Summary
Background
COVID-19 vaccine booster doses restore vaccine effectiveness lost from waning immunity and emerging variants. Fractional dosing may improve COVID-19 booster acceptability and uptake and will reduce the per-dose cost of COVID-19 booster programmes. We sought to quantify the immunogenicity, reactogenicity, and safety of a half-dose BNT162b2 (Pfizer-BioNTech) booster relative to the standard formulation.
Methods
This randomised, controlled, non-inferiority trial recruited adults in Mongolia primed with a two-dose homologous ChAdOx1 nCov-19 (Oxford-AstraZeneca, n = 129 participants), BBIBP-CorV (Sinopharm (Beijing), n = 399), or Gam-COVID-Vac (Gamaleya, n = 70) schedule. Participants were randomised (1:1) to receive a 15 μg (half-dose) or 30 μg (full-dose) BNT162b2 booster. Participants and study staff assessing reactogenicity were blinded up to day 28. Co-primary endpoints were Wuhan-Hu-1 anti-spike S1 IgG seroresponse 28 days post-boosting and reactogenicity within 7 days of boosting. The non-inferiority margin for the absolute difference in seroresponse was −10%. Differences in seroresponse were estimated from logistic regression with marginal standardisation. Geometric mean ratios of IgG were also estimated. ClinicalTrials.gov Identifier: NCT05265065.
Findings
Between May 27th and September 30th, 2022, 601 participants were randomized to full-dose BNT162b2 (n = 300) or half-dose (n = 301). 598 were included in safety analyses, and 587 in immunological analyses. The frequency of grade 3–4 reactions was similar between arms (half-dose: 4/299 [1.3%]; full-dose: 6/299 [2.0%]). Across all severity grades, half-dose recipients reported fewer local and systemic reactions (60% versus 72% and 25% versus 32%, respectively). Seroresponse was 84.7% (250/295) and 86.6% (253/292) in the half-dose and full-dose arms, respectively (Difference: −2.8%; 95% CI −7.7, 2.1). Geometric mean IgG titres were similar in those receiving full and half-dose boosters for the ChAdOx1 and BBIBP-CorV primed groups, but lower in the half-dose arm in Gam-COVID-Vac-primed participants (GMR: 0.71; 95% CI 0.54, 0.93).
Interpretation
Half-dose BNT162b2 boosting elicited an immune response that was non-inferior to a full-dose, with fewer reactions, in adults primed with ChAdOx1 nCov-19 or BBIBP-CorV. Half-dose boosting may not be suitable in adults primed with Gam-COVID-Vac. Half-dose BNT162b2 boosting may be considered in populations primed with ChAdOx1 nCov-19 or BBIBP-CorV.
Funding
Coalition for Epidemic Preparedness Innovations (CEPI).
Keywords: COVID-19, SARS-CoV-2, Vaccination, Fractional dose, Half-dose, Booster, BNT162b2, ChAdOx1, BBIP-CorV, Gam-COVID-Vac, Sputnik V
Research in context.
Evidence before this study
We searched Scopus for articles published up to February 3rd, 2023, in English, that provided evidence of the efficacy, immunogenicity, or effectiveness of fractional intramuscular BNT162b2 (Pfizer-BioNTech) booster vaccination in adults using the search terms: (TITLE-ABS-KEY (sars-cov-2 OR covid∗) AND TITLE-ABS-KEY (vaccin∗) AND TITLE-ABS-KEY ((fraction∗ W/4 dos∗) OR (half∗ PRE/1 dos∗) OR (reduced PRE/1 dos∗)) AND TITLE-ABS-KEY (efficac∗ OR immun∗ OR effective∗) AND NOT TITLE (intradermal∗)). Five studies compared fractional (half-dose/15 μg) versus standard doses of BNT162b2 (3 RCTs [primed with CoronaVac (Sinovac), ChAdOx1 nCov-19 (Oxford-AstraZeneca); or BNT162b2] and 2 cohort studies [CoronaVac-primed]). All studies demonstrated comparable immune responses in those receiving a half-dose, with fewer reactions. No studies had compared half-dose versus full-dose BNT162b2 in individuals primed with BBIBP-CorV (Sinopharm (Beijing)) or Gam-COVID-Vac (Gamaleya).
Added value of this study
COVID-19 vaccine effectiveness declines over time and can be temporarily restored with a booster dose. Heterologous boosting with BNT162b2 is more immunogenic than homologous boosting in adults primed with various non-mRNA COVID-19 vaccines but is also more reactogenic. Fractional doses may elicit a similar immune response with fewer side effects at reduced cost. Heterologous schedules, with or without fractional dosing, constitute off-label use in many countries and implementation of such schedules requires robust evidence of risks and benefits. This study provides crucial evidence for policymakers and immunisation advisory bodies on the use of fractional heterologous booster doses following various priming schedules. To the best of our knowledge, this is the first study to assess and compare the immunogenicity and reactogenicity of a half- versus full-dose BNT162b2 booster in adults primed with a two-dose homologous BBIBP-CorV or Gam-COVID-Vac schedule, both of which were widely used in low- and middle-income countries.
Implications of all the available evidence
Our results add to a growing body of evidence that half-dose BNT162b2 boosting elicits a similar immune response with fewer reactions the standard dose in ChAdOx1 nCov-19-primed populations, and indicate that this is generalisable to populations primed with BBIBP-CorV but not Gam-COVID-Vac. Our results diversify the evidence-base from which policymakers and immunisation advisory committees can draw upon to make flexible decisions regarding boosting schedules specific to various priming schedules.
Introduction
Evidence of declining vaccine effectiveness following COVID-19 vaccination due to waning immunity and emerging variants is well established.1 Booster doses can temporarily restore vaccine effectiveness and should be used within high-priority groups, even when overall coverage of the priming schedule remains low, to mitigate disease burden.1,2 Many countries are implementing booster (3rd) and second booster (4th) doses using currently authorised COVID-19 vaccines, including BNT162b2 (Pfizer-BioNTech, hereafter referred to as BNT) 30 μg.2, 3, 4
Heterologous BNT boosting is more immunogenetic than homologous boosting in adults primed with ChAdOx1 nCov-19 (Oxford-AstraZeneca, hereafter ChAd), BBIBP-CorV (Sinopharm (Beijing), hereafter BBIBP), or CoronaVac (Sinovac),4, 5, 6 but also more reactogenic.6 It is plausible that the authorised mRNA vaccines, including BNT 30 μg, are formulated at doses higher than required for effective boosting.7, 8, 9 Fractional dosing, a strategy used successfully for other diseases, was initially appealing due to global vaccine shortages.9 While supply issues are no longer a major global concern, hesitancy to receive mRNA booster vaccination (due to the level of reactogenicity) amongst fully primed individuals is of increasing concern.10 Fractional doses may elicit a similar immune response with fewer reactogenicity events, and thus improve COVID-19 booster acceptability and uptake.11 Additionally, fractional dosing has the potential to reduce costs despite recent per-dose price increases and protect against potential supply shocks triggered by new variants of concern or political, economic or humanitarian crises.12,13
Data on the immunogenicity and reactogenicity of heterologous fractional BNT booster schedules are sparse, particularly for a diverse range of priming schedules, including whole inactivated virus vaccines, which have been shown to confer lower levels of protection than mRNA and viral vector vaccines.4,14, 15, 16 As priming involves a range of epitopes, it is plausible that the different priming vaccines may vary in their efficiency. Data from the UK, Thailand, and Indonesia suggest that half-dose (15 μg) BNT [mRNA] boosting following either a ChAd [viral vector],17, 18, 19 BNT,18,19 or CoronaVac [whole inactivated virus]20, 21, 22 priming schedule elicits an immune response similar to or only marginally lower than the standard 30 μg dose, with fewer systemic reactions. However, this may not be generalisable to all priming schedules. To our knowledge, no studies have compared fractional versus standard doses of BNT in adults primed with BBIBP or Gam-COVID-Vac (Gamaleya, hereafter Gam) groups, which have been widely used in low- and middle-income countries.
