR12 rates according to the different types of previous DAA regimen, response rates were observed to be significantly lower in patients previously failing VEL/SOF (SVR 12: 90%, p = 0.01, Table 3 and Fig. 2). However, SVR rates of patients exposed to other previous DAA regimens were observed to be high with no significant differences between the groups: SVR12 rates were 92% in patients previously treated with daclatasvir/sofosbuvir, 98% in patients treated with LDV/SOF, 100% in patients failing simeprevir/SOF, 98% in patients failing 2D/3D (ombitasvir/paritaprevir/ritonavir±dasabuvir), 98% in patients failing elbasvir/grazoprevir and 92% in patients failing G/P (Table 3 and Fig. 2).
Fig. 2.
Rates of SVR according to different previous DAA treatments.
Categorial variables were compared using the χ2 or the Fisher’s exact test; continuous variables were compared using the Mann-Whitney U test or the Kruskall-Wallis test, when appropriate. 2D/3D, ombitasvir/paritaprevir/ritonavir/ombitasvir/paritaprevir/ritonavir+dasabuvir; DAA, direct-acting antiviral; DCV/SOF, daclatasvir/sofosbuvir; ELB/GZR, elbasvir/grazoprevir; G/P, glecaprevir/pibrentasvir; LDV/SOF, ledipasvir/sofosbuvir; SIM/SOF, simeprevir/sofosbuvir; SVR, sustained virologic response; VEL/SOF, velpatasvir/sofosbuvir.
Beyond that, we analyzed baseline factors significantly associated with treatment effectiveness of VOX/VEL/SOF in more depth. Regarding HCV GTs, significantly lower SVR rates were observed for patients with GT3 (SVR 12: 91%, p <0.001; Table 3). In contrast, treatment effectiveness was observed to be significantly higher in patients with GT1, whereas other HCV GTs did not impact treatment outcome (Table 3 and Fig. S1). Moreover, we not only analyzed treatment outcome according to the presence or absence of HCC, but also according to the temporal occurrence of HCC. The results showed that HCC onset during or after re-treatment with VOX/VEL/SOF was significantly associated with a lack of SVR: SVR12 rates were 79% vs. 97% in patients with or without HCC onset (p <0.001; Fig. 1). On the contrary, previous HCC history did not impact treatment effectiveness of VOX/VEL/SOF (p = 0.39; Table 3 and Fig. 1).
Moreover, treatment effectiveness was observed to be high in patients who carried rare sub/genotypes and chimera. Of 21 patients with rare geno/subtypes who completed follow-up, 20 (PP SVR12: 95%) had undetectable HCV RNA 12 weeks after the EOT and only one patient harboring HCV GT3b relapsed. Concerning treatment response of the three patients who had twice failed to achieve an SVR after being treated with DAAs, SVR12 could be detected in one of them after being re-treated. However, two patients (one carrying GT1a and one GT3a), both with cirrhosis, relapsed on re-treatment with VOX/VEL/SOF.
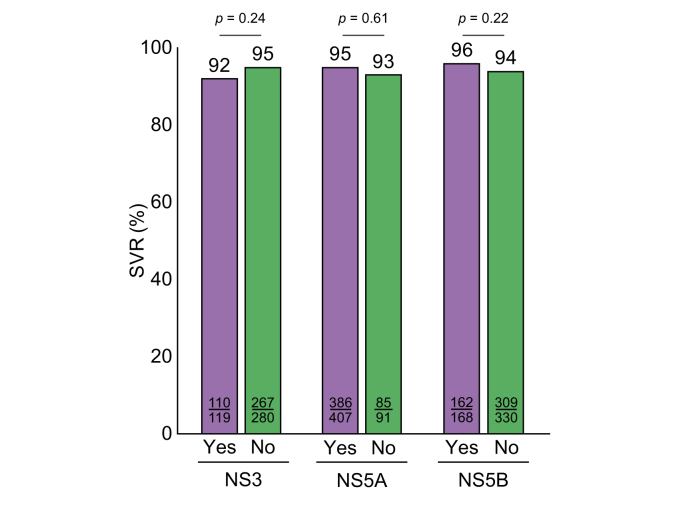
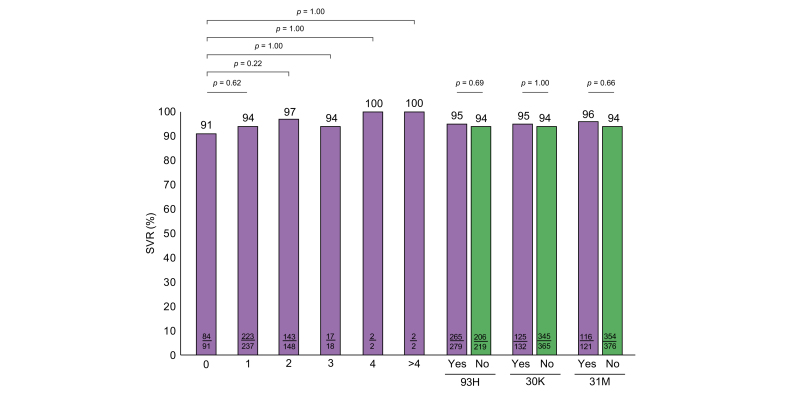
Among the 716 patients re-treated with VOX/VEL/SOF who had available treatment outcomes, 514 patients had detectable RASs prior to re-treatment: 30% had NS3, 82% NS5A and 34% had NS5B RASs. Combined RASs were detected in 167 of these patients (NS3 + NS5A in 17%; NS5A + NS5B in 17%, NS3 + NS5A + NS5B in 10%). However, despite the high prevalence of RASs prior to re-treatment, treatment outcome was not significantly associated with the presence of RASs in general nor with single or multiple baseline RASs (Table 3 and Fig. 3). Beyond that, the impact of NS5A RASs on treatment effectiveness of VOX/VEL/SOF was evaluated more precisely: SVR rates were analyzed according to different frequencies and major types of NS5A RASs. However, neither the number of NS5A RASs nor the presence of clinically relevant and major NS5A variants such as 93H (p = 0.69), 30K (p = 1.00) and 31M (p = 0.66) affected treatment outcomes on VOX/VEL/SOF (Fig. 4).
