Abstract
Mice with targeted deletion of the gene for interleukin-10 (IL-10) spontaneously develop enterocolitis when maintained in conventional conditions but develop only colitis when kept in specific-pathogen-free (SPF) environments. This study tested the hypothesis that enteric bacteria are necessary for the development of spontaneous colitis and immune system activation in IL-10-deficient mice. IL-10-deficient mice were maintained in either SPF conditions or germfree conditions or were populated with bacteria known to cause colitis in other rodent models. IL-10-deficient mice kept in SPF conditions developed colitis in all segments of the colon (cecum and proximal and distal colon). These mice exhibited immune system activation as evidenced by increased expression of CD44 on CD4+ T cells; increased mesenteric lymph node cell numbers; and increased production of immunoglobulin A (IgA), IgG1, and IL-12 p40 from colon fragment cultures. Mice populated with bacterial strains, including Bacteroides vulgatus, known to induce colitis in other rodent models had minimal colitis. Germfree IL-10-deficient mice had no evidence of colitis or immune system activation. We conclude therefore that resident enteric bacteria are necessary for the development of spontaneous colitis and immune system activation in IL-10-deficient mice.
Ulcerative colitis and Crohn’s disease, collectively known as inflammatory bowel diseases (IBD) in people, are chronic immune-mediated diseases of the intestinal tract with unknown etiologies. Various pathogenic mechanisms have been proposed, including an appropriate inflammatory response to a luminal pathogen or abnormal luminal constituent, autoimmunity, or an abnormal immune response to a normal luminal constituent such as ubiquitous intestinal bacterial or dietary antigens (43). The hypothesis that aberrant immune responses to nonpathogenic luminal bacteria can cause colitis is supported by clinical observations that decreasing intestinal bacterial concentrations by various techniques can lead to clinical improvement and decreased intestinal inflammation (42). The role of normal resident bacterial flora in the development of chronic intestinal inflammation has been further demonstrated in several rodent models of experimental colitis, both induced and spontaneous. For example, HLA-B27 transgenic rats raised under specific-pathogen-free (SPF) conditions spontaneously develop colitis, gastritis, and arthritis, whereas transgenic rats do not develop these lesions when maintained under germfree conditions (39, 50). Similarly, T-cell receptor-α knockout mice fail to develop colitis in the absence of normal bacteria (16). Colitis spontaneously develops in interleukin-2 (IL-2)-deficient mice under conventional housing conditions but is greatly attenuated in germfree conditions (12, 41, 45). Severe combined immunodeficient mice repopulated with CD4+CD45 RBhi T cells spontaneously develop a wasting syndrome and colitis, but when these T-cell-repopulated mice are treated with antibiotics, the wasting syndrome improves or resolves, presumably in conjunction with improvement in intestinal inflammation (35). Finally, Lewis rats treated with indomethacin and antibiotics develop less severe inflammation than rats treated with indomethacin alone (5, 55). All of these observations are consistent with intestinal bacteria having a role in either the initiation or perpetuation of chronic intestinal inflammation. Reconstitution studies of HLA-B27 transgenic rats and carrageenan-fed guinea pigs demonstrated that Bacteroides vulgatus was particularly important to the induction of colitis in these models (38, 39). Defined bacterial products may also have an important role in chronic enterocolitis, as evidenced by the chronic granulomatous inflammation that develops in response to subserosal injection of sterile peptidoglycan-polysaccharide complexes in susceptible Lewis rats (32).
IL-10 plays a critical role in the shaping of immune responses. Produced by a variety of cell types, but principally by activated macrophages and Th2 T cells, IL-10 generally promotes the development of humoral, Th2 cytokine-driven immune responses (reviewed in reference 34). Importantly, IL-10 inhibits the development of Th1 immune responses (19, 20), primarily by reducing the capacity of macrophages to produce IL-12, a potent inducer of Th1 immune responses (6, 7, 14, 47, 48). IL-10 has been proposed to exert a regulatory effect in intestinal mucosa (36, 44). The importance of IL-10 in shaping mucosal immune responses has been elegantly demonstrated by the spontaneous onset of inflammation in the IL-10-deficient mouse (27). IL-10-deficient mice spontaneously develop enterocolitis when housed in conventional environments, but when housed in SPF conditions IL-10-deficient mice develop inflammation limited to the colon, suggesting that resident enteric flora play a role in the development of spontaneous colitis in these mice. We addressed the hypothesis that spontaneous colitis in IL-10-deficient mice requires the presence of normal enteric bacterial flora and that various genetically engineered colitis models have a similar profile of dominant bacterial strains which preferentially induce inflammation.
MATERIALS AND METHODS
Mice.
Mice from a C57BL/6 × 129 Ola background, including wild-type and heterozygous mice and mice with a targeted deletion of the IL-10 gene, were generously provided from the breeding colony at DNAX, Palo Alto, Calif. (3, 27). The genotype of the mice was confirmed both before and after sacrifice by analysis of tail tip digests by PCR (27).
Intestinal bacterial population of mice.
Germfree (sterile) mice (heterozygous and IL-10 deficient) were Caesarian derived by one of the investigators (E.B.) and were maintained according to standard techniques (30) in Trexler flexible film isolators at the Gnotobiotic Animal Facility of the Center for Gastrointestinal Biology and Disease located at the Laboratory Animal Resources facility of the North Carolina State University College of Veterinary Medicine, Raleigh. SPF mice were maintained in a dedicated room at the North Carolina State University College of Veterinary Medicine or the University of North Carolina Laboratory Animal Resources facility. The germfree status was monitored every 2 weeks by aerobic and anaerobic culture and gram stain of stool samples and/or bedding material.