In Mongolia, COVID-19 vaccination of adults began in February 2021, most commonly with BBIBP (whole cell inactivated) but also with the ChAd (viral vector), Gam (viral vector) and BNT (mRNA) vaccines. Booster (third) doses began in August 2021, mainly with BNT. At recruitment (May 2022), 64% of the total Mongolian population had received two priming doses of a COVID-19 vaccine, and 31% had received a booster dose.
We sought to quantify the immunogenicity, reactogenicity, and safety of a half-dose BNT booster, relative to the standard dose, in adults in Mongolia primed with a two-dose homologous ChAd, BBIBP, or Gam schedule. We used a larger sample size for the BBIBP primed group because this is the most widely used priming vaccine in Mongolia, and because it is anticipated to have the poorest responses, potentially impacting booster responses.
Methods
Trial design
This is a 1:1 randomised, controlled, non-inferiority phase 3 trial comparing the immunogenicity, safety, and reactogenicity of a BNT half-dose (15 μg) versus BNT full-dose (30 μg) COVID-19 booster amongst adults in Mongolia primed with a two-dose homologous ChAd, BBIBP, or Gam schedule (ClinicalTrials.gov Identifier: NCT05265065). The trial will follow participants for 1 year, with blood draws pre-boosting and 28 days, 6 months, and 12 months post-boosting. This paper reports on data to the Day-28 visit. The trial was reviewed and approved by the Human Research Ethics Committee at the Royal Children's Hospital Melbourne (HREC/81800/RCHM-2021) and the Mongolian Ethics Committee of the Ministry of Health (Decision #273, April 5th, 2022). The study protocol is provided in Supplementary Material 2.
Participants
Participants were adults aged ≥18 years primed with a two-dose homologous BBIBP, ChAd, or Gam schedule ≥6 months before entering this study, recruited from vaccination clinics at three district health centres in Ulaanbaatar (Bayangol, Sukhbaatar and Songinokhairkhan) and two provincial hospitals (Arkhangai and Darkhan-Uul), and from public and private organisations by mobile recruitment teams in Ulaanbaatar (Table S1). Potential participants were included if they were willing and able to give written informed consent and complete the follow-up requirements, and excluded if they: had already received a third dose of a COVID-19 vaccine; were currently on immunosuppressive medication or anti-cancer chemotherapy; were known to be HIV positive; had a congenital immune deficiency syndrome; had received immunoglobulin or other blood products within the past three months; were study staff or their relatives; had a history of severe allergic reaction to any COVID-19 vaccine or other contraindications to further COVID-19 vaccination. All participants provided written informed consent.
Randomisation and blinding
An unblinded independent statistician from the Melbourne Children's Trial Centre provided a secure, password-protected, web-based randomisation schedule. Participants were randomised 1:1 into half- and full-dose arms using random blocks of permutated length, with stratification by priming schedule (BBIBP, ChAd, or Gam) and age group (<50 or ≥50 years). Recruitment of participants within each unique priming and age stratum ceased once pre-specified recruitment targets were met (200 BBIBP-primed, 100 ChAd-primed and 100 Gam-primed participants in each age group [<50/≥50 years], yielding a total of 800 participants). Details of the randomisation method are held securely in a REDCap database by the Clinical Epidemiology and Biostatistics Unit (CEBU) at MCRI.
Study staff involved in administering the vaccine were not blinded. The participants and the clinical study team assessing reactogenicity were blinded to the allocation until the Day-28 visit. Laboratory staff were blinded during the analysis of specimens. Statisticians and analysts were blinded during the Statistical Analysis Plan (SAP) development, and codes were developed using a dummy variable for treatment allocation.
Procedures
Potential participants were identified from national vaccination records and approached by phone, through vaccination clinics or mobile recruitment teams working with public and private organisations employing frontline workers (Table S1). Those interested in participating were given an information sheet and a consent form to review with trial staff and were screened for eligibility. The Day 0 enrolment visit included consent, allocation, baseline data collection, baseline peripheral blood draw (5 mL), and vaccination. Those who met the inclusion and exclusion criteria and provided written informed consent were randomly assigned to receive a half- or full-dose BNT booster. The full-dose BNT booster contained 30 μg of nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 in 0.3 mL; the half-dose contained 15 μg in 0.15 mL. Vaccines were administered via intramuscular injection into the upper arm; participants were observed for at least 15 min following vaccine administration, and immediate adverse events were documented. Participants were provided with a diary card (hard copy or an electronic REDCap form), ruler, and thermometer, and instructions for use for self-documentation of reactogenicity (i.e., solicited adverse events) over the following 7 days.
Participants were contacted by study staff on days 1 and 7 to review the diary card and document solicited and unsolicited adverse events. Participants were scheduled to return for an in-person visit between days 28 and 35, inclusive (hereafter the Day-28 visit). During the Day-28 visit, 5 mL of peripheral blood was drawn, and information on adverse events (including new information on prior adverse events) was documented. Participants were also asked about known COVID-19 episodes, including the positive test day and severity. Participants were also encouraged to contact the study team between scheduled visits if they developed COVID-19 symptoms, and then were referred for a PCR test; for those with a positive result, the study team sought to obtain a residual nasal specimen, maintained daily telephone contact throughout their illness, and recorded the severity of illness.
Blood samples were processed for serum and stored at −80 °C until use. Binding antibody to the S1 domain of the spike protein of SARS-CoV-2 (Wuhan-Hu-1) (hereafter ‘anti-spike IgG’) was measured using the SARS-CoV-2 QuantiVac S1 IgG ELISA kits (Euroimmun, Lübeck, Germany) and reported as relative units/ml (RU/ml) as per manufacturer's instructions and converted to binding antibody units (BAU/ml) using the WHO reference serum from NIBSC, UK. Percentage inhibition of the interaction between the SARS-CoV-2 receptor-binding domain (RBD) from Wuhan-Hu-1 or Omicron B.1.1.529 and host-cell angiotensin converting enzyme-2 (ACE2) by neutralising antibodies in participant sera was measured using the C-PASS surrogate virus neutralisation test (sVNT, Genscript, USA).23
Endpoints
The co-primary endpoints were seroresponse at 28 days post-boosting and reactogenicity within 7 days of boosting. Seroresponse was defined as anti-spike IgG levels at the Day-28 visit ≥4-times that at baseline if baseline levels were <200 BAU/ml, or ≥2-times that at baseline if baseline levels were ≥200 BAU/ml.24 Reactogenicity was defined as any solicited grade 3 or 4 local or systemic reactions within 7 days of vaccination, inclusive (solicited reactions and their grading are detailed in the protocol [Supplement 2]). Secondary immunological endpoints were the geometric mean ratio (half- versus full-dose) in anti-spike IgG (BAU/ml) and median percentage inhibition (for both Wuhan-Hu-1 and B.1.1.529 variants) at 28 days post-boosting. Safety endpoints were any adverse or serious adverse event (AE or SAE, respectively) to Day 28 (inclusive) by severity, causality, Medical Dictionary for Regulatory Activities (MedDRA) system organ class (SOC), and outcome. AEs of special interest (AESI) were defined according to the Brighton Collaboration (October 2022). A detailed list of all endpoints, including population summary measures, measures of association, and comparisons, is provided in the SAP [Supplement 3].
