Summary
Background
Ivermectin mass drug administration (MDA) is effective for controlling onchocerciasis and scabies, with evidence supporting its role in some species of soil-transmitted helminth (STH) infections. In the context of RISE, a cluster-randomised trial for scabies, this study evaluated the effectiveness of ivermectin MDA in reducing STH burden in the Western Province of Solomon Islands.
Methods
Twenty villages were randomised 1:1 to receive ivermectin MDA as one dose (IVM-1) or two doses (IVM-2) for scabies. The effectiveness of one and two doses in reducing STH prevalence and intensity was evaluated before (May 2019) and 21 months after (February 2021) MDA in May 2019. All residents aged 12 months or older in the study villages were eligible to participate and provide stool specimens. Species-specific STH infection and intensity were assessed using quantitative PCR. We compared prevalence and intensity of infection between baseline and 21 months in each intervention arm individually using cluster-level analysis (adjusted for clustering) and individual-level analysis (adjusted for sex, age, and clustering). The primary outcomes were the prevalence risk difference (RD) from the cluster-level analysis, and the change in adjusted odds of infection from the individual-level analysis. Secondary outcomes included change in incident rates of mean eggs per gram (epg) of stool from baseline to 21 months, relative risk difference in prevalence and relative change in odds of infection between arms at 21 months. Sex data (male/female) were self-reported.
Findings
Overall, STH infection was assessed in 830 participants from 18 villages at baseline and 1172 from 20 villages at follow-up. Females represented 58% (n = 478) of the sample at baseline and 59% (n = 690) at follow-up. We observed a reduction in Strongyloides spp. prevalence following two doses of ivermectin MDA in the cluster-level analysis from 7.0% (32/458 participants) to 1.2% (8/674 participants), corresponding to a RD of −0.07 (95% CI −0.14 to −0.01, p = 0.036), and in the individual-level analysis (OR 0.11, 95% CI 0.04–0.33, p < 0.001). T. trichiura prevalence decreased following one dose from 19.4% (74/372 participants) to 11.7% (56/505 participants) (OR 0.44, 95% CI 0.26–0.73, p = 0.0040), while egg count reduced in both arms (IVM-1: IRR 0.28, 95% CI 0.11–0.70, p = 0.0070; IVM-2: IRR 0.18, 95% CI 0.08–0.40, p < 0.001), in the individual-level analysis. We did not detect a significant difference in effect measures between the one- and two-dose arms for any species after 21 months.
Interpretation
Our study highlights the long-term benefits of ivermectin MDA in reducing the burden of Strongyloides spp. and T. trichiura. STH control programs should leverage the geographical overlap of NTDs, existing drug distribution channels, and broad-spectrum agents.
Funding
The National Health and Medical Research Council of Australia.
Keywords: Soil-transmitted helminths, Ivermectin, Preventive chemotherapy, Mass drug administration
Research in context.
Evidence before this study
We searched PubMed without language restrictions for publication up until February 26, 2023, with the terms “ivermectin” and “soil-transmitted helminths” and “mass drug administration” or “preventive chemotherapy”. We identified five key studies assessing the impact of mass drug administration (MDA) with ivermectin monotherapy on soil-transmitted helminths (STH) prevalence. Three studies assessed the impact of single dose ivermectin MDA. Two of these were cross-sectional studies, conducted in Ecuador and Nigeria, which observed lower prevalence in treated communities compared to non-treated communities for T. trichiura, but not with hookworm. The Ecuador study additionally observed an impact on A. lumbricoides prevalence, but the Nigeria study did not. A third study, also in Ecuador, evaluated the impact of single dose ivermectin MDA for onchocerciasis by comparing retrospectively collected baseline data to a follow-up survey six years later, observed no significant difference from baseline to follow-up in the prevalence of T. trichiura, A. lumbricoides, nor hookworm, but observed a significant reduction in S. stercoralis. Additionally, a before-after study conducted in Brazil assessed the impact of two-dose ivermectin MDA, for parasitic skin diseases, on STH infections and found a reduction in the prevalence of S. stercoralis, T. trichiura, and hookworm, 9 months after MDA. Finally, there was a study embedded within a cluster-randomised trial for scabies and impetigo comparing the effectiveness of MDA with ivermectin alone vs MDA with ivermectin and azithromycin, where the first dose of ivermectin was given at baseline and the second dose 7 days later only to individuals diagnosed with scabies at baseline. It included a before-after analysis on S. stercoralis combining both trial arms together and found a 44% reduction at 12 months.
Added value of this study
To our knowledge, this study is the first to show a lasting impact of ivermectin MDA (beyond 12 months) on STH using a rigorous cluster-randomised, before-after design, and the first to assess effectiveness based on PCR, highlighting the long-term benefits of ivermectin MDA in reducing the burden of Strongyloides spp. and T. trichiura. We observed a significant reduction in Strongyloides spp. prevalence 21 months following two doses of ivermectin MDA in the cluster-level analysis and, after adjusting for confounders, a reduction in the individual-level analysis. T. trichiura prevalence decreased following one dose, while infection intensity decreased in both arms, in the individual-level analysis. The study was not powered to detect a difference in the magnitude of effectiveness between one and two doses for any species.
Implications of all the available evidence
Current international guidelines pose a major challenge for STH control, where the WHO recommends targeted preventive chemotherapy using one of the benzimidazole anthelmintics, mebendazole or albendazole, both of which have inadequate efficacy against Strongyloides stercoralis and T. trichiura. The available evidence highlights the key role of ivermectin MDA in improving the control of STH and other neglected tropical diseases simultaneously. An STH control strategy should leverage the geographical overlap of NTDs, existing drug distribution channels, and broad-spectrum agents.
Introduction
Soil-transmitted helminths (STHs) are the most prevalent neglected tropical disease (NTD) globally, affecting an estimated 895 million people1 and contributing 1.9 million disability-adjusted life years.2 Children and women of childbearing age are at highest risk of morbidity,1 and the sequalae of chronic and heavy intensity infections include iron deficiency anaemia, stunted growth, and impaired cognitive development.3,4 STH infections are caused by a group of intestinal nematodes including Ascaris lumbricoides, Trichuris trichiura, hookworms (Necator americanus, Ancylostoma ceylanicum, Ancylostoma duodenale) and Strongyloides stercoralis.
