Abstract
Background:
ISCHEMIA-CKD reported an initial invasive strategy (INV) did not reduce the risk of death or nonfatal myocardial infarction (D/MI) compared with a conservative strategy (CON) in patients with advanced chronic kidney disease, stable coronary disease, and moderate or severe myocardial ischemia. The cumulative frequency of different MI type after randomization and subsequent prognosis have not been reported.
Methods:
MI classification was based on the Third Universal Definition for MI (UDMI). For procedural MI, the primary MI definition used CK-MB as the preferred biomarker whereas the secondary MI definition used cardiac troponin; both definitions included elevated biomarker-only events with higher thresholds than non-procedural MIs. The cumulative frequency of MI type according to treatment strategy was determined. The association of MI with subsequent all-cause death and new dialysis initiation was assessed by treating MI as a time-dependent covariate.
Results:
The 3-year incidence of type 1 or 2 MI with the primary MI definition was 11.2% in INV and 13.6% in CON (difference −2.39 [95% CI: −7.88, 3.10]) (HR 0.64, 95% CI 0.41, 1.01). Procedural MIs were more frequent in INV and accounted for 9.5% and 27.1% of all MIs with the primary and secondary MI definitions, respectively. Patients had an increased risk of all-cause death after type 1 MI (adjusted HR (aHR)=4.35; 95% 2.73, 6.93), and after procedural MI with the primary (aHR=2.75; 0.99,7.60) and secondary MI definitions (aHR=2.91; 1.73, 4.88). Dialysis initiation was increased after a type 1 MI (HR 6.45 [2.59, 16.08]) compared with patients without an MI.
Conclusions:
In ISCHEMIA-CKD, the invasive strategy had higher rates of procedural MIs, particularly with the secondary MI definition, and lower rates of type 1 and 2 MIs. Procedural MIs, type 1 and type 2 MIs were associated with increased risk of subsequent death. Type 1 MI increased the risk of dialysis initiation.
Keywords: Myocardial Infarction, Myocardial Ischemia, Myocardial Revascularization, Chronic Kidney Disease
Graphical Abstract
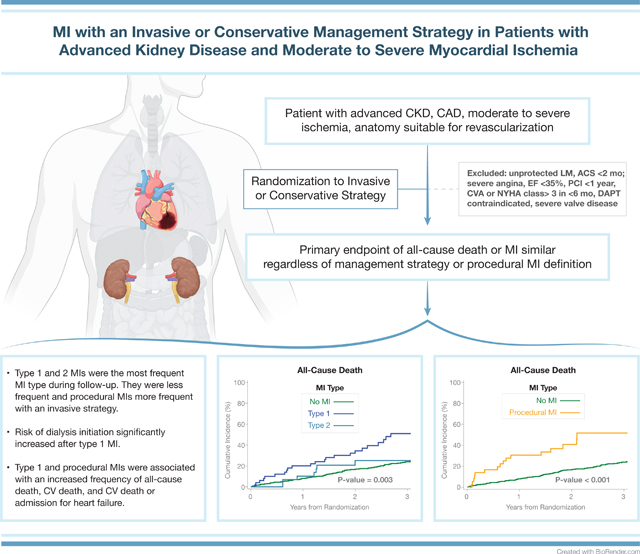
Introduction
Cardiovascular disease is the leading cause of death in patients with advanced CKD and associated with more rapid progression of coronary atherosclerosis and cardiovascular events compared with patients without this condition.1–7 Most prior studies in this population report MI as a single entity and do not report MIs by type as described in the UDMI. The UDMI classifies MI type according to pathophysiology, an important consideration when comparing an invasive to a conservative treatment strategy.8–10 The frequency of MI types, in-hospital complications after MI, and outcomes of all-cause death, cardiovascular death, or composite of cardiovascular death or admission for heart failure according to treatment strategy in this population with moderate to severe myocardial ischemia and contemporary guideline based medical management are unavailable.
In the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches—Chronic Kidney Disease (ISCHEMIA-CKD), 777 patients with advanced CKD and moderate or severe ischemia on non-invasive testing were randomized to an initial invasive strategy of coronary angiography and revascularization if suitable or a conservative strategy with coronary angiography reserved for use after adverse cardiovascular events or refractory symptoms.11 After a median follow-up of 2.2 years, there were no statistical differences observed between strategies in the primary endpoint of all-cause death or MI, or in the major secondary composite endpoint of death, nonfatal MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest.12 MIs accounted for slightly less than half of the primary endpoint events and the analysis plan prespecified that the primary and major secondary endpoints would be examined using two different MI definitions.11, 12 The specific aims of this report are to examine: (i) the incidence of MI and type of MI using two different procedural MI definitions, and (ii) to determine the prognostic implication of MI types on death and renal outcomes.
Methods
The ISCHEMIA-CKD Trial
The data were assembled and analyzed by the Statistical and Data Coordinating Center located at Duke Clinical Research Institute. The data that support the findings of this study are available from the corresponding author on reasonable request. Detailed descriptions of the ISCHEMIA-CKD trial design, protocol, baseline demographics, and outcomes have been published.11–14 The patient flow diagram is found in Figure S1. The protocol was approved by the Institutional Review Board at each institution. All patients provided written informed consent. The trial was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health with industry support providing some pharmacologic therapies and devices.
A total of 777 patients were randomized between April 29, 2014 and January 31, 2018. Follow-up after randomization was scheduled at 1.5, 3, 6, and 12 months and every 6 months thereafter.
Event Definitions
All primary endpoints were adjudicated by a Clinical Events Committee (CEC). The CEC process and definitions for cardiovascular death, non-cardiovascular death, resuscitated cardiac arrest, and hospitalizations for MI, unstable angina, and heart failure were identical to those used in the ISCHEMIA trial.12, 15, 16
Myocardial Infarction
MI types were classified according to the Third UDMI (Supplemental Material).8 The diagnosis of MI types 1, 2, 4b, and 4c used cardiac troponin (cTn) as the preferred biomarker (unless only CK-MB values were available) and included ancillary evidence of myocardial ischemia. The primary MI definition used the site-determined MI decision limit, and the secondary MI definition used the manufacturer’s recommended 99th percentile of the upper reference limit (URL) with specific clinical, angiographic, electrocardiographic (ECG), and imaging criteria as described in the supplement. For procedural MI, the primary MI definition used CK-MB as the preferred biomarker, whereas the secondary MI definition used cTn. A post-procedural biomarker value >5-fold the upper limit of normal (ULN) within 48 hours associated with specific ECG, angiographic, or imaging findings indicating myocardial ischemia defined a type 4a PCI-related MI. The ECG and angiographic criteria required either new ST segment depression of ≥1mm for the primary definition and ≥0.5 mm for the secondary definition or Q waves, or a new coronary dissection ≥NHLBI grade 3. For type 5 CABG-related MI, a post-procedural biomarker value >10-fold the ULN within 48 hours associated with new Q waves or persistent left bundle branch block defined an MI.. Both procedural MI definitions included a category of biomarker-only events with much higher thresholds (cTn >70-fold or >100-fold 99th percentile upper reference limit (URL) for type 4a and type 5 MI respectively). The CEC prospectively classified each suspected MI according to the primary and secondary MI definition blinded to treatment assignment as previously described.15 The definition of heart failure required the presence of heart failure symptoms, signs, and treatment.16
Statistical Analysis
Continuous variables are presented as medians (Q1, Q3) and categorical variables are presented as counts (percentages). The number of MI events (first MI events; overall and by type for primary and secondary definitions) are summarized with counts and percentages among all randomized patients. To account for the competing risk of any type of death in the analysis of individual non-fatal MI endpoints, cumulative incidence rates (95% CI) were estimated for the invasive and conservative strategies and Gray’s test was applied to compare incidence rates over the duration of follow-up.17
Cox proportional hazards regression modeling was used to characterize the association between randomized treatment strategy and time to first occurrence of an MI. Unadjusted and adjusted hazard ratios (95% CI) and p-values are reported for comparing invasive vs conservative strategies. Each model was adjusted for a set of pre-specified prognostically important baseline covariates that included age at randomization, sex, kidney function (dialysis status and eGFR in patients not receiving dialysis), left ventricular ejection fraction (LVEF), and diabetes. To maximize the amount of information each covariate provides to a covariate-adjusted analysis, multiple imputation, using the same approach as was used in the main trial was used to impute missing covariate data12 (Supplemental Material). To allow for non-linear covariate effects, the continuous variables of age, LVEF and eGFR were modeled as restricted cubic splines with knots at the approximate 10th, 50th, and 90th percentiles of each variable’s empirical distribution. The association of MI vs. no MI with subsequent events of death, cardiovascular death, hospitalization for heart failure, and initiation of new dialysis was characterized by reporting the adjusted hazard ratio (95% CI) and p-value from a Cox regression model in which MI during follow-up was treated as a time-dependent covariate. We extended this Cox model to include time-varying coefficients for different time intervals from the occurrence of an MI. Models were adjusted for age at randomization, sex, kidney function (dialysis status and eGFR in patients not receiving dialysis), LVEF, diabetes, and randomized treatment strategy.
