Abstract
Background & Aims
While cholangiocarcinoma (CCA) incidence and mortality rates are increasing globally, whether there are regional/temporal variations in these rates for different biliary tract cancer (BTC) subtypes, or whether they differ by sex, socioeconomic status, or route to diagnosis (RtD) remains unknown. In this work, we aimed to perform an in-depth analysis of data on the incidence, mortality, survival and RtD of CCA and other BTCs.
Methods
Data on all BTCs diagnosed in England between 2001 and 2018 were extracted from NHS Digital’s National Cancer Registration Dataset. Age-standardised incidence rates (ASRs), mortality rates (ASMRs) and net survival rates were calculated, and Kaplan-Meier overall survival estimates and RtD trends were analysed. Analyses were stratified by sex, socioeconomic deprivation, tumour subtype and region.
Results
The ASR for CCA rose from 2.9 in 2001-2003 to 4.6 in 2016-2018 and from 1.0 to 1.8 for gallbladder cancers (GBCs). ASMR trends mirror those of incidence, with most deaths due to iCCA. Over 20% of patients with CCA were under 65 years old. The ASRs and ASMRs were consistently higher in the most socioeconomically deprived group for CCA and GBC. The most common RtD was the emergency route (CCA 49.6%, GBC 46.2% and ampulla of Vater cancer 43.0%). The least deprived patients with CCA and ampulla of Vater cancer had better overall survival (p <0.001). Net survival rates rose for all BTCs, with 3-year net survival for CCA increasing from 9.2% in 2001 to 12.6% in 2016-2018. There was notable geographical variation in ASRs, ASMRs and net survival for all BTCs.
Conclusions
BTC incidence and mortality rates are increasing, with differences observed between tumour types, socioeconomic deprivation groups, RtDs and geographical regions. This highlights the need for targeted interventions, earlier diagnosis and better awareness of this condition amongst the public and healthcare professionals.
Impact and implications
Cholangiocarcinoma (CCA) incidence and mortality rates are rising globally, particularly for intrahepatic CCA. However, it has not previously been reported if, within a single country, there are temporal and regional differences in incidence, mortality and survival rates for different biliary tract subtypes, and whether these differ by sex, socioeconomic status, or route of diagnosis. In this study we show that mortality rates for patients with CCA continue to rise and are almost 40% higher in the most socioeconomically deprived compared to the least; additionally, we observed regional variation within England in incidence, mortality and survival. This study is relevant to researchers and policy makers as it highlights regional variation and inequality, as well as emphasising the need for earlier diagnosis and better awareness of this condition amongst the public and healthcare professionals.
Keywords: cholangiocarcinoma, national, incidence, survival, diagnosis, variation, deprivation
Graphical abstract

Highlights
-
•
BTC cases, of which CCA was the most common, doubled during the study period, with increases across all socioeconomic deprivation quintiles.
-
•
For patients with CCA, mortality rates were almost 40% higher in the most compared to the least socioeconomically deprived groups.
-
•
iCCA was associated with the highest incidence and the lowest survival rates.
-
•
Emergency presentation was the most common route of diagnosis for all BTC subtypes.
-
•
Net survival improved for all BTC subtypes during the study period.
Introduction
Cholangiocarcinoma (CCA) comprise a group of malignancies arising from the epithelium anywhere along the biliary tree.1 Together CCA, gallbladder cancer (GBC) and ampulla of Vater (AoV) cancer form the highly lethal group of malignancies known collectively as biliary tract cancers (BTCs). Historically, and for the purposes of coding, CCA are broadly sub-divided into intrahepatic CCA (iCCA) and extrahepatic CCA (eCCA), depending on their anatomical location. iCCA are located proximal to the second order bile ducts within the liver parenchyma. eCCA arise distal to the origin of the second order biliary ducts and, in clinical practice, are further sub-divided into perihilar CCA (pCCA) and distal CCA (dCCA). pCCA arise between the second-degree bile ducts and the insertion of the cystic duct into the common bile duct, while dCCA are located in the common bile duct below the cystic duct insertion and up to the AoV.1 CCA is the commonest biliary tract malignancy, and iCCA is the second commonest primary liver cancer after hepatocellular carcinoma.1 Each of the three subtypes exhibit differences in their epidemiological trends, underlying pathophysiology, relative susceptibility to risk factors, prognosis and approach to clinical management.[1], [2], [3], [4], [5]
Of note, many studies over the past few decades have reported an increasing incidence of CCA globally. These studies have employed international and national datasets, including the World Health Organisation database, US cancer registry, Japanese and other European registries.[6], [7], [8], [9], [10], [11], [12] A consistent pattern of rising incidence of iCCA, but relatively stable or decreasing incidence of eCCA has been reported from these various studies. The reasons for consistently rising rates of iCCA remain unclear and unexplained. Given that the overall prognosis for all CCA is relatively poor, with a 5-year overall survival rate of less than 10%,1,2,8 it is important to monitor and investigate these trends in detail. A recent study from the European Reference Network for the Study of Cholangiocarcinoma investigated the clinical course of 2,234 patients with CCA from 26 referral healthcare centres from 11 European countries over a 10-year period (from 2010).13 This arose from the ENSCCA Registry, a multicentre observational study.13 The authors found that CCA was frequently diagnosed at an advanced stage with almost 60% of patients presenting with locally advanced or metastatic disease. Furthermore, around 20% did not receive any specific cancer therapy, but best supportive care only.13 Although this was an important and large multicentre study, data was collected from self-selected high-volume centres of expertise and findings may not be representative of the whole population of individual participating countries. The study was not aimed at examining temporal trends either. Epidemiological studies published to date reporting changing rates of CCA in individual countries are lacking in several important areas. Regional variation within countries and the effect of socioeconomic deprivation status and their temporal trends have not previously been reported. Furthermore, the routes to diagnosis for CCA have not previously been reported. These are issues which may be important in providing insights to explain the changing epidemiological pattern of CCA.
The intention of this work was to perform the most in-depth study of the epidemiology of CCA and other BTC to date in England. The specific aims of the study were to explore incidence, mortality and survival statistics of subtypes of CCA and BTC diagnosed in England between 2001 and 2018, including an analysis of regional variation over time and socioeconomic deprivation status and routes to diagnosis. The hope is to identify factors which could be addressed to lead to improvements in outcomes in the future.
Patients and methods
Data
Data on all biliary tract invasive cancers diagnosed in England between 1st January 2001 and 31st December 2018 were extracted from the National Cancer Registration Dataset [AV2018],14 held by the National Cancer Registration and Analysis Service (NCRAS) at NHS Digital. We used the ICD10 codes in combination with the ICD for Oncology (second edition) (ICD-O2) to define the following biliary tract tumours: iCCA, ICD10 C22.1; eCCA, ICD10 C24.0; other CCA, ICD10 C220, C222-C229 with ICD-O2 8160 and ICD10 C248-249; GBC, ICD10 C23 and AoV, ICD10 C24.1. An exception was made for the mortality analysis where the other CCA group only consists of ICD10 C248-249 tumours. Malignant neoplasms of the liver and intrahepatic bile ducts ICD10 C220, C222-C229 with an ICD-O2 8160 morphology were included in the study in order to capture CCA tumours which may have been miscoded (Table 1). A small number of tumours with a morphology code of 8162/3 were included in the analysis (n = 561) and were classified depending on which ICD10 site code had been recorded. If they had a tumour site code of C221 they were included as iCCA, whereas if they were recorded with a site code of C240 they were included as eCCA.