The mainstay of STH public health control is targeted preventive chemotherapy using one of the benzimidazole anthelmintics, mebendazole or albendazole, delivered to school-aged children.5 Although these drugs are efficacious against A. lumbricoides and hookworm, they have poor efficacy against T. trichiura6 and S. stercoralis.7 Furthermore, concerns have been raised of the potential for anthelmintic resistance in humans,8 a phenomenon already well documented in livestock.9 Therefore, investigation into alternative medication regimens to be used in control programs is a priority. Ivermectin is a broad-spectrum antiparasitic drug that has high therapeutic efficacy against S. stercoralis and A. lumbricoides, moderate efficacy against T. trichiura, and poor efficacy against hookworms.6,10, 11, 12 The combination of albendazole and ivermectin has excellent efficacy against all species.6
While ivermectin alone has not been used in preventive chemotherapy programs for STH, it has been used for almost four decades in highly effective mass drug administration (MDA) campaigns against onchocerciasis13 and more recently against scabies.14,15 Evidence from cross-sectional studies evaluating the impact of ivermectin MDA for onchocerciasis suggests that ivermectin monotherapy has a potent impact on S. stercoralis prevalence and may also play a role in improving T. trichiura and A. lumbricoides control as a one-dose regimen16, 17, 18; however it is unclear whether two doses are more effective.19,20 There is mixed evidence on the impact of ivermectin monotherapy MDA on hookworm burden, but mostly limited impact has been observed.16, 17, 18, 19
Solomon Islands is an archipelago country situated in the Western Pacific region with a population of approximately 695,00021 and is divided into 10 administrative areas comprised of nine provinces and the capital city of Honiara. NTDs, including scabies22 and STHs,23 are major public health problems affecting the country. Although there are currently no control programs directly targeting STHs outside of Honiara, Solomon Islands Ministry of Health and Medical Services recently scaled up ivermectin MDA for scabies with support from the World Scabies Program24 in 2022. Given ivermectin's broad-spectrum activity and the high co-endemicity of NTDs, we identified an opportunity to document the concomitant effectiveness of NTD control programs on off-target conditions. In the context of a cluster-randomised trial for scabies,25 we evaluated the effectiveness of ivermectin MDA in reducing the prevalence and intensity of STH infections in Western Province of Solomon Islands.
Methods
Setting
The Solomon Islands is ranked 153 of 189 countries on the 2018 Human Development Index26 and with 12.7% of the population living below the National Poverty Line in 2017.27 According to WHO and the United Nations Children's Fund (UNICEF) Joint Monitoring Programme (JMP) for Water Supply, Sanitation and Hygiene, 77.9% of urban households and only 20.1% of rural households in the country had access to basic sanitation in 2017.28 The climate is subtropical, being hot and humid all year, with a dry season occurring from May to October and wet season from November to April. The average annual temperature is 27 °C, with small seasonal variation.29,30 Western Province is the third most populous province in Solomon Islands with an estimated population of 76,649 people.21
Study design and participants
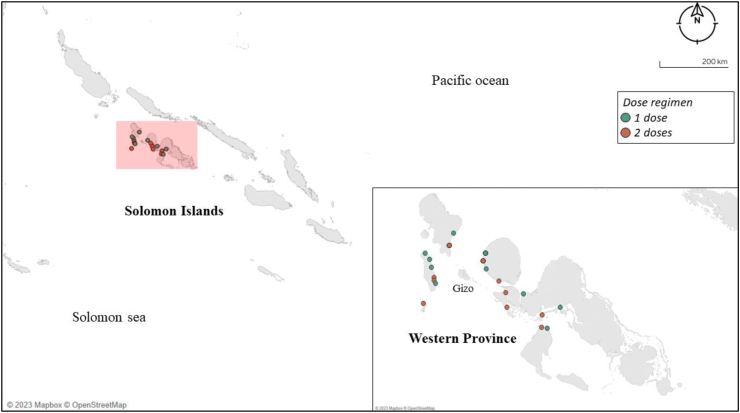
The study reported here was embedded within RISE, an open-label, cluster-randomised control trial of ivermectin MDA for scabies (Australian and New Zealand Clinical Trials Registry ACTRN12618001086257),31 which included impact on STH infections as a secondary outcome. Under the RISE design, 20 villages with a population size of between 180 and 300 people in Western province (Fig. 1) were selected randomly. These 20 villages were then randomly assigned in a 1:1 ratio by an independent statistician to receive MDA for scabies with ivermectin (150–250 μg/kg) given as one dose (IVM-1) or two doses given 7–14 days apart (IVM-2). Medications were purchased for the study using the study funding, procured from international suppliers, and met appropriate international quality standards including Good Manufacturing Practice (GMP). Adverse events were monitored through passive surveillance and are reported in the published RISE trial.25,31 Further details of the trial design can be found in the published trial protocol and findings.25,31
Fig. 1.
Study sites in Western Province. The figure shows the locations of the 20 villages included in the study.
We conducted a baseline STH cross-sectional survey of residents in 18 villages prior to MDA (May 2019) and a 21-month follow-up survey in 20 villages (February 2021). For logistical reasons, the STH survey could not be conducted in the first two villages at baseline. The follow-up survey was planned to take place at 12 months but was delayed due to coronavirus disease 2019 (COVID-19) travel restrictions. The RISE trial recorded MDA coverage. All residents aged 12 months or older in the study villages were eligible to participate in the STH assessment. Written informed consent was provided prior to STH related data collection, including obtaining signed consent from all adults and parents or guardians of children aged less than 18 years. Ethical approval was obtained from Solomon Islands Health Research and Ethics Review Board (HRE005/18) and the Royal Children's Hospital Human Research Ethics Committee, Melbourne, Australia (38099A).