Results
As previously reported, baseline characteristics of the study population were similar in both treatment groups (Table S1).12 A baseline history of diabetes (70.1% vs 54.6%; p=0.001), prior MI (27.6% vs 15.1%; p<0.001), and prior PCI (32.3% vs 16.2%; p<0.001) was more common in patients who had an MI during follow-up compared with patients who did not.
Frequency of MI by Type, Definition, and Management Strategy
The frequency of first MI events was greater in the conservative strategy using the primary MI definition and greater in the invasive strategy using the secondary MI definition (Figure 1; Table S2). The difference was related to the greater number of procedural MIs identified with the secondary MI definition: procedural MIs accounted for 9.5% of the total number of MIs during follow-up with the primary MI definition and 27.1% with the secondary MI definition. There were 83 type 1 or 2 MIs and 11 procedural MIs with the primary MI definition and 83 and 39 MIs respectively, with the secondary MI definition (Tables 1, 2). The number of types 1 and 2 MI exceeded the number of procedural MIs with both MI definitions regardless of treatment strategy; the difference was less in the invasive strategy.
Figure 1. Frequency distribution of first myocardial infarction (MI) by type and definition according to treatment strategy.
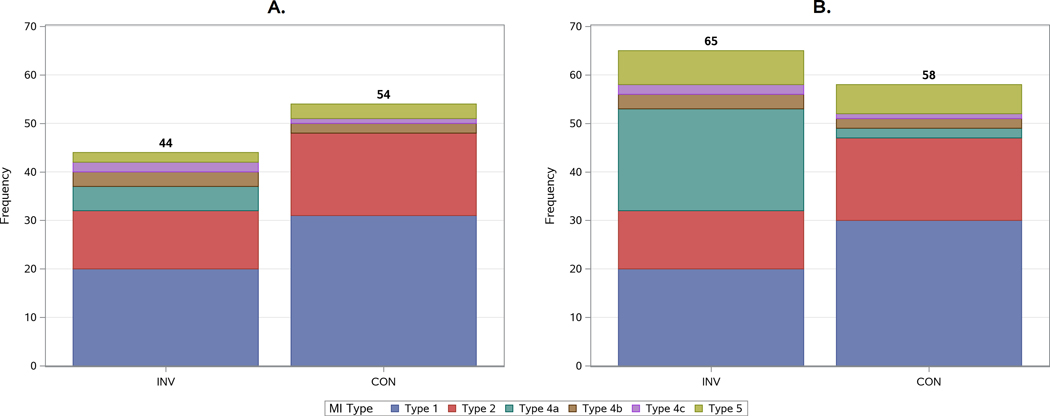
A) Primary MI Definition; B) Secondary MI Definition. Spontaneous type 1 (dark blue) and type 2 (red) MIs were less frequent in the invasive strategy regardless of which MI definition was used. Procedural MIs (type 4a-green; type 5- olive) were more common in the invasive strategy and with the secondary MI definition. Stent related type 4b (stent thrombosis-related and 4c MIs (in-stent restenosis–related) shown in brown/violet were more frequent in the invasive strategy. Silent MIs were infrequent (n=5) and are not shown.
Table 1:
Cumulative Incidence Rates and Hazard Ratios for MI Types: Primary MI Definition
| MI type* | INV (N=388) | CON (N=389) | Adjusted† Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Overall Definition | 0.84 (0.56, 1.24) | 0.372 | ||
| Number of Events | 46 | 56 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 3.39% (1.90%, 5.55%) | 4.12% (2.46%, 6.44%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 3.91% (2.29%, 6.20%) | 5.16% (3.26%, 7.68%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 6.34% (4.18%, 9.10%) | 8.26% (5.79%, 11.28%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 9.90% (7.09%, 13.26%) | 12.55% (9.41%, 16.16%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 15.04% (10.94%, 19.75%) | 15.92% (12.09%, 20.22%) | ||
| Type 1 | 0.64 (0.37, 1.09) | 0.098 | ||
| Number of Events | 22 | 35 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.30% (0.50%, 2.87%) | 3.09% (1.69%, 5.18%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.30% (0.50%, 2.87%) | 3.87% (2.26%, 6.13%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 2.92% (1.55%, 5.00%) | 5.94% (3.88%, 8.60%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 4.73% (2.86%, 7.29%) | 8.03% (5.54%, 11.10%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 7.77% (4.81%, 11.65%) | 8.99% (6.24%, 12.35%) | ||
| Type 2 | 0.64 (0.32, 1.30) | 0.215 | ||
| Number of Events | 13 | 20 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 0.26% (0.03%, 1.38%) | 0.77% (0.22%, 2.12%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.26% (0.03%, 1.38%) | 1.03% (0.35%, 2.48%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.34% (0.51%, 2.95%) | 1.55% (0.65%, 3.19%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 2.81% (1.44%, 4.93%) | 4.57% (2.76%, 7.04%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 3.93% (2.05%, 6.77%) | 6.02% (3.73%, 9.05%) | ||
| Type 1,2 | 0.66 (0.42, 1.02) | 0.061 | ||
| Number of Events | 33 | 50 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.56% (0.65%, 3.22%) | 3.87% (2.26%, 6.13%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.56% (0.65%, 3.22%) | 4.90% (3.05%, 7.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 3.72% (2.13%, 5.99%) | 7.23% (4.93%, 10.10%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 7.00% (4.65%, 9.98%) | 11.13% (8.19%, 14.56%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 11.17% (7.57%, 15.54%) | 13.56% (10.07%, 17.57%) | ||
| Type 4a | 6.95 (0.75, 64.75) | 0.088 | ||
| Number of Events | 5 | 1 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.30% (0.50%, 2.87%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.30% (0.50%, 2.87%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.30% (0.50%, 2.87%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.30% (0.50%, 2.87%) | 0.27% (0.03%, 1.41%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.30% (0.50%, 2.87%) | 0.27% (0.03%, 1.41%) | ||
| Type 4b | 2.97 (0.37, 23.91) | 0.307 | ||
| Number of Events | 3 | 2 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.26% (0.03%, 1.39%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 0.26% (0.03%, 1.39%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 0.26% (0.03%, 1.39%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.30% (0.34%, 3.64%) | 0.52% (0.11%, 1.75%) | ||
| Type 4c | 3.76 (0.38, 36.94) | 0.255 | ||
| Number of Events | 4 | 1 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.52% (0.11%, 1.77%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.07% (0.36%, 2.56%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.07% (0.36%, 2.56%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.07% (0.36%, 2.56%) | 0.43% (0.04%, 2.21%) | ||
| Type 4b/c | 2.32 (0.57, 9.40) | 0.237 | ||
| Number of Events | 6 | 3 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.79% (0.22%, 2.15%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.06% (0.36%, 2.54%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.06% (0.36%, 2.54%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 2.09% (0.82%, 4.45%) | 0.94% (0.25%, 2.64%) | ||
| Type 5 | 0.81 (0.13, 4.96) | 0.816 | ||
| Number of Events | 2 | 3 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 0.52% (0.11%, 1.76%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.52% (0.11%, 1.76%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 0.52% (0.11%, 1.76%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 0.52% (0.11%, 1.76%) | 0.83% (0.23%, 2.28%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 0.52% (0.11%, 1.76%) | 0.83% (0.23%, 2.28%) | ||
| Procedural (Type 4a/5) | 2.04 (0.59, 7.08) | 0.259 | ||
| Number of Events | 7 | 4 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.82% (0.81%, 3.57%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.82% (0.81%, 3.57%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.82% (0.81%, 3.57%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.82% (0.81%, 3.57%) | 1.10% (0.37%, 2.64%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.82% (0.81%, 3.57%) | 1.10% (0.37%, 2.64%) | ||
| Silent/Unrecognized | 0.61 (0.09, 3.86) | 0.595 | ||
| Number of Events | 2 | 3 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 0.56% (0.11%, 1.88%) | 0.64% (0.13%, 2.17%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 0.56% (0.11%, 1.88%) | 1.14% (0.30%, 3.15%) |
Each MI type is analyzed based on the time to the first event for that type. No MI events were Type 3.