For some studies reported here, adult mice were transferred from the germfree environment to either an SPF environment or to Trexler plastic isolators containing animals populated with either six bacterial species (Bacteroides vulgatus, Streptococcus faecium [Group D], Escherichia coli, Peptostreptococcus productus, Eubacterium contortum, and Streptococcus avium) isolated from a guinea pig with carrageenan-induced colitis (B. vulgatus) and patients with Crohn’s disease (S. faecum, E. coli, P. productus, E. contortum, and S. avium) and referred to as colitis-related flora, as previously described (39), or B. vulgatus only. Colonization of the transferred mice was achieved by placing soiled bedding in the mouse cages. Studies were approved by the North Carolina State University and University of North Carolina at Chapel Hill Institutional Animal Care and Use Committees.
Helicobacter PCR.
Fecal DNA was purified with the QIAamp Tissue Kit (Qiagen Inc., Chatsworth, Calif.) according to the manufacturer’s instructions and as previously described (2). Five microliters of the fecal DNA preparation was added to a PCR with Helicobacter-specific primers graciously provided by Richard Murray (DNAX). Purified Helicobacter DNA from pure cultures served as positive controls. PCR products were resolved and visualized on a 1.8% agarose gel stained with ethidium bromide. Fecal samples from SPF, germfree, and colitis-related-bacteria-colonized mice were tested in identical fashion.
Histopathology.
Sections of the stomach, duodenum, jejunum, ileum, and several regions of the large intestine (representing the cecum and proximal and distal colon) were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin for histologic scoring. Scoring was conducted in blinded fashion on a scale of 0 to 4 with 0 representing no inflammation and 4 representing severe inflammation characterized by widespread infiltration with inflammatory cells, distortion of architecture, and the presence of crypt abscesses and ulcers, as previously described and validated (39). The mean histologic colonic inflammatory score for each mouse was determined by adding the scores for each section of the large intestine examined (minimum of three sections per mouse) and dividing the total by the number of sections examined.
Lymphoid cell preparations and culture.
Mesenteric lymph nodes (MLN), which enlarge with colonic inflammation (8), were removed and single cell suspensions were prepared by gentle teasing. Cells were washed and resuspended in complete medium (RPMI 1640; Tissue Culture Facility, University of North Carolina Lineberger Cancer Center, Chapel Hill) supplemented with 5% heat-inactivated fetal calf serum (Irvine Scientific, Santa Ana, Calif.), 2 mM l-glutamine, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, 50 mg of gentamicin (Sigma, St. Louis, Mo.)/ml, and penicillin (100 U/ml)-streptomycin (100 mg/ml)-amphotericin B (0.25 mg/ml) (Gibco, Life Technologies, Grand Island, N.Y.).
Lymphoid cells were stimulated in vitro with immobilized anti-CD3. Cells were cultured at 106 cells/ml, 0.2 ml per culture, in 96-well flat-bottom plates (Corning Costar, Cambridge, Mass.) that were precoated with 10 μg of anti-CD3/ml (purified by protein A from supernatant of the hamster hybridoma 145-2C11) (28). In previous studies, no increased cellular proliferation or cytokine secretion was observed in cultures of cells stimulated with immobilized normal hamster immunoglobulin, thus ruling out nonspecific stimulation via Fc receptors rather than via CD3 ligation (52). Concanavalin A (Con A) (2 μg/ml; Sigma) and lipopolysaccharide (LPS) (25 μg/ml) (E. coli 055: B5) (Difco Laboratories, Detroit, Mich.) were used as polyclonal stimulators.
Lymphoid cell proliferation was measured by incorporation of [3H]thymidine for the final 18 h of a 90-h culture. Cells were cultured in triplicate with medium alone, immobilized anti-CD3, Con A, or LPS. For cytokine detection, duplicate supernatants of anti-CD3-stimulated cells were collected on day 3 and day 6 after culture initiation and stored at −20°C until being assayed.
Colon fragment cultures.
Cultures of colon fragments from segments of proximal, middle, and distal colon except the cecum were prepared following published methods (10). Briefly, colon segments were flushed with phosphate-buffered saline (PBS) to remove fecal contents, opened lengthwise, cut into 0.5- to 1-cm pieces, and shaken vigorously for 30 min in PBS. Tissue was then apportioned to wells (50 to 100 mg of tissue per well) of a 24-well tissue culture plate (Costar) in duplicate or triplicate, as the amount of tissue permitted, and cultured in 1 ml of complete medium containing antibiotics and the antimycotic agent as outlined above. The cultures were incubated at 37° for 18 h. Culture supernatants were collected and stored at −20°C until being assayed.
Lymphokine assays.
IL-12 p40 was measured by enzyme-linked immunosorbent assay (ELISA) with the commercially available antibodies C15.6 and biotinylated C17.8 (Pharmingen, San Diego, Calif.) for capture and detection, respectively, of the IL-12 p40 subunit. Bound antibodies were detected by using horseradish peroxidase (HRP)-labeled streptavidin (Kirkegaard and Perry Laboratories [KPL], Gaithersburg, Md.). Concentrations of IL-12 p40 were established by comparison of values against a standard curve generated for each assay with recombinant IL-12 p70 (Pharmingen). IL-12 p40 was measured in duplicate culture supernatants in each separate experiment.
Immunoglobulin isotype measurements.
Immunoglobulins (immunoglobulin A [IgA], IgG1, and IgG2a) elaborated into colon culture supernatants were measured by ELISA as previously described (51). Secreted immunoglobulins represented both newly synthesized and preformed proteins. Reagents for capture and detection of IgA, IgG1, and IgG2a were affinity purified unlabeled anti-IgA, anti-IgG1, and anti-IgG2a, and HRP-labeled polyclonal goat anti-IgA, anti-IgG1, and anti-IgG2a, respectively (Southern Biotechnology, Birmingham, Al.). Concentrations of immunoglobulins were determined by comparison with standard curves for purified mouse IgA, IgG1, and IgG2a (Pharmingen), respectively.
Flow cytometry.