Fig. 3.
Rates of SVR according to different RASs.
Categorial variables were compared using the χ2 or the Fisher’s exact test; continuous variables were compared using the Mann-Whitney U test or the Kruskall-Wallis test, when appropriate. RASs, resistance-associated substitutions; SVR, sustained virologic response.
Fig. 4.
Rates of SVR according to NS5A RASs.
(A) SVR rates by different numbers of NS5A RASs, (B) SVR rates according to different specific NS5A RASs. Categorial variables were compared using the χ2 or the Fisher’s exact test; continuous variables were compared using the Mann-Whitney U test or the Kruskall-Wallis test, when appropriate. RASs, resistance-associated substitutions; SVR, sustained virologic response.
Finally, we conducted logistic regression analysis to identify independent negative predictors of SVR12 following treatment with VOX/VEL/SOF. Based on PP univariate analysis, the presence of cirrhosis (p <0.001), HCC onset during or after treatment with VOX/VEL/SOF (p <0.001), HCV GT3 (p <0.001), and previous VEL/SOF treatment (p = 0.01) were significantly associated with a lack of SVR (Table 4). A multivariable analysis revealed that HCC onset during or after re-treatment with VOX/VEL/SOF (p = 0.002), cirrhosis (p = 0.02) and HCV GT3 (p = 0.004) had the largest effect on SVR and were the only independent predictive factors of treatment failure.
Table 4.
Univariate and multivariate analysis of factors associated with the achievement of SVR12 (n = 716).
| SVR12 rates | Univariate |
Multivariate |
||
|---|---|---|---|---|
| p value | OR (95% CI) | p value | ||
| Sex, n (%) | 0.56 | |||
| Males | 526/550 (96) | |||
| Females | 156/165 (95) | |||
| Age (years) | 56 (16-88) | 0.76 | ||
| ALT (IU/ml), median (range) | 54 (10-538) | 0.15 | ||
| HCC history, n (%) | 68/79 (86) | <0.001 | 10.02 (1.98-18.34) | 0.002 |
| Previous HCC history | 37/37 (100) | 1.00 | ||
| HCC onset during/after treatment | 22/28 (79) | <0.001 | ||
| Cirrhosis, n (%) | 250/275 (91) | <0.001 | 5.31 (1.19-8.86) | 0.02 |
| Liver transplant, n (%) | 16/19 (84) | 0.05 | ||
| Genotype 3, n (%) | <0.001 | 8.14 (0.44-0.86) | 0.004 | |
| Non-GT3 | 481/493 (98) | |||
| GT3 | 202/222 (91) | |||
| IFN experienced, n (%) | 166/174 (95) | 0.89 | ||
| Previous DAA combination, n (%) | 0.02 (0.38-3.04) | 0.71 | ||
| Sofosbuvir-based | 422/446 (95) | 0.18 | ||
| SIM/SOF | 4/4 (100) | 1.00 | ||
| DCV/SOF | 98/106 (92) | 0.11 | ||
| LDV/SOF | 202/207 (98) | 0.09 | ||
| VEL/SOF | 104/115 (90) | 0.006 | ||
| PTV/RTV, OMB ± DSB | 120/122 (98) | 0.11 | ||
| ELB/GZR | 78/80 (98) | 0.37 | ||
| G/P | 49/52 (92) | 0.28 | ||
| RBV use in previous DAA treatment, n (%) | 160/171 (94) | 0.18 | ||
| Baseline HCV RNA (log IU/ml), median (range) | 6.0 (1.8-7.9) | 0.93 | ||
| RBV use in VOX/VEL/SOF treatment, n (%) | 57/59 (97) | 0.64 | ||
| RASs, n (%) | ||||
| Any | 683/716 (95) | 0.63 | ||
| NS5A | 386/407 (95) | 0.59 | ||
| Y93H | 265/279 (95) | 0.65 | ||
| Multiple RAS | 275/289 (95) | 0.70 | ||
ALT, alanine aminotransferase; DCV, daclatasvir; DSB, dasabuvir; ELB, elbasvir; G/P, glecaprevir/pibrentasvir; GT, genotype; GZR, grazoprevir; IFN, interferon; LDV, ledipasvir; OMB, ombitasvir; OR, odds ratio; PTV, paritaprevir; RASs, resistance-associated substitutions; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir.
Characteristics of treatment failures
A total of 33 patients had detectable RNA 12 weeks after EOT with VOX/VEL/SOF. The main clinical and virological features of treatment failures are listed in Table 5. Twenty one patients (64%) were infected with HCV GT3, 8 (24%) with GT1a and two each with GT1b (6%) and GT4 (6%). 25 patients (76%) had cirrhosis, six of whom had a history of decompensation (CTP A: n = 13, CTP B: n = 5, CTP C: n = 1; n. a. n = 6) and three had received liver transplantation. HCC occurrence was observed in 11 out of 28 patients (39%) with available data. Information on the temporal occurrence of HCC was available in six patients, all of whom developed liver cancer during or after treatment with VOX/VEL/SOF. Except for one patient, whose previous DAA treatment was unknown, all patients who failed to achieve SVR12 had received a NS5A-containing regimen (SOF-based in 25 cases). RBV was administered in addition to VOX/VEL/SOF in three cases.
Table 5.