Hazard ratios compare INV vs. CON. The covariates used for adjustment include age, sex, eGFR, ejection fraction, diabetes and dialysis. Missing values are imputed through multiple imputation. Continuous variables are modeled as restricted cubic splines.
Table 2:
Cumulative Incidence Rates and Hazard Ratios for MI Types: Secondary MI Definition
| MI Type* | INV (N=388) | CON (N=389) | Adjusted† Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Overall Definition | 1.18 (0.83, 1.68) | 0.345 | ||
| Number of Events | 67 | 60 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 8.58% (6.05%, 11.65%) | 4.64% (2.85%, 7.06%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 9.36% (6.71%, 12.54%) | 5.41% (3.46%, 7.98%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 11.79% (8.79%, 15.26%) | 8.52% (6.01%, 11.57%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 15.33% (11.86%, 19.21%) | 13.59% (10.33%, 17.30%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 20.40% (15.86%, 25.35%) | 16.92% (13.01%, 21.28%) | ||
| Type 1 | 0.64 (0.37, 1.09) | 0.098 | ||
| Number of Events | 22 | 35 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.30% (0.50%, 2.87%) | 3.09% (1.69%, 5.18%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.30% (0.50%, 2.87%) | 3.87% (2.26%, 6.13%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 2.92% (1.55%, 5.00%) | 5.94% (3.88%, 8.60%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 4.73% (2.86%, 7.29%) | 8.03% (5.54%, 11.10%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 7.77% (4.81%, 11.65%) | 8.99% (6.24%, 12.35%) | ||
| Type 2 | 0.64 (0.32, 1.30) | 0.215 | ||
| Number of Events | 13 | 20 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 0.26% (0.03%, 1.38%) | 0.77% (0.22%, 2.12%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.26% (0.03%, 1.38%) | 1.03% (0.35%, 2.48%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.34% (0.51%, 2.95%) | 1.55% (0.65%, 3.19%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 2.81% (1.44%, 4.93%) | 4.57% (2.76%, 7.04%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 3.93% (2.05%, 6.77%) | 6.02% (3.73%, 9.05%) | ||
| Type 1,2 | 0.66 (0.42, 1.02) | 0.061 | ||
| Number of Events | 33 | 50 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.56% (0.65%, 3.22%) | 3.87% (2.26%, 6.13%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.56% (0.65%, 3.22%) | 4.90% (3.05%, 7.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 3.72% (2.13%, 5.99%) | 7.23% (4.93%, 10.10%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 7.00% (4.65%, 9.98%) | 11.13% (8.19%, 14.56%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 11.17% (7.57%, 15.54%) | 13.56% (10.07%, 17.57%) | ||
| Type 4a | 4.82 (1.81, 12.86) | 0.002 | ||
| Number of Events | 21 | 5 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 5.45% (3.48%, 8.04%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 5.45% (3.48%, 8.04%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 5.45% (3.48%, 8.04%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 5.45% (3.48%, 8.04%) | 1.04% (0.35%, 2.52%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 5.45% (3.48%, 8.04%) | 1.51% (0.55%, 3.40%) | ||
| Type 4b | 2.97 (0.37, 23.91) | 0.307 | ||
| Number of Events | 3 | 2 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.26% (0.03%, 1.39%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 0.26% (0.03%, 1.39%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 0.26% (0.03%, 1.39%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.30% (0.34%, 3.64%) | 0.52% (0.11%, 1.75%) | ||
| Type 4c | 3.76 (0.38, 36.94) | 0.255 | ||
| Number of Events | 4 | 1 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.52% (0.11%, 1.77%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.07% (0.36%, 2.56%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.07% (0.36%, 2.56%) | --- | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.07% (0.36%, 2.56%) | 0.43% (0.04%, 2.21%) | ||
| Type 4b/c | 2.32 (0.57, 9.40) | 0.237 | ||
| Number of Events | 6 | 3 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 0.79% (0.22%, 2.15%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.06% (0.36%, 2.54%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.06% (0.36%, 2.54%) | 0.52% (0.11%, 1.75%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 2.09% (0.82%, 4.45%) | 0.94% (0.25%, 2.64%) | ||
| Type 5 | 1.31 (0.43, 3.93) | 0.633 | ||
| Number of Events | 7 | 6 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 1.56% (0.65%, 3.22%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 1.82% (0.81%, 3.57%) | 0.26% (0.03%, 1.37%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 1.82% (0.81%, 3.57%) | 0.78% (0.22%, 2.12%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 1.82% (0.81%, 3.57%) | 1.61% (0.67%, 3.32%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 1.82% (0.81%, 3.57%) | 1.61% (0.67%, 3.32%) | ||
| Procedural (Type 4a/5) | 2.86 (1.42, 5.78) | 0.003 | ||
| Number of Events | 28 | 11 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | 7.02% (4.75%, 9.86%) | 0.52% (0.11%, 1.74%) | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | 7.28% (4.96%, 10.16%) | 0.52% (0.11%, 1.74%) | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | 7.28% (4.96%, 10.16%) | 1.29% (0.49%, 2.85%) | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 7.28% (4.96%, 10.16%) | 2.65% (1.36%, 4.67%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 7.28% (4.96%, 10.16%) | 3.11% (1.63%, 5.38%) | ||
| Silent/Unrecognized | 0.61 (0.09, 3.86) | 0.595 | ||
| Number of Events | 2 | 3 | ||
| Cumulative Incidence Rate (95% CI) at 3 Months | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 6 Months | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 1 Year | --- | --- | ||
| Cumulative Incidence Rate (95% CI) at 2 Years | 0.56% (0.11%, 1.88%) | 0.64% (0.13%, 2.17%) | ||
| Cumulative Incidence Rate (95% CI) at 3 Years | 0.56% (0.11%, 1.88%) | 1.14% (0.30%, 3.15%) |
Each MI type is analyzed based on the time to the first event for that type. No MI events were Type 3.