Table 1.
Site and morphology codes used to define each cancer type.
| Group name | Subgroup name | ICD10, topography code | ICD-O-2, morphology code |
|---|---|---|---|
| Cholangiocarcinoma overall | Intrahepatic cholangiocarcinoma | C221 (Intrahepatic bile duct carcinoma) | All morphologies |
| Extrahepatic cholangiocarcinoma | C240 (Extrahepatic bile duct carcinoma) | All morphologies | |
| Cholangiocarcinoma other | C248 (Overlapping lesion of biliary tract), C249 (Biliary tract, unspecified) | All morphologies | |
| C220, C222, C223, C224, C227, C229 | 8160 | ||
| Ampulla of Vater | C241 | All morphologies | |
| Gallbladder | C23 | All morphologies |
ICD10 was in use in England for the entire study period. The main results are presented for CCA overall which comprises iCCA, eCCA and other CCA subgroups.
Socioeconomic deprivation was measured by lower super output areas (geographic areas of a consistent size that cover a population of approximately 1,500 persons) of residence based on the income domain score of the English Indices of Multiple Deprivation.15 Lower super output areas were grouped into five socioeconomic deprivation quintiles, each containing 20% of the population of England. The least deprived quintile was labelled 1 and the most deprived 5. For the incidence, survival and routes to diagnosis analyses patients were assigned to a socioeconomic deprivation quintile based on their postcode of residence at the time of diagnosis using the appropriate index closest to the year of diagnosis, while for the mortality analyses patients were assigned to a deprivation quintile based on their postcode of residence at the time of death. The analysis of differences in incidence, mortality and survival rates between socioeconomically deprived groups was restricted to diagnosis years 2010-2018 due to mid-year population estimates by deprivation quintile not being available for the time period prior to 2010.
Each patient was assigned to one of the 21 Cancer Alliances based on their postcode of residence at the time of diagnosis. Cancer Alliances are partnerships of health and social care organisations with designated population catchment areas covering the whole of England. Our analysis was based on the 2021 Cancer Alliances’ definitions.14,15
A route to diagnosis was assigned to each tumour diagnosed between 2006 and 2017, as data were only available for tumours diagnosed during that time period. The NCRAS used an algorithm developed by Elliss-Brookes et al.16,17 to assign a route to diagnosis for each cancer. The algorithm used routine data to identify an inpatient or outpatient episode taking place within 6 months prior to the diagnosis date that most likely led to the cancer diagnosis. Patients were assigned to one of the following routes: emergency presentation, two week wait (TWW, urgent general practitioner [GP] referral with a suspicion of cancer), GP referral, inpatient elective, other outpatient, death certification only and unknown.
Mid-year population estimates, mortality data and information on death were obtained from the Office for National Statistics for each of the years 2001-2018. All mortality analyses are restricted to patients where ICD-10 code C221, C240, C241, C248, C249 or C23 was the cause of death recorded on the death certificate (there is no morphology information recorded on the death certificate). Therefore, for all mortality analyses the other CCA group only consists of ICD10 C248-249 tumours.
Statistical analysis
The distributions of the patient characteristics (age, sex, socioeconomic deprivation status at the time of diagnosis, route to diagnosis and diagnosis year) were tabulated among patients from each BTC subgroup (CCA, GBC and AoV cancer). We used the Pearson chi-squared test for heterogeneity to assess the differences in case-mix by cancer subtype. For 2006-2017, yearly numbers and proportions of patients presenting through each diagnosis route were calculated. Chi-squared tests were used to assess the differences in the proportions of patients presenting via emergency or TWW route vs. all other routes. Age-standardised incidence (ASRs) and mortality (ASMRs) rates per 100,000 person-years were calculated using the European Standard Population 2013,18 with estimates from 3-year rolling cohorts used for graph smoothing. We used Joinpoint regression modelling to assess trends in incidence and mortality rates and identify timepoints when significant changes occurred (Joinpoint Regression Program, Version 5.0, Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute). Annual percentage change (APC) was calculated for each segment and the average APC (AAPC) was calculated for the whole period.
Overall survival (OS) estimates were calculated using the Kaplan-Meier method with comparison between groups using log rank tests. One and three-year net survival was estimated, and the Brenner method of age-adjustment applied. All patients were followed up to 31 December 2019. Only persons between the ages of 15 and 99 years and only the first primary tumour of the cancer of interest for each patient were entered into the survival analysis, with persons with a death certificate only registration, missing NHS number or vital status, and any errors in birth, death, diagnosis or vital status dates excluded (n = 1,861). Survival estimates were suppressed if there were no deaths or data in at least one age band, less than 10 in a group, the standard error was greater than 0.2 and/or the follow-up time was insufficient in a cohort.
Incidence and mortality rates, and net survival estimates were calculated for 3-yearly rolling periods for analyses at national level, and for 4-year rolling periods for geographical comparison analyses at the Cancer Alliance level. Statistical analysis was performed using Stata version 15.1 (StataCorp, College Station, TX) and in R Version 4.0.5.19
Results
Patient and tumour characteristics (descriptive)
A total of 50,871 biliary tract tumours were diagnosed in England between 2001-2018. CCA was the most commonly diagnosed subtype (63.4%, n =32,251) followed by GBC (23.1%, n = 11,742) and AoV cancer (13.5%, n = 6,878) (Table 2). Of CCA, 74.5% were iCCA, 19.2% were eCCA and 6.3% were other type. An 8162 morphology code (“Klatskin”, an outdated term for perihilar CCA) was only recorded for 1.2% of cases. Over 95% of BTCs were diagnosed in patients aged 50 years or older with the median age at diagnosis being 75 (IQR 66-82) years for both CCA and GBC, and 73 (IQR 64-81) years for AoV cancer. Median age was similar for both sexes, with little variation by CCA subtype. Over 20% of patients with CCA were under 65 years old. More CCA and GBC cases were diagnosed in women (51.5% and 71.1%, respectively) and more AoV cancer cases were diagnosed in men (54.9%). The distribution of cases across the socioeconomic deprivation quintiles (based on socioeconomic deprivation status at the time of diagnosis) was different between the cancer sites, with a higher proportion of patients with GBC (compared to CCA or AoV cancer) living in the most deprived areas. Similarly, there was a significant difference in routes to diagnosis between cancer sites, with a higher proportion of patients with CCA presenting via the emergency route than among patients with GBC or AoV cancer.
Table 2.