Preparations of lymphocytes were incubated overnight at 4°C in culture medium alone or with either rat monoclonal anti-mouse CD44 (IM7.8.1) (53) or anti-mouse CD45RB (23G2) (4). Supernatants containing these antibodies were prepared in S. Tonkonogy’s laboratory from hybridoma cultures. For labeling of CD4 cells, cells were washed twice and incubated with 25 μg of fluorescein isothiocyanate (FITC)-labeled goat anti-rat IgG/ml, absorbed with mouse serum (KPL) for 30 min at 4°C, and then washed twice and incubated with Tricolor-labeled anti-CD4 (clone CT-CD4; Caltag, South San Francisco, Calif.). B cells were detected by first incubating the cells for 30 min at 4°C with 12.5 μg of FITC-labeled goat anti-mouse IgA-IgM-IgG (KPL)ml and then washing the cells twice before detection. Analyses were conducted using a FACScan (Becton Dickinson, San Jose, Calif.) flow cytometer.
Statistics.
Statistical comparison of group means was accomplished by performing one-way analysis of variance with a commercial software package (SigmaStat; Jandel Scientific, San Rafael, Calif.). Significance was set at a P value of <0.05, and differences among groups were identified by the Student-Newman-Keuls method. For nonnormally distributed data, comparison of median values was achieved with a Kruskal-Wallis one-way analysis of variance on ranks and group differences identified by Dunn’s multiple comparison procedure.
RESULTS
Normal resident luminal bacteria induce colitis in IL-10-deficient mice.
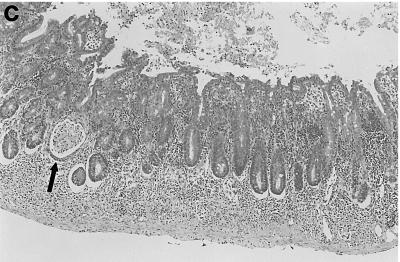
To determine the role of enteric bacterial flora in the pathogenesis of colitis in IL-10-deficient mice, mice were maintained in either an SPF or germfree environment. Consistent with previously reported results (3, 27), outbred IL-10-deficient mice reared in SPF conditions developed clinical signs of intestinal inflammation, as evidenced by weight loss and diarrhea beginning at 7 weeks of age. Some mice also developed rectal prolapse. Histologically, colitis was characterized primarily by mucosal hyperplasia, mononuclear infiltrates in the lamina propria, and loss of goblet cells and occasionally by crypt abscesses, crypt ulcers, and transmural inflammation (Fig. 1a). All regions of the colon were affected, confirming the observations of one earlier report (3) but not those of another in which inflammation was limited to the proximal colon (27). It should be noted that the distribution of colon lesions in a given mouse could be quite focal, with severely inflamed areas of mucosa adjacent to comparatively more mildly inflamed mucosa. As has been previously reported (3, 27), SPF IL-10-deficient mice had no evidence of small intestinal inflammation. Wild-type mice raised under SPF conditions had no clinical or histological evidence of gastroenteritis or colitis.
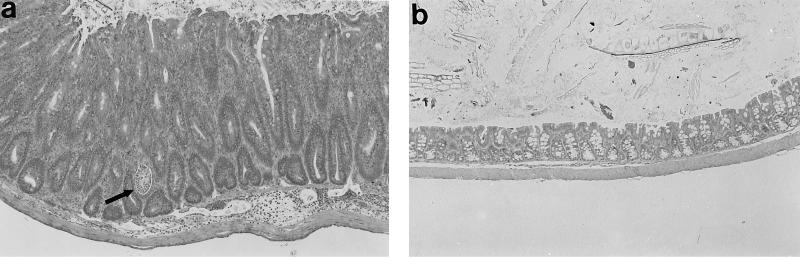
FIG. 1.
Resident enteric bacteria are required for colon inflammation in IL-10-deficient mice. Representative photomicrographs from the middle colon of (a) an IL-10-deficient SPF mouse, (b) an IL-10-deficient germfree mouse, and (c) a wild-type SPF mouse. Note the marked mucosal hyperplasia, lamina propria cellular infiltrates, and crypt abscess (arrow) shown in panel a that are absent in panels b and c. Magnification, ×58.
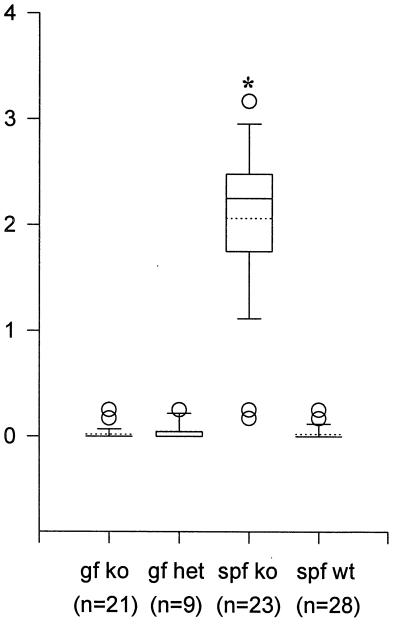
In contrast to IL-10-deficient mice reared under SPF conditions, germfree IL-10-deficient mice had no clinical or histological evidence of colitis (Fig. 1b) and were indistinguishable from wild-type SPF mice (Fig. 1c). Germfree IL-10-deficient mice were maintained for up to 6 months, and at all ages they were histologically indistinguishable from germfree wild-type mice. The mean histologic inflammatory score (Fig. 2) for IL-10-deficient SPF mice was 2.1, whereas for all other groups of mice, the mean histology scores were less than 0.5; the difference in histology scores between germfree and SPF IL-10-deficient mice was significant (P < 0.05). These results indicate that resident enteric bacterial flora are necessary for the spontaneous development of colitis in IL-10-deficient mice.
FIG. 2.