Fieldwork and data collection procedures
A local health promotion team conducted community awareness visits approximately one month prior to the commencement of the RISE trial including the STH assessment. Data collection procedures for the STH study were identical at both the baseline and follow-up surveys except for a water, sanitation, and hygiene (WASH) questionnaire that was only conducted at baseline (details below). One day prior to stool collection, trained field team members visited villages to seek permission from village leaders to conduct the STH research. Following approval, the team informed residents of the study either through household visits or community meetings at a central location, provided verbal instructions on how to provide a stool sample and offered the opportunity to ask questions. Each household member was given a stool collection kit containing a 70 ml plastic faeces specimen jar, gloves, a study information sheet, and written instructions on providing a sample. Residents were asked to self-collect a fresh stool sample the following day, and return it the same day to a pre-arranged central location where MDA was planned to take place, ensuring they did so before administration of ivermectin. A single aliquot of stool (3 g) per participant was fixed in 5% w/v potassium dichromate upon receipt and kept at room temperature for up to 10 weeks due to limited access to electricity and a refrigerator in the field.
At baseline only, participants were also asked to participate in a WASH questionnaire23 administered by local field staff after samples were collected. The questionnaire assessed access to drinking water sources and sanitation facilities, and hygiene behaviours, at the individual and household level.23 Multiple response options regarding drinking water sources were recategorized to match definitions of “improved” water sources (public tap/standpipe, protected spring, rainwater) and “unimproved” sources (unprotected spring, unprotected well) promulgated by the WHO and UNICEF JMP for Water Supply and Sanitation.28
Quantitative PCR analysis
The quantitative PCR methodology used in the present study is described elsewhere.23,32 Briefly, all samples were couriered to the University of Melbourne, Australia, where they were kept at 4 °C until DNA extraction. TaqMan probe-based multiplex qPCR was used to identify six STH species (N. americanus, A. ceylanicum, A. duodenale, T. trichiura, Strongyloides spp., A. lumbricoides) and quantify intensity of infection with N. americanus, T. trichiura, and A. lumbricoides through conversion of qPCR cycle-threshold (Ct) values into eggs per gram of stool (epg) using previously derived equations23,32, 33, 34, 35: A. lumbricoides epg = 10((Ct−30.048)/−3.2804); T. trichiura epg = 10((Ct−31.888)/−4.048); N. americanus epg = 10((Ct−32.657)/−3.878). Equine herpes virus (EHV-4) was used as an internal qPCR control and human 16S mitochondrial rRNA as a DNA extraction control. Given that samples were kept at room temperature in the field, STH eggs were embryonated, confirmed with microscopic examination of a random subset of positive samples. The conversion equations were therefore based on faecal seeding experiments where parasite-free human faeces were spiked with a serial dilution of known quantities of eggs purified from human and pig faeces that were allowed to embryonate for 10 weeks to mirror the embryonated state of the field samples.23,32 The epg values estimated from the conversion equations were classified into one of three infection intensity classes (light, moderate, heavy) according to WHO recommended thresholds.36
Statistical analysis and outcomes
The statistical analysis primarily aimed to evaluate the effectiveness of each intervention arm in reducing the prevalence and intensity of each STH species at 21 months.
Given that the RISE trial was powered to detect a difference in scabies prevalence between arms, we conducted post-hoc power analyses to determine the level of power the STH study had to detect observed effect sizes with the given sample size. We used a two-tailed, one-sample proportion test to calculate power in each arm individually and a two-sample proportion test to calculate power between arms. When incorporating information on the number of clusters, mean cluster size, and observed intra-cluster correlation coefficients for each STH species (ICCs: S. stercoralis 0.02 [95% CI 0.01–0.06], T. trichiura 0.30 [95% CI 0.19–0.45], N. americanus 0.16 [95% CI 0.09–0.27], A. lumbricoides 0.04 [95% CI 0.02–0.08]), the study had >80% power to detect observed differences in the prevalence of S. stercoralis (>99%) and T. trichiura (82%) in IVM-I, and in the prevalence of S. stercoralis (>99%) only in IVM-II. There was inadequate power (<70%) to detect the observed prevalence differences between arms for all species.
We conducted statistical analyses using both cluster-level summaries (villages) and individual-level observations (participants), as described below, to provide a richer understanding of the impact of MDA on STH outcomes. The primary outcomes of the present study were (1) the risk difference in prevalence between baseline and 21 months in each intervention arm individually from the cluster-level analysis and (2) changes in odds of infection from baseline to 21 months in each arm from the individual-level analysis. In the cluster-level analysis, we first calculated disease prevalence in each of the 20 clusters (villages) separately at baseline and follow-up for each intervention arm. To evaluate the effectiveness of each intervention arm individually, we compared the differences in proportion of infected individuals at follow-up (p2) compared to baseline (p1), and estimated 95% CIs for the risk difference (p2-p1) using a paired t-test based on cluster-level variations.37 In the individual-level analysis, we evaluated the effectiveness of the intervention using generalised linear mixed models with individual observations (participants), adjusting for household- and village-level clustering using random-effect terms, and sex and age group using fixed-effect terms, using a Bernoulli distribution and logit link function. To evaluate the effectiveness of each arm individually, we assessed the adjusted odds of infection between baseline and follow-up.
The secondary outcomes included (1) changes in incident rates of egg count (mean epg) from baseline to 21 months in each arm, (2) relative risk difference in prevalence between arms at 21 months (cluster-level analysis), and (3) relative change in adjusted odds of infection between arms at 21 months (individual-level analysis). Due to the overdispersion of egg count outcomes with inflated zeros, we analysed changes in mean epg in each arm using multilevel zero-inflated negative binomial models, which was favoured by the Vuong test over a standard negative binomial model for modelling T. trichiura (Z = 1.67, p = 0.048) and N. americanus (Z = 6.30, p < 0.001) epg. Zero-inflated models included household and village as random effects in both the count and zero-inflated components, with fixed effects including sex and age group in the count component, and age group for the zero-inflated component. A negative binomial distribution with a log link function was used for the model. To compare effectiveness between arms in the cluster-level analysis, we calculated the relative risk difference between arms at 21 months, defined as overall pooled prevalence ratio (p2/p1) in the IVM-1 arm minus the pooled prevalence ratio in the IVM-2 arm, and estimated 95% confidence intervals (CIs) using a two-sample t-test based on the cluster-period proportions.37 In the individual-level analysis, we assessed the relative change in adjusted odds of infection between arms at 21 months, derived from the interaction term of intervention arm by timepoint.