Patient and tumour characteristics.
| Total |
Cholangiocarcinoma |
Gallbladder cancer |
Ampulla of Vater cancer |
χ2 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| N | Col % | N | Col % | N | Col % | N | Col % | p value∗ | |
| Total | 50,871 | 32,251 | 63.4 | 11,742 | 23.1 | 6,878 | 13.5 | ||
| Age group | |||||||||
| 0-44 | 1,018 | 2 | 656 | 2 | 192 | 1.6 | 170 | 2.5 | |
| 45-54 | 2,914 | 5.7 | 1,783 | 5.5 | 631 | 5.4 | 500 | 7.3 | |
| 55-64 | 7,613 | 15 | 4,708 | 14.6 | 1,754 | 14.9 | 1,151 | 16.7 | |
| 65-74 | 13,846 | 27.2 | 8,621 | 26.7 | 3,224 | 27.5 | 2,001 | 29.1 | |
| 75-84 | 16,532 | 32.5 | 10,495 | 32.5 | 3,951 | 33.6 | 2,086 | 30.3 | 160.786 |
| >84 | 8,948 | 17.6 | 5,988 | 18.6 | 1,990 | 16.9 | 970 | 14.1 | p <0.0001 |
| Sex | |||||||||
| Males | 22,802 | 44.8 | 15,628 | 48.5 | 3,396 | 28.9 | 3,778 | 54.9 | 1,700.000 |
| Females | 28,069 | 55.2 | 16,623 | 51.5 | 8,346 | 71.1 | 3,100 | 45.1 | p <0.0001 |
| Socioeconomic deprivation quintile | |||||||||
| 1 - Least deprived | 9,472 | 18.6 | 6,088 | 18.9 | 2,035 | 17.3 | 1,349 | 19.6 | |
| 2 | 10,591 | 20.8 | 6,761 | 21 | 2,350 | 20 | 1,480 | 21.5 | |
| 3 | 10,536 | 20.7 | 6,680 | 20.7 | 2,384 | 20.3 | 1,472 | 21.4 | |
| 4 | 10,191 | 20 | 6,448 | 20 | 2,418 | 20.6 | 1,325 | 19.3 | 60.828 |
| 5 - Most deprived | 10,081 | 19.8 | 6,274 | 19.5 | 2,555 | 21.8 | 1,252 | 18.2 | p <0.0001 |
| Route to diagnosis | |||||||||
| DCO | 133 | 0.4 | 93 | 0.4 | 34 | 0.4 | 6 | 0.1 | |
| Emergency presentation | 17,781 | 48.1 | 11,718 | 49.7 | 3,985 | 46.4 | 2,078 | 43.1 | |
| GP referral | 8,147 | 22 | 4,839 | 20.5 | 2,111 | 24.6 | 1,197 | 24.8 | |
| Inpatient elective | 1,090 | 2.9 | 654 | 2.8 | 187 | 2.2 | 249 | 5.2 | |
| Other outpatient | 4,170 | 11.3 | 2,440 | 10.4 | 1,074 | 12.5 | 656 | 13.6 | |
| TWW | 4,780 | 12.9 | 3,253 | 13.8 | 959 | 11.2 | 568 | 11.8 | 334.013 |
| Unknown | 883 | 2.4 | 569 | 2.4 | 243 | 2.8 | 71 | 1.5 | p <0.0001 |
| Diagnosis year | |||||||||
| 2001 | 1,896 | 3.7 | 1,165 | 3.6 | 418 | 3.6 | 313 | 4.6 | |
| 2002 | 1,880 | 3.7 | 1,177 | 3.6 | 386 | 3.3 | 317 | 4.6 | |
| 2003 | 1,912 | 3.8 | 1,182 | 3.7 | 416 | 3.5 | 314 | 4.6 | |
| 2004 | 2,051 | 4 | 1,251 | 3.9 | 467 | 4 | 333 | 4.8 | |
| 2005 | 2,169 | 4.3 | 1,365 | 4.2 | 486 | 4.1 | 318 | 4.6 | |
| 2006 | 2,479 | 4.9 | 1,585 | 4.9 | 526 | 4.5 | 368 | 5.4 | |
| 2007 | 2,466 | 4.8 | 1,540 | 4.8 | 551 | 4.7 | 375 | 5.5 | |
| 2008 | 2,676 | 5.3 | 1,733 | 5.4 | 565 | 4.8 | 378 | 5.5 | |
| 2009 | 2,812 | 5.5 | 1,869 | 5.8 | 586 | 5 | 357 | 5.2 | |
| 2010 | 2,985 | 5.9 | 1,953 | 6.1 | 643 | 5.5 | 389 | 5.7 | |
| 2011 | 3,058 | 6 | 1,979 | 6.1 | 685 | 5.8 | 394 | 5.7 | |
| 2012 | 3,104 | 6.1 | 2,000 | 6.2 | 704 | 6 | 400 | 5.8 | |
| 2013 | 3,349 | 6.6 | 2,111 | 6.5 | 783 | 6.7 | 455 | 6.6 | |
| 2014 | 3,324 | 6.5 | 2,061 | 6.4 | 845 | 7.2 | 418 | 6.1 | |
| 2015 | 3,523 | 6.9 | 2,217 | 6.9 | 886 | 7.5 | 420 | 6.1 | |
| 2016 | 3,652 | 7.2 | 2,263 | 7 | 934 | 8 | 455 | 6.6 | |
| 2017 | 3,670 | 7.2 | 2,334 | 7.2 | 910 | 7.7 | 426 | 6.2 | 173.775 |
| 2018 | 3,865 | 7.6 | 2,466 | 7.6 | 951 | 8.1 | 448 | 6.5 | p <0.0001 |
∗p values calculated using Pearson's chi-squared test.
DCO, death certification only; GP, general practitioner; TWW, two week wait.