Statistical analysis
The sample size was based on the primary immunological endpoint, assuming an estimated seroresponse rate of 95% in both half- and full-dose arms, a non-inferiority margin of −10% (absolute difference), a one-sided significance level of 5%, and no loss to follow-up. Under this scenario, a sample size of 100 per arm provides 90% power to compare seroresponse rates between arms under the non-inferiority framework, allowing for subgroup analyses by priming schedule, and within the BBIBP-primed strata, subgroup analyses by age group. Recruitment targets were set to 200 BBIBP-primed, 100 ChAd-primed and 100 Gam-primed participants in each age group (<50/≥50 years), yielding a total sample size of 800.
We used the Estimand Framework to align the study objectives to the statistical analysis.25 Estimand-to-analysis tables, which relate each of the five estimand attributes to the analytical methods, were specified in the SAP for each endpoint (Supplement 3).26 The estimand for the primary immunogenicity endpoint is the risk difference in seroresponse 28 days after BNT boosting between half-dose (15 μg) and full-dose (30 μg) groups, in adults ≥18 years in Mongolia who have been primed through previous vaccination with either of the included priming schedules, irrespective of SARS-CoV-2 infection. SARS-CoV-2 infection at any time following trial vaccination was considered an intercurrent event and handled using the Treatment Policy strategy, with results interpreted within the context of infection rates.25 Supplementary analyses using the Hypothetical Strategy to handle breakthrough infections (those >14 days after boosting) were planned but not conducted due to only one event being detected.25 Missing data were handled via complete case analysis.
The reactogenicity and safety analysis populations included all randomised participants who received a study vaccine, according to the vaccine received (Fig. 1). The proportion of participants who experienced at least one solicited grade 3–4 local or systemic reaction within 7 days of boosting was estimated within each study arm with 95% Clopper-Pearson binomial confidence intervals. The proportion of participants who experienced each reaction by severity grading was presented, and the distribution of the day of onset for each reaction type was described. If a response was missing from the reactogenicity diary card, we assumed the participant did not experience the reaction that day. Unsolicited AEs starting ≤28 days post-vaccination were tabulated by severity and relationship. Grade 3–5 AEs and SAEs were tabulated by MedDRA SOC, outcome, and relationship.
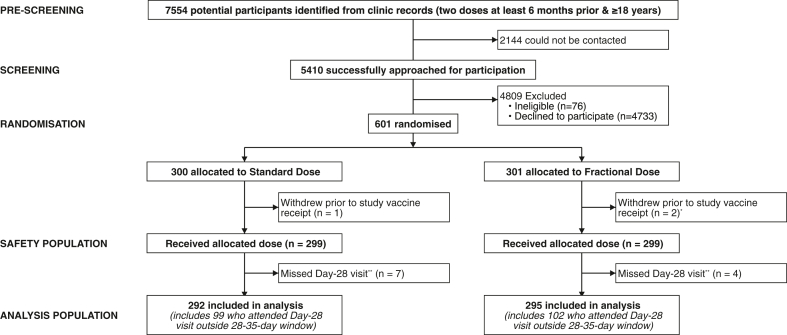
Fig. 1.
Trial profile. ∗Reasons for withdrawal on Day 0: Changed mind about participation due to a medical condition (n = 1); Changed mind about receiving vaccine that had been extended by the manufacturer, but the label incorrectly indicated that the expiry date had been passed (n = 2). ∗∗There were no missing immunological data for participants who attended their Day-28 visit, except for one participant with missing Day 0 and Day 28 sVNT data.
The analysis of immunogenicity endpoints included all participants with outcome data (Fig. 1). For the primary immunogenicity endpoint, the summary measure was the difference in seroresponse proportions (a marginal risk difference) between the half- and full-dose arms. To enable adjustment for covariates, this risk difference was estimated as the difference in predicted probabilities calculated from a logistic regression model. This was done using marginal standardisation (estimating the margins at values of covariates observed in the sample) using the ‘margins’ command in Stata software. Since we were interested in the difference within each priming stratum, a flexible logistic model was fitted by including interaction terms between arm and each covariate, allowing for marginal standardisation overall and within each priming stratum. The geometric mean ratio (GMR) in anti-spike IgG at day 28 was estimated by taking the antilogarithm of the mean difference (β1) from linear regression of the log-transformed anti-spike IgG levels. Percentage inhibition was plotted and described with median (25th–75th percentile) values. GMRs were adjusted for age group, priming schedule, duration between first and second dose, duration between second and third (study) dose, study day of blood draw, and baseline anti-spike IgG levels. The anti-spike IgG GMR was estimated within priming strata by fitting an interaction parameter between arm and priming strata in regression models. Exploratory subgroup analyses were conducted within each age stratum (<50/≥50 years), and by BMI group (<30/≥30 kg/m2).
Deviations from the SAP are listed and justified in Table S10. All statistical tests are 2-sided and performed using a 5% significance level, and all confidence intervals presented are 95% and two-sided. All analyses were performed using Stata version 17.0.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between May 27th and September 30th, 2022, 7554 potential participants were identified, of whom 5410 were approached for participation. Of those, 601 were recruited and randomised – 300 to receive a BNT full-dose (30 μg) booster and 301 to receive a BNT half-dose (15 μg) booster (Fig. 1). Recruitment targets were met for BBIBP (N = 401, of whom 206 were <50 years and 195 were ≥50 years [target = 200 per age group]) but not for ChAd (N = 130; 105 <50 years and 25 ≥50 years [target = 100 per age group]) or Gam (N = 70; 50 <50 years and 20 ≥50 years [target = 100 per age group]). Three participants withdrew before vaccination, and 598 received the allocated study vaccine and were included in the reactogenicity and safety analysis populations (Fig. 1). Eleven participants missed their Day-28 visit and were excluded from the immunogenicity analysis population (Fig. 1). No participants were excluded due to missing outcome or covariate data. Protocol deviations are described in Table S2. A substantial proportion (201/587; 34%) of participants attended their Day-28 visit outside the 28–35-day visit window (95% attended between days 21 and 35, inclusive); analyses were adjusted for the study day of blood draw, and exploratory analyses revealed little association between study day of blood draw and anti-spike IgG (Figs. S1 and S2).
Baseline characteristics are described in Table 1. No imbalances between study arms were noted (Table 1). The median age of the overall study population was 44 years (25th–75th percentile: 32–55 years) (Table 1). The proportion over 50 years of age in the study was 40%, but differed between the priming groups due to the vaccination policies that had been employed. The proportions over 50 years of age in the ChAd, BBIBP and Gam groups were 19%, 48% and 29% respectively. Thus the study population was relatively young, but this is indicative of the Mongolian population where the median age is 27 years and <5% of the population is over 65 years old. The median time between the second and third (study) dose among the total study population was 428 days (397–454 days) (Table 1). The time between the second and third (study) dose was slightly longer in ChAd-primed participants, and BBIBP-primed participants were slightly older (Table 1).
Table 1.