We investigated differences in sex and age between baseline and follow-up, and investigated potential imbalances between arms at baseline in demographic, infection prevalence, infection intensity, and WASH variables. Differences in the proportion of males sampled was assessed using a chi-squared test of independence. Differences in age was assessed using a two-sample t-test. We used generalised linear mixed models to investigate baseline imbalances in infection prevalence and intensity for each species, and the proportion of responses to the WASH questionnaire, while adjusting village clustering using random-effect terms.
Finally, we conducted an exploratory analysis to investigate the potential effect of WASH access on MDA effectiveness, using a previously described method.23 Briefly, we used mixed effects methods with backward stepwise variable selection to explore the additional impact of WASH covariates on changes in odds of infection beyond the effects of the intervention. The base model contained fixed effect terms including sex, age group, follow-up time and intervention arm as main effects, and an interaction term of follow-up time by intervention arm, and village-level clustering as a random effect term. All WASH covariates were entered as fixed effect terms and iteratively removed based on their p value and contribution to the model's predictive performance assessed with Akaike's Information Criterion, until all covariates in the model had a p value of less than 0.05. We handled missing WASH data with multiple imputation methods with chained equations, specifically using a chained regression model with 30 imputation iterations to increase the precision of the imputed values. We obtained the average of the 30 values as the final data point, then computed the village-level average at both baseline and follow-up.
For all models, two assumptions of mixed-effects logistic regression were tested: linearity between independent variables and log-odds of the outcome using locally weighted scatterplot smoothing curves, and the absence of multicollinearity among predictor variables using variance inflation factors (VIFs) with a criterion of VIF greater than 3 used to indicate multicollinearity. The significance level for all models was set at p < 0.05. To account for multiple testing of the two primary outcomes, p values were corrected using the Holm-Bonferroni method, maintaining the family wise error rate below 5%. Analyses were completed using Stata version 17.0 (Stata Corporation, College Station, Texas) using the meglm command and R version 4.2.1 using the glmmTMB package version 1.1.7.38
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Results
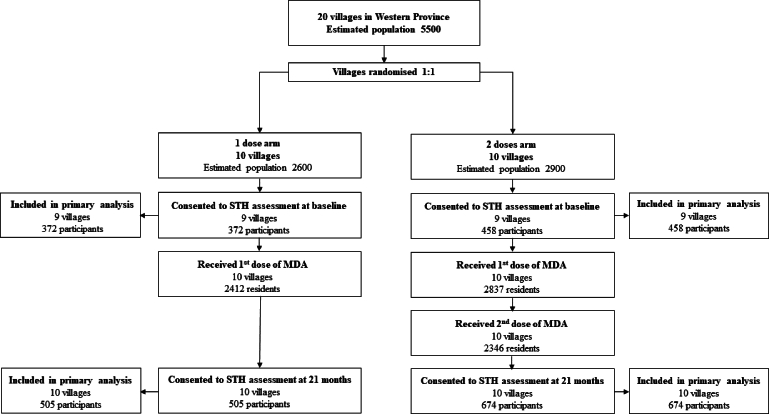
Overall, 830 participants out of an expected population of 4795 returned a stool sample from 18 villages at baseline (372 participants from 9 IVM-1 villages and 458 participants from 9 IVM-2 villages) and 1179 out of an expected population of 5520 from 20 villages participated at follow-up (505 participants from 10 IVM-1 villages and 674 from 10 IVM-2) (Table 1, Supplementary Table S1, Supplementary Figure S1). As per the larger scabies trial, MDA coverage was 92.8% in the IVM-1 arm, compared to 97.8% in the IVM-2 arm, with 80.9% the latter group receiving second dose (Supplementary Table S1). At baseline, the most prevalent species present was N. americanus, followed by T. trichiura, A. ceylanicum, then S. stercoralis in both arms (Table 2).
Table 1.
Demographic characteristics of participants at baseline and 21 months.
| Baseline |
21 months |
Baseline vs 21 months |
|||||
|---|---|---|---|---|---|---|---|
| IVM-I (N = 372) | IVM-II (N = 458) | All (N = 830) | IVM-I (N = 505) | IVM-II (N = 674) | All (N = 1179) | Differencea | |
| Sex—n (%) | |||||||
| Male | 153 (41.1) | 199 (43.5) | 352 (42.4) | 196 (38.8) | 293 (43.5) | 489 (41.5) | χ2 (1) = 0.17 p = 0.68 |
| Female | 219 (58.9) | 259 (56.6) | 478 (57.6) | 309 (61.2) | 381 (56.5) | 690 (58.5) | |
| Age group (years)—n (%) | |||||||
| 1–5 | 91 (24.5) | 121 (26.4) | 212 (25.5) | 83 (16.4) | 127 (18.8) | 210 (17.8) |
χ2(4) = 26.24 p < 0.0001 |
| 6–11 | 80 (21.5) | 121 (26.4) | 201 (24.2) | 91 (18.0) | 163 (24.2) | 254 (21.5) | |
| 12–17 | 51 (13.7) | 38 (8.3) | 89 (10.7) | 59 (11.7) | 84 (12.5) | 143 (12.1) | |
| 18–64 | 134 (36.0) | 163 (35.6) | 297 (35.8) | 240 (47.5) | 264 (39.2) | 504 (42.7) | |
| ≥65 | 16 (4.3) | 15 (3.3) | 31 (3.7) | 32 (6.3) | 36 (5.3) | 68 (5.8) | |
| Age (years)—mean (SD) | 22.7 (21.0) | 21.0 (20.2) | 21.7 (20.5) | 28.0 (22.1) | 24.0 (20.8) | 25.7 (21.4) |
t(2007) = 4.15 p < 0.0001 |
Difference in proportion of males and age group calculated using chi-square test of independence.
Bold text indicates a statistically significant difference at the alpha level of 0.05.
Difference in age (years) calculated using two-sample t-test.
Table 2.