Mean colon inflammatory scores of germfree or SPF IL-10-deficient mice, SPF wild-type mice, and germfree heterozygote mice. Sections of colon representing at least three areas per mouse were scored in a blinded manner on a scale from 0 (no inflammation) to 4 (severe inflammation), and the scores were averaged to calculate mean scores. Median values, 25th and 75th percentiles (boxes), 10th and 90th percentiles (error bars), outlying values (circles), and means (dashed lines) are represented by box plots. A statistically different (P < 0.05) mean (from all other means) is indicated by the asterisk. gf, germfree; ko, IL-10 deficient; het, heterozygous; wt, wild type.
Colonization of previously germfree adult mice changes the pattern of large intestinal inflammation.
We noted that some of our IL-10-deficient mice born in an SPF environment were colonized by Helicobacter hepaticus, detected by PCR analysis of stool. Helicobacter sp. has been implicated as a cause of colitis in some immunodeficient mouse models of colitis (54). To assess whether intestinal inflammation would develop in the absence of Helicobacter infection and whether the age of the host affects the mucosal inflammatory response of a previously germfree animal when transferred to a microbial environment, adult germfree IL-10-deficient and heterozygous mice were transferred to an SPF environment free of Helicobacter. When 20- to 24-week-old IL-10-deficient adult mice were moved from germfree conditions to SPF conditions free of Helicobacter sp., the pattern of inflammation changed compared to that for mice moved into an SPF environment at weaning (3 weeks). The former group of mice exhibited more severe cecal inflammation following at least 5 weeks of bacterial colonization (Fig. 3; Table 1). With bacterial colonization of IL-10-deficient mice at adulthood, cecal ulceration and submucosal inflammation were common, whereas such inflammation was significantly less in IL-10-deficient mice that were weaned into an SPF environment, even one in which Helicobacter was present (Table 1). Adult IL-10-deficient mice moved from germfree to SPF conditions had inflammation in other regions of the colon, though colonic inflammation was not as severe as cecal inflammation (data not shown). These results suggest that the age of IL-10-deficient mice at the time of bacterial colonization profoundly affects cecal inflammation. In addition, these results demonstrate that Helicobacter organisms were not essential for the pathogenesis of colitis in these mice. Some germfree IL-10-deficient mice were housed with bedding contaminated with Helicobacter-infected stools but failed to become positive for Helicobacter as determined by PCR detection of DNA in the stool in the 4 weeks before sacrifice (data not shown).
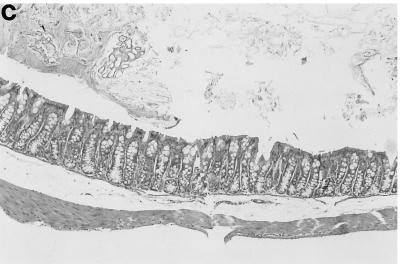
FIG. 3.
Cecal inflammation is more severe in germfree IL-10-deficient mice colonized as adults with a Helicobacter-negative SPF flora than in IL-10-deficient mice colonized at weaning. Representative histologies from (a) a wild-type SPF mouse, (b) an IL-10-deficient mouse (15 weeks old) maintained in SPF conditions since 3 weeks of age, (c) an IL-10-deficient mouse raised germfree and then moved to SPF conditions as an adult and sacrificed 5 to 7 weeks later are shown. Note the marked mucosal hyperplasia, transmural cellular infiltrates with aggressive submucosal inflammation, and crypt abscess (arrow) shown in panel c compared with the mild mucosal hyperplasia and infiltration limited to the lamina propria shown in panel b. Magnification, ×58.
TABLE 1.
Cecal histology scores for IL-10-deficient mice moved as adults from germfree conditions to Helicobacter-negative SPF conditions or introduced to an SPF environment as juveniles
| Mouse group | No. of mice examined | Age at colonization (weeks) | Duration of colonization (weeks) | Cecal scorea |
|---|---|---|---|---|
| IL-10 deficientb | 3 | 20–24 | 5 | 3.67 ± 0.33 |
| IL-10 deficient | 2 | 20–24 | 7 | 3.13 ± 0.125 |
| IL-10 deficient, raised SPF | 22 | 0c | 6–38 | 1.48 ± 0.17* |
Values are means ± standard errors of the mean. *, significantly different from other values (P < 0.05).
IL-10-deficient mice raised germfree and then colonized as adults with SPF bacteria documented to be free of Helicobacter.
Mice were colonized from birth.
Colonization with selected bacteria that cause colitis in other rodent models of colitis induces mild, delayed inflammation in IL-10-deficient mice.
To further explore the role of defined bacterial species in the development of spontaneous colitis in the IL-10-deficient mouse, adult (>20 weeks old) germfree mice were populated with six colitis-related bacterial species including B. vulgatus or were colonized with B. vulgatus alone. Colonization with these groups of bacteria has been previously shown to promote the development of spontaneous colitis in HLA-B27/β2 microglobulin transgenic rats (39). Colonization of the mice with these organisms was confirmed by examination of gram stains of fecal samples. In contrast to the severe inflammation observed in the IL-10-deficient mice after transfer of germfree adults to SPF conditions without Helicobacter, the germfree mice colonized with the colitis-related group of bacteria or B. vulgatus exhibited only mild inflammation (Table 2). Inflammation was not observed in the colitis-related flora group until the animals had been in this environment for 20 weeks. Even so, the inflammation observed in these mice was less severe than that observed in the germfree IL-10-deficient mice moved to SPF (without Helicobacter) at 5 weeks post-colonization. As observed in the mature mice moved from germfree to SPF conditions, mice moved from germfree to colitis-related flora conditions had more inflammation apparent in the cecum than other regions of the colon. Interestingly, two of these mice had overt gastric antral and duodenal inflammation not observed in the SPF colonized mice even at longer periods of colonization (data not shown). Adult mice populated with B. vulgatus for 32 weeks likewise developed only mild colonic inflammation (Table 2). These results show that hosts with different genetic abnormalities (IL-10 deficiency versus overexpression of HLA-B27 transgene) respond differently to the same defined bacterial stimuli.
TABLE 2.