Hazard ratios compare INV vs. CON. The covariates used for adjustment include age, sex, eGFR, ejection fraction, diabetes and dialysis. Missing values are imputed through multiple imputation. Continuous variables are modeled as restricted cubic splines.
Using the primary MI definition, the 3-year cumulative incidence of type 1 or 2 MIs was 11.2% with the invasive strategy and 13.6% with the conservative strategy (cumulative difference −2.39 [−7.88, 3.10]; the adjusted HR was 0.64 [0.41, 1.01]. The results were similar using the secondary MI definition. There was no statistical evidence of a reduction in the primary or major secondary composite endpoint (all-cause death, MI, resuscitated cardiac arrest, or hospitalization for unstable angina or heart failure) with the invasive strategy regardless of MI definition. (Figure 2).
Figure 2. Cumulative incidence of primary and secondary outcomes by randomized treatment group and by MI definition.
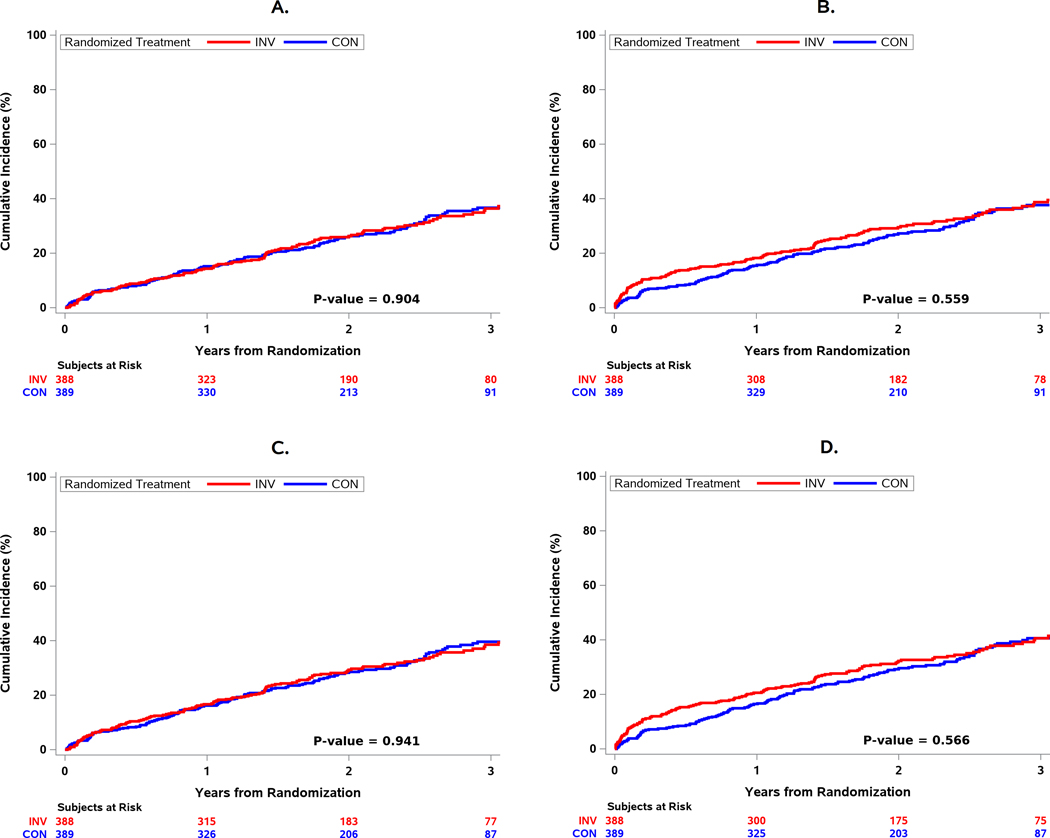
A) All-cause Death or MI (Primary MI Definition). B) All-cause Death or MI (Secondary MI Definition). C) All-cause Death, MI, or Hospitalization for UA, HF, or RCA (Primary MI Definition). D) All-cause Death, MI, or Hospitalization for UA, HF, or RCA (Secondary MI Definition). There were no treatment differences observed using the primary MI definition (Panel A) or secondary MI definition (Panel B). The results were similar for the major secondary composite endpoint of death, nonfatal myocardial infarction (MI), hospitalization for unstable angina (UA), heart failure (HF), or resuscitated cardiac arrest (RCA) (Panels C, D).
Prognostic Significance of MI Type
Type 1 MI
The cumulative incidence of all-cause death, cardiovascular death, and cardiovascular death or hospitalization for heart failure was significantly increased in patients with a type 1 MI compared with patients without an MI (Figures 3,4). During follow-up, 27.5% of patients with a type 1 MI died within 30 days of the event. Initiation of dialysis was increased after a type 1 MI (HR 6.45 [2.59, 16.08]). Compared with patients without an MI during follow-up, those with MI types 1 and 2 experienced significantly greater risk of all-cause death, cardiovascular death, and the composite of cardiovascular death or admission for heart failure (Figure 4). The risk of death was higher within 90 days following an MI as compared with 90 days or more after an MI.
Figure 3. MI type and relationship with subsequent all-cause death.
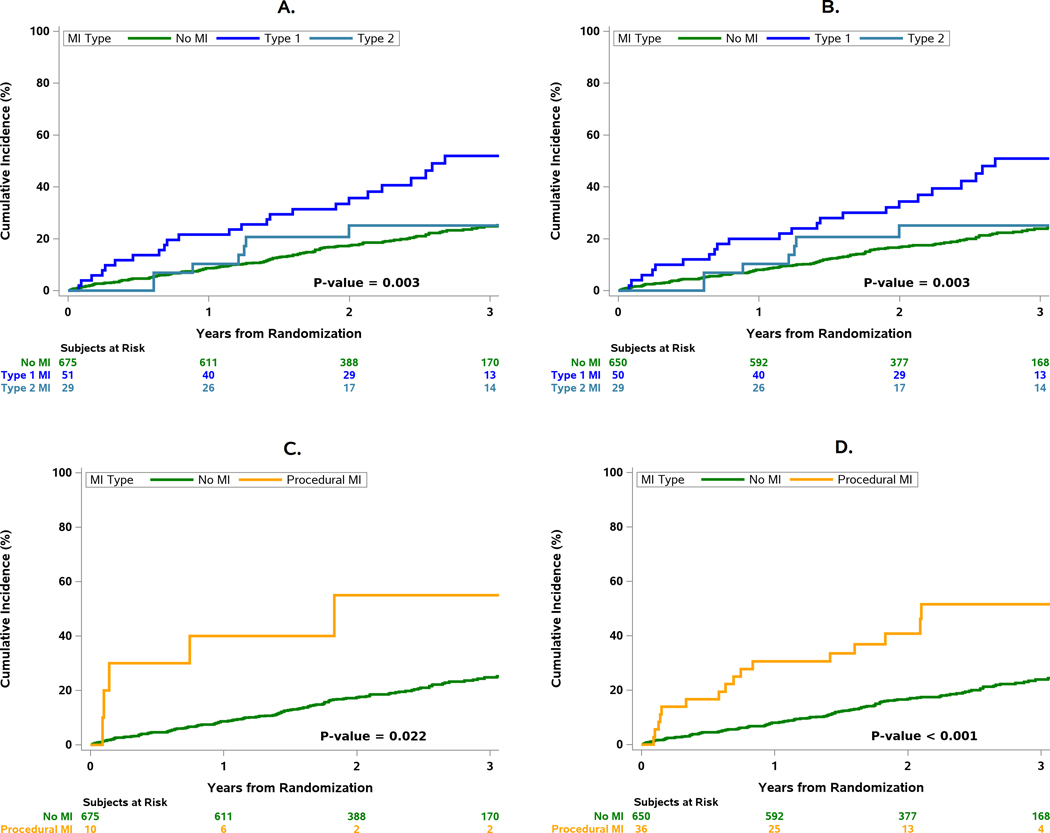
A) Cumulative incidence of subsequent all-cause death after types 1,2 MI and no MI for the primary MI definition; B) Cumulative incidence of subsequent all-cause death after types 1,2 MI and no MI for the secondary MI definition; C) All-cause Death (Primary MI Definition); D) All-cause Death (Secondary MI Definition). Procedural MIs were associated with an increased risk of death compared with those without an MI during follow-up regardless of MI definition.