Baseline characteristics by study vaccine allocation and priming strata.
| All priming strata |
Primed with ChAd |
Primed with BBIBP |
Primed with Gam |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total |
Standard |
Fractional |
Standard |
Fractional |
Standard |
Fractional |
Standard |
Fractional |
|
| N = 598 | N = 299 | N = 299 | N = 65 | N = 64 | N = 200 | N = 199 | N = 34 | N = 36 | |
| Age, years | 44 (32–55) | 44 (32–55) | 44 (33–55) | 34 (32–46) | 40 (34–50) | 48 (31–58) | 48 (32–57) | 43 (32–53) | 41 (35–50) |
| <50 years | 360 (60.2%) | 181 (60.5%) | 179 (59.9%) | 54 (83.1%) | 50 (78.1%) | 103 (51.5%) | 103 (51.8%) | 24 (70.6%) | 26 (72.2%) |
| ≥50 years | 238 (39.8%) | 118 (39.5%) | 120 (40.1%) | 11 (16.9%) | 14 (21.9%) | 97 (48.5%) | 96 (48.2%) | 10 (29.4%) | 10 (27.8%) |
| Biological sex assigned at birth | |||||||||
| Male | 273 (45.7%) | 132 (44.1%) | 141 (47.2%) | 32 (49.2%) | 33 (51.6%) | 85 (42.5%) | 86 (43.2%) | 15 (44.1%) | 22 (61.1%) |
| Female | 325 (54.3%) | 167 (55.9%) | 158 (52.8%) | 33 (50.8%) | 31 (48.4%) | 115 (57.5%) | 113 (56.8%) | 19 (55.9%) | 14 (38.9%) |
| BMI, kg/m2 | 25.2 (22.6–28.7) | 25.2 (22.7–28.9) | 25.1 (22.5–28.7) | 26.3 (23.7–30) | 25.4 (23.4–28.4) | 24.6 (22.0–28.7) | 24.8 (22.3–27.9) | 25.3 (24.2–28.7) | 25.8 (23.1–29.8) |
| Days between 1st and 2nd doses | 31 (24–45) | 30 (24–44) | 31 (24–46) | 43 (41–50) | 44 (41–51) | 28 (23–30) | 28 (23–33) | 58 (44–64) | 61 (58–64) |
| Days between 2nd dose and study (3rd) dose | 428 (397–454) | 432 (391–454) | 425 (400–454) | 494 (416–517) | 505 (410–519) | 422 (386–450) | 418 (397–450) | 419 (367–448) | 430 (388–450) |
| Reaction following 1st or 2nd dose | 93 (15.6%) | 45 (15.1%) | 48 (16.1%) | 21 (32.3%) | 18 (28.1%) | 17 (8.5%) | 24 (12.1%) | 7 (20.6%) | 6 (16.7%) |
| Of those with a reaction: | |||||||||
| Pain or fever medication taken | 21 (22.6%) | 9 (20.0%) | 12 (25.0%) | 7 (33.3%) | 8 (44.4%) | 1 (5.9%) | 4 (16.7%) | 1 (14.3%) | 0 (0.0%) |
| Medical advice sought | 3 (3.2%) | 1 (2.2%) | 2 (4.2%) | 1 (4.8%) | 1 (5.6%) | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) |
| Symptoms resolved | 53 (57.0%) | 28 (62.2%) | 25 (52.1%) | 11 (52.4%) | 5 (27.8%) | 10 (58.8%) | 15 (62.5%) | 7 (100%) | 5 (83.3%) |
| Comorbidities | |||||||||
| Obesity (BMI ≥30 kg/m2) | 115 (19.2%) | 55 (18.4%) | 60 (20.1%) | 13 (20.0%) | 12 (18.8%) | 37 (18.5%) | 39 (19.6%) | 5 (14.7%) | 9 (25.0%) |
| Diabetes mellitus | 25 (4.2%) | 17 (5.7%) | 8 (2.7%) | 2 (3.1%) | 1 (1.6%) | 10 (5.0%) | 5 (2.5%) | 5 (14.7%) | 2 (5.6%) |
| Cardiovascular disease | 56 (9.4%) | 26 (8.7%) | 30 (10.0%) | 2 (3.1%) | 5 (7.8%) | 20 (10.0%) | 23 (11.6%) | 4 (11.8%) | 2 (5.6%) |
| Hypertension | 166 (27.8%) | 80 (26.8%) | 86 (28.8%) | 14 (21.5%) | 14 (21.9%) | 59 (29.5%) | 60 (30.2%) | 7 (20.6%) | 12 (33.3%) |
| Cancer | 3 (0.5%) | 0 (0.0%) | 3 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 2 (5.6%) |
| Chronic obstructive pulmonary disease | 8 (1.3%) | 4 (1.3%) | 4 (1.3%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | 3 (1.5%) | 2 (5.9%) | 1 (2.8%) |
| Chronic kidney disease | 49 (8.2%) | 25 (8.4%) | 24 (8.0%) | 3 (4.6%) | 4 (6.2%) | 18 (9.0%) | 17 (8.5%) | 4 (11.8%) | 3 (8.3%) |
| Chronic liver disease | 19 (3.2%) | 9 (3.0%) | 10 (3.3%) | 1 (1.5%) | 3 (4.7%) | 6 (3.0%) | 4 (2.0%) | 2 (5.9%) | 3 (8.3%) |
| History of anaphylaxis (or carry an EpiPen) | 12 (2.0%) | 6 (2.0%) | 6 (2.0%) | 3 (4.6%) | 2 (3.1%) | 3 (1.5%) | 3 (1.5%) | 0 (0.0%) | 1 (2.8%) |
| Neurological disease (including stroke) | 6 (1.0%) | 3 (1.0%) | 3 (1.0%) | 1 (1.5%) | 1 (1.6%) | 2 (1.0%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) |
| On anticoagulant therapy | 33 (5.5%) | 17 (5.7%) | 16 (5.4%) | 4 (6.2%) | 2 (3.1%) | 9 (4.5%) | 11 (5.5%) | 4 (11.8%) | 3 (8.3%) |
| Immunocompromised | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Mastocytosis causing recurrent anaphylaxis | 1 (0.2%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) |
| Cigarette user | 125 (20.9%) | 66 (22.1%) | 59 (19.7%) | 16 (24.6%) | 17 (26.6%) | 37 (18.5%) | 34 (17.1%) | 13 (38.2%) | 8 (22.2%) |
| Currently pregnant | 1 (0.2%) | 1 (0.3%) | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Data are median (25th–75th percentile) or N (%). No data were missing for the variables reported. This table excludes the 3 participants who withdrew before receiving the study vaccine.
A high proportion (>70%) of participants had missing data for reactions that required measurement with a tape measure (redness, swelling, and hardness); missingness for other reactions was low (<5%) (Table S3). Grade 3–4 reactions to day 7 were fever (n = 3 [1.00%] half-dose; n = 3 [1.00%] full-dose), malaise/fatigue (n = 1 [0.33%] half-dose; n = 0 full-dose), joint pain (0 half-dose; n = 1 [0.33%] full-dose), and tenderness at the vaccination site (n = 0 half-dose; n = 2 [0.67%] full-dose) (Fig. 2). Combining all reaction types, 1.34% (95% CI: 0.37, 3.39) of participants in the half-dose arm experienced any Grade 3–4 reaction to day 7, compared to 2.01% (95% CI: 0.74, 4.32) in the full-dose arm. Including all severity grades, compared to full-dose participants, half-dose participants reported fewer local reactions (60% versus 72%; specifically, less pain, tenderness, and axillary lymphadenopathy), and fewer systemic reactions (25% versus 32%; specifically, less fever, vomiting, diarrhoea, headache, fatigue/malaise, joint pain, and muscle pain) (Fig. 2, Fig. S4, Table S4).