Mean colon scores for adult IL-10-deficient mice populated with defined bacterial flora or SPF flora (without Helicobacter)a
| Flora(s) | Duration of colonization (weeks) | No. of mice examined | Colon scoreb |
|---|---|---|---|
| Six defined bacterial species | 5 | 4 | 0.39 ± 0.1 |
| 9 | 3 | 0.25 ± 0.2 | |
| 13 | 3 | 0.48 ± 0.09 | |
| 20 | 3 | 1.17 ± 0.4 | |
| B. vulgatus | 32 | 4 | 0.58 ± 0.14 |
| SPF (without Helicobacter) | 5–7 | 5 | 2.23 ± 0.18* |
Mice were colonized with a mixture of B. vulgatus, S. faecium (Group D), E. coli, P. productus, S. avium and Eubacterium contortum or B. vulgatus alone or were put into a Helicobacter-free SPF environment as described in Materials and Methods.
Values are means ± standard errors of the mean. Colon scores are for both the cecum and other colon regions. ∗, significantly different from all other scores (P < 0.05).
Germfree IL-10-deficient mice have no evidence of immunologic stimulation or activation.
To assess the degree of immunological stimulation and activation of mice with different bacterial stimuli, several parameters were evaluated. Total numbers of lymphocytes obtained from the MLN of germfree IL-10-deficient mice were comparable to those obtained from the MLN of germfree heterozygous mice (Table 3). In contrast, SPF IL-10-deficient mice had significantly greater numbers of MLN cells than did SPF wild-type mice, consistent with the presence of an inflammatory response in the colon. Of note, SPF wild-type mice had over twice the number of MLN cells compared with those of germfree heterozygotes, indicating stimulation of the mucosal immune system by resident bacteria. Phenotypic analysis of MLN cells by flow cytometry demonstrated that germfree IL-10-deficient mice had proportions of CD4 and CD8 T cells that were comparable to those of SPF wild-type mice (Table 3). In contrast, SPF knockout mice had lower proportions of CD4 cells than either germfree IL-10 knockouts or wild-type SPF and had greater proportions of CD8 cells than SPF wild-type mice (Table 3). Additionally, the levels of CD44, a lymphocyte homing receptor whose expression is higher on activated and memory T cells than on resting and naive T cells (1, 29), is higher on CD4+ MLN from SPF IL-10-deficient mice than on CD4+ MLN of SPF wild-type mice (Table 3).
TABLE 3.
Mean numbers of MLN cells and proportions of CD4+ and CD8+ T cells obtained from IL-10-deficient mice, wild-type mice, and heterozygous mice kept in either SPF or germfree conditionsa
| Mouse group | No. of MLN cells (106 [no. of mice]) | % CD4+ T cells (no. of mice) | % CD8+ T cells (median)b (no. of mice) | CD44 level (median)bc (no. of mice) |
|---|---|---|---|---|
| SPF wild type | 22.7 ± 2.2 (29) | 36.6 ± 2.2 (15) | 15.4 ± 0.8 (15.3) (15) | 88.6 ± 5.99 (82.5) (12) |
| SPF IL-10 deficient | 33.4 ± 4.0* (25) | 28.4 ± 1.8* (12) | 22.8 ± 1.9 (24.1)** (12) | 130.3 ± 10.14 (127.5)** (14) |
| Germfree heterozygote | 9.5 ± 2.1 (6) | 36.7 ± 2.1 (7) | 21.4 ± 1.7 (21.1) (7) | 101 ± 4.56 (100) (7) |
| Germfree IL-10 deficient | 8.9 ± 1.2 (12) | 35.1 ± 1.6 (10) | 18.4 ± 1.6 (17.9) (10) | 97.5 ± 2.23 (98) (11) |
Except where indicated values are means ± standard errors of the mean. *, significantly different mean value compared to those for SPF wild-type, germfree heterozygote, and germfree IL-10-deficient mice (P < 0.05); **, significantly different median value compared to that for SPF wild-type mice (P < 0.05).
Median values were used for statistical comparisons because of nonparametric data.
Fluorescence intensity units.
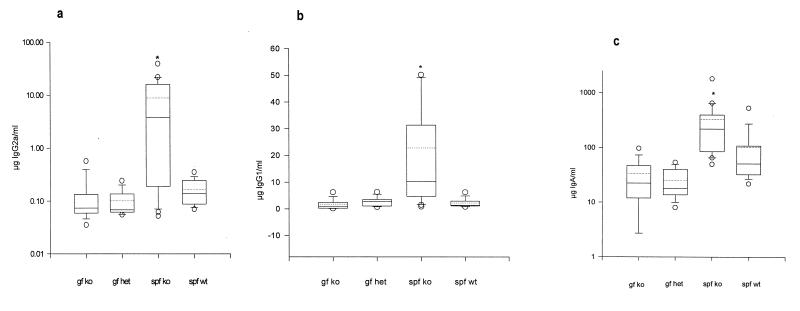
Similar to previous descriptions (3, 15), SPF IL-10 knockout mice with colitis had evidence of mucosal B-cell activation, as indicated by the elaboration of more IgA, IgG1, and IgG2a in supernatants of colon fragment cultures (Fig. 4) than those of wild-type mice without colitis. Similar patterns were observed in the sera of these groups of mice (data not shown). In contrast to the SPF mice, germfree IL-10-deficient mice had no significant differences in the concentrations of these immunoglobulins in colon fragment culture supernatants versus those of germfree and heterozygote SPF wild-type mice.
FIG. 4.
Mean immunoglobulin concentrations in supernatants of cultured colon fragments from mice with different bacterial stimulation. Segments of colon were minced and cultured, and supernatants were collected 18 h later for immunoglobulin quantitation by ELISA. All values are expressed on an equivalent weight basis. Median values, 25th and 75th percentiles (boxes), 10th and 90th percentiles (error bars), outlying values (circles), and means (dashed lines inside boxes) are represented by box plots. Statistically different medians (P < 0.05) (from all other medians in the respective panel) are indicated by asterisks (a) IgG2a concentrations; (b) IgG1 concentrations; (c) IgA concentrations. Abbreviations are the same as those in the legend for Fig. 2.