Incidence and mortality
Overall incidence and mortality trends
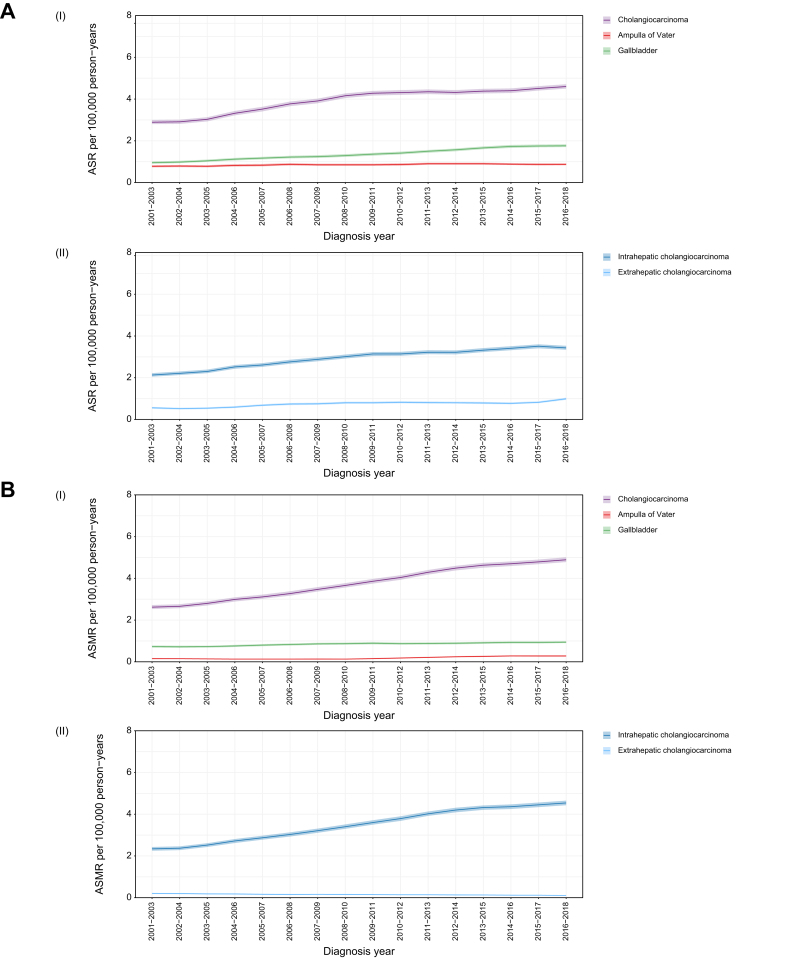
The number of cases diagnosed with CCA and GBC doubled between 2001 and 2018 and increased by more than 40% for AoV cancer (Table 2). The ASR for CCA rose from 2.9 (95% CI 2.8-3.0) in 2001-2003 to 4.6 (95% CI 4.5-4.7) in 2016-2018 and from 1.0 (95% CI 0.9-1.0) to 1.8 (95% CI 1.7-1.8) for GBC for the same time period. In comparison, the change in AoV cancer rates was less pronounced, rising from 0.8 (95% CI 0.7-0.8) to 0.9 (95% CI 0.8-0.9) (Fig. 1A(i)). The rise in CCA incidence was predominantly driven by the increase in iCCA (Fig. 1A(ii)) with ASRs increasing from 2.1 (95% CI 2.1-2.2) to 3.4 (95% CI 3.3-3.5) between 2001-2003 and 2016-2018, a statistically significant AAPC of 3.3% (95% CI 2.6-4.2). The iCCA rates increased between the years 2001 and 2009 (significant APC 5.4%, 95% CI 3.9–8.6) and plateaued between 2009 and 2018 (APC 1.5%, 95% CI -1.0 to 2.5). During the study’s time period the rise in eCCA was from 0.6 (95% CI 0.5-0.6) to 1.0 (95% CI 0.9-1.0) a statistically significant AAPC of 4.6% (95% CI 3.6-5.9). The eCCA rates increased between the years 2004 and 2007 (significant APC 16.5%, 95% CI 5.9–22.9) and between 2016 and 2018 (APC 25.7%, 95% CI 13.2–33.7), and plateaued between the years 2001 and 2004 (APC -4.5%, 95% CI -16.5 to 5.0) and between 2007 and 2016 (APC -0.1%, 95% CI -3.8 to 1.2). For CCA and AoV cancer the ASRs were higher in males than females, with both sexes having a similar trend during the study period. In contrast, GBC ASRs were higher in females than males.
Fig. 1.
ASRs and ASMRs by biliary tract cancer subtype.
(A) ASRs per 100,000 person-years and 95% CIs: (i) cholangiocarcinoma, gallbladder cancer and ampulla of Vater cancer; (ii) Intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma. (B) ASMRs and 95% CIs: (i) cholangiocarcinoma, gallbladder cancer and ampulla of Vater cancer; (ii) intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma. ASMR, age-standardised mortality rate; ASR, age-standardised incidence rate.
During 2001-2018 there were 40,215 deaths from BTC: 78.1% (n = 31,411) due to CCA (excluding ICD10 C220, C222-C229 with ICD-O2 8160 as morphology is not recorded on the death certificate), 18.1% (n = 7,257) due to GBC and 3.9% (n = 1,547) due to AoV cancer. The number of deaths from CCA and AoV cancer more than doubled during this period (2018 CCA, n = 2,603; AoV cancer, n = 139) and increased by 70% for GBC (2018 n = 516). The ASMR of CCA rose from 2.6 (95% CI 2.5-2.7) to 4.9 (95% CI 4.8-5.0) between 2001-2003 and 2016-2018 in parallel with the incidence rates, with a statistically significant AAPC of 3.9% (95% CI 3.6-4.2). The CCA mortality rates were stable between the years 2001 and 2003 (APC 0.3%, 95% CI -1.8 to 4.1) and increased faster between the years 2003 and 2013 (significant APC 5.5%, 95% CI 5.2–6.3) than between 2013 and 2018 (significant APC 2.0%, 95% CI 1.0–2.8). The ASMR for GBC followed a similar increasing trend and AoV cancer rates remained relatively stable (Fig. 1B(i)). The trends for eCCA and iCCA AMSR mirror those of incidence, with most deaths due to iCCA (Fig. 1B(ii)). Mortality rates for CCA and AoV cancer were slightly higher in males than females, with an accelerated increase in AoV cancer-related mortality in males in recent years. Women had consistently higher GBC mortality rates with the difference in rates between males and females increasing mildly over time.
Incidence and mortality by socioeconomic deprivation
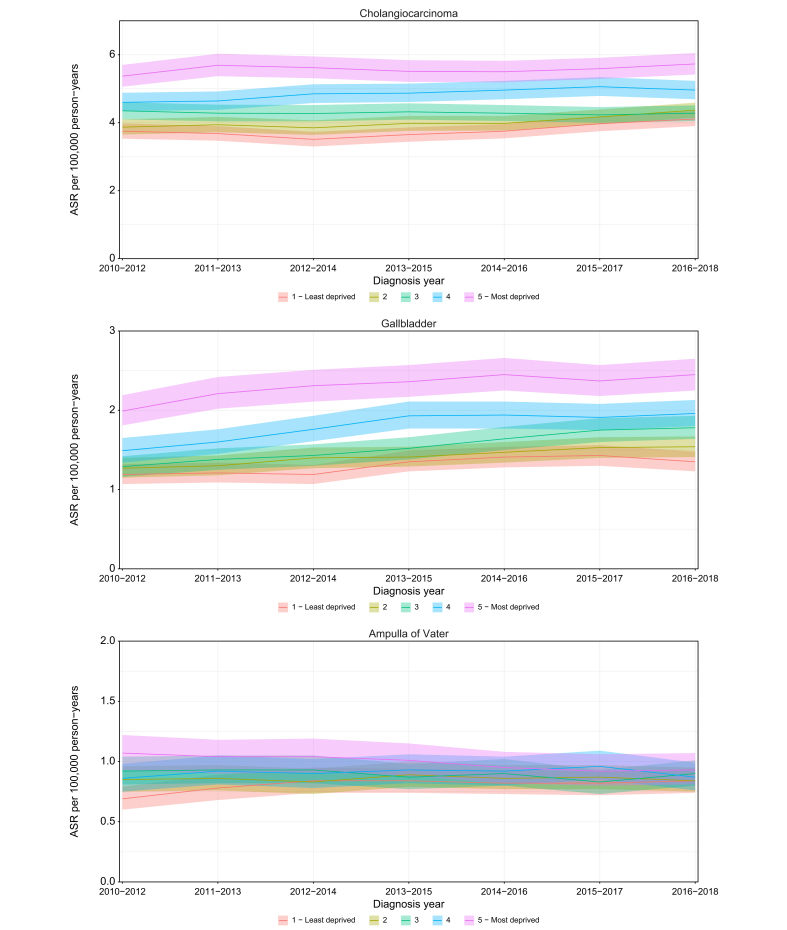
Between 2010 and 2018, ASRs were consistently higher in patients who were in the most socioeconomically deprived group at the time of diagnosis compared to the least deprived group for CCA and GBC, with the difference more pronounced for GBC, and similar rates across deprivation quintiles for AoV cancer (Fig. 2). Compared with the least deprived group, the iCCA incidence rates were higher in the most socioeconomically deprived group, while for the eCCA this was the case only in recent years when a steeper rise in rates for the most deprived group was observed. For the most recent 3-year diagnosis period, 2016-2018, the most socioeconomically deprived group of patients with CCA had an incidence rate of 5.7 (95% CI 5.4-6.1) compared to 4.1 (95% CI 3.9-4.3) in the least deprived group. The respective figures were 2.5 (95% CI 2.3-2.7) and 1.4 (95% CI,1.2-1.5) for GBC, and 0.9 (95% CI 0.8-1.1) and 0.8 (95% CI 0.7-0.9) for AoV cancer.