Features of patients with virological failure (n = 33).
| Center | Sex | Age (yrs) | Genotype | Cirrhosis | CTP score | HCC history | Previous DAA treatment | RBV use | Baseline RASs | Treatment-emergent RASs | Follow-up after VOX/VEL/SOF failure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spain | Male | 47 | 3 | Yes | 5 | No | DCV/SOF | No | n. a. | n. a. | No antiviral treatment |
| Spain | Male | 53 | 3 | Yes | 5 | No | VEL/SOF | No | n. a. | n. a. | Death |
| Spain | Male | 54 | 3 | No | n. a. | No | DCV/SOF | No | NS3: Y93H; NS5A: Y93H, A30K | n. a. | LTFU |
| Spain | Male | 63 | 3 | Yes | 7 | Yes | DCV/SOF | No | None | n. a. | HCC recurrence after treatment failure. Re-treatment with rescue therapy (G/P+SOF) over 16 weeks, death at SVR 4 |
| Spain | Male | 55 | 3 | Yes | 5 | Yes | DCV/SOF | No | NS3: D168G; NS5A: L28S, M31L; | n. a. | LTFU |
| Spain | Female | 50 | 4 | Yes | 5 | No | ELB/GZR | No | NS3: Y93H; NS5A: Y93H | n. a. | LTFU |
| Spain | Male | 62 | 3 | No | n. a. | No | DCV/SOF | No | NS5A: Y93H | n. a. | LTFU |
| Italy | Female | 71 | 1b | Yes | 5 | No | 2D/3D | Yes | None | None | No antiviral treatment |
| Italy | Male | 60 | 1a | Yes | 5 | Yes (peri-/post- treatment | LDV/SOF | Yes | NS3: 176S, NS5A: 30R, 31M | None | Rescue treatment with G/P+ SOF and RBV over 16 weeks, SVR12 |
| Italy | Male | 54 | 3 | Yes | 5 | No | DCV/SOF | No | n. a. | n. a. | LTFU |
| Italy | Male | 66 | 3 | No | n. a. | Yes (peri-/post- treatment) | VEL/SOF | No | NS5A: Y93H | n. a. | Rescue treatment with G/P/SOF and RBV over 16 weeks, SVR12 |
| Italy | Female | n. a. | 4 | Yes | 8 | No | LDV/SOF | No | n. a. | n. a. | Death |
| Italy | Male | n. a. | 1a | Yes | 5 | No | VEL/SOF | No | n. a. | n. a. | LTFU |
| Italy | Male | n. a. | 3a | Yes | 5 | No | n. a. | No | n. a. | n. a. | LTFU |
| Germany | Male | 58 | 3a | Yes | n. a. | No | VEL/SOF+RBV | No | NS5A: Y93H; NS5B: A150V | n. a. | LTFU |
| Germany | Male | 54 | 3a | Yes | 11 | Yes (peri-/post- treatment) | VEL/SOF | No | None | n. a. | HCC onset; no antiviral re-treatment |
| Germany | Male | 46 | 1a | N. a. | n. a. | None | ELB/GZR | No | NS3: Q80K | None | LTFU |
| Germany | Female | 61 | 3a | Yes | 8 | Yes (peri-/post-treatment) | VEL/SOF | No | NS5A: Y93H; NS5B: A150V | None | Rescue treatment with VEL/SOF and RBV over 24 weeks, SVR 12 |
| Germany | Male | 62 | 1a | No | No | LDV/SOF | No | NS3: Q80K | n. a. | LTFU | |
| Germany | Male | 40 | 3a | Yes | n. a. | n. a. | G/P | No | NS5A: A30K, Y93H | None | Rescue treatment with G/P+SOF over 16 weeks, SVR12 |
| Germany | Female | 63 | 1a | Yes | 6 | n. a. | G/P | No | NS5A: Q30H, L31 M/V, Y93H | None | Rescue treatment with G/P+SOF and RBV over 24 weeks, SVR12 |
| Germany | Female | 61 | 1b | Yes | 6 | n. a. | VEL/SOF | No | NS5A: Y93H | None | No antiviral treatment |
| Germany | Male | 63 | 1a | Yes | 8 | n. a. | VEL/SOF | No | NS5A: M28V | None | Death |
| Germany | Female | 57 | 3a | Yes | 6 | Yes (peri-/post-treatment) | LDV/SOF+RBV | No | NS5A: Y93H | None | No antiviral treatment |
| Germany | Male | 31 | 1a | No | No | 3D+RBV | No | NS5B: SS65G | NS3: Q80K; NS5B: SS65G |
No antiviral treatment | |
| Germany | Female | 69 | 1a | No | Yes | LDV/SOF | No | NS5A: Q30K, L31V | None | No antiviral treatment | |
| Germany | Female | 57 | 3a | Yes | n. a. | n. a. | DCV/SOF | No | NS5A: A30K | NS5A: A30K, Y93H | LTFU |
| Germany | Male | 60 | 3a | No | n. a. | VEL/SOF | No | NS3: D168K, NS5A: Y93H; NS5B: A150V, V321 A | None | Rescue treatment with G/P+SOF and RBV over 16 weeks, SVR12 | |
| Germany | Male | 60 | 3a | Yes | 8 | Yes (peri-/post-treatment) | VEL/SOF | No | n. a. | NS5A: Y93H; NS5B: A150I | Rescue treatment with VEL/SOF+ RBV over 24weeks, Relapse; antiviral re-treatment with G/P+SOF and RBV over 12 weeks, SVR12 |
| Germany | Female | 64 | 3a | Yes | 5 | Yes | VEL/SOF | No | NS3: Q168R; NS5A: Y93H | None | Rescue treatment with G/P+SOF over 12 weeks, SVR12 |
| Germany | Female | 53 | 3a | Yes | n. a. | No | VEL/SOF | No | NS5A: Y93H | None | Rescue treatment with G/P+SOF over 12 weeks, SVR12 |
| Germany | Male | 45 | 3b | Yes | n. a. | No | G/P | No | n. a. | NS5A: A30K, L31M | LTFU |
| Germany | Male | 55 | 3a | Yes | n. a. | Yes | DCV/SOF | No | NS5A: Y93H | None | Rescue treatment with G/P+SOF over 12 weeks, SVR12 |
CTP, Child-Turcotte-Pugh; DCV, daclatasvir; DSB, dasabuvir; ELB, elbasvir; G/P, glecaprevir/pibrentasvir; GZR, grazoprevir; LDV, ledipasvir; LTFU, long-term follow-up; OMB, ombitasvir; PTV, paritaprevir; RASs, resistance-associated substitutions; RBV, ribavirin; SOF, sofosbuvir; SVR(12), sustained virological response (12 weeks after the end of treatment); VEL, velpatasvir; VOX, voxilaprevir.