IL-10 plays an important role in the regulation of immune responses by inhibiting macrophage production of IL-12 (14, 47). We hypothesized that in the absence of IL-10, there would be more IL-12 produced by SPF IL-10-deficient mice than by other groups. Indeed, while the other groups of mice had essentially undetectable concentrations of IL-12 p40 in the colonic culture supernatants, IL-10-deficient SPF mice had elevated amounts of IL-12 p40 in the gut culture supernatants (Table 4). Though the level of the biologically active p70 heterodimer was not measured, these results support the possibility that, in the absence of IL-10, inflammation is driven by microbial stimulation of IL-12 production by activated macrophages. Furthermore, the combined data indicate that the absence of luminal bacterial stimulation is associated with no evidence of activation of the mucosal immune response, despite the lack of IL-10-mediated regulation.
TABLE 4.
Concentrations of IL-12 p40 subunit in supernatants of colon fragment cultures of IL-10-deficient mice wild-type mice, and heterozygous mice kept in either SPF or germfree conditions
| Mouse group | No. of mice examined | Mean IL-12 p40 concentration (pg/ml)a |
|---|---|---|
| SPF wild type | 23 | <1 |
| SPF IL-10 deficient | 25 | 1,037 ± 105* |
| Germfree heterozygote | 10 | <1 |
| Germfree IL-10 deficient | 21 | <1 |
*, significantly different from all other groups (P < 0.05); for statistical comparison, all other groups were assigned a mean value of 1.
DISCUSSION
IL-10 plays a crucial role in the modulation of immune responses and recently has been implicated as one of the cytokines mediating mucosal T-cell tolerance (17, 24) to bacterial antigens. Our results show that normal enteric bacteria are essential for the development of spontaneous colitis in IL-10-deficient mice since germfree IL-10-deficient mice had no histologically detectable colitis. In contrast, IL-10 knockout mice maintained in SPF environments had moderate colitis, comparable in character and severity to that of SPF IL-10-deficient mice from previous reports (3, 15, 27, 40). Unlike some previous descriptions in which inflammation was limited to the proximal colon of IL-10-deficient mice (27), inflammation in this study was found in all regions of the colon. Interestingly, severely inflamed areas could lie adjacent to nearly normal areas of mucosa, similar to the focal nature of the “skip lesions” of Crohn’s disease. Also, we present novel data regarding the absence of mucosal immune responses, including IL-12 p40 secretion, in the absence of normal luminal bacteria. These data also illustrate the importance of age at the time of bacterial colonization, the ability of Helicobacter-free bacteria to stimulate colitis in this model, and the different capacities of defined bacterial species to induce colitis in different genetically engineered rodents.
Germfree IL-10-deficient mice displayed none of the immune system activation observed in the SPF IL-10-deficient mice, indicating that subclinical colitis was not present in the germfree state. In the absence of viable bacteria, markers of T-cell activation and in vitro immunoglobulin secretion were not different from those of germfree heterozygote or SPF wild-type mice. Although IL-10-deficient SPF mice had increased concentrations of immunoglobulins in their colonic culture supernatants, B lymphocytes are not essential to the pathogenesis of colitis in IL-10-deficient mice (15). Therefore, elevated levels of circulating antibody and enhanced mucosal immunoglobulin production should be considered markers of immune system activation rather than effector molecules causing tissue damage. Recent observations that IL-10-deficient mice have anticolonic epithelial cell antibodies (15) thus may mean that these autoantibodies are a secondary result of epithelial damage in the setting of a dysregulated immune response. Moreover, the pattern of in vitro immunoglobulin secretion with abundant IgG2a in SPF IL-10-deficient mice is consistent with a Th1-gamma interferon (IFN-γ)-driven immune response (40, 49).
In keeping with a Th1 profile of cytokines, we found that IL-10-deficient mice with colitis had readily detectable amounts of IL-12 p40 in culture supernatants of inflamed colon fragments, presumably reflecting increases in biologically active IL-12 p70 heterodimer. It should be emphasized that these cultures had no in vitro stimuli to promote IL-12 production, suggesting that cells residing in the colon were activated in vivo to produce and secrete IL-12 p40. The production of IL-12 p40 mRNA and biologically active p70 IL-12 from macrophages or dendritic cells is upregulated in vitro in response to many stimuli. Live bacteria and bacterial products such as LPS and lipotechoic acid, as well as prokaryotic DNA, have all been demonstrated to increase production of IL-12 in vitro (9, 11, 22, 25, 26, 33, 48). Our results suggest that in vivo exposure to resident luminal bacterial components may likewise stimulate IL-12 production. IL-12 has recently been incriminated as a mediator of autoimmune disease induced by bacterial products (46) and loss of tolerance to resident luminal bacteria in experimental colitis (17). Furthermore, treatment with neutralizing antibodies to IL-12 prevents the development of colitis in IL-10-deficient mice if treatment is initiated at an early age before development of severe colitis (40), prevents the development of hapten-induced colitis in IL-2-deficient mice (18), and reverses established inflammation in trinitrobenzene sulfonic acid-treated mice (37). Because IL-10 promotes the development of Th2 responses by inhibiting macrophage production of IL-12 (14), it is not surprising that in the absence of IL-10, unregulated colonic production of IL-12 could occur in response to microbial stimulation.
An unexpected finding in this study was the increased severity of cecal inflammation observed in gnotobiotic IL-10-deficient mice that had been colonized with SPF bacteria as adults compared with those colonized at weaning with the same organisms. The difference in the intensity of inflammation may have been related to the age of the animals and the maturity of their mucosal immune system at the time of colonization. Not only is cecal inflammation more aggressive with submucosal inflammation and mucosal ulceration more common following colonization of older IL-10-deficient mice, but the onset of inflammation is more rapid (5 weeks) than in germfree IL-10-deficient mice colonized at weaning (8 to 12 weeks). The submucosal pattern of colitis is more aggressive than that seen in 3-month-old HLA-B27 transgenic rats colonized with SPF bacteria, which developed cecal inflammation confined to the mucosa which was not significantly worse than colitis in B27 transgenic rats born in an SPF environment (39).