Fig. 2.
ASRs and 95% CIs by socioeconomic deprivation quintiles for cholangiocarcinoma, gallbladder cancer and ampulla of Vater cancer.
ASR, age-standardised incidence rate.
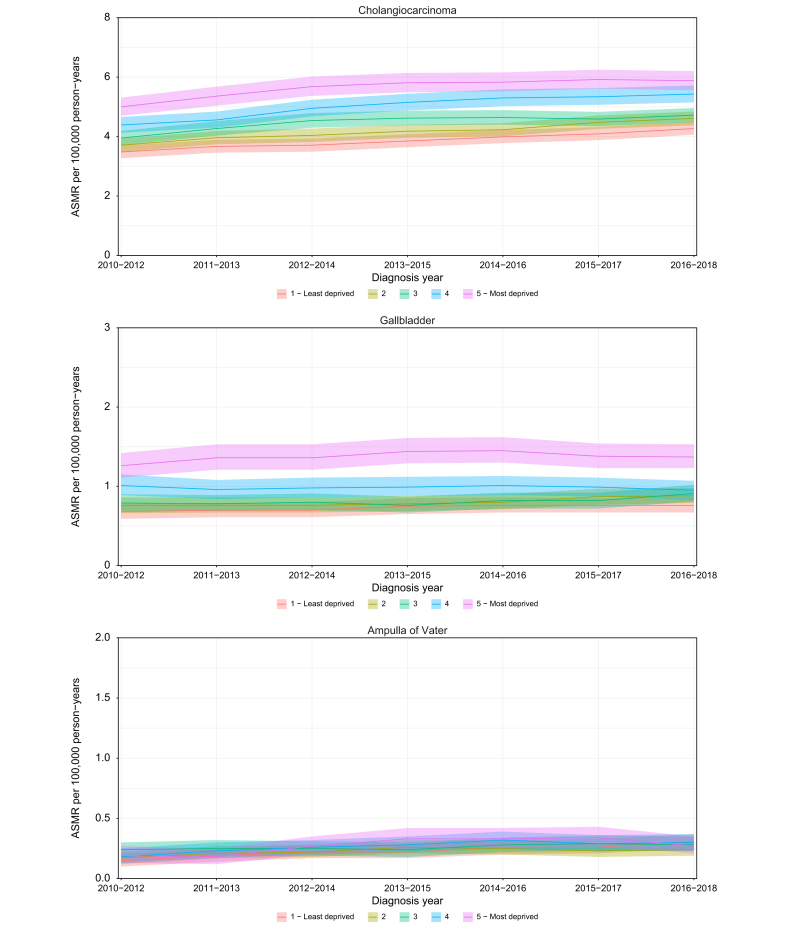
Mortality rates for CCA, GBC and AoV cancer follow a similar pattern to incidence. Based on the patients’ socioeconomic deprivation status at the time of death, the patients in the most deprived group had higher mortality rates compared to the least deprived group for CCA and GBC and no difference in rates was observed between deprivation quintiles for AoV cancer (Fig. 3). The ASMR for CCA has increased over time across all deprivation quintiles. Patients with iCCA from the most deprived group had higher mortality rates than those from the least deprived group, however the rates for eCCA were similar among the deprivation quintiles. In 2016-2018, the ASMRs were 5.9 (95% CI 5.6-6.2) and 4.3 (95% CI 4.1-4.5) in the most deprived and least deprived CCA groups, respectively, a difference of 37%. The corresponding values were 1.4 (95% CI 1.2-1.5) and 0.8 (95% CI 0.7-0.9) for GBC, and 0.3 (95% CI 0.2-0.4) and 0.3 (95% CI 0.3-0.4) for AoV cancer.
Fig. 3.
ASMRs and 95% CIs by socioeconomic deprivation quintiles for cholangiocarcinoma, gallbladder cancer and ampulla of Vater cancer.
ASMR, age-standardised mortality rate.
Routes to diagnosis
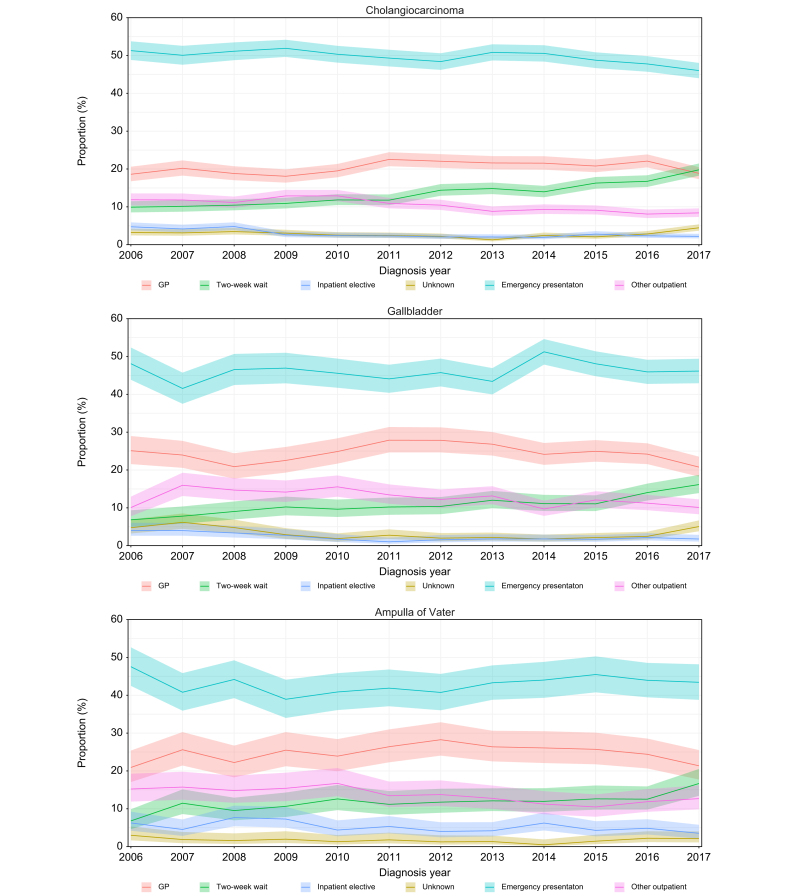
A total of 37,098 BTCs were included in the routes to diagnosis analysis for 2006-2017. The most common route to diagnosis was the emergency route (49.6% for CCA [iCCA 50.4%, eCCA 46.1], 46.2% for GBC and 43.0% for AoV cancer) followed by the GP referral route (20.5% for CCA [iCCA 20.2%, eCCA 21.8%], 24.5% for GBC and 24.8% for AoV cancer) and TWW referrals (13.8% for CCA [iCCA 13.9%, eCCA 13.4%], 11.1% for GBC and 11.8% for AoV cancer). Based on the socioeconomic deprivation status at the time of diagnosis, there were relatively more patients from the most deprived group diagnosed through an emergency route compared to all non-emergency routes for all three cancer sites (CCA p <0.001, GBC p = 0.001, AoV p <0.001). For CCA and AoV cancer relatively fewer patients who were in the most socioeconomically deprived group at the time of diagnosis were diagnosed via TWW compared to all non-TWW routes (CCA p = 0.011, AoV p <0.002), but there was no difference across the deprivation quintiles for GBC (p = 0.207). Between 2006 and 2017 there was a downward trend in the proportion of patients with CCA presenting via emergency route (decreasing from 51.3% in 2006 to 46.0% in 2017), but for GBC and AoV there was no clear trend (Fig. 4). In contrast, TWW referrals showed an increasing trend for all cancer sites, rising from 9.9% in 2006 to 19.8% in 2017 for CCA, from 6.8% to 16.2% for GBC and from 6.8% to 16.7% for AoV cancer.