Prevalence of STHs at baseline and 21 months (cluster analysis).
| Prevalence at baseline % (n) 95% CI |
Prevalence at 21 months % (n) 95% CI |
Prevalence risk difference (RD) RD (95% CI) p |
|||||
|---|---|---|---|---|---|---|---|
| IVM-I (N = 372) | IVM-II (N = 458) | IVM-I (N = 505) | IVM-II (N = 674) | IVM-Ib | IVM-IIb | IVM-I vs IVM-IIc | |
| T. trichiura | 19.9 (74) 16.1–24.3 | 21.4 (98) 17.9–25.4 | 11.1 (56) 8.6–14.1 | 12.0 (81) 9.8–14.7 | −0.10 (−0.24 to 0.04) p = 0.23 | −0.04 (−0.29 to 0.22) p = 0.83 | 0.27 (−0.24 to 0.78) p = 0.61 |
| Strongyloides spp. | 4.6 (17) 2.9–7.2 | 7.0 (32) 5.0–9.7 | 1.8 (9) 0.9–3.4 | 1.2 (8) 0.6–2.4 | −0.02 (−0.06 to 0.01) p = 0.20 | −0.07 (−0.14 to −0.01) p = 0.036 | −0.37 (0.92–0.18) p = 0.38 |
| N. americanus | 51.6 (180) 46.5–56.7 | 58.5 (268) 53.4–63.0 | 52.3 (241) 47.9–56.6 | 61.3 (413) 57.5–64.9 | 0.07 (−0.15 to 0.28) p = 0.55 | 0.01 (−0.17 to 0.21) p = 1 | 0.04 (−0.29 to 0.21) p = 0.77 |
| N. americanus MH intensitya | 5.9 (22) 3.9–8.8 | 9.0 (41) 6.7–11.9 | 6.5 (33) 4.7–9.1 | 6.2 (42) 4.6–8.3 | 0.01 (−0.05 to 0.07) p = 0.80 | −0.03 (−0.11 to 0.04) p = 0.42 | −0.40 (−1.00 to 0.20) p = 0.24 |
| A. ceylanicum | 19.9 (74) 16.1–24.3 | 14.4 (66) 11.5–17.9 | 18.8 (95) 15.6–22.5 | 19.6 (132) 16.8–22.8 | −0.01 (−0.09 to 0.05) p = 1 | 0.01 (−0.11 to 0.12) p = 1 | 0.19 (0.72–1.10) p = 1 |
| A. lumbricoides | 0.27 (1) 0.04–1.9 | 1.1 (5) 0.5–2.6 | 0.6 (3) 0.2–1.8 | 1.3 (9) 0.7–2.5 | NA | NA | NA |
p values corrected for multiple testing using the Bonferroni-Holm method. Bold text indicates a statistically significant difference at the alpha level of 0.05.
NA, not applicable due to insufficient observations.
Moderate-to-heavy intensity infections.
Reference category: baseline timepoint.
Reference category: baseline timepoint and IVM-I group.
In the cluster-level analysis, there was no significant reduction in the prevalence of any species in the IVM-1 arm (Table 2). In the IVM-2 arm, we observed a significant reduction in Strongyloides spp. prevalence only, from 7.0% (95% CI 5.0–9.7) at baseline to 1.2% (95% CI 0.6–2.4), corresponding to a risk difference of −0.07 (95% CI −0.14 to −0.01, p = 0.036).
In the individual-level analysis, after adjusting for sex, age, and clustering, we observed a significant reduction in the odds of infection of T. trichiura (prevalence from 19.4% to 11.7%, OR 0.44, 95% CI 0.26–0.73, p = 0.0040) and a marginally non-significant reduction in odds of infection of Strongyloides spp (prevalence from 4.3% to 1.6%, OR 0.33, 95% CI 0.12–0.89, p = 0.058) in the IVM-1 arm (Supplementary Table S3). Similar to the cluster-level analysis, there was a significant reduction in the odds of Strongyloides spp. infection in the IVM-2 arm after adjusting for confounders (OR 0.11 95% CI 0.04–0.33, p < 0.0001).
There was a significant reduction in T. trichiura mean epg in both the IVM-1 (from mean epg 253 (SD 794) to mean epg 65.8 (SD 101), IRR 0.28, 95% CI 0.11–0.70, p = 0.0070) and IVM-2 (from mean epg 648 (SD 1484) to mean epg 102 (SD 147), IRR 0.18, 95% CI 0.08–0.40, p < 0.001) arms (Table 3). There was no significant difference in N. americanus mean epg in either arm (Table 3), nor in the prevalence of moderate-to-heavy intensity infections (Table 2, Supplementary Table S3). Full output for zero-inflated negative binomial models, including outputs from the count and zero-inflated components of the model, are summarised in Supplementary Tables S5–S10.
Table 3.
STH infection intensity at baseline and 21 months (individual analysis).
| Eggs per gram in stool at baseline mean epg (SD) |
Eggs per gram in stool 21 months mean epg (SD) |
Difference in eggs per gram stool Incident rate ratio (95% CI) p |
|||||
|---|---|---|---|---|---|---|---|
| IVM-I | IVM-II | IVM-I | IVM-II | IVM-Ia | IVM-IIa | IVM-II vs IVM-Ib | |
| T. trichiura | 253 (794) | 648 (1484) | 65.8 (101) | 102 (147) | 0.28 (0.11–0.70)p = 0.0070 | 0.18 (0.08–0.40) p < 0.001 | 0.65 (0.20–2.16) p = 0.48 |
| N. americanus | 754 (1526) | 1040 (2010) | 924 (1938) | 854 (1925) | 1.28 (0.86–1.91) p = 0.23 | 0.83 (0.56–1.22) p = 0.34 | 0.71 (0.40–1.25) p = 0.23 |
| A. lumbricoides | 33,713 (0) | 58,375 (130,527) | 46.5 (37.6) | 7232 (11,847) | NA | NA | NA |
NA, not applicable due to insufficient observations. Bold text indicates a statistically significant difference at the alpha level of 0.05.
Reference category: baseline timepoint.
Reference category: baseline timepoint and IVM-I group.
We did not detect a statistically significant difference between the one dose and 2 dose arms at 21 months in either the cluster- or individual-level analysis (Table 2, Table 3, Supplementary Table S3).