Baseline RAS testing was available in 25 patients who failed to achieve SVR: three patients had no detectable baseline RAS and 13 patients carried one single RAS with NS5A being the most frequent one (single NS3 RASs, n = 2; single NS5A RASs, n = 7; single NS5B RASs, n = 1). Combined RASs were observed in 46% of patients (n = 9) with available data (NS3 + NS5A, n = 6; NS5A + NS5B, n = 2; NS5A + NS3 + NS5B, n = 1). Multiple NS5A RASs (≥3) were only detected in one patient, who had a GT1a infection. After VOX/VEL/SOF failure, RAS testing was available in 18 patients. At re-treatment failure, pre-existing NS3, NS5A and NS5B patterns were maintained in most patients: 14 patients harbored the same RASs compared to baseline. Minor changes in NS3 and NS5A patterns were observed in two patients after re-treatment failure: one patient with GT1a maintained the S65G variant in NS5B and selected Q80K within NS3 after VOX/VEL/SOF failure. One further patient with GT3a selected Y93H to the pre-existing A30K variant within NS5A after failing re-treatment with VOX/VEL/SOF. Serum samples were not available at baseline for two patients in whom VOX/VEL/SOF re-treatment failed: the first patient with GT3a harbored the NS5A Y93H and NS5B A150I variant after re-treatment failure. The second patient with GT3b had the NS5A A30K plus L31M double variant after failing VOX/VEL/SOF treatment.
Rescue treatment
After failing VOX/VEL/SOF re-treatment (n = 33), no rescue treatment was initiated in 21 cases (12 patients were lost-to-follow-up, two patients died, both of whom suffered from decompensated cirrhosis, and rescue treatment was not conducted in a further seven cases).
Finding or suspicion of advanced HCC and postponement of treatment due to patient’s request were found to be the major reasons for omitting initiation of rescue therapy.
Rescue treatment was initiated in 11 patients. Most of the patients (n = 9) received G/P+ SOF with (n = 5) or without RBV (n = 4) as third-line therapy over 12 (n = 3), 16 (n = 5) and 24 weeks (n = 1). One patient, who suffered from cirrhosis (CTP class B) and HCC, died after achieving SVR4. Accordingly, treatment outcome of third-line therapy with G/P + SOF was available in eight patients, all of whom achieved SVR12. Two further patients with GT 3a received re-treatment with VEL/SOF plus RBV over 24 weeks, one of whom achieved SVR12 (PP SVR12: 50%). The second patient who relapsed was subsequently re-treated with G/P+SOF plus RBV over 12 weeks and finally also achieved SVR12. The resulting overall SVR rate of patients treated with rescue therapy after failing VOX/VEL/SOF re-treatment was 91% (Fig. S2). The PP SVR12 rate of G/P + SOF and of VEL/SOF + RBV as rescue therapies was 100% (n = 9/9) and 50% (n = 1/2), respectively.
Discussion
To our knowledge, this is the largest real-world study on treatment effectivity of VOX/VEL/SOF in patients previously failing on DAAs. Our results, which derive from a multicenter cohort consisting of 746 patients from Germany, Austria, Switzerland, Belgium, Italy and Spain demonstrate that re-treatment with VOX/VEL/SOF is an effective salvage therapy with an overall PP SVR rate of 95%, thus supporting results reported in clinical trials and previously reported real-life experiences.6,13,17,28
As expected in the real-life setting of DAA failure, our study cohort consisted of a representative population of patients with difficult-to-treat cofactors such as GT3 and cirrhosis, who accounted for 32% and 39% of patients enrolled, respectively. Of the patients with cirrhosis, 25 patients (9%) had a history of decompensation. Although previous studies were highly heterogeneous with respect to distribution of HCV GTs and the rate of patients with cirrhosis, HCV GT3 and cirrhosis were observed to be the most prevalent features among patients with virological failure on VOX/VEL/SOF in the approval study as well as in several real-life settings.6,12,14,16,28 Nevertheless, owing the relatively small number of patients especially with difficult-to-treat cofactors in each study, SVR rates according to GT 3 and cirrhosis were highly variable in these studies ranging between 80-100% for GT3 and between 81-100% for cirrhosis, respectively.14,15,17 The results of our study now clearly establish that HCV GT3 and cirrhosis represent the predominant factors associated with VOX/VEL/SOF treatment failure (64% and 76% of patients, respectively). Beyond that, based on a large case number of patients with difficult-to-treat cofactors, our study results demonstrate that HCV GT3 and cirrhosis were not only significantly associated with treatment response but emerged to be independent negative predictors of a treatment response.