H. hepaticus has been recently shown to be associated with cecal inflammation in several immunodeficient mouse strains and has been advanced as a putative cause of experimental colitis in genetically engineered mice (54). However, the development of aggressive typhlitis, and to a lesser degree colitis, in a Helicobacter-free environment does not support an essential role of Helicobacter in the development of colitis in IL-10-deficient mice. However, a potential contribution of Helicobacter infection in the pathogenesis of colitis in IL-10-deficient mice is not excluded. Our observations that resident bacteria in the absence of Helicobacter can cause colitis are particularly important given the widespread colonization of IL-10-deficient mice with this opportunistic pathogen (39a).
The combination of the six colitis-related bacteria used in the present study readily provoked colitis in HLA-B27 transgenic rats which approximated that seen with SPF bacteria (39). The observation that adult germfree IL-10-deficient mice colonized with these same colitis-related flora had delayed onset of very mild colitis compared to that of the germfree mice moved to SPF conditions suggests that all bacterial strains do not have equal capacity to induce intestinal inflammation. Importantly, the mild nature of colitis induced in these mice colonized with the six identical organisms which induce aggressive colitis in the HLA-B27 transgenic rat further supports the notion that other factors, in particular the host genetic background, play a critical role in the development of experimental colitis in response to any given bacterial stimulus. Indeed, inbred C57BL/6 mice with the IL-10 deletion develop mild, delayed-onset colitis in striking contrast to the early and aggressive colitis of IL-10-deficient mice of the 129 Sv background (3). Similarly, we did not observe colonic adenocarcinomas in either the SPF or germfree IL-10-deficient outbred (C57BL/6 × 129) mice followed for up to 38 weeks of age, as were observed in 6-month-old 129 Sv mice (3). Alternatively to differences in genetic background, quantitative differences in the numbers of each bacterium (not measured in this study) may have influenced the development of inflammation, particularly if pro-inflammatory bacteria were limited in their proliferation by noninflammatory bacteria. Further studies will be necessary to determine the dominant enteric bacterial strains that induce or perpetuate colitis in IL-10-deficient mice.
These results point out the inflammatory consequences of unbalanced immune responses to resident nonpathogenic bacteria but do not completely explain the mechanisms by which inflammation is mediated. Possible unregulated mucosal production of IL-12 as suggested by our results could result in excess production of IFN-γ, a proinflammatory Th1 cytokine known to alter epithelial barrier integrity in vitro. Preliminary observations with IL-10-deficient mice suggest that even in the germfree state these mice have lower colonic mucosal electrical resistance than do wild-type mice (46a). Recombinant IL-10 has been shown to prevent IFN-γ-mediated damage to epithelial barrier integrity in cultured T84 cells (31). It thus is possible that IL-10-deficient mice have inherent defects in mucosal barrier function that allow endogenous luminal bacteria and/or bacterial products to induce colonic inflammation. Alternatively, IL-10-deficient mucosal macrophages exposed to bacterial antigens could have overly aggressive cytokine production and antigen-presenting activities in the absence of immunosuppressive IL-10, perhaps resulting in activation of T lymphocytes to endogenous bacterial antigens. Exacerbations of pathologic immune responses have been described in IL-10-deficient mice infected with Trypanosoma cruzi (23) or Toxoplasma gondii (21). These results have been attributed to an intrinsic lack of down regulation of Th1 immune responses, since administration of exogenous recombinant IL-10 or neutralizing anti-IL-12 antibodies abrogates pathologic responses in these models and disease in the infected mice is not attributable to uncontrolled proliferation of the infecting organism. Lastly, an unbalanced cytokine environment could alter antigen-presenting cell function (13) to lead to immune responses to normally nonimmunogenic antigens. Applying these observations to resident luminal bacteria, we speculate that in the genetically susceptible host, a dysregulated immune response could establish the cytokine milieu which promotes pathologic responses to normally nonpathogenic luminal bacterial antigens.