Fig. 2.
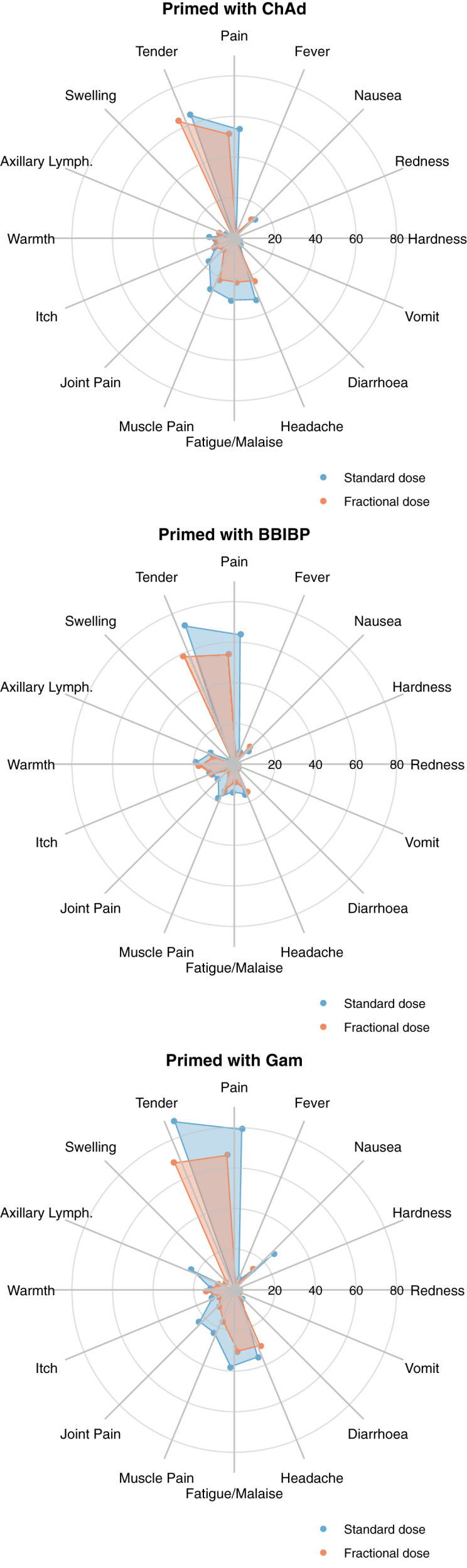
Percent of participants who experienced each solicited reaction at any severity grade, by study arm and priming schedule. Concentric circles indicate 20, 40, 60, and 80%.
Overall, 84.7% (95% CI: 80.1, 88.7) of half-dose participants seroresponded compared to 86.6% (95% CI: 82.2, 90.3) of full-dose participants (Fig. S3). The percentage difference in seroresponse in the half- versus full-dose arms was −2.9% and the 95% CI (−7.7, 2.0) excluded the non-inferiority margin of −10% (Fig. S3). Within priming strata, the percentage difference in seroresponse was −0.5% (95% CI: −17.1, 16.1) in Gam-primed participants, −3.1% (95% CI: −9.5, 3.2) in BBIBP-primed participants, and −2.4% (95% CI: −16.1, 11.3) in ChAd-primed participants (Fig. S3).
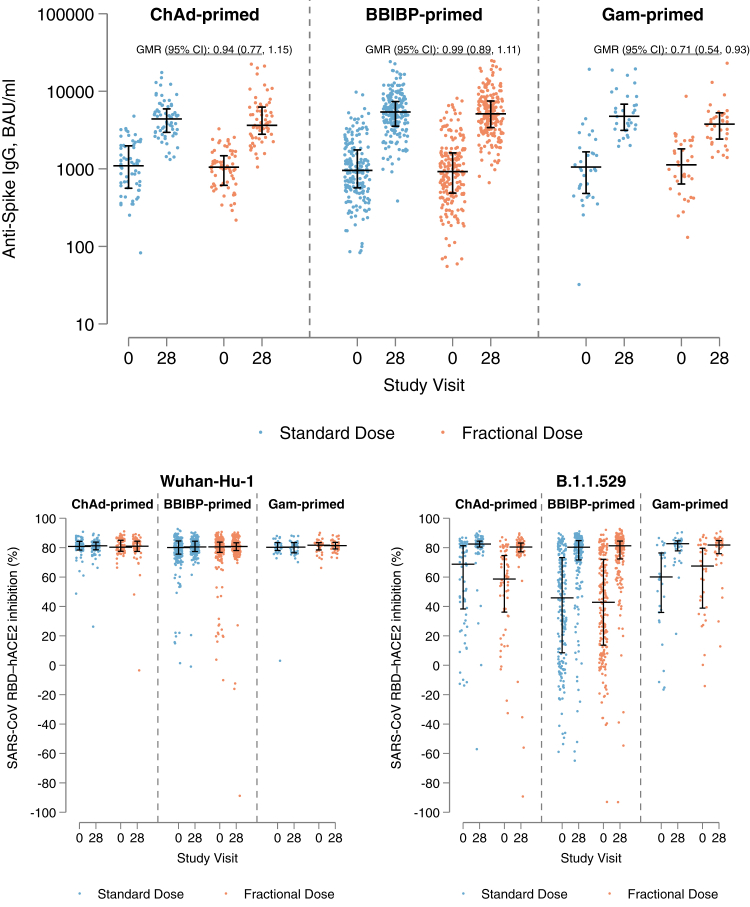
When day-28 anti-spike IgG was analysed on the continuous scale, the GMR between the half- and full-dose arms across all priming strata was 0.94 (95% CI: 0.86, 1.04; p = 0.228) (Table 2). The GMR was similar in ChAd-primed participants (0.94 [95% CI: 0.77, 1.15; p = 0.537]) and BBIBP-primed participants (0.99 [95% CI: 0.89, 1.11; p = 0.922]) (Table 2). In Gam-primed participants, the geometric mean anti-spike IgG was lower in the half-dose arm compared to the full-dose arm (GMR 0.71 [95% CI: 0.54, 0.93; p = 0.014]) (Table 2). GMRs were similar in each age stratum (<50/≥50 years) and BMI group (<30/≥30 kg/m2) for all priming schedules (Tables S5 and S6). Anti-spike IgG levels pre- and post-BNT boosting are presented by study arm and priming strata in Fig. 3.
Table 2.