There was a statistically significant difference in age between baseline and follow-up (t (2007) = 4.15, p < 0.0001), but not sex (χ2 (1) = 0.17, p = 0.68) (Table 1). At baseline, there was no significant differences in sex (χ2 (1) = 0.45, p = 0.50) or age (t (828) = 1.17, p = 0.12) between arms. Similarly, there were no significant differences at baseline between arms in infection prevalence with T. trichiura (p = 0.72), Strongyloides spp (p = 0.66), N. americanus (p = 0.20), A. ceylanicum (p = 0.45), nor in mean epg T. trichiura (p = 0.47) and N. americanus (p = 0.44). However, there was a significantly greater proportion of participants from the IVM-2 arm who had no toilet in their household (87.4%, 95% CI 83.4–90.6) compared to those in the IVM-1 arm (69.8%, 95% CI 64.0–75.0, p = 0.039) (Supplementary Table S2).
Due to the observed discrepancy in T. trichiura findings between dosage arms in the individual-level analysis (Supplementary Table S3), we conducted an exploratory analysis to investigate the potential effect of WASH access on MDA effectiveness for this species. The analysis did not show an additional impact of WASH variables in predicting changes in odds of T. trichiura infection beyond the effects of MDA alone (Supplementary Table S4).
Model assumptions were met, with visually linear relationships observed in the locally weighted scatterplot smoothing curves for independent variables and log-odds of the outcome, and all VIFs were below 1.5.
Discussion
To our knowledge, this study is the first to show a lasting impact of ivermectin MDA on Strongyloides spp. and T. trichiura using a rigorous cluster-randomised, before-after design, and PCR for STH detection. We observed a significant reduction in Strongyloides spp. prevalence 21 months following two doses of ivermectin MDA in both the cluster- and individual-level analyses and no significant differences following one dose. T. trichiura prevalence decreased following one dose, while infection intensity decreased in both arms, in the individual-level analysis. We did not detect any added benefit of two doses of ivermectin as part of MDA compared to one dose on STH prevalence or intensity after 21 months.
Drugs used in the WHO recommended strategy for STH control (albendazole and mebendazole) have limited efficacy against S. stercoralis7 and T. trichiura.6 Ivermectin monotherapy has high efficacy against S. stercoralis and A. lumbricoides, moderate efficacy against T. trichiura, and poor efficacy against hookworms.6,10, 11, 12 Our findings generally align with previous efficacy trials and cross-sectional studies comparing onchocerciasis-treated to untreated areas, that demonstrated substantial reductions in S. stercoralis prevalence and moderate reductions in T. trichiura prevalence and intensity, associated with one dose of ivermectin MDA.10, 11, 12,16, 17, 18The marginal non-significant prevalence reduction for Stronygloides spp. may be due to the longer follow-up period. Before-after studies have demonstrated significant reductions in the prevalence of both S. stercoralis19,20 and T. trichiura19 nine to twelve months after two doses. We found that such improvements were sustained for almost two years for Stronygloides spp. prevalence and T. trichiura infection intensity. We hypothesise that the lack of reduction in T. trichiura prevalence in the IVM-2 arm may be due to insufficient power or baseline imbalances in sanitation access. Notably, we detected poorer access to household toilets in the IVM-2 arm, conceivably resulting in greater exposure to environmental contaminants and therefore earlier reinfection following MDA in these villages, although this did not emerge as a significant predictor of T. trichiura infection beyond the effects of MDA alone. Our hookworm findings are broadly consistent with the existing body of evidence that generally demonstrates a limited impact of one dose of ivermectin on hookworm burden in onchocerciasis-treated areas.16, 17, 18 A study in Brazil showed a significant reduction in hookworm prevalence nine months after two doses,19 possibly suggesting that any reduction in the IVM-2 arm was nullified by the 21-month timepoint due to reinfection.39
Our study is the first to assess the effectiveness of ivermectin MDA against STHs using quantitative PCR, which is becoming more common for STH diagnostics, particularly in research studies.23,40 Due to its higher sensitivity, it has a role in monitoring progress of STH control strategies, especially in near elimination settings.41 Additionally, it has the advantage of allowing speciation of hookworm species, therefore identifying areas where A. ceylanicum is prevalent and One Health measures may be necessary.23 Finally, it may be useful for S. stercoralis identification.23,40 Microscopy techniques for this species are different from the conventional Kato-Katz method and have been shown to have low sensitivity and specificity.42 On the other hand, while serology is sensitive, it may underestimate intervention effectiveness given that it yields more false-positive results after treatment as sero-reversion of anti-NIE responses can take more than 12 months.20
Considering the reality of constrained resources and funding, a national STH control strategy could exploit the geographical co-endemicity of NTDs and existing drug distribution channels used in other control programs. In Solomon Islands, where ivermectin MDA for scabies is ongoing, our findings may be used to justify funding to add albendazole to the MDA. Following the cessation of this program, additional provision of ivermectin tablets would be needed to sustain control efforts for T. trichiura and S. stercoralis. Our findings may be used as evidence to justify the procurement of additional ivermectin tablets through drug donation programs and/or private channels where ivermectin can be secured from generic manufacturers at an affordable cost.43 More generally, integrated NTD control programs using combination MDA with ivermectin, diethylcarbamazine citrate, and albendazole may be considered in countries where STH, scabies, and onchocerciasis or lymphatic filariasis are co-endemic.40 Improving access to WASH facilities is also necessary for sustained STH control as it protects against exposure, as is adopting a One Health approach for the zoonotic A. ceylanicum in settings where it is endemic, such as Solomon Islands.23,44
There are limitations to our study. We assumed that the observed reductions in disease burden were attributable to MDA; however, we cannot rule out the effect of non-pharmaceutical or environmental confounders. For example, our study was conducted during the onset of the COVID-19 pandemic, where significant travel restrictions were imposed and social distancing and frequent handwashing were promoted, which could have affected infectious disease transmission dynamics. There was a significant difference in sex and age characteristics between timepoints, possibly due to the exclusion of two villages at baseline, although this was controlled for in statistical analyses. There was also imbalance in one of the WASH variables at baseline, despite the random allocation of villages to intervention arms, which may have influenced reinfection rates differently between arms. There was some sample selection bias present as villages included in this study were restricted to a population size of 180–300 people.22 The seasonal variation effects cannot be ruled out, as the baseline survey was conducted in the wet season while the follow-up was done in the dry season, and STH transmission is influenced by environmental factors.23,45 This sample represented roughly 20% of the surveyed villages’ anticipated population, and we cannot dismiss the potential difference in infection risk between participants and non-participants. Finally, our study was not designed to compare the comparative effectiveness of one vs two dose ivermectin MDA.