Figure 4. Forest Plots for Death/Hospitalization for Heart Failure.
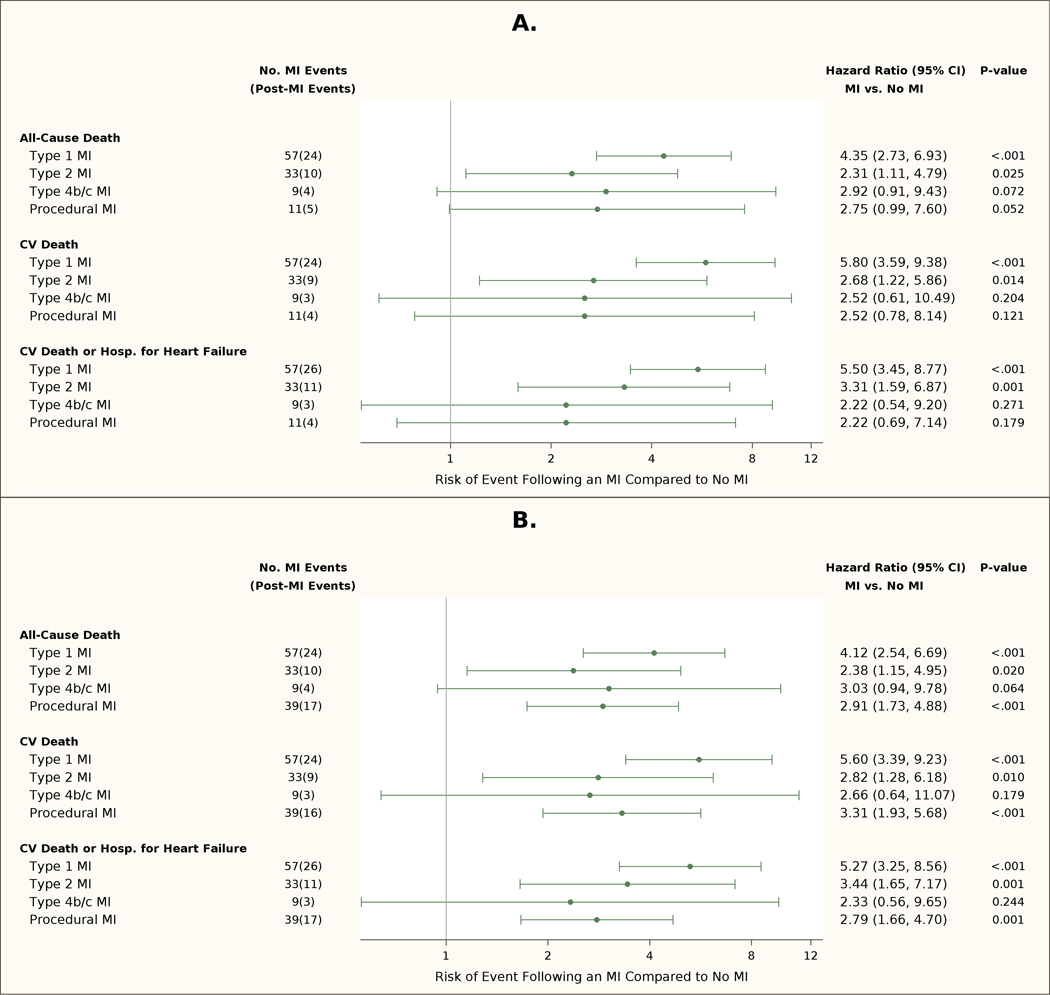
CV=cardiovascular; MI=myocardial infarction. Multivariate adjusted risks of MI on subsequent all-cause death, cardiovascular death, or cardiovascular death or heart failure admission, according to MI definition. A) Prognosis of MI Types in ISCHEMIA CKD (Primary MI Definition); B) Prognosis of MI Types in ISCHEMIA CKD (Secondary MI Definition). Total number of MI events and subsequent deaths are shown in the second column. The adjusted risk of subsequent all-cause death, cardiovascular death, or cardiovascular death or admission for heart failure was increased for patients that had a type 1 MI and type 2 MI and no procedural MI compared with patients that had no MI during follow-up. The risk for all 3 endpoints was increased for patients with a procedural MI (p=0.001) using the secondary MI definition. There were a limited number of procedural MIs using the primary MI definition.
Procedural MI
Procedural MIs were also associated with an increased risk of all-cause death, cardiovascular death, and the composite of cardiovascular death or hospitalization for heart failure. (Figures 3,4). The HR for subsequent all-cause and cardiovascular death was 2.75 [0.99, 7.60] and 2.52 [0.78, 8.14] for the primary MI definition, and 2.91 [1.73, 4.88] and 3.31 [1.93, 5.68] for the secondary MI definition. The relationship between preprocedural biomarkers and death is shown in Tables S3 and S4. After adjustment for elevated pre-procedure or missing biomarkers, the risks of all-cause and cardiovascular death after procedural MI remained significant with the secondary MI definition.
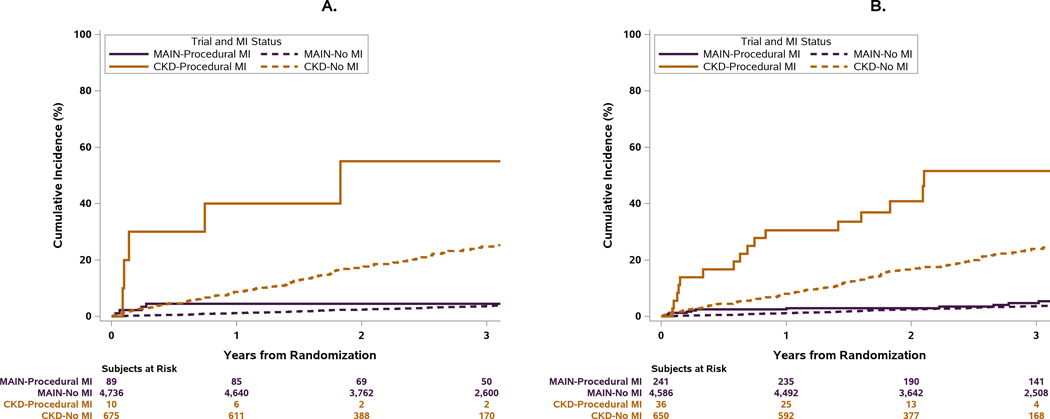
Cumulative Incidence of Death after Type 1 and Procedural MIs in the ISCHEMIA-CKD and ISCHEMIA trials
The cumulative incidence of all-cause death was greater in patients that had a type 1 MI compared with those that did not during follow-up in both trials regardless of MI definition used. The cumulative incidence of all-cause death was greater among patients that had a procedural MI, regardless of definition used compared with those that did not during follow-up in the ISCHEMIA-CKD trial. The incidence of all-cause death was similar among patients that had a procedural MI compared with those that did not during follow-up in the lower risk population enrolled in the ISCHEMIA trial.