Immunological parameters at the Day-28 visit by study arm and priming schedule.
| Anti-spike IgG, BAU/ml | |||
|---|---|---|---|
| Priming strata | GM IgG at Day-28 standard dose | GM IgG at Day-28 fractional dose | GMR |
| All | 4946 (4614, 5302) [N = 292] | 4619 (4292, 4970) [N = 295] | 0.94 (0.86, 1.04); p = 0.228 |
| ChAd | 4394 (3863, 4997) [N = 65] | 4167 (3550, 4892) [N = 62] | 0.94 (0.77, 1.15); p = 0.537 |
| BBIBP | 5109 (4678, 5580) [N = 194] | 4970 (4541, 5438) [N = 198] | 0.99 (0.89, 1.11); p = 0.922 |
| Gam | 5160 (4128, 6450) [N = 33] | 3662 (3016, 4447) [N = 35] | 0.71 (0.54, 0.93); p = 0.014 |
| Wuhan-Hu-1 SARS-CoV-2 RBD–ACE2 inhibition (%) | |||
|---|---|---|---|
| Priming strata | Median inhibition at Day-28 standard dose | Median inhibition at Day-28 fractional dose | |
| All | 81 (78–84) [N = 290] | 81 (78–84) [N = 295] | - |
| ChAd | 81 (78–84) [N = 64] | 81 (77–84) [N = 62] | - |
| BBIBP | 80 (77–84) [N = 193] | 81 (78–83) [N = 198] | - |
| Gam | 80 (76–84) [N = 33] | 81 (79–84) [N = 35] | - |
| B.1.1.529 SARS-CoV-2 RBD–ACE2 inhibition (%) | |||
|---|---|---|---|
| Priming strata | Median inhibition at Day-28 standard dose | Median inhibition at Day-28 fractional dose | |
| All | 82 (74–85) [N = 291] | 81 (75–84) [N = 295] | - |
| ChAd | 82 (80–84) [N = 65] | 80 (77–83) [N = 62] | - |
| BBIBP | 80 (72–85) [N = 193] | 81 (72–84) [N = 198] | - |
| Gam | 83 (78–85) [N = 33] | 82 (76–85) [N = 35] | - |
Numbers are GM (95% CI) [number included in analysis], Median (25th–75th percentile), or GMR (95% CI); p-value. The GMR is adjusted for age group, priming vaccine, duration between first and second dose, duration between second and third (study) dose, study day of blood draw, and baseline anti-spike IgG.
GM: Geometric Mean. GMR: Geometric Mean Ratio. Ref.: Reference Group.
Fig. 3.
Anti-spike IgG and SARS-CoV RBD–hACE2 inhibition (%) against Wuhan-Hu-1 and B.1.1.529 variants at Day-0 and Day-28 visits, by study arm and priming strata. Horizontal black lines with vertical bars indicate the median and 25th–75th percentiles. GMR: Geometric mean ratio in Day-28 levels between fractional and standard dose arms, adjusted for age group, priming vaccine, duration between first and second dose, duration between second and third (study) dose, study day of blood draw, and baseline anti-spike IgG.
In an exploratory analysis, we plotted day-28 anti-spike IgG levels across the range of baseline anti-spike IgG values; these plots show that: 1) response to BNT boosting was greater in individuals with lower baseline levels in all priming strata; 2) in ChAd- and BBIBP-primed strata, response to BNT boosting was similar between half- and full-dose arms regardless of baseline levels; 3) in the Gam-primed strata, the lower response to BNT boosting in the half-dose arm is more pronounced in individuals with lower baseline IgG levels; and 4) individuals who did not meet the definition for seroresponse all had very high baseline anti-spike IgG levels (median 2244 BAU/ml [25th–75th percentile: 1639–3370] compared to 836 BAU/ml [494–1373] in those who met the seroresponse definition) (Fig. S5).
In both half- and full-dose arms, neutralising antibody against Wuhan-Hu-1, measured as percentage inhibition using the sVNT assay, was high prior to boosting and similar after boosting in all priming strata (median percentage inhibition around 80% pre- and post-boosting across all groups) (Fig. 3, Table 2). Conversely, percentage inhibition against Omicron B.1.1.529 was lower prior to BNT boosting but increased to around 80% (median) in both half- and full-dose arms in all priming strata (Fig. 3, Table 2). Results were similar in each age stratum (<50/≥50 years) and BMI group (<30/≥30 kg/m2) for all priming schedules (Tables S5 and S6).
Three participants tested positive for SARS-CoV-2 by rapid antigen test or PCR within 14 days (inclusive) of study vaccination (two full-dose participants [on Day 1 and Day 3] and one half-dose participant [Day 2]) (Table S7). One asymptomatic breakthrough infection occurred on Day 18 in the half-dose arm (Table S7). Forty-three participants experienced an AE, including five who experienced an SAE (22 AEs including three SAEs in the full-dose arm and 21 AEs including two SAEs in the half-dose arm). None of the SAEs were related to the study. No differences in the frequency of AEs were noted between the study arms, even when events were categorised according to severity, relationship to study vaccine, outcome, or SOC (Table S8). There were no notable differences between arms in the duration of events or the study day of onset (Figs. S6–S9). There were no AESI.
Discussion
COVID-19 booster doses are required to restore vaccine effectiveness in the face of waning immunity and emerging variants. Fractional booster dosing addresses several issues, including poor acceptability of boosters (related to reactogenicity to prior doses), cost, and supply issues.
This randomised, controlled, non-inferiority trial sought to quantify the immunogenicity, reactogenicity, and safety of a half-dose BNT booster, relative to the standard 30 μg dose, in adults in Mongolia primed with a two-dose homologous ChAd, BBIBP, or Gam schedule. Both immunogenicity and reactogenicity are listed as co-primary endpoints. This was because these are the two endpoints that are most influential as governments decide on Covid-19 vaccination strategies. Immunogenicity is an indicator of effectiveness, while reactogenicity is a marker of acceptability. These are independent variables so statistical adjustment is not required, and the relative importance of these must be determined at country level. The safety of BNT vaccine is well documented.
We found that the humoral immune response to Wuhan-Hu-1 28-days post-boosting with BNT 15 μg was non-inferior to the standard 30 μg in adults primed with ChAd or BBIBP, while BNT 15 μg was associated with a lower humoral immune response in adults primed with Gam. Percentage inhibition against the Wuhan-Hu-1 and Omicron B.1.1.529 variants 28-days post-boosting was similar in the BNT 15 μg and BNT 30 μg groups with medians of around 80% across all priming strata. Those who received the 15 μg half-dose reported fewer reactions in the 7 days following vaccination.
In this trial, participant retention was high (98%), as was data completeness (100% for retained participants), except for solicited reactions that required measurement with a tape measure, for which we assumed the absence of a measurement indicated absence of the reaction. We used the Estimand Framework to align the study objectives to the statistical analysis and clearly define the treatment effect, including consideration of intercurrent events.25 Breakthrough infections were identified as intercurrent events and handled using the Treatment Policy strategy, which could theoretically inflate the immunogenicity of the fractional dose if it conferred less clinical protection. However, only three symptomatic and one asymptomatic breakthrough infections were detected, although there may have been more as subsequent investigation has revealed many undiagnosed infections. While a correlate of protection against COVID-19 is yet to be established, we considered both immunoglobulin (anti-spike IgG) titres and functional antibody responses (SARS-CoV RBD–ACE2 percentage inhibition, as a surrogate measure of virus neutralisation by neutralising antibodies).24 Use of the sVNT assay over the conventional virus neutralization test allowed for samples to be analysed in a low-resource setting.23 The sVNT assay was conducted against both the Wuhan-Hu-1 and Omicron B.1.1.529 variants, which adds additional information on humoral immunity; Omicron BA.5 was dominant in Mongolia at the time of this study.