Fig. 4.
Proportion of patients and 95% CIs by routes to diagnosis for cholangiocarcinoma, gallbladder cancer and ampulla of Vater cancer.
Survival
Overall survival
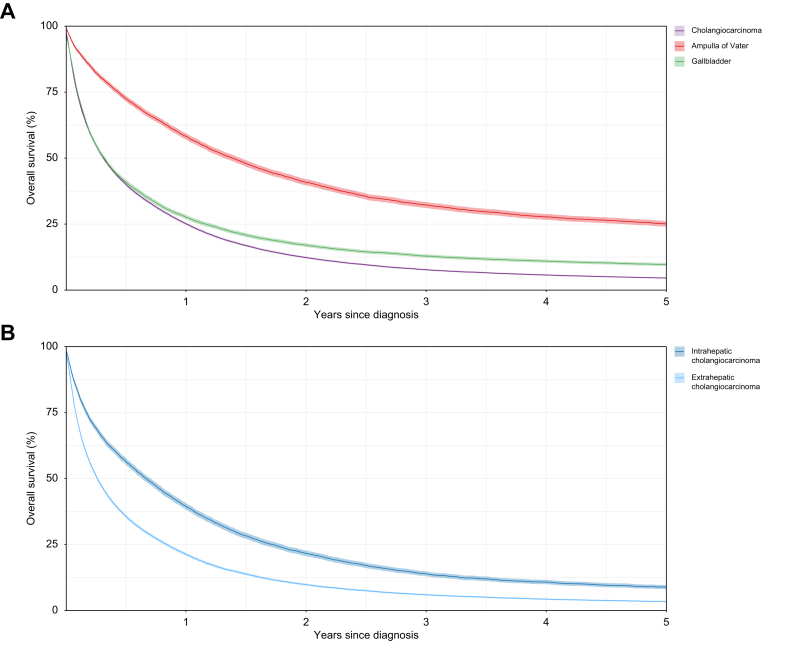
A total of 49,010 BTCs were included in the survival analysis. Patients with CCA had lower overall survival than those diagnosed with GBC or AoV (p <0.001) (Fig. 5). The Kaplan-Meier 1-year survival estimate for CCA was 25.1% (95% CI 24.7-25.6) compared to 27.6% (95% CI 26.8-28.4) for GBC and 58.3% (95% CI 57.1-59.5) for AoV cancer. At 3 years after diagnosis the overall survival estimates for CCA, GBC and AoV cancer were 7.7% (95% CI 7.4-8.1), 12.9% (95% CI 12.3-13.5), and 32.3% (95% CI 31.1-33.4), respectively. The overall survival for patients with eCCA was higher than for those with iCCA (p <0.001). One-year survival rates were 39.4% (95% CI 38.2–40.7) for eCCA and 21.3% (95% CI 20.8-21.8) for iCCA, and 3-year survival rates were 13.9% (95% CI 13.0-14.9) and 6.0% (95% CI 5.7–6.3), respectively. Males with CCA, GBC and AoV cancer had higher overall survival rates than female patients (p <0.001, p = 0.0193 and p = 0.0254, respectively). Younger patients with CCA had better overall survival (p <0.001), as did younger patients with GBC and AoV cancer (p <0.001 and p <0.001, respectively). Overall survival was better for patients with CCA (p <0.001) and AoV cancer (p <0.001) who were in the least socioeconomically deprived group at the time of diagnosis; however, survival was similar between deprivation quintiles for GBC (p = 0.1162).
Fig. 5.
Kaplan-Meier survival estimates with 95% Cls.
(A) Cholangiocarcinoma, gallbladder cancer and ampulla of Vater cancer; (B) Intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma.
Net survival
One and 3-year net survival was higher for AoV than for CCA and GBC, with similar improvement between 2001 and 2018 for all BTCs (Fig. S1). Patients with eCCA had better 1- and 3-year net survival than those with iCCA. One-year net survival for CCA increased from 27.9% (95% CI 26.3-29.4) in 2001-2003 to 33.6% (95% CI 32.5-34.7) in 2016-2018 and 3-year net survival increased from 9.2% (95% CI 8.2-10.2) to 12.6% (95% CI 11.7-13.6), respectively. A similar increase was also seen for GBC and to a lesser extent for AoV cancer. There was no difference in 1-year and 3-year net survival between males and females.
Based on the socioeconomic deprivation status at the time of diagnosis, one-year net survival for patients with CCA was significantly higher in the least deprived than the most deprived group (except in the beginning of the study period) (Fig. S2) and increased in both the least deprived (from 31.3% [95% CI 27.5%-35.1%] in 2001-2003 to 37.1% [95% CI 34.6%-39.7% in 2016-2018]), and the most deprived cohort (from 24.8% [95% CI 21.5%-28.3%] in 2001-2003 to 30.2% [95% CI 27.7%-32.8%] in 2016-2018). At 3 years after diagnosis a difference in net survival between the most deprived and the least deprived quintiles was only observed between 2005-2007 and 2010-2012. There was no significant difference in 1-year or 3-year net survival between the deprivation quintiles for GBC or AoV cancer.