Discussion
In this prespecified analysis from the ISCHEMIA-CKD trial, procedural MIs were more frequent with the invasive strategy, particularly with the secondary MI definition, and were associated with higher death rates compared with no MI during follow-up. There were fewer type 1 and 2 MIs with the invasive compared with the conservative strategy. Type 1 and 2 MIs were associated with increased all-cause death, cardiovascular death, and the composite of cardiovascular death or heart failure and for type 1 MIs, new initiation of dialysis compared with not having an MI during follow-up.
Impact of MI Type on Cardiovascular and Renal Outcomes
Type 1 MI increased the risk of cardiovascular death or heart failure hospitalization and the need for new dialysis confirming earlier observations of the adverse impact of an MI on cardiovascular and renal function.6 Overall survival in dialysis patients has improved in recent years, and survival after an acute MI has improved.1, 5 Although the death rate observed after spontaneous MI (type 1) at 1-year follow-up in ISCHEMIA-CKD was high, it was lower than earlier studies that did not report MI by type.2–4
Procedural MI
More procedural MIs in ISCHEMIA-CKD were identified using the secondary MI definition with the majority having ancillary evidence of myocardial ischemia. Procedural MIs were associated with an increased risk of all-cause death, cardiovascular death and the endpoint of cardiovascular death or heart failure compared with patients without an MI during follow-up. We observed particularly high frequencies of death in patients with elevated biomarkers pre-procedure similar to other reports.18 The definition of procedural MI used in the ISCHEMIA-CKD trial differs from the Third and Fourth UDMI by inclusion of biomarker-only criteria with markedly increased thresholds.8, 9 The threshold used in the ISCHEMIA and ISCHEMIA-CKD trials for biomarker-only criteria is substantially greater than the 5-fold threshold recommended by the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions (EAPCI) that define important acute myocardial injury associated with increased death regardless of presence or absence of overt myocardial ischemia.19 The use of a much higher biomarker threshold without overt evidence of myocardial ischemia used in ISCHEMIA and ISCHEMIA-CKD would be associated with even greater specificity for the diagnosis of severe acute myocardial injury, an adverse outcome that occurred more frequently in the invasive strategy.
The frequency of procedural MI and subsequent death in a general population of patients with chronic coronary disease undergoing a revascularization procedure can vary depending on the definition of MI used.19–24 Two recent studies of patients undergoing PCI or CABG using the UDMI, Academic Research Consortium-2 (ARC), and Society for Cardiovascular Angiography and Interventions (SCAI) showed more procedural MIs were detected using the UDMI definition compared with the ARC-2 or SCAI definition.23, 24 All three definitions were associated with increased death rates although death tended to be greater with the ARC-2 or SCAI definitions in patients undergoing PCI and similar in patients undergoing CABG.23, 24 Patients with advanced CKD are often underrepresented in studies of this type and additional research on different MI definitions and their subsequent impact on death and cardiovascular death are needed.
In the ISCHEMIA-CKD trial, the CEC prospectively classified all procedural MIs using the third UDMI definition with the added category of markedly elevated biomarkers. The primary MI definition for procedural MIs used CK-MB as the preferred biomarker and the secondary MI definition used cardiac troponin as the preferred biomarker for classification. The intent of having the CEC prospectively classify all procedural MIs using two different MI definitions was to allow an analysis of the primary trial endpoint (death or MI) with two commonly used procedural MI definitions at the time the trial was designed. The outcome showed that regardless of the MI definition used, the overall conclusions of the trial were similar (Figure 2).
MI Frequency and Prognosis in the ISCHEMIA-CKD and ISCHEMIA Trials
Spontaneous type 1 MIs were the most frequent type of MIs in the ISCHEMIA and ISCHEMIA-CKD trials with the primary MI definition. In ISCHEMIA-CKD, they were associated with a 2-fold increase in the rate of death compared with the rates observed in the ISCHEMIA trial using the same definitions and the same CEC (Table 3).15, 16 While both trials showed that type 1 MI were associated with subsequent death, CV death and CV death or HF hospitalization, the absolute rate of death was far higher in ISCHEMIA CKD. The higher risk nature of the ISCHEMIA-CKD cohort is illustrated by the fact that the cumulative incidence of death in patients after a type 1 MI or a procedural MI in ISCHEMIA-CKD exceeded that observed in the ISCHEMIA patients that had a type 1 MI or procedural MI In the ISCHEMIA-CKD population, procedural MI by both definitions was associated with increased risk of death or CV death, whereas in the ISCHEMIA trial, the cumulative risk of all-cause death after a procedural MI was similar to that of patients without an MI during follow-up (Figure 5a,5b).
Table 3:
Hazard Ratios for Death in patients with Type 1 and Procedural MI Compared to No MI in the ISCHEMIA-CKD and ISCHEMIA Trials
| Type of MI | ||||
|---|---|---|---|---|
|
| ||||
| Type 1 MI | Procedural MI | |||
|
| ||||
| ISCHEMIA | ISCHEMIA-CKD | ISCHEMIA | ISCHEMIA-CKD | |
| Primary MI Definition | ||||
| All-cause Death | 2.44 (1.54, 3.88) | 4.35 (2.73, 6.93) | 1.14 (0.43, 3.08) | 2.75 (0.99, 7.60) |
| Cardiovascular Death | 3.38 (2.03, 5.61) | 5.80 (3.59, 9.38) | 1.99 (0.73, 5.43) | 2.52 (0.78, 8.14) |
| Secondary MI Definition | ||||
| All-cause Death | 2.55 (1.60, 4.06) | 4.12 (2.54, 6.69) | 1.06 (0.56, 2.02) | 2.91 (1.73, 4.88) |
| Cardiovascular Death | 3.52 (2.11, 5.88) | 5.60 (3.39, 9.23) | 1.24 (0.57, 2.68) | 3.31 (1.93, 5.68) |
Figure 5. Relationship between Type 1 MI and Procedural MI vs No MI and all-cause death in the ISCHEMIA and ISCHEMIA-CKD trials.