Documenting the concomitant effectiveness of MDA on off-target conditions is key to accelerating WHO targets for the control and eventual elimination of NTDs. Our study demonstrates the long-term benefits of ivermectin MDA directed at scabies on reducing the burden of Strongyloides spp. and T. trichiura. There are opportunities for national STH control strategies to capitalize on the geographical overlap of NTDs, existing drug distribution channels, and broad-spectrum agents.
Contributors
All authors contributed to the design of the study. SVN conceived the STH component of the study. JK and SVN acquired funding for STH study. BL and NEC were co-primary coordinators of STH fieldwork and data collection. BL had primary responsibility for data curation, data analysis, writing up the manuscript, and is primary author of the manuscript. BL, HW, and NO designed the statistical analysis strategy and conducted the analyses. AS acquired funding for the larger scabies trial in which this study is embedded and led the trial. AS, LR, DE, JK, OS, TN, and DB conceived the scabies trial. SL coordinated the scabies trial. SFH and PAZH completed the qPCR analysis with support from RT. AB, EL, SW, and AK supported fieldwork activities and data collection at baseline. EL and SW supported fieldwork activities and data collection at follow-up. BL, HW, NO, and SVN has directly accessed and verified the underlying data in the study. All authors read, edited, and approved the final version of the manuscript, and had final responsibility for the decision to submit for publication.
Data sharing statement
Some restrictions will apply to accessing the data for this study. All relevant aggregated data are within the paper and its supporting information files. Individual data cannot be made public in compliance with the protocol approved by the research ethics board in order to respect participant privacy. Researchers may request approval to access deidentified data from the Solomon Islands Health Research and Ethics Review Board (HRE005/18) and the Royal Children's Hospital Human Research Ethics Committee, Melbourne, Australia (38099A).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
AS has received grants or contracts from the US National Institutes of Health and the Leducq Foundation, participated on a Data Safety Monitoring Board run by Griffith University, is Co-Director of the Australian Step A Vaccine Initiative, is Co-Chair of the Step A Vaccine Global Consortium, and is President of the Lancefield Society for Streptococci and Streptococcal Diseases. LR declares receiving a grant or contract by the Australian National Health and Medical Research Council (NHMRC) and is a member of the Executive team and Lead of Integration of the World Scabies Program. All remaining authors have no conflicts of interest to disclose.
Acknowledgements
We are indebted to all the villages and their leaders for allowing for the study to take place, and to all residents who participated in the study. We would like to express our gratitude to the local staff in Solomon Islands for contributing to fieldwork procedures and data collection.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100942.
Appendix A. Supplementary data
Supplementary Figure.
Recruitment flow diagram.
References
- 1.James S.L., Abate D., Abate K.H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyu H.H., Abate D., Abate K.H., et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell S.J., Nery S.V., D'Este C.A., et al. Investigations into the association between soil-transmitted helminth infections, haemoglobin and child development indices in Manufahi District, Timor-Leste. Parasites Vectors. 2017;10(1):192. doi: 10.1186/s13071-017-2084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pabalan N., Singian E., Tabangay L., Jarjanazi H., Boivin M.J., Ezeamama A.E. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1) doi: 10.1371/journal.pntd.0005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation (WHO) 2017. Preventive chemotherapy to control STH infection in at risk population groups. [PubMed] [Google Scholar]
- 6.Clarke N.E., Doi S.A.R., Wangdi K., Chen Y., Clements A.C.A., Nery S.V. Efficacy of anthelminthic drugs and drug combinations against soil-transmitted helminths: a systematic review and network meta-analysis. Clin Infect Dis. 2019;68(1):96–105. doi: 10.1093/cid/ciy423. [DOI] [PubMed] [Google Scholar]
- 7.Henriquez-Camacho C., Gotuzzo E., Echevarria J., et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;1:CD007745. doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercruysse J., Albonico M., Behnke J.M., et al. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist. 2011;1(1):14–27. doi: 10.1016/j.ijpddr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20(10):469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Marti H., Haji H.J., Savioli L., et al. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg. 1996;55(5):477–481. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]
- 11.Adenusi A.A., Oke A.O., Adenusi A.O. Comparison of ivermectin and thiabendazole in the treatment of uncomplicated human Strongyloides stercoralis infection. Afr J Biotechnol. 2003;2(11):465–469. [Google Scholar]
- 12.Belizario V.Y., Amraillo M.E., de Leon W.U., de los Reyes A.E., Bugayong M.G., Macatangay B.J.C. A comparison of the efficacy of single doses of ALB, IVM, and DBMZ alone or in combinations against Ascaris and Trichuris spp. Bull World Health Organ. 2003;81(1):35–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Omura S., Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30(9):445–455. doi: 10.1016/j.pt.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Romani L., Whitfeld M.J., Koroivueta J., et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373(24):2305–2313. doi: 10.1056/NEJMoa1500987. [DOI] [PubMed] [Google Scholar]
- 15.Hardy M., Samuela J., Kama M., et al. Community control strategies for scabies: a cluster randomised noninferiority trial. PLoS Med. 2021;18(11) doi: 10.1371/journal.pmed.1003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moncayo A.L., Vaca M., Amorim L., et al. Impact of long-term treatment with ivermectin on the prevalence and intensity of soil-transmitted helminth infections. PLoS Negl Trop Dis. 2008;2(9):e293. doi: 10.1371/journal.pntd.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anselmi M., Buonfrate D., Guevara Espinoza A., et al. Mass administration of ivermectin for the elimination of onchocerciasis significantly reduced and maintained low the prevalence of Strongyloides stercoralis in esmeraldas, Ecuador. PLoS Negl Trop Dis. 2015;9(11) doi: 10.1371/journal.pntd.0004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutman J., Emukah E., Okpala N., et al. Effects of annual mass treatment with ivermectin for onchocerciasis on the prevalence of intestinal helminths. Am J Trop Med Hyg. 2010;83(3):534–541. doi: 10.4269/ajtmh.2010.10-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heukelbach J., Winter B., Wilcke T., et al. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull World Health Organ. 2004;82(8):563–571. [PMC free article] [PubMed] [Google Scholar]