Therefore, and in the context of a lack of availability of further re-therapies in many countries, the question arises whether the effectiveness of therapy with VOX/VEL/SOF can be optimized by the additional administration of RBV or by an extension of treatment duration in patients with HCV GT3 or cirrhosis. This would be in line with current EASL and AASLD guidelines.5,29 However, patients did not receive RBV in addition to VOX/VEL/SOF in the approval study nor in several subsequent real-life studies.6,13,14,30 In other real-world studies, the addition of RBV to VOX/VEL/SOF was described. However, prescription was observed to be low, ranging between 3-25% (n = 2-38 patients) and no systematic analysis of the effect of RBV was possible.15,16,18,19,31 In our study, RBV was administered in 61 patients (8%) at baseline, who significantly more frequently carried RASs (p = 0.04). Although differences of additional characteristics were not statistically significant, these patients more frequently carried other difficult-to-treat factors at baseline in comparison to the remaining patients (GT 3: 61% vs. 31%; cirrhosis: 44% vs. 38%; HCC: 17% vs. 12%). The overall SVR rate in patients who received RBV was 97%. In the subgroups of patients with GT3 and cirrhosis, SVR rates were higher with add-on RBV (100% and 93%) compared to those without RBV (90% and 91%). Based on these numerical differences, the addition of RBV can be recommended for re-treatment following treatment failure in patients with GT3 and/or cirrhosis. Nevertheless, the effects of RBV in these difficult-to-treat subgroups were observed to be statistically insignificant, which is probably due to the small case number (cirrhosis with vs. without RBV: p = 1.00; GT3 with vs. without RBV: p = 0.14; n = 27 patients with cirrhosis and RBV, n = 23 patients with GT3 and RBV).
In addition to cirrhosis and HCV GT3, our study identified HCC as the other main clinical feature strongly associated with treatment failure. Subsequent analyses revealed that HCC onset during or after treatment with VOX/VEL/SOF impacted treatment outcome (SVR rates of 79%), while SVR rates were not affected by previous HCC history (100%). Moreover, HCC onset was not only observed to be strongly associated with treatment failure but was also identified as an independent negative predictor of treatment response in a consecutive multivariate analysis (odds ratio 10.02, 95% CI 1.98-18.34; p = 0.002). To date, only a few studies with small case numbers (n = 7-22) have investigated the relevance of HCC in treatment response to VOX/VEL/SOF with all of them showing considerably lower SVR rates in patients with HCC.12,16,17 Our results confirm these findings with larger case numbers and demonstrate that it is not a previous history of HCC but rather active HCC that affects treatment responses to VOX/VEL/SOF. The biological mechanisms of a diminished SVR in patients with active HCC are not entirely understood. One pathophysiological explanation is that HCC may serve as a reservoir for HCV replication, which might alter liver architecture, decrease drug delivery of DAAs and allow for development and survival of resistant HCV strains.32,33 A more recent study also reported a higher prevalence of RASs within tumoral tissues of resected HCC-affected livers or liver explants, even in the absence of mutations in paired plasma samples, providing another potential mechanism for DAA failure in patients with active tumors.34
One further issue, which is still under debate, is the treatment effectiveness of VOX/VEL/SOF in patients with prior VEL/SOF experience. Besides LDV/SOF, VEL/SOF represented the most frequent previous treatment in our study cohort, accounting for 17% (n = 123) of patients. Thus, it was possible to evaluate the influence of VEL/SOF on treatment effectiveness of VOX/VEL/SOF based on representative case numbers. Our results showed that treatment effectiveness was significantly affected by VEL/SOF pre-treatment. However, prior VEL/SOF failure was not identified as an independent predictor of lower response to VOX/VEL/SOF (p = 0.71). Therefore, triple therapy with the NS3/4 protease inhibitor VOX±RBV seems reasonable in VEL/SOF-experienced patients and the use of two new agents as second-line therapy should only be considered if VEL/SOF pre-treatment is accompanied by further difficult-to-treat-cofactors. Nevertheless, G/P+SOF might be, although not officially approved, the more effective treatment option in this subgroup of patients and further data on this topic are warranted.
Since resistance testing was available for most patients (n = 547; 73%), comprehensive analyses of the impact of RASs on outcomes of VOX/VEL/SOF treatment could be conducted. Overall, RASs were detected in 86% of patients, with NS5A RASs accounting for 79%. Y93H, which confers high-level resistance to all NS5A inhibitors, was present in 56% of patients presenting with RASs. In line with results of a post hoc analysis on VOX/VEL/SOF registration data and several real-world studies, the presence of baseline RASs was not associated with treatment outcome.8,16,17 Further assessment of the effect of NS5A RASs on treatment effectiveness of VOX/VEL/SOF showed neither single nor multiple RASs, nor specific RASs conferring high-level resistance against NS5A inhibitors, impacted SVR rates after VOX/VEL/SOF treatment. Regarding resistance analysis within NS3, NS5A and NS5B genes before and after VOX/VEL/SOF failure, only minor changes in RAS frequencies and profile were observed in our study cohort, consistent with previous observations: only two patients exhibited treatment-emergent RASs after VOX/VEL/SOF treatment failure. These data show that RAS testing is not mandatory before starting re-treatment with VOX/VEL/SOF. However, it can help to optimize therapy in certain subgroups of difficult-to-treat patients and may guide re-treatment in cases of highly resistant RAS patterns, especially in countries with limited availability of DAA regimens.