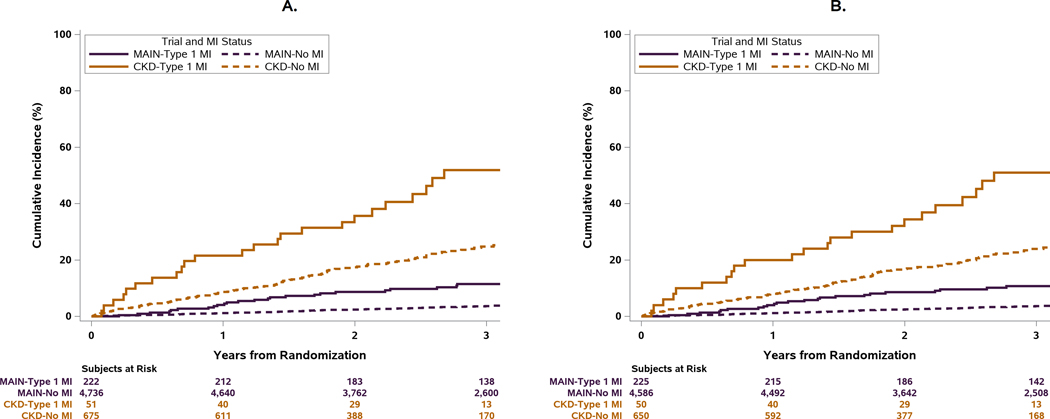
Main refers to the ISCHEMIA trial. CKD refers to the ISCHEMIA-CKD trial. A) All-cause Death (Primary MI Definition); B) All-cause Death (Secondary MI Definition). Top Panel: The cumulative incidence of all-cause death was greater in patients that had a type 1 MI compared with those that did not during follow-up in both trials regardless of MI definition used. The higher risk population in the ISCHEMIA-CKD trial is illustrated by the fact that the cumulative incidence of death in patients without an MI during follow-up exceeded that observed in the ISCHEMIA population with a type 1 MI.
Bottom Panel: The cumulative incidence of all-cause death after procedural MI was greater compared with those that did not have an MI during follow-up, regardless of MI definition used. In contrast, in the ISCHEMIA trial which enrolled a lower-risk population, the cumulative incidence of all-cause death after procedural MI was similar compared with those that did not have an MI during follow-up, regardless of MI definition used.
Limitations
The diagnosis of MI in the setting of advanced CKD is often challenging since many patients have chronic myocardial injury and diminished renal clearance of elevated cardiac troponins. The Third UDMI that was used in the ISCHEMIA trials requires a rising and/or falling pattern of cardiac troponin accompanied by ischemic symptoms, new ischemic ECG changes, or loss of viable myocardium on imaging, thus increasing the likelihood of an MI diagnosis.8 Acute myocardial injury patterns can also occur with acute heart failure exacerbations. For example, in the patients that had a type 2 MI in ISCHEMIA-CKD, the initial cTn value exceeded the 99th percentile URL in 90% of patients and 40% of the patients had heart failure on admission. Baseline ECGs were abnormal in 1/3 of the ISCHEMIA-CKD population. The diagnosis of heart failure in advanced CKD can also be difficult as can distinguishing volume overload from an acute heart failure episode.
Although ISCHEMIA-CKD is the largest trial of its type in advanced CKD, the number of events for some MI types was relatively small. Our results do not apply to patients who were excluded from participation in the ISCHEMIA-CKD trial such as those with left main coronary artery disease (CAD), LVEF <35%, or NYHA Class 3 or 4 heart failure.
Conclusions
An invasive strategy in patients with advanced CKD and moderate or severe myocardial ischemia did not reduce the risk of death or MI over a median 2.2 years of follow-up regardless of the definition of MI. Procedural MIs were more frequent with the invasive strategy, particularly with the secondary MI definition, and were associated with higher death rates. There were fewer type 1 and 2 MIs with the invasive compared with the conservative strategy which were associated with increased death, cardiovascular death or heart failure hospitalization and new dialysis initiation compared with no MI during follow-up.
Supplementary Material
Clinical Perspective.
WHAT IS KNOWN
In the ISCHEMIA-CKD trial, an initial invasive strategy in patients with stable coronary disease, moderate or severe ischemia, and advanced CKD, did not reduce the risk of death or nonfatal myocardial infarction (MI) compared with an initial conservative strategy.
WHAT THE STUDY ADDS
The most frequent type of MI after a median 2.2 year follow-up in patients with advanced CKD are type 1 and 2 MIs regardless of initial treatment strategy
Patients with advanced CKD randomized to an invasive strategy had fewer type 1 and 2 MIs but more procedural MIs, particularly with the secondary MI definition, compared with those randomized to a conservative strategy.
Both spontaneous and procedural MIs were associated with significant increase in subsequent all-cause death, cardiovascular death and composite of cardiovascular death or heart failure hospitalization.
Type 1 MI increased the risk of dialysis initiation
Sources of Funding:
NIH Grants U01HL117904 and U01HL1179050.
Other Support:
This project was supported by grants from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck Sharp & Dohme Corp. and Sunovion Pharmaceuticals
Disclosures
Dr. Bernard R. Chaitman reports grants from National Heart, Lung, and Blood Institute during the conduct of the study, personal fees from Merck, NovoNordisk, Sanofi, Lilly,Johnson and Johnson,Daiichi Sankyo,Tricida, Relypsa, Imbria, and Xylocor outside the submitted work.
Dennis F. Kunichoff reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Karen P. Alexander reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Radoslaw Pracoń reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Kevin R. Bainey reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Anoop Mathew reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Anjali Acharya reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Derek Cyr reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Jerome L. Fleg reports no conflict of interest
Dr. Renato D. Lopes received funding from Bayer; Boehringer Ingleheim ; Bristol-Myers Squibb; Daiichi Sankyo; Glaxo Smith Kline; Medtronic; Merck; Pfizer; Portola; Sanofi.
Dr. Mandeep S. Sidhu reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; personal fees from Astra Zeneca, personal fees from Sanofi-Regeneron, outside the submitted work.
Dr. Rebecca Anthopolos grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Frank W. Rockhold reports grants from the National Institutes of Health during the conduct of the study; grants and personal fees from Janssen; personal fees from Merck HeathCare KGaA, Merck Research Laboratories, Novo Nordisk, Kuala Lumpur Sports Medicine Centre, Aldeyra, and Rhythm; grants and personal fees from AstraZeneca; personal fees from Complexa; grants and personal fees from Eidos; other from Athira, Spencer Healthcare, and GlaxoSmithKline; and personal fees from Phathom, outside the submitted work.
Dr. Gregg Stone has received speaker or other honoraria from Cook and Terumo; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme, Cardiomech, Elucid Bio, Occlutech; and has Equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, Valfix.
Dr. David J. Maron reports support from National Heart, Lung, and Blood Institute during the conduct of the study.
Judith S. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Medtronic, Inc.; Abbott Vascular, Inc (formerly St. Jude Medical, Inc).; Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc; Espero BioPharma; and Amgen Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Dr. Sripal Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from Biotronik, Pfizer, Amgen, and Reata outside the submitted work.