Geographical variation
Incidence
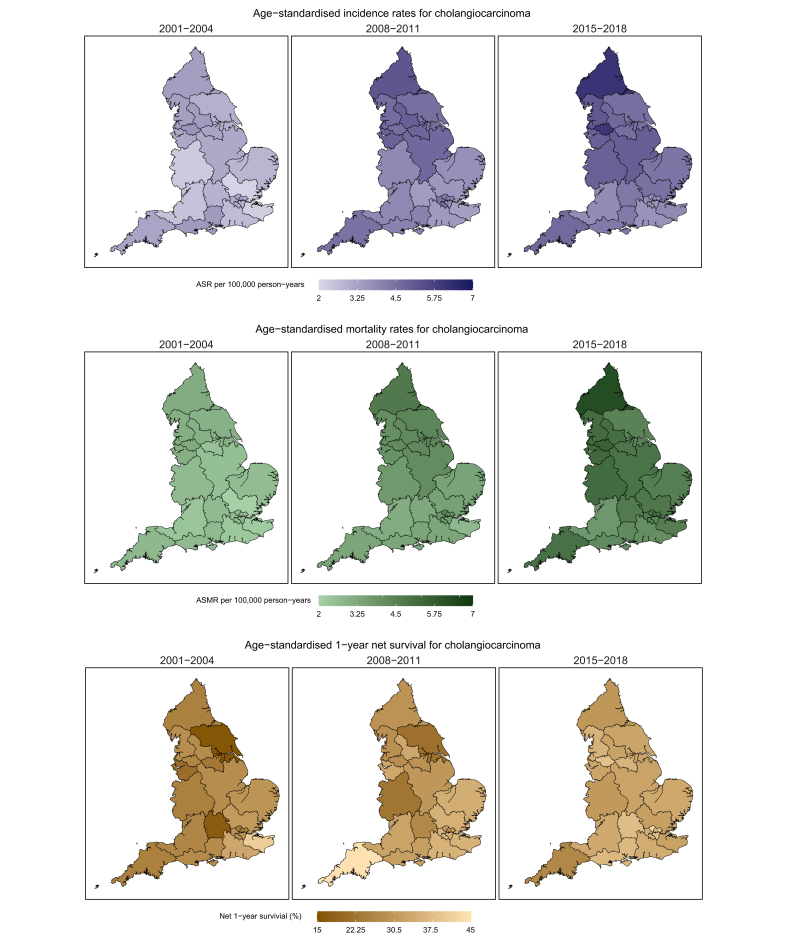
The geographical variation in the ASRs in the most recent years was similar to the variation in rates observed at the beginning of the study period for CCA, GBC, and AoV cancer. The CCA ASRs increased for most Cancer Alliances in England between 2001 and 2018, though some areas showed a decline in rates in the more recent years (South East London, North West and South West London) (Fig. 6). Northern and Greater Manchester Cancer Alliances were more often among those with the highest CCA incidence rates (2016-2018 ASRs of 6.1 [95% CI 5.7-6.6] and 6.1 [95% CI 5.6-6.6], respectively) with Northern, Greater Manchester and West Yorkshire and Harrogate Cancer Alliances having the most pronounced increase in rates. By 2015-2018 London Cancer Alliances were among those with the lowest rates. A similar geographical variation in ASRs was observed for GBC with most Cancer Alliances experiencing increasing incidence rates and some areas with the highest rates being found in the North of England. A relatively steep increase in GBC rates was observed from 2013 for the North East London Cancer Alliance, becoming the area with the highest incidence rate in 2015-2018 at 2.69 (95% CI 2.2-3.2). The incidence rates for AoV cancer were more stable, but similarly the Northern Cancer Alliance was the area with some of the highest rates during the study period.
Fig. 6.
Cholangiocarcinoma ASRs, ASMRs, and 1-year net survival by Cancer Alliance.
ASMR, age-standardised mortality rate; ASR, age-standardised incidence rate.
Mortality
The geographical variation in CCA mortality rates also widened between 2001-2004 (ASMR range [2.02-3.19]) and 2015-2018 (ASMR range [3.77-6.25]), with all Cancer Alliances seeing an increase in mortality rates. The highest mortality rates were seen in the Cancer Alliances with the highest incidence rates (Northern and Greater Manchester Cancer Alliances: 2016-2018 ASMRs of 6.3 [95% CI 5.8-6.7] and 5.5 [95% CI 5.1-6.1], respectively). Gallbladder mortality rates showed a wide geographical variation but were more stable over the study period. Deaths from AoV cancer were very rare at Cancer Alliance level, with mortality rate calculations not being possible for many Cancer Alliances for the earliest years of the study period. For the second half of the study period AoV mortality rates showed a similar pattern to GBC.
Net survival
The variability in 1 and 3-year net survival estimates for CCA at Cancer Alliance level by time period was wide but reduced between 2001 and 2018 (1-year net survival 2001-2004 range [16.9%-40.8%], 2015-2018 range [27.2%-42.4%]). Each Cancer Alliance demonstrated a general pattern of improving survival over time. In the most recent time period, 2015-2018, North Central London (42.4% [95% CI 34.0%-50.5%]), South East London (38.9% [95% CI 31.2%-46.4%]) were the Cancer Alliances with the highest 1 and 3-year net survival. One- and 3-year net survival generally improved for GBC and AoV cancer but varied widely between Cancer Alliances.
Discussion
Several epidemiological studies have been published over the past 20 years or so reporting rising incidence and/or mortality rates for iCCA in individual countries.[5], [6], [7], [8], [9], [10], [11], [12] This work is novel in several important areas. It is the first, to our knowledge, to explore regional variation and temporal trends across a nation, including a breakdown of CCA rates by socioeconomic or deprivation status, and changing patterns in the routes to diagnosis for CCA. As such, we believe this is the most in-depth national study of BTC rates to date worldwide.
Although all BTC cases doubled between 2001 and 2018, CCA remained the commonest type of BTC, almost 75% of which were iCCA. Although most cases occur in the elderly, almost a quarter (22%) of CCA cases occur in individuals aged under 65. Most of the rise in CCA incidence and mortality is found in iCCA but rates for eCCA have also increased, with similar trends in both sexes. CCA is becoming less rare and a substantial proportion of patients are not elderly. The reasons for this remain unclear and are unlikely to be simply related to improvements in diagnosis and awareness. Although several risk factors are recognised for CCA, and some of these may be increasing, such as type 2 diabetes and chronic liver disease,3 a substantial proportion of cases have no obvious risk factors. Furthermore, it should be considered that mortality rates for CCA are likely to be affected by the consistency and accuracy of cause of death as coded on death certification, given the comorbidities many of these patients have, as well as the frequent presence of underlying chronic liver disease. Nonetheless, further work on the aetiology and pathogenesis of CCA is urgently needed.
CCA is associated with lower overall survival than GBC or AoV cancer, and iCCA is associated with lower survival than eCCA. This may be due to a more non-specific presentation of iCCA, or a lack of effective therapies at presentation. Unsurprisingly, overall survival is better in younger age groups, who are likely to have less co-morbidity.
Of note, ASMRs for CCA have increased over time across all deprivation quintiles. The higher overall incidence and mortality rates for BTC and poorer overall survival in the most deprived socioeconomic groups is another cause for concern. This may reflect differences in the burden of pre-existing life-style and risk factors in different socioeconomic groups and/or access to healthcare, or other reasons. A previous study by NCRAS found that an increase in liver cancer incidence, including of iCCA, was largely driven by the most deprived areas and suggested that this might be due to the prevalence of known risk factors such as chronic hepatitis infection and excessive alcohol consumption.20 However, unlike hepatocellular carcinoma – the most common primary liver cancer globally – the majority of CCA cases are not associated with pre-existing chronic liver disease, alcohol excess or metabolic syndrome.1 Nonetheless, comorbidities may clearly affect incidence and survival; however, including these in our analyses was beyond the scope of this descriptive study. Further work is warranted in this area.