Our results build on evidence from previous studies on the immunogenicity and reactogenicity of fractional heterologous booster doses. Data from the UK, Thailand, and Indonesia show that half-dose (15 μg) BNT [mRNA] boosting following either a ChAd,17, 18, 19 BNT,18,19 or CoronaVac20, 21, 22 priming schedule elicits an immune response similar to or only marginally lower than the standard 30 μg dose, with fewer systemic reactions. Only two of these studies randomised participants into half- or full-dose groups, and neither adjusted for covariates. Strengths of this study in relation to these prior studies include the inclusion of BBIBP and Gam priming schedules, randomisation into half- and full-dose groups, adjustment for covariates, and use of the estimand framework to consider the impact of intercurrent events.
Due to the urgency of developing an effective COVID vaccine, a 30 μg dose was used in Phase 3 BNT studies because, at the time, the immunogenicity required for clinical protection was unknown. Thus the highest tolerated dose from Phase 2 studies was used (30 μg) to maximise the probability of a successful outcome (10 μg and 100 μg were also tested).7, 8, 9 It is therefore widely recognised that a lower dose than the currently authorised 30 μg is likely to elicit comparable or near-comparable protection, especially when in the context of boosting, and there remains a moral obligation from the pharmaceutical and scientific community to identify effective dose-sparing strategies, including fractional dosing, to ensure equitable and affordable access to vaccines.8 Heterologous schedules, with or without fractional dosing, constitute off-label use in many countries and implementation of such schedules requires robust data on risks and benefits.27 This study provides crucial evidence for policymakers and immunisation advisory bodies on the use of a half-dose BNT booster following various priming schedules. Nevertheless, it is difficult to generate relevant evidence in a post-authorisation environment due to the rapidly evolving nature of SARS-CoV-2 research and public health interventions. A vaccine development framework that incorporates evaluation of dose-sparing mechanisms as soon as safety has been established, in parallel with prelicensure phase 2 and 3 trials, will help to avert vaccine shortages, minimize reactogenicity, and improve inequities in future pandemics.8
This study had some limitations. We recruited 601 participants having approached 5410 individuals. Most refused, either because they were not interested in additional Covid-19 vaccine doses, or because they did not want to join the research. Those agreeing to join the study are the population from which we have been able to draw our conclusions. Anti-spike S1 IgG levels were only ascertained for Wuhan-Hu-1, while SARS-CoV RBD–hACE2 percentage inhibition was ascertained for Wuhan-Hu-1 and Omicron B.1.1.529, yet new variants continue to emerge. We also failed to meet our recruitment targets in the ChAd- and Gam-primed strata despite concerted efforts to find eligible participants, and seroresponse was lower than anticipated in our sample size calculations (∼85% versus 95%), resulting in a higher than anticipated level of uncertainty around stratum-specific estimates. Nevertheless, the lower bound of our confidence intervals in the unstratified analysis and within BBIBP-primed participants excludes the −10% non-inferiority margin, and our analysis of anti-spike IgG on the continuous scale excludes the non-inferiority margin recommended by WHO (GMR of 0.67) within both ChAd- and BBIBP-primed participants.24
Day-28 seroresponse with a −10% non-inferiority margin was chosen as the primary immunological endpoint because it is commonly used in COVID-19 immunobridging vaccine studies where the comparator vaccine is highly efficacious, and is recommended by the WHO.24 However, dichotomising outcome variables (including seroresponse) results in loss of information and reduces statistical efficiency.28,29 This is demonstrated by the apparent contradiction in results in Gam-primed participants, whereby there was no detectable difference in seroresponse proportions, while geometric mean anti-spike IgG was significantly lower in the half-dose arm. Exploration of anti-spike IgG levels pre- and post-boosting revealed that all ‘non-responders’ had very high pre-booster (Day-0) IgG levels, while those with lower pre-booster (Day-0) IgG levels far exceeded the seroresponse threshold. The significant difference in anti-spike IgG response to BNT boosting between the half- and full-dose arms in Gam-primed participants was more pronounced in those with lower baseline levels, who all met the binary seroresponse definition; dichotomisation into a binary seroresponse variable therefore obscured this difference in response.
Our study cohort will be followed to obtain further data at 6 and 12 months to answer key questions on long-term immunogenicity and safety, including the rate of waning and breakthrough infections.30 Cellular immunity is also likely to play an important role in clinical protection; additional samples were collected from 40% of participants in this study for T cell analysis which will be reported separately. Future research is needed to evaluate effectiveness against clinical endpoints, the optimal timing of booster vaccination, and the interval between repeated booster doses if required.30 Establishing a standardised correlate of protection for COVID-19 will expedite this process.13 Although our trial included individuals with comorbidities, further research is needed to determine if these results are generalisable to specific vulnerable populations.30
Our results add to a growing body of evidence that half-dose BNT162b2 boosting elicits a similar immune response with fewer reactions than the standard 30 μg dose in ChAdOx1 nCov-19-primed populations, and indicate that this is generalisable to populations primed with BBIBP-CorV but not Gam-COVID-Vac. Our results diversify the evidence-base from which policymakers and immunisation advisory committees can draw upon to make flexible decisions regarding boosting schedules specific to various priming schedules. Fractional dosing may improve COVID-19 booster acceptability and uptake and will reduce the per-dose cost of COVID-19 booster programmes, which is particularly important in low- and middle-income countries where BBIBP-CorV has been widely used for the priming schedule. Half-dose BNT162b2 boosting could be considered in populations primed with a two-dose homologous ChAdOx1 nCov-19 or BBIBP-CorV schedule to improve uptake of COVID-19 boosting and reduce costs. Subsequent data from this trial on cellular immune responses and rates of waning and breakthrough infections will provide additional insights.
Contributors
TBa, KMu, CvM, HT, BO, PVL, US, KBa, and GD conceptualised the study. KMu, TBa, and HT acquired funding. TBa, HT, BT, and FJ managed and coordinated the project. TBa, HT, OA, KJ, and LB recruited the participants, did the investigations, and collected the data. PVL, NM, KMa, SL, LAHD, BA, SJ, AT, TBu, and NA managed sample collection and laboratory analyses. HT, FJ, and JDH monitored the trial. EFGN managed and curated the databases. KAM and CDN wrote the statistical analysis plan. KAM analysed the data. KAM wrote the original draft of the manuscript. HT, CvM, FJ, and KBr provided supervision and oversight. JDH was the medical adviser. All authors reviewed the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
Anonymous participant data that underlie the results reported in this Article will be available on completion of the clinical trial. Data requests should be sent to the corresponding author. The requester must provide a scientifically sound proposal and data transfer agreement for the sponsors' and collaborators’ approval. On approval, data will be transferred through a secure online platform.
Declaration of interests
The National Centre for Communicable Diseases (NCCD) is part of the Mongolian Ministry of Health and is a focal point for WHO International Health Regulations. CDN receives funding from Merck Sharp & Dohme as a co-investigator/biostatistician on a Merck Investigator Studies Program grant on pneumococcal serotype epidemiology in children with empyema, and from Pfizer as a co-investigator/biostatistician on a clinical research collaboration on PCV vaccination in Mongolia. PVL receives funding from the Gates Foundation and the National Health and Medical Research Council (NHMRC, Australia). CvM is the principal investigator on a Pfizer clinical research collaborative grant on PCV vaccination in Mongolia. CvM has received honoraria from Pfizer and Merck for participation in expert panels. KMu was on the Data Safety Monitoring Board of a Novavax Covid-19 vaccine trial, which is now complete, and was funded to attend the October 2022 and March 2023 Strategic Advisory Group of Experts on Immunization (SAGE) meetings as a SAGE member. Other authors declare no competing interests.