- 20.Marks M., Gwyn S., Toloka H., et al. Impact of community treatment with ivermectin for the control of scabies on the prevalence of antibodies to Strongyloides stercoralis in children. Clin Infect Dis. 2020;71(12):3226–3228. doi: 10.1093/cid/ciaa584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon Islands National Statistics Office . 2019. Solomon Islands population: Solomon Islands goverment.https://www.statistics.gov.sb/statistics/social-statistics/population Available from: [Google Scholar]
- 22.Lake S.J., Engelman D., Sokana O., et al. Defining the need for public health control of scabies in Solomon Islands. PLoS Negl Trop Dis. 2021;15(2) doi: 10.1371/journal.pntd.0009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le B., Clarke N., Hii S.F., et al. Using quantitative PCR to identify opportunities to strengthen soil-transmitted helminth control in Solomon Islands: a cross-sectional epidemiological survey. PLoS Negl Trop Dis. 2022;16(5) doi: 10.1371/journal.pntd.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch Children's Research Institute (MCRI) 2019. World scabies program: MCRI.https://www.mcri.edu.au/research/projects/world-scabies-elimination-program Available from: [Google Scholar]
- 25.Lake S.J., Phelan S.L., Engelman D., et al. Protocol for a cluster-randomised non-inferiority trial of one versus two doses of ivermectin for the control of scabies using a mass drug administration strategy (the RISE study) BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-037305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United Nations Development Programme (UNDP) UNDP; New York, USA: 2018. Human development indices and indicators: 2018 statistical update. [Google Scholar]
- 27.Solomon Islands National Statistics Office . Solomon Islands Government; Solomon Islands: 2015. Solomon Islands poverty profile based on the 2012/13 household income and expenditure survey. Honiara. [Google Scholar]
- 28.World Health Organisation (WHO) WHO and UNICEF; Geneva, Switzerland: 2017. The United nations Children's Fund (UNICEF). Progress on drinking water, sanitation and hygiene: 2017. [Google Scholar]
- 29.Solomon Islands Government (Meteorological Services Division) 2020. Solomon Islands climate in brief.https://www.met.gov.sb/solomon-islands-climate-in-brief Available from: [Google Scholar]
- 30.Solomon Islands Meterological Service. Australian Beaureau of Metorology. Commonwealth Scientific and Industrial Research Organisation (CSIRO) 2011. Current and future climate of the Solomon Islands.https://www.pacificclimatechangescience.org/wp-content/uploads/2013/06/13_PCCSP_Solomon_Islands_8pp.pdf Available from: [Google Scholar]
- 31.Lake S.J., Engelman D., Zinihite J., et al. One versus two doses of ivermectin-based mass drug administration for the control of scabies: a cluster randomised non-inferiority trial. PLoS Negl Trop Dis. 2023;17(3) doi: 10.1371/journal.pntd.0011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zendejas-Heredia P.A., Colella V., Hii S.F., Traub R.J. Comparison of the egg recovery rates and limit of detection for soil-transmitted helminths using the Kato-Katz thick smear, faecal flotation and quantitative real-time PCR in human stool. PLoS Negl Trop Dis. 2021;15(5) doi: 10.1371/journal.pntd.0009395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hii S.F., Senevirathna D., Llewellyn S., et al. Development and evaluation of a multiplex quantitative real-time polymerase chain reaction for hookworm species in human stool. Am J Trop Med Hyg. 2018;99(5):1186–1193. doi: 10.4269/ajtmh.18-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij J.J., Canales M., Polman K., et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103(4):342–346. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett A.W., Traub R., Amaral S., et al. Comparison between quantitative polymerase chain reaction and sodium nitrate flotation microscopy in diagnosing soil-transmitted helminth infections. Am J Trop Med Hyg. 2021;105(5):1210–1213. [Google Scholar]
- 36.World Health Organisation (WHO) 2nd ed. 2011. Helminth control in school-age children: a guide for managers of control programmes. Geneva, Switzerland. [Google Scholar]
- 37.Hayes R.J., Moulton L.H. CRC Press; 2009. Cluster randomised trials. [Google Scholar]
- 38.R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2022. A language and environment for statistical computing. [Google Scholar]
- 39.Jia T.W., Melville S., Utzinger J., King C.H., Zhou X.N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6(5) doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le B., Monteiro M.A.A., Amaral S., et al. The impact of ivermectin, diethylcarbamazine citrate, and albendazole mass drug administration on the prevalence of scabies and soil-transmitted helminths in school-aged children in three municipalities in Timor-Leste: a before-after assessment. Lancet Glob Health. 2023;11(6):e924–e932. doi: 10.1016/S2214-109X(23)00134-1. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa M., Pilotte N., Kikuchi M., et al. What does soil-transmitted helminth elimination look like? Results from a targeted molecular detection survey in Japan. Parasites Vectors. 2020;13(1):6. doi: 10.1186/s13071-019-3875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker S.L., Piraisoody N., Kramme S., et al. Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Cote d'Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Trop. 2015;150:210–217. doi: 10.1016/j.actatropica.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Bisanzio D., Montresor A., French M., et al. Preventive chemotherapy for the control of strongyloidiasis in school-age children: estimating the ivermectin need. PLoS Negl Trop Dis. 2021;15(4) doi: 10.1371/journal.pntd.0009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traub R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43(12-13):1009–1015. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Wardell R., Clements A.C.A., Lal A., et al. An environmental assessment and risk map of Ascaris lumbricoides and Necator americanus distributions in Manufahi District, Timor-Leste. PLoS Negl Trop Dis. 2017;11(5) doi: 10.1371/journal.pntd.0005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.