Besides epidemic HCV subtypes, which have become prevalent globally and generally respond well to DAA therapies, there are more localized endemic GTs, which have been found to commonly harbor RASs that may confer resistance to DAAs.35 Accordingly, recent in vitro experiments and clinical data have shown that most of these rare subtypes, especially 1l, 3l and 4r, are associated with a reduced susceptibility to current DAA therapies.29,[36], [37], [38] To date, it is still unclear how patients with rare GTs respond to the triple therapy and whether RBV (or even other combinations) need to be added according to resistance testing. A subgroup of our German cohort, re-treated with VOX/VEL/SOF, carried rare subtypes and chimera. Of those, four patients were infected with the HCV GT3b, all of whom harbored the double NS5A RAS 30K/31M, which has been shown to confer high-level drug resistance to VEL.39,40 Interestingly, GT3b was associated with lower SVR rates in the approval studies in China for both VEL/SOF and G/P.41,42 Beyond that, the subtype 4r was found in four patients, three of whom harbored the 28M/30R/31M triple polymorphism, which was found to be associated with a resistant phenotype.36,43 However, overall treatment effectiveness was observed to be high in this subgroup: except for one patient with GT3b who relapsed, all patients with available data 12 weeks after the EOT achieved SVR12 (20/21; 95%). Thus, the triple combination with the protease inhibitor voxilaprevir seems to maintain its antiviral activity in patients with rare GTs and variants, which confer high-level drug resistance to VEL and SOF. However, due to the limited number of rare GTs, we cannot make a general treatment recommendation regarding re-treatment in this subgroup of patients and the combination of G/P+SOF might be comparably effective.
So far, only case reports have been published on re-treatment in patients with failure on VOX/VEL/SOF rescue therapy, with the exception of one larger analysis.[44], [45], [46] This study, which included a subgroup of our cohort, reported high SVR rates for multiple targeted therapies with second-line DAAs such as VOX/VEL/SOF or G/P+SOF.44 Rescue treatments in our study included G/P+SOF with or without RBV for 12-24 weeks and VEL/SOF plus RBV for 24 weeks in two patients with decompensated cirrhosis. SVR was reported in one out of two patients (50%) re-treated with VEL/SOF. The second patient experienced relapse, was subsequently re-treated with G/P+SOF, and finally achieved SVR12. Consistent with previous reports, treatment response to rescue therapy with G/P+SOF with and without RBV for 12 to 24 weeks was observed to be excellent with SVR12 rates of 100%.22,45,46 These results pose the question of whether G/P+SOF may be a more effective treatment option than VOX/VEL/SOF. In fact, G/P+SOF it is not only proposed as a third-line but also as a second-line treatment option in patients with difficult-treat cofactors by the international guidelines of AASLD and EASL. However, official approval of G/P+SOF as both second- and third-line therapy has not yet been granted, partly due to the limited data available.
A major limitation of our study is its retrospective design in the Italian and German sub-cohort. Diagnostic tests, such as biochemical values or liver imaging studies, as well as initiation of therapeutic regimens were at the physician’s discretion. Moreover, treatment-emergent adverse events were not systematically recorded in the German sub-cohort. Thus, the safety of VOX/VEL/SOF was not analyzed in our study. Beyond that, one further limitation is the heterogeneity of the included studies regarding not only the study design but also how treatment adherence was recorded. Evaluation of treatment adherence was a factor recorded in the Italian and Spanish cohort but not in the German one and was thus not included in our integrative analysis. Therefore, we cannot rule out with certainty that patients without RASs but with a relapse to VOX/VEL/SOF have a non-virologic treatment failure due to possible non-adherence. A sub-analysis for patients with RASs and for patients with NS5A RASs (Table S3-6) led to the identification of the same predictors of re-treatment failure as in the overall analysis. Therefore, our conclusions seem not to be significantly affected by possible non-adherence of patients without RASs in whom re-treatment failed. However, based on our analysis, we cannot state what effect adherence has on treatment failure.
To conclude, our study reports excellent effectiveness of VOX/VEL/SOF in a large cohort of patients with HCV and prior DAA failure treated in a European real-life setting. In a multivariate analysis, RAS prevalence as well as RAS features and previous DAA therapy including prior VEL/SOF experience did not impact on the effectiveness of triple therapy. Moreover, treatment effectiveness of VOX/VEL/SOF was found to be high in a subgroup of patients with rare GTs and chimera. HCV GT3, HCC and cirrhosis were identified as the only independent negative predictive factors of a SVR12 following treatment with VOX/VEL/SOF. The addition of RBV should be evaluated in these cases as it seems to enhance efficacy of the triple therapy, especially in patients with HCV GT3 infections.
Financial support
This study was supported by a DZIF (German Center for Infection Research) grant entitled ‘HCV Treatment Optimization’ to CS and JD (TTU 05.809).
Authors’ contributions
CG, CS and JD designed the study concept. Data collection was performed by RA, ED, JL, PL, MB, CS, SP, JV, GD, KHP, FF, SZ and CG. Analysis and interpretation of data was performed by CG, EH and CS. Draft of the manuscript was performed by CG, JD and CS. PL, MB, ED, RD and JL provided the critical revision of the manuscript.
Data availability statement
The authors confirm that the data supporting the findings of this study within the article and/or supplementary materials are available upon request.