Acknowledgements
This study was made possible by the generosity of the study participants – we thank them for their time and cooperation in the study procedures. We would like to acknowledge the invaluable contributions of: Dr. Chinburen Jigjidsuren (Member of Parliament); Dr. Ganzorig Dorjdagva (Ministry of Health); Dr. Otgonbold Jamyandorj (City Health Department); Dr. Urangoo Khurlee, Dr. Gentsenpilmaa Batbold, Dr. Gantuya Damdinsuren, and Dr. Altanshahai Boldbaatar (First Central Hospital of Mongolia); Dr. Tserendagva Dalkh (Mongolian National University of Medical Sciences); Dr. Davaalkham Jagdagsuren, Dr. Naranzul Tsedenbal, Tserendulam Bazarkhuu, and Ariunbileg Gankhuyag (National Centre for Communicable Diseases); Dr. Narantuya Namjil, Dr. Budkhand Ichinkhorloo, and Dr. Erdenebayar Tsedevsuren (Onoshmed Laboratory); Dr. Baldandugar Zeeren and Dr. Davaajav Tsend-Ayush (Bayangol District Health Department); Bat-Ireedui Purevbaatar and Dr. Gantuya Gansukh (Sukhbaatar District Health Department); Dr. Davaajargal Oyunsuren (Songinokhairkhan District Health Department); Dr. Gandiimaa Riimaadai, Dr. Tserendulam Dorjsuren and Dr. Ariunaa Jadambaa (Arkhangai Province Health Department); and Dr. Erkegul Sandalhan (Railway Central Hospital). This clinical trial is funded by CEPI, an innovative global partnership working to accelerate the development of vaccines against epidemic and pandemic threats so they can be accessible to all people in need. Grant management support is provided by PATH, an international non-profit global health organization. The Government of Mongolia provided the study vaccines. Kerryn Moore is supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (Grant Number APP1160936); the content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NHMRC. Authors affiliated with MCRI were supported by the Victorian Government's Operational Infrastructure Support Program.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100953.
Appendix A. Supplementary data
References
- 1.Menni C., May A., Polidori L., et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO SAGE roadmap for prioritizing uses of Covid-19 vaccines. 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2023.1 [Google Scholar]
- 3.Strategic Advisory Group of Experts on Immunization (SAGE) 2022. Good practice statement on the use of second booster doses for COVID-19 vaccines. [Google Scholar]
- 4.Tan C.Y., Chiew C.J., Lee V.J., Ong B., Lye D.C., Tan K.B. Comparative effectiveness of 3 or 4 doses of mRNA and inactivated whole-virus vaccines against COVID-19 infection, hospitalization and severe outcomes among elderly in Singapore. Lancet Reg Health West Pacific. 2022;29 doi: 10.1016/j.lanwpc.2022.100654. 100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghnieh R., Mekdashi R., El-Hassan S., et al. Immunogenicity and reactogenicity of BNT162b2 booster in BBIBP-CorV- vaccinated individuals compared with homologous BNT162b2 vaccination: results of a pilot prospective cohort study from Lebanon. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Shaw R.H., Stuart A.S.V., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020 doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 8.Roozen G.V.T., Roukens A.H.E., Roestenberg M. COVID-19 vaccine dose sparing: strategies to improve vaccine equity and pandemic preparedness. Lancet Glob Health. 2022 doi: 10.1016/S2214-109X(22)00075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling B.J., Lim W.W., Cobey S. Fractionation of COVID-19 vaccine doses could extend limited supplies and reduce mortality. Nat Med. 2021 doi: 10.1038/s41591-021-01440-4. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus J.V., Wyka K., White T.M., et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med. 2023 doi: 10.1038/s41591-022-02185-4. [DOI] [PubMed] [Google Scholar]
- 11.Al-Qerem W., Al Bawab A.Q., Hammad A., Ling J., Alasmari F. Willingness of the Jordanian population to receive a COVID-19 booster dose: a cross-sectional study. Vaccines (Basel) 2022 doi: 10.3390/vaccines10030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erman M. Pfizer expects to hike U.S. COVID vaccine price to $110-$130 per dose. Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-expects-price-covid-vaccine-110-130-per-dose-2022-10-20/
- 13.Więcek W., Ahuja A., Chaudhuri E., et al. Testing fractional doses of COVID-19 vaccines. Proc Natl Acad Sci U S A. 2022 doi: 10.1073/pnas.2116932119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premikha M., Chiew C.J., Wei W.E., et al. Comparative effectiveness of mRNA and inactivated whole-virus vaccines against coronavirus disease 2019 infection and severe disease in Singapore. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok C.K.P., Cohen C.A., Cheng S.M.S., et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2022 doi: 10.1111/resp.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rearte A., Castelli J.M., Rearte R., et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet. 2022 doi: 10.1016/S0140-6736(22)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nantanee R., Jantarabenjakul W., Jaru-Ampornpan P., et al. A randomized clinical trial of a fractional low dose of BNT162b2 booster in adults following AZD1222. Vaccines (Basel) 2022 doi: 10.3390/vaccines10060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munro A.P.S., Janani L., Cornelius V., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Munro A.P.S., Feng S., et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect. 2022 doi: 10.1016/j.jinf.2022.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadlyana E., Setiabudi D., Putri N.D., et al. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in I. Lancet Infect Dis. 2023 doi: 10.2139/ssrn.4185918. [DOI] [PubMed] [Google Scholar]
- 21.Angkasekwinai N., Niyomnaitham S., Sewatanon J., et al. [Preprint] The immunogenicity and reactogenicity of four COVID-19 booster vaccinations against SARS-CoV-2 variants of concerns (Delta, Beta, and Omicron) following CoronaVac or ChAdOx1 nCoV-19 primary series. medRxiv. 2022 doi: 10.1101/2021.11.29.21266947. [DOI] [PubMed] [Google Scholar]
- 22.Kanokudom S., Assawakosri S., Suntronwong N., et al. Comparison of the reactogenicity and immunogenicity of a reduced and standard booster dose of the mRNA COVID-19 vaccine in healthy adults after two doses of inactivated vaccine. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan C.W., Chia W.N., Qin X., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020 doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Considerations for evaluation of Covid19 vaccines. 2022. https://extranet.who.int/pqweb/sites/default/files/documents/considerations-who-evaluation-of-covid-vaccine_v25_112020.pdf [Google Scholar]
- 25.Committee for Medicinal Products for Human Use . 2020. ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials. [Google Scholar]
- 26.Kang M., Kendall M.A., Ribaudo H., et al. Incorporating estimands into clinical trial statistical analysis plans. Clin Trials. 2022 doi: 10.1177/17407745221080463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO . Interim recommendations for heterologous COVID-19 vaccine schedules: interim guidance. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-heterologous-schedules [Google Scholar]
- 28.Snapinn S.M., Jiang Q. Responder analyses and the assessment of a clinically relevant treatment effect. Trials. 2007;8:31. doi: 10.1186/1745-6215-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naggara O., Raymond J., Guilbert F., Roy D., Weill A., Altman D.G. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. Am J Neuroradiol. 2011 doi: 10.3174/ajnr.A2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Interim statement on dose-sparing strategies for COVID-19 vaccines (fractionated vaccine doses) 2021. https://www.who.int/news/item/10-08-2021-interim-statement-on-dose-sparing-strategies-for-covid-19-vaccines-(fractionated-vaccine-doses
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.