Our study also found evidence of regional variation in incidence, mortality and net survival for BTC, particularly for CCA, with relatively higher rates in Cancer Alliances in Northern England. These differences have persisted over the 17-year period of the study. Several theoretical explanations exist, such as possible differences in exposure to local risk factors [known and unknown]; tumour characteristics including stage and subtype; access to specialist healthcare; differences in clinical management and/or differences in data collection. This is, as yet, a relatively unexplored area of CCA research but further work is needed to explain the reasons behind regional differences.
A high proportion of CCA cases present via the emergency route, with less than 20% presenting via the NHS TWW urgent referral pathway. This is similar to other cancers with high mortality which are initially asymptomatic or present with non-specific symptoms, for example pancreatic cancer. Emergency presentations of cancers are generally associated with advanced stage disease at diagnosis and poorer outcomes. The approximate doubling in the proportion of BTC cases diagnosed via the TWW pathway during the study period is encouraging but it still accounts for a minority of cases. This emphasises the need to identify risk factors and high-risk groups, to develop better screening tools (as well as early and accurate biomarkers), and to improve awareness of CCA.
There is an important limitation in this and preceding studies on CCA epidemiology, which is infrequently acknowledged. What cannot be teased out in our analyses are the rates of pCCA specifically, as the main ICD coding system has historically lacked a specific code for pCCA, which has likely been mostly miscoded as iCCA in the past.21 Only 1.2% of cases had an 8162 morphology code recorded, which is used for “Klatskin” CCA, an outdated term for pCCA. This is an important issue as, in clinical practice and published data, pCCA is the most common subtype of CCA, accounting for at least 50% of CCA. The minority of CCA are truly iCCA or dCCA – other than in parts of Asia where iCCA is likely the predominant subtype.21 The lack of specific coding for pCCA is to be corrected in the subsequent version of ICD but this does not help with understanding the historical rates of pCCA distinct from iCCA and dCCA. Furthermore, as Selvadurai et al.’s recent retrospective clinical review of coding of CCA tumours at several hepatobiliary centres in England concluded, in some cases even after a careful multi-disciplinary review, it is not possible to definitively code to the correct original tumour site, for example large tumours involving both the hilum and the deeper liver parenchyma.21
Finally, although there has been an encouraging improvement in 1- and 3- year survival during the study period, these improvements are small and highlight an unmet need for earlier diagnosis and for more effective systemic therapies for advanced disease.
Financial support
This work was supported by funding from the AMMF: The Cholangiocarcinoma Charity.
Authors’ contributions
DT, SK, HM, LP and MBT designed the study. DT, RH and KW obtained the data and performed the statistical analyses. SK and DT drafted the original manuscript which was reviewed and amended with comments from all authors.
Data availability statement
Data used in this study is collated, maintained, held and quality assured by the National Disease Registration Service, which is part of NHS England.
Conflict of interest
The authors have no conflicts of interest to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors thank AMMF for funding this project and are grateful for contributions to this work from the National Cancer Registration and Analysis Service, NHS Digital. This work uses data that has been provided by patients and collected by the NHS as part of their care and support. The data is collated, maintained and quality assured by the National Cancer Registration Service, which is part of NHS England. We also thank Tracey Genus for starting the data collation for the project at its inception. SAK and MBT are additionally grateful for support from the UK National Institutes for Health Research (NIHR) Biomedical Facilities at Imperial College London. MBT’s Chair is supported in part by a donation from Marit Mohn to Imperial College London to support Population Child Health through the Mohn Centre for Children’s Health and Wellbeing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100983.
Contributor Information
Daniela Tataru, Email: daniela.tataru@nhs.net.
Shahid A. Khan, Email: shahid.khan@imperial.ac.uk.
Mireille B. Toledano, Email: m.toledano@imperial.ac.uk.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Brindley P., Bachini M., Ilyas S., et al. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;9;7(1):65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales J., Marin J., Lamarca A., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020 Jun 30 doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements O., Eliahoo J., Kim J.U., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol. 2020;72(1):95–103. doi: 10.1016/j.jhep.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Khan S.A., Tavolari S., Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019 doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 5.Khan S.A., Toledano M.B., Taylor-Robinson S.D. Epidemiology, risk factors and pathogenesis of cholangiocarcinoma. HPB. 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor-Robinson S.D., Toledano M.B., Arora S., et al. Increase in mortality rates for intrahepatic cholangiocarcinoma in England & Wales 1968-1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S.A., Taylor-Robinson S.D., Toledano M.B., et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours 1979-1997. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 8.Khan S.A., Taylor-Robinson S.D., Davidson B.R., et al. Cholangiocarcinoma: seminar. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 9.Khan S.A., Emadossadaty S., Ladep N., et al. Rising Trends in Cholangiocarcinoma: is the ICD Classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. Patel et al 2002. [DOI] [PubMed] [Google Scholar]
- 10.West J., Wood H., Logan R.F., et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006 Jun 5;94(11):1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertuccio P., Bosetti C., Levi F., et al. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013 Jun;24(6):1667–1674. doi: 10.1093/annonc/mds652. Epub 2013 Feb 1. [DOI] [PubMed] [Google Scholar]
- 12.Bertuccio P., Malvezzi M., Carioli G., et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019 Jul;71(1):104–114. doi: 10.1016/j.jhep.2019.03.013. Epub 2019 Mar 23. PMID: 30910538. [DOI] [PubMed] [Google Scholar]
- 13.Izquierdo-Sanchez L., Lamarca A., La Casta A., et al. J Hepatol. 2022;76(5):1109–1121. doi: 10.1016/j.jhep.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Henson K.E., Elliss-Brookes L., Coupland V.H., et al. Data resource profile: national cancer registration dataset in England. Int J Epidemiol. 2019 doi: 10.1093/ije/dyz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achieving world-class cancer outcomes: a strategy for England 2015-2020. 2015. https://www.england.nhs.uk/publication/achieving-world-class-cancer-outcomes-a-strategy-for-england-2015-2020/ [Google Scholar]
- 16.Elliss-Brookes L., McPhail S., Ives A., et al. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://geoportal.statistics.gov.uk/maps/4845c8f156fb41ac8766d0a3f2d146cc/explore?location=48.845927%2C0.545406%2C6.33 Accessed 1 August 2021.
- 18.Revision of the European standard population 2013 edition. European Union, Publications Office of the European Union; Luxembourg: 2013. [Google Scholar]
- 19.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: a language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 20.Konfortion J., Coupland V.H., Kocher H.M., et al. Time and deprivation trends in incidence of primary liver cancer subtypes in England. J Eval Clin Pract. 2014;20(4):498–504. doi: 10.1111/jep.12188. Epub 2014 Jun 5. [DOI] [PubMed] [Google Scholar]
- 21.Selvadurai S., Mann K., Mithra S., et al. Cholangiocarcinoma miscoding in hepatobiliary centres. Eur J Surg Oncol. 2020 doi: 10.1016/j.ejso.2020.09.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
Data used in this study is collated, maintained, held and quality assured by the National Disease Registration Service, which is part of NHS England.