Conflict of interest
Christiana Graf reports speaking and/or consulting fees from AbbVie and travel support from AbbVie and Gilead outside the submitted work. Roberta D’Ambrosio reports speaking and/consulting fees from AbbVie, Gilead and Takeda and research grant from AbbVie and Gilead outside the submitted work. Elisabetta Degasperi reports speaking and/or consulting fees from AbbVie, Gilead, MSD; research grants from Gilead and travel support from AbbVie outside the submitted work. Stefania Paolucci: no conflicts to disclose.Jordi Llaneras: no conflicts to disclose. Johannes Vermehren reports speaking and/or consulting fees from Abbott, AbbVie, Bristol-Myers, Squibb, Gilead, Medtronic, Merck/MSD and Roche outside the submitted work. Georg Dultz reports speaking and/or consulting fees from AbbVie and Gilead outside the submitted work. Kai-Henrik Peiffer: no conflicts to disclose. Fabian Finkelmeier: no conflicts to disclose. Eva Herrmann: no conflicts to disclose. Stefan Zeuzem reports speaking and/or consulting fees from Abbvie, BioMarin, Gilead, GSK, Ipsen, Janssen, Madrigal, Merck/MSD, NovoNordisk, SoBi, and Theratechnologies outside the submitted work. Maria Buti reports speaking and/or consulting fees from AbbVie, MSD and Gilead outside the submitted work. Pietro Lampertico reports speaking and/or consulting fees from AbbVie, BMS, Gilead, GSK, Janssen, MSD and Roche outside the submitted work. Julia Dietz reports research grants from Gilead outside the submitted work. Christoph Sarrazin reports speaking and consulting fees from Abbvie, MSD, Gilead, Merck/MSD and research support from AbbVie and Gilead outside the submitted work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100994.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Naggie S., Muir A.J. Oral combination therapies for hepatitis C virus infection: successes, challenges, and unmet needs. Annu Rev Med. 2017;68:345–358. doi: 10.1146/annurev-med-052915-015720. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Bacon B., Dieterich D., et al. Tu1033 evaluation of access to care in patients prescribed sofosbuvir-containing regimens: data from the TRIO network. Gastroenterology. 2015;148 S-1090. [Google Scholar]
- 3.Lawitz E., Yang J.C., Stamm L.M., et al. Characterization of HCV resistance from a 3-day monotherapy study of voxilaprevir, a novel pangenotypic NS3/4A protease inhibitor. Antivir Ther. 2018;23:325–334. doi: 10.3851/IMP3202. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 5.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatol Baltim Md. 2015;62:932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 6.Bourlière M., Gordon S.C., Flamm S.L., et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376:2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 7.Bourlière M., Gordon S.C., Schiff E.R., et al. Deferred treatment with sofosbuvir-velpatasvir-voxilaprevir for patients with chronic hepatitis C virus who were previously treated with an NS5A inhibitor: an open-label substudy of POLARIS-1. Lancet Gastroenterol Hepatol. 2018;3:559–565. doi: 10.1016/S2468-1253(18)30118-3. [DOI] [PubMed] [Google Scholar]
- 8.Sarrazin C., Cooper C.L., Manns M.P., et al. No impact of resistance-associated substitutions on the efficacy of sofosbuvir, velpatasvir, and voxilaprevir for 12 weeks in HCV DAA-experienced patients. J Hepatol. 2018;69:1221–1230. doi: 10.1016/j.jhep.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Dietz J., Müllhaupt B., Buggisch P., et al. Long-term persistence of HCV resistance-associated substitutions after DAA treatment failure. J Hepatol. 2023;78:57–66. doi: 10.1016/j.jhep.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Sun J.-H., O’Boyle D.R., et al. Persistence of resistant variants in hepatitis C virus-infected patients treated with the NS5A replication complex inhibitor daclatasvir. Antimicrob Agents Chemother. 2013;57:2054–2065. doi: 10.1128/AAC.02494-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyles David, Mangia Alessandra, Cheng Wendy, et al. Long-term persistence of HCV NS5A resistance-associated substitutions after treatment with the HCV NS5A inhibitor, ledipasvir, without sofosbuvir. Antivir Ther. 2018;23(3):229–238. doi: 10.3851/IMP3181. [DOI] [PubMed] [Google Scholar]
- 12.Degasperi E., Spinetti A., Lombardi A., et al. Real-life effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous DAA failure. J Hepatol. 2019;71:1106–1115. doi: 10.1016/j.jhep.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Belperio P.S., Shahoumian T.A., Loomis T.P., Mole L.A., Backus L.I. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70:15–23. doi: 10.1016/j.jhep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Llaneras J., Riveiro-Barciela M., Lens S., et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs. J Hepatol. 2019;71:666–672. doi: 10.1016/j.jhep.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Vermehren J., Serfert Y., Cornberg M., et al. Sofosbuvir, velpatasvir, and voxilaprevir for patients with failure of previous direct-acting antiviral therapy for chronic hepatitis C: results from the German Hepatitis C-Registry (DHC-R) Z Gastroenterol. 2020;58:841–846. doi: 10.1055/a-1217-7669. [DOI] [PubMed] [Google Scholar]
- 16.Papaluca T., Roberts S.K., Strasser S.I., et al. Efficacy and safety of sofosbuvir/velpatasvir/voxilaprevir for hepatitis C virus (HCV) NS5A-inhibitor experienced patients with difficult to cure characteristics. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;73:e3288–e3295. doi: 10.1093/cid/ciaa1318. [DOI] [PubMed] [Google Scholar]
- 17.Smith D.A., Bradshaw D., Mbisa J.L., et al. Real world SOF/VEL/VOX retreatment outcomes and viral resistance analysis for HCV patients with prior failure to DAA therapy. J Viral Hepat. 2021;28:1256–1264. doi: 10.1111/jvh.13549. [DOI] [PubMed] [Google Scholar]
- 18.Janjua N., Wilton J., Cook D., et al. Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir as a hepatitis C virus infection salvage treatment. J Hepatol. 2020;73:S356–S357. [Google Scholar]
- 19.Flamm S., Tsai N., Bacon B., et al. Pangenotypic therapies glecaprevir-pibrentasvir (G-P) and sofosbuvir-velpatasvir-voxilaprevir (S-V-V) after failure with interferon (IFN)-free direct-acting antiviral (DAA) treatment for hepatitis C. J Hepatol. 2020;73:S846–S847. [Google Scholar]