Nonstandard Abbreviations and Acronyms
- CKD
Chronic kidney disease
- CON
Conservative treatment strategy
- cTn
Cardiac troponin
- CAD
Coronary artery disease
- INV
Invasive treatment strategy
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- UDMI
Universal Definition of Myocardial Infarction
Footnotes
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
TRIAL REGISTRATION ClinicalTrials.gov Identifier: NCT01985360
References
- 1.Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, Gill JS, Hlatky MA, Jardine AG, Landmesser U, Newby LK, Herzog CA, Cheung M, Wheeler DC, Winkelmayer WC, Marwick TH and Conference P. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1823–1838. [DOI] [PubMed] [Google Scholar]
- 2.Herzog CA, Ma JZ and Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Littrell K, Arko C, Frederick PD and Blaney M. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: a collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation. 2007;116:1465–72. [DOI] [PubMed] [Google Scholar]
- 4.Szummer K, Lindhagen L, Evans M, Spaak J, Koul S, Akerblom A, Carrero JJ and Jernberg T. Treatments and Mortality Trends in Cases With and Without Dialysis Who Have an Acute Myocardial Infarction: An 18-Year Nationwide Experience. Circ Cardiovasc Qual Outcomes. 2019;12:e005879. [DOI] [PubMed] [Google Scholar]
- 5.Modi ZJ, Lu Y, Ji N, Kapke A, Selewski DT, Dietrich X, Abbott K, Nallamothu BK, Schaubel DE, Saran R and Gipson DS. Risk of Cardiovascular Disease and Mortality in Young Adults With End-stage Renal Disease: An Analysis of the US Renal Data System. JAMA Cardiol. 2019;4:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal N, Zelnick L, Bhat Z, Dobre M, He J, Lash J, Jaar B, Mehta R, Raj D, Rincon-Choles H, Saunders M, Schrauben S, Weir M, Wright J, Go AS and Investigators CS. Burden and Outcomes of Heart Failure Hospitalizations in Adults With Chronic Kidney Disease. J Am Coll Cardiol. 2019;73:2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shroff GR, Frederick PD and Herzog CA. Renal failure and acute myocardial infarction: clinical characteristics in patients with advanced chronic kidney disease, on dialysis, and without chronic kidney disease. A collaborative project of the United States Renal Data System/National Institutes of Health and the National Registry of Myocardial Infarction. Am Heart J. 2012;163:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force Members C, Thygesen K, Alpert JS, White HD, Biomarker S, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Subcommittee ECG, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging S, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification S, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Intervention S, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Trials, Registries S, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Trials, Registries S, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Trials, Registries S, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Trials, Registries S, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Guidelines ESCCfP, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document R, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P and Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98.22958960 [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD and Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial I. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 10.Chaitman BR, Reynolds HR, Maron DJ and Hochman JS. Response by Chaitman et al to Letter Regarding Article, “Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons”. Circulation. 2021;144:e14–e15. [DOI] [PubMed] [Google Scholar]
- 11.Bangalore S, Maron DJ, Fleg JL, O’Brien SM, Herzog CA, Stone GW, Mark DB, Spertus JA, Alexander KP, Sidhu MS, Chertow GM, Boden WE, Hochman JS and Group I-CR. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD): Rationale and design. Am Heart J. 2018;205:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, Berger JS, Mathew RO, Bockeria O, Broderick S, Pracon R, Herzog CA, Huang Z, Stone GW, Boden WE, Newman JD, Ali ZA, Mark DB, Spertus JA, Alexander KP, Chaitman BR, Chertow GM, Hochman JS and Group I-CR. Management of Coronary Disease in Patients with Advanced Kidney Disease. N Engl J Med. 2020;382:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog CA, Simegn MA, Xu Y, Costa SP, Mathew RO, El-Hajjar MC, Gulati S, Maldonado RA, Daugas E, Madero M, Fleg JL, Anthopolos R, Stone GW, Sidhu MS, Maron DJ, Hochman JS and Bangalore S. Kidney Transplant List Status and Outcomes in the ISCHEMIA-CKD Trial. J Am Coll Cardiol. 2021;78:348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briguori C, Mavromatis K, Huang Z, Mathew R, Hickson L, Lau WL, Ye Z, Mathew A, Mahajan S, Wheeler D, Claes K, Chen G, Nolasco F, Fleg J, Sidhu M, Chertow G, Hochman J, Maron D and Bangalore S. DIALYSIS INITIATION IN PATIENTS WITH STABLE ISCHEMIC HEART DISEASE AND SEVERE CHRONIC KIDNEY DISEASE IN THE ISCHEMIA-CKD TRIAL. J Am Coll Cardiol. 2021;77:10-10. [Google Scholar]
- 15.Chaitman BR, Alexander KP, Cyr DD, Berger JS, Reynolds HR, Bangalore S, Boden WE, Lopes RD, Demkow M, Piero Perna G, Riezebos RK, McFalls EO, Banerjee S, Bagai A, Gosselin G, O’Brien SM, Rockhold FW, Waters DD, Thygesen KA, Stone GW, White HD, Maron DJ, Hochman JS and Group IR. Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons. Circulation. 2021;143:790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini GBJ, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE Jr., Rockhold FW, Broderick S, Ferguson TB Jr., Williams DO, Harrington RA, Stone GW, Rosenberg Y and Group IR. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray RJ. A Class of $K$-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 18.Koskinas KC, Ndrepepa G, Raber L, Karagiannis A, Kufner S, Zanchin T, Hieber J, Hunziker L, Mayer K, Byrne RA, Heg D, Windecker S and Kastrati A. Prognostic Impact of Periprocedural Myocardial Infarction in Patients Undergoing Elective Percutaneous Coronary Interventions. Circ Cardiovasc Interv. 2018;11:e006752. [DOI] [PubMed] [Google Scholar]
- 19.Bulluck H, Paradies V, Barbato E, Baumbach A, Botker HE, Capodanno D, De Caterina R, Cavallini C, Davidson SM, Feldman DN, Ferdinandy P, Gili S, Gyongyosi M, Kunadian V, Ooi SY, Madonna R, Marber M, Mehran R, Ndrepepa G, Perrino C, Schupke S, Silvain J, Sluijter JPG, Tarantini G, Toth GG, Van Laake LW, von Birgelen C, Zeitouni M, Jaffe AS, Thygesen K and Hausenloy DJ. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: a Consensus Document of the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2021;42:2630–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregson J, Stone GW, Ben-Yehuda O, Redfors B, Kandzari DE, Morice MC, Leon MB, Kosmidou I, Lembo NJ, Brown WM 3rd, Karmpaliotis D, Banning AP, Pomar J, Sabate M, Simonton CA, Dressler O, Kappetein AP, Sabik JF 3rd, Serruys PW and Pocock SJ. Implications of Alternative Definitions of Peri-Procedural Myocardial Infarction After Coronary Revascularization. J Am Coll Cardiol. 2020;76:1609–1621. [DOI] [PubMed] [Google Scholar]
- 21.Silvain J, Zeitouni M, Paradies V, Zheng HL, Ndrepepa G, Cavallini C, Feldman DN, Sharma SK, Mehilli J, Gili S, Barbato E, Tarantini G, Ooi SY, von Birgelen C, Jaffe AS, Thygesen K, Montalescot G, Bulluck H and Hausenloy DJ. Procedural myocardial injury, infarction and mortality in patients undergoing elective PCI: a pooled analysis of patient-level data. Eur Heart J. 2021;42:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E and Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polzl L, Thielmann M, Cymorek S, Nagele F, Hirsch J, Graber M, Sappler N, Eder J, Staggl S, Theurl F, Abfalterer H, Reinstadler SJ, Holfeld J, Griesmacher A, Ulmer H, Grimm M, Bauer A, Ruttmann-Ulmer E, Ruhparwar A, Bonaros N and Gollmann-Tepekoylu C. Impact of myocardial injury after coronary artery bypass grafting on long-term prognosis. Eur Heart J. 2022;43:2407–2417. [DOI] [PubMed] [Google Scholar]
- 24.Ueki Y, Otsuka T, Bar S, Koskinas KC, Heg D, Haner J, Siontis GCM, Praz F, Hunziker L, Lanz J, Stortecky S, Pilgrim T, Losdat S, Windecker S and Raber L. Frequency and Outcomes of Periprocedural MI in Patients With Chronic Coronary Syndromes Undergoing PCI. J Am Coll Cardiol. 2022;79:513–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