REFERENCES
- 1.Aruffo A, Stamenkovic I, Melnick M, Underhill C B, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 2.Beckwith C S, Franklin C L, Hook R R, Jr, Besch-Williford C L, Riley L K. Fecal PCR assay for diagnosis of Helicobacter infection in laboratory rodents. J Clin Microbiol. 1997;35:1620–1623. doi: 10.1128/jcm.35.6.1620-1623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg D J, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach M W, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Investig. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkland M L, Johnson P, Trowbridge I S, Pure E. Changes in CD45 isoform expression accompany antigen-induced murine T cell activation. Proc Natl Acad Sci USA. 1989;86:6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjarnason I, Hayllar J, Smethurst P, Price A, Gumpel M J. Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropahy. Gut. 1992;33:1204–1208. doi: 10.1136/gut.33.9.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-γ production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 7.Cahn S H, Perrussia B, Gupta J W, Kobayashi M, Pospisil M, Young H A, Wolf S F, Young D, Clark S C, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter P B, Collins F M. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella M, Scheidegger D, Plamer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke S J, Stokes C R. The intestinal and serum humoral immune response to systematically and orally administered antigens in liposomes. I. The response to liposome-entrapped soluble proteins. Vet Immunol Immunopathol. 1992;32:125–138. doi: 10.1016/0165-2427(92)90074-z. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipotechoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD-14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contractor N V, Bassiri H, Reya T, Park A Y, Baumgart D C, Wasik M A, Emerson S G, Carding S R. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1997;160:385–394. [PubMed] [Google Scholar]
- 13.Cua D J, Coffman R L, Stohlman S A. Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J Immunol. 1996;157:2830–2836. [PubMed] [Google Scholar]
- 14.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin-10 inhibits human lymphocyte IFN-γ production by suppressing natural killer cell stimulatory factor/interleukin-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson N J, Leach M W, Fort M M, Thompson-Snipes L, Kühn R, Müller W, Berg D J, Rennick D M. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dianda L, Hanby A M, Wright N A, Sebesteny A, Hayday A C, Owen M J. T cell receptor-αβ-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 17.Duchmann R, Schmitt E, Knolle P, Meyer zum Büschenfelde K H, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 18.Ehrhardt R O, Ludviksson B R, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2 deficient mice. J Immunol. 1997;158:566–573. [PubMed] [Google Scholar]
- 19.Fiorentino D F, Zlotnik A, Mossman T R, Howard M H, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [Google Scholar]
- 20.Fiorentino D F, Zlotnik A, Vieira P, Mossman T R, Howard M, Moore K W, O’Garra A. IL-10 acts on the antigen presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 21.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 22.Halpern M D, Kurlander R J, Pisetsky D S. Bacterial DNA induced murine interferon-γ production by stimulation of interleukin-12 and tumor necrosis factor-α. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 23.Hunter C A, Ellis-Neyes L A, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 24.Khoo U Y, Proctor I E, Macpherson A J. CD4+ T cell down-regulation in human intestinal mucosa. J Immunol. 1997;158:3626–3634. [PubMed] [Google Scholar]
- 25.Kincy-Kain T, Bost K L. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- 26.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kämpgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 28.Leo O, Foo M, Sachs D, Samelson L, Bluestone J. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1986;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald H R, Budd R C, Cerottini J-C. Pgp-1 (Ly24) as a marker of murine memory T lymphocytes. Curr Top Microbiol Immunol. 1990;159:97–109. doi: 10.1007/978-3-642-75244-5_6. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald T T, Carter P B. Contact sensitivity in germfree mice. J Reticuloendothel Soc. 1978;24:287–293. [PubMed] [Google Scholar]
- 31.Madsen K L, Lewis S A, Tavernini M M, Hibbard J, Fedorak R N. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 32.McCall R D, Haskill S, Zimmerman E M, Lund P K, Thompson R C, Sartor R B. Tissue IL-1 and IL-1 receptor antagonist expression in rat strains variably susceptible to granulomatous enterocolitis. Gastroenterology. 1994;106:960–972. doi: 10.1016/0016-5085(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 33.Miller M A, Skeen M J, Ziegler H K. Non-viable bacterial antigens administered with IL-12 generate antigen-specific T cell responses and protective immunity against Listeria monocytogenes. J Immunol. 1995;155:4817–4828. [PubMed] [Google Scholar]
- 34.Moore K W, O’Garra A, deWaal Malefyt R, Vieira P, Mossman T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 35.Morrissey P J, Charrier K. Induction of wasting disease in SCID mice by the transfer of normal CD4+/CD45RBhi T cells and the regulation of this autoreactivity by CD4+/CD45RBlo T cells. Res Immunol. 1994;145:357–362. doi: 10.1016/s0923-2494(94)80200-9. [DOI] [PubMed] [Google Scholar]
- 36.Neissner M, Volk B A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neurath M F, Fuss I, Kelsall B L, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onderdonk A B, Franklin M L, Cisneros R L. Production of experimental ulcerative colitis in gnotobiotic guinea pigs with simplified microflora. Infect Immun. 1981;32:225–231. doi: 10.1128/iai.32.1.225-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rath H C, Herfarth H H, Ikeda J S, Grenther W B, Hamm T E, Balish E, Taurog J D, Hammer R E, Wilson K H, Sartor R B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis and arthritis in HLA-B27/human β2 microglobulin transgenic rats. J Clin Investig. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.Rennick, D. Unpublished observation.
- 40.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/− mice: an overview. J Leukocyte Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 41.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 42.Sartor R B. Treating IBD by altering luminal contents: rationale and response. In: Rachmilewitz D, editor. Inflammatory bowel diseases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 177–191. [Google Scholar]
- 43.Sartor R B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 44.Schreiber S, Heinig T, Thiele H-G, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 45.Schultz M, Sellon R K, Tonkonogy S L, Balish E, Sartor R B. IL-2 deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation and progressive loss of B cells. Gastroenterology. 1997;112:A1086. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- 46.Segal B M, Klinman D M, Shevach E M. Microbial products induce autoimmune disease by an IL-12-dependent pathway. J Immunol. 1997;158:5087–5090. [PubMed] [Google Scholar]
- 46a.Sellon, R. K., and A. Blikslager. Unpublished data.
- 47.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. Regulation of murine macrophage IL-12 production. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 48.Skeen M J, Ziegler H K. Activation of γδ T cells for production of IFN-γ is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 49.Snapper C M, Peschel C, Paul W E. IFN-γ stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 50.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhous W A, Fernandez-Sueiro J L, Balish E, Hammer R E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tonkonogy S L, Sartor R B. Immune system activation in C3H/HeJBir mice exhibiting spontaneous perianal ulceration. Inflamm Bowel Dis. 1997;3:10–19. [PubMed] [Google Scholar]
- 52.Tonkonogy S L, Swain S L. Distinct lymphokine production by CD4+ T cells isolated from mucosal and systemic lymphoid organs. Immunology. 1993;80:574–580. [PMC free article] [PubMed] [Google Scholar]
- 53.Trobridge I S, Lesley J, Schulte R, Hyman R, Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell-surface glycoprotein on lymphoid cells. Immunogenetics. 1982;15:299–312. doi: 10.1007/BF00364338. [DOI] [PubMed] [Google Scholar]
- 54.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 55.Yamada T, Sartor R B, Marshal S, Specian R D, Grisham M B. Mechanisms of acute and chronic inflammation induced by indomethacin. Inflammation. 1993;17:641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]