- 20.Sarrazin C. Treatment failure with DAA therapy: importance of resistance. J Hepatol. 2021;74:1472–1482. doi: 10.1016/j.jhep.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Dietz J., Di Maio V.C., de Salazar A., et al. Failure on voxilaprevir, velpatasvir, sofosbuvir and efficacy of rescue therapy. J Hepatol. 2021;74:801. doi: 10.1016/j.jhep.2020.11.017. 810. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q., Perales C., Soria M.E., et al. Deep-sequencing reveals broad subtype-specific HCV resistance mutations associated with treatment failure. Antivir Res. 2020;174 doi: 10.1016/j.antiviral.2019.104694. [DOI] [PubMed] [Google Scholar]
- 24.Soria M.E., García-Crespo C., Martínez-González B., et al. Amino acid substitutions associated with treatment failure for hepatitis C virus infection. J Clin Microbiol. 2020;58:019855. doi: 10.1128/JCM.01985-20. e2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Cehic D., Rando A., Rodriguez-Frias F., et al. Resistance-associated substitutions after sofosbuvir/velpatasvir/voxilaprevir triple therapy failure. J Viral Hepat. 2021;28:1319–1324. doi: 10.1111/jvh.13497. [DOI] [PubMed] [Google Scholar]
- 26.Dietz J., Susser S., Vermehren J., et al. Patterns of resistance-associated substitutions in patients with chronic HCV infection following treatment with direct-acting antivirals. Gastroenterology. 2018;154:976–988.e4. doi: 10.1053/j.gastro.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 27.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66:153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Onofrio F.Q., Cooper C., Borgia S.M., et al. Salvage therapy with sofosbuvir/velpatasvir/voxilaprevir in DAA-experienced patients: results from a prospective Canadian Registry. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;72:e799. doi: 10.1093/cid/ciaa1510. e805. [DOI] [PubMed] [Google Scholar]
- 29.Pawlotsky J.-M., Negro F., Aghemo A., et al. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Pearlman B., Perrys M., Hinds A. Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 2019;114:1550–1552. doi: 10.14309/ajg.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 31.Bacon B., Curry M., Flamm S., et al. THU-116-Effectiveness of the salvage therapy sofosbuvir-velpatasvir-voxilaprevir (SOF-VEL-VOX) in chronic hepatitis C: clinical practice experience from the TRIO Network. J Hepatol. 2019;70:e209. [Google Scholar]
- 32.Chtioui H. OATP1B1 and DAA treatment for hepatitis C in patients with hepatocellular carcinoma. Hepatol Baltim Md. 2017;66:2091. doi: 10.1002/hep.29556. [DOI] [PubMed] [Google Scholar]
- 33.Kushner T., Dieterich D., Saberi B. Direct-acting antiviral treatment for patients with hepatocellular carcinoma. Curr Opin Gastroenterol. 2018;34:132–139. doi: 10.1097/MOG.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 34.Sorbo M.C., Carioti L., Bellocchi M.C., et al. HCV resistance compartmentalization within tumoral and non-tumoral liver in transplanted patients with hepatocellular carcinoma. Liver Int Off J Int Assoc Study Liver. 2019;39:1986. doi: 10.1111/liv.14168. 1998. [DOI] [PubMed] [Google Scholar]
- 35.Shah R., Ahovegbe L., Niebel M., Shepherd J., Thomson E.C. Non-epidemic HCV genotypes in low- and middle-income countries and the risk of resistance to current direct-acting antiviral regimens. J Hepatol. 2021;75:462–473. doi: 10.1016/j.jhep.2021.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fourati S., Rodriguez C., Hézode C., et al. Frequent antiviral treatment failures in patients infected with hepatitis C virus genotype 4, subtype 4r. Hepatol Baltim Md. 2019;69:513–523. doi: 10.1002/hep.30225. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen D., Smith D., Vaughan-Jackson A., et al. Efficacy of NS5A inhibitors against unusual and potentially difficult-to-treat HCV subtypes commonly found in sub-Saharan Africa and South East Asia. J Hepatol. 2020;73:794–799. doi: 10.1016/j.jhep.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 38.Wei L., Lim S.G., Xie Q., et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4:127–134. doi: 10.1016/S2468-1253(18)30343-1. [DOI] [PubMed] [Google Scholar]
- 39.Smith D., Magri A., Bonsall D., et al. Resistance analysis of genotype 3 hepatitis C virus indicates subtypes inherently resistant to nonstructural protein 5A inhibitors. Hepatol Baltim Md. 2019;69:1861–1872. doi: 10.1002/hep.29837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L., Lim S.G., Xie Q., et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4:127–134. doi: 10.1016/S2468-1253(18)30343-1. [DOI] [PubMed] [Google Scholar]
- 41.Zeuzem S., Foster G.R., Wang S., et al. Glecaprevir–pibrentasvir for 8 or 12 Weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354–369. doi: 10.1056/NEJMoa1702417. [DOI] [PubMed] [Google Scholar]
- 42.Foster G.R., Afdhal N., Roberts S.K., et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 43.da Silva Filipe A., Sreenu V., Hughes J., et al. Response to DAA therapy in the NHS England Early Access Programme for rare HCV subtypes from low and middle income countries. J Hepatol. 2017;67:1348–1350. doi: 10.1016/j.jhep.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 44.Dietz J., Di Maio V.C., de Salazar A., et al. Failure on voxilaprevir, velpatasvir, sofosbuvir and efficacy of rescue therapy. J Hepatol. 2021;74:801–810. doi: 10.1016/j.jhep.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Bernhard B., Stickel F. Successful fourth line treatment of a relapse patient with chronic hepatitis C virus infection genotype 3a using sofosbuvir, glecaprevir/pibrentasvir, and ribavirin: a case report. Z Gastroenterol. 2020;58:451–455. doi: 10.1055/a-1131-8058. [DOI] [PubMed] [Google Scholar]
- 46.Fierer D.S., Wyles D.L. Re-Treatment of hepatitis C infection after multiple failures of direct-acting antiviral therapy. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa095. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7148001/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
The authors confirm that the data supporting the findings of this study within the article and/or supplementary materials are available upon request.